Abstract
Excessive gibberellin (GA) signalling, mediated through the DELLA proteins, has a negative impact on plant fertility. Loss of DELLA activity in the monocot rice (Oryza sativa) causes complete male sterility, but not in the dicot model Arabidopsis (Arabidopsis thaliana) ecotype Landsberg erecta (Ler), in which DELLA function has been studied most extensively, leading to the assumption that DELLA activity is not essential for Arabidopsis pollen development. A novel DELLA fertility phenotype was identified in the Columbia (Col-0) ecotype that necessitates re-evaluation of the general conclusions drawn from Ler.
Fertility phenotypes were compared between the Col-0 and Ler ecotypes under conditions of chemical and genetic GA overdose, including mutants in both ecotypes lacking the DELLA paralogues REPRESSOR OF ga1-3 (RGA) and GA INSENSITIVE (GAI).
Ler displays a less severe fertility phenotype than Col-0 under GA treatment. Col-0 rga gai mutants, in contrast with the equivalent Ler phenotype, were entirely male sterile, caused by post-meiotic defects in pollen development, which were rescued by the reintroduction of DELLA into either the tapetum or developing pollen.
We conclude that DELLA activity is essential for Arabidopsis pollen development. Differences between the fertility responses of Col-0 and Ler might be caused by differences in downstream signalling pathways or altered DELLA expression.
Keywords: Arabidopsis thaliana, Col-0, DELLA, ecotypic differences, gibberellin, Ler, male sterility, pollen development
Introduction
The phytohormone gibberellin (GA) regulates stamen development in numerous flowering plants (Pharis & King, 1985). GA is necessary for filament elongation and pollen development in Arabidopsis (Arabidopsis thaliana) and rice (Oryza sativa) (reviewed in Plackett et al., 2011): anther development arrests prematurely in mutants unable to synthesize or perceive bioactive GA in these two species and tomato (Nester & Zeevaart, 1988; Jacobsen & Olszewski, 1991; Goto & Pharis, 1999; Cheng et al., 2004; Aya et al., 2009). Expression analyses suggest that GA signalling occurs in the anther tapetum and developing microspores (Hirano et al., 2008; Hu et al., 2008). GA signalling in rice anthers acts through the transcription factor OsGAMYB (Aya et al., 2009), with downstream targets regulating tapetum secretory functions and programmed cell death (PCD). Two GAMYB homologues in Arabidopsis, MYB33 and MYB65, have similar functions (Millar & Gubler, 2005), suggesting that GA regulation of stamen development is conserved between monocots and dicots.
GA signalling acts through the degradation of DELLA proteins (reviewed in Harberd et al., 2009; Ueguchi-Tanaka & Matsuoka, 2010; Sun, 2010), a class of transcriptional regulators belonging to the GRAS (for GA INSENSITIVE (GAI), REPRESSOR OF ga1-3 (RGA) and SCARECROW (SCR)) superfamily (Pysh et al., 1999) that otherwise inhibit GA-dependent changes in the expression of downstream target genes (Cao et al., 2006; Zentella et al., 2007; Hou et al., 2008). DELLA loss-of-function mutants display constitutive growth responses which mimic treatment with exogenous GA (Silverstone et al., 1997; Ikeda et al., 2001). DELLA-dependent transcriptional regulation is mediated through protein–protein interactions with multiple classes of transcription factors, the first described example of this being the sequestration of PHYTOCHROME INTERACTING FACTOR (PIF)3 and PIF4 to inhibit hypocotyl elongation during photomorphogenesis (de Lucas et al., 2008; Feng et al., 2008).
Although most recent investigations have concentrated on the effects of GA deficiency or insensitivity on stamen development, excessive GA signalling also negatively affects Arabidopsis fertility. Wild-type Columbia (Col-0) and GA biosynthetic mutants set fewer seeds per silique when grown under exogenous GA treatment (Jacobsen & Olszewski, 1993; Rieu et al., 2008a,b). Arabidopsis possesses five DELLA paralogues (Dill & Sun, 2001), and studies on their function have been conducted mainly in Landsberg erecta (Ler). It has been shown recently that a basal level of fertility persists in the Ler ecotype, even in the global mutant, which carries mutations in all five DELLA paralogues (Fuentes et al., 2012). By contrast, loss-of-function mutants in the monocot species rice and barley (Hordeum vulgare), which each possess single DELLA orthologues, are reported as sterile (Lanahan & Ho, 1988; Ikeda et al., 2001).
In this article, we report the creation of a novel rga gai loss-of-function mutant in the Col-0 ecotype, rga-28 gai-td1, which display complete male sterility, contrary to the phenotype of Ler DELLA mutants. Plants in which DELLA activity was not impaired did not display such severe fertility phenotypes when grown under exogenous GA treatment. Loss of ERECTA (ER) function from the Col-0 DELLA loss-of-function mutant rga-28 gai-td1 did not restore fertility, and so cannot explain the phenotypic differences between Col-0 and Ler DELLA mutants. These findings highlight hitherto unsuspected ecotypic differences in floral developmental responses to GA, and imply potential differences in GA signal transduction or downstream transcriptional networks between Col-0 and Ler.
Microscopic analysis of rga-28 gai-td1 anthers identified post-meiotic developmental defects leading to the collapse of immature pollen. Reintroduction of RGA rescued male fertility, confirming the necessity of DELLA activity to pollen development in Col-0. Surprisingly, it was found that the expression of RGA separately in either the tapetum or developing microspores was sufficient to restore pollen development, raising the prospect of post-meiotic communication between these two distinct and physically separate cell lineages downstream of the DELLA proteins.
Materials and Methods
Plant material and growth conditions
Experiments were performed in either the Arabidopsis thaliana (L.) Heynh. Col-0 or Ler ecotype, as specified. Plant growth conditions and exogenous 100 µM GA3 treatment were as described in Rieu et al. (2008b). The rga-28 allele has been described previously in Tyler et al. (2004). rga-30 was identified from the SALK collection (SALK_137951) (Alonso et al., 2003), gai-td1 from the SAIL collection (SAIL_82_F06) (Sessions et al., 2002), and gai-td2 (SK14663) and gai-td3 (SK20878) from the SK population of activation tagged lines (Robinson et al., 2009) (Supporting Information Fig. S1). The rga-24,rga-t2 and gai-t6 alleles have been described previously (Peng et al., 1997; Dill & Sun, 2001; Lee et al., 2002). rga-28 gai-td1,rga-28 gai-td2,rga-28 gai-td3,rga-30 gai-td1,rga-30 gai-td2 and rga-30 gai-td3 double-mutant lines were established by crossing and were verified by genotyping PCR (Table S1).
Transgenic lines
The RGA CDS lacking a stop codon was cloned into pENTR11 as a SalI-NotI restriction fragment and subsequently fused in frame with green fluorescent protein (GFP) in the pGWB450 vector (Nakagawa et al., 2007) via Gateway LR recombination (Invitrogen, Carlsbad, CA, USA).
Published fragments of the LTP12 (Ariizumi et al., 2002) or LAT52 (Twell et al., 1989) promoter sequence were introduced as XbaI restriction fragments. Segregating rga-28 gai-td1/+ populations were transformed via Agrobacterium, with subsequent selection of T1 transformants by kanamycin resistance. T1 individuals homozygous for both rga-28 and gai-td1 were identified by genotyping PCR (Table S1). Single insertion transgenic lines descended from these individuals were identified in the T2 generation using 3 : 1 Mendelian segregation, with the presence of the transgene confirmed by PCR.
Phenotypic characterization
Growth characterization experiments were performed using a blocked split plot design (Gomez & Gomez, 1984) with GA treatment applied across whole trays (main plot) and genotypes being randomized within trays (split plot). Phenotypic measurements were performed as described in Rieu et al. (2008b), using a population of 144 plants (n = 12). Three siliques were harvested from the primary inflorescence of each plant for seed counts. The fertility of individual floral positions was scored on the basis of silique elongation by the end of flowering.
Microscopy
Pollen viability was assessed using Alexander cytoplasmic stain (Alexander, 1969) on whole anther squashes; pollen developmental progression was determined by staining isolated stamens using 2.0 μg µl−1 4′,6-diamidino-2-phenylindole (DAPI, Sigma) in aqueous solution (Tarnowski et al., 1991) and observed using a UV light microscope (Nikon, Tokyo, Japan). Embedding of inflorescence tissues for sectioning was performed as described in Vizcay-Barrena & Wilson (2006). Two-micrometre-thick sections were observed with a Zeiss Axiophot light microscope (Carl Zeiss Ltd, Welwyn Garden City, UK) with an attached Retiga EXi camera system (QImaging, Surrey, BC, Canada). Paclobutrazol (PAC)-treated inflorescences (1 mM PAC, 0.06% Tween-20) were used in confocal microscopy of transgenic lines to enhance DELLA stabilization and thus GFP intensity. Fluorescence was detected using a Leica SP2 confocal laser scanning microscope (Leica Microsystems, Wetzlar, Germany), exciting fluorescence at 488 nm with an argon laser. GFP excitation was collected between 500 and 515 nm, and chlorophyll excitation was collected between 660 and 700 nm. Images were processed using Leica SP2 Image Analysis software.
