Abstract
A single nucleotide polymorphism in PTPN22 is linked to increased disease susceptibility in a range of autoimmune diseases including systemic lupus erythematosus (SLE). PTPN22 encodes the Lyp phosphatase that dampens TCR signaling and is necessary for signaling downstream of toll-like receptors in myeloid cells. To understand these dual functions in disease, we examined the impact of deficiency in PTPN22 on a spontaneous murine model of SLE. Male PTPN22 KO mice carrying BXSB chromosome 1 and the Yaa disease accelerating factor, developed disease at a similar rate and severity as PTPN22 WT. In contrast, although female BXSB mice showed no differences in survival in the absence of PTPN22, autoantibody production was significantly increased and splenic populations associated with pathogenesis in this model were expanded in the PTPN22 KO group. These findings support the notion that when coupled with other predisposing autoimmunity genes, PTPN22 deficiency contributes to a predisposition to lupus pathogenesis.
Keywords: Systemic Lupus Erythematosus, PTPN22, Tolerance, T follicular helper cells, Autoantibodies, BXSB
1. Introduction
The autoimmunity associated allele of PTPN22, R620W (C1858T), has been linked to a number of autoimmune conditions in humans such as type I diabetes (T1D) [1], rheumatoid arthritis (RA) [2] and systemic lupus erythematosus (SLE) [3]. To investigate the role PTPN22 plays in these diseases numerous mouse models of autoimmunity in which PTPN22 has been deleted, overexpressed, knocked down or mutated are beginning to emerge [4-8].
For SLE, multiple studies have shown increased risk associations between the C1858T SNP in PTPN22 [3, 9-11] over a range of ethnic populations with odds ratios (OR) for the T allele varying between 1.32-2.56. Interestingly, one study showed a stronger relationship between the PTPN22 risk variant in pediatric-onset SLE in Mexican populations than in adult-onset SLE in Caucasians [12]. Recent reports have even suggested correlation of this SNP with distinct disease subclasses, for example those patients with anti-cardiolipin antibodies [13].
PTPN22 encodes a phosphatase known as lymphoid tyrosine phosphatase (LYP) in humans or PEST-enriched phosphatase (PEP) in mice. Its function is best characterized in T cells in which it functions to dephosphorylate proximal TCR signaling molecules (Lck, ZAP-70, Src family kinases) that regulate TCR driven activation [4, 14, 15]. Loss of PTPN22 on the B6 background results in accumulation of memory T cells, increased germinal centers and serum IgG although these mice do not exhibit more autoantibodies or autoimmunity, possibly due to increased Treg numbers and function, or the lack of other factors that contribute to autoimmune disease [4, 16, 17]. Studies have suggested a role for PTPN22 in B cell signaling [18-22], although the extent to which this may be a consequence of increased T cell help is unresolved [4, 23]. Recently a novel, nonphosphatase role for PTPN22 in myeloid cell activity has been described downstream of TLR signaling which is necessary for efficient type I IFN production [24].
Two recent reports in which mice were engineered to express a mutation (R619W) analogous to the human R620W variant have described a phenotype similar to that of the PTPN22 KO mouse [5, 19]. The most recent of these papers describes the breaking of tolerance in these mice manifesting in systemic autoimmunity when on a mixed 129/B6 genetic background, a phenotype that is lost with successive backcrosses to B6 [19]. PTPN22 KO or mutation is not sufficient to develop spontaneous autoimmunity so the use of established autoimmune models or multiple gene knock-outs/mutations have been used to study this gene in a disease specific context [6, 7, 17, 23, 25].
The BXSB mouse, a recombinant inbred strain derived from C57BL/6 and SB/Le mice, develops SLE [26, 27]. This disease is characterized by B cell hyperplasia in peripheral lymphoid organs. Male mice develop more rapid and severe disease compared to females due to the presence of a Y chromosome linked accelerating factor (Yaa) that is an X chromosome translocation resulting in duplication of at least 16 genes including the Tlr7 gene [28, 29]. Males typically die around 4-5 months of age and pathology includes immune complex mediated renal disease. Females typically have a 50% survival of approximately 19.4 months of age but develop detectable levels of autoantibodies earlier [26, 27]. BXSB susceptibility regions aside from the Yaa locus can be found on chromosomes 1, 3 and 13. Regions on chromosome 1 that have been shown to confer lupus phenotypes include Bxs1-4 [30, 31]. The Yaa locus alone is insufficient to cause disease on non-autoimmune prone backgrounds but accelerates disease on the lupus prone backgrounds through a TLR7/type I IFN mechanism [28, 29, 32]. Type I IFN is crucial to disease in both mouse models and human lupus [33, 34].
To investigate the effect of PTPN22 on SLE we introduced regions from chromosome 1 of BXSB on PTPN22 KO, this report is the first to describe the effect of PTPN22 on a classical, spontaneous mouse model of lupus.
