Abstract
The dendritic cells (DCs) present in lymphoid and non-lymphoid organs are generated from progenitors with myeloid-restricted potential. However, in the thymus a major subset of DCs expressing CD8α and langerin (CD207) appears to stand apart from all other DCs in that it is thought to derive from progenitors with lymphoid potential. Using mice expressing a fluorescent reporter and a diphtheria toxin receptor under the control of the cd207 gene, we demonstrated that CD207+CD8α+ thymic DCs do not share a common origin with T cells but originate from intrathymic precursors that express markers that are normally present on all (CD11c+ and MHCII molecules) or on some (CD207, CD135, CD8α, CX3CR1) DC subsets. Those intrathymic myeloid-type precursors correspond to CD44+CD25− double-negative 1c (DN1c) cells and are continuously renewed from bone marrow-derived canonical DC precursors. In conclusion, our results demonstrate that the earliest intrathymic precursors of CD8α+ thymic DCs correspond to myeloid-type DN1c cells and support the view that under physiological conditions myeloid-restricted progenitors generate the whole constellation of DCs present in the body including the thymus.
Keywords: CD207, Dendritic cells, Development, Precursors, Thymus
Introduction
T cells develop in the thymus through discrete stages defined on the basis of the expression of CD4 and CD8 molecules. Immature double-negative (DN) CD4−CD8− T cells give rise to double-positive (DP) CD4+CD8+ cells that reside in the cortical region of the thymus. DP cells expressing T-cell antigen receptor (TCR) capable of interacting with self-peptides bound to major histocompatibility complex (MHC) molecules mature into single-positive (SP) CD4+CD8− or CD4−CD8+ cells and migrate to the medullary region of the thymus [1]. Following this transition known as positive selection, SP cells expressing TCR with a high affinity for peptide-MHC (pMHC) complexes present on medullary cells are deleted, a process referred to as negative selection. The pool of thymic self-peptides available for TCR selection derives from proteins expressed by thymic epithelial cells (TECs) and by thymic DCs (tDCs). The TECs found in the cortex (cTECs) are primarily devoted to positive selection [2]. In contrast, the medulla is the major site of negative selection due to the ability of medullary TECs (mTECs) to ectopically express self-proteins that are normally found outside the thymus [3]. mTECs are short-lived and upon death can transfer their protein content to the dense network of tDCs present in the medulla [4–6]. The respective contribution of mTECs and tDCs in central tolerance induction and in the shaping of the T regulatory cell lineage remains to be established [7].
Three subsets of CD11c+ DCs coexist in mouse thymus [8]. One subset expresses CD45R and corresponds to plasmacytoid DCs (pDCs), whereas the two other tDC subsets are CD45R− and can be distinguished based on the differential expression of CD8α and CD172α (Sirpα). The CD8αlowCD172α+ tDCs arise from quasi-differentiated blood precursors that continuously enter the thymus [9, 10]. In contrast, the CD8αhighCD172α− tDCs develop intra-thymically and stand apart from all other DCs in that they are reported to originate from early T-cell progenitors (ETPs) [10–12]. Based on the expression of CD25 and CD44, DN cells can be organized according to the following developmental series: DN1 (CD44+CD25−)→DN2 (CD44+CD25+)→DN3 (CD44−CD25+)→ DN4 (CD44−CD25−) [13]. DN1 cells can be subdivided further into DN1a, b, c, d and e subsets based on the expression of CD24 and of the c-Kit tyrosine kinase receptor (also called CD117) [13–15]. DN1a (CD24−CD117hi) cells constitute the precursors of DN1b (CD24intCD117hi) cells that give rise to the T-cell lineage. DN1a plus DN1b thymocytes are termed ETPs [16, 17], and it is these ETPs that are thought to generate CD8αhighCD172α− tDCs in addition to T cells [10–12]. In contrast, the CD8αlowCD172α+ tDCs derive from bone marrow (BM)-derived DC progenitors. Such BM -derived DC progenitors are composed of the common macrophage-DC progenitors (MDP), the common DC precursors (CDP) and of pre-DCs that constitute an intermediate stage between CDPs and classical DCs [18, 19]. Therefore, CD8αhighCD172α− tDCs would constitute an exception among a universe of DCs that originate from myeloid-restricted precursors. Contradictory data have, however, suggested that CD8αhighCD172α− tDCs also derive from myeloid precursors [20], or from precursors that are unrelated to the T-cell lineage and that remain to be characterized [21].
To further explore the origin of CD8αhighCD172α− tDCs and characterize their earliest intrathymic precursors, we took advantage of knockin mice in which an enhanced green fluorescent protein (Langerin-EGFP mice) or a human diphtheria receptor (DTR) fused to an EGFP (Langerin-DTREGFP mice) was placed under the control of the gene coding for langerin (CD207) [22]. Langerin is a C-type lectin the expression of which was originally described on epidermal Langerhans cells (LC) and later identified on additional DC subsets [23]. Using the highly sensitive Langerin-EGFP reporter mice, we characterized the abundant CD207(EGFP) + DC population that is found in the thymus and showed that it corresponds entirely to CD8αhighCD172α− tDCs. Moreover, since Langerin-DTREGFP mice are particularly suitable to analyze the precursor-product relationship that exists between CD207+ DCs [24, 25], we used them to demonstrate that the earliest intrathymic precursors of CD207+ tDCs are not contained among ETPs but correspond to DN1c (CD24+CD117int) cells. DN1c cells have no potential to generate T cells and their developmental potential has remained unclear [14]. Consistent with the conclusion that the DN1c cells are the source of the CD207+CD8αhigh tDCs and are developmentally separated from the T-cell lineage, we showed that a mutation in the interferon-regulatory factor 8 (IRF-8) gene prevented the development of both DN1c cells and CD207+ CD8αhigh tDCs without affecting the ETPs and the T-cell lineage. Finally, to corroborate the myeloid origin of the CD207+CD8αhigh tDCs, we also showed that they can be generated via adoptive transfer of MDPs, CDPs or pre-DCs that represent the different stages of the developmental series leading to canonical DCs.
