Abstract
Background: Radioactive iodine (RAI) ablation is frequently performed after initial surgery for well-differentiated thyroid cancer (WDTC). We examined the frequency and timing of childbirth as well as nononcologic complications after RAI ablation for WDTC on a population level.
Methods: A retrospective cohort study of 25,333 patients (18,850 women) with WDTC was performed using the California Cancer Registry and California Office of Statewide Health Planning and Development database, 1999–2008. The primary outcomes were birthrate and median time to first live birth among women of childbearing age. Secondary outcomes were nononcologic diagnoses occurring outside the acute setting (>30 days) after ablation.
Results: RAI ablation did not affect birthrate among women in the full dataset. However, in subgroup analyses, birthrate among women age 35–39 was significantly decreased in those who received RAI versus those who did not (11.5 versus 16.3 births per 1000 woman-years, p<0.001). Median time to first live birth after diagnosis of WDTC was prolonged among women who received RAI compared to those who did not (34.5 versus 26.1 months; p<0.0001). When 5-year age groups were examined individually, delay to first live birth was observed in women age 20–39 (p<0.05). This remained significant after adjustment for tumor characteristics, socioeconomic status, and marital status. The only nononcologic, nonreproductive adverse effect associated with RAI ablation was an increased rate of nasolacrimal stenosis (RR 3.44, p<0.0001).
Conclusions: RAI ablation is associated with delayed childbearing in women across most of the reproductive lifespan, and with decreased birthrate in the late reproductive years. The underlying mechanism likely involves physician recommendation to delay pregnancy, as well as a potential impact of RAI on both reproductive choice and reproductive health. Further investigation is merited.
Introduction
Thyroid cancer accounted for 3.6% of new cancer diagnoses in the United States in 2013 (1). Radioactive iodine (RAI) ablation is frequently performed after initial surgery for well-differentiated thyroid carcinoma (WDTC) (2). However, recent data suggest that RAI ablation confers no benefit in low-risk patients, who represent the majority of WDTC cases (3,4).
Uncertainty regarding the risks and benefits of RAI is manifest in the wide variability in rates of RAI ablation for WDTC patients nationally (5–9). RAI ablation is associated with a small but statistically significant increase in the risk of secondary malignancies (10). Nononcologic complications of RAI ablation have been described, which include sialodenitis, xerostomia, dental caries, and nasolacrimal duct stenosis, but their frequency has not been systematically quantified on the population level (2,11). RAI also affects gonadal tissue, causing temporary secondary amenorrhea, oligomenorrhea, and/or earlier onset of menopause (12,13). Limited evidence suggests that RAI ablation is not associated with reduced fertility, although no large scale studies have been performed (12).
The California Cancer Registry (CCR) is a prospectively maintained statewide cancer database containing unique patient identifiers that permit longitudinal follow-up of WDTC patients. Patients in CCR are linked to ambulatory and inpatient hospital records maintained by the California Office of Statewide Health Planning and Development (OSHPD) database. We utilized the CCR to assess for unrecognized effects of RAI ablation on childbearing and other nononcologic health conditions. We hypothesized that RAI ablation would be associated with decreased childbearing in female patients.
Methods
Patients with thyroid cancer were abstracted from the CCR from 1999–2008 using ICD-O-2/3 code C739. Patients with WDTC (papillary and follicular histology) were identified using the histology codes 8050, 8260, 8330, 8331, 8332, 8335, 8340, 8342, 8343, 8344, and 9690. Tumor stage, tumor size, socioeconomic status (SES), and marital status were defined using CCR variables. SES quintiles were based upon the Yost index (14). Patients who received RAI were identified using the RADSUM variable, which codes for type of radiation therapy received only during the first course of treatment. CCR coding protocol classifies the first course of therapy as ending 12 months after diagnosis if no discrete endpoint is designated.
