Abstract
Objective
To identify age-related changes in human meibomian glands that may be associated with meibomian gland dysfunction (MGD).
Methods
Excess eyelid tissue from 36 patients (age range, 18–95 years, 19 female, 17 male) who underwent canthoplasty procedures were used. Dermatologic history, age, and presence of MGD were recorded. Samples were frozen, sectioned, and stained with specific antibodies against peroxisome proliferator–activated receptor γ(PPARγ) to identify meibocyte differentiation, Ki67 nuclear antigen to identify cycling cells, and CD45 to identify inflammatory cell infiltration.
Results
Staining for PPARγ showed cytoplasmic and nuclear localization in the 2 youngest subjects (ages, 18 and 44 years). Older individuals (>60 years) showed predominantly nuclear staining, with cytoplasmic staining limited to the basal acinar cells in 17 of 31 subjects. The number of Ki67 positively stained basal cells were significantly elevated in the younger compared with older subjects based on linear regression analysis (r2= 0.35; P <.001). There was also a significant correlation between MG expression grade and CD45 cell infiltration (r =0.414; P =.05).
Conclusions
These results indicate that aging human meibomian glands show decreased meibocyte differentiation and cell cycling that is associated with the development of MGD. Findings also suggest that altered PPARγ signaling may lead to acinar atrophy and development of an age-related hyposecretory MGD.
Clinical Relevance
Meibomian gland dysfunction and evaporative dry eye are common age-related eyelid disorders. Understanding the underlying mechanism of MGD may lead to the development of novel therapeutic strategies to treat this disease.
Meibomian gland dys-function (MGD) is a common eyelid disorder that has widespread prevalence of 39% to 50% in the US population,1 with the incidence increasing with age.2–4 It is also a major cause of evaporative dry eye disease, with loss of glands resulting in decreased tear film lipid, unstable tear film, increased aqueous tear evaporation,5 and increased tear film osmolarity,6 leading to ocular surface changes and blepharitis.7,8 Currently, 3 forms of MGD are recognized: hypersecretory MGD, hyposecretory MGD, and obstructive MGD, with the latter form considered to be the most common.9 Based on clinical and animal studies,10–14 obstructive MGD is thought to involve hyperkeratinization of the meibomian gland duct, leading initially to plugging of the meibomian gland orifice followed by cystic dilation of the duct and a disuse atrophy of the acini that is detected as gland dropout on infrared photography (meibography).9
While the risk of evaporative dry eye and MGD increases with age,15,16 there have been few histopathologic articles describing the effects of age on meibomian gland structure.10,17,18 These reports suggest that aging results in atrophy of the meibomian gland acini and decreased lipid expression from the gland.17,18 Additional changes that have been noted include focal hyperkeratinization of the ductal epithelium, cystic dilation, and lipogranulomatoses, although the association with aging is less clear.10 Recently, we identified specific age-related changes in the mouse meibomian gland that include decreased acinar cell proliferation, decreased meibomian gland size, and increased inflammatory cell infiltration that occur concurrently with altered localization of the peroxisome proliferator–activated receptor gamma (PPAR γ).19 The PPAR γ is a lipid-activated nuclear hormone receptor that regulates lipid synthesis and cell differentiation.20 It has also recently been shown to be a marker for meibocyte differentiation in the developing mouse meibomian gland.21 Because PPARγ is critical for sebocyte and adipocyte differentiation,22 these findings suggest that age-related meibomian gland atrophy in the mouse may involve altered PPARγ signaling.
To assess whether age-related human MGD may involve similar changes to that identified in the mouse, we have evaluated human eyelid tissue taken from oculo-plastic patients varying in age from 18 to 95 years. We report that human meibomian glands undergo similar age-related changes to those identified in mice and therefore hypothesize that age-related human MGD may involve altered PPARγ-regulated gene expression leading to downregulation of meibocyte differentiation, acinar atrophy, and hyposecretory MGD.