Statistical analysis
All statistical analysis was performed using the Genstat software package (2010, 13th edition, ©VSN International Ltd, Hemel Hempstead, UK). Phenotypic characters were analysed by analysis of variance (ANOVA), using a transformed scale where necessary (natural logarithm for vegetative internode and silique lengths), to meet the assumptions of a normal distribution and homogeneity of variance, and taking into account the blocked split plot design. The main effects of genotype and GA treatment and the interaction between these factors were assessed by F tests. GA treatment did not have a significant effect on flowering time (P = 0.096), but genotype was strongly significant (P < 0.001). For all other characters, a significant interaction (P < 0.001) was detected between genotype and GA treatment. The binary character of silique set was analysed by modelling the proportion of plants (n = 12) for each genotype by GA treatment combination having set a silique at each inflorescence position, using a generalized linear model (GLM) (McCullagh & Nelder, 1989) with a logit link function. Following ANOVA or GLM, comparisons were made between pairs of genotypes or GA treatment conditions within a single genotype using least significant differences (LSDs) based on the appropriate degrees of freedom (df) with a significance threshold of either 5% or 1%, as specified. Means (in figures) are otherwise presented with individual standard errors (SE).
Results
Loss of functional RGA and GAI causes complete sterility in the Col-0 ecotype, but not in Ler
Exogenous GA treatment is known to reduce seed set (per silique) in the Arabidopsis Col-0 ecotype (Jacobsen & Olszewski, 1993; Rieu et al., 2008a,b). In addition, we found that GA treatment reduces the frequency of silique set in Col-0 (Fig.1a,b), although this phenomenon was unpredictable, with flowers immediately adjacent to infertile positions often setting fully fertile siliques (Fig.1b). Unexpectedly, a novel DELLA loss-of-function double mutant generated in the Col-0 background, rga-28 gai-td1, was completely infertile under control growth conditions (Fig.1c). The equivalent mutant in the Ler ecotype, rga-24 gai-t6, has been shown previously to be fertile, albeit at reduced levels (Dill & Sun, 2001). The association of this phenotype with the loss of both RGA and GAI in the Col-0 background was confirmed by combining other rga and gai loss-of-function alleles. All combinations proved infertile (Fig. S1).
Figure 1.
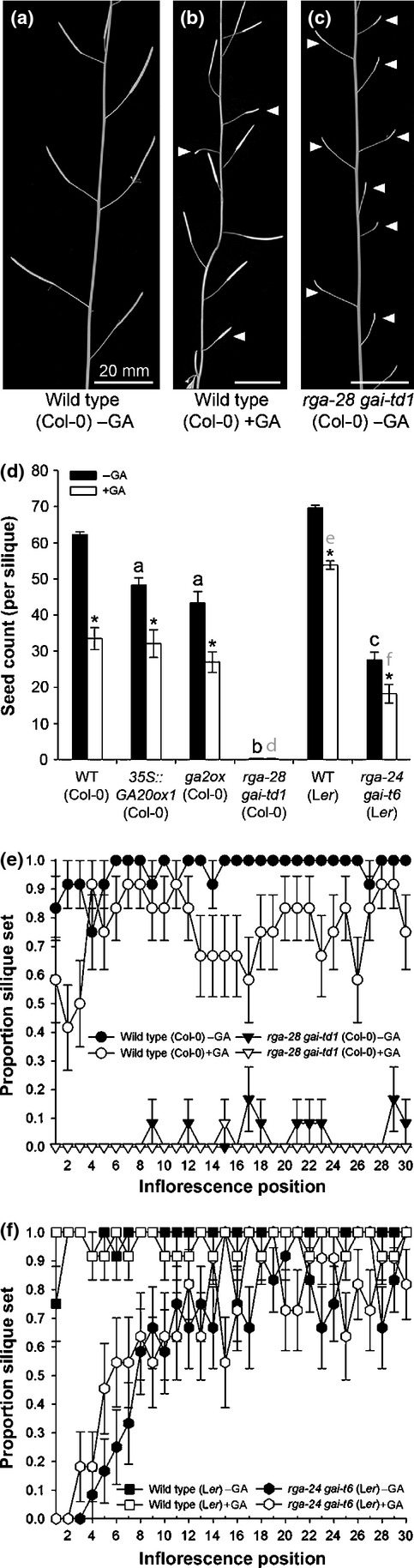
Differential fertility of Arabidopsis thaliana Col-0 and Ler rga gai mutants. (a–c) Primary inflorescences showing silique set of wild-type Col-0 under (a) control growth conditions and (b) 100 µM GA3 treatment, and (c) rga-28 gai-td1 (Col-0). White arrowheads indicate infertile silique positions. (d) Mean number of seeds per silique (n = 36, ± SE) under control conditions and GA3 treatment. Letters indicate significant difference (P < 0.01) of a genotype within a single GA treatment from control wild-type (black) and GA-treated wild-type (grey), respectively. Genotypes marked with different letters are significantly different from one another. Asterisks indicate significant difference (P < 0.01) between GA treatments within the same genotype. Pairwise comparisons were made using 1% least significant differences (LSDs) on a log-transformed scale (8.782 on 121 df between genotypes, 9.717 on 71 df between GA treatments). (e, f) Mean frequency of successful silique set on the primary inflorescence (n = 12, ± SE) of wild-type and rga gai mutant genotypes in Col-0 (e) and Ler (f) under control growth conditions and GA treatment.
To further investigate the interaction between GA overdose, ecotype and fertility, lines with reduced DELLA signalling (rga-28 gai-td1 and the comparable Ler mutant, rga-24 gai-t6; Dill & Sun, 2001) or altered GA biosynthesis (35S::GA20ox1 (Col-0) and the Col-0 ga2ox quintuple loss-of-function mutant, herein referred to as ga2ox; Coles et al., 1999; Rieu et al., 2008a) were characterized under both control growth conditions and GA treatment. Increasing bioactive GA through genetic manipulation reduced seed set (P < 0.01; Fig.1d), but neither this nor further exogenous GA treatment reduced seed set to the extent displayed by either rga gai mutant (P < 0.01). Further reductions in seed set in the two genetically GA-overdosed lines when under GA treatment (P < 0.01) suggest that GA responses in these lines are not fully saturated. rga-24 gai-t6 (Ler) was confirmed as partially fertile under our growth conditions compared with the wild-type (P < 0.01; Fig.1d). Seed set in this mutant was further reduced by GA treatment (P < 0.01), but still remained more fertile than rga-28 gai-td1 (Col-0; P < 0.01). The remaining capacity of the rga-24 gai-t6 (Ler) reproductive tissues to respond to GA could be explained by the presence of at least one additional DELLA paralogue in this ecotype.
Interestingly, although seed number was not significantly different between Col-0 and Ler wild-type under control growth conditions (P > 0.05; Fig.1d), Ler seed set under GA treatment was far more robust, being reduced to 77.30% of the untreated wild-type compared with 53.69% in Col-0 (P < 0.01), despite indications from the similarity in seed set between GA-treated Col-0 wild-type, 35S::GA20ox1 and ga2ox (P > 0.05) that GA treatment was sufficiently strong to saturate downstream responses. Similar conclusions can be drawn when considering the frequency of silique set across the primary inflorescence: GA treatment reduced the mean probability of silique set in Col-0 to 0.76 ± 0.02 compared with a control value of 0.97 ± 0.01 (Fig.1e), whereas the fertility of Ler was unaffected (0.96 ± 0.01 compared with 0.99 ± 0.01; Fig.1f). Genetic GA overdose induced similar reductions in Col-0 silique set to GA treatment (Fig. S2), none of which were as severe as the infertile rga-28 gai-td1 (Col-0) phenotype (Fig.1e). Rare rga-28 gai-td1 (Col-0) siliques contained single seeds that probably resulted from uncontrolled cross-pollination. By contrast, early infertility of rga-24 gai-t6 (Ler) quickly recovered (Fig.1f) to a mean silique set frequency of 0.83 ± 0.03 from the 12th flower position onward. GA treatment had no discernible effect on fertility in this Ler mutant. Other differential growth responses between Col-0 and Ler were identified in a broader phenotypic characterization (Table S2), most notably the number and elongation of vegetative internodes, with the loss of RGA and GAI affecting each ecotype differently and each rga gai mutant responding differently to GA treatment. This includes some phenotypic differences remaining under GA treatment.
