Abstract
Background: Mutations of v-raf murine sarcoma viral oncogene homolog B (BRAF) are commonly identified in papillary and anaplastic thyroid carcinoma and are associated with worse prognosis compared with the wild type. BRAF inhibition in papillary thyroid carcinoma cell lines and xenografts inhibits proliferation and decreases downstream phosphorylation. Our objectives were to analyze safety and efficacy of the selective BRAF inhibitor dabrafenib in patients with metastatic BRAF-mutant thyroid carcinoma.
Methods: We present the subset of patients with BRAF-mutant thyroid carcinoma enrolled in a larger phase 1 study, the main results of which are reported elsewhere.
Results: Fourteen patients with BRAFV600E-mutant thyroid carcinoma were enrolled, of whom 13 (93%) had received prior radioactive iodine. The median duration on treatment was 8.4 months, and seven (50%) patients received treatment for ≥10 months. The most common treatment-related adverse events were skin papillomas (n=8, 57%), hyperkeratosis (n=5, 36%), and alopecia (n=4, 29%), all of which were grade 1. Treatment-related adverse events grade ≥3 included grade 4 elevated lipase and grade 3 elevated amylase, fatigue, febrile neutropenia, and cutaneous squamous cell carcinoma (n=1 for each). Four (29%) partial responses were observed, and nine (64%) patients achieved at least 10% decrease. Only one responder progressed while on the study drug after a response duration of 9.3 months. The other three responders had not progressed, with response duration of 4.6+, 10.4+, and 21.4+ months. With seven (50%) patients showing no progression at the time of study completion, the median progression-free survival was 11.3 months.
Conclusions: Dabrafenib was well tolerated and resulted in durable responses in BRAF-mutant differentiated thyroid carcinoma patients.
Introduction
Effective standard therapies for patients with radioiodine-refractory thyroid carcinoma remain limited (1). Since the median progression free survival is approximately 1 year with sorafenib, new therapies are needed, especially as salvage therapy (2). In contrast to the 10-year survival rate of 92% in patients who achieve remission after treatment with radioactive iodine (131I), a 10-year survival rate of 29% is observed in patients who do not achieve remission and a 10-year survival rate of only 10% is seen in patients without any initial 131I uptake (1). The median overall survival of patients who develop distant metastases ranges from 3 to 6 years (3). Traditional cytotoxic chemotherapy regimens, such as doxorubicin monotherapy or doxorubicin in combination with cisplatin, have yielded low response rates of short duration and have been associated with significant toxicity (3).
The mitogen-activated protein kinase (MAPK) signaling cascade, which includes the kinases RAS, RAF, MEK, and ERK, regulates cell proliferation, differentiation, apoptosis, and cell survival (4). In 80% of patients with papillary thyroid carcinoma, activating mutations are identified in genes encoding signaling molecules within the MAPK pathway (3).
Constitutive activation of v-raf murine sarcoma viral oncogene homolog B (BRAF) by a somatic mutation results in oncogenic transformation of normal cells (5,6). Mutations in the RAF isoform BRAF typically entail substitution of valine with glutamic acid at amino acid residue 600 (V600E) and are seen in 36–86% of patients with papillary thyroid cancer and 20–25% of patients with anaplastic thyroid carcinoma (7–13). In these patients, the BRAFV600E mutation is associated with worse prognosis than wild type BRAF, including higher clinical stage, tumor size, extrathyroidal extension, lymph node metastasis, tumor recurrence, absence of tumor 131I avidity, and treatment failure of recurrent disease (8,14–17).
In cell lines of BRAF-mutant papillary thyroid cancer, selective targeting of BRAFV600E inhibited proliferation, decreased phosphorylation of ERK and MEK, induced a G(1) block, and altered expression of genes involved in the control of G(1)-S cell-cycle transition (18). Similar promising results were observed in a xenograft model of BRAF-mutant papillary thyroid cancer, in which selective targeting of BRAFV600E achieved tumor inhibition, as well as reduction of phospho-ERK and phospho-MEK levels (18).
Dabrafenib (GSK2118436), a potent ATP-competitive inhibitor of BRAF kinase, is selective for mutant BRAF in kinase panel screening, cell lines, and xenografts (19). We performed a phase 1 study of dabrafenib in patients with advanced solid malignancies. The study design and pharmacokinetic, pharmacodynamic, and efficacy data for dabrafenib in tumors other than thyroid cancer are reported elsewhere (20). In this article, we report the safety and antitumor activity of dabrafenib in the subset of patients with advanced thyroid cancer.
Materials and Methods
Study design
This report is a subanalysis of the BRAF-mutant thyroid cancer patients who enrolled in the first-in-human phase 1 trial of dabrafenib, in which safety, pharmacokinetic, pharmacodynamic, and antitumor activity endpoints were evaluated in patients with BRAF-mutant solid tumor malignancies (20). Dabrafenib doses administered orally to thyroid carcinoma patients included 150 mg twice daily or 100 mg three times daily. The protocol was approved by the institutional review boards of the participating institutions, and all patients provided written informed consent.