Results
CD207+ tDCs correspond to CD8αhighCD172α− tDCs
To characterize the phenotype of the CD207+ DCs that are found in adult mouse thymus [22], thymi from Langerin-EGFP mice were digested with collagenase and light-density cells were prepared using Optiprep gradient. After excluding CD11cintCD11bhigh eosinophils [26], CD11cintCD45R+ pDCs [27] and CD11cintNK1.1+ NK cells (Fig. 1), the remaining CD11c+CD45R–NK1.1− tDCs were analyzed for the expression of CD207(EGFP) and CD172α permitting us to distinguish CD207+CD172α− and CD207−CD172α+ subsets that represented 49.6±5.2 and 34.9 ± 4.6% of the CD45R− tDCs, respectively (Fig. 1). CD207+CD172α− were CD8αlow to high and CD207−CD172α+ tDCs were CD8α−to low (Fig. 1). Therefore, in contrast to CD8α that has a continuous density distribution on CD45R− tDCs, the bimodal distribution of CD207(EGFP) permitted unambiguous definition of CD207−CD172α+ and CD207+CD172α− tDC subsets. CD207+CD172α− tDCs were predominantly found in the medulla (data not shown) and corresponded to the previously described CD8αhighCD172α− tDCs [9] and are here referred to as CD207+CD8αhigh tDCs.
Figure 1.
CD207+ tDCs correspond to CD8αhighCD172α−tDCs. Single-cell suspensions were prepared from thymi digested with collagenase-DNase I, and light-density cells were separated by centrifugation over an Optiprep gradient. After excluding eosinophils (CD11cintCD11bhigh), pDCs (CD11cintCD45R+) and NK cells (CD11cintNK1.1+), the remaining CD11cinttohiCD45R−NK1.1− DCs were analyzed for the expression of CD207(EGFP) versus CD172α, CD8α versus CD172α and CD24 versus CD172α. Data are representative of at least ten independent experiments.
DN1c cells comprise CD207+ myeloid-type cells
To determine whether precursors of the CD207+CD8αhigh tDCs can be detected among the DN1a–b ETPs as previously suggested [10–12], we took advantage of the high sensitivity afforded by Langerin-EGFP reporter mice over staining with anti-CD207 antibodies. For the sake of consistency, thymi to be used for such analysis were digested with collagenase and subjected to isopycnic centrifugation on Optiprep solution as described for the isolation of light-density tDCs. The pellet of heavy-density cells that formed after centrifugation contained the whole constellation of thymic T cells including ETPs (see below) and, based on work performed on splenic DCs [28], should entail the earliest stages of CD207+ CD8αhigh tDC development. To enrich for DN cells, heavy-density cell pellets prepared from Langerin-EGFP mice were depleted of CD4+ T cells by complement-mediated killing. As will be described below, the earliest intrathymic precursors of the CD207+CD8αhigh tDCs correspond to DN1c cells and an ‘intermediate’ developmental stage links DN1c cells to the terminally differentiated CD207+CD8αhigh tDCs that are confined to the light-density cell fraction. Cells belonging to such intermediate stage start expressing CD8α (Fig. S1). To preserve them, CD8 antibodies were thus omitted from the complement-mediated killing step aimed at enriching DN cells, and residual CD8+ SP T cells were excluded from further analysis by staining with anti-CD3 and anti-CD5 antibodies and gating out CD3+CD5+ cells. Accordingly, on an operational basis our DN1c cells comprise both bona fide CD4−CD8− DN1c cells [14] and CD4−CD8αlow to + intermediate cells that links the DN1c cells to the terminally differentiated CD207+CD8αhigh tDCs.
Analysis of the cells remaining in the heavy-density cell fraction at the end of the enrichment steps showed that their CD25-CD44 profile corresponded to the one expected for DN cells [13] and that the CD11c+ cells they contained were exclusively found in the DN1 subset (Fig. 2A). Consistent with previous work [14], the analysis of the DN1 cells for CD24 and CD117 expression showed that they comprised DN1c (CD24+CD117int), DN1d (CD24+CD117−) and DN1e (CD24−CD117−) subsets in addition to the DN1a–b subsets (Fig. 2B). CD11c+ DN1 cells segregated into DN1c (58.0 ± 5.6%), DN1d (9.64 ± 2.3%) and DN1e (25.1 ± 5.3%) subsets (Fig. 2B). Importantly, the DN1c subset was primarily made of CD11c+ cells and included almost all the CD207(EGFP)+ cells found in the heavy-density cell fraction (Fig. 2C and D).
Figure 2.
DN1c cells express markers that are normally present on DCs. Thymocytes of Langerin-EGFP or CX3CR1-EGFP mice were subjected to Optiprep gradient separation, and the heavy-density cell fraction was isolated and depleted of DP and of CD4+ SP T cells. Residual T cells, B cells and pDCs were excluded from further analysis using a cocktail of anti-CD3ε, anti-CD5 and anti-CD45R antibodies. (A) The remaining CD3ε−CD5−CD45R− DN cells were analyzed for the expression of CD44 versus CD25. CD11c+ cells constituted 0.5% of the DN cells and were exclusively found among the DN1 (CD44+CD25∼) subset. (B) DN1 cells were further subdivided into DN1a–b, DN1c, DN1d and DN1e subsets using CD24 and CD117 expression [41]. CD11c+ cells fell in the DN1c, DN1d and DN1e gates. (C) Expression of CD11c, CD207(EGFP), CD135, CD8α, MHCII and CX3CR1(EGFP) among the four DN1 subsets defined in (B). Percentages of cells positive for the specified marker are indicated. (D) Characterization of the DN1c subset of Langerin-EGFP mice. CD11c+ DN1 cells were separated into a CD207(EGFP)+ and a CD207(EGFP)− fraction and analyzed for the expression of CD24 versus CD117. Gated CD207+ and CD207− DN1c cells were analyzed for the expression of CD11c, CD207(EGFP), CD135, CD8α, MHCII and for their size (FSC-A). The small fraction of CD135+ DN1a–b cells corresponded to the earliest thymus seeding progenitors [16, 17]. The DN1d and DN1e subsets contained small percentages of CD11c+CD207− cells that might correspond to CD207−CD172α+ tDC precursors. Percentages of cells positive for the specified marker are indicated. The mean FSC-A value is indicated. Data are representative of three independent experiments.