2. Materials and Methods
2.1 Mice
Experimental procedures were carried out according to the National Institutes of Health Guide for the care and use of laboratory animals and approved by the Scripps Institutional Animal Care and Use Committee. PTPN22 −/− mice were obtained from Dr. Andrew Chan (Genentech, San Francisco, CA) and have previously been described [4]. BXSB/Scr mice were obtained from Scripps breeding colony and bred to PTPN22 −/− mice. Male BXSB-Yaa were crossed to female PTPN22 −/− mice and the F1 were then bred to female BXSB mice until all selected microsatellite regions on chromosome 1 were homozygous for BXSB. BXSB PTPN22 +/- mice resulting from this cross were then interbred to yield BXSB PTPN22 +/+, BXSB PTPN22 +/− and PTPN22 −/− and used in subsequent assays. Microsatellite markers used to track BXSB desired regions were D1mit3, D1mit21, D1mit387 and D1mit206 (this includes chromosome 1 regions between 19.8 and 174.9 Mb) as described in [31].
2.2 Flow cytometry
Cells to be stained were resuspended in FACS buffer (HBSS containing 1% FCS) and incubated with the indicated antibodies for 15 minutes on ice. Cells were then washed in FACS buffer before acquisition on an LSR-II flow cytometer (BD Bioscience, Franklin Lakes, NJ) and analysis using Flowjo (Treestar). Antibodies (Biolegend, San Diego, CA unless otherwise stated) used were anti-mouse CD4 PerCP-Cy5.5, CD8 Pacific Blue/APC-cy7, PD-1 FITC, CXCR5-biotin (BD Bioscience), CD44 Pacific Blue, GL-7 FITC, FAS PE, CD138 APC, CD19 APC-cy7, CD23 PE, CD21 PerCP-Cy5.5, CD11b-biotin, CD11c Pacific Blue/APC, B220 PE, PDCA-1 Pacific Blue and streptavidin APC/FITC/PerCP. For intracellular staining of markers, an intracellular staining kit (Fix/Perm, eBioscience, San Diego CA) was used together with anti-mouse Foxp3 PE (eBioscience).
2.3 ELISA
Serum was collected from mice at the stated time points. Maxisorp plates (Nunc, Rochester, NY) were coated with 3.6μg/ml of chromatin overnight at 4°C. Plates were blocked in 1% gelatin (Sigma Aldrich) for an hour at 37°C. Plates were washed three times with wash buffer (HBSS with 0.1% Tween-20 (Sigma Aldrich)). Sera were diluted accordingly following optimization for each experiment in reagent buffer (HBSS containing 1% BSA, 0.1% Tween-20) and incubated on the plate in duplicate for 1 hour at 37°C. Plates were washed three times. Anti-mouse IgG alkaline phosphatase (AP) was then diluted and added to the wells for a further hour at 37°C (Jackson Immunoresearch). Plates were washed and then incubated with pNPP AP substrate (Sigma Aldrich). Plates were read using a Versamax plate reader (Molecular devices, Sunnyvale, CA) at 405 nm.
2.4 Anti-Nuclear Antibody staining
ANAs were detected on Hep2 slides (MBL Bion, Des Plains, IL) at 1/100 diluted sera and 1/200 diluted Alexa Fluor 488-conjugated anti-mouse IgG secondary antibody (Invitrogen) as described in [35].
2.5 Proteinuria
Proteinuria was measured by Bio-Rad protein assay (Bio-rad) according to the manufacturers protocol. Urine was diluted 1:100 and BSA serial dilutions were prepared for a standard curve (Sigma-Aldrich).
2.6 Histology
Sections of kidney, lung, liver, heart and spleen were collected from BXSB mice and zinc-formalin fixed. Sections were then stained with Periodic acid-Schiff (PAS) and hematoxylin (TSRI histology core) and scored blindly. For glomerulonephritis a clinical score on the scale of 1-4 was assigned to each [36].
2.7 Immunizations
6-week-old male BXSB-Yaa mice were immunized subcutaneously (s.c.) with 100μg of NP-KLH (Biosearch technologies, Novarto, CA) in complete Freund's adjuvant (CFA) (Difco, Detroit, MI). Draining lymph node and spleen were collected at 11 days post immunization.
2.8 Intracellular staining of IFNα
Splenocytes were stimulated in a 96 well plate with either Imiquimod (5μg/ml) or R848 (1μg/ml) in the presence of Brefeldin A (Sigma Aldrich) for 5 hours and 37°C. Following this incubation time, surface markers for pDCs (CD19- PDCA+ B220+) were stained and the cells were then fixed and permeabilized using the Cytofix/Cytoperm kit (BD bioscience) and stained for IFNα (PBL Assay Science). Cells were then analyzed by flow cytometry.
2.9 Statistics
Graphs were assembled and analyzed using Prism 5 software (Graphpad, San Diego, CA). For multiple group analyses, one-way ANOVA with Tukeys post-test was carried out. For comparison of two-group data sets, a two-tailed Students t-test was used. P<0.05 was considered significant.
