Abstract
Objective
In previous studies, we have shown a three to four times higher urban incidence of breast cancer and estrogen receptor-positive breast cancers in the Gharbiah Province of Egypt. We investigated the urban–rural incidence differences of gynaecologic malignancies (uterine, ovarian and cervical cancers) to explore if they show the same trend that we found for breast cancer.
Design
Cancer registry-based incidence comparison.
Setting
Gharbiah population-based cancer registry (GPCR), Tanta, Egypt.
Sample
All patients with uterine, ovarian and cervical cancer in GPCR from 1999 to 2002.
Methods
We calculated uterine, ovarian and cervical cancer incidence from 1999 to 2002. For each of the three cancers, we calculated the overall and age-specific rates for the province as a whole, and by urban–rural status, as well as for the eight districts of the province.
Results
Incidence of all three cancer sites was higher in urban than in rural areas. Uterine cancer showed the highest urban–rural incidence rate ratio (IRR = 6.07, 95% CI = 4.17, 8.85). Uterine cancer also showed the highest urban incidence in the oldest age group (70+ age category, IRR = 14.39, 95% CI = 4.24, 48.87) and in developed districts (Tanta, IRR = 4.14, 95% CI = 0.41, 42.04). Incidence rates by groups of cancer sites showed an increasing gradient of urban incidence for cancers related to hormonal aetiology, mainly of the breast and uterus (IRR = 4.96, 95% CI = 2.86, 8.61).
Conclusions
The higher urban incidence of uterine cancer, coupled with our previous findings of higher incidence of breast cancer and estrogen receptor positive breast cancer in urban areas in this region, may be suggestive of possible higher exposure to environmental estrogenic compounds, such as xenoestrogens, in urban areas.
Keywords: Egypt, gynaecologic cancers, urban–rural, xenoestrogens
Introduction
Malignancies specific to female organs, such as those of breast, uterus, and ovary, tend to have a hormone-related aetiology.1–3 Reproductive risk factors that increase the exposure of women to higher levels of endogenous estrogens seemingly lead to an increased risk of such cancers.1–3 The malignancies of these three organs also have higher incidence rates in more affluent or developed countries compared with the developing world.4 However, among these three organ sites, breast cancer is the most common cancer with the highest incidence in most populations across the world.4,5 This difference in incidence between various organ sites may be the result of differences in tissue structure of the organs and their anatomical site, and/or physiological function, which translates into differences in exposure.
Cervical cancer is also a malignancy that is specific to women, but has a risk profile and epidemiology quite unlike that of cancers of the breast, ovary, or uterus. Cervical cancer has mainly an infectious aetiology, and the human papillomavirus (HPV) is found to be implicated in most cases of cervical cancer.6 As is true for most infectious diseases, cervical cancer has a higher incidence in developing and more tropical countries.
Within developing countries, urban areas tend to be more affluent and developed compared with rural areas. This difference in development and industrialisation translates into differences in exposure to certain man-made chemicals called xenoestrogens that have been shown to act like natural hormones within the body, and have been implicated in numerous in vitro, animal, and human studies to increase the risk of breast cancer.7 Numerous studies across the world have shown that xenoestrogen presence and exposure is higher in urban areas of the world.8–14 Over the past several years we have explored the differences between developed and developing populations, with a special focus on Egypt, where distinct differences between urban and rural areas exist15,16 and may provide a unique setting for investigating the association between development and urbanisation, and differences in cancer incidence and distribution.
We have already published our hypotheses regarding the probable association between xenoestrogens and breast cancer,17 and in our previous studies in Gharbiah, Egypt, we also found a three to four times higher incidence of breast cancer and estrogen receptor positive (ER+) breast tumours in urban areas than in rural areas.18 Because of the hormonal aetiology of breast cancer and the likelihood that populations in urban areas might be exposed to xenoestogenic compounds, as shown in urban areas of other countries,8–14 we hypothesised that the incidence of other gynaecological malignancies such as those of the uterus and ovary must be higher in urban populations. At the same time, higher xenoestrogen exposure must not have any effect on creating urban–rural differences for cervical cancer, which does not have a hormonal aetiology. Thus, we examined the hypothesis that the incidence of uterine and ovarian cancer is higher in urban areas as compared with rural areas, whereas the incidence of cervical cancer is not significantly different between urban and rural areas in Gharbiah, Egypt. For these purposes we analysed the data from the population-based Gharbiah Cancer Registry for the 4-year period of 1999–2002 to assess differences in urban–rural incidence of uterine, ovarian and cervical cancers.
Methods
The methods of this study are similar to the methods published previously.18 Here we have provided the methods specific to this study in brief.