Thyroid cancer patients were enrolled at the University of Texas MD Anderson Cancer Center and at the University of Western Australia Sir Gairdner Hospital. Eligibility criteria of the parent study included patients who had a histologically confirmed solid tumor for which no curative treatment was available, age 18 years or older, Eastern Cooperative Oncology Group performance status of 1 or less, and adequate organ function (Supplementary Methods; Supplementary Data are available online at www.liebertpub.com/thy).
Procedures
Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (21). Safety assessments are described in the Supplementary Methods. Dermatological examination was performed at baseline and as clinically indicated. Disease assessments were completed using Response Evaluation Criteria in Solid Tumors (RECIST), version 1.0 (22). Baseline radiological assessment was performed within 35 days before initiation of treatment. Initial restaging occurred at nine weeks in the escalation cohorts and at six weeks in the expansion cohorts. Subsequent restaging occurred every nine weeks. Patients who received at least one dose of study drug were included in both the safety and efficacy analyses. Accredited assays were used for tumor BRAF genotyping (appendix). Central genotyping was mandatory in the expansion cohorts and was done by Response Genetics (Los Angeles, CA).
Statistical analysis
This report includes results based on the final study data on March 19, 2012. We calculated exact 95% confidence intervals (CIs) for the proportion of patients achieving a response. Progression-free survival was defined as the interval between first dose and date of disease progression or death due to any cause. When no progression or death occurred, it was censored at the date of last adequate disease assessment. Kaplan-Meier method was used to estimate progression-free survival, and Brookmeyer and Crowley's method was used to estimate the 95% CI for median progression-free survival (23). Duration on treatment was defined as the time from first dose to the date of last dose. For patients with confirmed response, duration of response was defined as the interval between first documented response and the first date of disease progression or death due to any cause. Censoring rule for duration of response were the same as for progression-free survival. SAS version 9.1 was used for all analyses. This study is registered with ClinicalTrials.gov, number NCT00880321.
Results
Between May 27, 2009, and March 2, 2011, 184 patients with various advanced malignancies were enrolled into the parent study. The pharmacokinetic and pharmacodynamic analysis of dabrafenib and adverse events in solid tumors are reported elsewhere (20). In this subanalysis, 14 patients with thyroid carcinoma were evaluated (Table 1). All 14 patients had BRAFV600E mutations and 13 (93%) patients had received prior radioactive iodine therapy. Nine of the 14 patients had not received systemic regimens in the metastatic setting, aside from 131I. Among the 5 patients who received systemic regimens in the metastatic setting, their best responses to their last lines of systemic therapy were partial response in one patient, stable disease in 2 patients, progressive disease in 1 patient and unknown in 1 patient. One patient had received prior treatment with a MEK inhibitor, and no patients had previously been treated with a selective BRAF inhibitor. Other prior systemic treatments included tamoxifen, sorafenib, tipifarnib, gemcitabine, docetaxel, interleukin-6, OPB31121 (STAT3 inhibitor), sunitinib, valproic acid, pazopanib, and axitinib.
Table 1.
Baseline Demographic and Clinical Characteristics
| Patients (N=14) | |
|---|---|
| Age (years) | |
| Median (range) | 61 (44–83) |
| Sex | |
| Male | 5 (36%) |
| Female | 9 (64%) |
| BRAFV600E mutation | 14 (100%) |
| ECOG PS | |
| 0 | 3 (21%) |
| 1 | 11 (79%) |
| Prior radioactive iodine treatment (131I) | 13 (93%) |
| Prior surgery | 13 (93%) |
| Prior external beam radiation | 1 (7%) |
| Prior non-131I systemic regimens in the metastatic setting | |
| 0 | 9 (64%) |
| 1 | 2 (14%) |
| 2 | 2 (14%) |
| >3 | 1 (7%) |
| Dabrafenib dose received | |
| 150 mg BID | 13 (93%) |
| 100 mg TID | 1 (7%) |
| Prior MEK inhibitor treatment | 1 (7%) |
| Histology | |
| Conventional (well differentiated)a | 9 (64%) |
| Poorly differentiatedb or high gradec | 3 (21%) |
| Follicular variantb | 2 (14%) |
| Oncocytic and Hürthle cell features | 1 (7%) |
Data are presented as n (%) except where noted.
One patient's histology was mistakenly entered as poorly differentiated into database and is correctly listed here as well differentiated.
One patient had concurrent poorly differentiated features and follicular variant histology.
Includes one patient with anaplastic features.
BID, twice a day; BRAF, v-raf murine sarcoma viral oncogene homolog B; ECOG, Eastern Cooperative Oncology Group; PS, performance status; TID, three times a day.