3. Results
3.1 Disease onset in male BXSB-Yaa is unaffected by PTPN22
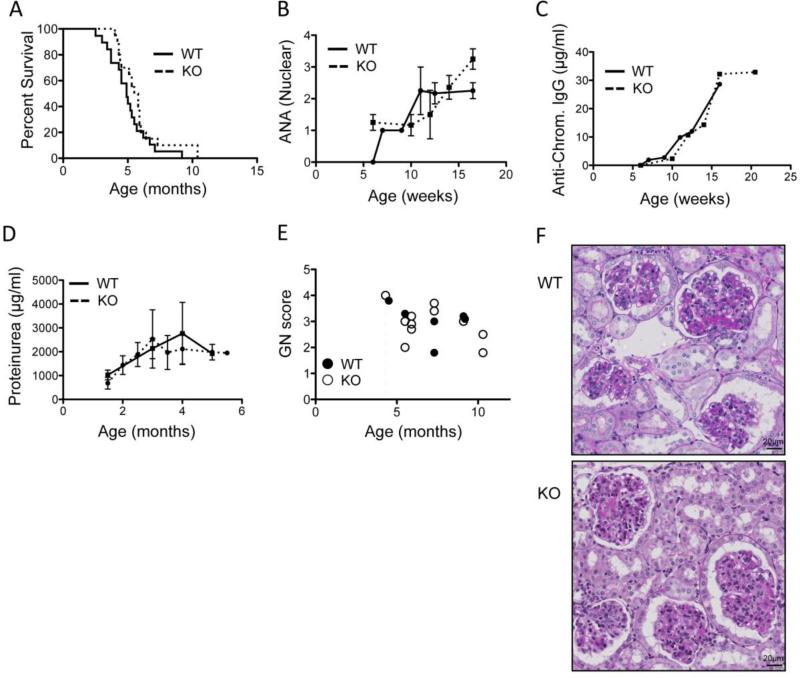
To study the effect of PTPN22 on SLE we backcrossed defined regions of the BXSB chromosome 1 onto the WT or PTPN22 KO B6 background until homozygosity (see methods 2.1 for more details). In addition, the Y chromosome was fixed for BXSB in all cases so as to include the lupus accelerating Yaa locus. Survival was found to be similar in both the WT and PTPN22 KO cohorts of male mice (median survival was 4.9 vs 5.6 months for WT and KO respectively) (Figure 1A). In addition anti-nuclear antibody and anti-chromatin antibody titers in the sera of both groups of mice was similar (Figure 1B and C). Proteinuria was also similar amongst WT and KO groups of animals (Figure 1D). Autopsies of these animals revealed the major cause of death was kidney disease with minimal lung or heart pathologies. Glomerulonephritis was scored and showed no difference between WT and KO mice (Figure 1E). Figure 4F shows representative images of kidney sections showing typical changes of immune complex-mediated glomerulonephritis including enlarged glomeruli, presence of deposits, increased cellularity, and inflammatory cell infiltrates. Overall, these data suggest that PTPN22 does not influence onset and severity of disease in the male BXSB-Yaa model of SLE.
Figure 1.
PTPN22 does not affect the onset of disease on the BXSB.Yaa background. A) shows male PTPN22 KO and WT BXSB.Yaa survival (WT n=19, KO n=20). B) shows anti-nuclear antibody levels in the serum (WT n=5, KO n=12) C) shows serum anti-chromatin IgG concentration as measured by ELISA for PTPN22 KO and WT BXSB.Yaa mice (WT n=5, KO n=12). D) shows proteinuria concentration measured by Bradford assay (WT n=10, KO n=17). E&F) kidneys were collected, sectioned and scored for glomerulonephritis (each data point represents 1 mouse). Images are representative of the median GN score for each genotype and are PAS and hematoxylin stained (magnification 40x).
Figure 4.
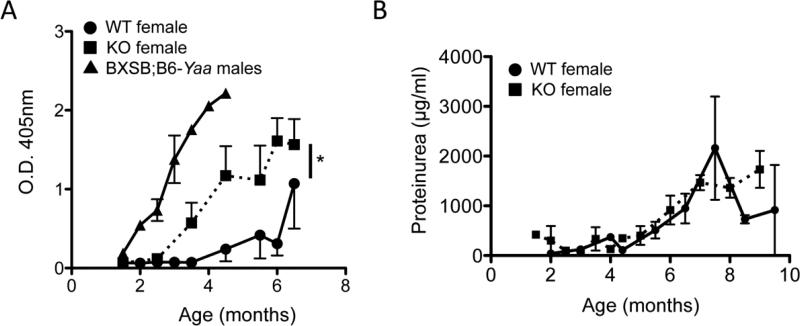
PTPN22 KO increases autoantibody production in female BXSB mice. A) Sera from PTPN2 KO (squares) and WT (circles) BXSB female mice were collected and anti-chromatin antibody levels were measured by ELISA (WT n=8, KO n=8). Part A also includes male BXSB.Yaa as a comparison (triangles). B) shows proteinuria concentration in PTPN22 KO and WT mice (WT n=8, KO n=7). * p<0.05.