Further characterization of the DN1c cells showed that they markedly differed from the DN1a–b, DN1d and DN1e subsets in that they expressed MHCII molecules and the Fms-like tyrosine kinase receptor 3 (Flt3, also called CD135; Fig. 2C), a receptor required for the development of most DC subsets and for their maintenance in lymphoid and non-lymphoid tissues [19, 29, 30]. DN1c cells were CCR7− (data not shown) and expressed low levels of the CX3CR1 chemokine receptor (Fig. 2C). Consistent with previous data [21], DN1c cells did not express detectable levels of the IL-7 receptor α chain (CD127) (data not shown). DN1c cells were composed of a minor CD207− fraction and of a major CD207+ fraction (Fig. 2C). When compared with CD11c+ CD207+ DN1c cells, CD11c+CD207−DN1c cells were smaller, expressed lower levels of MHCII molecules and only one-fourth expressed CD8α (Fig. 2D). CD11c+CD207+ DN1c cells were CX3CR1−, whereas CD11c+CD207−DN1c cells were CX3CR1low (see below). Therefore, DN1c cells expressed markers that are normally present on all (CD11c+ and MHCII molecules) or on some (CD207, CD135, CD8α, CX3CR1) DC subsets. In contrast, DN1a–b cells that have been thought to constitute the precursors of CD207+CD8αhigh tDCs were negative for all those markers (Fig. 2B and C).
DN1c cells are the earliest intrathymic CD207+ CD8αhigh tDC precursors
To determine the precursor-product relationship that exists between the distinct populations of CD11c+CD207+ cells that were found in the heavy- and light-density cell fraction, we followed their kinetics of restoration after injection of DT into Langerin-DTREGFP mice (Fig. 3). An almost complete depletion of the CD207+ DN1c cells was manifested 16 h after DT injection (Fig. 3). At 16 and 24 h post-DT injection, the few CD11c+ DN1c cells that were left had a CD207−CD8α−phenotype (Fig. 3C). CD11c+CD207+ DN1c cells reappeared around day 3 and their pool was gradually restored over the next 5 days. The first CD11c+CD207+ DN1c cells that reappeared were mainly CD8α− (Fig. 3C). Such protracted kinetics of CD8α expression as compared with that of CD207 suggests that the progeny of DN1C cells progresses through CD11c+CD207+CD8α− and CD11c+CD207+CD8α+ stages up to the CD11c+CD207+CD8αhigh tDCs found in the heavy-density cell fraction. Importantly, the analysis of CD11c–CD117 dot plots prior to and around the time of reappearance of the CD207+ DN1c cells showed that there was no streak of transitional cells connecting the CD11c−CD117hi DN1a–b cell cluster with the CD11c+CD117int DN1c cell cluster (Fig. 3A). Therefore, this last result suggests that the CD11c+ CD207−CD8α− DN1c cells constitute the direct source of the CD11c+CD207+CD8α− and CD11c+CD207+CD8α+ intermediate stages found in the light-density cell fraction (Fig. 3).
Figure 3.
Kinetics of reappearance of the CD11c+CD207+ cells found in the heavy-density cell fraction after DT treatment. (A) DN cells from the heavy-density cell fraction of Langerin-EGFP thymi were analyzed at various time points after the last DT injection for the expression of CD24 versus CD117 and CD11c versus CD117. Gates corresponding to the DN1a–b, DN1c, DN1d and DN1e subsets are as specified in Fig. 2B. (B) Gated CD11c+ DN1 cells were analyzed as in (A). (C) Gated CD11c+ DN1c cells were analyzed for CD207 versus CD8α expression at various time points after DT injection. Data are representative of three independent experiments and the percentages of cells found in each gate are indicated.
Further analysis of DT-treated Langerin-DTREGFP mice showed that the CD207+ tDCs found in the light-density cell fraction reappeared with delayed kinetics relative to that of the CD207+ cells found in the heavy-density cell fraction (Fig. 4A), an observation consistent with the view that the latter constituted the reservoir of the former. To further support that precursor–product relationship, Langerin-EGFP mice were continuously exposed to 5-bromo-2′ deoxyuridine (BrdU) for up to 12 days, and the kinetics of BrdU incorporation of the CD207+ tDCs found in the heavy and light fractions was determined (Fig. 4B). ‘Heavy’ and ‘light’ CD207+ tDCs had a high turnover resembling that of lymphoid-tissue resident DCs [19] and of migratory DCs prior to their migration to draining LNs [23]. Congruent with the view that the ‘light’ CD207+ tDCs were rapidly generated from the ‘heavy’ CD207+ tDCs, a one-day delay was observed in the ascending part of the BrdU labeling curve corresponding to the ‘light’ CD207+ tDCs as compared with that of the ‘heavy’ CD207+ tDCs (Fig. 4B). Altogether, these data demonstrate the existence of an intrathymic developmental series that starts with bona fide DN1c cells and that proceeds through CD207+CD8α− and CD207+CD8α+ intermediate stages to the CD207+CD8αhigh tDCs found in the light-density cell fraction.
Figure 4.
Kinetics of reconstitution of the CD207+ DCs found in the heavy-density and light-density cell fractions. (A) Kinetics of disappearance and reappearance of the CD207+ tDCs found in the heavy-density and light-density cell fractions of Langerin-DTREGFP thymi at various time points after the last DT injection. Absolute numbers of cells were determined using Flow count fluorospheres (Coulter) and normalized to the absolute numbers of cells present in thymi of Langerin-DTREGFP mice that received no DT. (B) BrdU was administered continuously for 12 days to a group of two to three B6 mice to compare the BrdU-labeling kinetics of the CD207+ tDCs found in the light-density and high-density fractions of an Optiprep gradient. Data in (A) are representative of at least three mice per time point and correspond to three independent experiments and data in (B) are representative of three to four independent experiments. Error bars correspond to the SEM.
IRF-8 is required for DN1c cell and CD207+CD8αhigh tDC development
BXH2 C57BL/6J C3H/Hej mice lack both CD8α+ lymphoid-tissue-resident DCs [31, 32] and CD8α+-type migratory DCs [30] due to a mutation in the irf8 gene [33]. Analysis of such mice should reveal whether the Irf8BXH2/BXH2 mutation also affects the development of the CD207+CD8αhigh tDCs and of the DN1c cells that we propose to be their immediate intrathymic precursors. Comparison of the DN cells found in the heavy-density cell fraction of wild-type and of Irf8BXH2/BXH2 thymi showed that DN1c cells were dramatically reduced in Irf8BXH2/BXH2 thymi (Fig. 5A). In contrast, the Irf8BXH2/BXH2 mutation had no demonstrable effect on the other DN1 subsets, including the DN1a–b ETPs. Further analysis of the CD45R− tDCs found in the light-density cell fraction of Irf8BXH2/BXH2 thymi showed that they lacked CD207+CD8αhigh tDCs (Fig. 5B). The Irf8BXH2/BXH2 mutation had, however, no effect on the development of the CD207− CD172α+ tDCs. Therefore, the analysis of Irf8BXH2/BXH2 mice demonstrates the developmental interdependence that exists between DN1c and CD207+CD8αhigh tDCs and conversely shows that DN1a–b ETPs and T cells develop independently of DN1c and CD207+CD8αhigh tDCs.
