SUMMARY
Colony stimulating factor-1 (Csf-1) receptor and its ligand Csf-1 control macrophage development, maintenance, and function. The development of both Langerhans cells (LCs) and microglia is highly dependent on Csf-1 receptor signaling but independent of Csf-1. Here we show that in both mice and humans, interleukin-34 (IL-34), an alternative ligand for Csf-1 receptor, is produced by keratinocytes in the epidermis and by neurons in the brain. Mice lacking IL-34 displayed a marked reduction of LCs and a decrease of microglia, whereas monocytes, dermal, and lymphoid tissue macrophages and DCs were unaffected. We identified IL-34 as a nonredundant cytokine for the development of LCs during embryo-genesis as well as for their homeostasis in the adult skin. Whereas inflammation-induced repopulation of LCs appears to be dependent on Csf-1, once inflammation is resolved, LC survival is again IL-34-dependent. In contrast, microglia and their yolk sac precursors develop independently of IL-34 but rely on it for their maintenance in the adult brain.
INTRODUCTION
In both mice and humans, the epidermis and the central nervous system (CNS) are colonized by homogenous and onto-genically unique populations of the mononuclear phagocyte system (MPS), namely Langerhans cells (LCs) and microglia. LCs are the dendritic cells (DCs) of the epidermis and form the first immune barrier to foreign antigens that breach the skin. Microglia represent the resident macrophages of the CNS and are considered the first line of defense in many CNS pathologies. Most lymphoid and nonlymphoid tissue DCs and macrophages are constantly repopulated by blood-derived circulating myeloid precursors. In contrast, epidermal LCs and microglia derive from embryonic precursors that take residence in the skin and brain prior to birth and are maintained locally throughout adult life in the steady state (Ginhoux et al., 2010; Hoeffel et al., 2012; Merad et al., 2002). Apart from the cytokine transforming growth factor β (TGF-β), which is essential for the maintenance of LCs (Kaplan et al., 2007), the local cutaneous growth factors that control LC homeostasis are only now being discovered (Wang et al., 2012). As for microglia, the factors that govern their development and maintenance remain largely unclear.
Csf-1 is a critical growth factor for the generation, differentiation, and survival of macrophages and monocytes (Dai et al., 2002; Wiktor-Jedrzejczak et al., 1990). Mice lacking Csf-1 receptor (Csf-1r) or bearing a spontaneous null mutation in Csf-1 (Csf1op/op) display greatly reduced macrophage populations in various tissues including osteoclasts, leading to multiple developmental defects such as impaired bone remodeling, osteopetrosis, and tooth eruption (Dai et al., 2002). In particular, mice deficient in Csf-1r also lack epidermal LCs and microglia (Ginhoux et al., 2006, 2010). Interestingly however, in contrast to Csf1r−/− mice, Csf1op/op mice display relatively normal LC and microglia development (Ginhoux et al., 2006), suggesting that an alternative ligand for Csf-1r controls the maintenance of these two cell populations in vivo. Interleukin-34 (IL-34) has been identified as an additional ligand for Csf-1r and was shown to bind Csf-1r with high affinity in vitro (Garceau et al., 2010; Lin et al., 2008). IL-34 is more conserved in mammalian and avian species than Csf-1 (Garceau et al., 2010). IL-34 and Csf-1 were shown to bind on different regions of the Csf-1r and share no overt sequence homology (Chihara et al., 2010). IL-34 can promote the survival and proliferation of human monocytes and stimulate macrophage colony formation of human bone marrow (BM) cells (Lin et al., 2008). However, the role of IL-34 in the development and maintenance of LCs and microglia in vivo is only now becoming clearer (Wang et al., 2012). Here, by generating IL-34 deficient reporter mice, we demonstrated that IL-34 is crucial for the embryonic development of LCs and their homeostasis in the adult in the steady state. During skin inflammation, IL-34 is not required for the recruitment of monocytes and their subsequent differentiation into LCs as was observed by Wang et al. (2012). However, here we found it to be critical for their maintenance after inflammation was resolved. In addition, IL-34 did not control the embryonic development of microglia but contributed to micro-glia homeostasis in the adult. Taken together, IL-34 is a stroma-derived homeostatic cytokine and growth factor specifically targeting two unique populations of the MPS, namely LCs and microglia.
RESULTS
Csf-1r Signaling Is Critical for LC Homeostasis
As previously shown, Csf1op/op mice display only slightly reduced numbers of LCs whereas Csf1r−/− mice are completely devoid of LCs (Figures 1A and 1B) (Ginhoux et al., 2006). However, in Csf1r−/− embryos, LC precursors are lacking already in the embryonic skin (Hoeffel et al., 2012). Thus, in order to circumvent such developmental defect and to determine whether Csf-1r signaling also controls LC homeostasis in the adult skin, we generated mice in which Csf-1r was specifically deleted in Langerin+ cells. Within the epidermis, Langerin expression is only acquired after birth and restricted to LCs (Merad et al., 2002; Tripp et al., 2004). We observed an almost complete loss of LCs in adult Langerin-Cre+ × Csf1rfl/fl mice (Figure 1C) demonstrating that Csf-1r not only controls LC embryonic development, but is also required for their maintenance throughout adult life. Neonatal LC precursors, which are Langerin−, were present at normal numbers on postnatal day 1 in Langerin-Cre+ × Csfr1fl/fl neonates (Figure 1D). Only when LC precursors had differentiated into LCs (Langerin+) was the Csf1r gene deleted, which led to the decrease in LC numbers (Figure 1D). To confirm that Csf-1r controls LC homeostasis in adult mice, we also treated wild-type (WT) mice with a neutralizing monoclonal antibody (mAb) to Csf-1r (AFS98) (Hashimoto et al., 2011; Sudo et al., 1995). Extending previous results (Mac-Donald et al., 2010), administration of anti-Csf-1r mAb in adult animals resulted in depletion of LCs, again demonstrating that Csf-1r signaling is critical for LC homeostasis in adult mice (Figure 1E). In addition, we used Rosa26-CreERT2+ × Csf1rfl/fl mice to inducibly delete the Csf1r gene in adult mice upon tamoxifen administration (Figure 1F). Also in this model, we found that conditional deletion of Csf-1r in the adult led to depletion of LCs indicating that Csf-1r signaling is required for the maintenance of fully differentiated LCs in healthy adult skin.
Figure 1. Csf-1r Signaling Is Critical for LC Homeostasis in Adult Skin.