3.2 Loss of PTPN22 on the male BXSB-Yaa background enhances T-dependent antigen responses
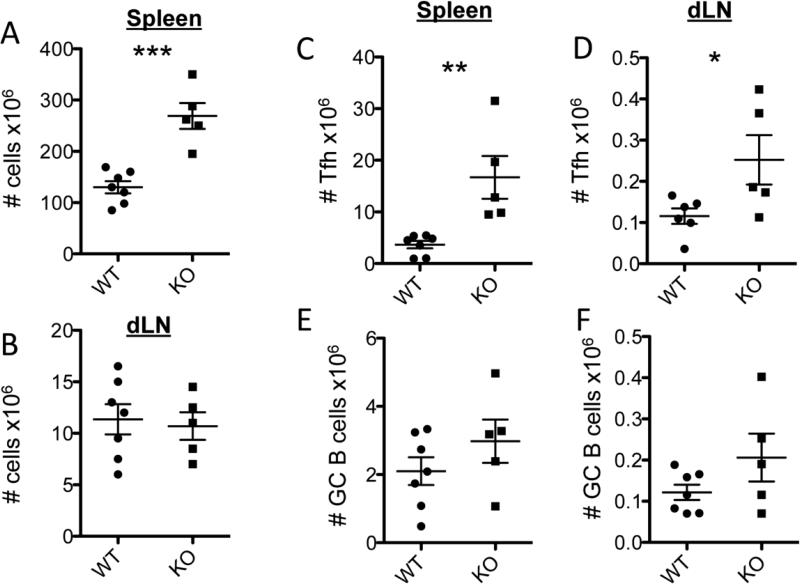
We have previously reported that PTPN22 KO mice on the B6 background have higher numbers and increased function of TFH cells leading to larger germinal center responses and antibody production. In light of the lack of disease enhancement in PTPN22 KO male BXSB-Yaa mice, we asked whether this phenotype was maintained on the BXSB background. At 6-weeks of age, which is prior to the onset of lupus symptoms, mice were immunized with NP-KLH in CFA, and 11 days later the spleen and draining LN (dLN) were collected and analyzed for germinal center activity (Figure 2). TFH numbers were significantly increased in both the spleen and dLN of PTPN22 KO mice compared to WT (Figure 2A and B). GC B cells numbers were increased in the spleens and LN of the KO mice compared to WT although these differences were not statistically significant (Figure 2 C and D). Overall these data show that PTPN22 KO on the BXSB-Yaa male background increases GC activity to a foreign antigen.
Figure 2.
PTPN22 KO BXSB.Yaa mice have increased T-dependent antigen responses. Six-week-old PTPN22 KO and WT BXSB.Yaa mice were immunized with NP-KLH in CFA s.c. and 11 days later the spleen and draining LN were harvested and stained for flow cytomteric analysis. Total numbers of cells is shown for the spleen (A) and dLN (B). Absolute numbers of TFH cells (CD4+ CD44hi CXCR5+ PD-1+) in are shown for the spleen (C) and dLN (D). Absolute numbers of germinal center B cells (CD19+ GL-7+ FAS+) are shown for the spleen (E) and dLN (F). All data points represent 1 mouse and graphs show pooled data from at least 3 independent experiments. * p<0.05; *** p<0.001.
3.3 PTPN22 KO mice have less IFNα producing plasmacytoid dendritic cells
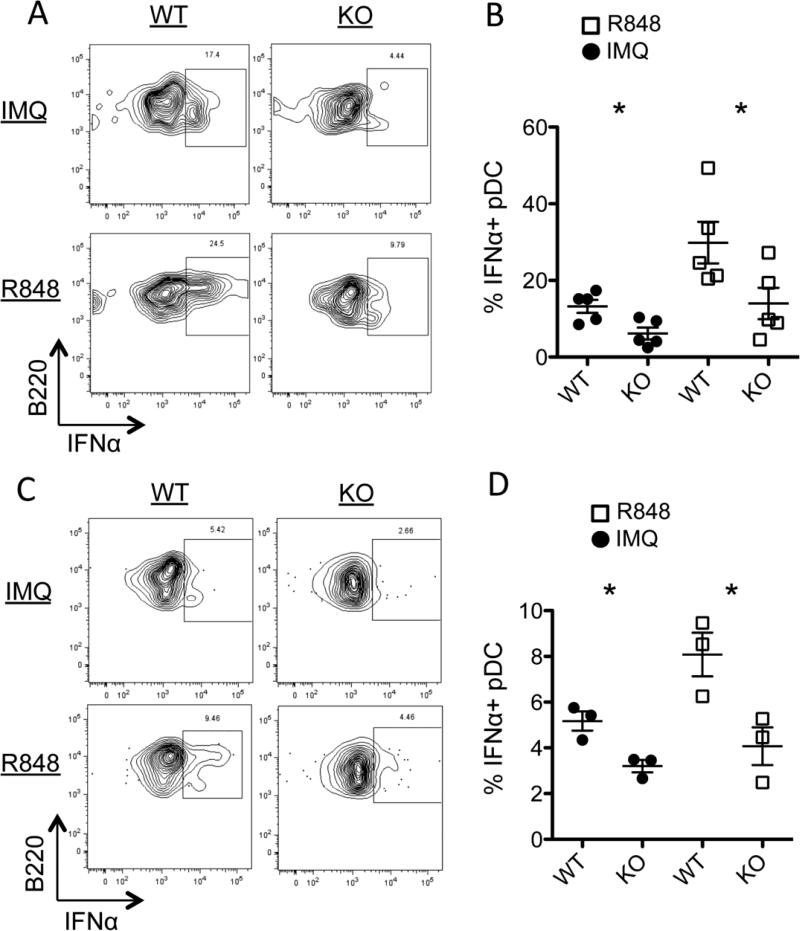
Type I IFN is a major driver of disease during the initiating stages of BXSB-Yaa due to a gene duplication of TLR7 in the Yaa locus [28, 29]. It has been reported that PTPN22 deificiency reduces production of IFNα by myeloid cells [24]. To explore the consequence of PTPN22 KO on type I IFN production we stimulated splenocytes in vitro with TLR7 agonists, Imiquimod or R848, and measured IFNα by intracellular flow cytometry. Figure 3A and B confirms results previously published showing that in response to TLR7 stimulation, plasmacytoid dendritic cells (pDCs) in B6 PTPN22 KO mice produce significantly less IFNα than WT B6 mice. Similarly on the BXSB-Yaa background, PTPN22 KO mice have significantly less IFNα producing pDCs compared to WT (Figure 3C and D). These BXSB-Yaa mice were all less than 8 weeks old, prior to the onset of disease. Overall these data show that PTPN22 is required for efficient IFNα production downstream of TLR7, but does not completely abolish its expression.