To determine female reproductive outcomes after RAI ablation, female patients less than 15 years of age and male patients were excluded. Patients who received external beam radiation or chemotherapy and patients who received RAI therapy more than 12 months after diagnosis were also excluded. Live births were abstracted from the OSPHD database using ICD-9 diagnosis codes for normal delivery (650-659.xx), complications related to pregnancy with delivery (640-648.xx, 650-677.xx), and outcome of delivery (V27 except V27.1, V27.4, V27.7, and V30-V39) excluding a fifth digit of 1 or 2 that indicates no delivery. We used CPT procedure codes for vaginal delivery (59400-59410, 59610, 59612, 59614) and cesarean delivery (59510-59515, 59618, 59620, 59622). Live births within 6 months after cancer diagnosis were considered to be preexisting pregnancies. These were compared between women who received RAI versus those who did not as indicator of reproductive choice and fertility. Preexisting pregnancies were then excluded from the principal analyses. In keeping with the data reporting structure established by the Centers for Disease Control and the National Center for Health Statistics, birthrate was calculated as the number of live births per 1,000 women-years, and women were stratified into 5-year age groups. Given the low number of births in women aged 45+, this group was collapsed into the 40–44 age group. To determine whether disease severity had an effect on reproductive outcomes, we determined overall birthrate and median time to first live birth between treatment groups with patients stratified by tumor size and stage.
To determine comorbidities positively associated with ablation, the OSHPD database was queried for primary diagnoses for all hospitalizations from 1999–2008 and all ambulatory data from 2005–2008. To exclude acute complications of RAI ablation, we excluded diagnosis codes within 30 days of ablation. For patients who did not receive RAI therapy, we excluded diagnosis codes within 83 days from the day of surgery (83 days was chosen by adding 30 days to the median time between surgery to RAI therapy [53 days] among patients who received RAI). Similarly, we determined the frequency of thyroid cancer-related hospitalizations by searching the OSHPD database for hospitalizations with primary ICD-9 diagnosis code 193 (malignant disease of thyroid gland). Hospitalizations occurring within 30 days of ablation, or within 83 days of initial surgery among patients that did not receive RAI, were excluded.
Statistical analysis
Negative binomial regression was used to compare the birthrates between patients who received ablation and those who did not. p Values were adjusted for tumor size, tumor stage, SES, and marital status (married versus all others) by including them as covariates in the negative binomial regression model. Time from presentation to first live birth was compared between groups using the Wilcoxon rank-sum test. p Values were adjusted for tumor size, tumor stage, SES, and marital status (married versus all others) by including them as covariates in a linear regression model with time to the first live birth log-transformed.
The occurrence rate of each primary diagnosis in patients who received RAI ablation was compared to those who did not using Fisher's exact test. We adjusted for inflation of type I error rate due to multiple comparisons by controlling the false discovery rate within 0.05. Analyses were performed using SAS 9.2 (SAS Institute, Inc., Cary, NC).
Results
WDTC patients
We identified 25,233 unique patients with papillary or follicular thyroid cancer between 1999–2008. Patients who received RAI were younger and had a lower mean Charlson comorbidity score than patients who did not receive RAI (p<0.0001, Table 1). Patients who received RAI also had a higher rate of regional disease and a larger mean tumor size in comparison to patients who did not (p<0.0001). There was no difference in the hospitalization rate for thyroid cancer between patients who received RAI and those who did not (p=0.54).
Table 1.
Baseline Demographics, All Patients
| No RAI (n=12022) | Yes RAI (n=13211) | p Value | |
|---|---|---|---|
| Age (years), mean±SD | 49.7±16.1 | 46.3±15.4 | <0.0001a |
| Tumor size, n (%) | <0.0001b | ||
| 0–9 mm | 4451 (40.0%) | 2047 (16.2%) | |
| 10–19 mm | 2680 (24.1%) | 4067 (32.1%) | |
| 20–39 mm | 2653 (23.8%) | 4663 (36.8%) | |
| 40+ mm | 1343 (12.1%) | 1884 (14.9%) | |
| Stage, n (%) | <0.0001b | ||
| Local | 9001 (74.9%) | 7440 (56.3%) | |
| Regional | 2387 (19.9%) | 5059 (38.3%) | |
| Remote | 634 (5.3%) | 712 (5.4%) | |
| Charlson comorbidity score excluding tumor, mean±SD | 0.52±1.15 | 0.38±0.89 | <0.0001c |
| Cancer-related hospitalization rate following initial therapy (# per patient-year) | 0.27 | 0.27 | 0.54d |
t test.
χ2 test.
Wilcoxon two-sample test.
Negative binomial regression.
RAI, radioactive iodine; SD, standard deviation.