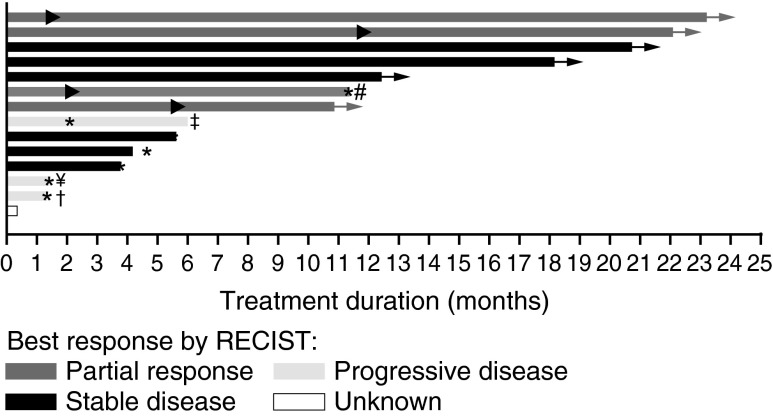
Thirteen patients received the recommended phase 2 dose of 150 mg twice daily, and one patient received 100 mg three times daily. The median duration on treatment was 8.4 months with range from 0.3 to 23.2 months, and 7 (50%) patients received treatment for longer than 10 months (Fig. 1). Table 2 shows the most common treatment-related adverse events noted at a total frequency of 10% or higher in patients with thyroid carcinoma. The toxicity profile observed in this analysis is similar to the adverse events observed in the parent study.
FIG. 1.
Duration on treatment for 14 patients with BRAFV600E-mutant thyroid cancer treated with dabrafenib. ▸, indicates the first occurrence of PR; *, indicates time of PD;→, patient continued treatment in the rollover study; ¥, patient received prior MEK inhibitor treatment; #, patient received 100 mg TID, all other subjects received 150 mg BID; †, patient with concurrent papillary and Hurthle cell features; ‡, patient with anaplastic histology. This patient had a 66% decrease in target lesions but developed a new lesion at first restaging. Note 1: Six patients had not developed progressive disease at the time of study completion and continued receiving treatment in a rollover study. At the time of this publication, all six of these patients are still receiving dabrafenib, and four of them have been receiving dabrafenib for more than 2.5 years. Note 2: One patient withdrew consent before having any post-baseline disease assessment. Note 3: Duration on treatment is defined as the time interval from the first dose to the last dose on the phase 1 study, regardless of dose interruption. BID, twice a day; PD, progressive disease; PR, partial response; SD stable disease; TID, three times a day; UNK, unknown response.
Table 2.
Treatment-Related Adverse Events with Total Frequency of 10 Percent or Higher in Patients with Thyroid Cancer
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total | |
|---|---|---|---|---|---|
| Skin papilloma | 8 (57%) | 0 | 0 | 0 | 8 (57%) |
| Hyperkeratosis | 5 (36%) | 0 | 0 | 0 | 5 (36%) |
| Alopecia | 4 (29%) | 0 | 0 | 0 | 4 (29%) |
| Arthralgia | 1 (7%) | 1 (7%) | 0 | 0 | 2 (14%) |
| Hair texture abnormal | 2 (14%) | 0 | 0 | 0 | 2 (14%) |
| Pyrexia | 1 (7%) | 1 (7%) | 0 | 0 | 2 (14%) |
| Seborrhoeic keratosis | 2 (14%) | 0 | 0 | 0 | 2 (14%) |
| Skin hypertrophy | 2 (14%) | 0 | 0 | 0 | 2 (14%) |
Data are presented as n (%).
The most common treatment-related adverse events observed were skin papillomas (n=8, 57%), hyperkeratosis (n=5, 36%), and alopecia (n=4, 29%), all of which were grade 1. Treatment-related adverse events of grade 3 or higher included grade 4 elevated lipase (n=1), grade 3 elevated amylase (n=1), grade 3 fatigue (n=1), grade 3 febrile neutropenia (n=1), and grade 3 cutaneous squamous cell carcinoma (n=1).
Treatment-related serious adverse events were noted in two patients, including febrile neutropenia (n=1) and squamous cell carcinoma (n=1). Adverse events that resulted in dose reduction occurred in two patients, including influenza like illness and pyrexia in one patient and arthralgia in one patient. Dose interruptions were necessary in 7 (50%) patients because of a total of 13 adverse events, of which the most common were fatigue (n=3, 21%) and chills (n=2, 14%). No patients discontinued treatment due to adverse event. No deaths resulted from adverse events, and 10 patients (71%) had no drug-related adverse events higher than grade 2. A full list of serious adverse events (both treatment-related and unrelated) observed in thyroid cancer patients treated on this trial are described in Supplementary Results.
