Abstract
Tissue resident stem cells are believed to exist in every organ, and their identification is commonly done using a combination of immunostaining for putative stem cell markers and label-retaining cell (LRC) strategy. In this study, we employed these approaches to identify potential stem cells in the penis. Newborn rats were intraperitoneally injected with thymidine analog, 5-ethynyl-2-deoxyuridine (EdU), and their penis was harvested at 7 h, 3 days, 1 week, and 4 weeks. It was processed for EdU stains and immunofluorescence staining for stem cell markers A2B5, PCNA, and c-kit. EdU-positive cells were counted for each time point and co-localized with each stem cell marker, then isolated and cultured in vitro followed by their characterization using flowcytometry and immunofluorescence. At 7 h post-EdU injection, 410±105.3 penile corporal cells were labeled in each cross-section (∼28%). The number of EdU-positive cells at 3 days increased to 536±115.6, while their percentage dropped to 25%. Progressively fewer EdU-positive cells were present in the sacrificed rat penis at longer time points (1 and 4 weeks). They were mainly distributed in the subtunic and perisinusoidal spaces, and defined as subtunic penile progenitor cells (STPCs) and perisinusoidal penile progenitor cells (PPCs). These cells expressed c-kit, A2B5, and PCNA. After culturing in vitro, only ∼0.324% corporal cells were EdU-labeled LRCs and expressed A2B5/PCNA. Therefore, labeling of penis cells by EdU occurred randomly, and label retaining was not associated with expression of c-kit, A2B5, or PCNA. The penile LRCs are mainly distributed within the subtunic and perisinusoidal space.
Introduction
It is generally believed that tissue-specific stem cells exist in every organ and tissue, and their function is to maintain tissue homeostasis by supplying new tissue-specific cells during normal tissue cycling and when existing tissue cells are lost due to injuries.
As an organ composed of multiple types of tissues, the penis itself contains a variety of stem cells. Two types of foreskin stem cells have been isolated to date, including skin-derived progenitors (SKPs) and mesenchymal stem cells (MSCs). A new and unique multipotent progenitor cell population derived from adult mammalian dermis, termed SKPs, has been isolated and expanded from rodent and human skin and differentiated into both neural and mesodermal progeny [1,2]. Meanwhile, MSCs were also defined, from low-temperature preserved human foreskin biopsies, by their adherent culture growth pattern. These cells could differentiate into mesodermal lineages, including adipocytes, osteocytes, and myocytes [3]. MSCs are antigenically distinct from SKPs, and when grown under the same conditions, they grow adherently (plastic adherence is one of the three hallmarks of MSC), while SKPs grow as floating spheres.
Vernet et al. investigated whether cells from normal tunica albuginea and Peyronie's disease (PD) plaques undergo osteogenesis, express stem cells markers, or give rise to other cell lineages via processes modulated by transforming growth factor-β1 (TGF-β1) [4]. In addition, penile shaft tissue sections from the rat and wild-type mouse were immunostained for Oct 4, an embryonic stem cell marker [5]. The results showed that Oct 4+ cells were detected in tunical and corporal tissues, and they could differentiate into smooth muscle cells (SMCs), myofibroblasts, or cardiomyocytes. This is the first report of isolation and characterization of embryonic-like endogenous stem cells in penile tissue. Although perivascular stem cells have been extracted from multiple organs, such as bone marrow, dental pulp, placenta, fat, and umbilical cord [6], the penis, as a part of the systematic circulation tree, has not yet received attention in this regard.
Due to the lack of specific markers, potential stem cells in the urinary bladder have tentatively been identified using the “label-retaining cell (LRC)” strategy [7]. In this study, the authors intraperitoneally injected thymidine analog, 5-bromo-2-deoxyuridine (BrdU), into 6-week-old rats daily for 4 consecutive days. However, the use of adult rats differs from the original and prevailing LRC protocol that calls for the use of newborn animals [8,9]. In addition, the immunohistochemical detection of BrdU-labeled cells is difficult due to the subtle color difference between BrdU and nuclear stains. More importantly, the use of strong acids and high temperature in the detection procedure degrades cellular proteins, rendering them unrecognizable by their cognate antibodies. Consequently, determination of stem cell marker expression in BrdU-labeled cells is often not possible.
