Abstract
Generation of functional organs from patients' own cells is one of the ultimate goals of regenerative medicine. As a novel approach to creation of organs from pluripotent stem cells (PSCs), we employed blastocyst complementation in organogenesis-disabled animals and successfully generated PSC-derived pancreas and kidneys. Blastocyst complementation, which exploits the capacity of PSCs to participate in forming chimeras, does not, however, exclude contribution of PSCs to the development of tissues—including neural cells or germ cells—other than those specifically targeted by disabling of organogenesis. This fact provokes ethical controversy if human PSCs are to be used. In this study, we demonstrated that forced expression of Mix-like protein 1 (encoded by Mixl1) can be used to guide contribution of mouse embryonic stem cells to endodermal organs after blastocyst injection. We then succeeded in applying this method to generate functional pancreas in pancreatogenesis-disabled Pdx1 knockout mice using a newly developed tetraploid-based organ-complementation method. These findings hold promise for targeted organ generation from patients' own PSCs in livestock animals.
Introduction
Recent developments in induced pluripotent stem cell (iPSC) technology permit establishment of individual patients' own PSCs [1,2]. However, current stem cell therapy mainly targets diseases that can be treated by cell transplantation. Faced with absolute deficiency of donor organs to treat patients with organ failure, regenerative medicine has the generation of organs as one of its ultimate goals. We propose that this be done using the patient's own PSCs, as represented by embryonic stem cells (ESCs), yielding organs that can be transplanted into the patient.
We recently demonstrated successful generation of PSC-derived pancreas and kidneys using blastocyst complementation in pancreatogenesis- or nephrogenesis-disabled mice [3,4]. We then succeeded in generating rat PSC-derived pancreas in mice by interspecific blastocyst complementation [3]. In an ancillary work, we developed pancreatogenesis-disabled pigs in which, through blastocyst complementation, we successfully generated exogenous-pig pancreata [5].
While these studies prepared us to examine the feasibility of generating human PSC-derived pancreata in pancreatogenesis-disabled pigs, some ethical issues on making such “admix chimeras” have yet to be solved. A part of the concern comes from the possibility that human iPSC-derived cells contribute to neural or germ cells in chimeric animals. To overcome this issue, in this study, we attempted to restrict differentiation of PSC-derived cells into endodermal organs by introducing a gene encoding the transcription factor Mixl1.
Mixl1 is a mouse homolog of a Mix family gene, originally discovered in Xenopus as a transcription factor inducing differentiation of pluripotent animal cap cells into the endoderm [6]. Mixl1 also regulates endoderm and paraxial mesoderm formation, a potential reason for death early in development when Mixl1 is defective [7]. Forced expression of Mixl1 during mouse ESC differentiation in vitro represses mesodermal fate determination and promotes endodermal fate [8,9]. We speculated that this transcription factor can autonomously induce ESCs to form endodermal cells after blastocyst injection.
Materials and Methods
Animals
C57BL/6NCrSlc, BDF1, DBA/2CrSlc, 129/Sv, and ICR mice were purchased from SLC Japan (Shizuoka, Japan). Pdx1-LacZ heterozygous mice [10], kindly provided by Dr. Y. Kawaguchi (Kyoto University) and Dr. C. V. Wright (Vanderbilt University), were crossed with C57BL/6-, DBA2-, or BDF1-strain mice. In the Dox(+) setting, mice were given drinking water containing 2 mg/mL Dox (Clontech, Palo Alto, CA) and 3.5% sucrose (Wako, Tokyo, Japan). All experiments were performed in accordance with the animal care and use committee guidelines of the Institute of Medical Science, University of Tokyo.
Culture of mouse ESCs/iPSCs
Undifferentiated mouse ESCs/iPSCs were maintained on gelatin-coated dishes without feeder cells in Glasgow's modified Eagle's medium (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (Nichirei Bioscience, Tokyo, Japan), 0.1 mM 2-mercaptoethanol (Invitrogen, San Diego, CA), 0.1 mM nonessential amino acids (Invitrogen), 1 mM sodium pyruvate (Invitrogen), 1% L-glutamine penicillin streptomycin (Sigma), 1,000 U/mL of mouse leukemia inhibitory factor (LIF) (Millipore, Bedford, MA) with or without 2 inhibitors [2i; 1 μM MEK inhibitor PD0325901 (Wako), and 3 μM GSK3 inhibitor CHIR99021 (Axon, Groeningen, The Netherlands)]. In the Dox(+) setting, Dox (2 μg/mL) was added to the culture medium. For differentiation of ESCs, retinoic acid (Sigma) was added to a concentration of 1 μM in the culture medium without LIF and 2i. DsRed-expressing mouse ESCs (EB3DR), kindly provided by Dr. H. Niwa (Center for Developmental Biology, RIKEN, Hyogo, Japan), were derived from EB3-ESCs [11] and carried CAG promoter-driven DsRed. Wild-type (WT) mouse ESCs (K3) were generated from hybrid blastocysts obtained after intercrossing of 129/Sv and C57BL6 mice (data not shown). Pdx1 knock-out (KO) iPSCs were generated from Pdx1 KO mouse-derived neonatal fibroblasts by introducing three mouse factors (Oct3/4, Klf4, and Sox2) in one retroviral vector (data not shown).
