Abstract
Although it has been previously reported that patients with inflammatory breast cancer (IBC) experience worse survival than patients with other breast cancer (BC) types, the socioeconomic and ethnic factors leading to this survival difference are not fully understood. The association between county-level percent of persons below the poverty level and BC-specific (BCS) survival for cases diagnosed from 1990 to 2008 in the Surveillance, Epidemiology, and End Results (SEER) database linked to census derived county attributes was examined. A sub-analysis of cases from 2000 to 2008 also examined BCS survival by an index combining percent below poverty and less than high school graduates as well as metropolitan versus non-metropolitan county of residence. The Kaplan–Meier estimator was used to construct survival curves by stage, inflammatory status, and county-level socioeconomic position (SEP). Stage and inflammatory status stratified proportional hazards models, adjusted for age, race/ethnicity, tumor and treatment characteristics were used to determine the hazard of BCS death by county-level SEP. Kaplan–Meier survival curves indicated IBC has worse survival than stage matched non-IBC, (stage III IBC median survival = 4.75 years vs. non-IBC = 13.4 years, p < 0.0001). Residing in a lower SEP, non-metro county significantly worsens BCS survival for non-IBC in multivariate proportional hazards models. African American cases appear to have worse survival than non-Hispanic Whites regardless of inflammatory status, stage, county-level SEP, tumor, or treatment characteristics. This is the first study to examine IBC survival by SEP in a nationwide population-based tumor registry. As this analysis found generally poorer survival for IBC, regardless of SEP or race/ethnicity, it is important that interventions that help educate women on IBC symptoms target women in various SEP and race/ethnicity groups.
Keywords: Inflammatory breast cancer, Socioeconomic position, Epidemiology, Breast cancer-specific survival
Introduction
Inflammatory breast cancer (IBC), a rare and aggressive form of the disease, has been shown to have worse survival than other types of breast cancer (BC), even after adjustment for numerous individual and tumor characteristics as well as treatment [1–7]. In clinical trials of neoadjuvant chemotherapy, IBC had worse prognosis than stage III non-IBC both for increased loco-regional and distant recurrence rates and overall survival [8].
Although a few hospital and population-based studies have examined IBC survival by patient and tumor characteristics [2, 4, 5, 7, 9–12], only one population based study examined IBC survival by socioeconomic position (SEP) and race/ethnicity [11]. This study included 935 incident cases of IBC (1998–2002) in the Florida Cancer Data System. The authors reported African American IBC cases had lower median survival than White IBC cases and also noted longer median survival for patients living in more affluent areas as classified by percentage of population living under the federal poverty line. However, this study only examined community poverty level and insurance status in addition to race/ethnicity as measures of SEP, and was limited to cases diagnosed in Florida, so may not be representative of IBC cases from across the United States [11]. Furthermore, hormone receptor (HR) status was not available, so the authors were unable to adjust their results for this important prognostic factor [11].
As IBC cases appear to have worse survival even after adjustment for individual, tumor, and treatment characteristics, this study sought to detect potential differences in stage III and IV IBC and non-IBC survival by SEP and metropolitan (metro) versus non-metro residence in the United States (US) Surveillance, Epidemiology, and End Results (SEER) database linked to 1990 and 2000 census derived county attributes. Examining IBC and non-IBC survival by SEP and area of residence will aid in elucidating factors associated with the survival difference and thus, this study has important implications for the targeting of BC education and health care resources.
Methods
Data source
The SEER 17 Registries database linked to 1990 and 2000 US county attributes was utilized for this analysis [13]. The US SEER database covers approximately 28 % of the US population, including ~ 25 % of Whites, 26 % of African Americans, 41 % of Hispanics, 43 % of American Indians and Alaska Natives, 54 % of Asians, and 71 % of Hawaiian/Pacific Islanders [14].
Individual-level measures
The outcome variable for this analysis was BC-specific survival in years, stratified by stage and inflammatory status (IBC vs. non-IBC). Cases were followed from date of diagnosis to date of death, loss to follow-up, or December 31, 2008, if still alive and not lost to follow-up. As IBC is automatically at Stage III B or higher (IV) if metastatic at diagnosis, this analysis was limited to first malignant primary American Joint Committee on Cancer (AJCC) Stage III and IV IBC and non-IBC in women aged 18+ years [15]. In order to be certain all IBC cases were captured, a comprehensive case definition was used where a BC case having any one of the following codes assigned to the SEER*stat (version 7.0.5, National Cancer Institute, Bethesda, MD) variables below was classified as IBC, while all other cases were considered non-IBC [2, 4, 6, 7, 16, 17]:
Histologic type ICD-O-3 (1990–2008) = 8530 (“Inflammatory Carcinoma”) [18, 19]
Stage-derived AJCC T, 6th ed (2004–2008) = T4d (“Inflammatory Carcinoma”) [15, 18]
- Extent of disease [EOD]-CS extension (2004–2008) = 71–73 [18, 20]
- 71: “Diagnosis of inflammatory carcinoma without a clinical description of inflammation, erythema, edema, peau d’orange, etc., of more than 50 % of the breast, with or without dermal lymphatic infiltration. Inflammatory carcinoma, NOS.”
- 72: “Diagnosis of inflammatory carcinoma with a clinical description of inflammation, erythema, edema, peau d’orange, etc., of less than or equal to 50 % of the breast, with or without dermal lymphatic infiltration.”
- 73: “Diagnosis of inflammatory carcinoma with a clinical description of inflammation, erythema, edema, peau d’orange, etc., of more than 50 % of the breast, with or without dermal lymphatic infiltration.”
Other individual variables included in the analysis were age at diagnosis (analyzed as continuous), race/ethnicity (Non-Hispanic White (NH White), African American, Hispanic White, Asian/Pacific Islander (API), and American Indian/Alaska Native (AI/AN)), HR status, grade, receipt of surgery at primary BC site (yes/no), and receipt of any type of radiation therapy as part of the initial treatment plan (yes/no). Cases that were estrogen and/or progesterone receptor positive were classified as hormone receptor positive (HRP), while cases not testing positive for either of these HRs were considered hormone receptor negative (HRN). Estrogen and progesterone receptor status was determined by assay results in the medical record abstracted by SEER registries. In cases where assay results were reported for more than one tumor specimen, the highest value was recorded (if any sample is positive, the receptor is recorded as positive). SEER abstractors record assay results from tumor specimens prior to receipt of neoadjuvant therapy, although if not available, post-treatment results are reported. Cases where either assay was not ordered or performed, borderline and undetermined whether positive or negative, ordered—results not in chart, or where there was no documentation/information in the patient record were excluded [22]. Grade was dichotomized into low grade (well differentiated grade I and moderately differentiated grade II tumors) vs. high grade tumors (poorly differentiated grade III and undifferentiated; anaplastic grade IV tumors).
County-level measures
Three county-level measures of SEP derived from the 1990 and 2000 US censuses were used in this analysis [23]. For both the 1990 and 2000 census, the percent of persons below the poverty level within a county was dichotomized to <20 % vs. ≥20 %. The 1990 poverty level and census result was applied to all cases diagnosed from 1990 to 1999, while the 2000 poverty level and census result was applied to cases diagnosed from 2000–2008.
SEP variation in survival was further examined in the subset of cases diagnosed from 2000 to 2008 by an index combining the percent of persons below the poverty level and the percent of persons who did not graduate from high school. The percent of adults greater than 25 years of age within a county who did not graduate from high school (based on 2000 US Census results) was divided into quartiles based on the distribution of the variable across all counties in the US using the SEER county attributes database [24]. These high school quartiles were then combined with the poverty variable to create high and lower SEP categories as follows: high SEP = <10.00 % poverty and ≤ 15.99 % less than high school graduate, Lower SEP = ≥10 % poverty and >15.99 % less than high school graduate. This dichotomized index is based on one previously used to examine IBC incidence and BC survival [17, 25].
