Abstract
Ex vivo gene therapy using stem cells transduced with viral vectors is a useful method for delivering a therapeutic protein to augment bone repair in animal models. However, the duration of cell-mediated protein production and the fate of the transduced cells are unknown. We constructed an adenoviral vector encoding Myc epitope tagged bone morphogenetic protein (BMP)-2 gene (AdBMP-2Myc). Rat bone marrow cells transduced with this vector produced biologically active BMP-2 protein, which was confirmed by Western blot analysis and alkaline phosphatase assay. Implantation of bone marrow cells infected ex vivo with AdBMP-2Myc caused orthotopic bone formation in mouse hindlimbs and bony union of critical-sized mouse radial defects. Immunohistochemical analysis revealed that rBMCs expressed Myc epitope-tagged BMP-2 protein for 14 days in vivo and became incorporated in the endochondral fracture callus. This novel adenovirus encoding for epitope-tagged BMP-2 can be used for immunohistochemical tracking of transduced cells in ex vivo gene therapy for bone repair.
Treatment of bone defects secondary to trauma, tumor, or infection often requires bone grafting. Bone grafting can be accomplished with autogenous bone graft, allograft bone from cadaveric donors, demineralized bone matrices, calcium-phosphate ceramics, or recombinant proteins. Autogenous bone graft from the iliac crest often is considered the first choice of bone-graft materials, but the supply is limited. In addition, harvesting autogenous bone graft can be associated with morbidity such as donor-site pain, nerve damage, and bleeding.1,5,18 Increased attention has focused on using recombinant proteins such as BMPs to induce bone repair.14,19 However, supraphysiologic doses of recombinant BMPs are required to induce bone formation in clinical cases.7,14,34 There is also concern that one dose of exogenous protein will not be sufficient to induce an adequate osteoinductive response in patients with large bone defects or with significant soft tissue injury.10 One potential alternative to direct recombinant protein delivery is to develop a biologic cellular delivery vehicle via gene therapy to enhance bone formation.8,13
Gene therapy strategies for bone repair include in vivo and ex vivo gene therapy. In vivo gene therapy involves delivery of the vector and gene directly via local injection. In vivo gene therapy has been used to induce bone repair in animal models using direct injection of adenoviral vectors encoding BMPs.2,3,33 However, in vivo injection of naked adenoviral vectors cannot target specific cells and is associated with significant immune response with concomitant transient protein production.2,27 In ex vivo regional gene therapy, target cells are transduced in vitro with a vector encoding the desired protein product. Transduced cells then are delivered to the patient or animal at the desired anatomic site, often with a carrier.4,22 Adenoviral vectors are attractive for in vivo and ex vivo bone repair strategies because they can be produced in high titers, and are known to infect dividing and nondividing cells.11 Because they are not incorporated into the host genome, they cannot alter the cellular genotype.11
Ex vivo gene therapy has been used successfully in various preclinical animal models to promote bone repair. Critical-sized femoral and calvarial defects have healed successfully using ex vivo gene therapy strategies with adenoviral and retroviral vectors.9,16,20,21 The duration of in vivo protein production and the fate of the infected cells in vivo still need to be defined. Do the transduced cells just act as cellular delivery vehicles or do they actively participate in the bone repair process? Bone marrow cells, adipose-derived stem cells, and muscle-derived stem cells, all of which can differentiate into bone under the appropriate stimuli, have been used as cellular delivery vehicles in ex vivo gene therapy strategies.12,15,17,21,28,30 A potential advantage of using osteoprogenitor cells is that these transduced cells not only produce the osteogenic protein (paracrine effect), but also have the ability to respond to the osteogenic signal (autocrine effect).17,21 This dual effect has the potential to produce a more robust biologic response. It has been difficult to confirm this hypothesis because of the inability to track transduced cells in vivo and the inability to discriminate between endogenous and virally produced BMPs.
We hypothesized that an epitope tag could be added to a BMP-2 adenoviral vector to allow identification of virally mediated protein in an ex vivo gene therapy strategy. We aimed to determine if this epitope tag on BMP-2 affected its biologic activity and ability to stimulate bone formation. We hypothesized that this novel adenoviral vector would allow immunohistochemical determination of cell location (ie, tracking) and duration of BMP-2 production.
MATERIALS AND METHODS
Rat bone marrow cells (rBMCs) were obtained from the femora of 3-month-old Lewis rats by flushing their intramedullary canals with Dulbecco's modified Eagle's medium (DMEM, Invitrogen, Carlsbad, CA) containing 15% fetal bovine serum (FBS). The cells were cultured in the conditioned media at 37°C in 5% CO2/95% air.21,23 The 293 cells were grown in DMEM with 10% FBS at 37°C in 5% CO2/95% air.