Study population
The study population consisted of all women diagnosed with uterine, ovarian and cervical cancer from 1999 to 2002, a total period of four years, in the Gharbiah population-based cancer registry (GPCR), Tanta, Egypt. The registry number, age at diagnosis, address, address code, smoking status, occupation, basis of diagnosis, tumour grade, stage, morphology, medical record number and place of reference of each woman were abstracted from routinely collected registry data.
Gharbiah population-based cancer registry
The Gharbiah population-based cancer registry was founded in 1998 as a part of the Middle East Cancer Consortium (MECC), and is located in Tanta, the capital of Gharbiah Province.19 This is an active registry and it collects cases from a number of sources in the governorate to determine cancer incidence. Most of the cancer cases for this study came from Tanta Cancer Center (40–50%) and Gharbiah Cancer Society (20–25%). The remaining cases came from pathology laboratories (10%), Mansoura Radiotherapy Hospital (3–4%), insurance hospitals (4–5%), National Cancer Institute (NCI), Cairo (2–3%) and mortality records (4–5%). Most of the cases were diagnosed by pathological confirmation.20 The WHO ICD-9 coding is used to determine the types of cancer. Cases were registered with Surveillance Epidemiology and End Results (SEER) staging information from 1999–2002, although all available records for patients from 1999 to 2002 was retrieved, and previous SEER staging was replaced by American Joint Committee on Cancer (AJCC) staging.
Gharbiah Province
Gharbiah Province is an administrative region located 90 km north of Cairo in the Nile Delta Region. It has eight districts, with Tanta being the capital of Tanta district as well as of the entire governorate. Gharbiah has a population of more than 4 million people, and 49% of them are women. Approximately 30% of the population resides in urban areas and almost 47% of the female population is below the age of 20, according to the 2006 Central Agency for Public Mobilization and Statistics (CAPMAS) census.21 Most of the residents residing in rural areas are part of an agricultural economy, but most people living in cities participate in industrial occupations, with most of the industries located in the two largest districts of Tanta and El Mehalla.
Census data
Census data for the female population in Gharbiah was obtained from the 1996 and 2006 CAPMAS census,21 and a constant growth of the population was assumed in order to project populations for the years in between using a linear regression model. The linear growth rates of eight districts were applied to the urban and rural populations within those districts to determine the urban and rural populations from 1999 to 2002. The census data consisted of 16 age categories at 5-year intervals. Six age categories were created from these by collapsing the age categories below 29 years, and by collapsing the age categories into 10-year intervals after that. These population figures formed the denominators to calculate the overall, age-specific, district-specific and urban–rural incidence rates for uterine, ovarian and cervical cancer in women.
Urban–rural classification
The urban–rural classification followed the CAPMAS coding of urban and rural areas. Urban areas consisted of all the capital cities of the eight districts of the governorate, whereas the remaining areas in the governorate were considered to be rural. Each case in the registry is assigned a residence code based on their residential address that follows the CAPMAS coding. This code was used to classify patients as being either urban or rural.
Statistical analyses
Descriptive statistics and rate analyses were completed using SAS 9.1.3 (SAS Institute, Cary, NC, USA). Univariate analyses were used to develop a descriptive profile using demographic and geographical indicators. Yearly raw and age-adjusted incidence rates for uterine, ovarian and cervical cancer were calculated for Gharbiah governorate, each of the eight districts, and urban and rural areas for the governorate and each district. Age-specific rates, overall and urban–rural, were calculated for each of six age categories. Raw incidence rates were calculated by taking the number of cases per year (1999–2002) divided by the person-year estimates for 1999–2002. Direct age-adjusted incidence rates were calculated by direct age-standardization for each district, and their urban and rural areas, using the world population as the standard.4 We also compared world age-standardised overall and urban–rural incidence rates with SEER incidence rates from the USA. Incidence rate ratios (IRRs) and P-values for the trends were calculated using negative binomial regression by the GENMOD procedure in SAS. Age, histology and stage at diagnosis could have been potential confounding factors. However, histology was uniform in distribution across urban–rural strata, and stage at diagnosis did not affect IRRs by more than 10%. Therefore, we have reported age-standardised IRRs and 95% confidence intervals (CIs).
As additional analyses following our initial results, we also compared urban–rural incidence of female leukaemia (a cancer with mostly genetic and some environmental aetiology, which will thus be most likely to have the least differences between urban and rural populations), all female cancers except breast and uterine cancer (two cancers with maximal links to hormonal risk factors in addition to other factors), all female cancers (including breast and uterine cancer), and hormonal cancers (breast and uterus).