Construction of vectors and gene introduction
For construction of pRosa26-tTA-Mixl1, a targeting vector for insertion of the single cassette Tet-Off regulatory system into the Rosa26 locus (a kind gift from Dr. J. Miyazaki; Osaka University, Osaka, Japan) was modified to insert Mixl1 as described [12]. For construction of pRosa26-TIN-TRE-Mixl1 in Fig. 2B, a splice-acceptor sequence amplified from a pSAβ-geo vector (a kind gift from Dr. P. Soriano; Fred Hutchinson Cancer Research Center, Seattle, WA); tdTomato amplified from ptdTomatoN1 (Clontech); IRES amplified from a GCDNsam vector (a kind gift from Dr. M. Onodera; National Research Institute for Child Health and Development, Tokyo, Japan); Neor-SV40pA amplified from a pcDNA3 vector (Invitrogen); TRE-Mixl1 amplified from pRosa26-tTA-Mixl1; and an insulator sequence [13] were inserted into the XbaI site of pRosa26-SwaI, a pRosa26-1 vector (a kind gift from Dr. P. Soriano), by addition of an SwaI site upstream of the 5′ homology arm. For construction of pCAG-tTA-IP in Supplementary Fig. S2A (Supplementary Data are available online at www.liebertpub.com/scd), a CAG promoter [14], HA-tagged tTA amplified from pTet-Off Advanced (Clontech), and IRES-Puror amplified from pCAG-Cre-IRES-Puror-pA (a kind gift from Dr. J. Miyazaki) were inserted into the multi-cloning site of pBluescript-SwaI, that is, into pBluescript KS(+) (Stratagene, La Jolla, CA) modified by addition of an SwaI site into the start and end positions of the multi-cloning site. For construction of pOct3/4-BAC-tTA-Venus, bacterial artificial chromosome recombineering technology was used [15]. Homology arms for recombineering were amplified from genomic DNA of mouse ESCs by polymerase chain reaction (PCR) using PrimeSTAR or PrimeSTAR GXL DNA polymerase (Takara Bio, Otsu, Japan) according to the manufacturer's protocol. The arms and tTA-IRES-Venus were subcloned into the multi-cloning site of the pBT-loxP2-Zeo vector, a pBT-loxP2 vector (a kind gift from Dr. R. Kaneko; Gunma University, Gunma, Japan) modified by replacing a gene conferring neomycin resistance with one conferring zeocin resistance.
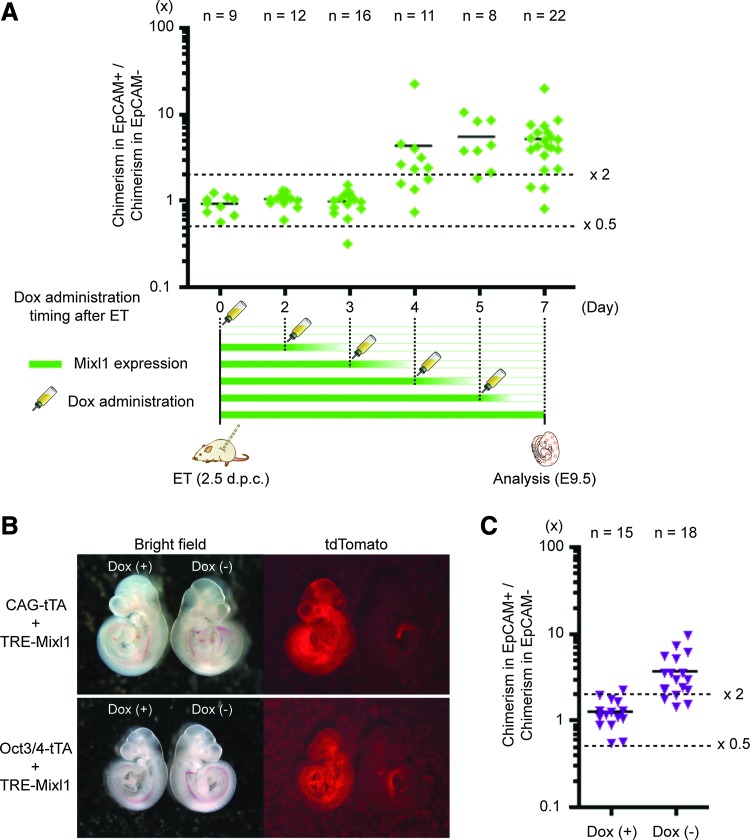
FIG. 2.
Forced expression of Mixl1 until the epiblast stage is necessary for endodermal induction. (A) Degrees of chimerism in embryonic endodermal tissues with varying periods of Dox administration. Chimerisms were analyzed at E9.5. After anti-EpCAM antibody staining, DsRed-expressing cells were sorted into EpCAM-expressing (EpCAM+) and EpCAM-lacking (EpCAM−) fractions by flow cytometry. Diamonds indicate values calculated by dividing percentage of chimerism in the EpCAM+ fraction by percentage of chimerism in the EpCAM− fraction for individual chimeric embryos. (B) Chimeric fetuses at 1 week after embryo transfer of CHT5- or OTiV1-ESC–injected wild-type blastocysts. In each image, the fetus at the viewer's left grew in the presence (+) of Dox and the fetus at the viewer's right grew in the absence (−) of Dox. (C) Degrees of chimerism in embryonic endodermal tissues in Dox(+) and Dox(−) settings. Triangles indicate values for individual chimeric embryos.