Delayed nononcologic complications
Data regarding diagnoses related to hospitalizations and ambulatory data was collected over 112,192 person-years of follow-up, with a median follow-up time of 4.1 years per patient. After excluding acute complications, 2325 diagnosis codes were identified. Nasolacrimal duct stenosis was the only diagnosis that occurred with greater frequency in patients who received RAI compared to those who did not (4.01 versus 1.16 cases per 1000 patients; RR 3.44, p<0.0001). The median time from RAI ablation to first documentation of nasolacrimal duct stenosis was 31.4 months.
Female reproductive outcomes
Our study cohort for reproductive outcomes consisted of 18,850 women, of whom 9883 (52.4%) received RAI ablation. Women in the study were followed for a total of 83,498 woman-years, with a median follow-up of 4.0 years. During the study period, 1179 live births occurred after 6 months following diagnosis of WDTC.
Women who received RAI were younger and had a lower Charlson comorbidity score versus women who did not (Table 2). With respect to SES, a higher percentage of women who received RAI belonged in the highest SES quintile (25.7% versus 25.4%, p<0.0001) and a smaller percentage in the lowest SES quintile (11.9 versus 14.7%, p<0.001, Table 2). A higher percentage of women who received RAI were married (62.8 versus 59.7%, p<0.0001). Women who received RAI had a higher proportion of larger tumor sizes as well as increased percentage of regional (36.3 versus 17.9%, p<0.0001) and metastatic disease (4.5 versus 3.0%, p<0.0001; Table 2).
Table 2.
Baseline Demographics, Female Patients Greater Than Fifteen Years of Age
| No RAI (n=8967) | Yes RAI (n=9883) | p Value | |
|---|---|---|---|
| Age (years), mean±SD | 48.5±15.6 | 45.5±15.0 | <0.0001a |
| Tumor size, N | <0.0001b | ||
| 0–9 mm | 3552 (42.4%) | 1580 (16.7%) | |
| 10–19 mm | 2078 (24.8%) | 3261 (34.4%) | |
| 20–39 mm | 1955 (23.3%) | 3491 (36.8%) | |
| 40+ mm | 790 (9.4%) | 1159 (12.2%) | |
| Stage, n (%) | <0.0001b | ||
| Local | 7087 (79.0%) | 5853 (59.2%) | |
| Regional | 1609 (17.9%) | 3590 (36.3%) | |
| Remote | 271 (3.0%) | 440 (4.5%) | |
| Charlson comorbidity score excluding tumor, mean±SD | 0.44±1.03 | 0.34±0.82 | 0.0003c |
| Yost (14) SES quintiles | <0.0001b | ||
| 1st quintile (lowest) | 1321 (14.7%) | 1178 (11.9%) | |
| 2nd quintile | 1551 (17.3%) | 1685 (17.1%) | |
| 3rd quintile | 1837 (20.5%) | 2087 (21.1%) | |
| 4th quintile | 1980 (22.1%) | 2391 (24.2%) | |
| 5th quintile (highest) | 2278 (25.4%) | 2542 (25.7%) | |
| Marital status (yes), n (%) | 5355 (59.7%) | 6202 (62.8%) | <0.0001b |
t-test
χ2 test.
Wilcoxon two-sample test
RAI, radioactive iodine; SD, standard deviation; SES, socioeconomic status.
There were 104 preexisting pregnancies at time of WDTC diagnosis that later resulted in live birth. There was no significant difference in the number of preexisting pregnancies resulting in birth between women who received RAI versus those who did not (45 versus 59 births per 1000 women, p=0.07).
Overall birthrate (all ages) did not differ significantly between women who received RAI and those who did not (p=0.81). However, in subgroup analyses, women age 35–59 who received RAI had a significantly reduced birthrate versus those who did not (11.5 versus 16.3 births per 1000 woman-years, p<0.001; Table 3). Birthrates did not significantly differ between women who received RAI and those who did not when stratified by tumor size and tumor stage after adjustment for confounding variables (Supplementary Tables S1 and S2; Supplementary Data are available online at www.liebertpub.com/thy).
Table 3.