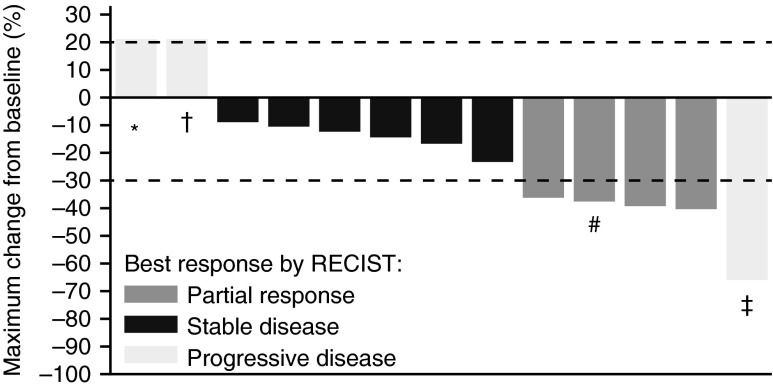
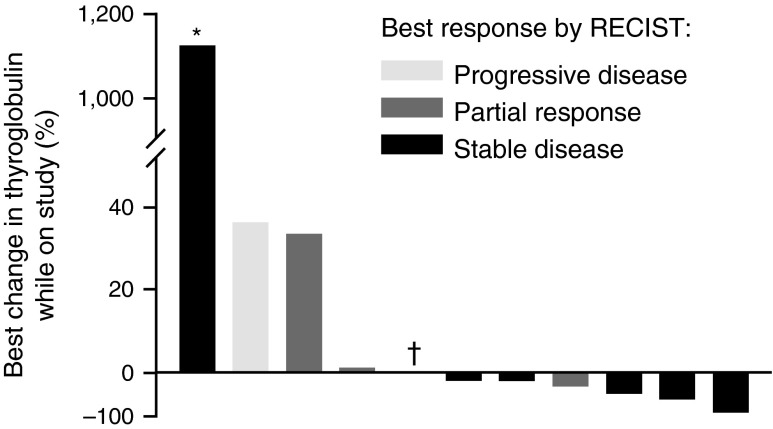
Among the 14 patients with thyroid cancer, four (29%; 95% CI=8–58%) partial responses were observed (Figs. 2, 3), 6 patients achieved stable disease, and 3 patients had progressive disease as best response. One patient withdrew consent after only 11 days of treatment, before having any post-baseline disease assessment. Among the 3 patients with progressive disease as best response, 1 patient had anaplastic thyroid cancer, 1 patient had concurrent papillary and Hürthle cell features, and 1 patient had prior MEK inhibitor treatment. No deaths occurred while on the study. Nine of the 14 patients achieved at least a 10% decrease by RECIST, including the four patients with partial responses. Thyroglobulin response data was available for 13 of the 14 patients (Fig. 4).
FIG. 2.
Change in tumor size in 14 patients with BRAFV600E-mutant thyroid cancer treated with dabrafenib. *, patient received prior MEK inhibitor treatment; †, patient with concurrent papillary and Hurthle cell features; #, patient received 100 mg TID. All other patients received 150 mg BID. ‡, patient with anaplastic histology. This patient had a 66% decrease in target lesions but developed a new lesion at first restaging. Note 1: One patient withdrew consent before having any post-baseline disease assessment, so was not included in the plot. Note 2: The patient with the largest reduction experienced reduction in her target lesion but disease progression in a non-target lesion at the same disease assessment. Subsequent biopsy of the progressing non-target lesion demonstrated anaplastic carcinoma histology, rather than papillary carcinoma.
FIG. 3.