Greater loss of fertility in rga gai mutants compared with that when manipulating bioactive GA content might reflect different underlying causes of infertility. Reduced fertility of two GA biosynthesis mutants, ga20ox1 ga20ox2 (Rieu et al., 2008b) and ga3ox1 ga3ox3 (Hu et al., 2008), has been attributed to mismatched growth between the stamens and pistil, creating a mechanical block to pollination. However, non-linear modelling of Col-0 floral organ growth during flower opening (Fig. S2, Table S3, Methods S1), comparing control growth conditions and GA treatment, found no effect on stamen length relative to the pistil (over 100% at flower opening), nor on the timing of anthesis (P > 0.05; 5.45 ± 0.91 h and 4.47 ± 1.37 h before flower opening under control and GA-treated conditions, respectively). Therefore, reduced fertility under GA treatment cannot be explained by a mechanical barrier to pollination.
It was noticed that anthers from GA-treated flowers appeared to be smaller than those in untreated flowers. Modelling Col-0 anther length (taken as a linear measurement from the anther–filament junction to the anther tip) in the same experimental population found that GA treatment caused a consistent significant reduction in anther size over the developmental period measured (P < 0.05; Fig. S2, Table S3), suggesting that GA treatment has a direct negative effect on anther development.
Sterility in rga-28 gai-td1 is caused by pollen lethality
Loss of RGA or GAI separately did not affect silique set when compared with wild-type Col-0 (Fig.2a), indicating that these paralogues act redundantly to promote fertility. In contrast with wild-type Col-0, rga-28 and gai-td1 flowers (Fig.2b–d, f–h), rga-28 gai-td1 double-mutant flowers exhibited a pollenless phenotype (Fig.2e,i). rga-28 gai-td1 flowers set siliques when pollinated manually with wild-type Col-0 pollen, indicating that infertility of rga-28 gai-td1 (Col-0) inflorescences is caused by male sterility. The same pollenless phenotype was observed when rga-28 gai-td1 was recapitulated in the gid1a-1 gid1b-1 gid1c-1 (Col-0) triple GA receptor mutant background (Griffiths et al., 2006; herein referred to as gid1; Fig.2m,q). Insensitivity of gid1 to GA signalling causes constitutive inhibition of GA responses by DELLA proteins, and thus the pollenless phenotype is necessarily dependent on downstream DELLA signalling. Infertility of gid1 stamens caused by arrested floral development (Fig.2j,n; Griffiths et al., 2006) was overcome by loss of RGA alone (Fig.2k,o), but not GAI (Fig.2l,p). Pistil, stamen and petal size were all reduced in rga-28 gai-td1 gid1 compared with rga-28 gai-td1 (Fig.2e,m), suggesting that other DELLA paralogues repress aspects of Arabidopsis floral growth and development. A separate gid1 triple mutant, gid1a-1 gid1b-1 gid1c-2, has been reported previously as non-flowering (Willige et al., 2007; Ragni et al., 2011), but, under our growth conditions, this mutant flowered at a similar time to gid1a-1 gid1b-1 gid1c-1 and displayed a very similar floral phenotype (Fig. S3).
Figure 2.

rga-28 gai-td1 (Arabidopsis thaliana Col-0) is male sterile. (a) Primary inflorescence phenotypes of rga-28 gai-td1 combinatorial mutant lines (as specified). White arrowheads indicate infertile silique positions. (b–i) Floral phenotypes of the mutant lines in (a), showing newly opened flowers (b–e) and whole anthers (f–i). Anthers have been stained to determine pollen viability (see the Materials and Methods section): dark red colouring indicates viable pollen. Black arrowheads indicate empty locules. (j–q) Floral phenotypes of rga-28 gai-td1 combinatorial mutant lines in the gid1 GA-insensitive mutant background (as specified), showing newly opened flowers (j–m) and whole anthers (n–q). Anthers have been stained to determine pollen viability: dark red colouring indicates viable pollen. Black arrowheads indicate empty locules.
Pollen development is negatively affected in rga gai mutants in both Col-0 and Ler
The partial fertility of Ler rga gai mutants (Fig.1d,f) was also investigated, examining the floral phenotypes of rga-24 gai-t6 (which also carries transparent testa 1,tt1) and the independent line rga-t2 gai-t6. The stigmas of newly opened mutant flowers bore visibly less pollen than wild-type Ler (Fig.3a–c), consistent with previous observations by Dill & Sun (2001). Under microscopic examination, individual empty locules were identified in rga-24 gai-t6 and rga-t2 gai-t6 anthers (Fig.3e,f). This indicates that pollen development is disrupted by the loss of RGA and GAI function in both ecotypes, but with a less severe impact on Ler male fertility. The expression of additional DELLA paralogues in Ler anther tissues could potentially explain this (see the Discussion section). However, the global DELLA (Ler) quintuple mutant, which is reported to lack functional DELLA protein (Feng et al., 2008), produces viable pollen and displays a similar degree of fertility to rga-24 gai-t6 (Fig. S4; Fuentes et al., 2012), suggesting that the differences between Col-0 and Ler regulating pollen development lie downstream of the DELLAs.
Figure 3.
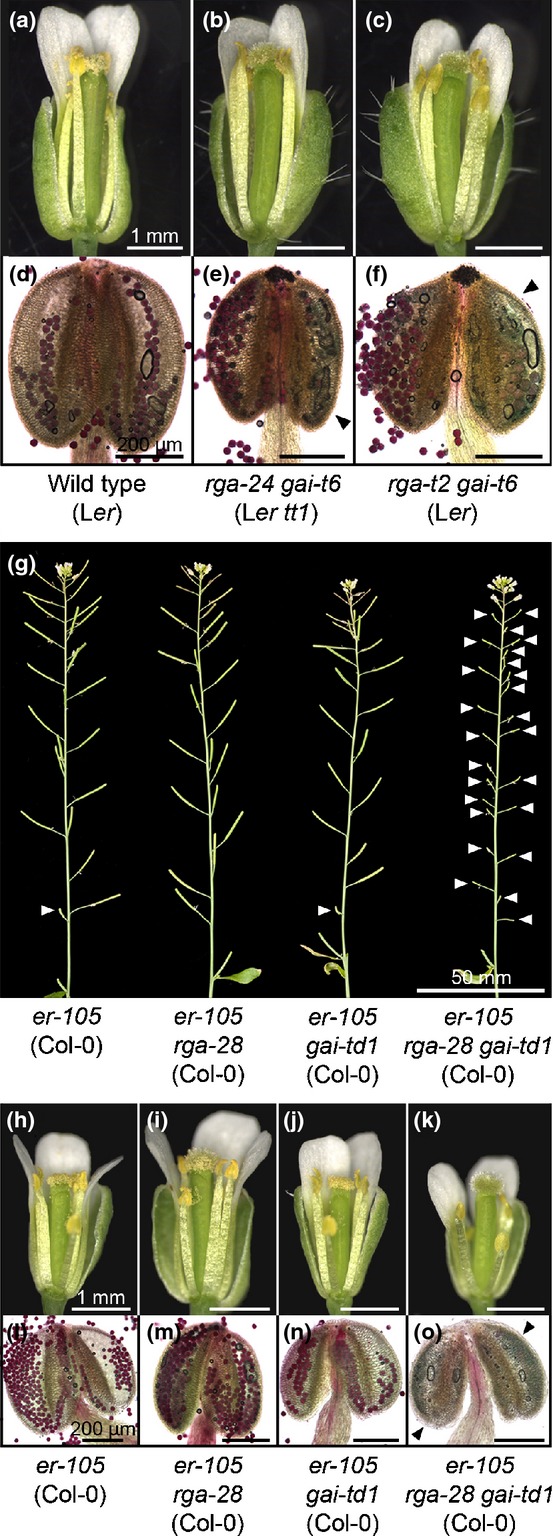
rga gai (Arabidopsis thaliana Ler) mutants retain male fertility. (a–f) Floral phenotypes of wild-type Ler,rga-24 gai-t6 and rga-t2 gai-t6, showing newly opened flowers (a–c) and whole anthers (d–f). Anthers have been stained to determine pollen viability: dark red colouring indicates viable pollen. Black arrowheads indicate empty locules. (g) Primary inflorescence phenotypes of er-105 rga-28 gai-td1 combinatorial mutant lines. White arrowheads indicate infertile silique positions. (h–o) Floral phenotypes of mutants described in (g), showing newly opened flowers (h–k) and whole anthers (l–o). Anthers have been stained to determine pollen viability: dark red colouring indicates viable pollen. Black arrowheads indicate empty locules.
