Abstarct
Background/Objectives: Among adolescents, obesity may increase the risk for premature cardiovascular disease (CVD). Lifestyle interventions may prevent or delay the onset of CVD through improvements in vascular health. The purpose of this study was to examine the effects of a 12-week lifestyle intervention on markers of vascular health in obese Latino youth.
Subjects/Methods: Fifteen obese Latino adolescents [body mass index (BMI) percentile=96.3±1.1%, 15.0±1.0 year, 8 females and 7 males] participated in a 12-week lifestyle intervention consisting of nutrition education and physical activity. Markers of vascular health included oxidized low-density lipoprotein (oxLDL), soluble intercellular adhesion molecule-1 (sICAM-1), soluble vascular cell adhesion molecule-1 (sVCAM-1), and soluble endothelial leukocyte adhesion molecule-1 (sE-Selectin).
Results: Relative to baseline data, the intervention resulted in lower oxLDL (−21.8%, P=0.001) and sE-Selectin (−13.3%, P=0.008) concentrations; sICAM-1 and sVCAM-1 did not change significantly. When examining overall responsiveness to change for each marker, oxLDL was reduced in 93.3%, sE-Selectin was reduced in 78.6%, and sICAM-1 was reduced in 71.4% of participants, respectively, whereas sVCAM-1 was reduced in only 42.9% of participants following lifestyle. Using a composite change score (summed change in four markers) for each participant there was an improvement in at least three of four markers among 64% of participants; this was confirmed by principal component analysis.
Conclusions: Therefore, although improvements in the vascular health of obese youth were observed, the vascular response to lifestyle intervention may be heterogeneous. Further investigation into the mechanisms mediating the heterogeneity in vascular response to lifestyle intervention is warranted.
Introduction
Cardiovascular disease (CVD) has its antecedents early in life and is progressive across the life span.1 Obese youth often have elevated CVD risk factors,2 which could predict future CVD outcomes.3,4 Therefore, reductions in CVD markers early in life may prevent or delay CVD in adulthood. Lifestyle intervention programs for obese youth often target weight loss as the primary goal,5 with limited data available on their effects on vascular health markers related to atherogenesis.6
An emerging area of interest is the use of novel vascular health biomarkers that may improve prediction of CVD risk and potentially aid in determination of treatment success.6 These markers include oxidized low-density lipoprotein (oxLDL), soluble intercellular adhesion molecule-1 (sICAM-1), soluble vascular cell adhesion molecule-1 (sVCAM-1), and soluble endothelial leukocyte adhesion molecule-1 (sE-Selectin). These vascular health markers may be induced by oxidative stress and/or inflammation, are elevated in obese youth,7–9 and are associated with the CVD process in adults.10,11 However, few studies have examined whether these markers are modifiable through lifestyle intervention.12,13
In addition to modification, there are no data examining individualized responses of vascular health markers in response to lifestyle intervention. The importance of determining individual heterogeneity is supported by studies of exercise,14 diet,15 and pharmacology,16 with all showing marked individual variability in responses to cardiometabolic health markers despite receiving the same intervention. To determine the success or failure of an intervention at the individual level, it is necessary to examine responses at the individual level rather than relying exclusively on group mean changes.17 From a clinical perspective, data on individual responses may facilitate translation of research findings into clinical practice at a more expedited rate. Therefore, the purpose of this study was to examine the effects of a 12-week lifestyle intervention on markers of vascular health in obese Latino youth and explore whether changes in behavior (e.g., physical activity and/or dietary patterns) may explain individual response.
Materials and Methods
Fifteen obese Latino adolescents [8 female, 7 male; body mass index (BMI) percentile=96.3±1.1%; 15.0±1.0 years] completed a 12-week community-based lifestyle intervention. The intervention included weekly nutrition education classes (60 min) focused on healthful dietary strategies, such as reducing dietary fat intake as well as increasing consumption of fruits and vegetables. In addition to nutrition education, three, 60-min group exercise sessions per week were delivered by a certified personal trainer. Sessions included a combination of structured and unstructured physical activities with the goal of eliciting a heart rate of 150 beats/min (bpm) on average throughout each exercise session. The intervention has been described in detail elsewhere.18 Data collection occurred at baseline and postintervention. The Arizona State University Institutional Review Board approved the study, and all participants as well as a parent/guardian provided written informed consent prior to enrollment.