BC survival by metro versus non-metro area of residence was also examined in the subset of cases diagnosed from 2000 to 2008. As in previous analyses, rural–urban continuum codes (RUCC) codes 1–3 (counties in metro areas of 1 million to fewer than 250,000) were defined as metro counties, while codes 4–9 (urban population of 20,000 or more, adjacent to a metro area to completely rural or <2,500 urban population, not adjacent to a metro area) defined non-metro counties [17, 25–28].
Statistical analysis
Breast cancer specific (BCS) survival stratified on inflammatory status and stage was examined through use of the Kaplan–Meier estimator to compare survival curves by county-level SEP variables, with the log-rank test used to detect differences between the survival curves. Separate proportional hazards models were fit for each of the three county-level SEP variables, stratified by inflammatory status and stage, in order to determine if the hazard of death from IBC as well as non-IBC differs by county-level SEP. All models were adjusted for age at diagnosis, race/ethnicity, HR status, tumor grade, and receipt of surgery and/ or radiation. An alpha level ≤0.05 was used to determine statistical significance. Proportionality of the hazard curves was assessed by examining log (−log S(t)) against log t plots. Analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC).
Results
76,644 Stage III and IV first malignant primary BCs diagnosed between 1990 and 2008 were available for this analysis. Six cases were excluded due to missing county-level information, 314 due to missing race/ethnicity, 13,827 due to missing HR status, 8,317 due to missing grade, 359 due to missing surgery information, and 2,148 due to missing radiation information, leaving 5,526 IBC and 46,147 non-IBC cases.
Table 1 provides the means and frequencies for the sociodemographic, tumor, and treatment characteristics of the study population. Both stages III and IV IBCs had lower mean ages than non-IBC: stage III IBC = 57.1 years versus non-IBC = 57.8 years; stage IV IBC = 56.8 years versus non-IBC = 61.2 years. A higher proportion of stage IV IBC was found in African Americans, Hispanic Whites, and AI/ANs as compared to stage IV non-IBC. Regardless of stage, IBC tended to have worse prognostic tumor characteristics compared to non-IBC, including less HRP and higher grade tumors. The majority of cases, regardless of stage and inflammatory status, resided in counties with <20 % poverty.
Table 1.
Sociodemographic, tumor, and treatment characteristics of the SEER study population, 1990–2008
| Inflammatory breast cancer n(%) |
Non-inflammatory breast cancer n (%) |
|||
|---|---|---|---|---|
| Stage III | Stage IV | Stage III | Stage IV | |
| Mean age at diagnosis (SD) | 57.1 (14.5) | 56.8 (13.3) | 57.8 (14.8) | 61.2 (14.4) |
| Race/ethnicity | ||||
| Non-Hispanic White | 3,124 (70.3) | 675 (62.2) | 22,221 (67.6) | 9,305 (70.1) |
| African American | 532 (12.0) | 197 (18.2) | 4,201 (12.8) | 1,863 (14.0) |
| Hispanic White | 518 (11.7) | 142(13.1) | 3,804 (11.6) | 1,180 (8.2) |
| Asian/Pacific Islander | 227 (5.1) | 62 (5.7) | 2,467 (7.5) | 850 (6.4) |
| Am. Indian/AK Native | 40 (0.90) | 9 (0.83) | 174 (0.53) | 82 (0.62) |
| Hormone receptor status | ||||
| Positive | 2,447 (55.1) | 618 (57.0) | 23,617 (71.9) | 9,655 (72.7) |
| Negative | 1,994 (44.9) | 467 (43.0) | 9,250 (28.1) | 3,625 (27.3) |
| Grade | ||||
| Low (MI) | 1,187 (26.7) | 287 (26.5) | 13,995 (42.6) | 5,704 (43.0) |
| High (III/IV) | 3,254 (73.3) | 798 (73.5) | 18,872 (57.4) | 7,576 (57.0) |
| Surgery at BC site | ||||
| Yes | 4,048 (91.2) | 597 (55.0) | 31,842 (96.9) | 7,788 (58.6) |
| No | 393 (8.8) | 488 (45.0) | 1,025 (3.1) | 5,492 (41.4) |
| Radiation therapy | ||||
| Yes | 2,659 (59.9) | 467 (43.0) | 18,717 (57.0) | 5,147 (38.8) |
| No | 1,782 (40.1) | 618 (57.0) | 14,150 (43.0) | 8,133 (61.2) |
| % Below poverty in county | ||||
| <20.00 % | 4,092 (92.1) | 1,005 (92.6) | 30,263 (92.1) | 12,193 (91.8) |
| ≥ 20.00 % | 349 (7.9) | 80 (7.4) | 2,604 (7.9) | 1,087 (8.2) |
| Sub-analysis of cases diagnosed from 2000 to 2008 | ||||
| Poverty-high school index | ||||
| High SEP | 768 (24.5) | 199 (22.4) | 6,741 (25.6) | 2,651 (26.9) |
| Lower SEP | 2,372 (75.5) | 691 (77.6) | 19,562 (74.4) | 7,194 (73.1) |
| Residence at diagnosisa | ||||
| Metro County | 2,799 (89.3) | 800 (90.1) | 23,821 (90.6) | 8,906 (90.5) |
| Non-Metro County | 334 (10.7) | 88 (9.9) | 2,457 (9.4) | 931 (9.5) |
42 Cases missing RUCC
For the sub-analysis of cases diagnosed from 2000 to 2008, 4,030 IBC and 36,148 non-IBC cases were included. Forty-two cases were missing RUCC, leaving 4,021 IBC and 36,115 non-IBC cases for the metro versus non-metro county analysis. The majority of cases, regardless of stage or inflammatory status, resided in metro and lower SEP counties.
For cases diagnosed from 1990 to 2008, the median BCS survival for stage III IBC was 4.75 years (range: 0–18.8 years), while the median BCS survival for stage III non-IBC was much higher (13.4 years, range: 0–18.9 years), with the log-rank test indicating significant differences between the survival curves between stage III IBC and non-IBC (p < 0.0001). The median BCS survival for both stage IV IBC and non-IBC were much lower (1.75 years, range: 0–15.7 and 2.3 years, range: 0–18.9, respectively), with the log-rank test indicating significant differences between the survival curves of stage IV IBC and non-IBC as well (p < 0.0001).