Birthrates by Age Group and Radioiodine Treatment Compared to National U.S. Birthrates
| No RAI | Yes RAI | ||||||
|---|---|---|---|---|---|---|---|
| Age group (years) | Women with WDTC (live births) | Live births per 1000 woman-years | Women with WDTC (live births) | Live births per 1000 woman-years | Unadjusted p value | Adjusted p valuea | 1999–2008 U.S. national birthrate (births per 1000 woman-years) (18) |
| 15–19 | 120 (24) | 44.8 | 218 (35) | 35.0 | 0.89 | 0.86 | 42.8±3.2 |
| 20–24 | 361 (106) | 64.7 | 483 (125) | 58.9 | 0.24 | 0.84 | 104.5±2.8 |
| 25–29 | 544 (145) | 57.9 | 756 (236) | 65.6 | 0.62 | 0.53 | 115.4±2.2 |
| 30–34 | 743 (136) | 39.3 | 1042 (212) | 42.1 | 0.49 | 0.14 | 95.0±4.3 |
| 35–39 | 977 (73) | 16.3 | 1222 (65) | 11.5 | <0.001 | <0.01 | 43.7±3.6 |
| 40+ | 6222 (11) | 0.4 | 6162 (11) | 0.4 | 0.68 | 0.69 | — |
Negative binomial regression, adjusted for tumor size, tumor stage, socioeconomic status (SES), marital status.
RAI, radioactive iodine; WDTC, well-differentiated thyroid cancer.
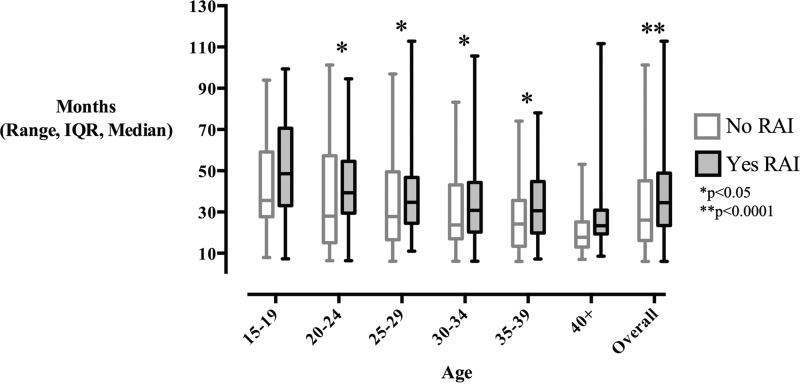
The overall median time to first delivery following initial presentation was significantly longer in women who received RAI (34.5 versus 26.1 months, p<0.0001, Fig. 1). When stratified by age group, women age 20–39 years old who received RAI experienced a significantly longer median time to first live birth: age 20–24, 39.3 versus 28.0 months, p<0.05; age 25–29, 34.7 versus 27.8 months, p<0.05; age 30–34, 30.8 versus 23.7 months, p<0.05; age 35–39, 30.6 versus 24.2 months, p<0.05 (Fig. 1). p Values remained significant after adjustment for tumor characteristics, socioeconomic status, and marital status. When stratified by tumor size and stage, RAI ablation was still associated with a significant delay in median time to first live birth after adjustment for confounding variables among patients with localized and regional disease (Supplementary Tables S3 and S4).
FIG. 1.
Median time from presentation to first live birth.
Discussion
Our analysis of reproductive outcomes in 18,850 women treated for WDTC demonstrate that RAI ablation was not associated with decreased overall birthrate. However, in subgroup analyses, we found a significant 29% reduction in birthrate among women age 35–39 who received RAI. Additionally, women age 20–39 who received RAI experienced a significant delay in median time to first live birth ranging from 6 to 11 months. To our knowledge, this is the largest population-based study to date examining the effects of RAI on childbirth. Our large sample size permitted age stratification while maintaining adequate statistical power. Given the known age-related changes in female fertility and reproductive choice, age stratification yielded findings that may otherwise not have been discernible.
The decreased birthrate observed among women 35–39 years old who received RAI may reflect an effect on reproductive choice and/or reproductive health, and is likely multifactorial. A previous meta-analysis found that although RAI ablation was not associated with a significant effect on overall fertility, 8–27% of women experienced absent menses and increased miscarriage rates in the first year after ablation, as well as earlier onset of menopause (12,15). Given the time frame spanned by our study, the great majority of women receiving RAI ablation would have done so after levothyroxine withdrawal. Levothyroxine withdrawal obligates a period of thyrotropin instability following ablation, during which fertility may be compromised or not recommended (16). Patients of older reproductive age may be less able to tolerate these physiologic effects of RAI ablation. A delay in median time to childbirth combined with early onset of menopause may extend female patients age 35–39 beyond a critical period of fertility, leading to decreased birthrate (17).