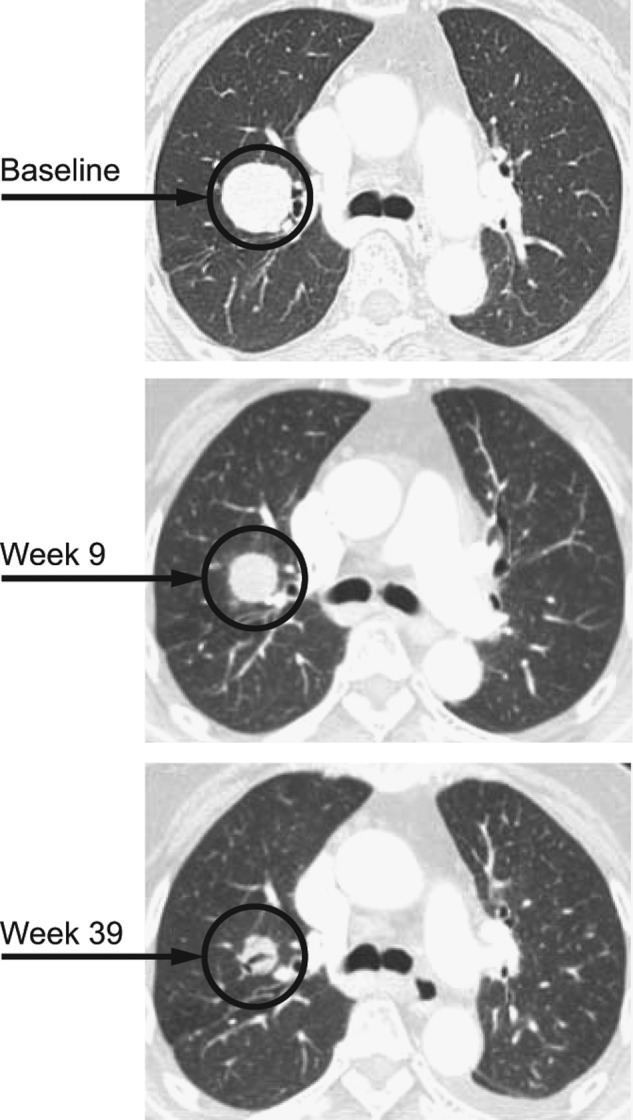
Partial response in a BRAFV600E-mutant papillary thyroid cancer treated with dabrafenib. This patient achieved a 38% decrease in tumor size and received treatment for 12 months before progressing. (A) Baseline. (B) After 9 weeks of treatment. (C) After 39 weeks of treatment.
FIG. 4.
Best percentage change in thyroglobulin in patients with BRAFV600E-mutant thyroid cancer treated with dabrafenib. *, patient experienced radiological progression on study, with thyroglobulin level increasing from 7 μg/L at baseline to 79 μg/L on progression; †, patient underwent total thyroidectomy; this patient had a best response by RECIST of PD. Note 1: Of the 14 patients in the study, thyroglobulin response data was not available in 1 patient with baseline thyroglobulin values only (best response by RECIST, unknown). Note 2: Two additional patients were excluded for reasons that might interfere with interpretation of thyroglobulin levels: one patient had a baseline thyroglobulin level that was assessed more than 3 months before starting dabrafenib (best response, PR), and one patient had anti-thyroglobulin antibodies (best response, SD).
In the patient with anaplastic thyroid cancer who had a best response of progressive disease, the patient's original pathology had a mixed histology, with both differentiated and anaplastic features. Soon after starting dabrafenib, all of the patient's palpable tumors began to decrease except one new lesion, which was biopsied and demonstrated anaplastic features. By RECIST, the patient had a 66% decrease in measurable tumor, but the best response was determined to be progressive disease because of the development of the new lesion. Because the anaplastic features were present before enrolling on the trial, the progressive disease from anaplastic thyroid carcinoma was not attributed to dabrafenib.
Among the responders, at the time of data cutoff only 1 responder developed progressive disease, with duration of response of 9.3 months, and the other 3 responders had not progressed, with duration of response 4.6+, 10.4+, and 21.4+ months. Six patients who had not developed progressive disease, including the 3 remaining patients with partial responses, were permitted to continue receiving treatment on a subsequent rollover study, which was created in order to permit for patients who were receiving clinical benefit to continue the study treatment. The median progression-free survival was 11.3 months with 95% CI lower limit of 2.1 months and upper limit that could not be determined. Among the 7 patients who had not progressed, 5 patients had censored value of progression-free survival >11.3 months.
The 4 patients with variant histologies (i.e., poorly differentiated/high grade, follicular variant, oncocytic features) had a trend of less response (response rate 0% vs. 40%) and smaller median percentage decrease in size (13% vs. 23%) compared with the 10 patients with conventional (well-differentiated) histology. All of the durable responses were observed in patients with differentiated histology.
Discussion
We report detailed efficacy and safety results of the largest group of BRAF-mutant thyroid carcinoma patients treated with a selective BRAF inhibitor. Our findings show that dabrafenib has good clinical activity in patients with metastatic radioiodine-refractory BRAF-mutant thyroid carcinoma, with a 29% response rate and 64% of patients achieving at least a 10% reduction in tumor size.
The antitumor activity observed in this study corroborates thyroid carcinoma preclinical models, which suggested that selective targeting of mutant BRAFV600E would inhibit cell proliferation, decrease phosphorylation of downstream MEK and ERK, induce a G(1) block, and alter expression of genes involved in the control of G(1)-S cell-cycle transition (18). Similar to BRAF-mutant melanoma cells, which display features of oncogene addiction in vitro, these data suggest that BRAF-mutant thyroid cancer cells may depend on activated BRAF for cell survival and growth (24).
This study expands the previous very limited published clinical experience of selective BRAF inhibitors used for the treatment of BRAF-mutant thyroid cancer. In the first-in-human phase 1 trial of the selective BRAF inhibitor vemurafenib, antitumor activity was observed in the 3 patients with papillary thyroid cancer who enrolled, including an objective response lasting 8 months in one patient (who was progression free for 12 months), and stable disease lasting 11 and 13 months in each of the other two patients (25,26).