Anthropometrics
Following an overnight fast (>10 hr), height (to the nearest 0.1 cm) and weight (to the nearest 0.1 kg) were measured in light clothing without shoes using a wall-mounted stadiometer and a medical balance beam scale. BMI was calculated (kg/m2), and BMI percentile was determined using the Centers for Disease Control and Prevention (CDC) definitions for age and gender. Waist circumference (WC) (to the nearest 0.1 cm) was measured at the umbilicus at the end of normal exhalation.
Vascular health markers
A fasting blood draw was collected with serum separated by centrifugation at baseline and postintervention and stored at −80°C until analysis. All assays were run in duplicate with baseline and postintervention samples measured at the same time. oxLDL concentrations were measured via enzyme-linked immunosorbent assay (ELISA) (Mercodia, Winston-Salem, NC) with an intra-assay precision of 5.5%–7.5%. The concentrations of sICAM-1, sVCAM-1, and sE-Selectin were measured via ELISA (R&D Systems, Minneapolis, MN) with an intra-assay precision of 3.7%–5.0%, 2.3%–3.6%, and 5.1%–6.9% respectively. Data for sICAM-1, sVCAM-1, and sE-Selectin were not available for one participant.
Physical activity and dietary intake
Physical activity was assessed by self-report using the 3-Day Physical Activity Recall (3DPAR). The 3DPAR assesses 30-min time blocks of physical activities that are typically performed by adolescents and assigns a corresponding intensity of each time block over the previous 3 days. The 3DPAR has been validated against accelerometery in adolescents.19 Dietary intake was measured using the Brief Dietary Assessment Tool for Hispanics.20 This screening tool was specifically designed using data from Mexican Americans participating in National Health and Nutrition Examination Survey (NHANES III) to assess fruit, vegetable, and fat intake (servings/day) among Hispanics in community settings.
Data analysis
Group data are represented as mean±standard error of the mean (SEM) along with percent change. Baseline to postintervention changes were tested using a paired-samples t-test. To examine the effect of the intervention on the markers of vascular health on the group as a whole, a composite vascular health score for each participant was computed and tested using a one-sample t-test. The individual composite vascular health score was created by summation of percent change for each vascular marker. To reduce type 1 error, principal components analysis of percent change in the four markers of vascular health (oxLDL, sE-Selectin, sICAM-1, and sVCAM-1) was performed. Principal components extraction was used to derive the fewest number of relevant and distinct factors. Visual analysis of the scree plot, communality estimates for individual vascular health markers, and eigenvalues of ≥1.00 for derived factors were used as criteria to identify relevant factors. Factor scoring coefficients from each vascular health marker were used to generate component scores of the derived factors for each individual participant. Negative component scores are reflective of overall improvement in vascular health markers, with positive component scores indicating worsening of vascular health markers. Changes in anthropometric and behavioral factors were compared using a repeated-measures analysis of variance (ANOVA) for those who improved three or more out of four vascular markers (responders) and those whose profile worsened (nonresponders). All analysis was conducted using SPSS version 21.0 (IBM, Armonk, NY). Significance was set at P≤0.05.
Results
Baseline and postintervention values in anthropometrics and vascular health markers are presented in Table 1. Significant reductions were noted for BMI percentile (−1.3%; P=0.02); these changes were shown in the absence of weight-loss (−0.001%; P=0.44) and are largely attributable to increases in height (+0.24%; P=0.05). Following the intervention, participants had significantly lower concentrations of oxLDL (−21.8%; P=0.001) and sE-Selectin (−13.3%; P=0.008), with no significant changes in sICAM-1 (−2.9%; P=0.42) and sVCAM-1 (+3.8%; P=0.25).
Table 1.