Electroporation for gene targeting and gene introduction was carried out as described [16,17]. In brief, 1–5×106 mouse ESCs suspended in PBS were mixed with vectors linearized by restriction-enzyme digestion and were transferred to a Gene Pulser cuvette (Bio-Rad, Richmond, CA). Electroporation was carried out at 230 V, 250 μF in Gene Pulser equipment (Bio-Rad). After electroporation, ESCs were seeded onto gelatin-coated dishes and 24–48 h later, drugs for selection were added to the culture medium.
Embryo culture and manipulation
Preparation of WT and Pdx1 heterozygous intercrossing diploid embryos and of WT and EGFP-Tg mouse-derived tetraploid embryos was carried out according to published protocols [18]. In brief, eight-cell/morula-stage diploid embryos were collected in Medium 2 (M2; Millipore) from oviduct and uterus of mice at 2.5 days postcoitum (dpc). These embryos were transferred into a potassium simplex optimized medium with amino acids (KSOM-AA; Millipore) and were cultured for 24 h for blastocyst injection. For production of tetraploid embryos, two-cell stage diploid embryos were collected in M2 from oviduct of mice 1.5 dpc. These embryos were washed thrice with medium containing 0.01% polyvinyl alcohol (Sigma), 280 mM Mannitol (Sigma), 0.5 mM Hepes (Wako), and 0.15 mM MgSO4 (Invitrogen). Electrofusion of blastomeres to produce tetraploid embryos was carried out using a DC pulse (100 V/mm, 30 μs, 1 time) followed by application of AC pulses (5 V/mm, 10 s) using ECM 2001 (BTX, Holliston, MA). These tetraploid embryos were transferred into KSOM-AA and were cultured for 24 h for four-cell/morula injection.
For micro-manipulation, ESCs were trypsinized and suspended in ESC culture medium. A piezo-driven micro-manipulator (Prime Tech, Tokyo, Japan) was used to drill zona pellucida and trophectoderm under the microscope, and 5–10 ESCs were introduced into blastocyst cavities near the inner cell mass of diploid blastocysts or the perivitelline space of four-cell/morula stage tetraploid embryos. After the injection, diploid blastocyst embryos underwent follow-up culture for 1–2 h. Four-cell/morula stage tetraploid embryos underwent follow-up culture for 24 h to achieve blastocyst stage. Blastocysts of either origin were then transferred into the uteri of pseudopregnant recipient ICR female mice (2.5 dpc).
Genotyping for targeting
DNA was extracted from picked-up PSCs using QIAamp DNA Mini Kits (Qiagen, Germantown, MD). For genotyping, PCR primers for amplification of the Rosa26-tTA-Mixl1 knock-in locus were Fw, 5′-CCTCGGCTAGGTAGGGGATCGGGACTCT-3′, and Rv, 5′-CGGAGAACCTGCGTGCAATCCATCTTGTTC-3′ for 5′ upstream and Fw, 5′-GGATCACTCTCGGCATGGACGAGCTGTAC-3′, and Rv, 5′-AGCCTTAAACAAGCACTGTCCTGTCCTCAAG-3′ for 3′ downstream. PCR primers for amplification of the Rosa26-TIN-TRE-Mixl1 knock-in locus were Fw, 5′-CCTCGGCTAGGTAGGGGATCGGGACTCT-3′, and Rv, 5′-GGGCCCTCACATTGCCAAAAGACGG-3′ for 5′ upstream.
Western blot analysis
Whole-cell extracts were prepared by resuspending ESCs in lysis buffer (NACALAI TESQUE, Kyoto, Japan). After electrophoresis, gels were transferred to nitrocellulose membranes. Membranes were blocked in Tris-buffered saline with Tween-20 containing 5% BSA and were treated with antibodies. Primary antibodies against FLAG (mouse IgG, Sigma; rat IgG, Novus Biologicals, Littleton, CO), Oct3/4 (mouse IgG; Santa Cruz Biotechnology, Dallas, TX), β-actin (mouse IgG; Cell Signaling Technology, Boston, MA), and Mixl1 (a kind gift from Dr. A. Elefanty; Monash University, Australia) were used. Horseradish-peroxidase–conjugated secondary antibodies used were directed against mouse or rat IgG. After antibody treatment, blots were developed using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Pittsburgh, PA).
Immunostaining of cells, fetuses, and sections
ESCs, embryos, fetuses, and organs were fixed in 4% paraformaldehyde. Fetuses and organs were embedded in Optimal Cutting Temperature compound (Sakura Finetek, Tokyo, Japan) for frozen sections. Each sample was incubated with primary antibody for 1–2 h at room temperature (RT) and with secondary antibody for 1 h at RT. Primary antibodies against EGFP (rabbit IgG, Invitrogen; rat IgG, NACALAI TESQUE; goat IgG, abcam, Cambridge, United Kingdom), FLAG, DsRed (rabbit IgG; Clontech), Foxa2 (goat IgG; Santa Cruz), EpCAM (rat IgG, Developmental Studies Hybridoma Bank at the University of Iowa), HA (rabbit IgG; Cell Signaling Technology), PECAM1(rat IgG; BD, San Diego, CA), Insulin (rabbit IgG; Cell Signaling Technology), and Mixl1were used. Secondary antibodies used were Alexa488-, Alexa 568-, and Alexa 647-conjugated and were directed against rabbit, rat, and goat IgG (Invitrogen). After antibody treatment, samples were stained with 4′,6-diamidino-2-phenylindole (DAPI) to mark nuclei and were observed under fluorescence microscopy or confocal laser scanning microscopy. Sections of embryos were also scanned for chimerism analysis with Cellomics ArrayScan VTI HCS Reader technology (Thermo Scientific), and the data were analyzed by FlowJo software (Tree Star, Ashland, OR).