METHODS
HUMAN SUBJECT SELECTION AND ASSESSMENT
This study was conducted in accordance with the Declaration of Helsinki and was approved by the institutional review board at the University of California, Irvine, Medical Center. All subjects signed an informed consent form prior to enrollment in the study. Lower lateral eyelid specimens not affected by any disease processes were collected from subjects who underwent canthoplasty for treatment of various oculoplastic disorders (Table). History of ocular and dermatological disorders was collected prior to surgery, and severity of MGD was evaluated in terms of gland expression and dropout. Meibomian gland expression was assessed in the region of the eyelid that was to be removed using a cotton-tipped applicator. Quality of expression was graded according to the degree of opacity and viscosity on a 0 to 4 scale based on Mathers et al23 in which 0in-dicatesnormal; 1,opaque, normal viscosity; 2,opaque, increased viscosity; 3,severe thickening (toothpaste); and 4,no expression, glands completely blocked. Gland dropout was evaluated on a 0 to 4 scale along the entire lower eyelid: 0,none; 1,25%; 2,26% to 50%; 3,51% to 75%; and 4,76% to 100%.24
Table.
Subject Clinical History, MGD Grade, and Immunohistochemistry Findings
| Patient Age, y | Surgical Indication | Rosacea | MGD Grade
|
Ki67 LI | Cytoplasmic PPAR γ a | CD45 Infiltrationb
|
||
|---|---|---|---|---|---|---|---|---|
| Expression | Dropout | Acini | Duct | |||||
| 18 | Orbit tumor | − | 0 | 0 | 37.2 | ++ | − | NA |
| 44 | Cicatricial ectropion | − | 1 | 1 | 21.9 | ++ | − | NA |
| 58 | Basal cell cancer | + | 1 | 1 | 13.3 | + | + | + |
| 60 | NA | NA | NA | NA | 19.4 | + | − | − |
| 65 | Trichiasis | + | 3 | 3 | 8.0 | − | − | + |
| 66 | Involutional ectropion | − | 1 | 1 | 8.2 | + | − | NA |
| 67 | Involutional ectropion | + | 2 | 2 | 20.8 | + | − | NA |
| 68 | Involutional ectropion | + | 3 | 2 | 8.3 | + | ++ | + |
| 68 | Involutional ectropion | + | 3 | 3 | 8.0 | NA | − | − |
| 69 | Involutional ectropion | − | 1 | 1 | 8.8 | + | − | NA |
| 71 | Blepharoplasty | + | 2 | 1 | 19.9 | − | − | − |
| 72 | Involutional ectropion | − | 2 | 2 | 10.0 | + | − | NA |
| 72 | Involutional ectropion | + | 2 | 1 | 12.3 | + | − | NA |
| 72 | Eyelid retraction | − | 3 | 3 | 13.6 | + | + | NA |
| 73 | Trichiasis | − | 2 | 2 | 5.1 | − | + | + |
| 74 | Involutional ectropion | NA | NA | NA | 8.9 | NA | + | NA |
| 74 | Thyroid ophthalmopathy | + | 2 | 2 | 8.0 | − | − | − |
| 74 | Involutional ectropion | + | 4 | 4 | 7.0 | + | + | NA |
| 79 | Involutional ectropion | + | 4 | 4 | 6.5 | + | ++ | + |
| 80 | Involutional ectropion | + | 3 | 3 | 14.3 | + | − | − |
| 81 | Dermatochalazis | + | 3 | 2 | 3.4 | − | + | − |
| 81 | Involutional ectropion | + | 3 | 3 | 0.0 | − | + | + |
| 81 | Involutional ectropion | − | 3 | 3 | 7.4 | − | + | NA |
| 82 | Involutional ectropion | + | 2 | 2 | 11.2 | + | − | NA |
| 82 | Involutional ectropion | + | 3 | 2 | 8.2 | + | + | + |
| 83 | Involutional ectropion | − | 3 | 1 | 14.4 | NA | − | NA |
| 84 | Involutional ectropion | + | 4 | 4 | 11.1 | − | − | + |
| 85 | Involutional ectropion | + | 3 | 3 | 11.0 | + | + | NA |
| 85 | Involutional ectropion | + | 3 | 3 | 16.3 | − | ++ | + |
| 85 | Involutional ectropion | + | 3 | 3 | 8.1 | − | + | + |
| 87 | Benign eyelid lesion | + | 4 | 4 | 7.0 | − | − | NA |
| 88 | Involutional ectropion | − | 4 | 4 | 13.7 | + | NA | NA |
| 89 | Involutional ectropion | + | 3 | 3 | 12.0 | + | ++ | + |
| 92 | Involutional ectropion | + | 4 | 3 | 2.5 | − | ++ | NA |
| 93 | Involutional ectropion | + | 3 | 2 | 18.4 | − | + | − |
| 95 | Involutional ectropion | + | 3 | 3 | 16.5 | − | − | + |
Abbreviation: LI, labeling index; MGD, meibomian gland dysfunction; NA, not available; PPAR γ, peroxisome proliferator–activated receptor.