In contrast to the antitumor activity observed with BRAF inhibition in this study, MEK inhibition monotherapy as a strategy to target the MAPK pathway has yielded only modest success. Despite preclinical studies of MEK inhibitors demonstrating potent inhibition of proliferation and tumor growth in BRAF-mutant thyroid cell lines and xenografts, clinical trials of MEK inhibitor monotherapy have not yet demonstrated robust response rates in thyroid cancer (27–30). However, a recent study demonstrated that the MEK inhibitor selumetinib can produce clinically meaningful increases in iodine uptake and retention in a subgroup of patients with iodine-refractory thyroid cancer (31).
Importantly, dabrafenib was well tolerated and resulted in mostly mild, manageable toxicity. Adverse events noted in patients with thyroid carcinoma mirrored those of the parent study (20). Proliferative skin lesions such as papillomas and hyperkeratosis, the most frequent treatment-related adverse events, were controlled with conservative measures for most individuals.
Even long-term treatment with dabrafenib was well tolerated, as evidenced by the six patients who had not developed progressive disease at the time of study completion and who have continued receiving dabrafenib treatment in a rollover study, which was created in order to permit patients who were receiving clinical benefit to continue the study treatment. At the time of this publication, all six of these patients are still receiving dabrafenib without progressive disease, and four of them have been receiving dabrafenib for more than 2.5 years. A side effect profile of minimal toxicity is an especially important feature of dabrafenib that may facilitate its development as a potential therapy for papillary thyroid cancer, in which future clinical trials may investigate the potential benefit of dabrafenib in the adjuvant and neoadjuvant settings. In addition, many patients with papillary thyroid carcinoma have an indolent, chronic natural history of their disease, which might require prolonged systemic therapy. Whereas such patients are typically reluctant to undergo aggressive treatment with conventional cytotoxic chemotherapy, dabrafenib has the potential to be an acceptable alternative that could be offered as chronic treatment for such patients.
Similar to melanoma, development of resistance to BRAF inhibitors in thyroid carcinoma may be mediated through various mechanisms, including acquired mutations, such as in MEK or NRAS, or via acquired bypass pathways, such as overexpression of COT or PDGFR-beta (32). Montero-Conde et al. recently demonstrated that inhibition of BRAF in thyroid cancer cell lines leads to a rebound in ERK signaling which is associated with HER2/HER3 expression (33). Translational studies designed to identify other mechanisms of resistance in thyroid carcinoma are needed and will provide the basis for future clinical trials that would combine BRAF inhibitors with other therapeutic agents.
The patient with anaplastic thyroid carcinoma who experienced a 66% decrease in target lesions by RECIST, but who developed a new lesion, highlights the difficulty of managing patients with mixed responses. Although mixed responses are common in clinical practice and have been attributed to heterogeneous clonal subsets within tumors, a systematic approach to studying the phenomenon on a large scale is needed. Considering the considerable decrease of size observed in the target lesions in this patient, strategies to maximize clinical benefit in similar, future patients could include radiation or surgical excision of a solitary, progressing lesion.
Our study is limited by small sample size, as is expected in this first-in-human phase 1 study, which was open to all BRAF-mutant solid tumor malignancies. Further trials to investigate dabrafenib's efficacy in thyroid cancer are underway, including (1) a randomized phase 2 trial study of dabrafenib with or without the MEK inhibitor trametinib in patients with recurrent thyroid cancer (NCT01723202) and (2) a clinical trial to investigate whether dabrafenib can re-sensitize iodine-refractory papillary thyroid cancer patients to radioactive iodine therapy (NCT01534897). Because of dabrafenib's known activity against melanoma brain metastases (20,34), further trials should also be considered to investigate dabrafenib activity against brain metastases in BRAF-mutant thyroid cancer, which although is a uncommon event occurring in approximately 1.2% of patients with differentiated thyroid cancer, is associated with a poor prognosis, including a median survival of 12.4 months and very limited treatment options (35).
In conclusion, the selective BRAF inhibitor dabrafenib is well tolerated and active against BRAF-mutant differentiated thyroid cancer, and it is a promising treatment for further development in this patient population. Further monotherapy trials, novel combination trials, and translational studies should be pursued to identify and overcome mechanisms of resistance. In addition, future trials may consider investigating the potential role for dabrafenib treatment in the adjuvant and neoadjuvant setting.
Supplementary Material
Acknowledgments
We thank the patients and their families. We also thank Rosa Mostorino, Quan Lin, and Judith Christmas for clinical trial coordination and Adrienne Howard for regulatory protocol assistance. This work was supported by the National Institutes of Health Clinical and Translational Science Award UL1 RR024148 and by the National Institutes of Health Cancer Center Support Grant award CA016672 to MD Anderson Cancer Center. The parent study NCT00880321 was sponsored by GlaxoSmithKline.