To overcome these difficulties, we recently introduced a new stem cell labeling and detection method in which BrdU was replaced with 5-ethynyl-2-deoxyuridine (EdU) [10,11]. In this study, we injected EdU into newborn rats and examined the time-dependent distribution of EdU-labeled cells in the penis. We also investigated the relationship between EdU-labeled cells and various stem cell and cellular markers in these penile tissues.
Materials and Methods
Animals
All animal experiments in this study were approved by the Institutional Animal Care and Use Committee at our institution. Pregnant Sprague–Dawley rats were purchased from Charles River Laboratories for a different project investigating childbirth-related urinary incontinence. A total of 24 male neonatal pups delivered by these primiparous rats were used for the EdU retaining study. Each pup received an intraperitoneal injection of EdU (50 mg/kg; Invitrogen) immediately after birth. Six rats were sacrificed at each of the four time points (7 h, 3 days, 1 week, and 4 weeks postinjection) for penile corpora cavernosa tissue harvest.
Isolation of penile corporal cells and culture
Penile corporal cells were isolated from rats at 3 days post-EdU injection by incubation in 0.75% collagenase at 37°C for 20 min. The isolated cells were then cultured in DMEM supplemented with 10% fetal bovine serum (FBS) in a 10-cm dish. Corporal cells at passage 1 were seeded into six-well plates at 70% confluence. Forty-eight hours later, the cells were fixed with ice-cold methanol for 8 min. Corporal cells at passage 1 and 2 were also used for flowcytometry to identify EdU-positive cells.
Flowcytometry
The penile corporal cells at passage 1 and 2 were washed with 3% bovine serum albumin and incubated with anti-PCNA antibody (Abcam) in 50 μL buffer (1% FBS and 0.1% Na3N in PBS) for 30 min on ice. The cells were then incubated with Click-iT reaction cocktail containing Alexa594-azide (Cat# C10339; Invitrogen) for 30 min at room temperature without light. Thereafter, the cells were washed with PBS, re-suspended in 2 mL PBS, and analyzed in a fluorescence-activated cell sorter (FACSVantage SE System; BD Biosciences). The resulting data were further analyzed with FlowJo software (Tree Star, Inc.) to determine the percentage of proliferate cell nuclear antigen (PCNA) and EdU-positive cells among 1×105 penile corporal cells.
Preparation of tissue sections
Freshly dissected penile corporal tissue was fixed for 4 h with cold 2% formaldehyde and 0.002% picric acid in 0.1 M phosphate buffer, followed by overnight immersion in buffer solution containing 30% sucrose. Tissues were frozen in optimum cutting temperature compound (Sakura Finetek) and stored at −80°C until use. Sections were cut at 5 μm, adhered to charged slides, and air dried for 5 min before staining.
Immunofluorescence staining
Frozen tissue sections or fixed cultured penile corporal cells on glass cover slides were placed in 0.3% H2O2/methanol for 10 min, washed twice in PBS for 5 min, and incubated with 3% horse serum in PBS/0.3% Triton X-100 for 30 min at room temperature. After draining the solution from the tissue section, the slides were incubated overnight at 4°C with primary antibodies (Table 1) followed by secondary antibody conjugated with FITC or Texas Red (Vector Labs). Nuclear staining was performed with 4′,6-diamidino-2-phenylindole (DAPI). For tracking EdU-positive cells, tissue sections were incubated with Click-IT reaction cocktail (Invitrogen) for 30 min at room temperature. Control tissue sections were similarly prepared except no primary antibody was added.
Table 1.
Antibodies Used in This Study
| Target protein | Supplier | Catalog no. |
|---|---|---|
| SMA | Sigma-Aldrich, St. Louis, MO | 099K4816 |
| c-Kit | Santa Cruz Biotech, Santa Cruz, CA | sc-168 |
| vWF | Abcam, Inc., Cambridge, MA | ab6994 |
| A2B5 | Abcam, Inc., Cambridge, MA | ab53521 |
| PCNA | Abcam, Inc., Cambridge, MA | ab18197 |
Image and statistical analysis
Tissue slides were examined with a Nikon Eclipse E600 fluorescence microscope and photographed with Retiga 1300 Q-imaging camera using the ACT-1 software (Nikon Instruments, Inc.). The images were then quantified using Image-Pro Plus image software (Media Cybernetics). For statistical analysis, three to five randomly selected fields per slides were examined and analyzed with Prism 5 (GraphPad Software, Inc.). Data were expressed as mean±standard deviation. One-way ANOVA was used and followed by post hoc analysis to determine significance (P<0.05).
