Abstract
Objective
This study assessed the baseline knowledge, perceptions, attitudes and behaviors of prediabetes patients in order to tailor a new technology-enhanced primary care-based lifestyle modification intervention.
Methods
Patients with a diagnosis of prediabetes were enrolled in a randomized, controlled pilot study, Avoiding Diabetes Thru Action Plan Targeting (ADAPT), a technology-based intervention to promote action plan discussions around patient-selected behavior change goals.
Results
A total of 54 adults (82% female) were enrolled in the pilot study. Most (89%) had comorbid conditions and mean BMI was 36. Participants exhibited high risk of diabetes knowledge (knowledge score 20 on a 32 point scale) and high levels of willingness to make changes to decrease diabetes risk. Number of daily steps was inversely correlated with perceived physical activity (r=−0.35082, p<0.001). Poorer scores on diet quality were inversely correlated with BMI.
Conclusion
Participants in this sample demonstrated requisite levels of knowledge, self-efficacy, motivation and risk perception for effective behavior change. These data suggest that primary care-based prediabetes interventions can move beyond educational goals and focus on enhancing patients’ ability to select, plan and enact action plans.
Keywords: Type II Diabetes Mellitus, Prediabetes, Behavior Modification, Self-Efficacy, Risk-Perception
INTRODUCTION
In the United States, 25.8 million adults have type 2 diabetes mellitus (T2DM), and another 79 million adults are affected by prediabetes.1 Data from the 2007–2009 National Health Interview Survey and National Patient Information Reporting System reveal a disproportionately higher prevalence of diabetes among African Americans, Hispanics, and Asian Americans.2 The rising incidence of diabetes in the US is a public health concern with serious medical and financial implications. In 2012, the cost of diabetes in the US was estimated at $245 billion.3 Without intervention, individuals with prediabetes are at high risk of developing diabetes and associated comorbid complications. Prediabetes alone has also been associated with increased risk of cardiovascular disease,4–7 chronic kidney disease,8 retinopathy,9 and neuropathy.10 Lifestyle changes in patients with elevated glucose levels are known to delay or prevent the progression of prediabetes to T2DM.11,12 Effective interventions include changes in diet alone, exercise alone, diet plus exercise, and weight loss.13,14 The Diabetes Prevention Program (DPP) demonstrated that a structured, intensive program of dietary and exercise counseling combined with a weight loss regimen effectively reduced the risk of developing T2DM by 58% over three years and was more effective than metformin in reducing risk of diabetes, with protective benefits persisting for at least 10 years.15–19 The efficacy of diabetes intervention programs has also been demonstrated in primary care settings.
Patient factors such as a lack of willingness to change, low self efficacy, poor disease understanding, and inappropriate perception of risk are often cited as key obstacles to successful execution of behavior change programs in primary care.20,21 Challenges described include participants’ mixed levels of knowledge regarding whether diabetes can be prevented and little knowledge of risk factors.22 Providers note an absence of patient motivation to change their unhealthy habits as an important barrier for effective lifestyle counseling.23 Since diabetes is largely a self-managed disease, patients’ social support, access to care, financial barriers, and attitudes and beliefs about their illness are crucial components that determine individual health behaviors. Pre-existing physical conditions and social demands can act as impediments to behavior change and necessitate a holistic approach to lifestyle intervention.24
Comprehensive lifestyle interventions like the DPP have the time and resources to intervene on all of these factors: educational, motivational and behavioral. However, primary care provider-led interventions must be more focused and smaller in scope. This creates some confusion as to where emphasis should be placed for behavior change. Do prediabetes patients in primary care have enough knowledge to enact behavior change plans, or do interventions first need to focus on improving knowledge, attitudes and beliefs about their disease and risk for diabetes? Can interventions presume sufficient levels of these factors among patients, and instead focus on tools to enable them to achieve their behavioral goals? This study seeks to answer these questions and evaluate the baseline knowledge, attitudes, beliefs and behaviors of prediabetes patients in a primary care setting in order to guide the focus, development and implementation of a technology-enhanced lifestyle intervention.
METHODS
A randomized, controlled pilot study, Avoiding Diabetes Thru Action Plan Targeting (ADAPT), introduced a novel tool to enhance physician counseling for patients with prediabetes in the primary care setting. The full details of the study design have been previously published, but in brief, the ADAPT system uses the electronic medical record, pedometers and the internet to embed prediabetes-specific action planning into primary care encounters.25 Patients were recruited from two urban, academic primary care practices, both affiliated with the Mount Sinai Hospital in New York. Recruitment followed practical trial methods such that patients were recruited from practice databases and all study procedures were situated within the context of previously scheduled clinical visits.26 Patients were eligible if they were age 18 or older, English-speaking, and had prediabetes defined as having a glycosylated hemoglobin (A1C) of 5.7–6.4% (40–48mmol/mol) or a fasting glucose of 100–125. Patients were excluded if they had a diagnosis of diabetes, had ever been prescribed a diabetic medication, were unable to walk, or did not have access to email since part of ADAPT involves email-based communication. Eligible patients from each practice were approached via phone by trained research assistants and invited to participate in the study. Patients who expressed interest in participating over the phone and wanted additional information were subsequently sent a follow-up email with an attached consent form. Patients who were sent an email received an additional follow-up phone call to schedule a meeting to officially enroll in the study by signing the consent form and receiving a pedometer.