The Myc epitope, EQKLISEEDL, was inserted at one residue upstream of the first conserved cysteine within the BMP-2 coding region. This epitope tag allows for immunohistochemical staining of virally produced BMP-2. To insert the Myc epitope, two PCR fragments were produced using the human BMP-2 coding cDNA fragment (Wyeth, Madison, NJ) as a template.6 One pair of primers, 5′-CCTAAAGGTCGACCATGGTGGC-3′ (the Sal I site was underlined) and 5′-CGAATTCGATATCAGCTTCTGCTCGTGTTTGGCTTGACGTTTTTC-3′ (encoding half of the Myc and BMP-2 sequences from the epitope insertion site, [EcoRV site is underlined]) were used to produce a PCR fragment encoding the BMP-2 N-terminal to the insertion site. The other pair, 5′-CGGAATTCGATATCCGAGGAGGACCTGAAACAGCGGAAACGCCTTAAG-3′ (encoding half of the Myc and BMP-2 sequences from the epitope insertion site, [EcoRV site is underlined]) and 5′-CGAAGCTTTAATTTTGCTGTACTAGCG-3′ (Hind III site is underlined) were used to produce a PCR fragment encoding the insertion site to BMP-2 C-terminal region. These two fragments were joined using their primer-derived EcoRV sites and cloned into pBlue-script II KS (+) (Stratagene, La Jolla, CA). Using a nearby primer, this region was sequenced to confirm that no other mutations were introduced.
To create a novel adenoviral vector (AdBMP-2Myc) expressing epitope-tagged BMP-2, a Myc epitope-tagged BMP-2 fragment was subcloned into the adenoviral vector (pACCMVpLpASR)21 between the SalI and HindIII sites (pACCMVpLpASBMP-2Myc). This pACCMVpLpASBMP-2Myc contained a cytomegalovirus promoter and Poly-A signal. The recombinant AdBMP-2Myc adenovirus was generated by cotransfection of 293 cells with pACCMVpLpASBMP-2Myc and pJM17, a plasmid containing the genome of adenovirus Type 5 with deletions in the E1-E3 regions.24 AdBMP-2Myc was plaque urified and picked to elute viruses. The viral elution was used to infect 293 cells at a multiplicity of infection (MOI) of 10. The 293 cells were collected and lysed by three freeze and thaw cycles when they showed complete cytopathic effect. The crude viral lysate was used for amplification by serial propagation in 293 cells, grown in a high titer stock, and then purified by a CsCl density gradient. After dialysis, the viral titer was evaluated by optical density at 260 nm (1.1 × 1012 virus per OD260 unit).25 The titer of the purified AdBMP-2Myc used in these experiments was 3.4 × 1012 particles/mL obtained from 6 × 107 293 cells.
Biologic activity of the virus was assessed using a standard alkaline phosphatase (ALP) assay. The 1 × 105 W-20-17 cells derived from mouse bone marrow stroma31 were infected with purified AdBMP-2Myc for 24 hours at 1.4 × 103 particles/cell (MOI = 100).23 An MOI of 100 with similar adenoviral vectors typically resulted in greater than 90% transduction efficiency. The cells were washed with phosphate-buffered saline (PBS) and lysed in H2O by two freeze and thaw cycles. The cell lysates were added to the substrate solution (50 nmol/L glycine, 0.05% Triton X-100, 4 mmol/L MgCl2, and 5 mmol/L p-nitrophenol phosphate [pH,10.3]) for the assay of ALP activity. The reaction was stopped after 20 minutes at 37°C, and the spectrophotometric absorbance at 405 nm was determined within 15 minutes by enzyme-linked immunosorbent assay (ELISA). P-nitrophenol was used for the ALP activity as the standard.31 Data reported are the mean of five identical preparations per group.
Western blot analysis was used to determine if rBMCs infected with AdBMP-2Myc produced BMP-2. Conditioned media from 5 × 105 cultured rBMCs infected at a MOI of 100 with the purified media was collected for 48 hours and incubated with heparin-sepharose beads (Amersham, Piscataway, NJ) overnight at 4°C. Beads were collected by centrifugation, and bound proteins were eluted by heating at 100°C for 5 minutes with protein-loading buffer. The extract was loaded on 12% sodium dodecyl sulfate-polyacrylamid gel electrophoresis (SDS-PAGE) under nonreducing conditions. Recombinant human BMP-2 (Wyeth, Madison, NJ) was used as a positive control. Proteins were transferred to an enhanced chemiluminescence (ECL) membrane (Amersham) after SDS-PAGE. Bone morphogenetic protein-2 was detected by monoclonal mouse anti-BMP-2 (Sigma, St Louis, MO), and a horseradish peroxidase (HPR)-labeled goat anti-mouse secondary antibody (Bio-Rad, Hercules, CA) according to the ECL method. To completely strip and remove the primary and secondary antibodies, this membrane was submerged in stripping buffer (100 mmol/L 2-mercaptoethanol, 2% sodium dodecyl sulphate, 62.5 mmol/L Tris-HCl, pH 6.7) and incubated at 50°C for 30 minutes with occasional agitation. After washing in PBS at room temperature, Western blot analysis was performed using monoclonal mouse antibody 9E10 (anti-Myc) (Sigma) and HPR-labeled goat anti-mouse secondary antibody (Bio-Rad).