Figure 3.
PTPN22 is necessary for efficient IFNα production by pDCs. Splenocytes were stimulated with either Imiquimod (IMQ) or R848 for 4 hours in vitro. The cells were then fixed and permeabilized and stained for IFNα. A) Representative flow cytometry plots of IFNα expression on B6 pDCs (gated on CD19- B220+ PDCA-1+). B) Shows combined data of B6 PTPN22 WT and KO IFNα+ pDCs. C) PTPN22 KO or WT BXSB-Yaa (younger than 2 months) splenocytes were stimulated for 4 hours in vitro and IFNα was stained and analyzed by flow cytometry. D) Shows combined data from PTPN22 WT and KO BXSB-Yaa mice. Each data point represents 1 mouse. Figure shows results of 2 independent experiments. * p<0.05.
3.4 PTPN22 KO BXSB females have increased anti-chromatin IgG
Female BXSB mice do not die from systemic autoimmunity until very late, however they do develop auto-antibodies. PTPN22 KO and WT BXSB females showed good survival throughout the 10-month study period of our experiments, with no symptoms or health concerns (data not shown). However, PTPN22 KO BXSB females exhibit significantly increased and earlier onset anti-chromatin IgG sera titers compared to WT BXSB female mice (Figure 4A). Male BXSB-Yaa sera were also included as a comparison and shows higher and earlier onset of anti-chromatin IgG compared to both female genotypes. Proteinuria was also measured and was unaffected by PTPN22 expression (Figure 4B), both female groups have lower levels of proteinuria than male BXSB-Yaa mice (Figure 1D). Collectively these data show that PTPN22 can affect auto-antibody production in BXSB females although disease onset is unaffected within the age range observed in this study.
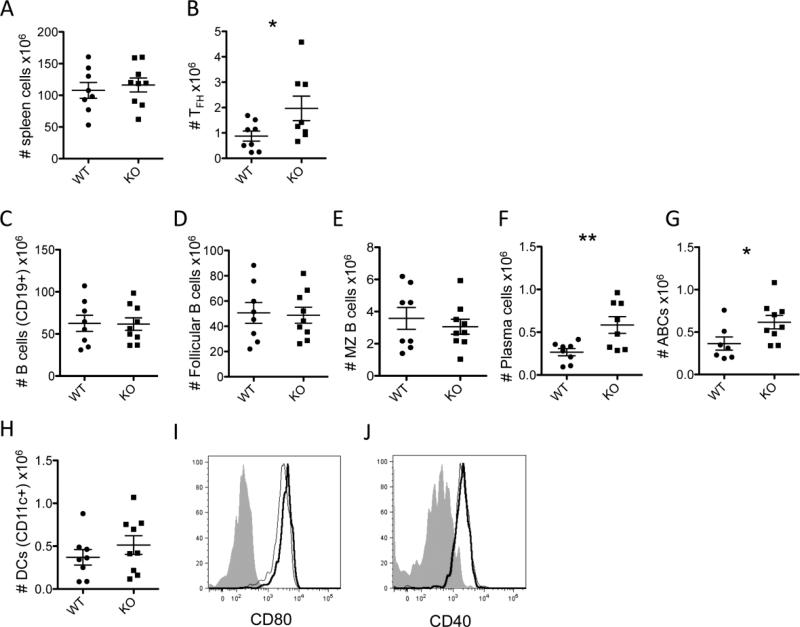
3.5 Spleens of female PTPN22 KO BXSB mice have increased numbers of disease associated lymphocyte populations
Spleens were collected from PTPN22 WT and KO BXSB females and stained for various T cell, B cell and antigen presenting cell populations at 4-months of age (Figure 5), when antibody titers differed significantly (Figure 4). The spleen cellularity overall was similar in KO mice compared to WT (Figure 5A), but TFH cells were significantly increased (Figure 5B). Total B cells as measured by CD19 expression were similar in PTPN22 KO BXSB mice compared to WT, and within this gate, follicular B cells and marginal zone B cells remained constant (Figure 5C-E). Plasma cells and age-associated B cells (ABCs) were both significantly increased in the spleens of PTPN22 KO BXSB mice compared to WT (Figure 5F and G). Dendritic cells were also slightly increased, although not to statistically significant levels in the KO mice compared to WT (Figure 5H). The expression of CD80 (Figure 5I) was marginally higher on KO DCs and CD40 (Figure 5J) was similar on WT and KO DCs. Overall these data suggest that PTPN22 deficiency amongst BXSB mice results in the expansion of lymphocytes that are commonly associated with lupus pathogenesis.
Figure 5.