Flow cytometry analysis
To analyze chimerism at E9.5, fetuses were trypsinized at 37°C for 10 min. The digestion products were stained with Alexa-647-conjugated anti-EpCAM antibody (rat IgG; Santa Cruz Biotechnology) and subjected to FACSCanto II flow cytometry (BD Bioscience).
Glucose tolerance testing
Blood was sampled via tail vein at intraperitoneal glucose administration (1 g/kg; 0 min) and at 15, 30, 60, and 120 min thereafter. Blood glucose values were determined using a Medisafe-Mini glucometer (Terumo, Tokyo, Japan).
Results
Contribution of Mixl1-inducible ESCs was limited to gut endoderm in chimeras
To control the in vivo expression of Mixl1, we introduced a single-cassette Tet-Off regulatory system [12] into the Rosa26 locus of mouse ESCs ubiquitously expressing DsRed (Supplementary Fig. S1A). Targeting was confirmed by PCR of genomic DNA using the primer pair described in the “Materials and Methods” section (Supplementary Fig. S1B). After excision of a Neo element flanked by loxP sites, FLAG-tagged Mixl1 expression and IRES-mediated EGFP expression were induced by removal of doxycycline (Dox) from culture medium (Supplementary Fig. S1C, D). We used these Mixl1-inducible ESCs (RT5-ESCs) for experiments.
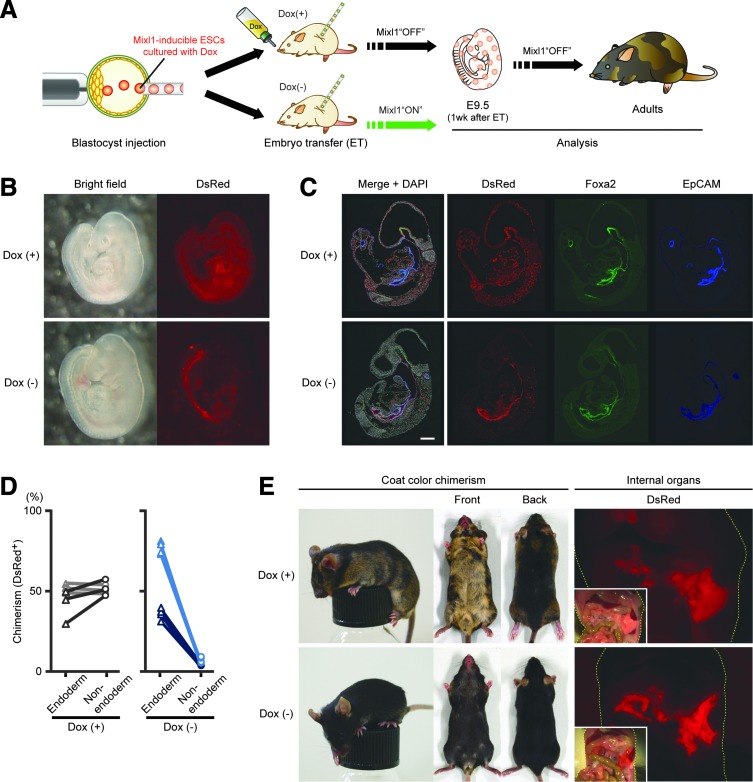
To check the effect of exogenous Mixl1 expression in early development, we cultured RT5-ESCs in the presence (+) of Dox [Dox(+)] and injected them into WT mouse blastocysts. The foster mothers were given drinking water with or without Dox during pregnancy (Fig. 1A). The transferred embryos were analyzed at one week after embryo transfer (ET). In Dox(+) settings, DsRed-expressing RT5-ESC–derived cells were ubiquitously present throughout the body, as in normal chimeric embryos (Fig. 1B). However, in the absence of Dox, most DsRed-expressing cells accompanied gut endoderm (Fig. 1B). Distributions of DsRed-expressing cells were also confirmed by immunostaining with antibodies against the endoderm markers Foxa2 and EpCAM [19,20].
FIG. 1.
Mixl1-inducible ESCs can preferentially contribute to gut endoderm after blastocyst injection. (A) Experimental schema for analysis of chimera generated by injection of Mixl1-inducible ESCs into wild-type blastocyst. (B) Chimeric fetuses at 1 week after transfer of RT5-ESC–injected wild-type blastocysts (developmental stage: E9.5 embryo) into foster-mother uteri. Foster mothers were given drinking water with (+) or without (−) Dox. (C) Distribution of RT5-ESC–derived cells in E9.5 chimeric fetus. In Dox(−) setting, to exclude the effect of EGFP expression on multi-color staining, Dox was given to foster mothers at 1 day before analysis. Sections were immunostained for DsRed (red) and for endodermal markers Foxa2 (green) and EpCAM (blue), with nuclear counterstaining (white) using DAPI. (D) Comparison of chimerism between endodermal and nonendodermal embryonic tissues in Dox(+) and Dox(−) settings. Sections immunostained in (C) were imaged by ArrayScan technology, with collected data analyzed by FloJo software (Supplementary Fig. S2). Both Foxa2- and EpCAM-expressing cells were defined as “endoderm (triangular dots)”; cells expressing neither were defined as “nonendoderm (circular dots).” Three or four sections from each of a pair of embryos were evaluated. Values obtained from the same sections are connected by lines. (E) Adult chimeric mice generated by injection of RT5-ESCs into wild-type blastocysts. In the Dox(−) setting, foster mothers were given drinking water with Dox except during 1 week after embryo transfer. Coat-color chimerism differences between host-blastocyst progeny (BDF1xB6-derived) and donor-ESC progeny (129ola-derived) are shown, as are macroscopic views of contributions of RT5-ESC–derived cells to internal organs. Yellow dashes outline bodies. Sections in (C) were observed under confocal laser scanning microscopy. Scale bars, 100 μm. DAPI, 4′,6-diamidino-2-phenylindole; ESCs, embryonic stem cells.