(A–F) Plots show the percentage and total cell number (±SEM) of LCs among CD45+ epidermal cells in adult mice (A–C, E and F) or neonates on postnatal day (p)1, p5, and p10 (D). Graphs represent data of pooled experiments. *p < 0.05, **p < 0.01, ***p < 0.001 (Student's t test, unpaired). (A) Csf1r−/− and control mice (Csf1r+/− or Csf1r+/+) (n ≥ 3). (B) Csf1op/op and control mice (Csf1op/+ or Csf1+/+) (n = 5). (C) Langerin (Lang) Cre+ × Csf1rfl/fl or control mice (LangCre+ × Csf1rfl/+ or LangCre− × Csf1rfl/fl) (n = 4). (D) LangCre+ × Csf1rfl/fl or LangCre− × Csf1rfl/fl neonates. On p1, R1 represents LC precursors (Langerin−) and R2 represents LCs (Langerin+). p2: n = 6, p5: n ≥ × p10: n ≥ 6. (E) Anti-(α)Csf-1r treated or isotype treated WT mice (n = 6). One representative of at least three individual experiments is shown.
(F) Rosa26-CreERT2+ (CreERT2+) × Csf1rfl/fl and CreERT2− × Csf1rfl/fl mice were treated with tamoxifen and the skin was analyzed 7 days post treatment (n = 2). One representative of two individual experiments is shown.
IL-34 Is Expressed by Keratinocytes in the Epidermis and Is Critical for the Development of LCs
IL-34 is an alternative ligand for Csf-1r (Lin et al., 2008) and its activity could explain the phenotypic discrepancy between Csf1op/op and Csf1r−/− mice. We found that IL-34 was highly expressed in the skin and in the brain compared to other nonlymphoid and lymphoid tissues e.g., liver, lung, and spleen (Figure 2A), as previously described (Wei et al., 2010). Within the skin, Il34 was the only Csf-1r ligand expressed in the epidermis, whereas both Il34 and Csf1 were expressed in the dermis (Figure 2B). In order to specifically trace IL-34 expression in vivo, we generated an IL-34 reporter mouse strain by targeting a LacZ cassette into the endogenous IL-34 locus (Il34LacZ/+). Within the skin, IL-34 was highly expressed by keratinocytes in the epidermis (Figures 2C and 2D). We also detected weak IL-34 reporter expression in the dermis localized to hair follicles (Figure 2C). The high expression of IL-34 within the epidermis suggests that IL-34 plays a role for the development or maintenance of LCs.
Figure 2. IL-34 Is Expressed by Keratinocytes in the Epidermis and Controls the Development of LCs.
(A) Quantitative RT-PCR analysis of Il34 mRNA expression of liver, brain, lung, spleen, and skin in WT mice. Data were normalized to the expression of RNA polymerase 2 (n = 2). Shown is one representative of three individual experiments.
(B) Quantitative RT-PCR analysis of Il34 and Csf1 mRNA expression of WT epidermis and dermis. Data were normalized to the expression of RNA polymerase 2 (n = 2). One representative of two individual experiments is shown.
(C) X-gal staining (blue) of skin sections from Il34LacZ/+ and Il34+/+ mice (scale bar represents 40 mm). HF, Hair follicle.
(D) Skin sections of Il34+/+ mice and Il34LacZ/+ mice were stained with X-gal (blue) and mAb against K14 (red) (scale bar represents 20 μm).
(E) Plots show the percentage and total cell number (±SEM) of LCs (CD11b+MHCII+) among CD45+ epidermal cells in adult Il34LacZ/LacZ and Il34LacZ/+ mice (n = 5). Pooled data of several individual experiments. *p < 0.05, **p < 0.01, ***p < 0.001 (Student's t test, unpaired).
(F) Images display anti-MHCII (LCs) and anti-CD3 (DETCs) staining on ear-derived epidermal sheets from adult Il34LacZ/LacZ and Il34LacZ/+ mice (scale bar represents 50 μm).
In order to determine whether IL-34 controls LC homeostasis in vivo, we generated mice deficient in IL-34 by crossing the knockin reporter allele to homozygosity (Il34LacZ/LacZ). In contrast to Csf1op/op mice (Dai et al., 2002), we did not detect overt growth abnormalities or bone malformations in Il34LacZ/LacZ mice. Also, the mice were born at normal Mendelian distribution. In contrast, adult mice lacking IL-34 displayed a drastic reduction of LCs (Figures 2E and 2F). This, however, did not lead to any overt macroscopic changes in the skin or fur, nor did we observe signs of skin inflammation. Also, as previously reported (Taveirne et al., 2011), the lack of LCs did not affect the numbers or morphology of the epidermal resident population of γδ T cells (dendritic epidermal gd T cells, DETCs) (Figure 2F).
With the exception of migratory LCs, which were missing from the skin draining lymph nodes in Il34LacZ/LacZ mice (see Figure S1 available online), DCs in spleen, liver, and dermis as well as DCs in skin draining lymph nodes were largely unaffected. Circulating Ly6Chi and Ly6Clo monocytes, spleen macrophages, lung alveolar macrophages, and macrophages in the liver and dermis were also not affected in the absence of IL-34. Thus, stroma-derived IL-34 within the skin is required for the development of LCs.
IL-34 Contributes to the Maintenance of Microglia in the Adult
Similar to the LC population, Csf1r−/− mice lack microglia but mice lacking Csf-1 display normal numbers of microglia (Ginhoux et al., 2010). Given that IL-34 is highly expressed in the brain (Figure 2A), we hypothesized that IL-34-mediated Csf-1r signaling may be involved in the development and/or maintenance of microglia. IL-34 reporter expression revealed that within the brain, IL-34 is highly expressed predominantly in the cortex, the anterior olfactory nucleus, and the hippocampus (Figure 3A), whereas no or very low expression could be detected in the brain stem and cerebellum (data not shown). IL-34 was specifically expressed by neurons (Figure 3B) as previously suggested (Mizuno et al., 2011). In adult Il34LacZ/LacZ mice, microglia were partially reduced, most pronounced in areas correlating with high IL-34 expression (Figures 3C–3E). Thus, IL-34 contributes to microglia homeostasis in specific areas of the brain.
Figure 3. IL-34 Contributes to the Homeostasis of Adult Microglia.
(A) X-gal staining (blue) of brain sections from Il34LacZ/+ and Il34+/+ mice (scale bar represents 200 μm).