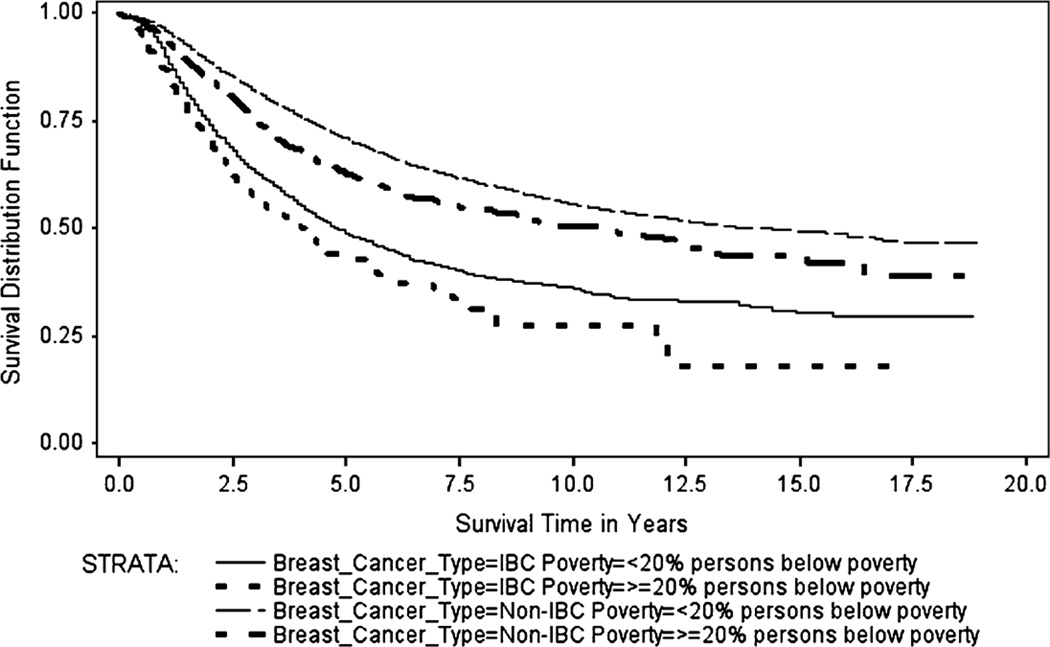
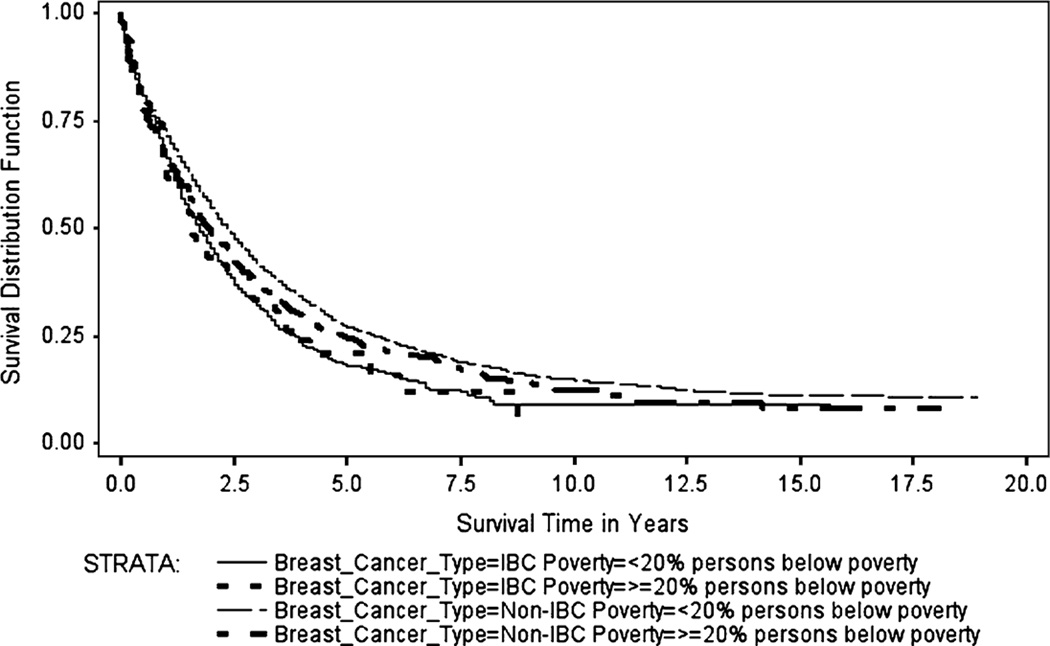
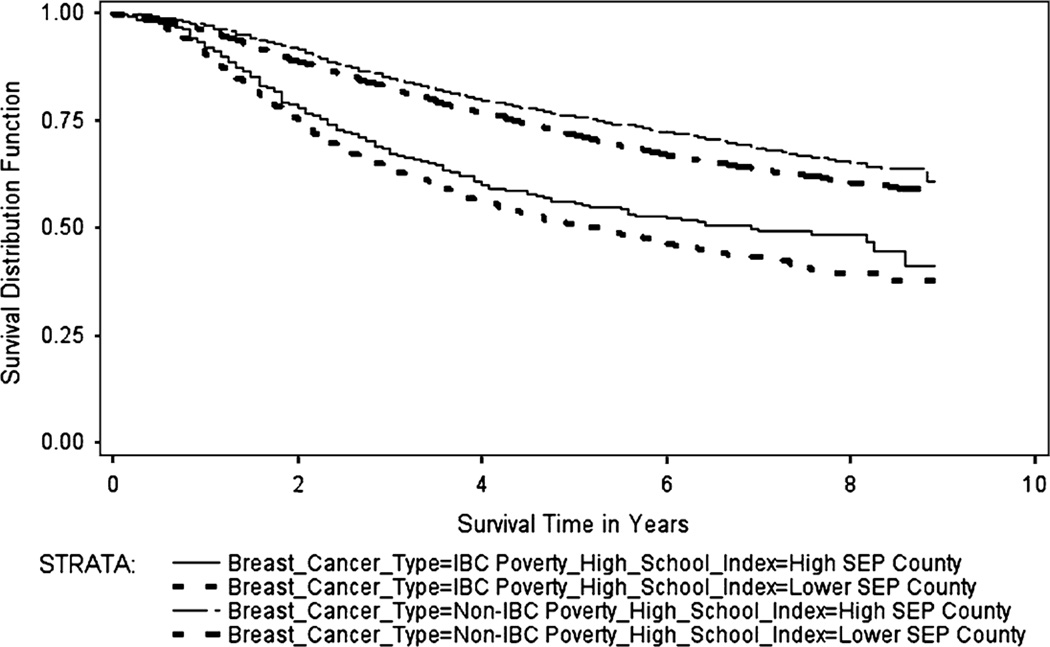
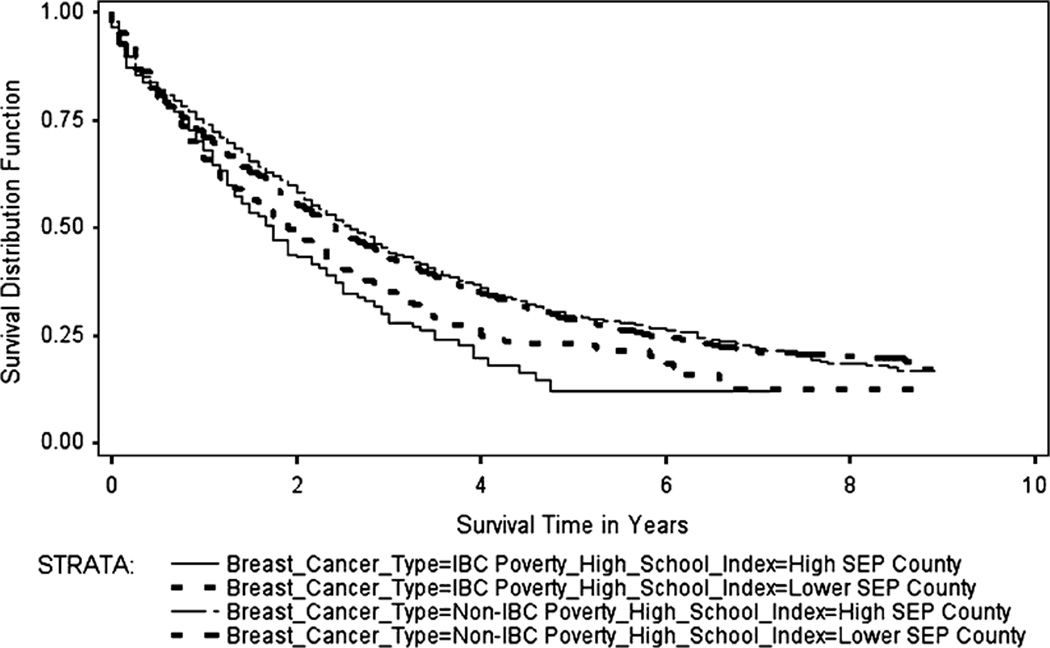
The Kaplan–Meier survival curves (Figs. 1, 2, 3, 4) generally showed non-IBC cases residing in low poverty, high SEP, metro counties had better overall survival, with survival differences between IBC and non-IBC and SEP groups appearing to be larger for stage III cancers. Log-rank test statistics indicated significant survival differences (p < 0.0001) between the survival curves shown on each figure.
Fig. 1.
Breast cancer specific Kaplan–Meier survival curves for stage III IBC and non-IBC by % persons below poverty in county, 1990–2008
Fig. 2.
Breast cancer specific Kaplan–Meier survival curves for stage IV IBC and Non-IBC by % persons below poverty in county, 1990–2008
Fig. 3.
Breast cancer specific Kaplan–Meier survival curves for stage III IBC and non-IBC by poverty-high school index, 2000–2008
Fig. 4.