Results
Development of the rat penis from 7 h to 4 weeks
The mean length of the rats penis was 2+0.2 mm, 3+0.8 mm, 5+0.65 mm, and 7+0.8 mm, (N=6) at 7 h, 3 days, 1 week, and 4 weeks after birth, respectively. The growth of the rat penis was rapid during the first 2 weeks after birth, but growth slowed down after that point.
Distribution of EdU retaining cells in the penis during development
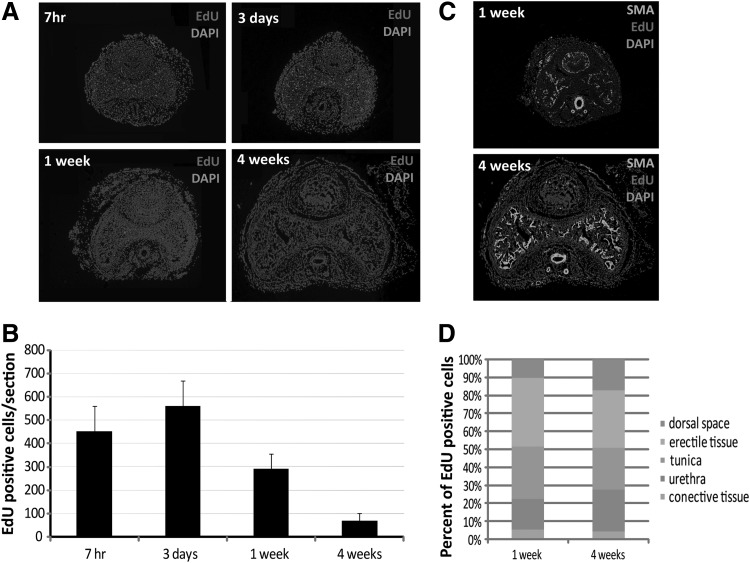
An intraperitoneal injection of EdU into newborn rats resulted in a high rate of penile corporal cells labeling (Fig. 1A). At 7 h post-EdU injection, 410±105.3 corporal cells were labeled in each cross-section (∼28%). These EdU-positive cells increased to 536±115.6, while their percentage dropped to 25% at 3 days. Progressively fewer EdU-positive cells were present in the sacrificed rat penis at longer time points (1 and 4 weeks) (Fig. 1B).
FIG. 1.
Distribution of 5-ethynyl-2-deoxyuridine (EdU) label-retaining cell (LRCs) in rats' penis. (A) EdU-labeled penile LRCs at different time points at 7 h, 3 days, 1 week, and 4 weeks post-EdU injection (original at ×40). (B) Number of EdU-labeled penile LRCs (P<0.01). (C) Location of EdU-labeled LRCs within the penis at 1 and 4 weeks post-EdU injection (original at×40). (D) percentage of EdU penile LRCs at different locations within the penis.
One week post-EdU injection, the location of EdU-labeled cells could be recognized with the help of SMA staining. Most labeling occurred in the penile erectile tissue, tunica, and urethral tissue, while some EdU-labeled cells were found in the connective tissue and dorsal vessel space (Fig. 1C, D). The EdU-labeling rates within the penile tissues just mentioned were similar at 1 and 4 weeks.