(B) Brain sections of Il34LacZ/+ mice were stained with X-gal (blue) and NeuN (brown) (scale bar represents 50 μm).
(C) Plots show the percentage and total cell number (±SEM) of microglia (CD45+CD11b+F4/80+) in adult Il34LacZ/LacZ and Il34LacZ/+ mice (n = 12). Pooled data of at least four different experiments are shown. *p < 0.05, **p < 0.01, ***p < 0.001 (Student's t test, unpaired).
(D and E) Brain sections of Il34LacZ/LacZ and Il34LacZ/+ adult mice were stained with anti-Iba-1 (scale bar represents 200 μm).
(E) Mean number of microglia (Iba-1+ cells) per mm2 (n = 2).
IL-34 Controls the Embryonic Development of LCs but Not Microglia
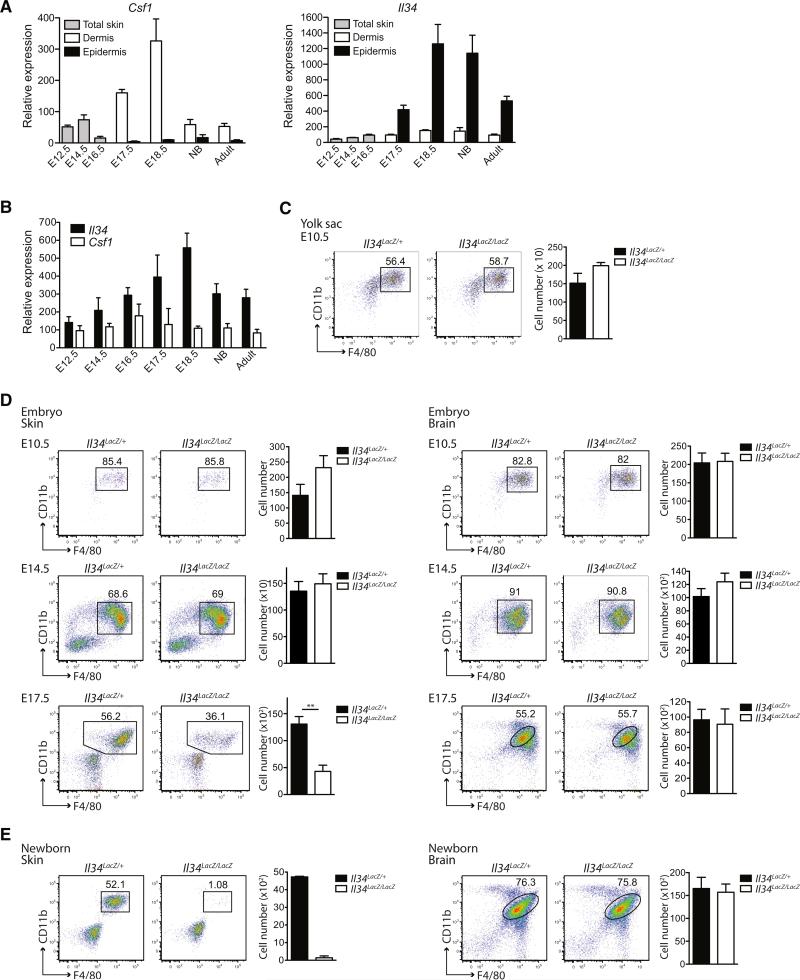
LCs arise from a pool of circulating precursors that take residence in the skin prior to birth. We have recently shown that adult LCs derive predominantly from fetal liver monocytes, which seed the skin around E14.5, but a fraction of LCs also derives from yolk sac macrophages that are recruited to the skin at around E10.5 (Hoeffel et al., 2012). To assess whether IL-34 also controls the differentiation of LC precursors in the embryo and their recruitment to the skin rudiment, we first examined the expression profiles of Il34 and Csf1 during embryogenesis in the developing skin and brain rudiment (Figures 4A and 4B). E17.5 represents the earliest time point at which the epidermis can be physically separated from the dermis (Hoeffel et al., 2012). For time points before E17.5, we analyzed limb buds, which represent embryonic skin as previously described (Hoeffel et al., 2012). We found that Csf1 and Il34 were both expressed in the CNS throughout embryogenesis as well as in newborns and adult mice while Il34 was expressed at higher levels (Figure 4B). In the developing skin, Csf1 was upregulated in the dermis at E17.5 but not detectable in the epidermis. Conversely, Il34 transcripts increased specifically in the epidermis at E17.5 and remained at high levels after E17.5 at all later time points (Figures 2B and 4A). Before E17.5, both cytokines were expressed at low levels in the skin. These data demonstrated that IL-34 is not only the only Csf-1r ligand present in the adult epidermis but also in the embryonic epidermis, suggesting a critical role for IL-34 in LC development.
Figure 4. IL-34 Controls the Development of LCs during Embryogenesis.
(A and B) Quantitative RT-PCR analysis of Il34 and Csf1 mRNA expression in the developing skin (A) and the developing brain (B) at different time points during embryogenesis and in newborn (NB) and adult WT mice. Total skin is shown from E12.5–E16.5, and epidermis and dermis are shown after E17.5. Data were normalized to the expression of HPRT1 (n = 3). Shown are pooled data of two individual experiments.
(C) Plots show the percentage (among CD45+ cells) and total cell number (±SEM) of primitive macrophages (CD11b+F4/80+) in the yolk sac at E10.5 (n ≥ 3).
(D) Plots show the percentage and total cell number (±SEM) (among CD45+ cells) of microglia precursors (CD11b+F4/80+) in the brain at E10.5, E14.5, and E17.5 and of LC precursors (CD11b+F4/80+) in the skin (limb buds) at E10.5 and E14.5 and in the epidermis at E17.5 in Il34LacZ/LacZ and Il34LacZ/+ embryos (for E10.5: n ≥ 3, for E14.5: n ≥ 4 and for E17.5: n = 5). Data are pooled of 1–2 different experiments.
(E) Plots show the percentage and total cell number (±SEM) of epidermal LC precursors and microglia (both CD11b+F4/80+) among CD45+ cells in newborn Il34LacZ/LacZ and Il34LacZ/+ mice (LC precursors: n = 2, microglia: n = 6). One representative of three individual experiments is shown. (C–E) *p < 0.05, **p < 0.01, ***p < 0.001 (Student's t test, unpaired).