Col-0 and Ler demonstrate distinct growth habits, including striking differences in floral cluster architecture (Fig. S5), attributed, in part, to loss of the ER leucine-rich repeat receptor-like kinase (LRR-RLK) in Ler (Torii et al., 1996). ER promotes stamen development in conjunction with ERECTA-LIKE (ERL)-1 and -2 (Shpak et al., 2004). To determine whether fertility differences between Col-0 and Ler rga gai mutants relate to the ER locus, the Col-0 null allele er-105 (Torii et al., 1996) was introgressed into rga-28 gai-td1 (Col-0). The introduction of er-105 resulted in an inflorescence phenotype resembling Ler in all mutant combinations (Figs3g, S5), but er-105 rga-28 gai-td1 remained infertile (Fig.3g). Flowers of er-105 (Fig.3h,l), er-105 rga-28 (Fig.3i,m) and er-105 gai-td1 (Fig.3j,n) produced pollen and were self-fertilizing, but er-105 rga-28 gai-td1 flowers were pollenless (Fig.3k,o). Fertility differences between Col-0 and Ler rga gai mutants do not appear to be associated with ER.
rga-28 gai-td1 (Col-0) male sterility is caused by a post-meiotic defect in pollen development
Anther and pollen development in rga-28 gai-td1 (Col-0) were examined microscopically to identify the cause of pollen lethality. Pollen mother cells (PMCs) were visible in both wild-type and mutant anthers (Fig.4a,b). Successful tetrad formation and microspore release were observed in wild-type anthers (Fig.4c,m), whereas, by contrast, although some tetrads in rga-28 gai-td1 anthers apparently progressed successfully to the free microspore stage (Fig.4n), abnormal meiotic products were frequently observed (Figs4d, S6). Post-meiotic rga-28 gai-td1 pollen development was also abnormal: wild-type microspores progressed through pollen mitosis to maturity (Fig.4o,q,s,u), but microspore nuclear polarization before pollen mitosis was not observed in rga-28 gai-td1 (Fig.4p), and microspores subsequently degenerated (Fig.4r,t).
Figure 4.
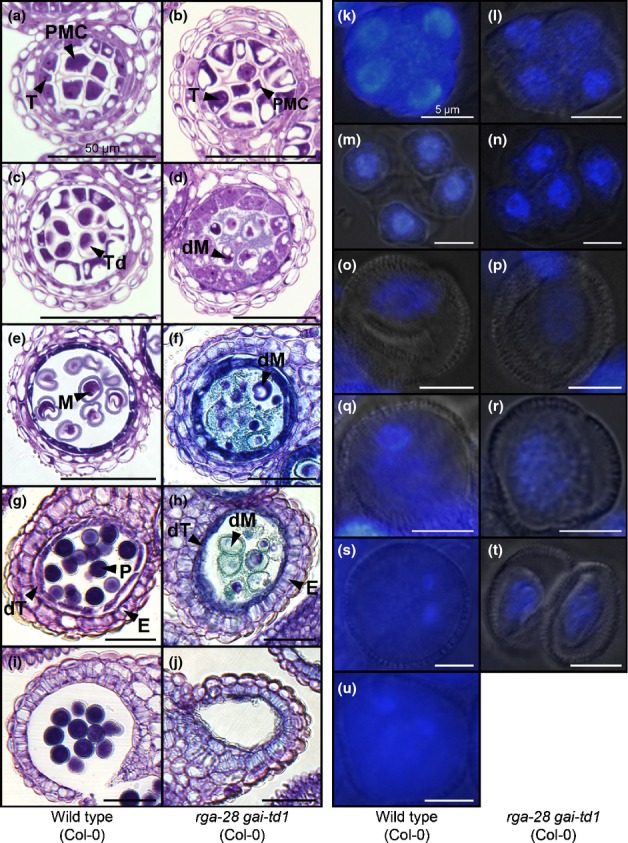
Microscopic analysis of rga-28 gai-td1 (Arabidopsis thaliana Col-0) pollen development. (a–j) Sections through wild-type (a, c, e, g, i) and rga-28 gai-td1 (Col-0) (b, d, f, h, j) anthers, encompassing developmental stages 5–6 (a, b), 7 (c, d), 8–9 (e, f), 11 (g, h) and 13 (i, j), as defined by Sanders et al. (1999). E, endothecium; dM, degenerating microspore; dT, degenerating tapetum; M, microspore; P, pollen; PMC, pollen mother cell; T, tapetum; Td, tetrad. (k–u) 4′,6-Diamidino-2-phenylindole (DAPI) fluorescence imaging of pollen nuclei in wild-type (k, m, o, q, s, u) and rga-28 gai-td1 (Col-0) (l, n, p, r, t) during development, showing tetrad formation (k–n), free unicellular microspores (o, p), polarized microspores (q), bicellular pollen (s) and mature tricellular pollen (u). rga-28 gai-td1 (Col-0) microspores do not polarize (p), and subsequently degenerate (r, t).
At the free microspore stage of anther development (stages 8–9; Sanders et al., 1999), large amounts of particulate material accumulated in rga-28 gai-td1 locules (Fig.4f,h), suggestive of defective pollen wall formation. rga-28 gai-td1 tapetal cells were enlarged relative to their wild-type equivalents (Fig.4a–d), and the onset of tapetal breakdown appeared to be slightly delayed (Fig.4e,f). Both microspores and tapetum had degenerated in rga-28 gai-td1 anthers by stage 11 (Fig.4h). The endothecium underwent apparently normal secondary thickening in rga-28 gai-td1 anthers (Fig.4h,j). We also observed that synchronous development between locules within the same anther was lost in some rga-28 gai-td1 stamens (Fig. S6), suggesting that DELLA activity might act to coordinate development between locules.
Pollen lethality in rga-28 gai-td1 is complemented by the reintroduction of RGA into either the tapetum or developing microspore
To confirm the requirement for DELLA activity for successful pollen development, and to identify the site of DELLA activity within the developing anthers, complementation of the rga-28 gai-td1 phenotype was attempted by expressing GFP-tagged RGA (Fig.5a) in two separate anther tissues under the tapetally expressed Arabidopsis LIPID TRANSFER PROTEIN 12 (LTP12) promoter (Ariizumi et al., 2002) or the tomato LAT52 promoter (Twell et al., 1989), expressed in developing pollen (Twell et al., 1990; Eady et al., 1994; Roy et al., 2011). A total of 27 LTP12::RGA::GFP and 29 LAT52::RGA::GFP transgenic T1 plants were isolated in the rga-28 gai-td1 background from three separate transformations with each construct. To our surprise, T1 plants expressing either construct in the rga-28 gai-td1 homozygous mutant background exhibited self-fertility. Three independent homozygous lines carrying single T-DNA insertions were established for each construct and the expression of each transgene in inflorescence tissue was verified by reverse transcription-polymerase chain reaction (RT-PCR) (Fig. S7).
Figure 5.
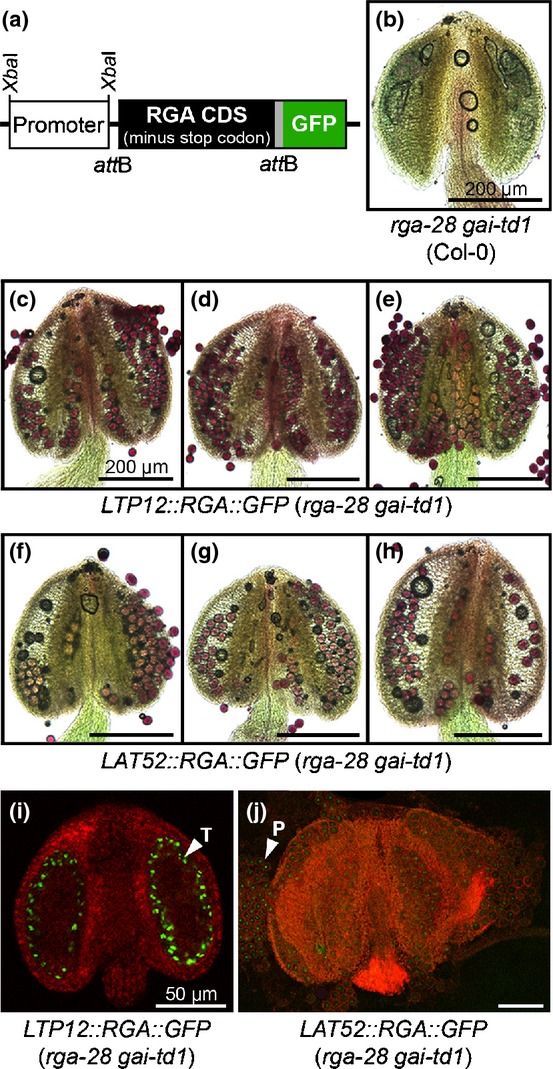
Reintroduction of REPRESSOR OF ga1-3 (RGA) rescues Arabidopsis thaliana pollen development in rga-28 gai-td1. (a) Schematic diagram of RGA-green fluorescent protein (GFP) transgenic fusion protein. (b) rga-28 gai-td1 anther phenotype. (c–e) Anther phenotype of three independent LTP12::RGA::GFP (rga-28 gai-td1) transgenic lines. (f–h) Anther phenotype of three independent LAT52::RGA::GFP (rga-28 gai-td1) transgenic lines. Anthers were stained to determine pollen viability: dark red colouring indicates viable pollen. (i, j) Anther tissue localization of RGA-GFP under the LTP12 (i) and LAT52 (j) promoters. P, pollen; T, tapetum.