Unlike in Dox+ settings, when Dox was lacking, DsRed expression colocalized with that of both Foxa2 and EpCAM in most cells (Fig. 1C). Quantitative data obtained using flow cytometry or image analysis also indicated preferential contribution of RT5-ESCs to gut endoderm (Figs. 1D and 2A and Supplementary Fig. S2).
We then examined chimerism in adulthood. In Dox(+) settings, chimeras showed clear chimerism of coat color between donor-derived cells (129ola-derived) and host-blastocyst–derived cells (BDF1xB6-derived; Fig. 1E). They also showed internal-organ chimerism as judged by DsRed expression (Fig. 1E). On the other hand, in the absence of Dox, coat-color chimerism was slight or unrecognizable (Fig. 1E), but internal-organ chimerism was apparent, especially in endoderm-derived organs such as the pancreas (Fig. 1E). These data strongly suggest that forced expression of Mixl1 in ESC progeny during development directed the fate of ESC-derived cells toward endodermal lineages.
Forced expression of Mixl1 until postimplantation epiblast stage suffices for endodermal induction
While we found that the forced expression of Mixl1 can guide ESC fate toward endodermal lineages after blastocyst injection, the period required for induction was still unknown. Especially for future applications, exogenous expression of the transgene should be minimized and tightly controlled by using optimal promoters. Furthermore, if a precise period is established, we might replace transgene use by other methods such as protein or RNAs delivery during a short period, thereby preventing transgene integration into genomic DNA. Thus, to identify the period required for endodermal induction by forced in vivo expression of Mixl1, we administered Dox at various time points (days 0, 2, 3, 4, 5, and 7 after ET) and then analyzed individual embryos at day 7 after ET by flow cytometry, using antibodies against the endodermal marker EpCAM (Fig. 2A). Although the degree of chimerism for cells that expressed EpCAM or failed to express EpCAM did not differ significantly until day 3 after ET (Fig. 2A), chimerism for EpCAM-expressing cells rose significantly when Dox administration began on day 4 after ET or thereafter (Fig. 2A). These data suggest that for endodermal induction, forced expression of Mixl1 is necessary until 4 days after ET, corresponding to the developmental stage of E6.5 embryos.
Based on these results, we attempted to regulate the expression of exogenous Mixl1 under the control of an early development marker, Oct3/4, that should enable timely, autonomous expression of exogenous Mixl1 without using Dox. For this purpose, we introduced an expression unit composed of a tetracycline transactivator (tTA) under the control of an Oct3/4 promoter (Oct3/4-tTA) and a tet-response element (TRE) with Mixl1 (TRE-Mixl1) into ESCs, thereby yielding OTiV-ESCs (Supplementary Fig. S4A). Unlike ESCs carrying an expression unit composed of tTA under the control of a CAG promoter (CAG-tTA) instead of Oct3/4-tTA (hereafter, CHT5-ESCs, Supplementary Fig. S3), OTiV-ESCs express exogenous Mixl1 under the proper control of an Oct3/4 promoter in the absence of Dox (Supplementary Fig. S4B–D). We investigated the effect on chimerism after an injection of OTiV1-ESCs into blastocysts. In embryos, one week after ET, maternal ingestion of Dox permitted OTiV1-ESCs to differentiate into all embryonic lineages equally in the absence of exogenous expression of Mixl1 (Left embryo in Fig. 2B). However, as expected, in the absence of Dox, OTiV1-ESCs preferentially differentiated into gut endoderm, and the same result was obtained when using RT5- or CHT5-ESCs (Fig. 2B, C). These data suggest that expression of exogenous Mixl1 for endodermal induction is required until the epiblast stage of early development and permits the inference that the period of forced expression of Mixl1 can be minimized during development.