Results
The number of cases was highest for ovarian cancer, followed by uterine and cervical cancer, respectively (Table 1). The numbers of cases of ovarian and uterine cancers were fairly constant across the years 1999–2002. There was some variation seen in the number of cervical cancer cases, with only 13 cases seen in 2001, but with 38 cases registered in 2000. For most organ sites and for most ages, the number of urban cases was higher, except for ovarian cancer (1999 and 0–29 age category). Among districts, most cases for all cancer sites came from Tanta, the largest district. Most of the cases were diagnosed microscopically.
Table 1.
Characteristics of women with uterine, ovarian and cervical cancer by urban–rural status in Gharbiah, Egypt, from 1999 to 2002
| Variable | Descriptive Category | Uterus
|
Ovary
|
Cervix
|
|||
|---|---|---|---|---|---|---|---|
| Urban No. (%) | Rural No. (%) | Urban No. (%) | Rural No. (%) | Urban No. (%) | Rural No. (%) | ||
| Total cases | 101 (73.19) | 37 (26.81) | 148 (53.62) | 128 (46.38) | 60 (58.25) | 43 (41.75) | |
| Year of diagnosis | 1999 | 17 (62.96) | 10 (37.04) | 29 (46.03) | 34 (53.97) | 15 (53.57) | 13 (46.43) |
| 2000 | 27 (72.97) | 10 (27.03) | 34 (55.74) | 27 (44.26) | 25 (65.79) | 13 (34.21) | |
| 2001 | 24 (68.57) | 9 (25.71) | 38 (50.67) | 37 (49.33) | 9 (56.25) | 7 (43.75) | |
| 2002 | 33 (80.49) | 8 (19.51) | 47 (61.04) | 30 (38.96) | 11 (52.38) | 10 (47.62) | |
| Age | 0–29 | 1 (100) | 0 | 16 (45.71) | 19 (54.29) | 1 (100) | 0 |
| 30–39 | 4 (100) | 0 | 26 (63.41) | 15 (48.39) | 5 (62.50) | 3 (37.50) | |
| 40–49 | 16 (66.67) | 8 (33.33) | 33 (55.00) | 27 (45.00) | 11 (50.00) | 11 (50.00) | |
| 50–59 | 30 (68.18) | 14 (31.82) | 34 (47.22) | 38 (52.78) | 18 (60.00) | 12 (40.00) | |
| 60–69 | 32 (72.73) | 12 (27.27) | 26 (55.32) | 21 (44.68) | 20 (64.52) | 11 (35.48) | |
| 70+ | 18 (85.71) | 3 (14.29) | 13 (61.91) | 8 (38.10) | 5 (45.46) | 6 (54.54) | |
| District | Tanta | 51 (80.95) | 12 (19.05) | 67 (62.62) | 40 (37.38) | 30 (69.77) | 13 (30.23) |
| El-Mehalla | 25 (71.43) | 10 (28.57) | 39 (59.09) | 27 (40.91) | 19 (73.08) | 7 (26.92) | |
| Kafr El-Zayat | 6 (66.67) | 3 (33.33) | 10 (52.63) | 9 (47.37) | 3 (50) | 3 (50) | |
| Zefta | 8 (66.67) | 4 (33.33) | 10 (58.82) | 7 (41.18) | 1 (25) | 3 (75) | |
| Samanoud | 2 (100) | 0 | 5 (41.67) | 7 (58.33) | 2 (28.57) | 5 (71.43) | |
| El Santa | 3 (60) | 2 (40) | 7 (25.93) | 20 (74.07) | 2 (28.57) | 5 (71.43) | |
| Kotoor | 3 (37.5) | 5 (62.5) | 3 (30) | 7 (70) | 2 (25) | 6 (75) | |
| Basyoon | 3 (75) | 1 (25) | 7 (38.89) | 11 (61.11) | 1 (100) | 0 | |
| Basis of diagnosis | Microscopic | 90 (72) | 35 (28) | 132 (54.1) | 112 (45.9) | 59 (58.42) | 42 (41.58) |
| Non-microscopic | 1 (100) | 0 | 7 (30.44) | 16 (69.56) | 1 (100) | 0 | |
| Death certificate | 10 (83.33) | 2 (16.67) | 9 (100) | 0 | 0 | 1 (100) | |
Crude incidence per 100 000 women for all three cancers was low, with cervical cancer having the lowest incidence (uterus, 1.91; ovary, 3.83; cervix, 1.43) (Table 2). However, the urban incidence of all three cancers was higher than the rural incidence – the highest difference being seen for uterine cancer (IRR = 6.07, 95% CI = 4.17, 8.85). Age-standardised rates for all cancer sites were much lower than US SEER (white) rates for all three cancer sites.
Table 2.