To verify whether IL-34 is required for the development of LCs, we analyzed whether absence of IL-34 affected the differentiation of LC precursors (F4/80+CD11b+) at E10.5, E14.5, and E17.5. We found no difference in primitive macrophages and LC precursors between Il34lacZ/lacZ and Il34LacZ/+ embryos in the yolk sac at E10.5 or in the limb buds at E10.5 or E14.5, respectively (Figures 4C and 4D). However the absence of IL-34 strongly reduced the number of LC precursors within the skin rudiment at E17.5 (Figure 4D) and the number of epidermal LCs in newborns (Figure 4E). The disappearance of LCs in the absence of IL-34 coincided with the increased Il34 expression observed in the epidermis of WT embryos at this time point (Figure 4A). Circulating monocytes, fetal liver, splenic, and dermal monocytes, as well as macrophages in the spleen, lung, dermis, and liver, were unaffected in embryos deficient in IL-34 at E17.5 (Figure S2). This finding demonstrated that IL-34 specifically controls LC differentiation locally in the skin during embryonic development.
In contrast to LCs, which predominantly arise from fetal liver precursors (Hoeffel et al., 2012), microglia are exclusively derived from primitive yolk sac macrophages that arise before embryonic age E8 (Ginhoux et al., 2010). Microglia genesis is highly dependent on Csf-1r signaling (Ginhoux et al., 2010). In order to determine whether microglia precursors require IL-34 signaling during embryogenesis, we analyzed the brain rudiment of Il34LacZ/LacZ embryos at E10.5, E14.5, and E17.5 (Figures 4C and 4D). Microglia precursors were present at normal numbers in the absence of IL-34 throughout embryonic development. Similar results were obtained in neonatal Il34LacZ/LacZ mice where no difference could be detected in microglia numbers compared to control mice (Figure 4E). These data indicated that IL-34 is dispensable for the embryonic development of microglia but contributes to microglia homeostasis in certain regions within the CNS in the adult.
IL-34 Does Not Control the Inflammation-Induced Repopulation of LCs but Is Crucial for Their Maintenance In Vivo
Under physiological conditions, adult LCs self-renew independently of blood-derived precursors (Merad et al., 2002). During skin inflammation, however, the pool of resident LCs is replenished by circulating Ly6Chi monocytes. Repopulation of resident LCs under inflammatory conditions is highly dependent on Csf-1r signaling (Ginhoux et al., 2006). To determine whether Csf-1r signaling is also required for the maintenance of adult monocyte-derived LCs, we generated mixed BM chimeras. Recipient mice were lethally irradiated and reconstituted with a 1:1 mixture of CD45.1 WT: CD45.2 Langerin-Cre+ × Csf1rfl/fl BM cells or CD45.1 WT: CD45.2 WT BM cells. Mixed chimeras were then exposed to UV light and analyzed for the presence of LCs (CD45.1+ versus CD45.2+) once the inflammation is resolved, which usually occurs in about 2 weeks post UV exposure in our model (Merad et al., 2002). Langerin-Cre+ × Csf1rfl/fl LCs were greatly reduced compared to WT LCs in the same BM chimera and compared to CD45.1 WT: CD45.2 WT BM chimeric animals (Figure S3A). Of note, there was no difference in the chimerism of blood monocytes before UV treatment in both groups of chimeras (data not shown). These data indicate that Csf-1r signaling is critical for the homeostasis and/or survival of the newly repopulated LCs. We have previously shown that the repopulation of LCs by Ly6Chi monocytes in the UV-exposed skin is delayed but not aborted in Csf1op/op mice (Gin houx et al., 2006), suggesting that IL-34-mediated Csf-1r signaling could be involved in the repopulation of LCs in inflamed skin. Here, we explored whether monocytes are recruited to the inflamed skin and can give rise to LCs in the absence of IL-34. To this end, Il34LacZ/+ and Il34LacZ/LacZ mice were exposed to UV light. We found that at the peak of acute skin inflammation (day 7 after UV exposure), numbers of skin-infiltrating monocytes were comparable between Il34LacZ/+ and Il34LacZ/LacZ mice (Figure 5A). At this time point, resident epidermal LCs were drastically decreased compared to naive Il34lacZ/+ mice (Figure 5A). However, 21 days after UV exposure, the epidermis of Il34LacZ/LacZ mice harbored fewer LCs in comparison to control mice, whereas at 45 days and 90 days after UV exposure, LCs were severely reduced in Il34LacZ/LacZ mice compared to Il34LacZ/+ (Figure 5B). Because Csf-1r is required for the differentiation of monocytes into LCs in the inflamed skin, these results suggest that Csf-1 might compensate for the lack of IL-34 allowing the recruitment of monocytes and their subsequent differentiation into LCs in the inflamed epidermis of IL-34-deficient mice, but that in the steady state, the only Csf-1r ligand that can maintain LC homeostasis is IL-34.
Figure 5. IL-34 Is Not Critical for the Repopulation of LCs in Inflammation but for Their Maintenance.
(A and B) Il34LacZ/LacZ and Il34LacZ/+ mice were exposed to UV light. (A) Plots show the percentage and total cell number (±SEM) of monocytes (CD11b+Ly6C+) among ear skin cell suspensions (CD45+ Ly6G−) and of LCs (Epcam+Langerin+) among CD45+ epidermal cells 7 days (d) after UV treatment or in untreated control mice (n = 4 for UV-treated groups and n = 3 for untreated control mice). Data are pooled from two individual experiments. *p < 0.05, **p < 0.01, ***p < 0.001 (Student's t test, unpaired). (B) Plots show the percentage and total cell number (±SEM) of LCs (Epcam+Langerin+) among CD45+ epidermal cells on day 21 (n = 7), day 45 (n = 7) and day 90 (n = 5) after UV exposure. Pooled data of two individual experiments. *p < 0.05, **p < 0.01, ***p < 0.001 (Student's t test, unpaired). (C) Quantitative RT-PCR analysis of Il34 and Csf1 mRNA levels in the epidermis of UV exposed WT mice at different time points post UV exposure or in naive WT mice (d 0). Data were normalized to the expression of RNA polymerase 2 (n = 3 for d 7, d 21 and d 42, n = 2 for d 0).
(D and E) Il34LacZ/LacZ and Il34LacZ/+ mice were treated with Calcipotriol (CP) for 2 weeks and epidermal cells were analyzed 21 days post treatment. (D) Plots show the percentage of LCs among CD45+ epidermal cells. Graph displays the total cell number (±SEM) of LCs of CP-treated mice (n = 6). Pooled data of two individual experiments are shown. (E) Images depict anti-MHCII staining on ear-derived epidermal sheets of Il34LacZ/LacZ and Il34LacZ/+ mice 21 days after CP treatment (scale bar represents 100 μm).