Mixl1-inducible ESCs permit generation of functional pancreata via blastocyst complementation
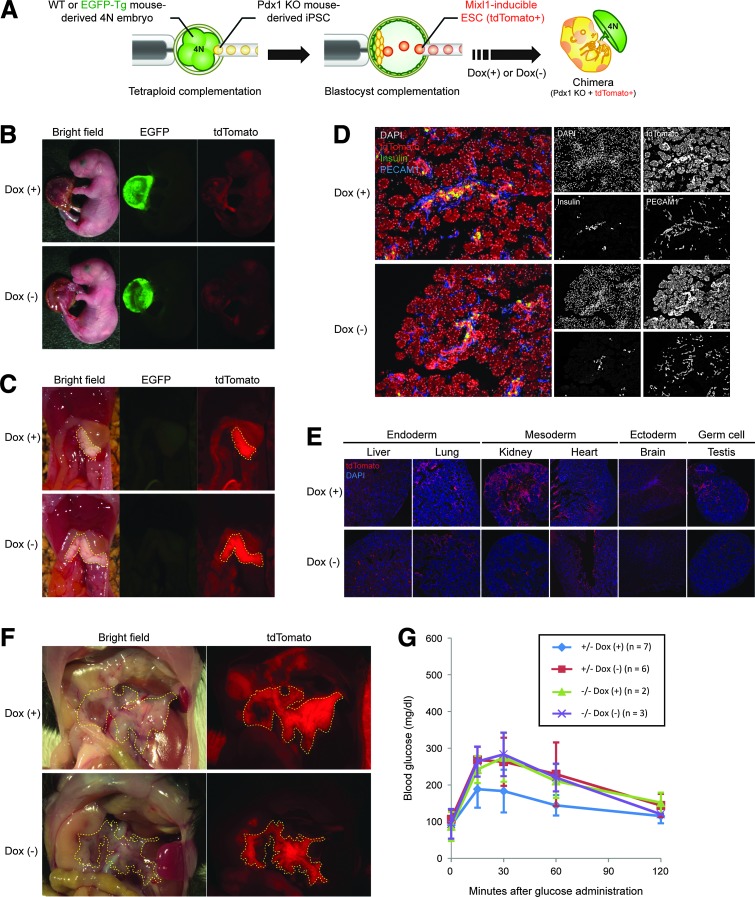
Finally, we tried to confirm the generation of organs from these Mixl1-inducible ESCs through blastocyst complementation. For this purpose, we developed a novel “tetraploid-based organ-complementation method.” In conventional blastocyst complementation, Mixl1-inducible ESCs are injected into pancreatogenesis-disabled Pdx1 KO mouse-derived blastocysts. Crossing heterozygous parental mice generates apancreatic Pdx1 KO mouse embryos only at a rate of one in four. In this tetraploid-based organ-complementation method, instead of injecting Mixl1-inducible ESCs into Pdx1 KO mouse-derived blastocysts, both Mixl1-inducible ESCs and Pdx-1 KO mouse-derived iPSCs are injected into WT or EGFP-labeled tetraploid embryos (Fig. 3A). This enables us to generate apancreatic Pdx1-KO mice and to perform organ complementation more steadily and efficiently. We, therefore, used this technique for confirmation of Mixl1-inducible ESC-derived organ generation.
FIG. 3.
Blastocyst complementation using Mixl1-inducible cells enables generation of functional pancreata in vivo. (A) Schema of novel blastocyst complementation system using tetraploid complementation and Mixl1-inducible ESCs. (B) Neonates generated via method shown in (A). To make tetraploid embryos, two-cell stage embryos derived from transgenic mice that ubiquitously express EGFP (EGFP-Tg) were electrofused. In the Dox(−) setting, foster mothers were given drinking water without Dox until 4 days after embryo transfer and were then given water with Dox. (C) Macroscopic views of internal organs of neonates shown in (B). Yellow dashes outline pancreata entirely composed of CHT5-ESC–derived cells. (D) Microscopic views of distribution of CHT5-ESC–derived cells in pancreata of newborn pups. Sections were immunostained for DsRed (red), insulin (green), and PECAM-1 (blue), with nuclear counterstaining using DAPI (white). (E) Microscopic views of distribution of CHT5-ESC–derived cells in various tissues of newborn pups. Sections were immunostained for DsRed (red), with nuclear counterstaining using DAPI (blue). (F) Macroscopic views of adult pancreata generated by an injection of CHT5-ESCs into Pdx1−/− blastocysts. In both Dox(+) and Dox(−) settings, mice have pancreata completely derived from CHT5-ESCs expressing tdTomato. (G) Results of glucose tolerance testing in Pdx1−/− and Pdx1+/− mice complemented with CHT5-ESCs in Dox(+) or Dox(−) settings (see insert). Blood was sampled via tail vein at intraperitoneal glucose administration (1 g/kg; 0 min) and at 15, 30, 60, and 120 min thereafter. Sections in (D, E) were observed under confocal laser scanning microscopy.
Reproductive rates are shown in Table 1. In neonates, EGFP expression was seen only in placentas of tetraploid embryos and not in embryos proper (Fig. 3B). When Dox had been given, contributions of CHT5-ESC progeny were observed throughout the body, except the pancreas; only the pancreas was completely composed of CHT5-ESC derivatives (Fig. 3B–D), as shown earlier [3,4]. On histologic study, contributions of CHT5-ESCs were observed not only in endodermal organs but also in the other germ-layer–derived organs such as kidney, heart, brain, and testis (Fig. 3E). In contrast, a few progeny of CHT5-ESCs were observed throughout the body in embryos not exposed to Dox until 7 days after ET (Fig. 3B). However, pancreata were completely composed of CHT5-ESC derivatives (Fig. 3C, D). These findings were also supported by a histologic study with the exception of the heart, where significant numbers of CHT5-ESC–derived cells were found (Fig. 3E). The reason for this unexpected contribution to heart tissues is not clear.
Table 1.