++ Indicates staining in all acinar cells; +, staining in basal cells; −, no staining.
− Indicates no infiltration; +, fewer than 5 cells; ++, more than 5 cells acini or duct staining with CD45.
IMMUNOHISTOCHEMISTRY
Specimens were fixed in 2% paraformaldehyde in phosphate buffered saline (PBS, pH 7.4) overnight, then placed in 15% sucrose in PBS for 4 hours and transferred to 30% sucrose in PBS overnight. Lids were then embedded in Tissue-Tek OCT (optimal cutting temperature) (Sakura Finetek USA, Inc, Torrance, California) freezing medium, frozen in liquid nitrogen, and sectioned using a Leica CM1850 Cryotome (Leica, Wetzlar, Germany). Because samples varied in the amount of meibomian gland tissue, not all could be evaluated for every antibody.
Sections were first hydrated with PBS and then placed in freezing acetone (−20°C) for 3 minutes. Tissue sections were then blocked with 1% bovine serum albumin in PBS for 30 minutes at 37°C, and then incubated with primary antibodies including rabbit polyclonal anti-PPARγ (1:100; Abcam, Cambridge, Massachusetts), rabbit polyclonal anti-Ki67 (1:100; Abcam), and fluorescein isothiocyanate–conjugated antihuman common leukocyte antigen (CD45, clone HLe-1 [2D1], 1:100; R&D Systems, Minneapolis, Minnesota). Sections were then incubated for 1 hour at 37°C, washed with PBS, and reacted with fluorescein isothiocyanate–conjugated secondary antibodies against mouse, rabbit, or goat IgG (Invitrogen, Carlsbad, California) for 1 hour at 37°C. All samples were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (300 nM) in PBS for 15 minutes. For negative controls, sections were either unstained, incubated with irrelevant IgG, or incubated with secondary antibodies alone. Sections were then evaluated and imaged using a Nikon Eclipse E600 fluorescence microscope (Nikon, Melville, New York).
MEASUREMENT OF KI67 LABELING INDEX
Digital images of anti-Ki67 stained sections from all specimens were analyzed using Metamorph Image analysis software (Molecular Devices, Downingtown, Pennsylvania) by an operator masked to the clinical diagnosis of the subject. Regions of the gland containing acini were first identified and the number of nuclei positively stained with anti-Ki67 antibodies marked and recorded using the cell count subroutine. The total number of nuclei within the basal cell layer was then counted using the same subroutine and the number recorded. Data were downloaded to an Excel spreadsheet (Microsoft, Redmond, Washington) and the labeling index calculated by dividing the number of Ki67-positive cell nuclei by the total number of acinar basal cells and multiplying by 100. All acini were counted in 3 different sections from each eyelid, and the average for each subject was recorded.
ANALYSIS OF LEUKOCYTE INFILTRATION
Digital images of sections stained with antibodies against CD45 were evaluated for the presence, localization, and degree of leukocyte infiltration. Each eyelid sample was assigned a − in the absence, + in the presence of a few cells (1–4), and ++ in the presence of high/marked (5 or more) cells infiltrating the acini and/or duct of the meibomian gland by CD45 positively stained cells.
STATISTICAL ANALYSIS
The relationship between age and other variables was determined using Sigma Stat (Systat Software, Inc, Point Richmond, California) stepwise linear regression analysis and calculation of Pearson correlation coefficient. P < .05 was considered statistically significant.
RESULTS
Specimens from 36 subjects were collected; the medical history and MGD grades for 2 subjects were not available with the tissue sample (Table). The mean age was 75 years with a range of 18 to 95 years. There were 19 women (53%) and 17 men (47%). The surgical indications for the subjects are summarized in the Table. Of the 34 subjects with complete medical histories, 25 presented with rosacea (66.6%). Grading of MG expression identified 1 subject with normal secretions (2.9%), 4 exhibiting grade 1 secretions (11.8%) with opaque excretion but normal viscosity, 7 exhibiting grade 2 (20.6%) with opaque and increased viscosity, 16 (47.1%) exhibiting grade 3 with severe thickening, and 6 (17.6%) exhibiting grade 4 with no expression. There was a significant correlation between age and MG expression grade (r =0.749, P <.001), with older subjects displaying significantly higher grades (Table).