Figure 5.
IRF-8 is required for both DN1c and CD207+CD8αhigh tDC development. (A) DN cells from the heavy-density cell fraction of wild-type (WT) and irf8BHX2/BXH2 mice were analyzed for the expression of CD24 versus CD117. Gates corresponding to the DN1a–b, DN1c, DN1d and DN1e subsets are as specified in Fig. 2B. Numbers indicate the percentages of cells within the specified gates. (B) tDCs from the light-density cell fraction of wild-type (WT) and irf8BHX2/BXH2 mice were analyzed for the expression of CD24 versus CD172α. In (A), a cocktail of antibodies directed against CD3ε, CD25, CD45R, NK11 and CD11b permitted to exclude cells positive for those markers and to focus on ‘lineage negative’ (Lin) cells. Data in (A) and (B) are representative of four mice per genotype and correspond to two independent experiments. Numbers indicate the percentages of cells within the specified gates.
MDPs, CDPs and pre-DCs give rise to CD207+CD8αhigh thymic DCs
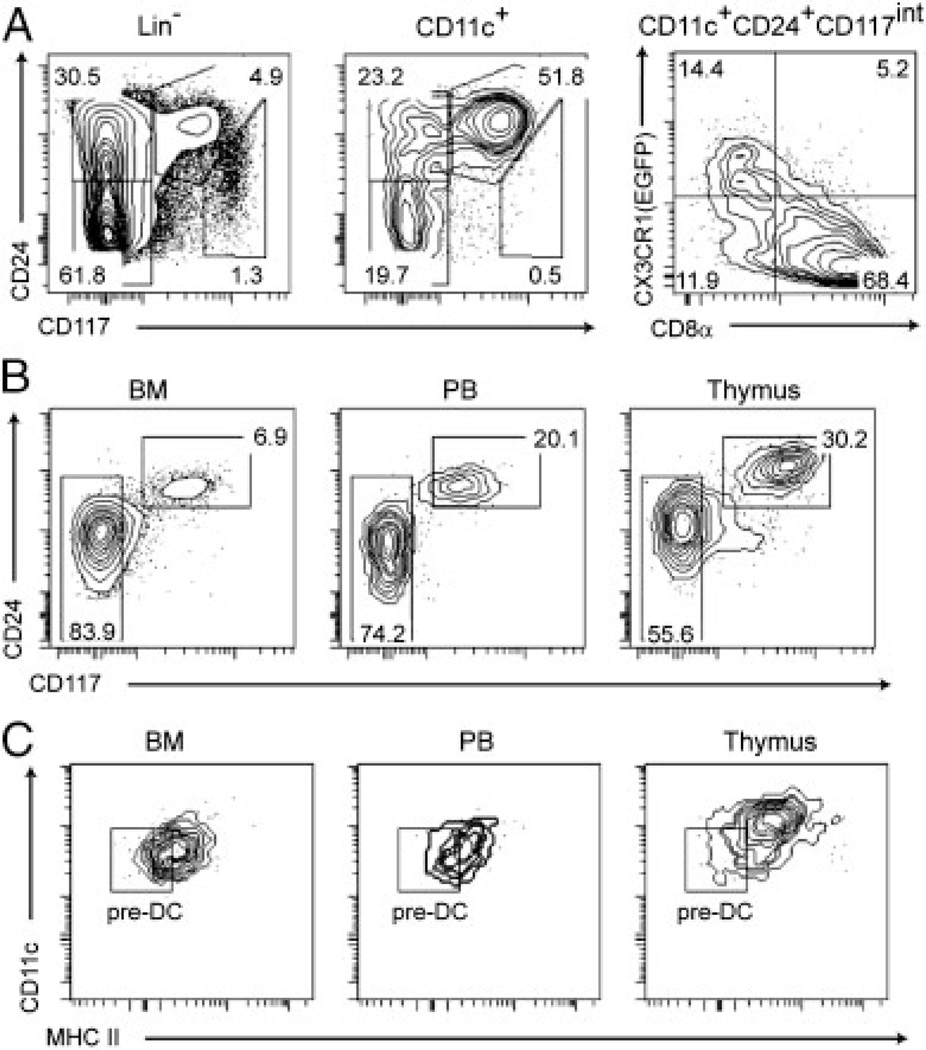
The CD8α− CCR7−CX3CR1lowMHCIIlow phenotype of the CD11c+ CD207− DN1c cells (Fig. 2) resembles that of the pre-DCs that are found in the BM and the blood [34–36] and suggests that they directly derive from them. Analysis of bona fide CD8α−DN1c cells of CX3CR1-EGFP mice showed that they express low levels of CX3CR1(EGFP) and lose it as they progress to the CD8αlow to + intermediate stage that link DN1c cells to the CD207+CD8αhigh tDCs (Fig. 6A). Interestingly, analysis of the CD11c+Lin−CX3-CR1(EGFP)low cells present in the BM and in peripheral blood of CX3CR1-EGFP mice for the expression of CD24 and CD117 showed the presence of cells resembling bona fide thymic DN1c (Fig. 6B). Thymic CD11c+CD24+CD117int DN1c cells expressed higher levels of MHCII molecules as compared with the pre-DCs found in the BM and the blood (Fig. 6C), suggesting that they are more mature than pre-DCs.
Figure 6.
Cells resembling thymic DN1c cells can be found in the bone marrow and the blood. (A) Lin− DN1 cells of CX3CR1-EGFP mice were prepared as described in Fig. 2. DN1 and CD11c+ DN1 cells were analyzed for CD24 and CD117 expression. The pattern of CX3CR1(EGFP) versus CD8α is shown for CD11c+ DN1c (CD24+CD117int) cells. (B) CD11c+ cells from bone marrow (BM) peripheral blood (PB) and thymus of CX3CR1-EGFP mice were enriched by MACS separation. After removing cells expressing CD25, CD45R, NK1.1, CD3e, CD19, Gr-1, CD115, CD172a, Sca-1 or CD8α, the remaining Lin−CX3CR1(EGFP)low cells were analyzed for the expression of CD24 versus CD117. (C) CD24+CD117int cells were prepared from bone marrow (BM) peripheral blood (PB) and thymus of CX3CR1-EGFP mice as defined in (B) and analyzed for CD11c versus MHCII molecules. Gate has been set up using the MHCII−CD24lowCD117− cells shown in panel (B). Results are representative of two independent experiments.