The fertility of T3 individuals was variable, but generally reduced compared with the wild-type early in flowering, with silique set recovering more robustly later (Fig. S8). T1 individuals generally set copious siliques and seed, and therefore reduced fertility exhibited by subsequent generations might be caused by silencing. In contrast with pollenless rga-28 gai-td1 anthers (Fig.5b), viable pollen was observed in mature anthers of all lines (Fig.5c–h). Tissue-specific expression of RGA-GFP was tested by fluorescence microscopy. In LTP12 lines, strong post-meiotic GFP fluorescence was detected exclusively in the tapetum (Fig.5i), with no visible signal in the developing pollen or other anther tissues. GFP intensity under LAT52 expression was far weaker than in LTP12, even with stabilization of RGA-GFP using PAC treatment (see the Materials and Methods section). GFP fluorescence in LAT52 transgenic lines was detected only in the nuclei of developing pollen (Fig.5j), supported by comparison against autofluorescence in wild-type anthers under the same confocal settings (Fig. S9). LAT52::RGA::GFP anthers were examined for tapetal GFP expression in earlier anther developmental stages, but no fluorescence above background levels was observed (Fig. S9). Segregating fluorescence in the pollen of hemizygous T2 individuals (Fig. S9) supports the assumption of exclusively post-meiotic expression under LAT52. These data suggest that independent expression of DELLA protein in either developing pollen or the surrounding tapetal cells is sufficient to rescue pollen development and fertility of rga-28 gai-td1 (Col-0).
Discussion
Loss of the DELLA paralogues RGA and GAI affects Arabidopsis fertility more seriously in the Col-0 ecotype than in Ler
The majority of past genetic analysis of Arabidopsis DELLA function has been conducted using loss-of-function mutants in the Ler ecotype, the results of which suggested that DELLA activity is not strictly required to maintain fertility (Dill & Sun, 2001; Fuentes et al., 2012) and that DELLA proteins act simply to inhibit Arabidopsis floral and stamen development until alleviated by GA signalling (Dill & Sun, 2001; Cheng et al., 2004; Tyler et al., 2004). In this article, we describe a novel, male-sterile phenotype associated with loss of functional RGA and GAI in the Col-0 ecotype, in contrast with published rga gai mutants in Ler, which retain some, albeit reduced, fertility (Dill & Sun, 2001).
The Col-0 phenotype corresponds closely to that in rice and barley, where loss of the sole DELLA orthologue results in sterility (Lanahan & Ho, 1988; Ikeda et al., 2001). The barley mutant, slender1, has been described as pollenless (Lanahan & Ho, 1988), which was also found to be the cause of sterility in the Col-0 mutant rga-28 gai-td1. This suggests a conserved requirement for DELLA activity in maintaining pollen development between dicots and monocots that was not previously appreciated based solely on the analysis of Ler phenotypes. A closer inspection of two Ler rga gai mutants identified a partial sterility phenotype, with individual anther locules devoid of pollen, suggesting that RGA and GAI regulate the same anther developmental processes in both Col-0 and Ler, but their loss in Ler has far less consequence for fertility. A similar phenomenon was observed under exogenous GA treatment, with the fertility of wild-type Ler inflorescences remaining much higher than those of Col-0. The GA responses of Col-0 and Ler also differed in other developmental pathways, most obviously in inflorescence architecture. Although some differential GA responses between Col-0 and Ler might be explicable through starting differences in the levels of endogenous GA, their phenotypes remained significantly different under otherwise saturating GA concentrations (as indicated by the phenotypic similarity of GA-treated Col-0, 35S::GA20ox1 and ga2ox). Furthermore, altered GA content cannot explain the continued fertility of the Ler DELLA global mutant (Fuentes et al., 2012), where all DELLA repression of downstream GA signalling has supposedly been lost.
Neither GA treatment nor genetic manipulation of GA biosynthesis replicated the phenotypic severity of rga gai mutants, probably because the DELLA protein is never completely depleted by GA signalling in planta, a hypothesis supported by past experimental studies (Tyler et al., 2004; Feng et al., 2008). GA signal transduction and GA biosynthesis are linked through homeostatic regulation (Zentella et al., 2007), with DELLA expression up-regulated by GA signalling in both rice and Arabidopsis (Itoh et al., 2002; Ariizumi et al., 2008). The hypothesis that reduced fertility under GA treatment can be explained by a purely mechanical barrier to pollination was not supported by the modelling of Col-0 floral organ growth. Instead, it was found that anther size is specifically reduced under GA treatment, suggesting that disrupted anther development could underlie the fertility effects of both GA overdose and loss of DELLA function. DELLA loss-of-function mutants, including the Ler global mutant, also demonstrate a reduction (but not complete loss) specifically in female fertility (Dorcey et al., 2009; Fuentes et al., 2012). As such, the reductions in seed set observed outside of male-sterile lines in this study were probably caused both by impaired male and female reproductive processes.
DELLA expression differs between Col-0 and Ler stamen tissues
Arabidopsis carries five DELLA paralogues, the expression patterns of which are unknown. The differences in fertility between Col-0 and Ler rga gai mutants might be caused by the expression of at least one of the remaining DELLA paralogues, RGA-LIKE (RGL)1,RGL2 or RGL3, in Ler anthers to maintain pollen development. The additional presence of RGL1 and RGL2 (but not RGL3) in developing Ler pollen is supported by transcriptome data (Honys & Twell, 2004; Table S4), and loss of RGL2 has been shown to reduce pollen number in Ler anthers and also to significantly rescue pollen production in the partially GA-insensitive gai-1 (Ler) background (Kay et al., 2013).
A comparison of past analyses highlights differences in the functional importance of DELLA paralogues between Col-0 and Ler during floral development. In the GA-deficient ga1-3 (Ler) background, loss of functional RGA and GAI is not sufficient to overcome microspore developmental arrest (Dill & Sun, 2001; King et al., 2001; Cheng et al., 2004), requiring the combined loss of RGA, RGL1 and RGL2 (Cheng et al., 2004). By contrast, loss of RGA alone was sufficient to overcome arrested microspore development in ga1-3 (Col-0) (Tyler et al., 2004), suggesting greater functional redundancy between DELLA paralogues during Ler stamen development. Successful pollen production by the rga-28 gid1 quadruple mutant, in contrast with pollenless rga-28 gai-td1 gid1, argues for a function for GAI in Col-0 pollen development redundant with RGA. Reduced floral organ growth in rga-28 gai-td1 gid1 relative to rga-28 gai-td1, including the stamen filament, suggests that at least one other DELLA paralogue regulates Col-0 floral development. RGA and GAI were found to be the dominant DELLA paralogues regulating Col-0 vegetative development (Fig. S10), as also observed in Ler (Dill & Sun, 2001; Cheng et al., 2004; Tyler et al., 2004).
Differences apparently exist between the GA responses of Col-0 and Ler beneath the level of DELLA activity
The Ler DELLA global mutant, which carries mutations in all five paralogues, but nevertheless remains partially fertile (Fuentes et al., 2012), suggests that at least some differences in the rga gai pollen phenotype observed between Col-0 and Ler are caused by genetic variation downstream of the DELLA proteins. Successful pollen production by the global mutant, albeit reduced, was confirmed by this study (Fig. S4). The theoretical possibility remains that the global mutant expresses some DELLA protein: the T-DNA insertion in the rgl1-1 allele disrupts the promoter region, but not the coding sequence (Lee et al., 2002). As yet, no Col-0 equivalent to the global mutant is available for comparison.
Significant genomic variation has been reported between Col-0 and Ler, including altered gene transcripts and gene expression patterns (Gan et al., 2011), some of which could potentially relate to GA signal transduction or downstream response pathways. ER protein itself indirectly regulates responses downstream of GA signalling, antagonizing SHORT INTERNODES (SHI), a zinc-finger transcription factor that negatively regulates GA responses (Fridborg et al., 2001). However, loss of functional ER did not rescue fertility or pollen development in the rga-28 gai-td1 (Col-0) background.