All but 1 subject showed some degree of MG dropout, with 7 subjects (20.6%) having grade 1, 9 (26.5%) having grade 2, 12 (35.3%) having grade 3, and 5 (14.7%) having grade 4 MG dropout. Meibomian gland dropout was also significantly correlated with age (r = 0.618; P <.001). Moreover, MG dropout was highly correlated with MG expression grade (r =0.882; P <.001), indicating that higher MG expression grades showed significantly higher levels of MG dropout.
There was also a significant correlation between rosacea and MGD expression grade (r=0.419; P=.05), MG dropout (r=0.355; P=.05), and age (r=0.391; P=.05). However, when the 2 youngest subjects were removed from the study, the these correlations were no longer significant. It should be noted that the correlation between age and MGD expression grade or MG dropout remained significant after exclusion of the 2 youngest subjects.
MEIBOCYTE DIFFERENTIATION AND PROLIFERATIVE POTENTIAL
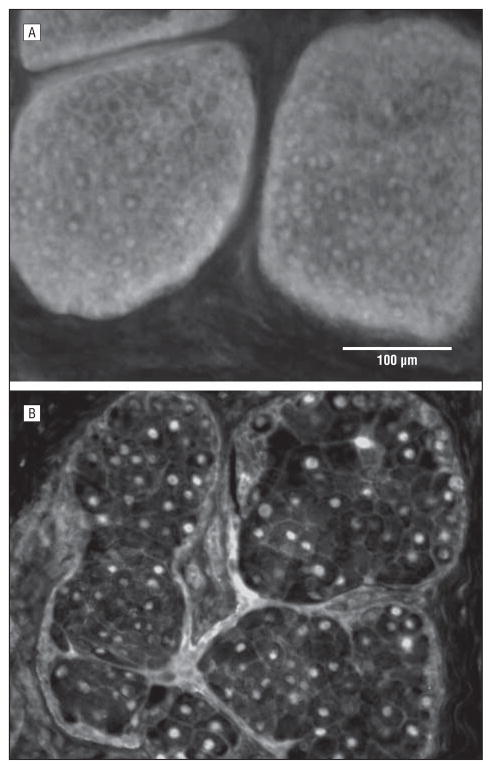
The PPARγ has been shown to be a marker for meibocyte differentiation in the developing mouse and shows a cytoplasmic/vesicular staining pattern in lipid-producing meibocytes in developing and adult glands that is lost in mice older than 1 year.19,21 The immunostaining of human eyelid tissue also showed 2 patterns in the localization of PPARγ, cytoplasmic/vesicular and nuclear, that varied in proportion depending on sample age. Figure 1A shows the cytoplasmic and nuclear localization obtained for the 44-year-old meibomian gland. Note that this pattern was only observed in the 2 subjects younger than 50 years (18 and 44 years; Table) and is similar to that observed in young mice.19 In contrast, Figure 1B shows predominantly nuclear localization of the PPARγ in a 72-year-old gland that appeared similar to what was observed in older mice. This pattern was observed in all the samples from 58 to 95 years, with some showing cytoplasmic staining limited to the basal cell layer. When PPARγ staining was scored based on cytoplasmic staining for all acinar cells (++), basal acinar cells (+), or no cytoplasmic staining (−), there was a significant correlation with age (r =0.664; P <.001), MGD expression grade (r=0.484; P =.005), and MG dropout (r =0.401; P =.02). However, stepwise linear regression analysis showed that age was the only variable predictive of PPARγ staining (r2=0.441). Furthermore, the correlation between age and PPARγ remained significant when the 2 youngest subjects were excluded from the analysis.
Figure 1.
Peroxisome proliferator–activated receptor γ localization in tissue obtained from 44- (A) and 72-year-old (B) subjects. Note the cytoplasmic acinar cell staining in the tissue from the younger subject that is lost in the older gland.