All study activities were conducted within the context of real clinical activities and were scheduled around or within upcoming primary care visits. As such, all participants completed a 60-item multiple-choice pre-enrollment survey administered by a research assistant by telephone or in-person prior to their primary care visit. Items included socio-demographics, medical history, and family history. Participants were asked about current physical activity and attempts to change physical activity. Similar questions were asked about individual body weight. In addition to inquiring about confidence/self-efficacy to change eating habits and physical activity, the survey assessed participants’ current stage of change in these behaviors according to the Transtheoretical Model of Stages of Change.27 Pre-diabetes knowledge and T2DM risk perception were measured as well.28,29 Symptoms of depression and anxiety were assessed using the Patient Health Questionnaire (PHQ-8) and the General Anxiety Disorder (GAD-2) scale, respectively.30,31 Participants answered questions related to the locus of responsibility for making changes to their lifestyle and the effectiveness of their provider in helping them change behaviors. Finally, self-reported diet behavior was assessed using the short Rapid Eating Assessment for Patients (REAP-S) tool, a 16-item instrument to address dietary intake and behavior.32,33
Participants were required to wear a pedometer (portable activity monitor, Omron HJ-720ITC) to measure their daily steps for one week prior to their first study visit. To be considered valid, a participant’s pedometer data needed to consist of (1) at least 10 hours of non-zero activity per day and (2) at least 3 days of activity.41 Hours of activity and days worn could be continuous or interrupted. Steps-per-day were then calculated for each patient for each valid day. On average, participants wore the pedometer for 5 weekdays and 1 weekend day for final analyses. At the initial baseline visit, height, A1c, and fasting lipid panels were measured.
This study was approved by the Institutional Review Board of the Mount Sinai Hospital.
Statistical Analysis
Descriptive statistics were performed for all baseline characteristics. Univariate analysis was performed to evaluate the association between number of daily steps, body mass index (BMI, kg/m2), stages of change, level of physical activity, Rapid Eating Assessment for Participants (REAPS) score, diabetes knowledge, and diabetes risk perception. For all tests, a p-value less than 0.05 was used for statistical significance. Data analyses were conducted using SAS (v9.1) software for Windows (SAS Institute, Cary, North Carolina).
RESULTS
Socio-demographics
A total of 63 patients were enrolled in the study. Five individuals presented with a baseline A1C less than 5.7 or greater than 6.4 and four individuals failed to complete all entry requirements leaving 54 participants for final analyses (Table 1).
Table 1.
Baseline socio-demographic and clinical characteristics of participants enrolled in ADAPT (Avoiding Diabetes Thru Action Plan Targeting)
| Variable | Overall (N= 54) |
|---|---|
|
| |
| Mean age (in years) (SD) | 45.7 (10.9) |
|
| |
| Gender | Female = 44 (81.5%) |
|
| |
| Race | |
| White | 6 (11.1%) |
| Black | 21 (38.9%) |
| Hispanic | 22 (40.7%) |
| Asian | 4 (7.4%) |
|
| |
| Insurance * | |
| Any Medicaid | 9 (17.6%) |
| Any Commercial | 41 (80.4%) |
| Only Medicare | 4 (7.8%) |
|
| |
| Education | |
| ≤ HS degree | 10 (18.5%) |
| Some college/trade or technical school | 21 (38.9%) |
| College graduate/professional training | 22 (40.7%) |
|
| |
| Mean Weight (kg) (SD) | 96.2 (23.0) range = 59.4 – 153.3 |
|
| |
| Mean BMI (kg/m2) (SD) | 35.7 (8.3) |
|
| |
| Mean A1C (SD) | 5.9 (0.2) |
| mmol/mol | 40–48 |
|
| |
| Lipids (mean (SD)) | |
| Total Cholesterol (normal < 200) | 187 (33) |
| LDL (normal < 100) | 110 (27) |
| HDL (normal > 60) | 56 (14) |
| TG (normal < 150) | 111 (77) |
|
| |
| In general how is your health? N (%) | |
| Excellent/Very Good | 11 (20.8%) |
| Good | 28 (52.8%) |
| Fair/Poor | 13(26.4%) |
|
| |
| Comorbidities | |
| 0 | 6 (11.1%) |
| 1–2 | 28 (51.9%) |
| 3+ | 20 (37.0% |
|
| |
| Family History of Diabetes | |
| Yes | 41 (75.9%) |
|
| |
| PHQ-8 (median) | 4.0 |
|
| |
| GAD-2 (anxiety) | |
| Not at all | 29 (61.7%) |
| Several days | 14 (29.8%) |
| Nearly every day | 4 (8.5%) |
|
| |
| GAD-2 (worrying) | |
| Not at all | 33 (67.3%) |
| Several days | 6 (12.2%) |
| Nearly every day | 10 (20.4%) |
Table 1 displays the socio-demographic and clinical characteristics of our sample. Most participants were female (82%) and either Black (39%) or Hispanic (41%), which was representative of the urban primary care practice from which they were recruited. Prediabetes was an isolated condition in only 11% of the sample, while co-morbid hypertension (45%), hyperlipidemia (29%), and arthritis (30%) were common. Most participants reported a family history of diabetes (76%), in addition to numerous friends with diabetes (74%). Eighty-one percent had some college education and 70% reported an annual income over $30,000. Mean BMI was nearly 36 (SD 8.3) and mean A1c was 5.9% (SD 0.2) (40–48mmol/mol).