To determine whether canonical DC precursors can give rise to the CD207+CD8αhigh tDCs, MDPs, CDPs and pre-DCs were purified from BM and injected into non-irradiated congenic mice. One week after adoptive transfer, their capacity to differentiate into CD8αhigh and CD8αlow tDCs was measured. Donor-derived DCs were detectable in the thymus of mice injected with MDPs, CDPs and pre-DCs and segregated into both CD8αhigh and CD8αlow subsets (Fig. 7A). The smaller generative potential of pre-DCs as compared with that of MDPs and CDPs is likely due to their more limited division potential [30]. As expected on the basis of previous studies [36], MDPs, CDPs and pre-DCs were also capable of giving rise to the CD8αhigh and CD8αlow DCs found in the spleen (Fig. 7B). These data together with the fact that mice deprived of Irf8 (Fig. 5) or of Batf3 [37] transcription factor lacked both splenic and thymic CD8α+ DCs highlights their related differentiation program. Therefore, these results formally demonstrate the myeloid origin of the CD8αhigh tDCs and corroborate our data showing that the earliest signs of intrathymic CD207 expression occurred in DN1c cells and coincided with the expression of markers restricted to the myeloid lineage.
Figure 7.
Thymic and splenic CD8α+ DCs arise from MDPs, CDPs and pre-DCs. MDPs, CDPs and pre-DCs purified from the BM of CD45.2+ mice were adoptively transferred into unconditioned CD45.1+ congenic host. Thymus (A) and spleen (B) were analyzed a week after adoptive transfer. CD11c+ cells were enriched using magnetic beads coated with anti-CD11c antibody and CD11c+MHCII+ DCs were identified on a CD11c versus MHCII dot plot. CD11c+MHCII+ DCs were subsequently analyzed and separated into CD8αhigh and CD8αlow fractions. Percentages of host-derived (CD45.1+) and donor-derived (CD45.2+) cells are shown for the CD11c+CD8αhigh and CD11c+CD8αlow fractions. Results are representative of three independent experiments.
Discussion
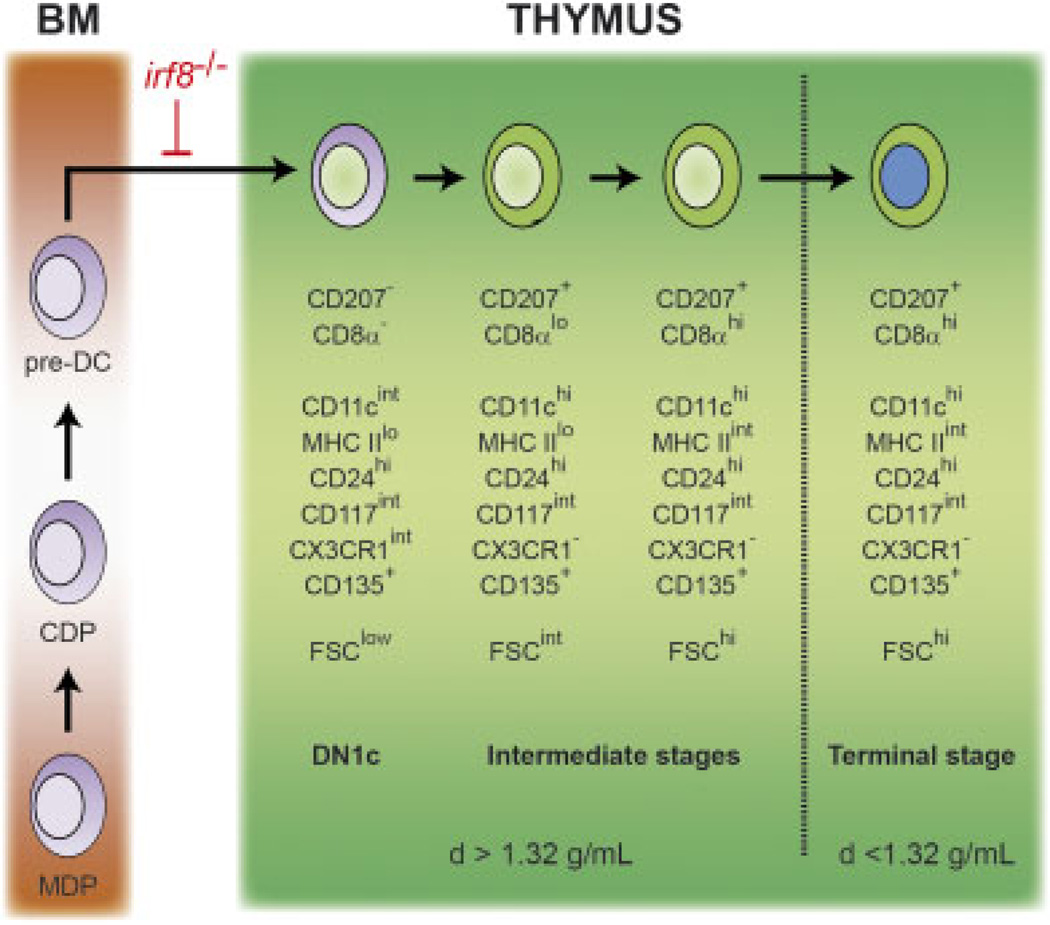
The CD8α+ DCs found in the thymus have been thought to have a lymphoid origin [10–12]. Considering that CD207 is not expressed outside of the DC lineage [22, 38] and that CD8αhigh tDCs correspond to CD207+ tDCs (this study), we used CD207(EGFP) expression to revisit the origin of the CD207+CD8αhigh tDCs. By visualizing the onset of CD207(EGFP) expression in the thymus of Langerin-EGFP mice, we showed that the intrathymic precursors of the CD207+CD8αhigh tDCs do not derive from ETPs but unexpectedly originate from DN1c cells. In contrast to ETPs, DN1c cells expressed markers that are normally present on all (CD11c+ and MHCII molecules) or on some (CD207, CD135, CD8α, CX3CR1) DC subsets. DN1c were found to have limited T lineage potential [14] and their developmental potential remained unclear prior to the present study. In addition, using Langerin-DTREGFP mice, we documented that the developmental series leading to CD207+ CD8αhigh tDCs starts with CD11c+CD207−CD8α−MHCIIlow DN1c cells proceeds through the immature CD207+CD8α+MHCIIint stage and ends up with the terminally differentiated CD207+ CD8α+MHCIIhi tDCs (Fig. 8).