(F and G) Ears of Il34LacZ/LacZ and Il34LacZ/+ mice were treated with Aldara cream for 9 days (n = 4). Shown is one representative of two individual experiments. (F) Kinetics of Aldara-induced skin inflammation measured by increase in ear thickness. (G) Plots show the percentage and total cell number (±SEM) of LCs among CD45+ epidermal cells of Aldara treated ears on day 12 (n = 7). *p < 0.05, **p < 0.01, ***p < 0.001 (Student's t test, unpaired).
To confirm this notion, we examined the expression of IL-34 and Csf-1 in the epidermis of WT mice at different time points post UV exposure (Figure 5C). Accordingly, we found that during severe skin inflammation (day 7), IL-34 was decreased but the levels of Csf-1 were highly increased in the epidermis. After the inflammation was resolved (day 21 and day 42), IL-34 levels increased again to levels observed in uninflamed skin (Figure 5C). Clearly IL-34 is not needed for the influx of monocyte-derived LCs during inflammation. However, after resolution of inflammation, monocyte-derived LCs require IL-34 for their maintenance.
To confirm these findings in a different skin inflammation model, we administered Calcipotriol locally to the skin. Calcipotriol is a Vitamin-D3 analog that induces proliferation of keratinocytes and inflammation when applied to healthy skin (Li et al., 2006b). We found that similar to UV-induced skin inflammation, Calcipotriol leads to the depletion of resident LCs and, this cellular niche is subsequently replaced by BM-derived circulating precursors that proliferate locally and differentiate into LCs (Figures S3B and S3C). Upon Calcipotriol-induced inflammation, LCs were repopulated in Il34LacZ/LacZ mice (Figures 5D and 5E). However, similar to what we observed in the UV inflammation model, 3 weeks after Calcipotriol treatment, the total number of monocyte-derived LCs in Il34LacZ/LacZ mice was reduced compared to Il34LacZ/+ mice. No difference in phenotypic markers or morphology of LCs could be observed between Il34LacZ/LacZ and Il34LacZ/+ mice at this time point (Figure 5E). Thus, the recruitment of monocytes and their subsequent differentiation into LCs under inflammatory conditions is independent of IL-34, but once the inflammation resolves, the maintenance of these newly recruited LCs requires IL-34 signaling.
LCs Are Not Involved in Aldara-Induced Psoriasiform Inflammation
Psoriasis is a common relapsing and remitting inflammatory skin disease manifested by scaly erythematous plaques with silvery-white scaling and acanthosis. The psoriasiform skin lesions are characterized by hyperproliferative keratinocytes, infiltration of macrophages, DCs, neutrophils, and T cells (Perera et al., 2012). Although the pathogenesis of psoriasis is not clearly defined, IL-23-induced IL-17 secretion is essential for the psoriasiform plaque formation in mice as well as humans (van der Fits et al., 2009). We and others have recently demonstrated that skin-invading γδ T cells and innate lymphocytes producing the “TH17 cell–signature cytokines” (IL-17A, IL-17F, and IL-22) rather than TH17 cells, as sufficient and necessary in Aldara-induced skin inflammation (Cai et al., 2011; Pantelyushin et al., 2012). Even though LCs produce IL-23 (Igyártó et al., 2011) and reside in close proximity to keratinocytes, their contribution to the pathogenesis of psoriasiform inflammation is not known. In order to address the role of LCs in the development of Aldara-induced psoriasiform lesions, we exposed the skin of Il34LacZ/LacZ mice to Aldara cream as described previously (Pantelyushin et al., 2012). Ears of Il34LacZ/+ and Il34LacZ/LacZ mice were treated with Aldara cream for 9 days and the kinetics of inflammation was assessed by increase in ear thickness. No difference in the kinetics of Aldara-induced skin inflammation could be detected between Il34LacZ/+ and Il34LacZ/LacZ mice (Figure 5F). Analysis of skin infiltrating cells e.g., γδ T cells or DCs also revealed no difference between Il34LacZ/+ and Il34LacZ/LacZ mice (data not shown). In addition, similar to Calcipotriol and UV-induced skin inflammation, in Aldara-induced skin inflammation, LCs were repopulated in Il34LacZ/LacZ mice although at significantly lower numbers compared to Il34LacZ/+ mice (Figure 5G). These data indicate that LCs are dispensable for the formation and the pathogenesis of psoriasiform plaques but confirm that IL-34 controls LC homeostasis in adult epidermis.
IL-34 Is Expressed in Human Skin and Brain
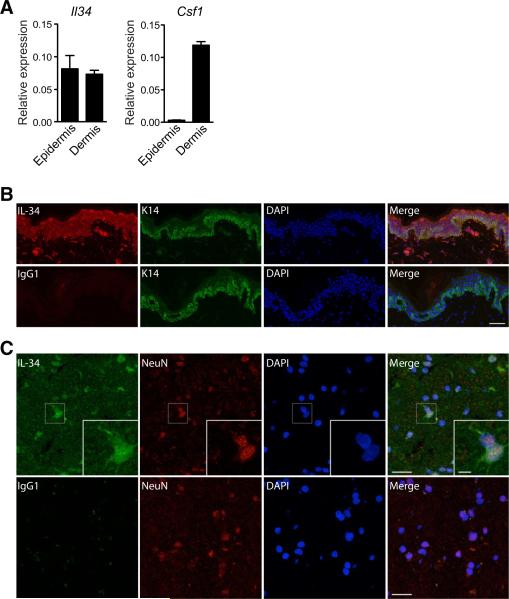
To determine whether our findings could also be translated to humans, we investigated human brain tissue obtained from intractable epilepsy patients undergoing selective amygdalahippocampectomy and skin tissue obtained from patients undergoing reductive mammoplasty. Il34 messenger RNA (mRNA) was previously shown to be expressed in several human tissues including lung, liver, kidney, brain, spleen, thymus, testis, ovary, and gut (Lin et al., 2008). However, the expression of IL-34 protein and its localization within the skin and the brain has not been previously reported. We found that IL-34 was the only Csf-1r ligand expressed in the human epidermis where it was produced by keratinocytes mirroring our finding in rodents, whereas both IL-34 and Csf-1 were expressed in the human dermis (Figures 6A and 6B). In the human brain, IL-34 protein was also readily detectable in the temporal lobe as shown in Figure 6C. These results establish that the expression pattern of IL-34 is comparable between species, indicating that IL-34 is an evolutionary highly conserved, nonredundant factor controlling microglia and LC homeostasis.