Change in Anthropometrics and Vascular Health Markers Among Obese Latino Youth (n=15) Following a Lifestyle Intervention
| Baseline | Postintervention | % change | P value | |
|---|---|---|---|---|
| Height (cm) | 165.8±3.1 | 166.2±3.2 | 0.24 | 0.05 |
| Weight (kg) | 90.6±6.8 | 89.9±7.2 | −0.001 | 0.44 |
| BMI (kg/m2) | 32.5±1.6 | 32.0±1.7 | −1.5 | 0.06 |
| BMI percentile (%) | 96.3±1.1 | 95.0±1.5 | −1.3 | 0.02 |
| Oxidized LDL (U/L) | 58.7±2.7 | 45.9±4.5 | −21.8 | 0.001 |
| sE-Selectin (ng/mL)a | 65.5±4.5 | 56.8±4.9 | −13.3 | 0.008 |
| sICAM-1 (ng/mL)a | 223.6±14.2 | 217.1±17.0 | −2.9 | 0.42 |
| sVCAM-1 (ng/mL)a | 713.9±42.1 | 741.3±50.7 | 3.8 | 0.25 |
Data are represented as mean±standard error (SE).
Paired t-tests were used to determine differences between baseline and postintervention values.
Denotes n=14.
BMI, body mass index; LDL, low-density lipoprotein; sICAM, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular cell adhesion molecule-1.
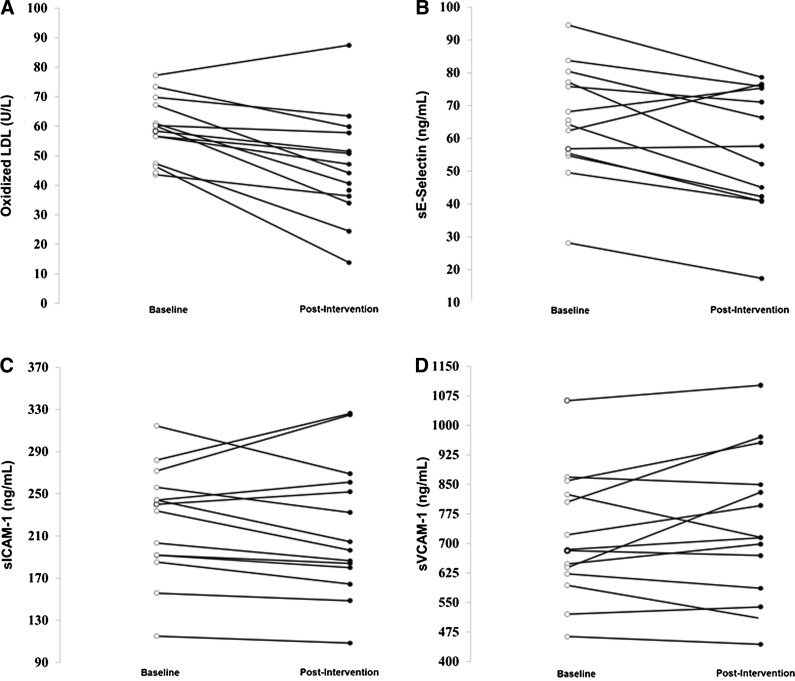
Figure 1 displays individual changes in vascular health markers from baseline to postintervention. Improvements were noted in 14 of 15 (93.3%) participants for oxLDL, 11 of 14 (78.6%) participants for sE-Selectin, 10 of 14 participants for sICAM-1 (71.4%), and six of 14 (42.9%) participants for sVCAM-1.
FIG. 1.
Individual changes in oxidized low-density lipoprotein (oxLDL) (A), soluble endothelial leukocyte adhesion molecule-1 (sE-Selectin) (B), soluble intercellular adhesion molecule-1 (sICAM-1) (C), and soluble vascular cell adhesion molecule-1 (sVCAM-1) (D) among obese Latino youth (n=15) following a lifestyle intervention.