Figure 8.
A model of CD207+CD8αhigh tDC development. Common macrophage-DC progenitors (MDP), common DC precursor (CDP) and classical DC-restricted precursors (pre-DCs) present in the bone marrow (BM) generate CD207+CD8αhigh tDCs. The earliest stage of the intrathymic developmental series corresponds to the DN1c (CD11c+ CD207−CD8α−MHCIIlow) cells previously identified by Porritt and colleagues [14]. The DN1c cells are found in the heavy-density cell fraction (d41.32g/mL) of an Optiprep gradient and progress through CD11c+CD207+CD8α− and CD11c+CD207+CD8α+ intermediate stages to the mature CD207+CD8αhigh stage that is found in the light-density cell fraction (d< 1.32 g/mL) of an Optiprep gradient. The developmental block observed in mice expressing a mutation in the interferon-regulatory factor 8 (irf8) gene is shown. Our findings do not formally rule out the possibility that, under some experimental conditions, early T-cell precursors can give rise to CD207+CD8αhigh tDCs. As recently stressed [42], it is important to distinguish physiological fate choices - as documented in the present study - from cell fates that are possible experimentally.
To corroborate the myeloid origin of CD207+CD8αhigh tDCs, we showed that MDPs, CDPs and pre-DCs isolated from the BM gave rise to CD207+CD8αhigh tDCs. In addition, we demonstrated that a mutation in the irf8 gene prevented the development of both DN1c cells and CD207+CD8αhigh tDCs without affecting the ETPs and the T-cell lineage. Altogether, our data demonstrate that the CD207+CD8αhigh tDCs have a myeloid origin and are thus congruent with fate mapping studies visualizing the history of Il7r expression in the thymus [21] and with other observations [39, 40] that indirectly suggested a lack of developmental relationship between tDCs and T cells. Consistent with our view that DN1c cells are of myeloid origin and developmentally insulated from the T-cell lineage, conditional ablation of the Notch ligand Delta-like 4 (Dll4) in thymic epithelium affected DN1a–b cells but spared DN1c cells [41]. The previous claim that CD8αhigh tDCs derive from an intrathymic T-DC bi-potent precursor might stem from the fact that CD11c+CD8α−MHCIIlow cells that constitute the precursors of the CD8αhigh tDCs were present among the sorted CD117+ DN cells that were used in previous studies [11]. In conclusion, using knockin mice in which an EGFP reporter or a human DTR was placed under the control of the cd207 gene, we have identified the earliest intrathymic precursors of CD207+ CD8αhigh tDCs and showed that they correspond to myeloid-type DN1c cells and not to ETPs. These results are consistent with recent fate mapping experiments that showed that tDCs have no lymphoid past [42]. Altogether, those results support the view that myeloid-restricted progenitors generate the whole constellation of DCs present in the body including the thymus.
Materials and methods
Mice
Mice were housed under specific pathogen-free (SPF) conditions and used between 6 and 8 weeks of age. Lang-EGFP and Lang-DTREGFP mice have been described [22] and were backcrossed onto B6 CD45.2 mice for at least ten generations. A single copy of Langerin-DTREGFP is sufficient to render CD207+ DCs susceptible to DT. Since the Langerin-EGFP allele provides higher levels of EGFP fluorescence than the Langerin-DTREGFP allele, Langerin-EGFP–Langerin-DTREGFP heterozygous mice were used to facilitate depletion monitoring. Hemizygous Cx3cr1gfp mice [43] were used to allow the expression of CX3CR1 from the wild-type allele. BXH2 C57BL/6J C3H/Hej mice that carry a spontaneous mutation (R924C) in the irf8 gene [33] were purchased from The Jackson Laboratories. All experiments were done in accordance with the French and European guidelines for animal care (DDSV des Bouches-du-Rhône).
In vivo depletion of CD207+ tDCs
For systemic in vivo depletion of CD207+ tDCs, mice were injected twice and 15h apart with 1µg diphtheria toxin (DT) (Calbiochem). Mice were analyzed for the reappearance of CD207+ tDCs at different time points after the last DT injection.
Cell preparation
DCs were isolated from lymphoid organs as previously described [44]. Briefly, organs were first cut into small pieces and incubated for 20 min in RPMI-1640 medium with 2% fetal calf serum containing 1 mg/mL of type II collagenase (Worthington Biochemical) and 0.15 mg/mL of DNAse I (Sigma-Aldrich). The resulting cell suspension was treated with 5 mM EDTA for 5 min at room temperature to disrupt DC–T-cell conjugates and light-density cells that include DCs were enriched by isopycnic centrifugation on an Optiprep solution (d = 1.32 g/mL, Abcys). The pellet of high-density cells that forms after Optiprep gradient centrifugation contains immature tDCs among other cells [28]. To enrich for immature tDCs, the pellet was incubated for 20 min at room temperature with rat IgM directed against mouse CD4 (clone RL172.4), followed by the addition of a 1:10 dilution of Low-Tox rabbit complement (Cedarlane Laboratories). After a 30-min incubation at 37°C with frequent gentle agitation, viable cells were recovered through centrifugation on a Ficoll gradient (d = 1.077 g/mL, Pharmacia). As previously demonstrated [15], the IgM anti-CD4 antibody used in our study does not deplete CD4low DN1 and DN2 cells.
MACS purification
Beads coated with anti-CD11c antibody (Milteny Biotec) were used to isolate CD11c–expressing cells from bone-marrow, blood and collagenase-DNAse I treated thymus. A sensitive mode of separation (Posselds) was used on the autoMACSPro separator to ensure a high yield of both CD11cint and CD11chi DCs. To purify CD11c–expressing cells from peripheral blood, blood samples were collected from adult mice by direct heart puncture. Erythrocytes were lysed with a NH4Cl solution prior to incubation with beads coated with anti-CD11c antibody.