To determine if AdBMP-2Myc could induce orthotopic bone formation, transduced cells were implanted in a SCID mouse hindlimb model. We used 8-week-old male SCID mice weighing approximately 25 g. These mice were housed under pathogen-free conditions. All research was conducted in accordance with a protocol approved by the Chancellor's Animal Research Committee. The SCID mice were anesthetized with an intramuscular injection of ketamine (1.5 mg) and xylazine (0.3 mg). A 1-cm skin incision was made in the hindlimbs to make a muscle pouch.23 Five million rBMCs were infected with AdBMP-2Myc at a MOI of 100 or with Adβ-gal, which was constructed from the same background adenoviral vector as AdBMP-2Myc but expressed Escherichia coli β-galactosidase instead of BMP-2Myc. The rBMCs infected with these adenoviruses were harvested after 16 hours, and impregnated onto a 4 × 4-mm collagen sponge (Helistat, Integra LifeSciences Co, Plainsboro, NJ) after concentrating the cells into 10 μL of PBS. The cells were allowed to adhere to the sponge for 10 minutes. The same number of noninfected rBMCs also was collected and embedded in a collagen sponge as a control. AdBMP-2Myc-infected rBMCs were implanted into the hindlimbs of 16 SCID mice. The Adβ-gal–infected rBMCs and nontreated rBMCs were implanted into eight SCID mice each as controls. The mice were sacrificed for radiographic examination and histologic analysis at 3, 7, 10, 14, 21, 28, 42, and 56 days after implantation.
To determine if AdBMP-2Myc could be tracked in a defect model, transduced cells were implanted in a SCID mouse radial-defect model. Twenty-seven mice had surgery to create a critical-sized defect in the radius 3 mm long.17 Twelve mice had implantation of a collagen sponge containing 2.5-million cultured Lewis rat bone marrow cells transduced with AdBMP-2Myc (Group 1). Twelve mice had implantation of a collagen sponge containing 2.5-million cultured Lewis rat bone marrow cells transduced with AdBMP-2Control (Group 2). This adenovirus codes for nontagged BMP-2 and served as a positive control for healing and a negative control for tracking. Three mice in each group were sacrificed at four times: 3 days, 7 days, 14 days, and 21 days. The remaining three mice had a collagen sponge implanted without cells and were sacrificed at 21 days to serve as a negative control (Group 3).
To create a radial defect, a 5-mm skin incision was made over the radial aspect of the left foreleg of an adult SCID mouse. The radius was exposed by identifying the interval between the extensors and flexors of the wrist. A 3-mm tooth forceps was used to grip the radius at the junction between the proximal and middle thirds. The radius was cut on each side of the forceps with a sharp-tipped scissors, taking care not to fracture the ulna. The intact ulna served as a splint so the mouse could bear full weight immediately postoperatively. A collagen sponge (Helistat) soaked in the desired cell mixture then was inserted into the radial defect. We then used 5-0 Vicryl sutures to close the muscle and skin in layers over the defect.
The specimens were fixed for 24 hours in 10% formalin and decalcified with 10% ethylenediaminetetraacetic acid (EDTA) solution for 5–7 days. After decalcification, the specimens were dehydrated through with ethanol, embedded in paraffin, and sectioned at a thickness of 5 μm on a microtome. Immunohistochemical staining was performed with a polyclonal goat BMP-2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at a dilution of 1:1000, or a monoclonal antibody 9E10 (anti-Myc) (Sigma) at a dilution of 1:2000. Observation of bound antibodies was performed using VectaStain Elite ABC kit (Vector Laboratories, Burlingame, CA) and DAB substrate kit for peroxidase (Vector Laboratories). The slides were counterstained with hematoxylin.2 Sections of each specimen also were stained with hematoxylin and eosin and orange G.
A two-tailed Student's t test with a significance level of 0.05 was used to compare the mean ALP activity in each group, assuming a parametric distribution of data.