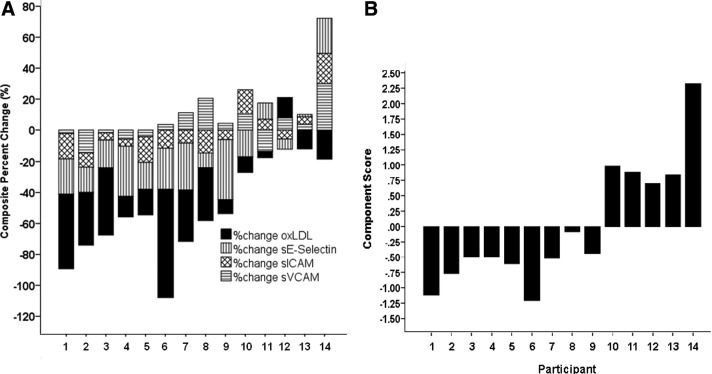
Figure 2A depicts a composite of percent change of the four vascular health markers for each individual participant. Five participants exhibited decreases in all four markers, four participants exhibited decreases in three markers, three participants exhibited decreases in two markers, and two participants exhibited decreases in only one marker. An improvement in the majority (≥3 of 4) of markers was noted in nine of 14 participants (64.3%). For each participant, percent change for each marker was summed as a composite. A one-sample t-test on composite percent change score showed a significant overall improvement in vascular health (i.e., decrease) following the lifestyle intervention [−38.0±11.8% change; 95% confidence interval (CI) (−63.5 to −12.6% change), P=0.007].
FIG. 2.
Descriptive representation (A) and principal component analysis (B) of the percent change in vascular markers of health by individual participants. oxLDL, oxidized low-density lipoprotein; sE-Selectin, soluble endothelial leukocyte adhesion molecule-1; sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular cell adhesion molecule-1.
Principal component analysis of the percent change in oxLDL, sE-Selectin, sICAM-1, and sVCAM-1 yielded one factor with an eigenvalue ≥1.0 (eigenvalue=2.1), which explained 52.4% of the variance in the four vascular health markers. All four vascular health markers loaded on this factor with factor coefficient scores of 0.61 for oxLDL, 0.82 for sE-Selectin, 0.88 for sICAM-1, and 0.52 for sVCAM-1 and communality estimates of 0.78, 0.68, 0.77, and 0.84, respectively. To determine the heterogeneity of individual responses between component scores we plotted the component score for each participant in Fig. 2B. A negative component score represents an overall reduction (i.e., improvement) in the vascular markers, while a positive value represents an overall increase (i.e., worsening) in the vascular markers. Using principal component analysis, we confirmed the findings from Fig. 2A, showing an improvement in markers of vascular health in nine of 14 participants. From this, we labeled the nine participants who improved the majority of their markers as “responders” and those whose vascular profile worsened as “nonresponders.”
Responders and nonresponders did not differ with regard to moderate-to-vigorous physical activity (MVPA), physical inactivity, screen time, dietary fat intake, or fruit/vegetable consumption, whereas a trend toward significance was noted for WC (Table 2). Vascular health responders exhibited a 6.1% reduction in WC whereas no changes were observed in nonresponders (P=0.07 for group·time interaction).
Table 2.
Anthropometric and Behavioral Differences Between Responders and Nonresponders to Vascular Markers of Health
| P value | ||||||
|---|---|---|---|---|---|---|
| Baseline | Postintervention | Time | Group | Time·group | ||
| Waist circumference (cm) | Responders (n=9) | 100.4±4.9 | 94.3±4.8 | 0.08 | 0.02 | 0.07 |
| Nonresponders (n=5) | 119.7±7.0 | 119.8±8.7 | ||||
| MVPA (30-min blocks/day) | Responders | 2.6±0.8 | 3±0.5 | 0.90 | 0.36 | 0.45 |
| Nonresponders | 2.1±0.9 | 2.7±1.2 | ||||
| Physical inactivity (30-min blocks/day) | Responders | 14.8±1.3 | 11.7±1.0 | <0.001 | 0.90 | 0.70 |
| Nonresponders | 15.4±1.1 | 10.9±0.9 | ||||
| Screen time (30-min blocks/day) | Responders | 4.6±1.3 | 3.1±1.0 | 0.001 | 0.98 | 0.62 |
| Nonresponders | 5.3±2.5 | 1.4±0.7 | ||||
| Dietary fat intake (servings/day) | Responders | 3.5±0.5 | 2.2±0.4 | 0.002 | 0.57 | 0.94 |
| Nonresponders | 3.1±0.4 | 1.8±0.3 | ||||
| Fruit and vegetable intake (servings/day) | Responders | 2.4±0.5 | 2.5±0.4 | 0.43 | 0.14 | 0.33 |
| Nonresponders | 4.3±1.3 | 3.1±0.9 | ||||
Data are presented as mean±standard error of the mean (SEM).