Overall, urban and rural incidence, crude and age-standardised to the world population, and urban–rural incidence rate ratios for uterine, ovarian and cervical cancer in Gharbiah, Egypt
| Organs | Incidence (per 100 000 women) and incidence rate ratios
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude* | Crude urban | Crude rural | IRR (95% CI) | ASW | ASW urban | ASW rural | ASW IRR | ASW US SEER (white) | |
| Uterus | 1.91 | 4.52 | 0.74 | 6.07 (4.17, 8.85) | 2.94 | 6.63 | 1.17 | 5.68 | 18.4 |
| Ovary | 3.83 | 6.62 | 2.57 | 2.57 (2.03, 3.26) | 5.02 | 8.15 | 3.50 | 2.33 | 13.2 |
| Cervix | 1.43 | 2.68 | 0.86 | 3.11 (2.10, 4.59) | 2.09 | 3.68 | 1.31 | 2.80 | 6.8 |
ASW, age-standardised to the world population; IRR, incidence rate ratio.
Crude incidence rate.
The overall age-specific incidence of the three cancers shows that these are diseases of old age, and that the incidence increases with increasing age (Table 3). A peak in incidence was seen for the age group of 50–59 years for ovarian and 60–69 years for uterine and cervical cancers. A comparison of age-specific urban and rural incidence showed some interesting features for all three cancers. For all cancer sites, urban incidence was higher than rural incidence from an early age, and urban incidence kept increasing with age, with the highest incidence seen in the 70+ age category for urban areas, except for cervical cancer. Rural incidence, however, peaked at 60–69 years and then declined. The differences in incidence between urban and rural areas were much wider for uterine cancer in most age groups compared with the other two cancer sites, with the highest difference seen in the 70+ age group (IRR = 14.39, 95% CI = 4.24, 48.87).
Table 3.
Overall and urban–rural age-specific incidence rates*, and urban-rural incidence rate ratios of uterine, ovarian and cervical cancers in Gharbiah, Egypt
| Age groups | Uterus
|
Ovary
|
Cervix
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Urban | Rural | IRR (95% CI) | Overall | Urban | Rural | IRR (95% CI) | Overall | Urban | Rural | IRR (95% CI) | |
| 0–29 | 0.02 | 0.07 | 0.00 | – | 0.77 | 1.19 | 0.59 | 2.02 (1.04, 3.93) | 0.02 | 0.07 | 0.00 | – |
| 30–39 | 0.39 | 1.18 | 0.00 | – | 4.03 | 7.66 | 2.21 | 3.47 (1.84, 6.55) | 0.79 | 1.47 | 0.44 | 3.33 (0.80, 13.95) |
| 40–49 | 3.25 | 5.95 | 1.70 | 3.50 (1.50, 8.17) | 8.12 | 12.28 | 5.75 | 2.14 (1.29, 3.55) | 2.98 | 4.09 | 2.34 | 1.75 (0.76, 4.03) |
| 50–59 | 10.12 | 20.17 | 4.89 | 4.12 (2.19, 7.78) | 16.55 | 22.86 | 13.28 | 1.72 (1.08, 2.73) | 6.90 | 12.10 | 4.19 | 2.89 (1.39, 5.99) |
| 60–69 | 14.53 | 32.60 | 5.84 | 5.59 (2.88, 10.85) | 15.52 | 26.49 | 10.21 | 2.59 (1.46, 4.61) | 10.24 | 20.38 | 5.35 | 3.81 (1.83, 7.95) |
| 70+ | 14.01 | 40.81 | 2.84 | 14.39 (4.24, 48.87) | 14.01 | 29.47 | 7.56 | 3.90 (1.62, 9.41) | 7.34 | 11.34 | 5.67 | 2.00 (0.61, 6.55) |
IRR, incidence rate ratio.
All incidence rates are per 100 000 women.
Among the districts, Tanta had the highest incidence for all three cancer sites (Table 4). For ovarian cancer the incidence in Tanta was slightly higher compared with Basyoon (IRR = 1.56, 95% CI = 0.45, 5.49), whereas the incidence was almost similar in the other districts. For uterine (Figure 1) and cervical cancers, the incidence in Tanta was much higher compared with Basyoon (Uterus, IRR = 4.14, 95% CI = 0.41, 42.04; Cervix, IRR = 11.31, 95% CI = 0.15, 867.4) (Table 4). However, because of the very low number of cases these estimates had large standard errors, and incidence rates may not be too different between the districts.
Table 4.