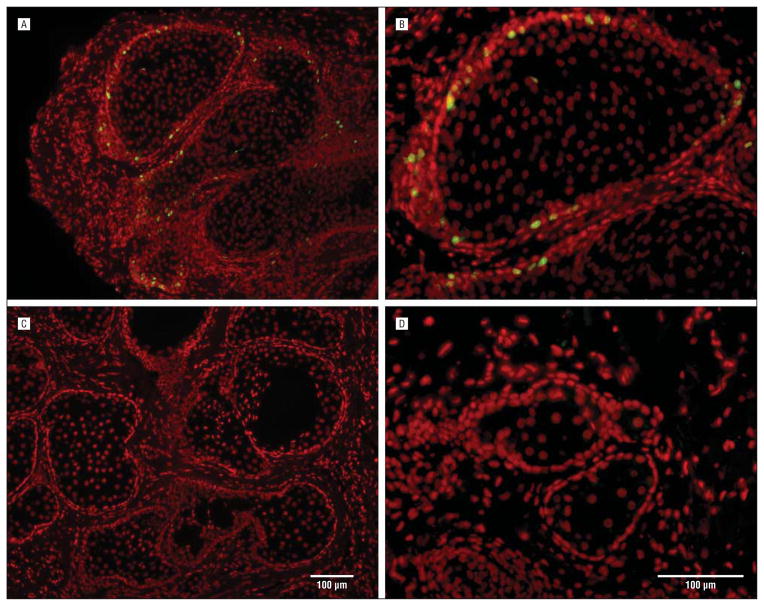
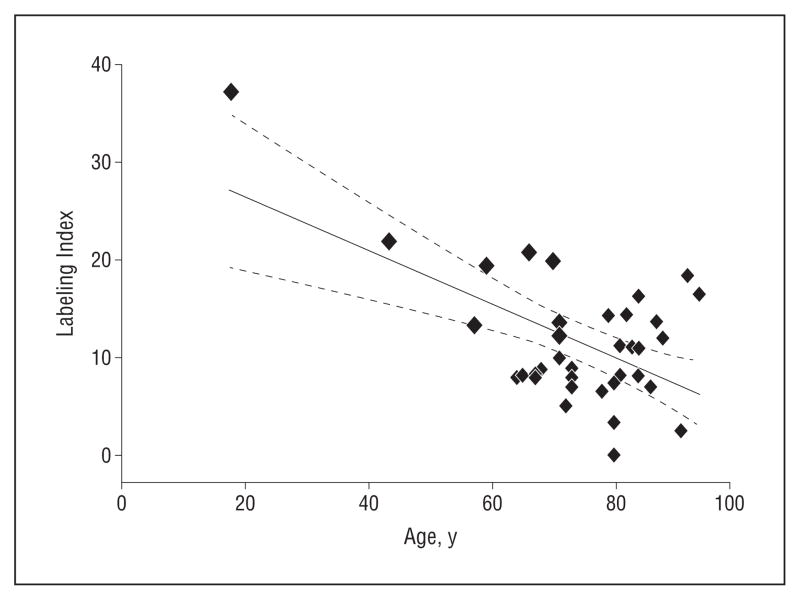
To evaluate proliferative potential, we stained the samples with Ki67, a marker of cell cycle entry. There was a high labeling index for basal cells in the samples obtained from the subjects aged 44 and 18 years (Figure 2; Table) that was similar in magnitude to the labeling index reported for young mice.19 Furthermore, samples from older subjects show substantially reduced labeling. Overall, there was a significant negative correlation between the Ki67 labeling index and age, with a correlation coefficient of −0.591, (r2=0.35; P<.001; Figure 3). However, the correlation was not significant if the youngest 2 subjects were excluded from the analysis.
Figure 2.
Staining with Ki67 of tissue from the 44- (A and B) and 72-year-old (C and D) subjects. Note that the younger gland show marked Ki67 labeling that is absent in the older the gland.
Figure 3.
Regression analysis of Ki67 labeling index with age of subject. Note that younger subjects showed a significantly higher labeling index than older subjects (r 2= 0.35; P <.001).