RESULTS
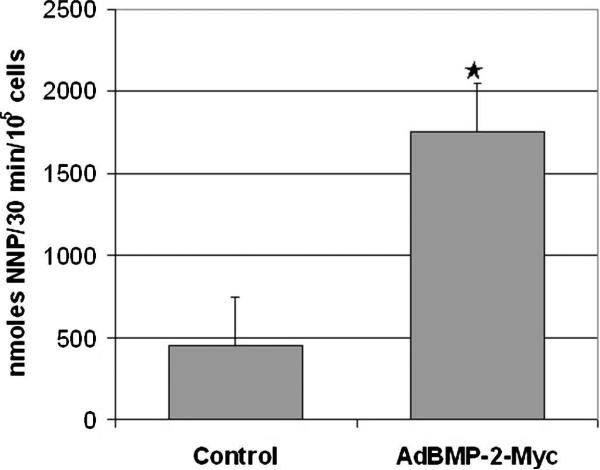
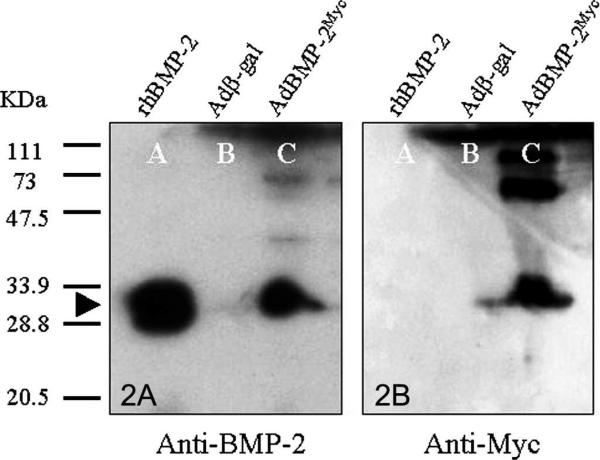
The novel adenoviral vector (AdBMP-2Myc) produced Myc-tagged BMP-2 and was osteogenic in ALP assay. Western blot analysis detected a 30-kDa band under nonreducing conditions with the BMP-2 antibody. This protein size was the same as the human recombinant BMP-2. The band detected by the BMP-2 antibody also was detected by the Myc antibody. No signal was detected from the conditioned media from Adβ-gal–infected cells (Fig 1). These results showed that rBMCs transduced with AdBMP-2Myc produced BMP-2 tagged with the Myc epitope. The W-20-17 cells differentiated into osteoblasts and expressed ALP after transduction with AdBMP-2Myc.31 Alkaline phosphastase activity was increased (p = 0.01) in infected W-20-17 cells infected with AdBMP-2Myc compared with noninfected cells (Fig 2). Based on ALP activity, the osteogenic activity of the cells transduced with the novel adenovirus compared favorably with activity of historical controls.21,23 These data showed that AdBMP-2Myc-infected cells produced active BMP-2 protein capable of stimulating the osteoprogenitor cells to express an osteoblast phenotype.
Fig 1.
A chart shows that the biologic activity of ALP was significantly increased in the AdBMP-2Myc-transduced W-20-17 cells when compared with nontransduced control cells (p = 0.01).
Fig 2.
(A) A Western blot is shown using BMP-2 antibody-assessed protein production of rBMCs transduced with AdBMP-2Myc. Lane A represents recombinant human BMP-2 (60 ng). Lanes B and C contained the conditioned medium from rBMCs infected with Adβ-gal and AdBMP-2Myc, respectively. The 30-kDa band was detected on Lanes A and C by the BMP-2 antibody under nonreducing conditions (black arrow). No signal was detected from Lane B. (B) The same membrane also was analyzed with the Myc antibody after pretreated BMP-2 antibodies were completely stripped. In Lane C the same band, which was detected by BMP-2 antibodies, also was detected by the Myc antibody confirming production of virally mediated, epitope-tagged BMP-2.
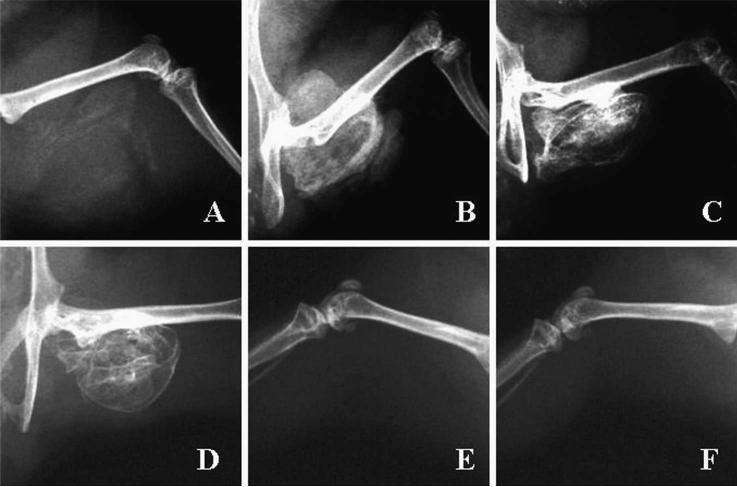
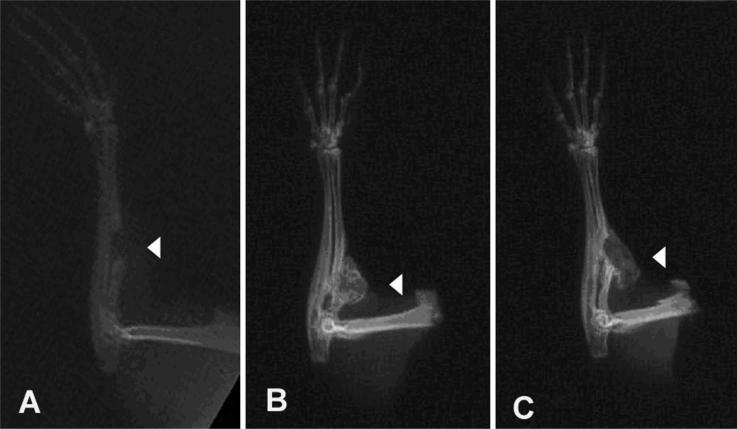
Implantation of cells transduced with AdBMP-2Myc stimulated bone formation in the mouse hindlimb and mouse radial-defect models. In mouse hindlimbs, orthotopic bone formation could be seen radiographically in all animals by Day 7 after implantation of cells transduced with AdBMP-2Myc. Increased bone formation and bony consolidation was observed by Day 21. No additional increase in bone formation was seen between Days 21 and 56. The bony consolidation was seen mainly in the area of the hindlimb where the collagen sponge had been surgically implanted. No significant mineralization was observed in implants receiving rBMCs infected with Adβ-gal or rBMCs alone by Day 56 (Fig 3). All defects treated with AdBMP-2Myc-transduced cells showed bony union at 21 days (Fig 4). Two of three defects treated with AdBMP-2Control cells healed. In the mouse that did not achieve bony union, the collagen sponge had migrated proximally out of the defect, but still formed ample bone that was seen on plain radiographs and with histologic analysis. None of the three defects in the animals treated with a collagen sponge alone showed union.