Flow cytometry
Pacific blue-conjugated anti-CD8α (53–6.7, BD Biosciences), anti-CD11b (M1/70, BD Biosciences) and anti-CD24 (M1/69, eBioscience); fluorescein isothiocyanate-conjugated anti-CD172α (P84, BD Biosciences); peridinin–chlorophyll–protein complex indodicarbocyanine (Cy5.5)-conjugated anti-CD11b (M1/70, BD Biosciences); phycoerythrin-conjugated anti-CD4 (RM4–5, BD Biosciences), anti-CD117 (2B8, BD Biosciences) and anti-CD172α (P84, BD Biosciences); phycoerythrin–indodicarbocyanine (Cy5)-conjugated anti-CD24 (M1/69, eBioscience), anti-Sca-1 (D7, eBioscience); phycoerythrin–indodicarbocyanine (Cy5.5)-conjugated anti-CD11c (N418, eBioscience); phycoerythrin–indotricarbocyanine (Cy7)-conjugated anti-CD8α (53–6.7, BD Biosciences), anti-CD11c (N418, eBioscience), anti-CD44 (IM7, eBioscience); allophycocyanin-conjugated anti-CD117 (2B8, BD Biosciences); Alexa-700 anti-MHC II (M5/114, eBioscience); allophycocyanin-H7-conjugated anti-CD25 (PC64, BD Biosciences), anti-CD45R (RA3–6B2, BD Biosciences) and anti-CD161c (PK136, BD Bios-ciences); biotin-conjugated anti-CD135 (A2F10, eBioscience), anti-CD45.1 (A20, BD Biosciences) and anti-CD45.2 (104, eBioscience). Biotin-conjugated antibodies were detected using streptavidin conjugated with Quantum-Dot605 (Invitrogen). Unless stated, a lineage cocktail (‘Lin’) consisting of APC-Cy7-conjugated anti-CD25, anti-CD45R and anti-CD161c was systematically used in all staining mixes to exclude CD117+ DN2 cells, pDCs and NK cells, respectively. Detection of intracellular langerin (CD207) was performed on permeabilized cells using a BD Cytofix/Cytoperm kit (BD Biosciences). Langerin was detected with an Alexa647-conjugated anti-CD207 antibody (929F3, Dendritics). Before staining, cells were pre-incubated 10 min on ice with the 2.4G2 antibody to block Fc receptors. In all experiment, Sytox Blue (Invitrogen) was used to exclude dead cells from the analysis. Multiparameter FACS acquisition was performed on a LSRII SORP system (BD Biosciences). Analysis was performed using FACSDiva 6.3 (BD Biosciences) and FlowJo7 (Tree Star) software. Doublets were systematically excluded based on side scatter (SSC) and forward scatter (FSC) parameters.
BrdU incorporation
Mice were injected intraperitoneally (i.p.) with 1.5 mg BrdU (Sigma-Aldrich) to ensure its immediate availability, and their drinking water was supplemented for the indicated time with 0.8 mg/mL of BrdU and 2% glucose and changed daily. Twelve days after continuous BrdU labeling, some mice received BrdU-free drinking water for an additional 2–3 weeks. The amount of BrdU incorporation was determined using the BrdU labeling Flow kit (BD Biosciences).
Adoptive transfer of DC precursors
For adoptive transfer experiment, MDPs, CDPs and pre-DCs were isolated as described [30]. Purified precursor cells (from 1 × 105 to 4 × 105) were injected intravenously and their contribution to splenic and tDCs was analyzed a week after adoptive transfer.
Statistical analysis
All results are expressed as the mean ± SEM. Statistical tests were performed using a two-tailed Student’s t-test.
Supplementary Material
Acknowledgements
We thank Marie Malissen, Martin Guilliams, Samira Tamoutounour, Marc Dalod, Claude Grégoire, Sho Yamasaki, Philippe Naquet, Lionel Poulin, Anne-Marie Schmitt-Verhulst and Lee Leserman for discussions and Dan Littman for the CX3CR1-EGFP mice. We thank Marc Barad, Pierre Grenot and Atika Zouine for assistance with cell sorting. This work was supported by CNRS, INSERM, European Communities Framework Program 7 (MASTERSWITCH Integrating Project; HEALTH-F2-2008-223404), INCA (Melan-Imm project), ARC and by postdoctoral fellowship from CNRS (H. L.) and SYBILLA Collaborative Project (H. L.).
Abbreviations
- CDP
common DC precursors
- DN
double negative
- DP
CD4+/CD8+ double positive
- DT
diphtheria toxin
- ETPs
early T-cell progenitors
- IRF-8
interferon-regulatory factor 8
- MDP
macrophage-DC progenitors
- mTECs
medullary TECs
- pDCs
plasmacytoid DCs
- SP
CD4+ or CD8+ single positive
- tDC
hymic dendritic cells
- TECs
thymic epithelial cells
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Petrie HT, Zuniga-Pflucker JC. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu. Rev. Immunol. 2007;25:649–679. doi: 10.1146/annurev.immunol.23.021704.115715. [DOI] [PubMed] [Google Scholar]
- 2.McCaughtry TM, Baldwin TA, Wilken MS, Hogquist KA. Clonal deletion of thymocytes can occur in the cortex with no involvement of the medulla. J. Exp. Med. 2008;205:2575–2584. doi: 10.1084/jem.20080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat. Rev. Immunol. 2009;9:833–844. doi: 10.1038/nri2669. [DOI] [PubMed] [Google Scholar]
- 4.Gray D, Abramson J, Benoist C, Mathis D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J. Exp. Med. 2007;204:2521–2528. doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koble C, Kyewski B. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. J. Exp. Med. 2009;206:1505–1513. doi: 10.1084/jem.20082449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallegos AM, Bevan MJ. Central tolerance: good but imperfect. Immunol. Rev. 2006;209:290–296. doi: 10.1111/j.0105-2896.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- 7.Klein L, Hinterberger M, von Rohrscheidt J, Aichinger M. Autonomous versus dendritic cell-dependent contributions of medullary thymic epithelial cells to central tolerance. Trends Immunol. 2011;32:188–193. doi: 10.1016/j.it.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Wu L, Shortman K. Heterogeneity of thymic dendritic cells. Semin. Immunol. 2005;17:304–312. doi: 10.1016/j.smim.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Proietto AI, van Dommelen S, Zhou P, Rizzitelli A, D’Amico A, Steptoe RJ, Naik SH, et al. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc. Natl. Acad. Sci. USA. 2008;105:19869–19874. doi: 10.1073/pnas.0810268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Park J, Foss D, Goldschneider I. Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. J. Exp. Med. 2009;206:607–622. doi: 10.1084/jem.20082232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ardavin C, Wu L, Li CL, Shortman K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature. 1993;362:761–763. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- 12.Corcoran L, Ferrero I, Vremec D, Lucas K, Waithman J, O’Keeffe M, Wu L, et al. The lymphoid past of mouse plasmacytoid cells and thymic dendritic cells. J. Immunol. 2003;170:4926–4932. doi: 10.4049/jimmunol.170.10.4926. [DOI] [PubMed] [Google Scholar]