LEUKOCYTE INFILTRATION
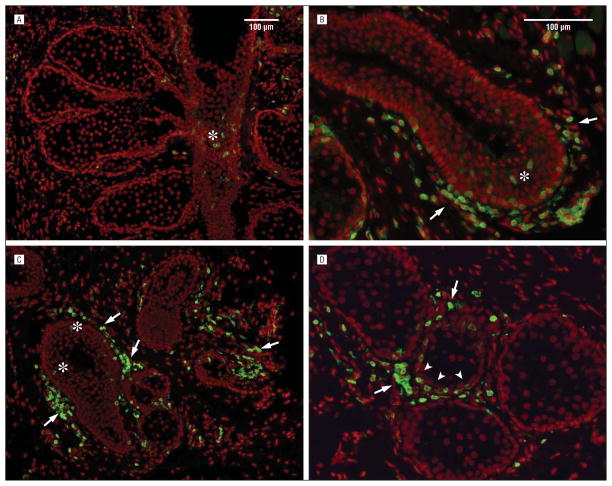
Immunofluorescent staining using antibodies to CD45 leukocyte marker showed variable amounts of CD45+ leukocyte infiltration of the meibomian gland within the acinar, ductal, and interstitial regions of the gland (Figure 4). Some samples showed positive CD45-stained cells in the ductal epithelium and interstitial tissue (Figure 4, A, B, and C), with no CD45-stained cells within the acini. Other samples showed CD45+ cells within the acini but not the portion of the duct present in the section (Figure 4D). It was therefore not possible to ascertain whether any portion of the gland was preferentially infiltrated by CD45+ cells. To assess the relationship between gland infiltration and MGD, the intensity of gland infiltration for each sample was noted as either negative (−), present (+), or marked (++). Because not all samples contained a section through the meibomian gland duct, analysis was limited to just the infiltration into the acini. Overall, CD45 leukocyte infiltration into the meibomian gland acini was significantly associated with the severity of MG expression grade (r = 0.414; P =.05), MG dropout (r =0.361; P =.05), and patient age (r =0.341; P =.05). When the 2 youngest subjects were excluded from the analysis, the only correlation that remained significant was between acinar infiltration and MGD expression grade. The presence of rosacea showed no correlation with gland infiltration.
Figure 4.
Staining with CD45 of tissue showing meibomian gland duct (asterisks) and interstitial tissue infiltration (arrows) (A–C). D, Note the infiltration of CD45 cells into the acinus (arrowheads) adjacent to CD45 stained cells within the interstitial tissue (arrows).
COMMENT
Given an asymmetric age distribution, the data from this study suggests that human meibomian glands show age-related changes in acinar cell proliferation and localization of the lipogenesis factor, PPARγ. These distinct age-related changes are similar to those identified in aging mice that showed significant atrophy of meibomian glands in association with decreased acinar proliferation and altered PPARγ localization.19 Additionally, meibomian glands exhibited variable amounts of leukocyte infiltration that are significantly correlated with the severity of MG expression grade but not with age. Taken together, the findings suggest that age-related MGD may involve altered PPARγ localization, suggesting altered regulation of PPARγ response genes that potentially lead to decreased meibocyte differentiation, acinar atrophy, decreased lipid synthesis, and the development of hyposecretory MGD.
However, it should be noted that these samples were obtained from subjects who underwent canthoplasty procedures for various underlying oculoplastic conditions and that tissue collection was limited to the lateral lower eyelid, which may introduce selection bias. In addition, there was an asymmetric age distribution with unequal sampling of younger subjects, which may also have biased these results. While the overall findings were consistent with findings in aging mice, evaluation of younger subjects is needed to substantiate these results. Verification of the of the relationship between meibomian gland atrophy and PPAR γ localization using eyelid samples from cadaveric sources with a more complete age distribution would be helpful, although evaluating the relationship between clinical MGD and PPARγ localization, gland proliferation, and gland infiltration may be more difficult in these tissues.