Fig 3.
Radiographic examinations of orthotopic bone in SCID mice hindlimbs are shown. Orthotopic bone formation was seen after implantation of rBMCs transduced with AdBMP-2Myc at (A) At Day 7, orthotopic bone is beginning to form. (B) At Day 14, bone formation has peaked. At (C) Day 21, and (D) Day 56, additional maturation of the orthotopic bone has occurred. Radiographs taken on Day 56 of mice receiving implants containing (E) rBMCs transduced with Adβ-gal or (F) rBMCs alone show no mineralization or orthotopic bone formation.
Fig 4.
Radiographs obtained 21 days postoperatively show SCID mice with radial defects treated with: (A) collagen sponge alone, (B) AdBMP-2Myc-transduced cells, or (C) AdBMP-2Control-transduced cells. Arrowheads in each figure identify location of persistent radial defects (A) and area of bony union (B) and (C).
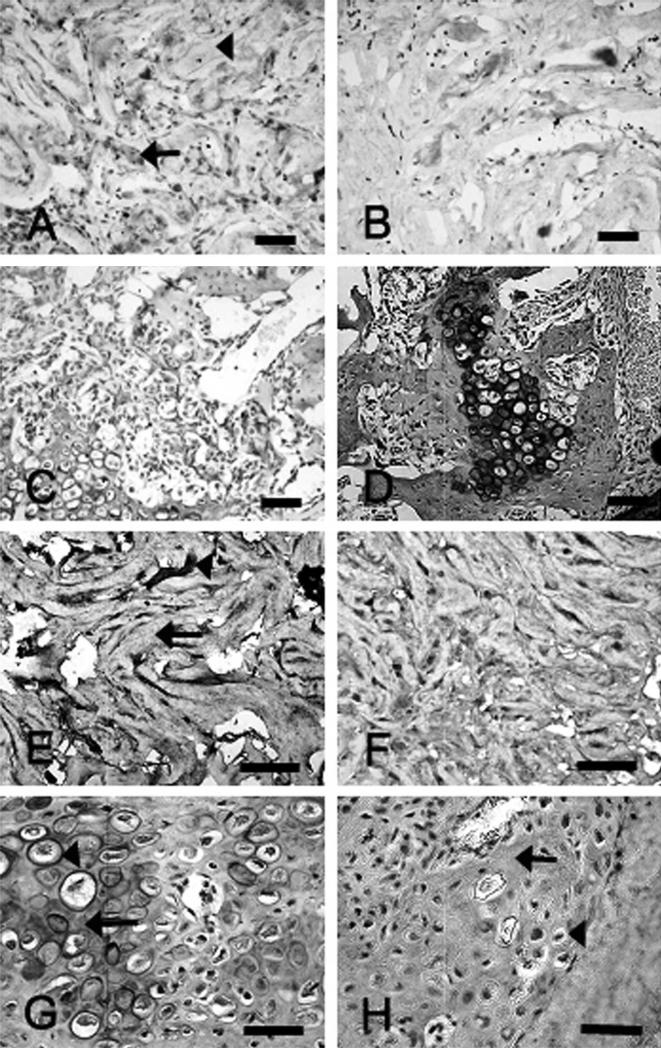
Immunohistochemical staining using Myc antibody and BMP-2 antibody successfully localized transduced cells in both models (Fig 5). At Day 3, the Myc-positive cells were localized in the region of the implanted collagen sponge. At Day 7, chondrocytes and premature osteocytes in early fracture callus stained positive with Myc and BMP-2 antibodies. Early woven bone formation could be seen surrounding the area of the implant. Immature osteocytes at Day 10 reacted to the c-Myc and BMP-2 antibodies. By Day 14, osteocytes stained positively with both antibodies, and more woven bone formation was seen. The c-Myc and BMP-2 expression peaked between Days 7 and 14, but these positive stains became fainter as the cells matured and became osteocytes. By Day 21, Myc- and BMP-2 positive cells were scarce in the area of mature bone. Myc-positive staining was absent at all times in the AdBMP-2Control group, whereas BMP-2–positive staining paralleled that of the AdBMP-2Myc group. These immunohistochemical data suggest that transduced cells differentiated into osteocyte-like cells and incorporated into new bone.
Fig 5.