Results of Tetraploid-Based Organ-Complementation Method
| 1st cell | 2nd cell | Tetraploid embryo | Dox | TE | Pups (% TE) | Analysis as neonates | Chimeras | With PSC pancreas |
|---|---|---|---|---|---|---|---|---|
| Pdxl KO iPSC | — | WT | — | 147 | 24 (16) | 24 | — | 0 |
| EB3DR | WT | — | 201 | 32 (16) | 9 | 9 | 9 | |
| RT5 | WT | (+) | 60 | 16 (27) | 16 | 12 | 11 | |
| RT5 | WT | (−) | 78 | 12 (15) | 12 | 7 | 7 | |
| CHT5 | WT or EGFP Tg | (+) | 122 | 8 (7) | 7 | 3 | 3 | |
| CHT5 | WT or EGFP Tg | (−) | 248 | 19 (8) | 18 | 6 | 5 | |
| K3 | — | WT | — | 82 | 14 (17) | 5 | — | — |
iPSC, induced pluripotent stem cell; PSC, pluripotent stem cell; TE, transferred embyros; WT, wild type.
Finally, to assess the function of pancreata composed of CHT5-ESC progeny, we conducted glucose tolerance testing in adult mice generated by an injection of CHT5-ESCs into blastocysts obtained by crossing of Pdx1+/− mice. In adults, after glucose administration, blood glucose levels of mice with tdTomato-expressing pancreata were normally maintained whether or not Dox had been supplied during development (Fig. 3F, G). These data suggest that pancreata derived from Mixl1-inducible ESCs can normally function in vivo.
Discussion
While generation of a functional organ via blastocyst complementation in pigs is an important step toward generation of human organs in large animals, several issues remain. First, whether this approach can be considered for humans and pigs is still a big challenge. These two species are evolutionarily far wider diverged from one another than are mouse and rat. Second, most human ESCs or PSCs are believed to be epiblast-stage PSCs with chimerism competency that is extremely limited [21,22], because their stage is developmentally more advanced (“primed” status) than is that of rodent PSCs (“naïve” status) [23]. However, recent reports provide crucial data on long-term maintenance of naïve-status human PSCs [24–26]. These findings hold promise for generation of organs derived from patients' own PSCs. Third, the generation of human-pig chimeras arouses ethical concerns, of which one is the possibility that a chimeric pig may have human PSC-derived cells in tissues other than the target organ. It is, therefore, desirable to guide differentiation of human PSC-derived cells toward the target organ. There are several ways to do this. One approach is genetically to manipulate human PSCs to induce apoptosis on differentiation toward neurons or germ cells by introducing a construct containing an apoptosis gene under control of a tissue-specific promoter. Another approach is to transplant committed progenitor cells into postimplantation embryos instead of injecting PSCs into blastocysts. In this study, we exogenously induced expression of Mixl1 in order to restrict differentiation of ESC-derived cells. The results showed that most of the cells exogenously expressing Mixl1 preferentially contributed to endoderm derivatives. In particular, almost no contribution of ESC-derived cells to coat color was found, while internal organs included ESC contributions. However, contributions to tissues in other lineages also were observed in some cases. This might occur because the expression pattern of exogenous Mixl1 is not completely homogeneous among developing ESCs after blastocyst injection. Indeed, even when undifferentiated ESCs were used, immunostaining revealed that exogenous Mixl1 and EGFP are expressed at different levels after removal of Dox (Supplementary Fig. S1B, D). This could also be caused by silencing of TRE inserted into the Rosa26 locus. Consequently, exogenous Mixl1 expression below a certain level might fail to shift the fate of ESC progeny toward the endoderm. Nonetheless, the system described here should significantly decrease the contribution of PSC-derived cells to nonendodermal organs and help reduce ethical concerns associated with the generation of human-pig chimeras.
We demonstrated that when directing lineage contributions, expression of Mixl1 is required only during the first 4 days after embryo transfer. If a drug-inducible promoter is used to drive expression of Mixl1, drug administration is required only during those first 4 days. Alternatively, as shown in this study, incorporation of a cell-intrinsic system in which Milx1 expression is controlled by an Oct4 promoter yields expression only during the critical time period.
In conclusion, we present a novel way to limit and to control PSC fate in vivo. Inducible expression of a lineage-specifier gene such as Mixl1 for the endoderm, in early PSC derivatives, might also assist studies of specific germ layers or cell lineages in vivo. Furthermore, as discussed earlier, this approach may find application in targeted generation of organs derived from patients' own PSCs.
Supplementary Material
Acknowledgments
The authors thank Dr. A. Knisely for a critical reading of this article. This work was supported by grants from Japan Science and Technology Agency (JST), a KAKENHI (23700507) Grant-in-Aid for Young Scientists (B) from Japan Society for the Promotion of Science (JSPS), and the Ministry of Education, Culture, Sport, Science, and Technology (MEXT).
Author Disclosure Statement
HN is a founder, shareholder of iCELL Inc. and ChimaERA Corporation.