Differences between means were determined via a repeated measures analysis of variance (ANOVA).
MVPA, moderate-to-vigorous physical activity.
Discussion
The findings from the present study support that reductions in oxLDL and sE-Selectin may be achieved through lifestyle intervention without changes in body weight among obese Latino adolescents. A novel contribution of the current study is that additional and potentially important information may be obtained by examination of individual data. From a clinical perspective, individual responses are essential for determining the success or failure of treatment and exploring potential factors that influence individual variability may facilitate translation of research findings into clinical practice. Our data suggest that dietary and physical activity behaviors (Table 2) cannot, by themselves, explain the variability in response because responders and nonresponders did not differ on any physical activity or dietary measure. However, there was a trend toward WC being reduced in responders. These data suggest a potential mechanism for future research to explore as a contributor to reductions in markers of vascular health.
We observed significant improvements in oxLDL, which results from oxidative modification of either the phospholipids or apolipoprotein B-100 on the LDL particle. There is evidence to support the direct role of oxLDL in the atherosclerotic process.21 oxLDL in circulation, as measured in this study, is associated with the presence of CVD and risk of CVD events in adults10,22 along with CVD risk factors in Latino youth.9 Kelishadi et al. reported decreases in oxLDL following a 6-week diet and exercise intervention in obese adolescents.13 Contrary to our findings, changes in oxLDL in that study were observed in the background of significant weight loss (57.1±10.2 to 54.7±9.8 kg, P=0.02). The present study demonstrates that oxLDL can be reduced in the absence of weight loss and may be the most permeable vascular health marker to change, with improvements observed in 14 of 15 participants. Tjonna et al.23 did not observe changes in oxLDL after 3 months of aerobic interval training or in a multidisciplinary lifestyle approach (group meetings with physician, psychologist, nutritionist, etc.) in overweight and obese adolescents. Therefore, exercise alone may not be a sufficient stimulus to alter this marker. Our findings support that weight loss per se may not be necessary to improve oxLDL status and support the synergistic effect of diet and exercise for improvements in this vascular health marker. Furthermore, our data suggest that oxLDL may be a robust clinical biomarker of vascular health improvements following lifestyle because it was decreased ubiquitously.
In addition to the marked improvements in oxLDL, we observed significant reductions in sE-Selectin as a group and improvements in 11 out of 14 participants. sE-Selectin is an inflammatory-induced ligand on the endothelial cell surface that initiates leukocyte recruitment to sites of vascular inflammation and allows for monocytes to begin to “roll” on the endothelial cell surface, a process that is viewed as a critical early mechanism contributing to atherogenesis.24,25 sE-Selectin is elevated in obese youth,7 is increased in the presence of CVD,11 and prospectively predicts CVD events in adults.26 Roberts et al.12 reported significant reductions in sE-Selectin following an intensive 2-week diet and exercise intervention in overweight youth. In a 6-month lifestyle intervention consisting of once-weekly physical activity and family-focused behavioral and lifestyle modification sessions, Huang and colleagues27 reported significant reductions in sE-Selectin. However, the observed improvements in sE-Selectin were only noted in participants who exhibited decreases in BMI, whereas participants who remained BMI stable did not decrease sE-Selectin. Our findings confirm that reductions in sE-Selectin can be observed without weight loss in obese youth.