Loss of DELLA activity in Arabidopsis anthers disrupts post-meiotic pollen development
The defects in pollen production in both Col-0 and Ler rga gai mutants suggest that DELLA activity in stamens does not merely inhibit development, but is also necessary for successful pollen production. Potentially, DELLA proteins might coordinate development between different anther tissues through inhibition at developmental ‘checkpoints’ until the necessary conditions to proceed are met. Pollen is heavily dependent on the surrounding tapetum for nutrition and the production of pollen wall components (Scott et al., 2004), and disruption of tapetal PCD results in pollen abortion (Kawanabe et al., 2006). Experiments in rice have demonstrated that GA signalling regulates both tapetal PCD and pollen wall synthesis (Aya et al., 2009). Cheng et al. (2004) identified malformed pollen in the ga1-3 rga-t2 gai-t6 rgl1-1 rgl2-1 (Ler) mutant, which they suggested was caused by the overproduction of pollen wall material. Coordination of anther development by DELLA is supported by our observation of desynchronized development between locules in rga-28 gai-td1 anthers (Fig. S7), which might explain the locule specificity of male sterility in rga gai (Ler) mutants. DELLA activity might also positively regulate pollen development directly: in floral tissues, 43% of DELLA transcriptional targets were up-regulated by the induction of DELLA expression (Cao et al., 2006).
rga-28 gai-td1 anthers displayed defects in both microspore and tapetum developmental processes. Phenotypically normal tetrads and free microspores were observed, although other defective meiotic products suggest perturbation to meiosis in some instances. Aberrant pollen development in rga-28 gai-td1 first becomes visible at the polarized microspore stage. Although the precise cause of pollen lethality in rga-28 gai-td1 could not be determined, the accumulation of free material in mutant locules is suggestive of defective pollen wall formation through aberrant tapetum function. However, whether this phenomenon is causative remains unclear, and potential intrinsic defects in microspore development (or a combination of both microspore and tapetal defects) should not be excluded. In rice pollen, SLENDER RICE1 (SLR1) is up-regulated specifically during meiosis and in tetrads (Hirano et al., 2008; Tang et al., 2010), suggesting that GA signalling is tightly regulated during meiosis. Similar resolution is not yet available for Arabidopsis, but AtDELLA expression has been identified in both unicellular and bicellular Ler pollen (Honys & Twell, 2004; Table S4). Arabidopsis pollen development was rescued by the reintroduction of RGA under two separate promoters, LTP12 and LAT52, both of which first express in post-meiotic tissues at or before the point at which the mutant pollen phenotype manifests, during the unicellular microspore stage (LTP12, Ariizumi et al., 2002) and in individual microspores within tetrads (LAT52, Francis et al., 2007). These indicate that, at the least, microspore development can be rescued by the reintroduction of DELLA signalling beyond the completion of meiosis. Other anther wall processes, such as endothecium secondary thickening and stomium development, occurred in a timely fashion in the rga-28 gai-td1 locule, suggesting that anther wall maturation is not exclusively dependent on RGA and GAI, but the possibility cannot be excluded that normal anther wall development was maintained by other DELLA paralogues.
Expression of RGA in either the tapetum or developing pollen rescued rga-28 gai-td1 fertility
Suppression of pollen lethality in rga-28 gai-td1 under either promoter was surprising, as each has been shown previously to express exclusively in separate tissues, LTP12 in the tapetum (Ariizumi et al., 2002) and LAT52 in developing pollen (Twell et al., 1990; Eady et al., 1994; Francis et al., 2007; Roy et al., 2011). Although the expression domains of native DELLAs in Arabidopsis anther tissues are unresolved, in rice, SLR1 is expressed simultaneously in both the tapetum and pollen (Hirano et al., 2008; Tang et al., 2010). No fluorescence or phenotypic evidence (e.g. dwarfing; Fig. S8) was found to suggest that RGA was expressed outside of the previously published expression patterns for LTP12 or LAT52. Nevertheless, the possibility cannot be entirely discounted that the observed complementation of defective pollen development under either promoter is an artefact caused by leaky basal expression in other anther tissues, and that DELLA activity is only required in the tapetum or pollen. One foreseeable mechanism for this could be inheritance by otherwise mutant microspores of DELLA protein from pre-meiotic anther cell lineages. A similar phenomenon has been hypothesized to explain the successful creation of theoretically lethal GA-insensitive gid1 mutants (Griffiths et al., 2006). That said, segregation of LAT52-driven fluorescence was observed in pollen produced by hemizygous T2 individuals (Fig. S9), suggesting that LAT52-expressed RGA is not present in PMCs.
If the independence of LTP12- and LAT52-driven expression is accepted, the rescue of pollen development by RGA in either the tapetum or pollen suggests that communication occurs between these two cell types downstream of DELLA activity. Such communication has been demonstrated during cell fate specification, where the PMC-secreted ligand TAPETUM DETERMINANT 1 (TPD1) interacts with the tapetum receptor EXTRA SPOROGENOUS CELLS/EXCESS MICROSPOROCYTES (EXS/EMS) (Yang et al., 2003, 2005; Jia et al., 2008) and, later in development, microspores may communicate with surrounding somatic cells via the extracellular protein MTR1 (Tan et al., 2012). The GAI transcript itself has been reported as a mobile signal (Ruiz-Medrano et al., 1999; Haywood et al., 2005).
The outcome of these comparative analyses is that it can no longer be assumed that experimental results from one ecotype are necessarily representative of all. Ecotypic differences also represent an additional and useful tool for the elucidation of genetic pathways, such as those underlying GA regulation of Arabidopsis pollen development.
Acknowledgments
This work was supported by a Rothamsted quota studentship to A.R.G.P. and by grants P16508 and BB/J001295/1, funded by the Biotechnology and Biological Sciences Research Council of the UK, which also provides strategic support to Rothamsted Research. We thank T-P. Sun, N. Harberd and C. Schwechheimer for supplying the mutant lines rga-24 gai-t6,rga-t2 gai-t6 and gid1a-1 gid1b-1 gid1c-2, respectively. The LAT52 promoter was kindly donated by D. Twell. We are grateful to I. Pearman, A. Griffin and other glasshouse staff for plant material. We are also grateful to the Rothamsted Visual Communications Unit for photographs, the Rothamsted Bioimaging Unit for microscopy resources and to M. Bennett for helpful discussions. The data in Table S3 are taken from transcriptomic data published in Honys & Twell (2004), with permission from the publishers, BioMed Central.
Supporting Information
Please note: Wiley Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Loss of REPRESSOR OF ga1-3 (RGA) and GIBBERELLIN INSENSITIVE (GAI) causes infertility in the Col-0 background.
Fig. S2 Effect of gibberellin (GA) treatment on silique set and floral organ growth.
Fig. S3 Comparison of gid1a-1 gid1b-1 gid1c-1 and gid1a-1 gid1b-1 gid1c-2 phenotypes.
Fig. S4 The Ler DELLA global mutant retains pollen viability.
Fig. S5 Loss of ERECTA (ER) in the rga-28 gai-td1 (Col-0) background phenocopies the Ler growth habit.
Fig. S6 Additional anther and pollen phenotypes of rga-28 gai-td1.
Fig. S7 Reverse transcription-polymerase chain reaction (RT-PCR) analysis of REPRESSOR OF ga1-3-green fluorescent protein (RGA-GFP) expression in LTP12::RGA::GFP and LAT52::RGA::GFP transgenic lines.
Fig. S8 Vegetative and reproductive phenotypes of LTP12::RGA::GFP and LAT52::RGA::GFP transgenic lines.
Fig. S9 Fluorescence analysis of LTP12::RGA::GFP and LAT52::RGA::GFP expression.
Fig. S10 Genetic analysis of rga gai gid1 vegetative phenotypes.
Table S1 PCR primer sequences
Table S2 Col-0 and Ler respond differently to chemical and genetic gibberellin (GA) overdose
Table S3 Modelling the effect of gibberellin (GA) treatment on floral organ growth in wild-type Col-0
Table S4 Expression of gibberellin (GA) biosynthetic and signalling genes during wild-type Ler pollen development
Methods S1 Non-linear modelling of floral organ growth.