The penis contains long-term DNA LRCs
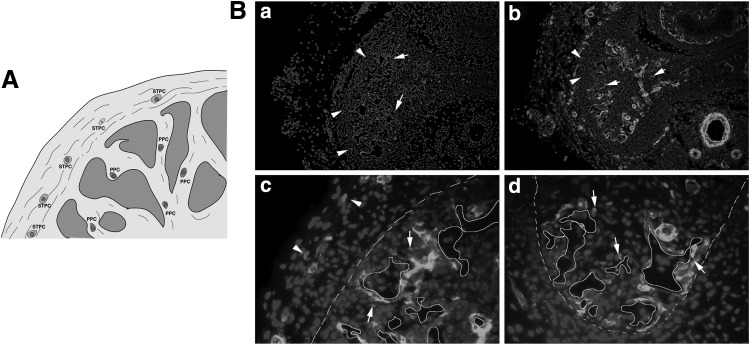
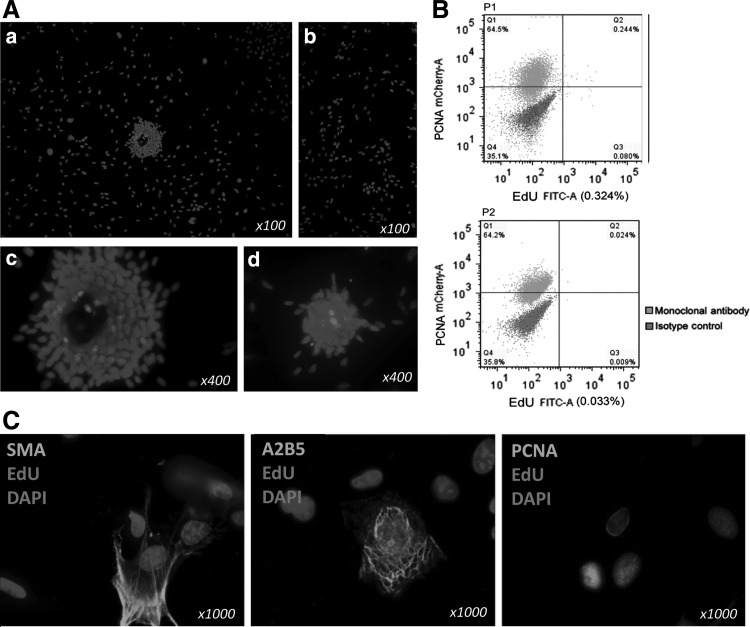
To detect long-term EdU retaining cells, the rats' penile tissues at 1 and 4 weeks post-EdU injection were used. At these time points, the label was mainly detectable in the perisinusoidal and subtunic tissue. We defined the EdU long-term LRCs as perisinusoidal penile progenitor cells (PPCs) and subtunic penile progenitor cells (STPCs) (Fig. 2A, B).
FIG. 2.
Localization of EdU penile LRCs. (A) Hypothetical scheme of the penile LRCs. (B) Location of STPC (arrowhead) and PPC (arrow). (a and b ×100, c and d ×400). STPC, subtunic penile progenitor cells; PPC, perisinusoidal penile progenitor cells.
Characterization of penile LRCs
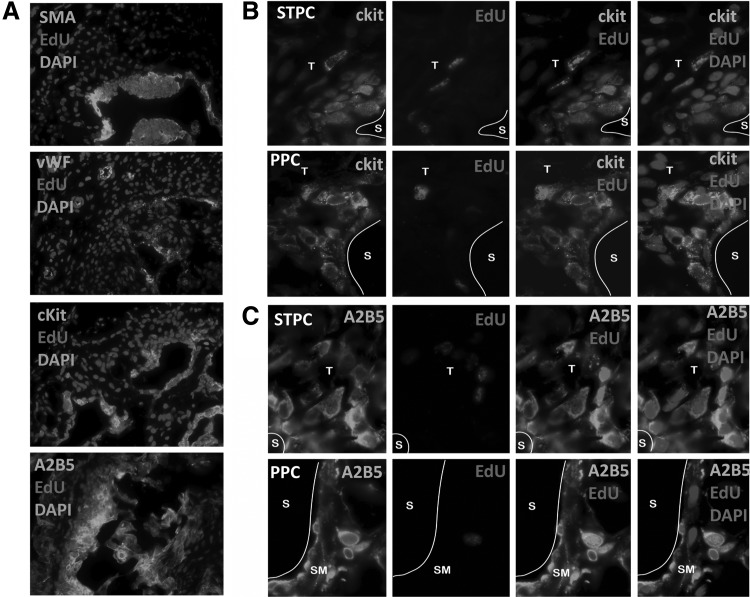
To characterize those penile LRCs, several cellular markers were used to screen LRCs at 4 weeks, including SMA, vWF, c-kit, A2B5, and PCNA (Fig. 3A). In this study, c-kit was detected in both STPCs and PPCs (Fig. 3B). In both compartments, the percentage of EdU-positive cells among c-kit-positive cells was largely constant (10%) at 4 weeks after EdU injection. The percentage of EdU-positive cells among A2B5-positive cells was also constant at 3.5. On the other hand, the percentage of A2B5-positive cells among EdU-positive cells was 99% (Fig. 3C).