Knowledge/attitudes
Table 2 displays measures of participant knowledge, attitudes, and perceptions. Participants were knowledgeable about their health and about prediabetes (diabetes knowledge score mean=20.1, SD=1.1 out of 32) and recognized the hardship diabetes would place on them (88% reporting life with diabetes would be “difficult”). They understood that being overweight, having low levels of physical activity, and having a family history of diabetes increase their chances for developing diabetes. They also recognized that lifestyle changes, including weight loss and exercise, could lower their risk for diabetes. Overall, patients exhibited positive mental health attitudes with no symptoms of depression, and over 60% reported that they are not at all anxious or worried on any given day.
Table 2.
Baseline cognitive characteristics of participants enrolled in ADAPT (Avoiding Diabetes Thru Action Plan Targeting)
| Stages of change, N (%) | |||||
|---|---|---|---|---|---|
| Pre-contemplation | Contemplation | Preparation | Action | Maintenance | |
| Diet | 2 (3.8%) | 11 (21.2%) | 24 (46.2%) | 11 (21.2%) | 4 (7.7%) |
| Exercise | 0 (0.0%) | 3 (6.0%) | 20 (40.0%) | 20 (40.0%) | 7 (14.0%) |
| Low | Average | High | |||
|
5-year risk perception (maximum 28) Compared to other people your age, your risk of getting diabetes over the next 5 years is… |
4 (8.3%) | 18 (37.5%) | 26 (54.2%) | ||
|
Complications (maximum 32) If you had diabetes the chance of having a complication like a heart attack or stroke is… |
31 (67.4%) | 3 (6.5%) | 12 (26.1%) | ||
| Mean Self-Efficacy | |||||
|
Diet (0–10 scale) How confident are you that you can successfully change your eating to prevent diabetes? |
7.7 | ||||
|
Exercise (0–10 scale) How confident are you that you can successfully change your physical activity to prevent diabetes? |
7.7 | ||||
Participants demonstrated appropriate risk perception, scoring an average 13.3 (SD 4.5) on a 20 point risk scale. The majority (78%) reported being worried about getting diabetes and felt that with diabetes they would be likely to have a complication such as a heart attack or stroke. However, they recognized that they were not invariably destined to have diabetes and overall felt that they could do something to stop the progression to diabetes.
Participants reported dissatisfaction with their current level of physical activity. Over half considered themselves to be somewhat active (59%), though nearly everyone desired to be more active (94%). Similarly, 92% of patients considered themselves to be overweight and wanted to weigh less (96%). Eighty percent said that it was very important to extremely important to become more physically active and lose weight.
Participants felt that they were most responsible for making changes to their diet and exercise. Many participants viewed their providers (94%), family (80%), and friends (64%) as valuable partners in helping them reach their goals for lifestyle modifications The majority of participants saw their providers as somewhat to very effective in helping them change their diet (94%) and physical activity (96%), and nearly all (96%) viewed their providers as effective at helping them lower their chances of developing diabetes. Of note, participants tended to view their friends and family as less responsible than providers in helping them to make lifestyle changes to lower their risk of developing diabetes.