Figure 6. IL-34 Is Expressed in Human Skin and Brain.
(A) Quantitative RT-PCR analysis of Il34 and Csf1 mRNA expression of human epidermis and dermis. Data were normalized to the expression of GAPDH. One of two independent experiments is shown.
(B) Human skin sections were stained with mAbs against K14 and IL-34 (or isotype, mouse IgG1) and counterstained with DAPI (scale bar represents 50 μm).
(C) Human brain sections of the temporal lobe were stained with mAbs against IL-34 (or isotype, mouse IgG1) and NeuN and counterstained with Dapi (scale bar left represents 20 μm, scale bar right represents 5 μm).
DISCUSSION
Csf-1r and its ligand Csf-1 have long been recognized as mandatory factors for the differentiation, maintenance, and function of tissue macrophages. Interestingly however, mice lacking Csf-1r display significantly more severe developmental abnormalities than mice lacking the ligand Csf-1. Specifically, LCs and micro-glia are absent in Csf1r−/− mice, whereas they display only a slight reduction in numbers in Csf1op/op mice. By specifically ablating Csf-1r on fully differentiated LCs, we could demonstrate that Csf-1r signaling is not only required for the development of LCs during embryogenesis but is also a prerequisite for the homeostasis of LCs in the adult skin. Consequently, these findings imply that another ligand apart from Csf-1 controls LC homeostasis in the adult skin.
This phenotypic divergence between Csf1r−/− and Csf1op/op mice could potentially be explained by the recent discovery of an alternative ligand for Csf-1, named IL-34. Here, we analyzed the role of IL-34 in the development of LCs and microglia. By using IL-34-deficient reporter mice, we found that within the skin, IL-34 was almost exclusively produced by keratinocytes in the epidermis and by neurons in the brain. During the preparation of this manuscript, a report by Wang et al. (2012) also demonstrated that adult Il34LacZ/LacZ mice displayed a drastic reduction of LCs, indicating that IL-34 is a key regulator for LCs in agreement with our study. Similar to mice, IL-34 was also the only Csf-1r ligand expressed in human epidermis.
In both mouse and human skin, we found IL-34 expression also in the dermis. The expression of dermal IL-34 was localized mainly to hair follicles in mice suggesting that hair follicle IL-34 expression could play a role in LC entry as demonstrated by Nagao et al. (2012).
In the brain, the deficiency of IL-34 only led to a decrease in microglia cell numbers by approximately 50% in a region-specific manner. This phenomenon suggests that within the CNS, Csf-1 might compensate for the loss of IL-34. Such compensatory Csf-1 expression was not observed within the epidermis, in agreement with the absence of Csf-1 expression in the epidermis. The fact that DCs and macrophages in other lymphoid and nonlymphoid tissues were unchanged in the absence of IL-34, demonstrates the tissue and target specificity of stroma-derived IL-34 on the MPS.
Wang et al. (2012) also observed developmental defects in LCs as well as microglia as illustrated by the lack of both cell populations in Il34Lac//LacZ neonatal mice. We observed the same phenotype in the neonatal skin; however, we failed to observe such a reduction of microglia in IL-34-deficient neonates. We thus extended these studies and analyzed the development of microglia and LC precursors during embryogenesis. In the developing skin at E10.5 and E14.5, LC precursors were present at normal numbers in the absence of IL-34. However, in the developing epidermis at E17.5, LC precursors were significantly reduced in Il34Lac//LacZ embryos compared to control littermates. This reduction of LC precursors in E17.5 Il34Lac//LacZ embryos correlated with the upregulated IL-34 expression in E17.5 epidermis in WT embryos. No differences were found in myeloid cell numbers in other organs or in micro-glia precursors in the developing brain throughout embryogenesis. These data indicate that LC precursors in the embryonic epidermis required IL-34 locally for their survival and/or differentiation into LCs, whereas the development of microglial cells was not exclusively dependent on IL-34 signaling. As mentioned above, we hypothesize that the expression of Csf-1 in the CNS parenchyma compensates for the loss of IL-34.
In three different skin inflammation models, we could show that the recruitment of monocytes and their subsequent development into LCs was not inhibited in the absence of IL-34, which is in agreement with the study published by Wang et al. (2012). However, upon longitudinal analysis, we found that after resolution of inflammation, the total number of LCs was significantly reduced and declined over time in Il34LacZ/LacZ mice. During acute skin injury induced by UV light, we found Il34 to be present at low levels in the epidermis compared to the steady state. Conversely, Csf1, which is absent from the epidermis in the steady state, was highly upregulated after UV exposure. These findings indicate that LC differentiation in an inflamed setting could occur independently of IL-34 but that the homeostasis of monocyte-derived LCs in the adult skin requires IL-34.
Taken together, our data revealed that IL-34 represents a unique cytokine, in that its expression is highly restricted to stromal cells of the epidermis and brain in both mice and humans. The fact that the expression pattern of IL-34 is comparable between these species is also indicative of a highly conserved evolutionary pathway. The identification of the IL-34 encoding gene and protein in avian species also adds credence to this prospect (Garceau et al., 2010). Thus, IL-34 represents a cytokine essential for the development and maintenance of LCs and homeostasis of microglia.
EXPERIMENTAL PROCEDURES
Mice
To generate IL-34-deficient mice, targeted JM8.N4 embryonic stem cells were obtained from EUCOMM (IKMC Project - ID: 33127). In brief, the L1L2_Bact_P cassette was placed upstream of exons 3–5. In this constellation (termed Il34LacZ), usage of the En2 (engrailed 2) splice acceptor will result in a fusion message between the first two exons and the LacZ reporter sequence, resulting in a truncated Il34 message. Csf1rfl/fl mice were provided by Jeffrey Pollard (Li et al., 2006a) (Albert Einstein, New York, NY). Langerin-Cre mice were provided by Daniel Kaplan (Kaplan et al., 2007) (University of Minnesota, Minneapolis, MN). All animal experiments performed in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of Mount Sinai School of Medicine and by the Swiss Veterinary Office.