References
- 1.Takahashi K. and Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676 [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K. and Yamanaka S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872 [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi T, Yamaguchi T, Hamanaka S, Kato-Itoh M, Yamazaki Y, Ibata M, Sato H, Lee YS, Usui J, et al. (2010). Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell 142:787–799 [DOI] [PubMed] [Google Scholar]
- 4.Usui J, Kobayashi T, Yamaguchi T, Knisely AS, Nishinakamura R. and Nakauchi H. (2012). Generation of Kidney from Pluripotent Stem Cells via Blastocyst Complementation. Am J Pathol 180:2417–2426 [DOI] [PubMed] [Google Scholar]
- 5.Matsunari H, Nagashima H, Watanabe M, Umeyama K, Nakano K, Nagaya M, Kobayashi T, Yamaguchi T, Sumazaki R, Herzenberg LA. and Nakauchi H. (2013). Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs. Proc Natl Acad Sci U S A 110:4557–4562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henry GL. and Melton DA. (1998). Mixer, a homeobox gene required for endoderm development. Science 281:91–96 [DOI] [PubMed] [Google Scholar]
- 7.Ng ES, Azzola L, Sourris K, Robb L, Stanley EG. and Elefanty AG. (2005). The primitive streak gene Mixl1 is required for efficient haematopoiesis and BMP4-induced ventral mesoderm patterning in differentiating ES cells. Development 132:873–884 [DOI] [PubMed] [Google Scholar]
- 8.Lim SM, Pereira L, Wong MS, Hirst CE, Van Vranken BE, Pick M, Trounson A, Elefanty AG. and Stanley EG. (2009). Enforced expression of Mixl1 during mouse ES cell differentiation suppresses hematopoietic mesoderm and promotes endoderm formation. Stem Cells 27:363–374 [DOI] [PubMed] [Google Scholar]
- 9.Izumi N, Era T, Akimaru H, Yasunaga M. and Nishikawa S. (2007). Dissecting the molecular hierarchy for mesendoderm differentiation through a combination of embryonic stem cell culture and RNA interference. Stem Cells 25:1664–1674 [DOI] [PubMed] [Google Scholar]
- 10.Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL. and Wright CV. (1996). PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development 122:983–995 [DOI] [PubMed] [Google Scholar]
- 11.Niwa H, Miyazaki J. and Smith AG. (2000). Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 24:372–376 [DOI] [PubMed] [Google Scholar]
- 12.Miyazaki S, Miyazaki T, Tashiro F, Yamato E. and Miyazaki J. (2005). Development of a single-cassette system for spatiotemporal gene regulation in mice. Biochem Biophys Res Commun 338:1083–1088 [DOI] [PubMed] [Google Scholar]
- 13.Bell AC, West AG. and Felsenfeld G. (1999). The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell 98:387–396 [DOI] [PubMed] [Google Scholar]
- 14.Niwa H, Yamamura K. and Miyazaki J. (1991). Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193–199 [DOI] [PubMed] [Google Scholar]
- 15.Copeland NG, Jenkins NA. and Court DL. (2001). Recombineering: a powerful new tool for mouse functional genomics. Nat Rev Genet 2:769–779 [DOI] [PubMed] [Google Scholar]
- 16.Hirabayashi M, Kato M, Sanbo M, Kobayashi T, Hochi S. and Nakauchi H. (2010). Rat transgenesis via embryonic stem cells electroporated with the Kusabira-orange gene. Mol Reprod Dev 77:474. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi T, Kato-Itoh M, Yamaguchi T, Tamura C, Sanbo M, Hirabayashi M. and Nakauchi H. (2012). Identification of rat Rosa26 locus enables generation of knock-in rat lines ubiquitously expressing tdTomato. Stem Cells Dev 21:2981–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagy A, Gertsenstein M, Vintersten K. and Behringer R. (2003). Manipulating the Mouse Embryo: A Laboratory Manual, Third Edition. Cold Spring Harbor Laboratory, New York [Google Scholar]
- 19.Sherwood RI, Jitianu C, Cleaver O, Shaywitz DA, Lamenzo JO, Chen AE, Golub TR. and Melton DA. (2007). Prospective isolation and global gene expression analysis of definitive and visceral endoderm. Dev Biol 304:541–555 [DOI] [PubMed] [Google Scholar]
- 20.Sherwood RI, Chen TY. and Melton DA. (2009). Transcriptional dynamics of endodermal organ formation. Dev Dyn 238:29–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tachibana M, Sparman M, Ramsey C, Ma H, Lee HS, Penedo MC. and Mitalipov S. (2012). Generation of chimeric rhesus monkeys. Cell 148:285–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James D, Noggle SA, Swigut T. and Brivanlou AH. (2006). Contribution of human embryonic stem cells to mouse blastocysts. Dev Biol 295:90–102 [DOI] [PubMed] [Google Scholar]
- 23.Nichols J. and Smith A. (2009). Naive and primed pluripotent states. Cell Stem Cell 4:487–492 [DOI] [PubMed] [Google Scholar]
- 24.Gafni O, Weinberger L, Mansour AA, Manor YS, Chomsky E, Ben-Yosef D, Kalma Y, Viukov S, Maza I, et al. (2013). Derivation of novel human ground state naive pluripotent stem cells. Nature 504:282–286 [DOI] [PubMed] [Google Scholar]
- 25.Chan YS, Goke J, Ng JH, Lu X, Gonzales KA, Tan CP, Tng WQ, Hong ZZ, Lim YS. and Ng HH. (2013). Induction of a human pluripotent state with distinct regulatory circuitry that resembles preimplantation epiblast. Cell Stem Cell 13:663–675 [DOI] [PubMed] [Google Scholar]
- 26.Theunissen TW, Powell BE, Wang H, Mitalipova M, Faddah DA, Reddy J, Fan ZP, Maetzel D, Ganz K, et al. (2014). Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell [Epub ahead of print]; DOI: 10.1016/j.stem.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.