Analysis of 4-month-old spleen populations of female BXSB mice. Spleens were collected from PTPN22 WT and KO BXSB female mice at 4 months of age and stained for various populations for analysis by flow cytometry. A) total spleen cellularity. Absolute numbers of TFH cells (B). Absolute numbers of CD19+ B cells (C), CD19+ CD21int CD23++ follicular B cells (D), CD19+ CD21+ CD23- marginal zone B cells (E), CD19low CD138+ plasma cells (F) and CD19+ CD11b+ CD11c+ age-associated B cells (G). Absolute numbers of dendritic cells (CD19- CD11c+) are shown in panel H and CD80 (I) and CD40 (J) expression on these cells is shown in the histograms (WT thin line, KO thick line, isotype control filled grey). All data points represent 1 mouse and graphs show pooled data from at least 3 independent experiments.
4. Discussion
The presence of the R620W allele of PTPN22 has been associated with multiple autoimmune diseases including SLE [1-3]. This paper is the first report to describe the effects of PTPN22 on a spontaneous mouse model of lupus. By using the BXSB model we were able to investigate the contribution of PTPN22 on both highly and moderately susceptible backgrounds because of the presence of the lupus-promoting Yaa in males, but not females.
Surprisingly, PTPN22 deficiency resulted in no change in disease severity or lifespan of male BXSB-Yaa mice. Despite the observation that PTPN22 KO BXSB mice still exhibited increased TFH and germinal center responses to foreign antigen as evidenced by the response to NP-KLH, this had no consequence on disease in this model.
A complication in the male BXSB-Yaa model is the effect of PTPN22 on myeloid cell TLR7 signaling and type I IFN production. DCs require PTPN22 for efficient TRAF3 auto-ubiquitination downstream of TLR4, 7 and 9 signaling in a non-phosphatase dependent manner [24]. As a result PTPN22 KO DCs are impaired in their type I IFN producing capacity. The male BXSB-Yaa model is highly dependent on TLR7 signaling and type I IFN [28, 37]. Early type I IFN blockade is beneficial in attenuating disease in the BXSB male model, although it is less effective when administered late, suggesting that the subsequent adaptive immune response is sufficient to cause death in this model. In the case of PTPN22 KO BXSB male mice, there are 2 opposing effects; the early type I IFN production by DCs may be lowered but the later germinal center response is amplified. The observation that disease is essentially unchanged by loss of PTPN22 may reflect these two opposing processes.
In the case of human SLE, the behavior of the lupus predisposing PTPN22 R620W variant in leading to reduced type I IFN production in a predominantly type I IFN driven disease is paradoxical [24, 38]. However it has been shown that target tissues of SLE are characterized by pro-inflammatory cytokines that are suppressed by type I IFN e.g. IL-1β and TNF-α [39, 40]. This, together with reported defects in B cell central and peripheral tolerance checkpoints associated with the R620W mutation in humans, could predispose individuals to inflammation and disease [21]. Despite the increased risk associated with the PTPN22 R620W mutation and SLE, the odds ratio is lower than that for RA and T1D where PTPN22 ranks 2nd and 3rd respectively amongst genetic risk variants [41]. As such the effect PTPN22 has on SLE is likely to be smaller than for RA and T1D.
Female BXSB mice live longer than males and all of the female mice in our experiments survived the 10-month study period. As we only bred BXSB chromosome 1 onto the B6 background, this could have resulted in less severe disease than is usually expected in female BXSB mice. PTPN22 KO BXSB females did not exhibit increased severity of disease or the time of onset within the period of observation, but PTPN22 deficiency did increase several factors associated with pathogenesis. Autoantibody production was significantly higher in PTPN22 KO BXSB female mice compared to WT BXSB with detectable anti-chromatin antibodies at 3 months compared to greater than 4 months in WT BXSB mice. In addition female PTPN22 KO BXSB mice had significantly increased numbers of TFH, plasma cells and ABCs at 4 months compared to WT. These results confirm similar reports of ABC accumulation in the PTPN22R619W mouse as well as previous reports of increased TFH activity in the PTPN22 KO mouse [19, 23]. ABCs, as defined in this report as CD19+ CD11b+ CD11c+, have been recently described to accumulate in female strains of autoimmune prone mice at a young age [42]. In the NZB/W F1 model of lupus, ABCs are detectable at 3 months and accumulate to high levels in the spleen at 8-10 months of age. They secrete anti-chromatin antibodies and are dependent on TLR7 for their function. The association between their increased numbers and increased amounts of anti-chromatin antibodies in PTPN22 KO BXSB is an area that will be further studied in this model as well as other autoimmune strains lacking PTPN22.
Despite these differences between WT and PTPN22 KO BXSB females, proteinuria and kidney histology was similar. It is possible that even in PTPN22 KO females, levels of autoantibodies do not reach the threshold level necessary to induce disease within the time frame of our observation. Other pro-autoimmune alleles may be required to push the immune system over the threshold needed for end-organ disease.
6. Conclusion
In summary, the results in this report demonstrate that the disease kinetics of the male BXSB-Yaa model was probably too severe to analyze the effect of PTPN22 on spontaneous lupus, while in less susceptible female BXSB mice, PTPN22 deficiency played a role in autoantibody production, but this was not sufficient for progression to kidney pathology. Further investigation of PTPN22 in other spontaneous models of lupus with more favorable kinetics is warranted.
Highlights.