Behaviors
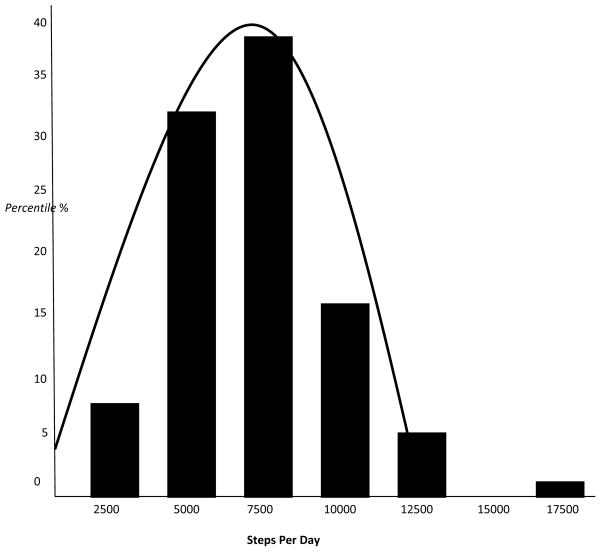
Participants were moderately active at baseline, with average daily steps of 7,074 (SD=2,716; range 2,437 – 16,406).34 Ninety percent of the group failed to meet recommendations of 10,000 steps per day (Figure 1).35
Figure 1.
Steps-per-day at baseline
When asked about making lifestyle adjustments with regard to diet and exercise, participants asserted confidence that they could successfully make changes to their diet (mean=7.7 out of 10 point scale) and exercise (7.7 out of 10 point scale) to prevent diabetes. Nearly all respondents were in the preparation or action phases of the Stages of Change model for diet (75%) and exercise (94%). Seventy-six percent reported spending over 4 hours a day sitting down and using electronics. Although 96% of patients have tried to lose weight and become more physically active in the past 12 months, only 10% reported being successful at these attempts. Participants generally demonstrated poor eating habits and unhealthy diets as evidenced by their responses to food specific inquiries (Appendix A). Nearly 75% of patients ate less than 2 servings of fruits or vegetables a day and half of patients reported eating more than 4 meals per week from restaurants. Additionally, 34% reported skipping breakfast regularly. Still, nearly all participants (98%) answered that they were willing to make changes to their eating habits to be healthier.
Univariate Analysis
Participants who reported greater physical activity on the pre-enrollment survey recorded fewer daily steps on pedometer measurement; the negative correlation was moderate in strength (r = −0.35, p < 0.001). On the REAPS assessment, a tool used to assess diet related to U.S. dietary guidelines, participants reported moderately healthy overall intake (mean score 16.4 (SD 5.2) on a 39 point scale where higher scores are associated with poorer nutrition). Poorer scores on the REAPS measure of diet quality was associated with lower BMI; this negative correlation was also moderate in strength (r = −0.42, p = 0.0017). Number of daily steps and BMI were not correlated with other variables of interest, such as stages of change, diabetes knowledge, and diabetes risk perception.
DISCUSSION
Our data suggest that contrary to common assumptions, patients enrolling in primary care-based prediabetes interventions are well-informed about their condition and exhibit high levels of risk perception, self-efficacy and motivation to change. Moreover, participants reported poor eating and physical activity habits despite their well-recognized risk for diabetes and positive attitudes towards diabetes prevention. These observations are particularly relevant considering the diversity of the sample and their high burden of diabetes risk factors including obesity, family history of diabetes, and cardiovascular comorbidities. These findings suggests that the sample, recruited using practical trial methods, represents an ideal target for a prediabetes lifestyle intervention and would likely resemble the typical patient seen in urban primary care practices. The data were used to redirect the development of the ADAPT intervention away from enhancing patient knowledge, perceptions and attitudes and instead leverage the participants’ existing positive attitudes to replace less healthy behaviors with more healthy ones. Barriers often cited for patients being unable to modify behaviors to prevent disease include the absence of health education, poor understanding of risk of disease and complications, and lack of motivation to change.36–38 Individual cultural beliefs, lack of community connection, and misperceptions about diabetes education programs also pose challenges to lifestyle change.39–41 However, this group of patients demonstrated adequate knowledge about their disease, appropriate risk perception for developing diabetes and its accompanying complications, and an overall high level of awareness of their unhealthy behaviors. Overall, patients had an accurate perception of the natural progression of prediabetes and diabetes. The fact that despite these factors they have been unsuccessful at making appropriate lifestyle changes emphasizes that cognitive awareness is not necessarily correlated with behavioral changes. ADAPT was therefore targeted to primary care patients presenting with adequate levels of knowledge and motivation, but who need a structured path to enact change.