We recognize the potential impact of thyroid cancer and treatment on reproductive choice. The overall and age-specific birthrates found in our study population were lower than contemporaneous U.S national birthrates for women who received RAI ablation and those who did not (Table 3) (18). This is consistent with prior studies demonstrating reduced childbearing in a number of chronic diseases (19,20). Thus, factors universally related to chronic disease that negatively impact reproductive choice, such as increased financial burden or psychological stress and anxiety, likely also affect patients with thyroid cancer. Nonetheless, decreased birthrates compared to the general population following thyroid cancer diagnosis have not been previously observed. Further studies are indicated to address whether factors specific to thyroid cancer affect reproductive choice, fertility, or reproductive health. To address potential confounding of increased disease severity on reproductive choice, we stratified patients by tumor size and stage. We demonstrate no difference in birthrate between women who received RAI and those who did not across all tumor sizes and stages after adjusting for confounding variables; this suggests that disease severity did not significantly impact our findings. Moreover, the analyses were adjusted for tumor size and stage, and by excluding patients who required chemotherapy or external beam radiation. The analyses were also adjusted for marital status and SES, both of which are known to influence reproductive choice.
The pattern of delayed childbearing we observed among women receiving RAI ablation is not entirely explained by compliance with physician recommendations to defer pregnancy after RAI ablation. Clinicians commonly recommend delaying pregnancy by 6 months after RAI ablation, in keeping with the 2009 American Thyroid Association guidelines (21). In clinical practice, the waiting period recommended by clinicians may extend to 12 months, and may account for the observed delayed childbearing. The median time to first live birth among women who did not receive RAI was 26.1 months, implying that attempts to conceive generally occurred 14 to 17 months after diagnosis (22). Assuming equal desire for pregnancy, a 6- or even 12-month physician-recommended waiting period for women receiving RAI ablation would still leave ample time for them to conceive and deliver a child within 26.1 months, thereby “keeping pace” with the non-RAI group. The 8.4-month delay observed in women receiving RAI involves attempts to conceive well beyond the physician-recommended waiting period, suggesting that factors beyond adherence to physician recommendations are at play. These factors remain to be identified.
The observed delay in childbearing after RAI ablation is likely unrelated to increasing disease severity. When stratified by tumor size, a significant increase in time to first live birth was still noted among all tumor sizes, including tumors <1 cm, after adjusting for confounding variables. Moreover, when stratified by tumor stage, patients with localized or regional disease both demonstrated a significantly increased median time to first birth. Although subgroup analysis of patients with metastatic disease found no significant difference in time to first live birth, this is likely attributable to the small number of live births (n=2) were observed among women who did not receive RAI for metastatic thyroid cancer.
Nononcologic complications of RAI ablation are limited and carry a smaller negative health impact than oncologic complications. In our study, the only significant nononcologic complication identified was nasolacrimal stenosis, which occurred in less than 1% of patients who received ablation. Other series have reported nasolacrimal stenosis in 2–5% patients receiving RAI for thyroid cancer (23,24). We did not observe other common, previously identified complications of RAI, namely xerostomia, sialadenitis, and xerophthalmia (2,24,25). This was likely due to incomplete capture of conditions managed primarily on an outpatient basis. In contrast, oncologic complications of RAI are well captured by cancer registries; RAI is associated with a modest increase in the rate of salivary gland tumors and leukemias, as well as a modest increased relative risk (1.5–2.3) for gastric, renal, and breast cancers compared to the general population (10,26).
Our study is limited by its retrospective design and 10-year study period, because late complications occurring beyond 10 years after RAI therapy were not assessed. Because outpatient data were only incorporated after 2005, whereas California begun to allow outpatient RAI treatment in 1998, patients receiving RAI on an outpatient basis may be incompletely captured. Furthermore, the CCR and OSPHD databases did not document the dose of RAI received or any subsequent RAI administration after the first course of therapy, prohibiting use of these factors as predictor variables. A lack of detailed reproductive health data in CCR and OSPHD databases barred us from controlling for contraceptive use, previous births/miscarriages, and pre-existing gonadal dysfunction, all of which can influence childbirth (27). Finally, suppression of thyrotropin levels in women can lead to mild menstrual disturbances that may affect time to childbirth (28). The absence of biochemical data in CCR and OSPHD did not permit us control for degree of thyrotropin suppression between treatment groups.