- PTPN22 is associated with increased risk to multiple autoimmune diseases including systemic lupus erythematosus
- Crossing of PTPN22 KO to the BXSB model of lupus allowed us to study the effect on disease
- PTPN22 KO does not affect disease onset or severity on the male BXSB-Yaa background
- PTPN22 KO increases autoantibody production in female BXSB mice but does not accelerate disease
Acknowledgements
We would like to thank the TSRI histology core for preparation of stained organ sections. L.A.S. was supported by NIH grant #DK050824-20. D.H.K. was supported by grant #HL114408. K.M.P. was supported by grant #ES022625 and ES021464.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare that no conflict of interest exists
References
- 1.Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet. 2004;36:337–8. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- 2.Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, Alexander HC, et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. American journal of human genetics. 2004;75:330–7. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyogoku C, Langefeld CD, Ortmann WA, Lee A, Selby S, Carlton VE, et al. Genetic association of the R620W polymorphism of protein tyrosine phosphatase PTPN22 with human SLE. American journal of human genetics. 2004;75:504–7. doi: 10.1086/423790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasegawa K, Martin F, Huang G, Tumas D, Diehl L, Chan AC. PEST domain-enriched tyrosine phosphatase (PEP) regulation of effector/memory T cells. Science. 2004;303:685–9. doi: 10.1126/science.1092138. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Zahir N, Jiang Q, Miliotis H, Heyraud S, Meng X, et al. The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness. Nat Genet. 2011;43:902–7. doi: 10.1038/ng.904. [DOI] [PubMed] [Google Scholar]
- 6.Zheng P, Kissler S. PTPN22 silencing in the NOD model indicates the type 1 diabetes-associated allele is not a loss-of-function variant. Diabetes. 2013;62:896–904. doi: 10.2337/db12-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeh LT, Miaw SC, Lin MH, Chou FC, Shieh SJ, Chuang YP, et al. Different modulation of Ptpn22 in effector and regulatory T cells leads to attenuation of autoimmune diabetes in transgenic nonobese diabetic mice. Journal of immunology. 2013;191:594–607. doi: 10.4049/jimmunol.1203380. [DOI] [PubMed] [Google Scholar]
- 8.Wu DJ, Zhou W, Enouz S, Orru V, Stanford SM, Maine CJ, et al. Autoimmunity-associated LYP-W620 does not impair thymic negative selection of autoreactive T cells. PLoS One. 2014;9:e86677. doi: 10.1371/journal.pone.0086677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gateva V, Sandling JK, Hom G, Taylor KE, Chung SA, Sun X, et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nature genetics. 2009;41:1228–33. doi: 10.1038/ng.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lea WW, Lee YH. The association between the PTPN22 C1858T polymorphism and systemic lupus erythematosus: a meta-analysis update. Lupus. 2011;20:51–7. doi: 10.1177/0961203310381774. [DOI] [PubMed] [Google Scholar]
- 11.Zheng J, Ibrahim S, Petersen F, Yu X. Meta-analysis reveals an association of PTPN22 C1858T with autoimmune diseases, which depends on the localization of the affected tissue. Genes Immun. 2012;13:641–52. doi: 10.1038/gene.2012.46. [DOI] [PubMed] [Google Scholar]
- 12.Baca V, Velazquez-Cruz R, Salas-Martinez G, Espinosa-Rosales F, Saldana-Alvarez Y, Orozco L. Association analysis of the PTPN22 gene in childhood-onset systemic lupus erythematosus in Mexican population. Genes Immun. 2006;7:693–5. doi: 10.1038/sj.gene.6364350. [DOI] [PubMed] [Google Scholar]
- 13.Namjou B, Kim-Howard X, Sun C, Adler A, Chung SA, Kaufman KM, et al. PTPN22 association in systemic lupus erythematosus (SLE) with respect to individual ancestry and clinical sub-phenotypes. PLoS One. 2013;8:e69404. doi: 10.1371/journal.pone.0069404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanford SM, Krishnamurthy D, Falk MD, Messina R, Debnath B, Li S, et al. Discovery of a novel series of inhibitors of lymphoid tyrosine phosphatase with activity in human T cells. J Med Chem. 2011;54:1640–54. doi: 10.1021/jm101202j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vang T, Liu WH, Delacroix L, Wu S, Vasile S, Dahl R, et al. LYP inhibits T-cell activation when dissociated from CSK. Nat Chem Biol. 2012;8:437–46. doi: 10.1038/nchembio.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brownlie RJ, Miosge LA, Vassilakos D, Svensson LM, Cope A, Zamoyska R. Lack of the phosphatase PTPN22 increases adhesion of murine regulatory T cells to improve their immunosuppressive function. Sci Signal. 2012;5:ra87. doi: 10.1126/scisignal.2003365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maine CJ, Hamilton-Williams EE, Cheung J, Stanford SM, Bottini N, Wicker LS, et al. PTPN22 Alters the Development of Regulatory T Cells in the Thymus. J Immunol. 2012;188:5267–75. doi: 10.4049/jimmunol.1200150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arechiga AF, Habib T, He Y, Zhang X, Zhang ZY, Funk A, et al. Cutting edge: the PTPN22 allelic variant associated with autoimmunity impairs B cell signaling. J Immunol. 2009;182:3343–7. doi: 10.4049/jimmunol.0713370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai X, James RG, Habib T, Singh S, Jackson S, Khim S, et al. A disease-associated PTPN22 variant promotes systemic autoimmunity in murine models. J Clin Invest. 