Interestingly, participants who reported higher levels of physical activity were in fact walking less according to pedometer data. This discrepancy between impressions of versus actual physical activity level illustrates that patients often have inaccurate perceptions of their physical activity throughout the day.42 Additionally, participants who reported healthier eating habits (lower REAPS) had higher BMIs than those who reported poor eating habits. Again, this finding suggests that participants are either not good at judging their diet change efforts or are not accurately reporting their behaviors. These findings suggest a gap between patient intentions or self-perceptions about their behavior and their actual behaviors. The ADAPT tool may be valuable in bridging this gap between intentions to exercise and eat healthfully and taking action to adopt healthy behaviors.
Participants demonstrated high levels of self-efficacy and asserted their confidence in their ability to successfully change their eating habits and physical activity to prevent getting diabetes. Despite that, they have not been successful at making changes in the past. This disconnect between how confident people feel and how successful they have been highlights the need for an intervention such as ADAPT to support people in implementing change. Goal setting, action planning and other simple cognitive behavioral change methods are designed to help link intentions and actions.43 The SMART (specific, measurable, attainable, relevant, time-bound) goals based action planning used in ADAPT can help patients focus their intentions into concrete actions to achieve their goals.44–46
ADAPT incorporates the self-management support component of the Chronic Care Model, which includes goal setting, action planning, and problem solving,47,48 and features a patient-centered approach to care that facilitates patient-selected short-term, specific goals coupled with provider feedback, which has been effective for patients at risk for or suffering from chronic diseases.43,49–51 ADAPT is valuable to providers and patients because it provides the framework for shared goal setting and implementation during the clinical encounter. Barriers to patient adoption of lifestyle changes are also commonly attributed to the provider side of the patient-provider interaction. Effective counseling is challenging and providers often lack knowledge and successful strategies for helping their patients change behavior; there is much room for improvement of behavioral counseling rates.52–56 Data from the 2005–2006 National Health and Nutrition Examination Survey showed that only 34.6% of adults with prediabetes receiving health care reported that they had been told by their physician in the past year to control or lose weight; 36.8% reported being told to reduce calories in their diet and 39.4% reported being told to increase physical activity.57 Less than half of adult patients with diabetes are receiving provider advice on how to reduce risk. Clearly, providers could more actively counsel healthy lifestyle changes, especially in a group similar to this study population who are in the preparation/action stages of change. ADAPT presents providers with a workflow embedded simple shared goal setting platform and framework to guide patients who are ready for change through an iterative action planning process.
Study limitations include small sample size, single institution, and urban setting, thus generalizability is limited. Participants were recruited randomly from a clinical database and so there was a significant degree of selection bias; patients who answered their phones, responded to emails, had active insurance and ultimately enrolled in the study were likely more motivated at baseline to change their behaviors to prevent diabetes than the average person. However, this selection process was designed to identify the type of primary care patients who are sufficiently engaged with their healthcare team to benefit from a primary care based behavior change program.
ADAPT promotes action plan discussions between patients and providers for patient-selected behavior change goals during the outpatient visit. This study demonstrated that the recruited sample were already knowledgeable about their health and risk for developing diabetes, demonstrated high levels of self efficacy, placed value on being more active and losing weight, and strongly felt that they could successfully make lifestyle changes to prevent diabetes. This suggests that typical primary care patients are candidates for simple and efficient primary care based action planning interventions. The ADAPT tool was tailored to meet this population’s needs to structure/facilitate behavior change to reduce diabetes risk.
Acknowledgments
Funding: This study was supported by a grant from the National Institute for Diabetes, Digestive and Kidney Diseases (5K23DK081665)
Abbreviations
- T2DM
Type II Diabetes Mellitus
- ADAPT
Avoiding Diabetes Thru Action Plan Targeting
Appendix A. REAPS (Rapid Eating Assessment for Participants – Shortened) Questions
| In an average week how often do you: | Usually | Often | Rarely/Never |
|---|---|---|---|
| Skip breakfast? | |||
| Eat 4 or more meals from sit-down or take out restaurants? | |||
| Eat less than 2 servings of whole grain products or high fiber starches a day? | |||
| Eat less than 2 servings of fruit a day? | |||
| Eat less than 2 servings of vegetables a day? | |||
| Eat or drink less than 2 servings of milk, yogurt, or cheese a day? | |||
| Eat more than 8 ounces of meat, chicken, turkey, or fish per day? | |||
| Use regular processed meats (like bologna, salami, corned beef, hotdogs, sausage or bacon) instead of low fat processed meats (like roast beef, turkey, lean ham, low fat cold/cuts, hotdogs? | |||
| Eat fried foods such as fried chicken, fried fish, French fries, fried plantains, tostones or fried yucca? | |||
| Eat regular potato chips, nacho chips, corn chips, crackers, regular popcorn, nuts instead of pretzels, low fat chips or low fat crackers, or air-popped popcorn? | |||
| Add butter, margarine, or oil to bread, potatoes, rice or vegetables at the table? | |||
| Eat sweets like cake, cookies, pastries, donuts, muffins, chocolate and candies more than 2 times a day? | |||
| Drink 16 ounces or more of non-diet soda, fruit drunk/punch or Kool-Aid a day? |
Footnotes
Author contributions: Devin Mann and Jenny Lin were responsible for project conception, data analyses, and manuscript drafting. Jennifer Kolb and Nicole Kitos analyzed data and drafted the manuscript. Ambili Ramachandran edited manuscript drafts.