Immunohistochemical analyses show the defect site for radial defects treated with AdBMP-2Myc or AdBMP-2control-transduced cells on a collagen sponge. (A). Myc staining of the AdBMP-2Myc cell group at Day 3 revealed positively stained cells (arrow) in the collagen sponge (arrowhead) (Stain, Myc immunostaining; original magnification, ×20). (B) Myc staining was negative in the control group at Day 3 (Stain, Myc immunostaining; original magnification, ×20). (C) Myc staining at Day 14 in the AdBMP-2Myc group revealed positive staining of cells in the area of the bone defect (Stain, Myc immunostaining; original magnification, ×20). Positive cells were visible in areas of endochondral ossification in the defect. (D) Myc-positive staining was not observed in the AdBMP-2Control group at Day 14 (Stain, Myc immunostaining; original magnification, ×20). (E) In the AdBMP-2Myc group at Day 3, BMP-2 immunostaining was strongly positive in cells (arrowhead) in the collagen sponge (arrow) (Stain, BMP-2 immunostaining; original magnification, ×40). (F) Bone morphogenetic protein-2 immunostaining was similarly positive in the AdBMP-2Control group at Day 3 (Stain, BMP-2 immunostaining; original magnification, ×40). In the (G) AdBMP-2Myc and (H) AdBMP-2control group at 14 days, BMP-2 immunostaining was positive in hypertrophic chondrocytes (arrowheads) in endochondral fracture callus (arrows) (scale bars, 50 μmol/L) (Stain, BMP-2 immunostaining; original magnification, ×40).
Histologic analysis using hematoxylin and eosin and orange G stains at 3 days revealed placement of the collagen sponges in the radial defect in both groups. At 7 days, the collagen sponge was beginning to break down but still was visible in the defect. At 14 days, substantial ossification of endochondral fracture callus was observed in all defects with bone formation. At 21 days, healed defects showed bony union with some neocortex formation. Histologic analysis of untreated control radial defects revealed fibrous nonunion.
DISCUSSION
In successful animal models of ex vivo gene therapy for bone repair, the fate of transduced cells and duration of protein production often is not reported. It was not clear whether the transduced cells were bystanders solely producing BMPs, or if they differentiated into osteogenic cells and became incorporated into bone. In our study, the novel adenoviral vector allowed immunohistochemical tracking of transduced cells, and epitope-tagged, virally mediated BMP-2 was identified 14 days after implantation. This epitope tag on BMP-2 did not affect its biologic activity and ability to stimulate bone formation. Transduced cells differentiated into hypertrophic chondrocytes and incorporated into newly forming bone.
Our study has several limitations. First, a SCID mouse model was chosen to minimize the effect of immune response on the study. The SCID mouse does not produce an immune response to the transduced cells or adenovirus. The results of the same study in an immunocompetent animal model likely would be different, as a vigorous immune response to adenoviral vectors has been reported. Second, the immunohistochemical data were evaluated in a qualitative fashion only. Documenting cell number per area and quantifying the amount of BMP-2 production would strengthen future tracking studies using this novel adenoviral vector. In addition, because only positive cells are tracked using this method, the percentage of cells implanted that actually produce BMP-2 remains unknown. The SCID mouse models did not represent the clinical situation of massive bone loss and poor vascularity where gene therapy for bone repair likely would be of clinical benefit.
There have been few studies assessing the fate of transduced cells in musculoskeletal gene therapy. Lee et al20 transduced male muscle-derived cells with an adenovirus encoding the BMP-2 gene, and implanted the cells with a collagen sponge in calvarial defects in female SCID mice. They identified the cells in vivo using a Y-chromosome-specific fluorescent in situ hybridization (FISH) technique and determined that most cells were located not only on the surfaces of newly formed bone, but also in the osteocyte lacunae of the new bone.20 Implanted cells found in the new bone stained positive for osteocalcin, indicating that they differentiated in vivo into osteogenic cells.20 However, bone repair in a calvarial defect heals by intramembranous ossification which differs from the healing that occurs in a radial defect. Gazit et al17 and Turgeman et al31 studied the histologic fate of mesenchymal stem cells in a SCID mouse radial defect model. The C3H10T1/2 clone cells expressing rhBMP-2 also were infected with a vector coding for the lac Z gene. The cells were identified in vivo by the coproduction of β-gal and BMP-2 and were observed to differentiate spontaneously into osteogenic cells in vitro and to differentiate into cells with chondrocytic morphologic features in vivo that were incorporated into new bone.17,31 However, in our study the marker gene and the BMP gene were contained in two separate vectors. The cDNA for myc-tagged BMP-2 was contained in one vector, which allowed us to definitively identify the location of transduced cells and duration of BMP expression.