FIG. 3.
Characterization of penile LRCs in tissue. (A) Cellular markers of penile LRCs (original at ×200). (B) Expression of c-Kit in STPC and PPC (original at ×1000). (C) Expression of A2B5 in STPC and PPC (original at ×1000).
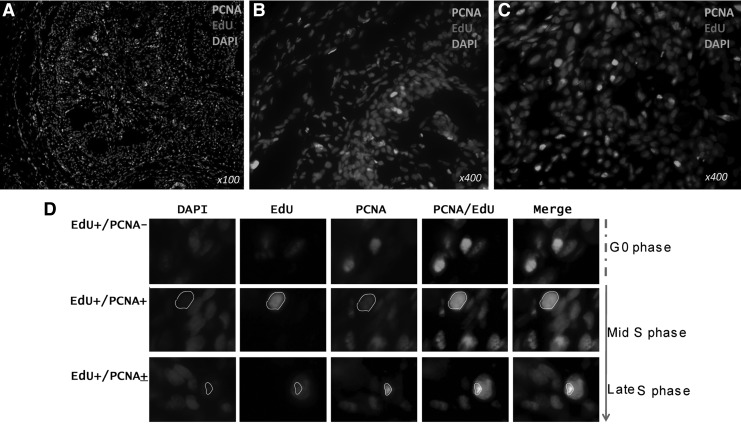
To determine the ratio of proliferating cells during penis development, expression of PCNA was checked. The nuclear antigen PCNA is a marker of proliferation and is expressed only in cycling cells. During the cell cycling at G0 phase, the PCNA was evenly distributed in the entire cell nucleus and the expression level decreased at Mid-S phase. The PCNA became condensate at the late-S phase. Eighty percent of EdU LRCs were at G0 phase and did not express PCNA, while only 10% of EdU LRCs expressed PCNA and were at Mid-S/Later-S phase (Fig. 4).
FIG. 4.
Expression of PCNA by penile LRCs (A), including the STPC (B) and PPC (C). (D) Cell cycling of EdU penile LRCs (original at ×1000). PCNA, proliferate cell nuclear antigen.
Characterization of long-term LRCs in vitro
Based on the immunohistochemistry analyses, we next examined whether cultured penile LRCs expressed A2B5 and PCNA. EdU-labeled penile LRCs were harvested and cultured until passage 1 and 2, and were noted to possess the ability to form clones in culture dishes, while some of the cells grew individually (Fig. 5A). A total of 0.324% corporal cells were EdU-labeled LRCs at passage 1, and this percentage dropped to 0.033% at passage 2 (Fig. 5B). The cultured LRCs expressed A2B5 and PCNA but did not express SMA (Fig. 5C).
FIG. 5.
Characterization of EdU-labeled penile LRCs in vitro. (A) (a–d) Clone formation in vitro. (B) Flow cytometric assay on EdU penile LRCs. (C) Cellular markers of EdU-labeled penile LRCs.
Discussion
The motivation for this work comes from the need to understand the stem cell population(s) of the penis in greater depth, in order to begin to dissect the molecular regulation of stem/progenitor cell function in greater detail. Identifying and locating potential stem/progenitor cells in the penis might facilitate future stem cell therapies targeting erectile dysfunction and PD.
A pulse of the thymidine analog BrdU given to young animals at the optimal time can identify the location of slow-cycling long-term LRCs, putative adult stem cells, within the adult tissue [12,13], while EdU could also fulfill this screening method for stem/progenitor cells [11]. This study reassesses the penile LRC profile by employing EdU label whose detection is completely devoid of ambiguity. With regard to histology, we examined the entire penis and isolated the LRCs and cultured them in vitro.