References
- 1.Centers for Disease Control and Prevention. [Accessed Retrieved 10/25/2011];National Diabetes Surveillance System. Available online at: http://apps.nccd.cdc.gov/DDTSTRS/default.aspx.
- 2.Permutt MA, Wasson J, Cox N. Genetic epidemiology of diabetes. The Journal of clinical investigation. 2005 Jun;115(6):1431–1439. doi: 10.1172/JCI24758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes A. Economic costs of diabetes in the U.S. in 2012. Diabetes care. 2013 Apr;36(4):1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes care. 1999 Feb;22(2):233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 5.Meigs JB, Nathan DM, D’Agostino RB, Sr, Wilson PW Framingham Offspring S. Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes care. 2002 Oct;25(10):1845–1850. doi: 10.2337/diacare.25.10.1845. [DOI] [PubMed] [Google Scholar]
- 6.Smith NL, Barzilay JI, Shaffer D, et al. Fasting and 2-hour postchallenge serum glucose measures and risk of incident cardiovascular events in the elderly: the Cardiovascular Health Study. Archives of internal medicine. 2002 Jan 28;162(2):209–216. doi: 10.1001/archinte.162.2.209. [DOI] [PubMed] [Google Scholar]
- 7.Erdogan D, Yucel H, Uysal BA, et al. Effects of prediabetes and diabetes on left ventricular and coronary microvascular functions. Metabolism. 2013 Aug;62(8):1123–1130. doi: 10.1016/j.metabol.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Plantinga LC, Crews DC, Coresh J, et al. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clinical journal of the American Society of Nephrology : CJASN. 2010 Apr;5(4):673–682. doi: 10.2215/CJN.07891109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathan DM. The prevalence of retinopathy in impaired glucose tolerace and recent onset diabetes in the Diabetes Prevention Program. Diabetic medicine : a journal of the British Diabetic Association. 2007;24:137–144. doi: 10.1111/j.1464-5491.2007.02043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith AG, Singleton JR. Impaired glucose tolerance and neuropathy. The neurologist. 2008 Jan;14(1):23–29. doi: 10.1097/NRL.0b013e31815a3956. [DOI] [PubMed] [Google Scholar]
- 11.Aroda VR, Ratner R. Approach to the patient with prediabetes. The Journal of clinical endocrinology and metabolism. 2008 Sep;93(9):3259–3265. doi: 10.1210/jc.2008-1091. [DOI] [PubMed] [Google Scholar]
- 12.Fonseca V. Identification and treatment of prediabetes to prevent progression to type 2 diabetes. Clinical cornerstone. 2007;8:19–20. doi: 10.1016/s1098-3597(09)60004-1. [DOI] [PubMed] [Google Scholar]
- 13.Baker MK, Simpson K, Lloyd B, Bauman AE, Singh MA. Behavioral strategies in diabetes prevention programs: a systematic review of randomized controlled trials. Diabetes research and clinical practice. 2011 Jan;91(1):1–12. doi: 10.1016/j.diabres.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 14.Narayan KM, Williamson DF. Prevention of type 2 diabetes: risk status, clinic, and community. Journal of general internal medicine. 2010 Feb;25(2):154–157. doi: 10.1007/s11606-009-1148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England journal of medicine. 2002 Feb 7;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diabetes Prevention Program Research G. Knowler WC, Fowler SE, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009 Nov 14;374(9702):1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes care. 1997 Apr;20(4):537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 18.Norris SL, Zhang X, Avenell A, Gregg E, Schmid CH, Lau J. Long-term non-pharmacological weight loss interventions for adults with prediabetes. Cochrane database of systematic reviews. 2005;(2):CD005270. doi: 10.1002/14651858.CD005270. [DOI] [PubMed] [Google Scholar]
- 19.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. The New England journal of medicine. 2001 May 3;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 20.Onwudiwe NC, Mullins CD, Winston RA, et al. Barriers to self-management of diabetes: a qualitative study among low-income minority diabetics. Ethnicity & disease. 2011 Winter;21(1):27–32. [PubMed] [Google Scholar]
- 21.Snoek FJ. Breaking the barriers to optimal glycaemic control--what physicians need to know from patients’ perspectives. International journal of clinical practice. Supplement. 2002 Jul;(129):80–84. [PubMed] [Google Scholar]
- 22.Rosal MC, Benjamin EM, Pekow PS, Lemon SC, von Goeler D. Opportunities and challenges for diabetes prevention at two community health centers. Diabetes care. 2008 Feb;31(2):247–254. doi: 10.2337/dc07-0746. [DOI] [PubMed] [Google Scholar]
- 23.Vermunt PW, Milder IE, Wielaard F, et al. Implementation of a lifestyle intervention for type 2 diabetes prevention in Dutch primary care: opportunities for intervention delivery. BMC family practice. 2012;13:79. doi: 10.1186/1471-2296-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penn L, Moffatt SM, White M. Participants’ perspective on maintaining behaviour change: a qualitative study within the European Diabetes Prevention Study. BMC public health. 2008;8:235. doi: 10.1186/1471-2458-8-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mann DM, Lin JJ. Increasing efficacy of primary care-based counseling for diabetes prevention: rationale and design of the ADAPT (Avoiding Diabetes Thru Action Plan Targeting) trial. Implement Sci. 2012;7:6. doi: 10.1186/1748-5908-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Challenges in Clinical Research. 2010 http://www.ncbi.nlm.nih.gov/books/NBK50888.