In a critical-sized femoral defect model in rats and a radial defect model in mice, transduced mesenchymal cells produced more robust bone formation than a large dose of recombinant protein.17,21,26 One hypothesis is that these transduced cells not only secrete the protein but can respond to the osteogenic signal in an autocrine fashion, producing a more vigorous osteogenic response.17,32 Our findings and the results reported by Gazit et al,17 Turgeman et al,31 and Lee et al20 support this hypothesis. Pluripotential stem cells transduced with an osteogenic viral vector likely stimulate local host mesenchymal stem cell differentiation. In addition, the transduced cells are stimulated in an autocrine fashion to differentiate into osteocyte-like cells and incorporate into new bone.29 Future studies using this novel adenoviral construct to track transduced cells after implantation at a tissue site will provide useful information regarding the lifespan, differentiation, and relative effectiveness of various cell types as delivery vehicles of osteogenic proteins in larger animal models. This information is essential for the development of cell-based regional gene therapy for use in humans.
Acknowledgments
One or more of the authors (JRL) has received funding from a grant from the National Institutes of Health, RO1: AR46789.
Each author certifies that his or her institution has approved animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Ahlmann E, Patzakis M, Roidis N, Shepherd L, Holtom P. Comparison of anterior and posterior iliac crest bone grafts in terms of harvest-site morbidity and functional outcomes. J Bone Joint Surg Am. 2002;84:716–720. doi: 10.2106/00004623-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Alden TD, Pittman DD, Hankins GR, Beres EJ, Engh JA, Das S, Hudson SB, Kerns KM, Kallmes DF, Helm GA. In vivo endochondral bone formation using a bone morphogenetic protein 2 adenoviral vector. Hum Gene Ther. 1999;10:2245–2253. doi: 10.1089/10430349950017220. [DOI] [PubMed] [Google Scholar]
- 3.Baltzer AW, Lattermann C, Whalen JD, Wooley P, Weiss K, Grimm M, Ghivizzani SC, Robbins PD, Evans CH. Genetic enhancement of fracture repair: healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene Ther. 2000;7:734–739. doi: 10.1038/sj.gt.3301166. [DOI] [PubMed] [Google Scholar]
- 4.Baltzer AW, Lieberman JR. Regional gene therapy to enhance bone repair. Gene Ther. 2004;11:344–350. doi: 10.1038/sj.gt.3302195. [DOI] [PubMed] [Google Scholar]
- 5.Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity: a statistical evaluation. Spine. 1995;20:1055–1060. doi: 10.1097/00007632-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Basler K, Edlund T, Jessell TM, Yamada T. Control of cell pattern in the neural tube: regulation of cell differentiation by dorsalin-1, a novel TGF beta family member. Cell. 1993;73:687–702. doi: 10.1016/0092-8674(93)90249-p. [DOI] [PubMed] [Google Scholar]
- 7.Boden SD, Zdeblick TA, Sandhu HS, Heim SE. The use of rhBMP-2 in interbody fusion cages: definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25:376–381. doi: 10.1097/00007632-200002010-00020. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y. Orthopedic applications of gene therapy. J Orthop Sci. 2001;6:199–207. doi: 10.1007/s007760100072. [DOI] [PubMed] [Google Scholar]
- 9.Cheng SL, Lou J, Wright NM, Lai CF, Avioli LV, Riew KD. In vitro and in vivo induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene. Calcif Tissue Int. 2001;68:87–94. [PubMed] [Google Scholar]
- 10.Cook SD, Wolfe MW, Salkeld SL, Rueger DC. Effect of recombinant human osteogenic protein-1 on healing of segmental defects in non-human primates. J Bone Joint Surg Am. 1995;77:734–750. doi: 10.2106/00004623-199505000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Danthinne X, Imperiale MJ. Production of first generation adeno-virus vectors: a review. Gene Ther. 2000;7:1707–1714. doi: 10.1038/sj.gt.3301301. [DOI] [PubMed] [Google Scholar]
- 12.Dragoo JL, Choi JY, Lieberman JR, Huang J, Zuk PA, Zhang J, Hedrick MH, Benhaim P. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res. 2003;21:622–629. doi: 10.1016/S0736-0266(02)00238-3. [DOI] [PubMed] [Google Scholar]
- 13.Evans CH, Robbins PD. Genetically augmented tissue engineering of the musculoskeletal system. Clin Orthop Relat Res. 1999;367(suppl):S410–S418. doi: 10.1097/00003086-199910001-00040. [DOI] [PubMed] [Google Scholar]
- 14.Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, Yin S. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83(suppl 1):S151–S158. [PMC free article] [PubMed] [Google Scholar]
- 15.Gafni Y, Turgeman G, Liebergal M, Pelled G, Gazit Z, Gazit D. Stem cells as vehicles for orthopedic gene therapy. Gene Ther. 2004;11:417–426. doi: 10.1038/sj.gt.3302197. [DOI] [PubMed] [Google Scholar]