Blocking Csf-1r Signaling
Csf-1r mAb (clone AFS98) was purified from culture supernatant of AFS98 hybridoma (gift from S. Nishimura, New York) as previously described (Hashimoto et al., 2011). Mice were injected intraperitoneally (i.p.) with AFS98 or isotype (rat IgG2a, clone 2A3, Bio X Cell) at doses of 2 mg/mouse on day 0 and 1 mg on days 2 and 4 and analyzed on day 12 after treatment start. Rosa26-CreERT2 × Csf1rfl/fl were injected i.p. with 2.5 mg tamoxifen (Sigma T5648) dissolved in corn oil three times every other day as previously described (Greter et al., 2012).
Cell Suspension Preparations
Cell suspensions were prepared as previously described (Ginhoux et al., 2009). Epidermis and dermis were separated after Dispase digestion, cut into small pieces, and incubated for 2.5 hr in 0.2 mg/ml collagenase type IV (Sigma-Aldrich) and passed through a 19G syringe to obtain a homogeneous cell suspension. CNS, spleen, lung, lymph nodes, and liver were incubated in collagenase type IV and subsequently passed through a19G syringe to obtain a homogeneous cell suspension. Liver and CNS cell suspensions were further enriched by a Percoll gradient as previously described (Ginhoux et al., 2009, 2010). Analysis was carried out by flow cytometry as described below.
Flow Cytometry
Flow cytometry was performed using an LSRII or LSRII Fortessa (Becton Dickinson) and analyzed with FlowJo software (Tree Star). Fluorochromeconjugated monoclonal antibodies (mAbs) specific for mouse MHC class II I-A/I-E (clone M5/114.15.2), CD11b (clone M1/70), CD11c (clone N418), CD45 (clone 30-F11), CD115 (clone AFS98), Ly6C (clone AL-21), Ly6G (clone 1A8), CD103 (clone 2E7), CD8a (clone 53-6.7), CD24 (clone M1/69), and Epcam (clone G8.8) were purchased either from BD Biosciences, eBioscience, or Biolegend. Anti-F4/80 (clone Cl:A3-1) mAb was purchased from AbD Serotec. Anti-Langerin Ab (clone E-17) and donkey-anti-goat IgG-PE were purchased from Santa Cruz.
Quantitative RT-PCR
Total RNA was extracted from tissues using the RNeasy Mini kit (QIAGEN) and reverse-transcribed into cDNA using MML-V reverse transcriptase (Invitrogen) or Superscript II RT (Invitrogen) followed by PCR amplification with Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen) or SYBR Green I Master Mix (Roche). The following primers were used: mouse IL-34 forward: ACTCA GAGTGGCCAACATCACAAG, mouse IL-34 reverse: ATTGAGACTCACCAA GACCCACAG, mouse Csf-1 forward: TACAAGTGGAAGTGGAGGAGCCAT, mouse Csf-1 reverse: AGTCCTGTGTGCCCAGCATAGAAT, mouse pol2 forward: CTGGTCCTTCGAATCCGCATC, mouse pol2 reverse: GCTCGATAC CCTGCAGGGTCA, human Csf-1 forward: GTTTGTAGACCAGGAACAGTT GAA, human Csf-1 reverse: CGCATGGTGTCCTCCATTAT, human GAPDH forward: ATGGGGAAGGTGAAGGTCG, human GAPDH reverse: GGGTCAT TGATGGCAACAATATC. For the embryo analysis (Figure 4), the following primers were used: mouse IL-34 forward, GGGTCATGGAACTGCTGTACT, mouse IL-34 reverse: ATCAAGGACCCCGGACTG, mouse Csf-1 forward: CTGCTTCACCAAGGACTATGAG, mouse Csf-1 reverse: GAAGTTCTTGATCT TCTCCAGCA, mouse HPRT1 forward: TCCTCCTCAGACCGCTTTT, and mouse HPRT1 reverse: CCTGGTTCATCATCGCTAATC.
X-gal Staining and Immunohistochemistry X-gal Staining
Tissues were fixed in 2% Formaldehyde/0.2% Glutaraldehyde for 1 hr and then stained in X-gal solution (1 mg/ml X-gal (Sigma), 2 mM MgCl2, 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 0.02% NP-40, 0.01% sodium deoxycholate) for 14–16 hr at 37°C. Tissues were then fixed in HOPE® I (DCS Innovative Diagnostik-System) for 72 hr and embedded in paraffin. Sections were stained with antibodies against K14 (Abcam, clone LL002) or NeuN (Millipore, clone A60) and counterstained with Nuclear fast red solution (Sigma) or Berlinerblau.
Epidermal Sheets
Epidermal sheets were prepared as previously described (Ginhoux et al., 2006) and stained with mAb against MHCII (clone M5/114.15.2) and CD3 (clone 145-2C44).
Human Brain and Skin
Snap-frozen temporal lobe tissue specimen was obtained from patients with chronic pharmacoresistant epilepsy undergoing selective amygdalohippocampectomy (University Hospital Zürich, Switzerland) and embedded in OCT. Human skin obtained from breast reduction (University of Zürich, Switzerland) was embedded fresh in OCT and snap frozen. Sections (10 μm) of brain or skin tissue were stained with primary antibodies against IL-34 (Abcam, clone 1D12) or isotype (mouse IgG1, Sigma, clone MOPC21), NeuN (Millipore, clone A60) or K14 (Abcam, clone LL002) followed by staining with secondary fluorochrome-conjugated anti-mouse antibodies and counterstaining with DAPI.
All procedures were conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Canton Zurich. Informed written consent was obtained from all patients.
Mouse Brain
Sections (10 μm) of paraffin embedded brains were stained with anti-Iba-1antibody (Wako) followed by staining with secondary HRP-conjugated anti-rabbit antibodies and counterstaining with hematoxylin.
Images were acquired with a Leica SP5 confocal microscope or with a brightfield microscope (Olympus) and were processed using ImageJ Software (NIH).
Skin Inflammation
Aldara Treatment
A daily dose of 5 mg of Aldara cream (5% IMQ cream, 3M Pharmaceuticals) was applied onto the ear for 9 days as previously described (Pantelyushin et al., 2012).
Calcipotriol Treatment
Calcipotriol (Sigma) (10 nmol) diluted in ETOH was applied every other day onto the ear for 14 days.
UV Treatment
Mice were exposed to UV light (254 nm, 28 cm from the target) for 25 min as previously described (Merad et al., 2002).
Statistical Analysis
Mean values, SEM values, and Student's t test (unpaired) were calculated with Prism (GraphPad software). *p < 0.05, **p < 0.01, ***p < 0.001 (Student's t test, unpaired).