Women younger than 45 years old account for one-third of new thyroid cancer diagnoses (1). The effects of RAI on female reproductive outcomes described herein may supplement other clinico-pathologic factors influencing the decision to proceed with RAI ablation after surgery for WDTC. A growing body of evidence supports limited/selective use of RAI in low-risk thyroid cancer patients (10). Further studies are indicated to evaluate the effect of RAI ablation on women of advanced maternal age in whom we observed reduced childbearing rates. These findings may be potentially attributable to either an inability to tolerate a delay in childbearing, either due to physician recommendation or impact of RAI on reproductive choice, or an additive or threshold effect of RAI upon reduced childbearing potential.
Supplementary Material
Acknowledgments
The authors thank Drs. Christiaan Schiepers and Stephanie Smooke for their critical review of the manuscript. This study was in part supported by NIH/NCRR/NCATS UCLA CTSI grant number UL1TR000124, NIH 7K23HD068552 (AML), and H.H. Lee Research Program. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Author Disclosure Statement
Dr. Leung has received research support from Genzyme Corporation for thyroid cancer registry development. For all other authors, no competing financial interests exist.
References
- 1.National Cancer Institute. Bethesda, MD: SEER Stat Fact Sheets: Thyroid Cancer. Available at: http://seer.cancer.gov/statfacts/html/thyro.html (Last accessed November16, 2013) [Google Scholar]
- 2.Van Nostrand D.2009The benefits and risks of I-131 therapy in patients with well-differentiated thyroid cancer. Thyroid 19:1381–1391 [DOI] [PubMed] [Google Scholar]
- 3.Hay ID, Thompson GB, Grant CS, Bergstralh EJ, Dvorak CE, Gorman CA, Maurer MS, McIver B, Mullan BP, Oberg AL, Powell CC, van Heerden JA, Goellner JR.2002Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg 26:879–885 [DOI] [PubMed] [Google Scholar]
- 4.Nixon IJ, Ganly I, Patel SG, Palmer FL, Di Lorenzo MM, Grewal RK, Larson SM, Tuttle RM, Shaha A, Shah JP.2013The results of selective use of radioactive iodine on survival and on recurrence in the management of papillary thyroid cancer, based on Memorial Sloan-Kettering Cancer Center risk group stratification. Thyroid 23:683–694 [DOI] [PubMed] [Google Scholar]
- 5.Haymart MR, Banerjee M, Yang D, Stewart AK, Sisson JC, Koenig RJ, Doherty GM, Griggs JJ.2013Variation in the management of thyroid cancer. J Clin Endocrinol Metab 98:2001–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haymart MR, Muenz DG, Stewart AK, Griggs JJ, Banerjee M.2013Disease severity and radioactive iodine use for thyroid cancer. J Clin Endocrinol Metab 98:678–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuessler KM, Banerjee M, Yang D, Stewart AK, Doherty GM, Haymart MR.2013Surgeon training and use of radioactive iodine in stage I thyroid cancer patients. Ann Surg Oncol 20:733–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papaleontiou M, Banerjee M, Yang D, Sisson JC, Koenig RJ, Haymart MR.2013Factors that influence radioactive iodine use for thyroid cancer. Thyroid 23:219–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haymart MR, Banerjee M, Stewart AK, Koenig RJ, Birkmeyer JD, Griggs JJ.2011Use of radioactive iodine for thyroid cancer. JAMA 306:721–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iyer NG, Morris LGT, Tuttle RM, Shaha AR, Ganly I.2011Rising incidence of second cancers in patients with low-risk (T1N0) thyroid cancer who receive radioactive iodine therapy. Cancer 117:4439–4446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander C, Bader JB, Schaefer A, Finke C, Kirsch CM.1998Intermediate and long-term side effects of high-dose radioiodine therapy for thyroid carcinoma. J Nucl Med 39:1551–1554 [PubMed] [Google Scholar]