Incidence rates* and incidence rate ratios of uterine, ovarian and cervical cancer by districts of Gharbiah, Egypt
| Districts | Uterus
|
Ovary
|
Cervix
|
|||
|---|---|---|---|---|---|---|
| Incidence | IRR (95% CI) | Incidence | IRR (95% CI) | Incidence | IRR (95% CI) | |
| Tanta | 3.68 | 4.14 (0.41, 42.04) | 6.25 | 1.56 (0.45, 5.49) | 2.51 | 11.31 (0.15, 867.37) |
| El-Mehalla | 1.89 | 2.12 (0.17, 26.46) | 3.56 | 0.89 (0.21, 3.71) | 1.40 | 6.31 (0.07, 555.26) |
| Kafr El-Zayat | 1.30 | 1.47 (0.10, 21.78) | 2.75 | 0.69 (0.15, 3.20) | 0.87 | 3.92 (0.04, 414.13) |
| Zefta | 1.55 | 1.75 (0.13, 23.73) | 2.20 | 0.55 (0.11, 2.85) | 0.52 | 2.33 (0.02, 336.7) |
| Samanoud | 0.37 | 0.42 (0.01, 19.27) | 2.23 | 0.56 (0.11, 2.87) | 1.30 | 5.85 (0.06, 527.23) |
| El Santa | 0.76 | 0.85 (0.04, 18.29) | 4.09 | 1.02 (0.26, 4.06) | 1.06 | 4.77 (0.05, 462.93) |
| Kotoor | 1.51 | 1.70 (0.12, 23.36) | 1.88 | 0.47 (0.08, 2.67) | 1.51 | 6.79 (0.08, 584.62) |
| Basyoon | 0.89 | 1.00 | 4.00 | 1.00 | 0.22 | 1.00 |
IRR, incidence rate ratio.
All incidence rates are per 100 000 women.
Figure 1.
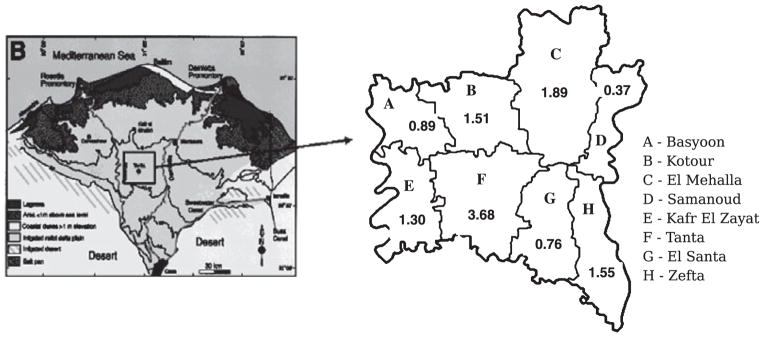
Location of the eight districts of Gharbiah in the Nile Delta Region of Egypt, and the incidence of uterine cancer (per 100 000 women) by district.
We also looked at female leukaemia and other female cancers in the groups to observe any gradients in terms of urban–rural differences in incidence in Gharbiah (Table 5). We found that leukaemia had the lowest urban–rural differences (overall IRR = 2.24, 95% CI = 0.55, 9.18), followed by all female cancers except those with the most pronounced hormonal aetiology (breast and uterus) (overall IRR = 2.81, 95% CI = 1.91, 4.14). Finally, when we included the cancer sites with hormonal aetiology, the urban–rural incidence difference increased further (overall IRR = 3.50, 95% CI = 2.56, 4.79). Looking at only hormonal cancer sites (breast and uterus), the urban–rural incidence was much higher than any other cancer group (overall IRR = 4.96, 95% CI = 2.86, 8.61).
Table 5.
Incidence rates* and incidence rate ratios of female cancers by groups in Gharbiah, Egypt
| Sites | 1999
|
2000
|
2001
|
2002
|
Overall
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urban | Rural | IRR | Urban | Rural | IRR | Urban | Rural | IRR | Urban | Rural | IRR | Urban | Rural | IRR (95% CI) | |
| Female leukaemia | 6.94 | 3.12 | 2.22 | 5.04 | 2.67 | 1.89 | 6.77 | 2.55 | 2.65 | 6.30 | 2.83 | 2.23 | 6.26 | 2.79 | 2.24 (0.55, 9.18) |
| All female cancers except breast and uterus | 96.98 | 34.44 | 2.82 | 96.67 | 33.53 | 2.88 | 96.91 | 36.25 | 2.67 | 103.71 | 35.91 | 2.89 | 98.61 | 35.05 | 2.81 (1.91, 4.14) |
| All female cancer sites | 175.70 | 48.83 | 3.60 | 170.48 | 47.54 | 3.59 | 171.03 | 53.25 | 3.21 | 185.91 | 51.24 | 3.63 | 175.84 | 50.24 | 3.50 (2.56, 4.79) |
| Breast and uterus | 75.25 | 14.39 | 5.23 | 72.73 | 14.09 | 5.16 | 71.62 | 16.61 | 4.31 | 81.15 | 15.56 | 5.22 | 75.22 | 15.17 | 4.96 (2.86, 8.61) |
IRR, incidence rate ratio.