Breast cancer specific Kaplan–Meier survival curves for stage IV IBC and non-IBC by poverty-high school index, 2000–2008
Figure 1 shows the BCS Kaplan–Meier survival curves for stage III IBC and non-IBC by county-level percent of persons below the poverty level for cases diagnosed from 1990 to 2008. The median BCS survival for stage III IBC cases residing in counties with <20 % poverty was 4.8 years (range: 0–18.8 years), while median BCS survival for stage III IBC cases in counties with ≥20 % poverty was 4.1 years (range: 0–17.2 years). The corresponding values for non-IBC were 14.0 years (range: 0–18.9 years) and 10.6 years (range: 0–18.75 years), respectively.
Figure 2 shows the BCS Kaplan–Meier survival curves for stage IV IBC and non-IBC by county-level percent of persons below the poverty level for cases diagnosed from 1990 to 2008. The median BCS survival for stage IV IBC cases residing in counties with <20 % poverty was 1.75 years (range: 0–15.7 years), while median BCS survival for stage IV IBC cases residing in counties with ≥20 % poverty was 1.6 years (range: 0–8.75 years). The corresponding values for non-IBC were 2.3 years (range: 0–18.9 years) and 2.0 years (range: 0–18.1 years), respectively.
Figure 3 shows the BCS Kaplan–Meier survival curves for stage III IBC and non-IBC by the county-level poverty-high school index for cases diagnosed from 2000 to 2008. The median BCS survival for stage III IBC cases residing in high SEP counties was 6.9 years (range: 0–8.9 years), while median BCS survival for stage III IBC cases in lower SEP counties was 5.2 years (range: 0–8.9 years). The median survival time for stage III non-IBC cases for both SEP groups had not been reached and therefore not estimated (range for both groups: 0–8.9 years).
Figure 4 shows the BCS Kaplan–Meier survival curves for stage IV IBC and non-IBC by the county-level poverty-high school index for cases diagnosed from 2000 to 2008. The median BCS survival for stage IV IBC cases residing in high SEP counties was 1.75 years (range: 0–7.2 years), while median BCS survival for stage IV IBC cases residing in lower SEP counties was 1.9 years (range: 0–8.75 years). The corresponding values for non-IBC were 2.6 years (range: 0–8.9 years) and 2.4 years (range: 0–8.9 years), respectively.
The median BCS survival for stage III IBC cases residing in metro counties was 5.5 years (range: 0–8.9 years), while median BCS survival for stage III IBC cases in non-metro counties was 5.9 years (range: 0–8.9 years). The median survival time for stage III non-IBC cases for both SEP groups had not been reached and therefore not estimated (range for both groups: 0–8.9 years). The median BCS survival for stage IV IBC cases residing in metro counties was 1.8 years (range: 0–8.75 years), while median BCS survival for stage IV IBC cases residing in non-metro counties was 2.3 years (range: 0–6.75 years). The corresponding values for non-IBC were 2.5 years (range: 0–8.9 years) and 2.2 years (range: 0–8.9 years), respectively (metro vs. non-metro Kaplan-Meier survival curves not shown).
Table 2 shows the Cox proportional hazards regression results for the hazard of BC death by inflammatory status, stage, and county-level percent below poverty. In general, residing in a county with ≥20 % persons below the poverty level appeared to increase the hazard of death, though the results were statistically significant only for stage III and IV non-IBC. Cases older in age and of African American race/ethnicity (as compared to NH White) also experienced a significantly higher hazard of death regardless of stage or inflammatory status. There was no significant difference in the Hispanic white, API, and AI/AN race/ethnicity groups hazard of death as compared to NH Whites, except among stage III non-IBC AI/AN cases (HR = 1.40, 95 % CI = 1.06–1.86). High grade and HRN cancers both carried a significantly elevated hazard of death, while not receiving surgery at the BC site and not receiving radiation as part of the first course of treatment both elevated the hazard of BC death, though the radiation result was not significant among stage IV IBC cases.
Table 2.
Hazard ratios (95 % CI) from four Cox proportional hazards regression models examining the relationship between county-level percent of persons below the poverty level, breast cancer type, stage and the hazard of breast cancer death, SEER Program, 1990–2008
| Variable | Model 1: stage III IBC HR (95 % CI) |
Model 2: stage III non-IBC HR (95 % CI) |
Model 3: stage IV IBC HR (95 % CI) |
Model 4: stage IV non-IBC HR (95 % CI) |
|---|---|---|---|---|
| County-Level % | ||||
| Below poverty | ||||
| <20.00 %a | 1.00 | 1.00 | 1.00 | 1.00 |
| ≥20.00 % | 1.13 (0.97–1.32) | 1.13 (1.05–1.22) | 1.05 (0.79–1.39) | 1.12 (1.03–1.21) |
| Age at diagnosis | 1.01 (1.00–1.01) | 1.02 (1.01–1.02) | 1.01 (1.01–1.02) | 1.02 (1.02–1.02) |
| Race/ethnicity | ||||
| NHWhitea | 1.00 | 1.00 | 1.00 | 1.00 |
| African American | 1.46 (1.29–1.66) | 1.41 (1.32–1.50) | 1.36 (1.12–1.64) | 1.23 (1.15–1.31) |
| Hispanic White | 0.99 (0.85–1.14) | 1.00 (0.92–1.08) | 0.95 (0.75–1.21) | 0.99 (0.91–1.07) |
| API | 0.87 (0.71–1.08) | 0.94 (0.86–1.03) | 1.01 (0.71–1.42) | 0.93 (0.85–1.02) |
| AI/AN | 0.96 (0.61–1.50) | 1.40 (1.06–1.86) | 1.35 (0.56–3.29) | 1.19 (0.91–1.57) |
| Hormone receptor | ||||
| Status | ||||
| Positivea | 1.00 | 1.00 | 1.00 | 1.00 |
| Negative | 1.73 (1.58–1.89) | 1.88 (1.79–1.97) | 1.67 (1.43–1.95) | 1.68 (1.60–1.77) |
| Grade | ||||
| Low (I/II)a | 1.00 | 1.00 | 1.00 | 1.00 |
| High (III/IV) | 1.58 (1.42–1.77) | 1.64 (1.56–1.73) | 1.41 (1.17–1.71) | 1.36 (1.30–1.43) |
| Surgery at BC site | ||||
| Yesa | 1.00 | 1.00 | 1.00 | 1.00 |
| No | 2.06 (1.79–2.37) | 2.75 (2.49–3.03) | 1.72 (1.47–2.01) | 1.76 (1.68–1.84) |
| Radiation therapy | ||||
| Yesa | 1.00 | 1.00 | 1.00 | 1.00 |
| No | 1.37 (1.25–1.50) | 1.39 (1.32–1.45) | 1.10 (0.94–1.29) | 1.07 (1.02–1.12) |
All variables in each column are mutually adjusted for each other
Referent Category
Table 3 shows the Cox proportional hazards regression results for the hazard of BC death by inflammatory status, stage, and the county-level poverty-high school index for the subset of cases diagnosed between 2000 and 2008. The only group for which residing in a lower SEP county increased the hazard of death significantly was among stage III non-IBC cases. Similar to the results for percent below poverty, cases older in age and of African American race/ethnicity had a significantly higher hazard of death regardless of stage or inflammatory status, with no significant difference in the hazard of death for the other race/ ethnicity groups. Having HRN cancer, high grade cancer, not receiving surgery at the BC site, and not receiving radiation as part of the first course of treatment all significantly increased the hazard of death, though the radiation result was not significant among stage IV IBC cases.
Table 3.