- 12.Sawka AM, Lakra DC, Lea J, Alshehri B, Tsang RW, Brierley JD, Straus S, Thabane L, Gafni A, Ezzat S, George SR, Goldstein DP.2008A systematic review examining the effects of therapeutic radioactive iodine on ovarian function and future pregnancy in female thyroid cancer survivors. Clin Endocrinol (Oxf) 69:479–490 [DOI] [PubMed] [Google Scholar]
- 13.Sawka AM, Lea J, Alshehri B, Straus S, Tsang RW, Brierley JD, Thabane L, Rotstein L, Gafni A, Ezzat S, Goldstein DP.2008A systematic review of the gonadal effects of therapeutic radioactive iodine in male thyroid cancer survivors. Clin Endocrinol (Oxf) 68:610–617 [DOI] [PubMed] [Google Scholar]
- 14.Yost K, Perkins C, Cohen R, Morris C, Wright W.2001Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 12:703–711 [DOI] [PubMed] [Google Scholar]
- 15.Garsi JP, Schlumberger M, Rubino C, Ricard M, Labbe M, Ceccarelli C, Schvartz C, Henri-Amar M, Bardet S, de Vathaire F.2008Therapeutic administration of 131I for differentiated thyroid cancer: radiation dose to ovaries and outcome of pregnancies. J Nucl Med 49:845–852 [DOI] [PubMed] [Google Scholar]
- 16.Koutras DA.1997Disturbances of menstruation in thyroid disease. Ann NY Acad Sci 816:280–284 [DOI] [PubMed] [Google Scholar]
- 17.Ceccarelli C, Bencivelli W, Morciano D, Pinchera A, Pacini F.2001131I therapy for differentiated thyroid cancer leads to an earlier onset of menopause: results of a retrospective study. J Clin Endocrinol Metab 86:3512–3515 [DOI] [PubMed] [Google Scholar]
- 18.Martin JA, Hamilton BE, Ventura SJ, Osterman MJK, Mathews TJ.2013Births: Final data for 2011. Natl Vital Stat Rep 62:1–69, 72. [PubMed] [Google Scholar]
- 19.Wallenius M, Skomsvoll JF, Irgens LM, Salvesen KA, Nordvag BY, Koldingsnes W, Mikkelsen K, Kaufmann C, Kvien TK.2012Parity in patients with chronic inflammatory arthritides childless at time of diagnosis. Scand J Rheumatol 41:202–207 [DOI] [PubMed] [Google Scholar]
- 20.Erichsen MM, Husebye ES, Michelsen TM, Dahl AA, Lovas K.2010Sexuality and fertility in women with Addison's disease. J Clin Endocrinol Metab 95:4354–4360 [DOI] [PubMed] [Google Scholar]
- 21.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, Haugen BR, Hauger BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM.2009Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 22.Joffe M.2000Time trends in biological fertility in Britain. Lancet 355:1961–1965 [DOI] [PubMed] [Google Scholar]
- 23.Kloos RT, Duvuuri V, Jhiang SM, Cahill KV, Foster JA, Burns JA.2002Nasolacrimal drainage system obstruction from radioactive iodine therapy for thyroid carcinoma. J Clin Endocrinol Metab 87:5817–5820 [DOI] [PubMed] [Google Scholar]
- 24.Rosario PW, Calsolari MR.2013Salivary and lacrimal gland dysfunction after remnant ablation with radioactive iodine in patients with differentiated thyroid carcinoma prepared with recombinant human thyrotropin. Thyroid 23:617–619 [DOI] [PubMed] [Google Scholar]
- 25.Sun GEC, Hatipoglu B.2013Epiphora after radioactive iodine ablation for thyroid cancer. Thyroid 23:243–245 [DOI] [PubMed] [Google Scholar]
- 26.Metso S, Auvinen A, Huhtala H, Salmi J, Oksala H, Jaatinen P.2007Increased cancer incidence after radioiodine treatment for hyperthyroidism. Cancer 109:1972–1979 [DOI] [PubMed] [Google Scholar]
- 27.Baxter NN, Sutradhar R, DelGuidice ME, Forbes S, Paszat LF, Wilton AS, Urbach D, Rabeneck L.2013A population-based study of rates of childbirth in recurrence-free female young adult survivors of Non-gynecologic malignancies. BMC Cancer 13:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krassas GE, Poppe K, Glinoer D.2010Thyroid function and human reproductive health. Endocr Rev 31:702–755 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.