All incidence rates are per 100 000 women.
Discussion
This study showed a higher incidence of uterine, ovarian and cervical cancers in urban areas compared with rural areas in the Gharbiah Province of Egypt. Furthermore, the most striking finding was the almost six times higher incidence of uterine cancer in urban areas than in rural areas of Gharbiah. We also found a gradient of increasing urban–rural difference for all female cancers. Cancers such as leukaemia with mainly genetic and some environmental risks (which will likely lead to minimal differences between urban and rural populations) had the lowest IRR, followed by the urban–rural IRR seen for female cancers excepting cancers with hormonal malignancies. On the inclusion of cancers with hormonal malignancies in the group analyses, the IRR increased by almost 70%. This urban–rural difference increased further by 146% when we looked at only hormonal cancers. In our previous studies we have found a three or four times higher incidence of breast cancer and ER+ breast cancer in urban areas of the Gharbiah Province.18 These urban–rural differences seen for breast cancer, in addition to a six times higher incidence of uterine cancer in urban areas, clearly show that women in urban areas experience a much higher exposure to hormonal risk factors of cancers.
In preparation for fertilisation, the uterus undergoes cyclical changes every month mainly under the influence of estrogen. Thus, the endometrium, which is rich in estrogen receptors, shows the highest proliferation rate during the first 18 days of the menstrual cycle.22 The ‘unopposed estrogens’ hypothesis (long-term exposure to estrogens, not counterbalanced by the presence of progesterone) is the most widely accepted hypothesis on the aetiology of endometrial cancer.22 Given the fact that urban and rural women in Egypt are genetically similar, and that most of the risk factors of uterine cancer are environmental, it can be inferred that urban women in Egypt have a higher exposure to environmental estrogens compared with rural women. It is clear from large surveys such as the Egyptian Demographic and Health Survey (EDHS) that in Egypt differences in reproductive factors are not substantial between urban and rural women.23 For example, the total fertility rate (TFR) for urban women and rural women is 2.7 and 3.0, respectively, in Lower Egypt (the area of Egypt in which Gharbiah is located).23 Also, as oral contraceptive use (which is protective for uterine cancer, and is most likely to be used by urban women) is quite low among Egyptian women,23 there are probably other environmental estrogenic factors that are leading to the higher urban incidence of uterine cancer.
The presence of and exposure to xenoestrogens are much higher in urban areas than in rural areas, a fact that has been seen in many populations across the world.8–14 Because of the high rate of development of the urban centres of Egypt, the exposure of women to xenoestrogens in urban Egypt might be high. There have been very few studies looking at the effect of xenoestrogens on uterine cancer in humans. However, animal studies show clearly that xenoestrogens are quite capable of causing uncontrolled uterine proliferation, usually through the same pathways by which endogenous estrogens act.24,25 There are more studies related to the link between breast cancer and xenoestrogens, and we have already hypothesised that the higher urban incidence of breast cancer is possibly a result of a higher exposure to xenoestrogens.17
Obesity is the other leading risk factor of uterine cancer worldwide, and has been known to explain 40% of endometrial cancer incidence.26 However, the differences between urban and rural women in terms of obesity are minimal, with urban and rural women having a mean body mass index (BMI) of 31.2 and 30.4, respectively, in Lower Egypt, according to EDHS.23 Also, the percentage of obese urban and rural women (as defined by a BMI ≥ 30) in Lower Egypt was 56.4 and 50.6%, respectively.23 Thus, differences in BMI cannot possibly explain the large urban–rural differences in uterine cancer incidence. It is also likely that uterine bleeding, the only way in which uterine cancer is detected, is much more easily detectable in urban areas. However, primary healthcare coverage in rural Egypt is 100%,27 and the remotest rural area in Gharbiah is not more than 50 km away from the capital city of the province. Thus, access to health care in Gharbiah is not too different between urban and rural areas. Also, the coverage of the Gharbiah registry is quite high, and given the multiple quality checks in the registry it is unlikely that many rural cases are missed.