Our results indicate that labeling of the neonatal rat penis by EdU occurred at a high rate, but the number of labeled cells dropped sharply within 1 week. These observations are consistent with most LRC studies and generally reflect the rapid cell cycling in most neonatal animal tissues [8,9,14]. It is noteworthy that the distribution of the labeled cells was mainly within the subtunic and perisinusoidal space. To examine stem cell marker expression in the penis, particularly LRCs, four well-characterized markers that relate to endothelial, SMC, cell proliferates, and neural stem cells were investigated. Both endothelial and SMCs were not co-localized with EdU.
Neural stem cell marker A2B5 was strongly expressed in penile Edu-labeled LRCs, including STPCs and PPCs. The expression of hematopoietic stem cell (HSC) marker c-kit in the penis has been extensively studied. While it is expressed in both mast cells and interstitial cells of Cajal (ICCs), most attention has been given to the latter as these cells are believed to be the pacemakers of detrusor contraction [15]. In this study, we detected c-kit expression in the LRCs near the penile sinusoids. Although the rate of co-localization in c-kit expression and EdU labeling is not higher, the location is quite important as it may play an important role in the relaxation and contraction of penile SMCs. While the significance of these findings is presently unknown, it should be pointed out that in the bone marrow, c-kit-expressing HSCs have been found to cycle rapidly from proliferative to quiescent state [16], and c-kit has been shown to regulate the maintenance of quiescent HSC [17]. In addition, c-kit expression has been associated with quiescent hepatic satellite cells [18], and ICCs in the adult small intestine have been found to lack proliferative activity. Therefore, the c-kit-expressing LRCs in the penis are probably quiescent ICCs.
It has been noted that PCNA expression detected by MAb may define different cell subpopulations in different cell types relative to those incorporating BrdU [19]. It has been widely used for the evaluation of cell proliferation in rat tissues with BrdU, PCNA, and Ki-67(MIB-5) Immunohistochemistry [20,21]. In our current data, it was noted that most EdU-labeled LRCs did not express PCNA, which indicates that most of these cells are quiescent cells at M0 phase.
After their isolation and culture in vitro, the EdU-labeled LRCs grew slow and formed cell clones. Only 0.33% of penile corporal cells were EdU-labeled LRCs, which is lower than the in situ rate in the penile tissue. This may be due to the difference of proliferation between the EdU-labeled LRCs and other types of corporal cells.
Conclusions
Labeling of penile corporal cells by EdU occurred randomly, and label retaining was not associated with expression of c-kit, A2B5, or PCNA. The penile LRCs are mainly distributed within the subtunic and perisinusoidal space.
Acknowledgment
This work was supported by a grant from the U.S. National Institutes of Health (DK045370).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Toma JG, Akhavan M, Fernandes KJ, Barnabe-Heider F, Sadikot A, Kaplan DR. and Miller FD. (2001). Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol 9:778–784 [DOI] [PubMed] [Google Scholar]
- 2.Toma JG, McKenzie IA, Bagli D. and Miller FD. (2005). Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells 6:727–737 [DOI] [PubMed] [Google Scholar]
- 3.Bartsch G, Yoo JJ, De Coppi P, Siddiqui MM, Schuch G, Pohl HG, Fuhr J, Perin L, Soker S. and Atala A. (2005). Propagation, expansion, and multilineage differentiation of human somatic stem cells from dermal progenitors. Stem Cells Dev 3:337–348 [DOI] [PubMed] [Google Scholar]
- 4.Vernet D, Nolazco G, Cantini L, Magee TR, Qian A, Rajfer J. and Gonzalez-Cadavid NF. (2005). Evidence that osteogenic progenitor cells in the human tunica albuginea may originate from stem cells: implications for peyronie disease. Biol Rep 6:1199–1210 [DOI] [PubMed] [Google Scholar]