References
- Alexander MP. Differential staining of aborted and non-aborted pollen. Stain Technology. 1969;44:117–122. doi: 10.3109/10520296909063335. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;30:653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- Ariizumi T, Amagai M, Shibata D, Hatakeyama K, Watanabe M, Toriyama K. Comparative study of promoter activity of three anther-specific genes encoding lipid transfer protein, xyloglucan endotransglucosylase/hydrolase and polygalacturonase in transgenic Arabidopsis thaliana. Plant Cell Reports. 2002;21:90–96. [Google Scholar]
- Ariizumi T, Murase K, Sun T-P, Steber CM. Proteolysis-independent downregulation of DELLA repression in Arabidopsis by the gibberellin receptor GIBBERELLIN INSENSITIVE DWARF1. Plant Cell. 2008;20:2447–2459. doi: 10.1105/tpc.108.058487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aya K, Ueguchi-Tanaka M, Kondo M, Hamada K, Yano K, Nishimura M, Matsuoka M. Gibberellin modulates anther development in rice via the transcriptional regulation of GAMYB. Plant Cell. 2009;21:1453–1472. doi: 10.1105/tpc.108.062935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao DN, Cheng H, Wu W, Soo HM, Peng JR. Gibberellin mobilizes distinct DELLA-dependent transcriptomes to regulate seed germination and floral development in Arabidopsis. Plant Physiology. 2006;142:509–525. doi: 10.1104/pp.106.082289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Qin LJ, Lee SC, Fu XD, Richards DE, Cao DN, Luo D, Harberd NP, Peng JR. Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development. 2004;131:1055–1064. doi: 10.1242/dev.00992. [DOI] [PubMed] [Google Scholar]
- Coles JP, Phillips AL, Croker SJ, García-Lepe R, Lewis MJ, Hedden P. Modification of gibberellin production and plant development in Arabidopsis by sense and antisense expression of gibberellin 20-oxidase genes. Plant Journal. 1999;17:547–556. doi: 10.1046/j.1365-313x.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- Dill A, Sun T-P. Synergistic derepression of gibberellin signaling by removing RGA and GAI function in Arabidopsis thaliana. Genetics. 2001;159:777–785. doi: 10.1093/genetics/159.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorcey E, Urbez C, Blázquez M, Carbonell J, Perez-Amador M. Fertilization-dependent auxin response in ovules triggers fruit development through the modulation of gibberellin metabolism in Arabidopsis. Plant Journal. 2009;58:318–332. doi: 10.1111/j.1365-313X.2008.03781.x. [DOI] [PubMed] [Google Scholar]
- Eady C, Lindsey K, Twell D. Differential activation and conserved vegetative cell-specific activity of a late pollen promoter in species with bicellular and tricellular pollen. Plant Journal. 1994;5:543–550. [Google Scholar]
- Feng SH, Martinez C, Gusmaroli G, Wang Y, Zhou JL, Wang F, Chen LY, Yu L, Iglesias-Pedraz JM, Kircher S. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature. 2008;451:475–479. doi: 10.1038/nature06448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis KE, Lam SY, Harrison BD, Bey AL, Berchowitz LE, Copenhaver GP. Pollen tetrad-based visual assay for meiotic recombination in Arabidopsis. Proceedings of the National Academy of Sciences, USA. 2007;104:3913–3918. doi: 10.1073/pnas.0608936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridborg I, Kuusk S, Robertson M, Sundberg E. The Arabidopsis protein SHI represses gibberellin responses in Arabidopsis and barley. Plant Physiology. 2001;127:937–948. [PMC free article] [PubMed] [Google Scholar]
- Fuentes S, Ljung K, Sorefan K, Alvey E, Harberd NP, Østergaard L. Fruit growth in Arabidopsis occurs via DELLA-dependent and DELLA-independent gibberellin responses. Plant Cell. 2012;24:3982–3996. doi: 10.1105/tpc.112.103192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan XC, Stegle O, Behr J, Steffen JG, Drewe P, Hildebrand KL, Lyngsoe R, Schultheiss SJ, Osborne EJ, Sreedharan VT. Multiple reference genomes and transcriptomes for Arabidopsis thaliana. Nature. 2011;477:419–423. doi: 10.1038/nature10414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez KA, Gomez AA. Statistical procedures for agricultural research. New York, NY, USA: John Wiley & Sons; 1984. [Google Scholar]
- Goto N, Pharis RP. Role of gibberellins in the development of floral organs of the gibberellin-deficient mutant, ga1-1, of Arabidopsis thaliana. Canadian Journal of Botany. 1999;77:944–954. [Google Scholar]
- Griffiths J, Murase K, Rieu I, Zentella R, Zhang ZL, Powers SJ, Gong F, Phillips AL, Hedden P, Sun T-P. Genetic characterisation and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell. 2006;18:3399–3414. doi: 10.1105/tpc.106.047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harberd NP, Belfield E, Yasumura Y. The angiosperm gibberellin-GID1-DELLA growth regulatory mechanism: how an “inhibitor of an inhibitor” enables flexible response to fluctuating environments. Plant Cell. 2009;21:1328–1339. doi: 10.1105/tpc.109.066969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood V, Yu TS, Huang NC, Lucas WJ. Phloem long-distance trafficking of GIBBERELLIC ACID-INSENSITIVE RNA regulates leaf development. Plant Journal. 2005;42:49–68. doi: 10.1111/j.1365-313X.2005.02351.x. [DOI] [PubMed] [Google Scholar]
- Hirano K, Aya K, Hobo T, Sakakibara H, Kojima M, Shim RA, Hasegawa Y, Ueguchi-Tanaka M, Matsuoka M. Comprehensive transcriptome analysis of phytohormone biosynthesis and signaling genes in microspore/pollen and tapetum of rice. Plant and Cell Physiology. 2008;49:1429–1450. doi: 10.1093/pcp/pcn123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honys D, Twell D. Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biology. 2004;5:R85. doi: 10.1186/gb-2004-5-11-r85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou XL, Hu WW, Shen L, Lee LYC, Tao Z, Han JH, Yu H. Global identification of DELLA target genes during Arabidopsis flower development. Plant Physiology. 2008;147:1126–1142. doi: 10.1104/pp.108.121301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JH, Mitchum MG, Barnaby N, Ayele BT, Ogawa M, Nam E, Lai WC, Hanada A, Alonso JM, Ecker JR. Potential sites of bioactive gibberellin production during reproductive growth in Arabidopsis. Plant Cell. 2008;20:320–336. doi: 10.1105/tpc.107.057752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda A, Ueguchi-Tanaka M, Sonoda Y, Kitano H, Koshioka M, Futsuhara Y, Matsuoka M, Yamaguchi J. slender rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAIRGARHTD8. Plant Cell. 2001;13:999–1010. doi: 10.1105/tpc.13.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Ueguchi-Tanaka M, Sato Y, Ashikari M, Matsuoka M. The gibberellin signaling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant Cell. 2002;14:57–70. doi: 10.1105/tpc.010319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen SE, Olszewski NE. Characterization of the arrest in anther development associated with gibberellin deficiency of the gib-1 mutant of tomato. Plant Physiology. 1991;97:409–414. doi: 10.1104/pp.97.1.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen SE, Olszewski NE. Mutations at the SPINDLY locus of Arabidopsis alter gibberellin signal transduction. Plant Cell. 1993;5:887–896. doi: 10.1105/tpc.5.8.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia GX, Liu XD, Owen HA, Zhao DZ. Signaling of cell fate determination by the TPD1 small protein and EMS1 receptor kinase. Proceedings of the National Academy of Sciences, USA. 2008;105:2220–2225. doi: 10.1073/pnas.0708795105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanabe T, Ariizumi T, Kawai-Yamada M, Uchimiya H, Toriyama K. Abolition of the tapetum suicide program ruins microsporogenesis. Plant and Cell Physiology. 2006;47:784–787. doi: 10.1093/pcp/pcj039. [DOI] [PubMed] [Google Scholar]
- Kay P, Groszmann M, Ross JJ, Parish RW, Swain SM. Modifications of a conserved regulatory network involving INDEHISCENT controls multiple aspects of reproductive tissue developments in Arabidopsis. New Phytologist. 2013;197:73–87. doi: 10.1111/j.1469-8137.2012.04373.x. [DOI] [PubMed] [Google Scholar]
- King KE, Moritz T, Harberd NP. Gibberellins are not required for normal stem growth in Arabidopsis thaliana in the absence of GAI and RGA. Genetics. 2001;159:767–776. doi: 10.1093/genetics/159.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanahan MB, Ho THD. Slender barley: a constitutive gibberellin-response mutant. Planta. 1988;175:107–114. doi: 10.1007/BF00402887. [DOI] [PubMed] [Google Scholar]
- Lee SC, Cheng H, King KE, Wang WF, He Y, Hussain A, Lo J, Harberd NP, Peng JR. Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes & Development. 2002;16:646–658. doi: 10.1101/gad.969002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lucas M, Daviere JM, Rodríguez-Falcón M, Pontin M, Iglesias-Pedraz JM, Lorrain S, Fankhauser C, Blázquez MA, Titarenko E, Prat S. A molecular framework for light and gibberellin control of cell elongation. Nature. 2008;451:480–484. doi: 10.1038/nature06520. [DOI] [PubMed] [Google Scholar]
- McCullagh P, Nelder JA. Generalised linear models. 2nd edn. London, UK: Chapman and Hall; 1989. [Google Scholar]