Nevertheless, we saw around a two times higher incidence of ovarian cancer and leukaemia in urban areas, and a almost three times higher incidence of cervical cancer in urban areas in Gharbiah. Apart from differences in the urban–rural distribution of risk factors, it is likely that there might still be slight differences in healthcare access and behaviour between urban and rural areas responsible for the higher urban incidence of female cancers. In terms of aetiology, ovarian cancer is not really a hormonally related cancer, as it is not under direct stimulatory effects of estrogen. Ovarian cancer development is more related to risk factors that lead to chronic inflammation, related to ‘incessant ovulation’.28,29 Thus, the observation of a lack of any large urban–rural differences with regards to ovarian cancer is explainable. Cervical cancer on the other hand is a cancer that is much more closely related to sexual behaviour than other cancers.6 The detection of cervical cancer is also related to women’s access to gynaecological clinics, and as such a higher urban incidence is possible. However, cervical cancer has a very low incidence in Egypt, and given the low number of cases it is much more difficult to draw clear inferences regarding this site in the context of our study.
Overall, in this study we found an approximately six times higher incidence of uterine cancer in urban areas, and, in addition, the evidence from our recent studies showed an almost four times higher urban incidence of breast cancer and ER+ breast cancer.18 Thus, it is likely that women in urban areas have a higher exposure to environmental hormonal risk factors, possibly xenoestrogens. This is especially the case in light of there being no substantial differences between urban and rural women with regards to known risk factors of uterine and breast cancer. Xenoestrogens are a preventable cause of cancer, and more research at the individual level is required to clearly enumerate a possible association between xenoestrogens with uterine and breast cancers.
Acknowledgments
Funding
This work was supported by the Middle East Cancer Consortium, National Cancer Institute, Bethesda [R25 CA112383, R03 CA117350, 5 P30 CA46592], the Burroughs Wellcome Fund (SDM) and the Breast Cancer Research Foundation (SDM), a Block Grant of the Department of Epidemiology, University of Michigan School of Public Health and the Travel Grant of the Rackham Graduate School of the University of Michigan (S Dey).
We are grateful to Dr H Gad, Mr K Daboos and other personnel of Tanta Cancer Center and Gharbiah Cancer Society for the valuable assistance they provided for this project.
Footnotes
Disclosure of interests
The authors declare that they have no conflicts of interest associated with this manuscript.
Contribution to authorship
SD contributed to the study design, data collection, data analyses, writing and editing of the manuscript. AH, IAS, KI, MR and HEH contributed to the data collection, study execution and editing of the manuscript. MLW, PB, JH and SDM contributed to the editing of the manuscript. ASS contributed to the study design, study execution and editing of the manuscript.
Details of ethics approval
The use of human subject data was approved by the University of Michigan Institutional Review Board on 13 May 2009 (reference no. HUM00020758).
References
- 1.Honig SF. Incidence, trends and the epidemiology of breast cancer. In: Spear SL, Little JW, Lippman ME, Wood WC, editors. Surgery of the Breast: Principles and Art. Philadelphia: Lippincott-Raven; 1998. pp. 3–22. [Google Scholar]
- 2.La Vecchia C. Epidemiology of ovarian cancer: a summary review. Eur J Cancer Prev. 2001;10:125–9. doi: 10.1097/00008469-200104000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Purdy DM, Green AC. Epidemiology of endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2001;15:341–54. doi: 10.1053/beog.2000.0180. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer Incidence in Five Continents. VIII. Lyon: International Agency for Research on Cancer; 2002. [Google Scholar]
- 5.Boyle P, Levin B. World Cancer Report. Lyon: International Agency for Research on Cancer; 2008. [Google Scholar]
- 6.Castellsague X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol. 2008;110:S4–7. doi: 10.1016/j.ygyno.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 7.Evans N. State of Evidence. What is the Connection Between Environment and Breast Cancer? 2. San Francisco, CA: Breast Cancer Fund and Breast Cancer Action; 2006. [Google Scholar]
- 8.Gouin T, Jantunen L, Harner T, Blanchard P, Bidleman T. Spatial and temporal trends of chiral organochlorine signatures in Great Lakes air using passive air samplers. Environ Sci Technol. 2007;41:3877–83. doi: 10.1021/es063015r. [DOI] [PubMed] [Google Scholar]