- 27.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. American journal of health promotion : AJHP. 1997 Sep-Oct;12(1):38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- 28.Weymiller AJ, Montori VM, Jones LA, et al. Helping patients with type 2 diabetes mellitus make treatment decisions: statin choice randomized trial. Archives of internal medicine. 2007 May 28;167(10):1076–1082. doi: 10.1001/archinte.167.10.1076. [DOI] [PubMed] [Google Scholar]
- 29.Walker EA, Caban A, Schechter CB, et al. Measuring comparative risk perceptions in an urban minority population: the risk perception survey for diabetes. The Diabetes educator. 2007 Jan-Feb;33(1):103–110. doi: 10.1177/0145721706298198. [DOI] [PubMed] [Google Scholar]
- 30.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of general internal medicine. 2001 Sep;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroenke K, Spitzer RL, Williams JB, Monahan PO, Lowe B. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Annals of internal medicine. 2007 Mar 6;146(5):317–325. doi: 10.7326/0003-4819-146-5-200703060-00004. [DOI] [PubMed] [Google Scholar]
- 32.Gans KM, Risica PM, Wylie-Rosett J, et al. Development and evaluation of the nutrition component of the Rapid Eating and Activity Assessment for Patients (REAP): a new tool for primary care providers. Journal of nutrition education and behavior. 2006 Sep-Oct;38(5):286–292. doi: 10.1016/j.jneb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Gans KM, Ross E, Barner CW, Wylie-Rosett J, McMurray J, Eaton C. REAP and WAVE: new tools to rapidly assess/discuss nutrition with patients. The Journal of nutrition. 2003 Feb;133(2):556S–562S. doi: 10.1093/jn/133.2.556S. [DOI] [PubMed] [Google Scholar]
- 34.Storti KL, Arena VC, Barmada MM, et al. Physical activity levels in American-Indian adults: the Strong Heart Family Study. American journal of preventive medicine. 2009 Dec;37(6):481–487. doi: 10.1016/j.amepre.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tudor-Locke C, Craig CL, Brown WJ, et al. How many steps/day are enough? For adults. The international journal of behavioral nutrition and physical activity. 2011;8:79. [Google Scholar]
- 36.Mann DM, Ponieman D, Leventhal H, Halm EA. Misconceptions about diabetes and its management among low-income minorities with diabetes. Diabetes care. 2009 Apr;32(4):591–593. doi: 10.2337/dc08-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim C, McEwen LN, Piette JD, Goewey J, Ferrara A, Walker EA. Risk perception for diabetes among women with histories of gestational diabetes mellitus. Diabetes care. 2007 Sep;30(9):2281–2286. doi: 10.2337/dc07-0618. [DOI] [PubMed] [Google Scholar]
- 38.Aljasem LI, Peyrot M, Wissow L, Rubin RR. The impact of barriers and self-efficacy on self-care behaviors in type 2 diabetes. The Diabetes educator. 2001 May-Jun;27(3):393–404. doi: 10.1177/014572170102700309. [DOI] [PubMed] [Google Scholar]
- 39.Barnes L, Moss-Morris R, Kaufusi M. Illness beliefs and adherence in diabetes mellitus: a comparison between Tongan and European patients. The New Zealand medical journal. 2004 Jan 30;117(1188):U743. [PubMed] [Google Scholar]
- 40.Graziani C, Rosenthal M, Diamond J. Diabetes education program use and patient-perceived barriers to attendance. Family medicine. 1999;31:358–363. [PubMed] [Google Scholar]
- 41.Satterfield DW, Lofton T, May JE, et al. Learning from listening: common concerns and perceptions about diabetes prevention among diverse American populations. Journal of public health management and practice : JPHMP. 2003 Nov;(Suppl):S56–63. [PubMed] [Google Scholar]
- 42.Thoolen B, de Ridder D, Bensing J, Gorter K, Rutten G. Beyond Good Intentions: the development and evaluation of a proactive self-management course for patients recently diagnosed with type 2 diabetes. Health education research. 2008 Feb;23(1):53–61. doi: 10.1093/her/cyl160. [DOI] [PubMed] [Google Scholar]