Hazard Ratios (95 % CI) from four Cox proportional hazards regression models examining the relationship between the county-level poverty-high school index, breast cancer type, stage and the hazard of breast cancer death, SEER Program, 2000–2008
| Variable | Model 1: stage III IBC HR (95 % CI) |
Model 2: stage III non-IBC HR (95 % CI) |
Model 3: stage IV IBC HR (95 % CI) |
Model 4: stage IV non-IBC HR (95 % CI) |
|---|---|---|---|---|
| Poverty-high school | ||||
| Index | ||||
| High SEPa | 1.00 | 1.00 | 1.00 | 1.00 |
| Lower SEP | 1.09 (0.95–1.25) | 1.10 (1.02–1.18) | 0.86 (0.69–1.06) | 1.04 (0.98–1.11) |
| Age at diagnosis | 1.01 (1.01–1.01) | 1.02 (1.02–1.02) | 1.01 (1.01–1.02) | 1.02 (1.02–1.02) |
| Race/ethnicity | ||||
| NHWhitea | 1.00 | 1.00 | 1.00 | 1.00 |
| African American | 1.57 (1.34–1.85) | 1.36 (1.25–1.47) | 1.50 (1.20–1.87) | 1.27 (1.18–1.37) |
| Hispanic White | 0.98 (0.82–1.18) | 1.02 (0.92–1.12) | 1.12 (0.85–1.48) | 1.06 (0.96–1.16) |
| API | 0.94 (0.71–1.24) | 0.93 (0.82–1.05) | 0.90 (0.59–1.37) | 0.88 (0.78–1.00) |
| AI/AN | 0.82 (0.43–1.59) | 1.00 (0.64–1.57) | 1.36 (0.50–3.66) | 1.13 (0.81–1.56) |
| Hormone receptor | ||||
| Status | ||||
| Positivea | 1.00 | 1.00 | 1.00 | 1.00 |
| Negative | 2.05 (1.82–2.32) | 2.25 (2.11–2.40) | 1.68 (1.40–2.01) | 1.75 (1.65–1.86) |
| Grade | ||||
| Low (I/II)a | 1.00 | 1.00 | 1.00 | 1.00 |
| High (III/IV) | 1.51 (1.30–1.75) | 1.74 (1.62–1.86) | 1.52 (1.22–1.90) | 1.37 (1.29–1.45) |
| Surgery at BC site | ||||
| Yesa | 1.00 | 1.00 | 1.00 | 1.00 |
| No | 2.05 (1.72–2.45) | 2.96 (2.64–3.33) | 1.89 (1.57–2.28) | 1.90 (1.79–2.00) |
| Radiation therapy | ||||
| Yesa | 1.00 | 1.00 | 1.00 | 1.00 |
| No | 1.43 (1.27–1.62) | 1.47 (1.38–1.56) | 1.06 (0.88–1.29) | 1.06 (1.00–1.12) |
All variables in each column are mutually adjusted for each other
Referent category
Table 4 shows the Cox proportional hazards regression results for the hazard of BC death by inflammatory status, stage, and metro vs. non-metro county of residence for the subset of cases diagnosed between 2000 and 2008. Although residing in a non-metro county appeared to increase the hazard of BC death, the result was significant only for stage III and IV non-IBC. Similar to the results for the other two county-level SEP measures, cases older in age and of African American race/ethnicity had a significantly higher hazard of death regardless of stage or inflammatory status, with no significant difference between the hazard of death for the other race/ethnicity groups. Also similar to previous results, having HRN cancer, high grade cancer, not receiving surgery at the BC site, and not receiving radiation as part of the first course of treatment all significantly increased the hazard of death, except for stage IV IBC cases.
Table 4.
Hazard ratios (95 % CI) from four Cox proportional hazards regression models examining the relationship between metro vs. non-metro county of residence, breast cancer type, stage and the hazard of breast cancer death, SEER Program, 2000–2008
| Variable | Model 1: stage III IBC HR (95 % CI) |
Model 2: stage III non-IBC HR (95 % CI) |
Model 3: stage IV IBC HR (95 % CI) |
Model 4: stage IV non-IBC HR (95 % CI) |
|---|---|---|---|---|
| County of residence | ||||
| Metroa | 1.00 | 1.00 | 1.00 | 1.00 |
| Non-metro | 1.07 (0.89–1.29) | 1.20 (1.09–1.32) | 1.02 (0.76–1.38) | 1.21 (1.11–1.33) |
| Age at diagnosis | 1.01 (1.01–1.01) | 1.02 (1.02–1.02) | 1.01 (1.01–1.02) | 1.02 (1.02–1.02) |
| Race/ethnicity | ||||
| NH Whitea | 1.00 | 1.00 | 1.00 | 1.00 |
| African American | 1.61 (1.37–1.89) | 1.39 (1.29–1.51) | 1.45 (1.17–1.80) | 1.30 (1.21–1.40) |
| Hispanic White | 1.00 (0.84–1.20) | 1.05 (0.96–1.16) | 1.08 (0.83–1.42) | 1.08 (0.98–1.19) |
| API | 0.95 (0.72–1.26) | 0.94 (0.83–1.06) | 0.88 (0.58–1.34) | 0.89 (0.79–1.01) |
| AI/AN | 0.57 (0.24–1.38) | 1.01 (0.62–1.65) | 0.90 (0.22–3.64) | 1.14 (0.81–1.61) |
| Hormone receptor | ||||
| Status | ||||
| Positivea | 1.00 | 1.00 | 1.00 | 1.00 |
| Negative | 2.06 (1.83–2.33) | 2.25 (2.11–2.40) | 1.68 (1.40–2.02) | 1.75 (1.65–1.86) |
| Grade | ||||
| Low (I/II)a | 1.00 | 1.00 | 1.00 | 1.00 |
| High (III/IV) | 1.50 (1.29–1.73) | 1.74 (1.62–1.87) | 1.52 (1.22–1.90) | 1.37 (1.29–1.45) |
| Surgery at BC site | ||||
| Yesa | 1.00 | 1.00 | 1.00 | 1.00 |
| No | 2.07 (1.73–2.46) | 2.98 (2.65–3.34) | 1.90 (1.58–2.29) | 1.91 (1.80–2.01) |
| Radiation therapy | ||||
| Yesa | 1.00 | 1.00 | 1.00 | 1.00 |
| No | 1.44 (1.27–1.62) | 1.48 (1.39–1.57) | 1.06 (0.88–1.29) | 1.06 (1.00–1.12) |
All variables in each column are mutually adjusted for each other
Referent category
Discussion
Similar to the Yang et al. [11] study, this analysis showed that while unadjusted median survival generally appeared to be lower for those residing in lower SEP counties, after adjustment for age at diagnosis, race/ethnicity, tumor, and treatment characteristics, these survival differences were not significant for IBC. This analysis also similarly found that African American race/ethnicity was an independent predictor of the hazard of BC death for all cases [11].
IBC is a rare disease, comprising ~ 2 % of all BC in the SEER database [2, 4], and is difficult to detect using standard mammography techniques [29–31]. It is therefore possible that, regardless of SEP, women and their health care providers tend to know less about this disease, and therefore it may be undetected and/or untreated for longer intervals in all SEP groups. In this regard, non-IBC cancers may be more likely to be detected earlier leading to a diagnostic biopsy and treatment. Therefore, women residing in lower SEP and more rural counties may experience a diagnostic and treatment delay leading to worse non-IBC survival if they have less access to health care. For example, in addition to less receipt of surgery, lower income, less educated women as well as those without private insurance are less likely to receive breast conserving surgery and more likely to receive mastectomies than their higher SEP counterparts [32–34]. Furthermore, a recent study examining receipt of adjuvant chemotherapy and hormonal therapy adhering to National Comprehensive Cancer Network Clinical Practice Guidelines among women with loco-regional BC found lack of insurance and residence in high poverty and/or low education areas was associated with receipt of non-guideline chemotherapy regimens, while living in a high poverty area was associated with receipt of non-guideline hormonal therapy [35]. It should also be noted only stage III and IV cancers are examined in this analysis. SEP may play a more important role in earlier stage survival due to its association with early detection and treatment.