We also measured levels of sICAM-1 and sVCAM-1, which are part of the immunoglobin superfamily and lie on the endothelial cell surface. sICAM-1 and sVCAM-1 allow for firm adhesion of monocytes to the endothelial cell wall, making monocytes immobile and able to migrate across the endothelium into the subendothelial space, where they can become macrophages and contribute to atherogenesis.25 Contrary to what has been reported by others,12 we observed no significant group changes in sICAM-1. Roberts et al.12 reported significant reductions in sICAM-1 after 2 weeks of intensive lifestyle intervention in obese youth, but their intervention had a higher volume of exercise, a more robust dietary modification, and also resulted in weight loss. Despite the lack of overall change in sICAM-1, we observed a reduction in 10 of 14 participants, representing important individual responses that are not evident in the group data. Thus, the sICAM-1 results in the present study support the argument that relying solely on group changes may miss important individual changes that accompany intervention. It should be noted that our study was not the first to present nonsignificant improvements in sICAM-1 due to lifestyle intervention. In a study of overweight and obese adolescent girls, Nassis et al. found no change in sICAM-1 due to 12-weeks of aerobic exercise training.28 Unfortunately, Nassis et al. did not present individual data, so it is plausible that improvements were observed in the majority of participants despite not reaching significance as a whole. Nevertheless, it is possible that the robust nature of the intervention by Roberts and colleagues12 was sufficient to reduce sICAM-1 and that the less prescriptive dietary program of the current intervention was insufficient for reducing this marker of vascular inflammation.
In agreement with several other lifestyle interventions in youth, we noted no changes in sVCAM-1.28 This was the CVD risk marker most resistant to change, showing a reduction in only six of 14 participants. Changes in sVCAM-1 have been reported in studies of adults, but only in studies involving specific dietary modification (e.g., soy nuts and olive oil).29,30 Exercise has been shown to acutely increase expression of sVCAM-1.31 Thus, it is possible the lack of reduction in sVCAM-1 may be due to a carryover effect of the last bout of exercise performed 24–48 hr prior to posttesting.
While changes in physical inactivity, screen time, and dietary fat intake were observed, these changes did not differ between responders and nonresponders, suggesting that these behaviors do not explain the observed heterogeneity in vascular health markers. In addition, it could also be due to measurement error, because we used self-reported dietary and physical activity measures. A trend toward significance was noted for decreases in WC among those who improved their vascular health markers compared to those who did not. Previously, we have reported abdominal adiposity to be associated with oxLDL in Latino youth,9 suggesting a potential for reductions in WC to improve this marker. Further investigation is warranted to determine whether changes in regional fat distribution drive changes in vascular health markers and whether the changes observed are sustainable long term.
Strengths of the present investigation include the focus on a population of youth at high risk for CVD, inclusion of multiple vascular health biomarkers, and examining group as well as individual responses. Additionally, it is interesting to note that when we compared our baseline levels of sE-Selectin, sICAM-1, sVCAM-1, and oxLDL with an age- and gender-matched normal weight comparison group, we observed differences between sE-Selectin and oxLDL only. This suggests that these markers are not only permeable to change but also able to potentially differentiate risk as baseline from apparently healthy normal weight youth. However, this will require further investigation.
Limitations include the small sample size, lack of measurement of pubertal maturation, and the lack of a control group. Although we observed decreases in biomarkers that are predictive of CVD in adults and associated with the CVD process, it is unknown whether these reductions are clinically meaningful because normative data for these markers are currently unavailable. Further investigation is needed to determine the acute response to exercise for these markers in obese adolescents because there may be a potential “carryover” effect of previous exercise bouts. Additionally, comparative effectiveness trails could be used to determine if exercise or weight loss is responsible for the reduction in markers of vascular health. Additionally, whether the improvements observed in markers of vascular health are sustainable and lead to long-term improvements in cardiovascular health requires further investigation. It is also impossible for us to detect whether the response observed were influenced by adherence because the lifestyle intervention lacked a gold-standard measures of behavior to infer adherence. Nonetheless, we have shown that lifestyle intervention can improve biomarkers of vascular health among obese Latino youth and that individual variability in responses is not explained by physical activity and dietary factors that include MVPA, physical inactivity, screen time, dietary fat intake, or fruit and vegetable consumption.