- 16.Gamradt SC, Lieberman JR. Genetic modification of stem cells to enhance bone repair. Ann Biomed Eng. 2004;32:136–147. doi: 10.1023/b:abme.0000007798.78548.b8. [DOI] [PubMed] [Google Scholar]
- 17.Gazit D, Turgeman G, Kelley P, Wang E, Jalenak M, Zilberman Y, Moutsatsos I. Engineered pluripotent mesenchymal cells integrate and differentiate in regenerating bone: a novel cell-mediated gene therapy. J Gene Med. 1999;1:121–133. doi: 10.1002/(SICI)1521-2254(199903/04)1:2<121::AID-JGM26>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 18.Goulet JA, Senunas LE, DeSilva GL, Greenfield ML. Autogenous iliac crest bone graft: complications and functional assessment. Clin Orthop Relat Res. 1997;339:76–81. doi: 10.1097/00003086-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, Aro H, Atar D, Bishay M, Borner MG, Chiron P, Choong P, Cinats J, Courtenay B, Feibel R, Geulette B, Gravel C, Haas N, Raschke M, Hammacher E, van der Velde D, Hardy P, Holt M, Josten C, Ketterl RL, Lindeque B, Lob G, Mathevon H, McCoy G, Marsh D, Miller R, Munting E, Oevre S, Nordsletten L, Patel A, Pohl A, Rennie W, Reynders P, Rommens PM, Rondia J, Rossouw WC, Daneel PJ, Ruff S, Ruter A, Santavirta S, Schildhauer TA, Gekle C, Schnettler R, Segal D, Seiler H, Snowdowne RB, Stapert J, Taglang G, Verdonk R, Vogels L, Weckbach A, Wentzensen A, Wisniewski T. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84:2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Lee JY, Musgrave D, Pelinkovic D, Fukushima K, Cummins J, Usas A, Robbins P, Fu FH, Huard J. Effect of bone morphogenetic protein-2-expressing muscle-derived cells on healing of critical-sized bone defects in mice. J Bone Joint Surg Am. 2001;83:1032–1039. doi: 10.2106/00004623-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Lieberman JR, Daluiski A, Stevenson S, Wu L, McAllister P, Lee YP, Kabo JM, Finerman GA, Berk AJ, Witte ON. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am. 1999;81:905–917. doi: 10.2106/00004623-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Lieberman JR, Ghivizzani SC, Evans CH. Gene transfer approaches to the healing of bone and cartilage. Mol Ther. 2002;6:141–147. doi: 10.1006/mthe.2000.0663. [DOI] [PubMed] [Google Scholar]
- 23.Lieberman JR, Le LQ, Wu L, Finerman GA, Berk A, Witte ON, Stevenson S. Regional gene therapy with a BMP-2-producing murine stromal cell line induces heterotopic and orthotopic bone formation in rodents. J Orthop Res. 1998;16:330–339. doi: 10.1002/jor.1100160309. [DOI] [PubMed] [Google Scholar]
- 24.McGrory WJ, Bautista DS, Graham FL. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology. 1988;163:614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- 25.Mittereder N, March KL, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moutsatsos IK, Turgeman G, Zhou S, Kurkalli BG, Pelled G, Tzur L, Kelley P, Stumm N, Mi S, Muller R, Zilberman Y, Gazit D. Exogenously regulated stem cell-mediated gene therapy for bone regeneration. Mol Ther. 2001;3:449–461. doi: 10.1006/mthe.2001.0291. [DOI] [PubMed] [Google Scholar]
- 27.Musgrave DS, Bosch P, Ghivizzani S, Robbins PD, Evans CH, Huard J. Adenovirus-mediated direct gene therapy with bone morphogenetic protein-2 produces bone. Bone. 1999;24:541–547. doi: 10.1016/s8756-3282(99)00086-1. [DOI] [PubMed] [Google Scholar]
- 28.Peng H, Huard J. Muscle-derived stem cells for musculoskeletal tissue regeneration and repair. Transpl Immunol. 2004;12:311–319. doi: 10.1016/j.trim.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Scaduto AA, Lieberman JR. Gene therapy for osteoinduction. Orthop Clin North Am. 1999;30:625–633. doi: 10.1016/s0030-5898(05)70115-2. [DOI] [PubMed] [Google Scholar]
- 30.Thies RS, Bauduy M, Ashton BA, Kurtzberg L, Wozney JM, Rosen V. Recombinant human bone morphogenetic protein-2 induces osteoblastic differentiation in W-20-17 stromal cells. Endocrinology. 1992;130:1318–1324. doi: 10.1210/endo.130.3.1311236. [DOI] [PubMed] [Google Scholar]
- 31.Turgeman G, Pittman DD, Muller R, Kurkalli BG, Zhou S, Pelled G, Peyser A, Zilberman Y, Moutsatsos IK, Gazit D. Engineered human mesenchymal stem cells: a novel platform for skeletal cell mediated gene therapy. J Gene Med. 2001;3:240–251. doi: 10.1002/1521-2254(200105/06)3:3<240::AID-JGM181>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 32.van Griensven M, Lobenhoffer P, Barke A, Tschernig T, Linden-maier W, Krettek C, Gerich TG. Adenoviral gene transfer in a rat fracture model. Lab Anim. 2002;36:455–461. doi: 10.1258/002367702320389134. [DOI] [PubMed] [Google Scholar]
- 33.Yoon ST, Boden SD. Osteoinductive molecules in orthopaedics: basic science and preclinical studies. Clin Orthop Relat Res. 2002;395:33–43. doi: 10.1097/00003086-200202000-00005. [DOI] [PubMed] [Google Scholar]