- 43.Bodenheimer T, Handley MA. Goal-setting for behavior change in primary care: an exploration and status report. Patient Educ Couns. 2009 Aug;76(2):174–180. doi: 10.1016/j.pec.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns. 2002 Oct-Nov;48(2):177–187. doi: 10.1016/s0738-3991(02)00032-0. [DOI] [PubMed] [Google Scholar]
- 45.Langford AT, Sawyer DR, Gioimo S, Brownson CA, O’Toole ML. Patient-centered goal setting as a tool to improve diabetes self-management. The Diabetes educator. 2007 Jun;33(Suppl 6):139S–144S. doi: 10.1177/0145721707304475. [DOI] [PubMed] [Google Scholar]
- 46.Ammerman AS, Lindquist CH, Lohr KN, Hersey J. The efficacy of behavioral interventions to modify dietary fat and fruit and vegetable intake: a review of the evidence. Prev Med. 2002 Jul;35(1):25–41. doi: 10.1006/pmed.2002.1028. [DOI] [PubMed] [Google Scholar]
- 47.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health affairs. 2001 Nov-Dec;20(6):64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 48.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. The Milbank quarterly. 1996;74(4):511–544. [PubMed] [Google Scholar]
- 49.MacGregor K, Handley M, Wong S, et al. Behavior-change action plans in primary care: a feasibility study of clinicians. Journal of the American Board of Family Medicine : JABFM. 2006 May-Jun;19(3):215–223. doi: 10.3122/jabfm.19.3.215. [DOI] [PubMed] [Google Scholar]
- 50.Chunchu K, Mauksch L, Charles C, Ross V, Pauwels J. A patient centered care plan in the EHR: improving collaboration and engagement. Families, systems & health : the journal of collaborative family healthcare. 2012 Sep;30(3):199–209. doi: 10.1037/a0029100. [DOI] [PubMed] [Google Scholar]
- 51.Dickman K, Pintz C, Gold K, Kivlahan C. Behavior changes in patients with diabetes and hypertension after experiencing shared medical appointments. Journal of the American Academy of Nurse Practitioners. 2012 Jan;24(1):43–51. doi: 10.1111/j.1745-7599.2011.00660.x. [DOI] [PubMed] [Google Scholar]
- 52.Fontaine KR, Haaz S, Bartlett SJ. Are overweight and obese adults with arthritis being advised to lose weight? Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2007 Feb;13(1):12–15. doi: 10.1097/01.rhu.0000256168.74277.15. [DOI] [PubMed] [Google Scholar]
- 53.Galuska DA, Will JC, Serdula MK, Ford ES. Are health care professionals advising obese patients to lose weight? JAMA : the journal of the American Medical Association. 1999 Oct 27;282(16):1576–1578. doi: 10.1001/jama.282.16.1576. [DOI] [PubMed] [Google Scholar]
- 54.Whitlock EP, Orleans CT, Pender N, Allan J. Evaluating primary care behavioral counseling interventions: an evidence-based approach. American journal of preventive medicine. 2002 May;22(4):267–284. doi: 10.1016/s0749-3797(02)00415-4. [DOI] [PubMed] [Google Scholar]
- 55.Kripalani S, Osborn CY, Vaccarino V, Jacobson TA. Development and evaluation of a medication counseling workshop for physicians: can we improve on ‘take two pills and call me in the morning’? Medical education online. 2011:16. doi: 10.3402/meo.v16i0.7133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keller S, Donner-Banzhoff N, Kaluza G, Baum E, Basler HD. Improving physician-delivered counseling in a primary care setting: lessons from a failed attempt. Education for health. 2000;13(3):387–397. doi: 10.1080/135762800750059507. [DOI] [PubMed] [Google Scholar]
- 57.Geiss LS, James C, Gregg EW, Albright A, Williamson DF, Cowie CC. Diabetes risk reduction behaviors among U.S. adults with prediabetes. American journal of preventive medicine. 2010 Apr;38(4):403–409. doi: 10.1016/j.amepre.2009.12.029. [DOI] [PubMed] [Google Scholar]