Acknowledgments
We would like to thank all of the youth and families who participated in this study. The project was supported by Phoenix Children's Hospital and by the ASU Southwest Interdisciplinary Research Center, an Exploratory Center of Excellence for Health Disparities Research and Training through a grant from the National Institutes of Health, National Center on Minority Health and Health Disparities (P20MD002316). Data management support was provided by grant number UL1-RR-024150 from the Mayo Clinic to use Research Electronic Data Capture (REDCap).
Author Disclosure Statement
No competing financial interests exist.
J.R.R. was responsible for hypothesis development, data analysis, data interpretation, and manuscript preparation; S.V.L. was responsible for data interpretation and manuscript revisions; G.A.G. was responsible for data interpretation and manuscript revisions; M.P.B. was responsible for data analysis and manuscript revisions; G.Q.S. was responsible for study design, funding, supervision, hypothesis development, data interpretation, and manuscript revisions.
References
- 1.Berenson GS, Wattigney WA, Tracy RE, et al. , Atherosclerosis of the aorta and coronary arteries and cardiovascular risk factors in persons aged 6 to 30 years and studied at necropsy (the Bogalusa Heart Study). Am J Cardiol 1992;70:851–858 [DOI] [PubMed] [Google Scholar]
- 2.Freedman DS, Mei Z, Srinivasan SR, et al. . Cardiovascular risk factors and excess adiposity among overweight children and adolescents: The Bogalusa Heart Study. J Pediatr 2007;150:12–17 [DOI] [PubMed] [Google Scholar]
- 3.Franks PW, Hanson RL, Knowler WC, et al. . Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med 2010;362:485–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nadeau KJ, Maahs DM, Daniels SR, et al. . Childhood obesity and cardiovascular disease: Links and prevention strategies. Nat Rev Cardiol 2011;8:513–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynn MAT, McNeil DA, Maloff B, et al. , Reducing obesity and related chronic disease risk in children and youth: A synthesis of evidence with ‘best practice’ recommendations. Obes Rev 2006;7(Suppl 1):7–66 [DOI] [PubMed] [Google Scholar]
- 6.Balagopal P, de Ferranti SD, Cook S, et al. . Nontraditional risk factors and biomarkers for cardiovascular disease: Mechanistic, research, and clinical considerations for youth. Circulation 2011;123:2749–2769 [DOI] [PubMed] [Google Scholar]
- 7.Glowinska B, Urban M, Peczynska J, et al. . Soluble adhesion molecules (sICAM-1, sVCAM-1) and selectins (sE selectin, sP selectin, sL selectin) levels in children and adolescents with obesity, hypertension, and diabetes. Metabolism 2005;54:1020–1026 [DOI] [PubMed] [Google Scholar]
- 8.Kelly AS, Jacobs DR, Jr, Sinaiko AR, et al. . Relation of circulating oxidized LDL to obesity and insulin resistance in children. Pediatr Diabetes 2010;11:552–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryder JR, Vega-Lopez S, Djedjos CS, et al. . Abdominal adiposity, insulin resistance, and oxidized low-density lipoproteins in Latino adolescents. Diabetol Metab Syndr 2013;5:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holvoet P, Mertens A, Verhamme P, et al. . Circulating oxidized LDL is a useful marker for identifying patients with coronary artery disease. Arterioscler, Thromb Vasc Biol 2001;21:844–848 [DOI] [PubMed] [Google Scholar]
- 11.Hwang S-J, Ballantyne CM, Sharrett AR, et al. . Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cses: The Atherosclerosis Risk In Communities (ARIC) Study. Circulation 1997;96:4219–4225 [DOI] [PubMed] [Google Scholar]
- 12.Roberts CA, Barnard RJ, Effect of a short-term diet and exercise intervention in youth on atherosclerotic risk factors. Atherosclerosis 2007;191:98–106 [DOI] [PubMed] [Google Scholar]