- 13.Ceredig R, Rolink T. A positive look at double-negative thymocytes. Nat. Rev. Immunol. 2002;2:888–897. doi: 10.1038/nri937. [DOI] [PubMed] [Google Scholar]
- 14.Porritt HE, Rumfelt LL, Tabrizifard S, Schmitt TM, Zuniga-Pflucker JC, Petrie HT. Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity. 2004;20:735–745. doi: 10.1016/j.immuni.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Laurent J, Bosco N, Marche PN, Ceredig R. New insights into the proliferation and differentiation of early mouse thymocytes. Int. Immunol. 2004;16:1069–1080. doi: 10.1093/intimm/dxh108. [DOI] [PubMed] [Google Scholar]
- 16.Sambandam A, Maillard I, Zediak VP, Xu L, Gerstein RM, Aster JC, Pear WS, Bhandoola A. Notch signaling controls the generation and differentiation of early T lineage progenitors. Nat. Immunol. 2005;6:663–670. doi: 10.1038/ni1216. [DOI] [PubMed] [Google Scholar]
- 17.Tan JB, Visan I, Yuan JS, Guidos CJ. Requirement for Notch1 signals at sequential early stages of intrathymic T cell development. Nat. Immunol. 2005;6:671–679. doi: 10.1038/ni1217. [DOI] [PubMed] [Google Scholar]
- 18.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat. Immunol. 2007;8:578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- 20.Manz MG, Traver D, Miyamoto T, Weissman IL, Akashi K. Dendritic cell potentials of early lymphoid and myeloid progenitors. Blood. 2001;97:3333–3341. doi: 10.1182/blood.v97.11.3333. [DOI] [PubMed] [Google Scholar]
- 21.Schlenner SM, Madan V, Busch K, Tietz A, Laufle C, Costa C, Blum C, et al. Fate mapping reveals separate origins of T cells and myeloid lineages in the thymus. Immunity. 2010;32:426–436. doi: 10.1016/j.immuni.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, et al. Dynamics and function of Langerhans cells in vivo dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Henri S, Poulin LF, Tamoutounour S, Ardouin L, Guilliams M, de Bovis B, Devilard E, et al. CD207+CD103+dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J. Exp. Med. 2010;207:189–206. doi: 10.1084/jem.20091964. S181-S186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+dendritic cells that develop and function independently of epidermal Langerhans cells. J. Exp. Med. 2007;204:3119–3131. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, Hogquist KA. Identification of a novel population of Langerin+dendritic cells. J. Exp. Med. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aguado E, Richelme S, Nunez-Cruz S, Miazek A, Mura A-M, Richelme M, Guo X-J, et al. Induction of T helper type 2 immunity by a point mutation in the LAT adaptor. Science. 2002;296:2036–2040. doi: 10.1126/science.1069057. [DOI] [PubMed] [Google Scholar]
- 27.Ferrero I, Held W, Wilson A, Tacchini-Cottier F, Radtke F, MacDonald HR. Mouse CD11c(+) B220(+) Gr1(+) plasmacytoid dendritic cells develop independently of the T-cell lineage. Blood. 2002;100:2852–2857. doi: 10.1182/blood-2002-01-0214. [DOI] [PubMed] [Google Scholar]
- 28.Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O’Keeffe M, Shortman K. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat. Immunol. 2006;7:663–671. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- 29.Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. Flt3 ligand regulates dendritic cell development from Flt3+lymphoid and myeloid-committed progenitors to Flt3+dendritic cells in vivo. J. Exp. Med. 2003;198:305–313. doi: 10.1084/jem.20030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, Price J, et al. The origin and development of nonlymphoid tissue CD103+DCs. J. Exp. Med. 2009;206:3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki S, Honma K, Matsuyama T, Suzuki K, Toriyama K, 1Akitoyo I, Yamamoto K, et al. Critical roles of interferon regulatory factor 4 in CD11bhighCD8alpha- dendritic cell development. Proc. Natl. Acad. Sci. USA. 2004;101:8981–8986. doi: 10.1073/pnas.0402139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamura T, Tailor P, Yamaoka K, Kong HJ, Tsujimura H, O’Shea JJ, Singh H, Ozato K. IFN regulatory factor-4 and −8 govern dendritic cell subset development and their functional diversity. J. Immunol. 2005;174:2573–2581. doi: 10.4049/jimmunol.174.5.2573. [DOI] [PubMed] [Google Scholar]
- 33.Turcotte K, Gauthier S, Tuite A, Mullick A, Malo D, Gros P. A mutation in the Icsbp1 gene causes susceptibility to infection and a chronic myeloid leukemia-like syndrome in BXH-2 mice. J. Exp. Med. 2005;201:881–890. doi: 10.1084/jem.20042170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, Carotta S, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat. Immunol. 2007;8:1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- 35.Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt31M–CSFR1 plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat. Immunol. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- 36.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, et al. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atibalentja DF, Murphy KM, Unanue ER. Functional redundancy between thymic CD8alpha+ and Sirpalpha+ conventional dendritic cells in presentation of blood-derived lysozyme by MHC class II proteins. J. Immunol. 2011;186:1421–1431. doi: 10.4049/jimmunol.1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valladeau J, Ravel O, Dezutter-Dambuyant C, Moore K, Kleijmeer M, Liu Y, Duvert-Frances V, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 39.Rodewald HR, Brocker T, Haller C. Developmental dissociation of thymic dendritic cell and thymocyte lineages revealed in growth factor receptor mutant mice. Proc. Natl. Acad. Sci. USA. 1999;96:15068–15073. doi: 10.1073/pnas.96.26.15068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radtke F, Ferrero I, Wilson A, Lees R, Aguet M, MacDonald HR. Notch1 deficiency dissociates the intrathymic development of dendritic cells and T cells. J. Exp. Med. 2000;191:1085–1094. doi: 10.1084/jem.191.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hozumi K, Mailhos C, Negishi N, Hirano K, Yahata T, Ando K, Zuklys S, et al. Delta-like 4 is indispensable in thymic environment specific for T cell development. J. Exp. Med. 2008;205:2507–2513. doi: 10.1084/jem.20080134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlenner SM, Rodewald HR. Early T cell development and the pitfalls of potential. Trends Immunol. 2010;31:303–310. doi: 10.1016/j.it.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J. Immunol. 2000;164:2978–2986. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.