Furthermore, most recent studies suggest IBC is a distinct biologic entity from other BCs, with these biologic differences likely contributing to poorer IBC survival [6, 7, 36–43]. For instance, typical characteristics of IBC include wide dissemination of tumor cells throughout the breast and associated dermal lymphatics leading to acute onset of clinical signs such as erythema, edema, and breast tenderness and/or pain, as compared to most non-advanced non-IBC’s which do not usually carry these same symptoms [9]. Other IBC histologic/biologic features such as tumors that are rapidly progressive, highly angiogenic and angioinvasive and of high histologic grade with atypical mitotic figures, likely lead to IBC’s propensity for metastasis and otherwise worse prognosis than non-IBC tumors [39].
As in this analysis, African Americans have previously been found to have worse BC and specifically, IBC survival [4, 5, 44–46]. Interestingly, the effect of African American race/ethnicity on poorer survival held even after adjustment for county-level SEP, age at diagnosis, tumor and treatment characteristics. Some previous studies have shown the effect of African American race/ethnicity on BC survival is largely eliminated after adjustment for SEP, while others have shown the effect is independent of certain SEP measures [44, 46–49]. A 2002 study of BC cases in the Metro Detroit SEER registry linked to Michigan Medicaid enrollment files found that after adjustment for age at diagnosis, marital status, stage, Medicaid enrollment, census tract percent below poverty, and surgery type, being African American was no longer associated with poorer survival as compared to White women [49]. However, a 2002 meta-analysis of 14 studies found African American race/ethnicity was an independent predictor of BC mortality after adjustment for SEP, stage, and age at diagnosis [46].
SEP encompasses a large array of characteristics, some easily measured and accounted for and others which can only readily be analyzed through proxy measures. It is possible this analysis does not use the SEP measure(s) most associated with IBC and African American race/ethnicity BC survival. Although there are a multitude of SEP measures and indices available, in the US, poverty level and education have the advantage of being easily understood and comparable across time and geographic areas [50]. Furthermore, the poverty level cut-point and poverty-high school index used in this analysis have been used in previous studies of cancer survival, with areas having ≥20 % poverty generally considered “poverty areas” in census publications [25, 51–53].
Numerous studies have found African American women are more often diagnosed with adverse prognostic characteristics, such as diagnosis at later stage, younger age, and having tumors that are more likely to be estrogen receptor negative and high grade [54–62]. While these characteristics are correlated with the inferior survival seen in African American women, it is also likely that SEP-related factors, such as reproductive history, diet, physical activity, medical co-morbidities, and access to BC screening and treatment also play an important role [46].
No studies have examined IBC vs. non-IBC BCS survival by SEP in a nation-wide, population based tumor registry. A major strength of this study is the use of the SEER database linked to 1990 and 2000 US census derived county-level attributes. The SEER program is considered to be the “gold” standard for data quality worldwide, with rigorous quality control, reliability, and completeness of data recorded timely and uniformly [63]. As IBC is a rare form of BC, the SEER data linked to county attributes offers a unique opportunity to examine IBC survival stratified on county-level SEP characteristics, as well as other tumor and treatment characteristics included in the database. Furthermore, as the SEER program oversamples US minority groups, analyses are also able to be stratified on multiple race/ethnicity groups [14].
Another strength of this study is the use of a comprehensive IBC case definition. IBC is primarily a clinical diagnosis [29–31, 64, 65]. Previous studies have used only the ICD-O 8530 histology code to identify IBC cases, which does not consider clinical skin changes and is not consistent with current AJCC staging guidelines [2, 5, 66, 67]. In this analysis, we used SEER EOD codes for cases prior to 2004 as well as the SEER derived AJCC staging for cases 2004 forward in order to help ensure cases meeting AJCC staging clinical criteria are included, as previous studies using only the ICD-O histologic code 8530 may have underestimated the number of IBC cases [2, 5, 66, 67].
This study has several limitations which should be noted. As the SEER database does not contain information on chemotherapy or hormonal treatment, we were unable to adjust for these important prognostic factors. It is also likely that changes in treatment over time have improved survival for those more recently diagnosed. Major advances in chemotherapy, surgery, and radiotherapy have been implemented during the period from 1990 to 2008 encompassing our analysis [29–31, 64, 65, 68]. However, it is unknown how quickly each of these therapies have been implemented in the community setting. The sub-analysis from 2000–2008 examining the poverty-high school index and metro versus non-metro residence had similar results to the poverty analysis and involved a shorter time period, and thus is less likely to be affected by secular treatment trends. Furthermore, the SEER program is considered to accurately represent the US cancer population treated in various academic and community settings across the country, and therefore can provide an overall picture of BC survival in the US [69].
Another limitation of this study is the inherent ecologic bias when county-level SEP is interpreted as individual-level SEP. An individual’s SEP may have a different effect on BC survival than that seen for county-level SEP measures [70]. Therefore, these results are better viewed as the effect of residing in a county with a particular SEP measure on BCS survival rather than that of an individual’s SEP. However, a previous study found census-level SEP measures have a similar association with individual-level health outcomes as individual SEP [71].
In conclusion, our results indicate IBC has worse survival than non-IBC, most pronounced for stage III cancers, and that residing in a lower SEP, non-metro county may worsen BCS survival, though this result was only significant for non-IBC in multivariate proportional hazards models. African Americans appear to have worse BCS survival regardless of inflammatory status, stage, county-level SEP, tumor, or treatment characteristics. Future research should examine multiple SEP measures and indices, both at the individual and community level, in order to better elucidate potential relationships between SEP and BC survival that will greatly aid in the targeting of BC, and specifically IBC, education, screening, and treatment programs. As this analysis and others have found generally poorer survival for IBC, regardless of SEP or race/ethnicity, it is important that IBC education interventions target women in various SEP and race/ethnicity groups. Finally, as this study and others indicate that African Americans experience poorer BC survival, programs designed specifically for African American women would be especially helpful.
Acknowledgments
The authors thank Dr. William Anderson at the Division of Cancer Epidemiology and Genetics, National Cancer Institute, for providing expertise on IBC and the SEER database, as well as the merged race-ethnicity and stage SEER*stat variables used in this analysis. This work was supported by a Rackham Merit Fellowship (JAS) from the University of Michigan and the Cancer Epidemiology Education in Special Populations Program of the University of Michigan (CA R25 112383). Additional funding was received from the Avon Foundation (AS and SDM), the Breast Cancer Research Foundation (SDM), and the Debbie Strange-Browne Inflammatory Breast Cancer Foundation (SDM).
Footnotes
Note: This study includes the essential elements of the REMARK guidelines for reporting of tumor marker studies in breast cancer research [72].
Conflict of interest None.
Contributor Information
Jennifer A. Schlichting, Department of Epidemiology, University of Michigan School, of Public Health, 109 Observatory St., Ann Arbor, MI 48109-2029, USA, jschlic@umich.edu
Amr S. Soliman, Department of Epidemiology, University of Michigan School, of Public Health, 109 Observatory St., Ann Arbor, MI 48109-2029, USA
Catherine Schairer, Division of Cancer Epidemiology and Genetics, National Cancer Institute National Institutes of Health, Department of Health and Human Services, Bethesda, MD 20892, USA.
David Schottenfeld, Department of Epidemiology, University of Michigan School, of Public Health, 109 Observatory St., Ann Arbor, MI 48109-2029, USA; Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI 48109, USA.
Sofia D. Merajver, Department of Epidemiology, University of Michigan School, of Public Health, 109 Observatory St., Ann Arbor, MI 48109-2029, USA Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI 48109, USA; Center for Global Health, University of Michigan, Ann Arbor, MI 48104, USA.