- 13.Kelishadi R, Hashemi M, Mohammadifard N, et al. . Association of changes in oxidative and proinflammatory states with changes in vascular function after a lifestyle modification trial among obese children. Clin Chem 2008;54:147–153 [DOI] [PubMed] [Google Scholar]
- 14.Bouchard C, Rankinen T. Individual differences in response to regular physical activity. Med Sci Sports Exerc 2001;33(Suppl 6):S446–S451; discussion S452–S443 [DOI] [PubMed] [Google Scholar]
- 15.Masson LF, McNeill G, Avenell A. Genetic variation and the lipid response to dietary intervention: A systematic review. Am J Clin Nutr 2003;77:1098–1111 [DOI] [PubMed] [Google Scholar]
- 16.Kang ES, Park SY, Kim HJ, et al. . The influence of adiponectin gene polymorphism on the rosiglitazone response in patients with type 2 diabetes. Diabetes Care 2005;28:1139–1144 [DOI] [PubMed] [Google Scholar]
- 17.Petronis A. Epigenetics as a unifying principle in the aetiology of complex traits and diseases. Nature 2010;465:721–727 [DOI] [PubMed] [Google Scholar]
- 18.Shaibi GQ, Konopken Y, Hoppin E, et al. . Effects of a culturally grounded community-based diabetes prevention program for obese Latino adolescents. Diabetes Educ 2012;38:504–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMurray RG, Ring KB, Treuth MS, et al. . Comparison of two approaches to structured physical activity surveys for adolescents. Med Sci Sports Exerc 2004;36:2135–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wakimoto P, Block G, Mandel S, et al. . Development and reliability of brief dietary assessment tools for Hispanics. Prev Chronic Dis 2006;3:A95. [PMC free article] [PubMed] [Google Scholar]
- 21.Steinberg D, Witztum JL. Oxidized Low-density lipoprotein and atherosclerosis. Arterioscler Thromb Vasc Biol 2010;30:2311–2316 [DOI] [PubMed] [Google Scholar]
- 22.Holvoet P, Kritchevsky SB, Tracy RP, et al. . The metabolic syndrome, circulating oxidized LDL, and risk of myocardial infarction in well-functioning elderly people in the health, aging, and body composition cohort. Diabetes 2004;53:1068–1073 [DOI] [PubMed] [Google Scholar]
- 23.Tjonna AE, Stolen TO, Bye A, et al. , Aerobic interval training reduces cardiovascular risk factors more than a multitreatment approach in overweight adolescents. Clin Sci 2009;116:317–326 [DOI] [PubMed] [Google Scholar]
- 24.Tedder TF, Steeber DA, Chen A, et al. . The selectins: Cascular adhesion molecules. FASEB J 1995;9:866–873 [PubMed] [Google Scholar]
- 25.Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol 2007;27:2292–2301 [DOI] [PubMed] [Google Scholar]
- 26.Blankenberg S, Rupprecht HJ, Bickel C, et al. . Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation 2001;104:1336–1342 [DOI] [PubMed] [Google Scholar]
- 27.Huang F, del-Rio-Navarro BE, de Castro GT, et al. . Weight loss induced by 6-month lifestyle intervention improves early endothelial activation and fibrinolysis in obese adolescents. Child: Care, Health Dev 2011;37:377–384 [DOI] [PubMed] [Google Scholar]
- 28.Nassis GP, Papantakou K, Skenderi K, et al. . Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. Metabolism 2005;54:1472–1479 [DOI] [PubMed] [Google Scholar]
- 29.Nasca MM, Zhou J-R, Welty FK. Effect of soy nuts on adhesion molecules and markers of inflammation in hypertensive and normotensive postmenopausal women. Am J Cardiol 2008;102:84–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez-Martinez P, Lopez-Miranda J, Blanco-Colio L, et al. , The chronic intake of a Mediterranean diet enriched in virgin olive oil, decreases nuclear transcription factor κB activation in peripheral blood mononuclear cells from healthy men. Atherosclerosis 2007;194:e141–e146 [DOI] [PubMed] [Google Scholar]
- 31.Shephard RJ. Adhesion molecules, catecholamines and leucocyte redistribution during and following exercise. Sports Med. 2003;33:261–284 [DOI] [PubMed] [Google Scholar]