- 5.Nolazco G, Kovanecz I, Vernet D, Gelfand RA, Tsao J, Ferrini MG, Magee T, Rajfer J. and Gonzalez-Cadavid NF. (2008). Effect of muscle-derived stem cells on the restoration of corpora cavernosa smooth muscle and erectile function in the aged rat. BJU Int 9:1156–1164 [DOI] [PubMed] [Google Scholar]
- 6.Ergun S, Tilki D. and Klein D. (2011). Vascular wall as a reservoir for different types of stem and progenitor cells. Antioxid Redox Signal 4:981–995 [DOI] [PubMed] [Google Scholar]
- 7.Kurzrock EA, Lieu DK, Degraffenried LA, Chan CW. and Isseroff RR. (2008). Label-retaining cells of the bladder: candidate urothelial stem cells. Am J Physiol Renal Physiol 6:F1415–F1421 [DOI] [PubMed] [Google Scholar]
- 8.Bickenbach JR. and Chism E. (1998). Selection and extended growth of murine epidermal stem cells in culture. Exp Cell Res 1:184–195 [DOI] [PubMed] [Google Scholar]
- 9.Duvillie B, Attali M, Aiello V, Quemeneur E. and Scharfmann R. (2003). Label-retaining cells in the rat pancreas: location and differentiation potential in vitro. Diabetes 8:2035–2042 [DOI] [PubMed] [Google Scholar]
- 10.Lin G, Huang YC, Shindel AW, Banie L, Wang G, Lue TF. and Lin CS. (2009). Labeling and tracking of mesenchymal stromal cells with EdU. Cytotherapy 7:864–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Lin G, Qiu X, Ning H, Banie L, Lue TF. and Lin CS. (2011). Label retaining and stem cell marker expression in the developing rat urinary bladder. Urology 3:746e1–746e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishikawa Y, Ida-Yonemochi H, Nakakura-Ohshima K. and Ohshima H. (2012). The relationship between cell proliferation and differentiation and mapping of putative dental pulp stem/progenitor cells during mouse molar development by chasing BrdU-labeling. Cell Tissue Res 1:95–107 [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa Y, Ida-Yonemochi H, Suzuki H, Nakakura-Ohshima K, Jung HS, Honda MJ, Ishii Y, Watanabe N. and Ohshima H. (2010). Mapping of BrdU label-retaining dental pulp cells in growing teeth and their regenerative capacity after injuries. Histochem Cell Biol 3:227–241 [DOI] [PubMed] [Google Scholar]
- 14.Baumhakel M, Werner N, Bohm M. and Nickenig G. (2006). Circulating endothelial progenitor cells correlate with erectile function in patients with coronary heart disease. Eur Heart J 18:2184–2188 [DOI] [PubMed] [Google Scholar]
- 15.McCloskey KD. (2011). Interstitial cells of Cajal in the urinary tract. In: Handbook of Experimental Pharmacology. Andersson KE, Michel MC, eds. Springer, Berlin, Germany, pp 233–254 [DOI] [PubMed] [Google Scholar]
- 16.Venezia TA, Merchant AA, Ramos CA, Whitehouse NL, Young AS, Shaw CA. and Goodell MA. (2004). Molecular signatures of proliferation and quiescence in hematopoietic stem cells. PLoS Biol 10:e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thoren LA, Liuba K, Bryder D, Nygren JM, Jensen CT, Qian H, Antonchuk J. and Jacobsen SE. (2008). Kit regulates maintenance of quiescent hematopoietic stem cells. J Immunol 4:2045–2053 [DOI] [PubMed] [Google Scholar]
- 18.Jiang W, Ma A, Wang T, Han K, Liu Y, Zhang Y, Dong A, Du Y, Huang X, et al. (2006). Homing and differentiation of mesenchymal stem cells delivered intravenously to ischemic myocardium in vivo: a time-series study. Pflugers Arch 1:43–52 [DOI] [PubMed] [Google Scholar]
- 19.Coltrera MD. and Gown AM. (1991). PCNA/cyclin expression and BrdU uptake define different subpopulations in different cell lines. J Histochem Cytochem 1:23–30 [DOI] [PubMed] [Google Scholar]
- 20.Valero J, Weruaga E, Murias AR, Recio JS. and Alonso JR. (2005). Proliferation markers in the adult rodent brain: bromodeoxyuridine and proliferating cell nuclear antigen. Brain Res 3:127–134 [DOI] [PubMed] [Google Scholar]
- 21.Muskhelishvili L, Latendresse JR, Kodell RL. and Henderson EB. (2003). Evaluation of cell proliferation in rat tissues with BrdU, PCNA, Ki-67(MIB-5) immunohistochemistry and in situ hybridization for histone mRNA. J Histochem Cytochem 12:1681–1688 [DOI] [PubMed] [Google Scholar]