2013;123:2024–36. doi: 10.1172/JCI66963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Habib T, Funk A, Rieck M, Brahmandam A, Dai X, Panigrahi AK, et al. Altered B cell homeostasis is associated with type I diabetes and carriers of the PTPN22 allelic variant. J Immunol. 2012;188:487–96. doi: 10.4049/jimmunol.1102176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menard L, Saadoun D, Isnardi I, Ng YS, Meyers G, Massad C, et al. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J Clin Invest. 2011;121:3635–44. doi: 10.1172/JCI45790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rieck M, Arechiga A, Onengut-Gumuscu S, Greenbaum C, Concannon P, Buckner JH. Genetic variation in PTPN22 corresponds to altered function of T and B lymphocytes. J Immunol. 2007;179:4704–10. doi: 10.4049/jimmunol.179.7.4704. [DOI] [PubMed] [Google Scholar]
- 23.Maine CJ, Marquardt K, Cheung J, Sherman LA. PTPN22 controls the germinal center by influencing the numbers and activity of T follicular helper cells. Journal of immunology. 2014;192:1415–24. doi: 10.4049/jimmunol.1302418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Shaked I, Stanford SM, Zhou W, Curtsinger JM, Mikulski Z, et al. The autoimmunity-associated gene PTPN22 potentiates toll-like receptor-driven, type 1 interferon-dependent immunity. Immunity. 2013;39:111–22. doi: 10.1016/j.immuni.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zikherman J, Hermiston M, Steiner D, Hasegawa K, Chan A, Weiss A. PTPN22 deficiency cooperates with the CD45 E613R allele to break tolerance on a non-autoimmune background. J Immunol. 2009;182:4093–106. doi: 10.4049/jimmunol.0803317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 27.Dixon FJ, Andrews BS, Eisenberg RA, McConahey PJ, Theofilopoulos AN, Wilson CB. Etiology and pathogenesis of a spontaneous lupus-like syndrome in mice. Arthritis Rheum. 1978;21:S64–7. doi: 10.1002/art.1780210909. [DOI] [PubMed] [Google Scholar]
- 28.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–72. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 29.Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A. 2006;103:9970–5. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haywood ME, Rogers NJ, Rose SJ, Boyle J, McDermott A, Rankin JM, et al. Dissection of BXSB lupus phenotype using mice congenic for chromosome 1 demonstrates that separate intervals direct different aspects of disease. Journal of immunology. 2004;173:4277–85. doi: 10.4049/jimmunol.173.7.4277. [DOI] [PubMed] [Google Scholar]
- 31.Kono DH, Haraldsson MK, Lawson BR, Pollard KM, Koh YT, Du X, et al. Endosomal TLR signaling is required for anti-nucleic acid and rheumatoid factor autoantibodies in lupus. Proc Natl Acad Sci U S A. 2009;106:12061–6. doi: 10.1073/pnas.0905441106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hudgins CC, Steinberg RT, Klinman DM, Reeves MJ, Steinberg AD. Studies of consomic mice bearing the Y chromosome of the BXSB mouse. Journal of immunology. 1985;134:3849–54. [PubMed] [Google Scholar]
- 33.Crow MK. Type I interferon in the pathogenesis of lupus. Journal of immunology. 2014;192:5459–68. doi: 10.4049/jimmunol.1002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kono DH, Baccala R, Theofilopoulos AN. TLRs and interferons: a central paradigm in autoimmunity. Current opinion in immunology. 2013;25:720–7. doi: 10.1016/j.coi.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollard KM, Hultman P, Toomey CB, Cauvi DM, Hoffman HM, Hamel JC, et al. Definition of IFN-gamma-related pathways critical for chemically-induced systemic autoimmunity. Journal of autoimmunity. 2012;39:323–31. doi: 10.1016/j.jaut.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, et al. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. The Journal of experimental medicine. 1978;148:1198–215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baccala R, Gonzalez-Quintial R, Schreiber RD, Lawson BR, Kono DH, Theofilopoulos AN. Anti-IFN-alpha/beta receptor antibody treatment ameliorates disease in lupus-predisposed mice. Journal of immunology. 2012;189:5976–84. doi: 10.4049/jimmunol.1201477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niewold TB. Interferon alpha as a primary pathogenic factor in human lupus. J Interferon Cytokine Res. 2011;31:887–92. doi: 10.1089/jir.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ostendorf B, Iking-Konert C, Kurz K, Jung G, Sander O, Schneider M. Preliminary results of safety and efficacy of the interleukin 1 receptor antagonist anakinra in patients with severe lupus arthritis. Ann Rheum Dis. 2005;64:630–3. doi: 10.1136/ard.2004.025858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Postal M, Appenzeller S. The role of Tumor Necrosis Factor-alpha (TNF-alpha) in the pathogenesis of systemic lupus erythematosus. Cytokine. 2011;56:537–43. doi: 10.1016/j.cyto.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 41.Stanford SM, Bottini N. PTPN22: the archetypal non-HLA autoimmunity gene. Nat Rev Rheumatol. 2014 doi: 10.1038/nrrheum.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, et al. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood. 2011;118:1305–15. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]