- Millar AA, Gubler F. The Arabidopsis GAMYBlike genes, MYB33 and MYB65, are microRNA-regulated genes that redundantly facilitate anther development. Plant Cell. 2005;17:705–721. doi: 10.1105/tpc.104.027920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Suzuki T, Murata S, Nakamura S, Hino T, Maeo K, Tabata R, Kawai T, Tanaka K, Niwa Y. Improved gateway binary vectors: high-performance vectors for creation of fusion constructs in transgenic analysis of plants. Bioscience Biotechnology and Biochemistry. 2007;71:2095–2100. doi: 10.1271/bbb.70216. [DOI] [PubMed] [Google Scholar]
- Nester JE, Zeevaart JAD. Flower development in normal tomato and a gibberellin-deficient (ga-2) mutant. American Journal of Botany. 1988;75:45–55. [Google Scholar]
- Peng JR, Carol P, Richards DE, King KE, Cowling RJ, Murphy GP, Harberd NP. The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes & Development. 1997;11:3194–3205. doi: 10.1101/gad.11.23.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharis RP, King RW. Gibberellins and reproductive development in seed plants. Annual Review of Plant Physiology. 1985;36:517–568. [Google Scholar]
- Plackett ARG, Thomas SG, Wilson ZA, Hedden P. Gibberellin control of stamen development: a fertile field. Trends in Plant Science. 2011;16:568–578. doi: 10.1016/j.tplants.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Pysh LD, Wysocka-Diller JW, Camilleri C, Bouchez D, Benfey PN. The GRAS gene family in Arabidopsis: sequence characterisation and basic expression analysis of the SCARECROW-LIKE genes. Plant Journal. 1999;18:111–119. doi: 10.1046/j.1365-313x.1999.00431.x. [DOI] [PubMed] [Google Scholar]
- Ragni L, Nieminen K, Pacheco-Villalobos D, Sibout R, Schwechheimer C, Hardtke CS. Mobile gibberellin directly stimulates Arabidopsis hypocotyl xylem expansion. Plant Cell. 2011;23:1322–1336. doi: 10.1105/tpc.111.084020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieu I, Eriksson S, Powers SJ, Gong F, Griffiths J, Woolley L, Benlloch R, Nilsson O, Thomas SG, Hedden P. Genetic analysis reveals that C19-GA 2-oxidation is a major gibberellin inactivation pathway in Arabidopsis. Plant Cell. 2008a;20:2420–2436. doi: 10.1105/tpc.108.058818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieu I, Ruiz-Rivero O, Fernandez-Garcia N, Griffiths J, Powers SJ, Gong F, Linhartova T, Eriksson S, Nilsson O, Thomas SG. The gibberellin biosynthetic genes AtGA20ox1 and AtGA20ox2 act, partially redundantly, to promote growth and development throughout the Arabidopsis life cycle. Plant Journal. 2008b;53:488–504. doi: 10.1111/j.1365-313X.2007.03356.x. [DOI] [PubMed] [Google Scholar]
- Robinson SJ, Tang LH, Mooney BAG, McKay SJ, Clarke WE, Links MG, Karcz S, Regan S, Wu YY, Gruber MY. An archived activation tagged population of Arabidopsis thaliana to facilitate forward genetics approaches. BMC Plant Biology. 2009;9:101. doi: 10.1186/1471-2229-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B, Copenhaver GP, von Arnim AG. Fluorescence-tagged transgenic lines reveal genetic defects in pollen growth- application to the Eif3 complex. PLoS ONE. 2011;6:e17640. doi: 10.1371/journal.pone.0017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Medrano R, Xoconostle-Cázares B, Lucas WJ. Phloem long-distance transport of CmNACP mRNA: implications for supracellular regulation in plants. Development. 1999;126:4405–4419. doi: 10.1242/dev.126.20.4405. [DOI] [PubMed] [Google Scholar]
- Sanders PM, Bui AQ, Weterings K, McIntire KN, Hsu YC, Lee PY, Truong MT, Beals TP, Goldberg RB. Anther developmental defects in Arabidopsis thaliana male sterile mutants. Sexual Plant Reproduction. 1999;11:297–322. [Google Scholar]
- Scott RJ, Spielman M, Dickinson HG. Stamen structure and function. Plant Cell. 2004;16:S46–S60. doi: 10.1105/tpc.017012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions A, Burke E, Presting G, Aux G, McElver J, Patton D, Dietrich B, Ho P, Bacwaden J, Ko C. A high-throughput Arabidopsis reverse genetics system. Plant Cell. 2002;14:2985–2994. doi: 10.1105/tpc.004630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpak ED, Berthiaume CT, Hill EJ, Torii KU. Synergistic interaction of three ERECTA-family receptor-like kinases controls Arabidopsis organ growth and flower development be promoting cell proliferation. Development. 2004;131:1491–1501. doi: 10.1242/dev.01028. [DOI] [PubMed] [Google Scholar]
- Silverstone AL, Mak PYA, Martínez EC, Sun T-P. The new RGA locus encodes a negative regulator of gibberellin response in Arabidopsis thaliana. Genetics. 1997;146:1087–1099. doi: 10.1093/genetics/146.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T-P. Gibberellin-GID1-DELLA: a pivotal regulatory module for plant growth and development. Plant Physiology. 2010;154:567–570. doi: 10.1104/pp.110.161554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Liang W, Hu J, Zhang D. MTR1 encodes a secretory fasciclin glycoprotein required for male reproductive development in rice. Developmental Cell. 2012;22:1127–1137. doi: 10.1016/j.devcel.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Tang X, Zhang ZY, Zhang WJ, Zhao XM, Li X, Zhang D, Liu QQ, Tang WH. Global gene profiling of laser-captured pollen mother cells indicates molecular pathways and gene subfamilies involved in rice meiosis. Plant Physiology. 2010;154:1855–1870. doi: 10.1104/pp.110.161661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnowski BI, Spinale FG, Nicholson JH. DAPI as a useful stain for nuclear quantitation. Biotechnic and Histochemistry. 1991;66:297–302. [PubMed] [Google Scholar]
- Torii KU, Mitsukawa N, Oosumi T, Matsuura Y, Yokoyama R, Whittier RF, Komeda Y. The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. Plant Cell. 1996;8:735–746. doi: 10.1105/tpc.8.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twell D, Wing R, Yamaguchi J, McCormick S. Isolation and expression of an anther-specific gene from tomato. Molecular Genetics and Genomics. 1989;217:240–245. doi: 10.1007/BF02464887. [DOI] [PubMed] [Google Scholar]
- Twell D, Yamaguchi J, McCormick S. Pollen-specific gene expression in transgenic plants: coordinate regulation of two different tomato gene promoters during microsporogenesis. Development. 1990;109:705–713. doi: 10.1242/dev.109.3.705. [DOI] [PubMed] [Google Scholar]
- Tyler L, Thomas SG, Hu JH, Dill A, Alonso JM, Ecker JR, Sun T-P. DELLA proteins and gibberellin-regulated seed germination and floral development in Arabidopsis. Plant Physiology. 2004;135:1008–1019. doi: 10.1104/pp.104.039578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Matsuoka M. The perception of gibberellins: clues from receptor structure. Current Opinion in Plant Biology. 2010;13:1–16. doi: 10.1016/j.pbi.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Vizcay-Barrena G, Wilson ZA. Altered tapetal PCD and pollen wall development in the Arabidopsis ms1 mutant. Journal of Experimental Botany. 2006;57:2709–2717. doi: 10.1093/jxb/erl032. [DOI] [PubMed] [Google Scholar]
- Willige BC, Ghosh S, Nill C, Zourelidou M, Dohmann EMN, Maier A, Schwechheimer C. The DELLA domain of GA INSENSTIVE mediates the interaction with the GA INSENSITIVE DWARF1A gibberellin receptor of Arabidopsis. Plant Cell. 2007;19:1209–1220. doi: 10.1105/tpc.107.051441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SL, Jiang LX, Puah CS, Xie LF, Zhang XQ, Chen LQ, Yang WC, Ye D. Overexpression of TAPETUM DETERMINANT1 alters the cell fates in the Arabidopsis carpel and tapetum via genetic interaction with EXCESS MICROSPOROCYTES1/EXTRA SPOROGENOUS CELLS. Plant Physiology. 2005;139:186–191. doi: 10.1104/pp.105.063529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SL, Xie LF, Mao HZ, Puah CS, Yang WC, Jiang LX, Sundaresan V, Ye D. TAPETUM DETERMINANT1 is required for cell specialization in the Arabidopsis anther. Plant Cell. 2003;15:2792–2804. doi: 10.1105/tpc.016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentella R, Zhang ZL, Park M, Thomas SG, Endo A, Murase K, Fleet CM, Jikumaru Y, Nambara E, Kamiya Y. Global analysis of DELLA direct targets in early gibberellin signalling in Arabidopsis. Plant Cell. 2007;19:3037–3057. doi: 10.1105/tpc.107.054999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Loss of REPRESSOR OF ga1-3 (RGA) and GIBBERELLIN INSENSITIVE (GAI) causes infertility in the Col-0 background.
Fig. S2 Effect of gibberellin (GA) treatment on silique set and floral organ growth.
Fig. S3 Comparison of gid1a-1 gid1b-1 gid1c-1 and gid1a-1 gid1b-1 gid1c-2 phenotypes.
Fig. S4 The Ler DELLA global mutant retains pollen viability.
Fig. S5 Loss of ERECTA (ER) in the rga-28 gai-td1 (Col-0) background phenocopies the Ler growth habit.
Fig. S6 Additional anther and pollen phenotypes of rga-28 gai-td1.
Fig. S7 Reverse transcription-polymerase chain reaction (RT-PCR) analysis of REPRESSOR OF ga1-3-green fluorescent protein (RGA-GFP) expression in LTP12::RGA::GFP and LAT52::RGA::GFP transgenic lines.
Fig. S8 Vegetative and reproductive phenotypes of LTP12::RGA::GFP and LAT52::RGA::GFP transgenic lines.
Fig. S9 Fluorescence analysis of LTP12::RGA::GFP and LAT52::RGA::GFP expression.
Fig. S10 Genetic analysis of rga gai gid1 vegetative phenotypes.
Table S1 PCR primer sequences
Table S2 Col-0 and Ler respond differently to chemical and genetic gibberellin (GA) overdose
Table S3 Modelling the effect of gibberellin (GA) treatment on floral organ growth in wild-type Col-0
Table S4 Expression of gibberellin (GA) biosynthetic and signalling genes during wild-type Ler pollen development
Methods S1 Non-linear modelling of floral organ growth.