Supplementary Material
ACKNOWLEDGMENTS
B.B. is supported by the Swiss National Science Foundation and the European Union FP7 project TargetBraIn (279017). M.M. is supported by NIH grants CA112100, HL086899, and AI080884. M.G. is supported by the Forschungskredit of the University of Zurich. F.G. is supported by the Singapore Immunology Network core grant. We also acknowledge the Neuroscience Center Zurich, University of Zurich, and ETH Zurich, Switzerland. We thank Andrew L. Croxford for critical reading of the manuscript.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes three figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi. org/10.1016/j.immuni.2012.11.001.
REFERENCES
- Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Jala VR, Zhang HG, Wang T, Zheng J, Yan J. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihara T, Suzu S, Hassan R, Chutiwitoonchai N, Hiyoshi M, Motoyoshi K, Kimura F, Okada S. IL-34 and M-CSF share the receptor Fms but are not identical in biological activity and signal activation. Cell Death Differ. 2010;17:1917–1927. doi: 10.1038/cdd.2010.60. [DOI] [PubMed] [Google Scholar]
- Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, Sylvestre V, Stanley ER. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- Garceau V, Smith J, Paton IR, Davey M, Fares MA, Sester DP, Burt DW, Hume DA. Pivotal Advance: Avian colony-stimulating factor 1 (CSF-1), interleukin-34 (IL-34), and CSF-1 receptor genes and gene products. J. Leukoc. Biol. 2010;87:753–764. doi: 10.1189/jlb.0909624. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, Stanley ER, Randolph GJ, Merad M. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, Price J, Yin N, Bromberg J, Lira SA, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J. Exp. Med. 2009;206:3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greter M, Helft J, Chow A, Hashimoto D, Mortha A, Agudo-Cantero J, Bogunovic M, Gautier EL, Miller J, Leboeuf M, et al. GM-CSF controls nonlymphoid tissue dendritic cell homeostasis but is dispensable for the differentiation of inflammatory dendritic cells. Immunity. 2012;36:1031–1046. doi: 10.1016/j.immuni.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto D, Chow A, Greter M, Saenger Y, Kwan WH, Leboeuf M, Ginhoux F, Ochando JC, Kunisaki Y, van Rooijen N, et al. Pretransplant CSF-1 therapy expands recipient macrophages and ameliorates GVHD after allogeneic hematopoietic cell transplantation. J. Exp. Med. 2011;208:1069–1082. doi: 10.1084/jem.20101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, Low D, Oller G, Almeida F, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med. 2012;209:1167–1181. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igyártó BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, Zurawski SM, Malissen B, Zurawski G, Berman J, Kaplan DH. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35:260–272. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DH, Li MO, Jenison MC, Shlomchik WD, Flavell RA, Shlomchik MJ. Autocrine/paracrine TGFbeta1 is required for the development of epidermal Langerhans cells. J. Exp. Med. 2007;204:2545–2552. doi: 10.1084/jem.20071401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chen K, Zhu L, Pollard JW. Conditional deletion of the colony stimulating factor-1 receptor (c-fms proto-oncogene) in mice. Genesis. 2006a;44:328–335. doi: 10.1002/dvg.20219. [DOI] [PubMed] [Google Scholar]
- Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc. Natl. Acad. Sci. USA. 2006b;103:11736–11741. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, Halenbeck R, Wu G, Zhou A, Behrens D, et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320:807–811. doi: 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- MacDonald KP, Palmer JS, Cronau S, Seppanen E, Olver S, Raffelt NC, Kuns R, Pettit AR, Clouston A, Wainwright B, et al. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood. 2010;116:3955–3963. doi: 10.1182/blood-2010-02-266296. [DOI] [PubMed] [Google Scholar]
- Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, Weissman IL, Cyster JG, Engleman EG. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Doi Y, Mizoguchi H, Jin S, Noda M, Sonobe Y, Takeuchi H, Suzumura A. Interleukin-34 selectively enhances the neuroprotective effects of microglia to attenuate oligomeric amyloid-β neurotoxicity. Am. J. Pathol. 2011;179:2016–2027. doi: 10.1016/j.ajpath.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao K, Kobayashi T, Moro K, Ohyama M, Adachi T, Kitashima DY, Ueha S, Horiuchi K, Tanizaki H, Kabashima K, et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat. Immunol. 2012;13:744–752. doi: 10.1038/ni.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelyushin S, Haak S, Ingold B, Kulig P, Heppner FL, Navarini AA, Becher B. Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J. Clin. Invest. 2012;122:2252–2256. doi: 10.1172/JCI61862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera GK, Di Meglio P, Nestle FO. Psoriasis. Annu. Rev. Pathol. 2012;7:385–422. doi: 10.1146/annurev-pathol-011811-132448. [DOI] [PubMed] [Google Scholar]
- Sudo T, Nishikawa S, Ogawa M, Kataoka H, Ohno N, Izawa A, Hayashi S, Nishikawa S. Functional hierarchy of c-kit and c-fms in intra-marrow production of CFU-M. Oncogene. 1995;11:2469–2476. [PubMed] [Google Scholar]
- Taveirne S, De Colvenaer V, Van Den Broeck T, Van Ammel E, Bennett CL, Taghon T, Vandekerckhove B, Plum J, Clausen BE, Kaplan DH, Leclercq G. Langerhans cells are not required for epidermal Vgamma3 T cell homeostasis and function. J. Leukoc. Biol. 2011;90:61–68. doi: 10.1189/jlb.1010581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp CH, Chang-Rodriguez S, Stoitzner P, Holzmann S, Stössel H, Douillard P, Saeland S, Koch F, Elbe-Bürger A, Romani N. Ontogeny of Langerin/CD207 expression in the epidermis of mice. J. Invest. Dermatol. 2004;122:670–672. doi: 10.1111/j.0022-202X.2004.22337.x. [DOI] [PubMed] [Google Scholar]
- van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, Lubberts E. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, Barrow AD, Diamond MS, Colonna M. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol. 2012;13:753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Nandi S, Chitu V, Yeung YG, Yu W, Huang M, Williams LT, Lin H, Stanley ER. Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells. J. Leukoc. Biol. 2010;88:495–505. doi: 10.1189/jlb.1209822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW, Jr., Ahmed-Ansari A, Sell KW, Pollard JW, Stanley ER. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc. Natl. Acad. Sci. USA. 1990;87:4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.