Author Disclosure Statement
GSF has received research funding and travel reimbursement from GlaxoSmithKline. MM has participated in advisory boards for GlaxoSmithKline. DH, AN, SP-P, and SGW have no conflict of interest to declare. MEC has received grant funding for her institute from Roche, Exelixis, and Eisai and has received personal fees (e.g., honoraria, consultancy fees, etc.) from Exelixis and Eisai. SIS has received grant funding to his institute from Genzyme and Pfizer and has received personal fees from Exelixis, Bayer, Eisai, AstraZeneca, Roche, Genzyme, Pfizer, and Veracyte. BM is an employee of GlaxoSmithKline. MC and VG are employees of, and hold stocks of, GlaxoSmithKline. RK has received research funding from GlaxoSmithKline.
References
- 1.Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire F, Schlumberger M.2006Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 91:2892–2899 [DOI] [PubMed] [Google Scholar]
- 2.Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI, Smit JW, Chung J, Kappeler C, Peña C, Molnár I, Schlumberger MJ; on behalf of the DECISION investigators. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 14:60421–60429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlumberger M, Sherman SI.2012Approach to the patient with advanced differentiated thyroid cancer. Eur J Endocrinol 166:5–11 [DOI] [PubMed] [Google Scholar]
- 4.Roberts PJ, Der CJ.2007Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 26:3291–3310 [DOI] [PubMed] [Google Scholar]
- 5.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA.2002Mutations of the BRAF gene in human cancer. Nature 417:949–954 [DOI] [PubMed] [Google Scholar]
- 6.Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ, Barford D, Marais R.2004Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116:855–867 [DOI] [PubMed] [Google Scholar]
- 7.Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA.2003High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res 63:1454–1457 [PubMed] [Google Scholar]
- 8.Lee JH, Lee ES, Kim YS.2007Clinicopathologic significance of BRAF V600E mutation in papillary carcinomas of the thyroid: a meta-analysis. Cancer 110:38–46 [DOI] [PubMed] [Google Scholar]
- 9.Henderson YC, Shellenberger TD, Williams MD, El-Naggar AK, Fredrick MJ, Cieply KM, Clayman GL.2009High rate of BRAF and RET/PTC dual mutations associated with recurrent papillary thyroid carcinoma. Clin Cancer Res 15:485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakayama H, Yoshida A, Nakamura Y, Hayashi H, Miyagi Y, Wada N, Rino Y, Masuda M, Imada T.2007Clinical significance of BRAF (V600E) mutation and Ki-67 labeling index in papillary thyroid carcinomas. Anticancer Res 27:3645–3649 [PubMed] [Google Scholar]
- 11.Caronia LM, Phay JE, Shah MH.2011Role of BRAF in thyroid oncogenesis. Clin Cancer Res 17:7511–7517 [DOI] [PubMed] [Google Scholar]
- 12.Santarpia L, El-Naggar AK, Cote GJ, Myers JN, Sherman SI.2008Phosphatidylinositol 3-kinase/akt and ras/raf-mitogen-activated protein kinase pathway mutations in anaplastic thyroid cancer. J Clin Endocrinol Metab 93:278–284 [DOI] [PubMed] [Google Scholar]
- 13.Takano T, Ito Y, Hirokawa M, Yoshida H, Miyauchi A.2007BRAF V600E mutation in anaplastic thyroid carcinomas and their accompanying differentiated carcinomas. Br J Cancer 96:1549–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Rhoden KJ, Carson KA, Vasko V, Larin A, Tallini G, Tolaney S, Holt EH, Hui P, Umbricht CB, Basaria S, Ewertz M, Tufaro AP, Califano JA, Ringel MD, Zeiger MA, Sidransky D, Ladenson PW.2005BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab 90:6373–6379 [DOI] [PubMed] [Google Scholar]
- 15.Kim TH, Park YJ, Lim JA, Ahn HY, Lee EK, Lee YJ, Kim KW, Hahn SK, Youn YK, Kim KH, Cho BY, Park do J.2012The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis. Cancer 118:1764–1773 [DOI] [PubMed] [Google Scholar]
- 16.Li C, Lee KC, Schneider EB, Zeiger MA.2012BRAF V600E mutation and its association with clinicopathological features of papillary thyroid cancer: a meta-analysis. J Clin Endocrinol Metab 97:4559–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM, Robenshtok E, Fagin JA, Puxeddu E, Fugazzola L, Czarniecka A, Jarzab B, O'Neill CJ, Sywak MS, Lam AK, Riesco-Eizaguirre G, Santisteban P, Nakayama H, Tufano RP, Pai SI, Zeiger MA, Westra WH, Clark DP, Clifton-Bligh R, Sidransky D, Ladenson PW, Sykorova V. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 14:1493–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salerno P, De Falco V, Tamburrino A, Nappi TC, Vecchio G, Schweppe RE, Bollag G, Santoro M, Salvatore G.2010Cytostatic activity of adenosine triphosphate-competitive kinase inhibitors in BRAF mutant thyroid carcinoma cells. J Clin Endocrinol Metab 95:450–455 [DOI] [PubMed] [Google Scholar]