References
- 1.Anderson WF, Chen BE, Jatoi I, et al. Effects of estrogen receptor expression and histopathology on annual hazard rates of death from breast cancer. Breast Cancer Res Treat. 2006;100:121–126. doi: 10.1007/s10549-006-9231-y. [DOI] [PubMed] [Google Scholar]
- 2.Anderson WF, Schairer C, Chen BE, et al. Epidemiology of inflammatory breast cancer (IBC) Breast Dis. 2005;22:9–23. doi: 10.3233/bd-2006-22103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang S, Alderfer JR, Asmar L, et al. Inflammatory breast cancer survival: the role of obesity and menopausal status at diagnosis. Breast Cancer Res Treat. 2000;64:157–163. doi: 10.1023/a:1006489100283. [DOI] [PubMed] [Google Scholar]
- 4.Hance KW, Anderson WF, Devesa SS, et al. Trends in inflammatory breast carcinoma incidence and survival: the Surveillance, Epidemiology, and End Results program at the National Cancer Institute. J Natl Cancer Inst. 2005;97:966–975. doi: 10.1093/jnci/dji172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang S, Parker SL, Pham T, et al. Inflammatory breast carcinoma incidence and survival: the Surveillance, Epidemiology, and End Results program of the National Cancer Institute, 1975–1992. Cancer. 1998;82:2366–2372. [PubMed] [Google Scholar]
- 6.Anderson WF, Chu KC, Chang S. Inflammatory breast carcinoma and noninflammatory locally advanced breast carcinoma: distinct clinicopathologic entities? J Clin Oncol. 2003;21:2254–2259. doi: 10.1200/JCO.2003.07.082. [DOI] [PubMed] [Google Scholar]
- 7.Dawood S, Ueno NT, Valero V, et al. Differences in survival among women with stage III inflammatory and noninflammatory locally advanced breast cancer appear early: a large population-based study. Cancer. 2011;117:1819–1826. doi: 10.1002/cncr.25682. [DOI] [PubMed] [Google Scholar]
- 8.Merajver SD, Weber BL, Cody R, et al. Breast conservation and prolonged chemotherapy for locally advanced breast cancer: the University of Michigan experience. J Clin Oncol. 1997;15:2873–2881. doi: 10.1200/JCO.1997.15.8.2873. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Angulo AM, Hennessy BT, Broglio K, et al. Trends for inflammatory breast cancer: is survival improving? Oncologist. 2007;12:904–912. doi: 10.1634/theoncologist.12-8-904. [DOI] [PubMed] [Google Scholar]
- 10.Levine PH, Steinhorn SC, Ries LG, et al. Inflammatory breast cancer: the experience of the Surveillance, Epidemiology, and End Results (SEER) program. J Natl Cancer Inst. 1985;74:291–297. [PubMed] [Google Scholar]
- 11.Yang R, Cheung MC, Hurley J, et al. A comprehensive evaluation of outcomes for inflammatory breast cancer. Breast Cancer Res Treat. 2009;117:631–641. doi: 10.1007/s10549-009-0312-6. [DOI] [PubMed] [Google Scholar]
- 12.Dawood S, Ueno NT, Valero V, et al. Identifying factors that impact survival among women with inflammatory breast cancer. Ann Oncol. 2012;23(4):870–875. doi: 10.1093/annonc/mdr319. [DOI] [PubMed] [Google Scholar]
- 13. Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence-SEER 17 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2009 Sub (1973–2007 varying)-Linked To County Attributes-Total U.S., 1969–2007 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2010, based on the November 2009 submission.
- 14.Surveillance Epidemiology and End Results Program. [Accessed 02 Apr 2012];Overview of the SEER Program. 2011 http://seer.cancer.gov/about/overview.html.
- 15.American Joint Committee on Cancer. Breast. In: Edge SB, Byrd DR, Compton CR, et al., editors. AJCC cancer staging manual. 7th edn. New York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 16.Schairer C, Brown LM, Mai PL. Inflammatory breast cancer: high risk of contralateral breast cancer compared to comparably staged non-inflammatory breast cancer. Breast Cancer Res Treat. 2011;129(1):117–124. doi: 10.1007/s10549-010-1324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlichting JA, Soliman AS, Schairer C, et al. Association of inflammatory and noninflammatory breast cancer with socioeconomic characteristics in the Surveillance, Epidemiology, and End Results database, 2000–2007. Cancer Epidemiol Biomarkers Prev. 2012;21:155–165. doi: 10.1158/1055-9965.EPI-11-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Surveillance Epidemiology and End Results Program. [Accessed 02 Apr 2012];SEER research record description: cases diagnosed in 1973–2008. 2011 http://seer.cancer.gov/manuals/TextData.FileDescription.pdf.
- 19.SEER Program Quality Control Section. [Accessed 02 Apr 2012];ICD-O-3 SEER site/histology validation list. 2009 http://seer.cancer.gov/icd-o-3/sitetype.icdo3.d20091204.pdf.
- 20.Johnson CH, editor. SEER program coding and staging manual, revision 1. NIH Publication Number 04-5581. Bethesda, MD: National Cancer Institute; 2004. [Google Scholar]
- 21.Fritz A, Ries L. SEER extent of disease 1988: codes and coding instructions. 3rd edn. Bethesda, MD: U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health; 1998. Cancer Statistics Branch, Surveillance Program, Division of Cancer Control and Population Sciences, National Cancer Institute (eds) Breast: C50.0-C50.6, C50.8-C50.9. [Google Scholar]
- 22.Johnson CH, Adamo M, editors. NIH Publication Number 07-5581, 2008 revision. Bethesda, MD: National Cancer Institute; SEER program coding and staging manual 2007. [Google Scholar]
- 23.Surveillance, Epidemiology, and End Results Program. [Accessed 02 Apr 2012];County attributes. 2009 http://seer.cancer.gov/seerstat/variables/countyattribs/index.html.
- 24.Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat database: county attributes— total U.S., 1969–2007 counties ( www.seer.cancer.gov/seerstat/variables/countyattribs) National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; [Google Scholar]
- 25.Yu XQ. Socioeconomic disparities in breast cancer survival: relation to stage at diagnosis, treatment and race. BMC Cancer. 2009;9:364. doi: 10.1186/1471-2407-9-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crowell RE, Goetz T, Wiggins C, et al. Regional disparities in treatment and survival of early stage non-small cell lung cancer. Ethn Dis. 2007;17:358–364. [PubMed] [Google Scholar]
- 27.Reid-Arndt SA, Cox CR. Does rurality affect quality of life following treatment for breast cancer? J Rural Health. 2010;26:402–405. doi: 10.1111/j.1748-0361.2010.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein J, Ji M, Rea NK, et al. Differences in male breast cancer stage, tumor size at diagnosis, and survival rate between metropolitan and nonmetropolitan regions. Am J Mens Health. 2011;5(5):430–437. doi: 10.1177/1557988311400403. [DOI] [PubMed] [Google Scholar]
- 29.Dawood S, Merajver SD, Viens P, et al. International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol. 2011;22(3):515–523. doi: 10.1093/annonc/mdq345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dushkin H, Cristofanilli M. Inflammatory breast cancer. J Natl Compr Cane Netw. 2011;9:233–240. doi: 10.6004/jnccn.2011.0018. [DOI] [PubMed] [Google Scholar]
- 31.Robertson FM, Bondy M, Yang W, et al. Inflammatory breast cancer: the disease, the biology, the treatment. CA Cancer J Clin. 2010;60:351–375. doi: 10.3322/caac.20082. [DOI] [PubMed] [Google Scholar]
- 32.Kotwall CA, Covington DL, Rutledge R, et al. Patient, hospital, and surgeon factors associated with breast conservation surgery. A statewide analysis in North Carolina. Ann Surg. 1996;224:419–426. doi: 10.1097/00000658-199610000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dolan JT, Granchi TS. Low rate of breast conservation surgery in large urban hospital serving the medically indigent. Am J Surg. 1998;176:520–524. doi: 10.1016/s0002-9610(98)00255-4. [DOI] [PubMed] [Google Scholar]