- 9.Kitada Y, Kawahata H, Suzuki A, Oomori T. Distribution of pesticides and bisphenol A in sediments collected from rivers adjacent to coral reefs. Chemosphere. 2008;71:2082–90. doi: 10.1016/j.chemosphere.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Chai Z, Sun H. Human hair as a potential biomonitor for assessing persistent organic pollutants. Environ Int. 2007;33:685– 93. doi: 10.1016/j.envint.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Pentamwa P, Oanh NT. Levels of pesticides and polychlorinated biphenyls in selected homes in the Bangkok metropolitan region, Thailand. Ann N Y Acad Sci. 2008;1140:91–112. doi: 10.1196/annals.1454.005. [DOI] [PubMed] [Google Scholar]
- 12.Jafari A, Moeckel C, Jones KC. Spatial biomonitoring of persistent organic pollutants in Iran: a study using locally produced butter. J Environ Monit. 2008;10:861–6. doi: 10.1039/b802061b. [DOI] [PubMed] [Google Scholar]
- 13.Kumari B, Singh J, Singh S, Kathpal TS. Monitoring of butter and ghee (clarified butter fat) for pesticidal contamination from cotton belt of Haryana, India. Environ Monit Assess. 2005;105:111–20. doi: 10.1007/s10661-005-3159-2. [DOI] [PubMed] [Google Scholar]
- 14.Pant N, Shukla M, Kumar Patel D, Shukla Y, Mathur N, Kumar Gupta Y, et al. Correlation of phthalate exposures with semen quality. Toxicol Appl Pharmacol. 2008;231:112–6. doi: 10.1016/j.taap.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Soliman AS, Vulimiri SV, Kleiner HE, Shen J, Eissa S, Morad M, et al. High levels of oxidative DNA damage in lymphocyte DNA of premenopausal breast cancer patients from Egypt. Int J Environ Health Res. 2004;14:121–34. doi: 10.1080/0960312042000209534. [DOI] [PubMed] [Google Scholar]
- 16.Soliman AS, Wang X, DiGiovanni J, Eissa S, Morad M, Vulimiri S, et al. Serum organochlorine levels and history of lactation in Egypt. Environ Res. 2003;92:110–17. doi: 10.1016/s0013-9351(02)00056-7. [DOI] [PubMed] [Google Scholar]
- 17.Dey S, Soliman AS, Merajver SD. Xenoestrogens may be the cause of high and increasing rates of hormone receptor positive breast cancer in the world. Med Hypotheses. 2009;72:652–6. doi: 10.1016/j.mehy.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 18.Dey S, Soliman AS, Hablas A, Seifeldin IA, Ismail K, Ramadan M, et al. Urban–rural differences in breast cancer incidence by hormone receptor status across 6 years in Egypt. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0427-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freedman LS, Edwards BK, Ries LAG, Young JL, editors. Cancer Incidence in Four Member Countries (Cyprus, Egypt, Israel, and Jordan) of the Middle East Cancer Consortium (MECC) compared with US SEER. Bethesda, MD: National Cancer Institute; 2006. NIH Pub. No. 06-5873. [Google Scholar]
- 20.Ibrahim AS, Ismail K, Hablas A, Hussein H, Elhamzawy H, Ramadan M, editors. Cancer in Egypt, Gharbiah. Triennial Report of 2000–2002. Tanta, Egypt: Gharbiah Population-Based Cancer Registry; 2007. [Google Scholar]
- 21. [Accessed 17 November 2009];CAPMAS Reports. www.msrintranet.capmas.gov.eg/pls/fdl/tst12e?action=&lname=
- 22.Key TJ, Pike MC. The dose-effect relationship between ‘unopposed’ oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk. Br J Cancer. 1988;57:205– 12. doi: 10.1038/bjc.1988.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Zanaty F, Way A. Egypt Demographic and Health Survey 2005. Cairo, Egypt: Ministry of Health and Population, National Population Council, El-Zanaty and Associates, and ORC Macro; 2006. [Google Scholar]
- 24.Papaconstantinou AD, Umbreit TH, Fisher BR, Goering PL, Lappas NT, Brown KM. Bisphenol A-induced increase in uterine weight and alterations in uterine morphology in ovariectomized B6C3F1 mice: role of the estrogen receptor. Toxicol Sci. 2000;56:332–39. doi: 10.1093/toxsci/56.2.332. [DOI] [PubMed] [Google Scholar]
- 25.Goloubkova T, Ribeiro MFM, Rodrigues LP, Cecconello AL, Spritzer PM. Effects of xenoestrogen bisphenol A on uterine and pituitary weight, serum prolactin levels and immunoreactive prolactin cells in ovariectomized Wistar rats. Arch Toxicol. 2000;74:92–8. doi: 10.1007/s002040050658. [DOI] [PubMed] [Google Scholar]
- 26.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11:1531–43. [PubMed] [Google Scholar]
- 27.EMRO. World Health Organization Country Profiles. Egypt: [Accessed: 18 September 2008]. www.emro.who.int/emrinfo/index.asp?Ctry=egy. [Google Scholar]
- 28.Fathalla MF. Factors in the causation and incidence of ovarian cancer. Obstet Gynecol Surv. 1972;27:751–68. doi: 10.1097/00006254-197211000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Casagrande JT, Louie EW, Pike MC, Roy S, Ross RK, Henderson BE. ‘Incessant ovulation’ and ovarian cancer. Lancet. 1979;2:170–3. doi: 10.1016/s0140-6736(79)91435-1. [DOI] [PubMed] [Google Scholar]