- 19.Laquerre S, Arnone M, Moss K, Yang J, Fisher KE, Kane-Carson LS, Smitheman K.2009A selective Raf kinase inhibitor induces cell death and tumor regression of human cancer cell lines encoding B-RafV600E mutation. Mol Cancer Ther 8:B88 (Abstract) [Google Scholar]
- 20.Falchook GS, Long GV, Kurzrock R, Kim KB, Arkenau TH, Brown MP, Hamid O, Infante JR, Millward M, Pavlick AC, O'Day SJ, Blackman SC, Curtis CM, Lebowitz P, Ma B, Ouellet D, Kefford RF.2012Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet 379:1893–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Cancer Institute 2011 Common terminology criteria for adverse events (CTCAE), version 3.0. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf (accessed December2, 2012)
- 22.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG.2000New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216 [DOI] [PubMed] [Google Scholar]
- 23.Brookmeyer R, Crowley J.1982A confidence interval for the mean survival time. Biometrics 38:29–41 [Google Scholar]
- 24.Karasarides M, Chiloeches A, Hayward R, Niculescu-Duvaz D, Scanlon I, Friedlos F, Ogilvie L, Hedley D, Martin J, Marshall CJ, Springer CJ, Marais R.2004B-RAF is a therapeutic target in melanoma. Oncogene 23:6292–6298 [DOI] [PubMed] [Google Scholar]
- 25.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O'Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB.2010Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 363:809–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim KB, Cabanillas ME, Lazar AJ, Williams MD, Sanders DL, Ilagan JL, Nolop K, Lee RJ, Sherman SI.2013Clinical responses to vemurafenib in patients with metastatic papillary thyroid cancer harboring BRAF(V600E) mutation. Thyroid 23:1277–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu D, Xing J, Trink B, Xing M.2010BRAF mutation-selective inhibition of thyroid cancer cells by the novel MEK inhibitor RDEA119 and genetic-potentiated synergism with the mTOR inhibitor temsirolimus. Int J Cancer 127:2965–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henderson YC, Chen Y, Frederick MJ, Lai SY, Clayman GL.2010MEK inhibitor PD0325901 significantly reduces the growth of papillary thyroid carcinoma cells in vitro and in vivo. Mol Cancer Ther 9:1968–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayes DN, Lucas AS, Tanvetyanon T, Krzyzanowska MK, Chung CH, Murphy BA, Gilbert J, Mehra R, Moore DT, Sheikh A, Hoskins J, Hayward MC, Zhao N, O'Connor W, Weck KE, Cohen RB, Cohen EE.2012Phase II efficacy and pharmacogenomic study of Selumetinib (AZD6244; ARRY-142886) in iodine-131 refractory papillary thyroid carcinoma with or without follicular elements. Clin Cancer Res 18:2056–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friday BB, Adjei AA.2008Advances in targeting the Ras/Raf/MEK/Erk mitogen-activated protein kinase cascade with MEK inhibitors for cancer therapy. Clin Cancer Res 14:342–346 [DOI] [PubMed] [Google Scholar]
- 31.Ho AL, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, Pentlow KS, Zanzonico PB, Haque S, Gavane S, Ghossein RA, Ricarte-Filho JC, Dominguez JM, Shen R, Tuttle RM, Larson SM, Fagin JA.2013Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med 368:623–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, Kehoe SM, Johannessen CM, MacConaill LE, Hahn WC, Meyerson M, Garraway LA.2011Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol 29:3085–3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montero-Conde C, Ruiz-Llorente S, Dominguez JM, Knauf JA, Viale A, Sherman EJ, Ryder M, Ghossein RA, Rosen N, Fagin JA.2013Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov 3:520–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long GV, Trefzer U, Davies MA, Kefford RF, Ascierto PA, Chapman PB, Puzanov I, Hauschild A, Robert C, Algazi A, Mortier L, Tawbi H, Wilhelm T, Zimmer L, Switzky J, Swann S, Martin AM, Guckert M, Goodman V, Streit M, Kirkwood JM, Schadendorf D.2012Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol 13:1087–1095 [DOI] [PubMed] [Google Scholar]
- 35.Chiu AC, Delpassand ES, Sherman SI.1997Prognosis and treatment of brain metastases in thyroid carcinoma. J Clin Endocrinol Metab 82:3637–3642 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.