- 34.Roetzheim RG, Gonzalez EC, Ferrante JM, et al. Effects of health insurance and race on breast carcinoma treatments and outcomes. Cancer. 2000;89:2202–2213. doi: 10.1002/1097-0142(20001201)89:11<2202::aid-cncr8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 35.Wu XC, Lund MJ, Kimmick GG, et al. Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol. 2012;30:142–150. doi: 10.1200/JCO.2011.36.8399. [DOI] [PubMed] [Google Scholar]
- 36.Brouwers B, Paridaens R, Lobelle JP, et al. Clinicopathological features of inflammatory versus noninflammatory locally advanced nonmetastatic breast cancer. Tumour Biol. 2008;29:211–216. doi: 10.1159/000152938. [DOI] [PubMed] [Google Scholar]
- 37.Chang S, Buzdar AU, Hursting SD. Inflammatory breast cancer and body mass index. J Clin Oncol. 1998;16:3731–3735. doi: 10.1200/JCO.1998.16.12.3731. [DOI] [PubMed] [Google Scholar]
- 38.Dawood S, Broglio K, Gong Y, et al. Prognostic significance of HER-2 status in women with inflammatory breast cancer. Cancer. 2008;112:1905–1911. doi: 10.1002/cncr.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Houchens NW, Merajver SD. Molecular determinants of the inflammatory breast cancer phenotype. Oncology. 2008;22:1556–1561. [PubMed] [Google Scholar]
- 40.Kleer CG, Zhang Y, Pan Q, et al. WISP3 and RhoC guanosine triphosphatase cooperate in the development of inflammatory breast cancer. Breast Cancer Res. 2004;6:R110–R115. doi: 10.1186/bcr755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le MG, Arriagada R, Bahi J, et al. Are risk factors for breast cancer similar in women with inflammatory breast cancer and in those with non-inflammatory breast cancer? Breast. 2006;15:355–362. doi: 10.1016/j.breast.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 42.Lo AC, Georgopoulos A, Kleer CG, et al. Analysis of RhoC expression and lymphovascular emboli in inflammatory vs. noninflammatory breast cancers in Egyptian patients. Breast. 2009;18:55–59. doi: 10.1016/j.breast.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turpin E, Bieche I, Bertheau P, et al. Increased incidence of ERBB2 overexpression and TP53 mutation in inflammatory breast cancer. Oncogene. 2002;21:7593–7597. doi: 10.1038/sj.onc.1205932. [DOI] [PubMed] [Google Scholar]
- 44.Newman LA. Breast cancer in African-American women. Oncologist. 2005;10:1–14. doi: 10.1634/theoncologist.10-1-1. [DOI] [PubMed] [Google Scholar]
- 45.Martinez SR, Tseng WH, Canter RJ, et al. Do radiation use disparities influence survival in patients with advanced breast cancer? Cancer. 2012;118:196–204. doi: 10.1002/cncr.26231. [DOI] [PubMed] [Google Scholar]
- 46.Newman LA, Mason J, Cote D, et al. African-American ethnicity, socioeconomic status, and breast cancer survival: a meta-analysis of 14 studies involving over 10,000 African-American and 40,000 White American patients with carcinoma of the breast. Cancer. 2002;94:2844–2854. doi: 10.1002/cncr.10575. [DOI] [PubMed] [Google Scholar]
- 47.Freeman HP, Wasfie TJ. Cancer of the breast in poor black women. Cancer. 1989;63:2562–2569. doi: 10.1002/1097-0142(19890615)63:12<2562::aid-cncr2820631234>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 48.Liberman L, Freeman HP, Chandra S, et al. Carcinoma detection at the breast examination center of Harlem. Cancer. 2002;95:8–14. doi: 10.1002/cncr.10640. [DOI] [PubMed] [Google Scholar]
- 49.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94:490–496. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 50.Krieger N, Chen JT, Waterman PD, et al. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter?: the Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156:471–482. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]
- 51.Singh GK, Miller BA, Hankey BF, Edwards BK. NIH Publication No. 03-5417. Bethesda, MD: National Cancer Institute; 2003. Area socioeconomic variations in U.S. cancer incidence, mortality, stage, treatment, and survival, 1975–1999. NCI cancer surveillance monograph series, number 4. [Google Scholar]
- 52.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 53.Bishaw A. Census 2000 special reports CENSR-16. Washington, DC: U.S. Department of Commerce, Economics and Statistics Administration, U.S. Census Bureau; 2005. [Accessed 02 Apr 2012]. Areas with concentrated poverty: 1999. http://www.census.gov/prod/2005pubs/censr-16.pdf. [Google Scholar]
- 54.Howlader N, Noone A, Krapcho M, et al. [Accessed 02 Apr 2012];SEER cancer statistics review, 1975–2008. 2011 http://seer.cancer.gov/csr/1975_2008/. Based on November 2010 SEER data submission, posted to SEER website, 2011.
- 55.Elledge RM, Clark GM, Chamness GC, et al. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J Natl Cancer Inst. 1994;86:705–712. doi: 10.1093/jnci/86.9.705. [DOI] [PubMed] [Google Scholar]
- 56.Kovi J, Mohla S, Norris HJ, et al. Breast lesions in black women. Pathol Annu. 1989;24(Pt 1):199–218. [PubMed] [Google Scholar]
- 57.Mohla S, Enterline JP, Sampson CC, et al. A predominance of poorly differentiated tumors among black breast cancer patients: management and screening implications. Prog Clin Biol Res. 1982;83:249–258. [PubMed] [Google Scholar]
- 58.Mohla S, Sampson CC, Khan T, et al. Estrogen and progesterone receptors in breast cancer in Black Americans: correlation of receptor data with tumor differentiation. Cancer. 1982;50:552–559. doi: 10.1002/1097-0142(19820801)50:3<552::aid-cncr2820500328>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 59.Chen VW, Correa P, Kurman RJ, et al. Histological characteristics of breast carcinoma in blacks and whites. Cancer Epidemiol Biomark Prev. 1994;3:127–135. [PubMed] [Google Scholar]
- 60.Newman LA, Alfonso AE. Age-related differences in breast cancer stage at diagnosis between black and white patients in an urban community hospital. Ann Surg Oncol. 1997;4:655–662. doi: 10.1007/BF02303751. [DOI] [PubMed] [Google Scholar]
- 61.Swanson GM, Lin CS. Survival patterns among younger women with breast cancer: the effects of age, race, stage, and treatment. J Natl Cancer Inst Monogr. 1994;16:69–77. [PubMed] [Google Scholar]
- 62.Newman LA, Bunner S, Carolin K, et al. Ethnicity related differences in the survival of young breast carcinoma patients. Cancer. 2002;95:21–27. doi: 10.1002/cncr.10639. [DOI] [PubMed] [Google Scholar]
- 63.National Cancer Institute. SEER: Surveillance, Epidemiology, and End Results Program brochure. [Accessed 02 Apr 2012];NIH Publication No. 05-4772. 2005 http://seer.cancer.gov/about/SEER_brochure.pdf.
- 64.Dawood S, Cristofanilli M. Inflammatory breast cancer: what progress have we made? Oncology (Williston Park) 2011;25(264–70):273. [PubMed] [Google Scholar]
- 65.Dawood S. Biology and management of inflammatory breast cancer. Expert Rev Anticancer Ther. 2010;10:209–220. doi: 10.1586/era.09.90. [DOI] [PubMed] [Google Scholar]
- 66.Wingo PA, Jamison PM, Young JL, et al. Population-based statistics for women diagnosed with inflammatory breast cancer (United States) Cancer Causes Control. 2004;15:321–328. doi: 10.1023/B:CACO.0000024222.61114.18. [DOI] [PubMed] [Google Scholar]
- 67.Taylor SH, Walters R. Potential impact of tumor registry rule changes for recording inflammatory breast cancer. Cancer. 2010;116:2745–2747. doi: 10.1002/cncr.25174. [DOI] [PubMed] [Google Scholar]
- 68.Woodward WA, Cristofanilli M. Inflammatory breast cancer. Semin Radiat Oncol. 2009;19:256–265. doi: 10.1016/j.semradonc.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 69.Yu JB, Gross CP, Wilson LD, et al. NCI SEER public-use data: applications and limitations in oncology research. Oncology (Williston Park) 2009;23:288–295. [PubMed] [Google Scholar]
- 70.Morgenstern H. Ecologic studies. In: Rothman KJ, Greenland S, Lash TL, editors. Modern epidemiology. 3rd edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 71.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies (REMARK) J Clin Oncol. 2006;100(23):9067–9072. doi: 10.1200/JCO.2004.01.0454. [DOI] [PubMed] [Google Scholar]