The PPARγ is a fatty acid–activated nuclear transcription factor expressed predominantly in adipocytes and sebocytes, where it regulates the expression of genes involved in lipogenesis and is critical for appropriate cell differentiation to form fat and sebaceous glands.22,25 A diverse range of polyunsaturated fatty acids are thought to be the natural ligands for PPARγ, including linoleic and linolenic acid, eicosapentaenoic acid, oxidized metabolites 9- and 13-hydroxyoctadecadienoic acid, 15-hydroxyeicosatetraenoic acid, and 15-deoxy-Δ12,14-prostaglandin J2.26 Synthetic ligands for PPARγ, ie, thiazolidinediones, have been shown to have important anti–type 2 diabetic actions in controlling insulin resistance as well as beneficial effects on atherosclerosis and hypertension.27
Binding of ligands to PPARγ result in heterodimerization with retinoic acid X receptors followed by binding to target gene promoters including aP2, a marker of terminal adipocyte differentiation, adiponectin, adipocyte differentiation–related protein, perilipin, and acetyl Co-A synthase.27 The PPARγ can also serve as a molecular switch and recruit corepressors nuclear receptor corepressor and silencing mediator for retinoid and thyroid receptors when nonliganded PPARγ is bound to some target promoters.28 There are also reports that PPARγ signaling may be modified by androgen receptors based on findings that androgen receptor–null mice show late-onset obesity with decreased PPARγ expression.29 Distinct sex differences in the expression of PPARγ in some tissues have also been identified30 as well as cell culture studies showing a synergistic activity between androgens and PPARγ ligands in immortalized sebocytes.31
Finally, PPARγ activation is known to have pleiotropic effects on inflammatory cytokine expression in many organ systems. Recent studies have shown that activation of PPARγ by rosiglitazone, a thiazolidine-diones class member, can reduce circulating interleukin 1 (IL-1) receptor antagonist when given to patients with metabolic syndrome while decreasing IL-1β and increasing IL-1Ra expression by THP-1 (human acute monocytic leukemia cell ine) monocytes in cultures.32 Similar effects have been shown for synovial fibroblasts treated with rosiglitazone.33 Furthermore, successful treatment of inflammatory bowel disease, psoriasis, and obstructive pulmonary disease and the downregulation of proinflammatory mediators associated with these chronic diseases have also been reported for synthetic PPARγ agonist.34
Together, the reported actions of PPARγ appear to be relevant to many of the known ocular surface changes involved in age-related MGD. First, androgens have been shown to effect meibomian gland gene expression,35–38 and women with androgen insensitivity show altered meibomian gland lipid profiles that might act through downstream PPARγ gene regulation.39–41 Second, some improvement in dry eye disease has been reported using dietary supplements containing omega 3 and omega 6 fatty acids,42 the natural ligands for PPARγ. Third, ocular surface disease has been shown to be associated with upregulation of proinflammatory cytokines such as IL-1 and tumor necrosis factor α,43–45 the expression of which has been reported to be modulated by PPARγ during chronic disease.32–34 Finally, Accutane therapy, which acts through retinoic acid X-receptor binding, the heterodimeric partner of PPARγ, is known to cause dry eye disease and hyposecretory MGD.46–48 While it is not known if PPARγ influences these ocular surface disease pathways, future studies are clearly needed to identify the role of PPAR γ in regulating meibomian gland function and it’s influence on the development of MGD.
Overall, the findings in this study suggest that age-related MGD may be associated with hyposecretory MGD that differs from the accepted obstructive MGD mechanism (Figure 5).9,49 In obstructive MGD, hyperkeratinization of the meibomian gland orifice is thought to lead to cystic ductal dilation and downstream disuse atrophy of the meibomian gland acini. This hypothesis is based on findings in animal models showing that chemically induced gland hyperkeratinization leads to initial blockage of the orifice.11,12,14 A few histopathologic studies have also described regional hyperkeratinization in human glands.10,18 However, it should be noted that a hallmark of MGD in subjects is gland dropout, as seen clinically by slitlamp examination and infrared meibography.23,50 By comparison, hyperkeratinized animal eyelids show swelling of the gland and increased transillumination defects starting at the gland orifice.11,13
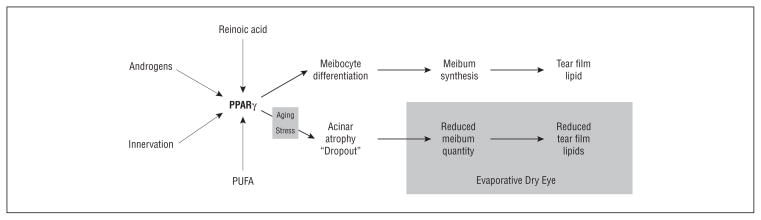
Figure 5.
Proposed mechanisms for the development of age-related, hyposecretory meibomian gland dysfunction (MGD). Expression of peroxisome proliferator–activated receptor (PPARγ) response genes controlling meibocyte differentiation and meibum synthesis and secretion onto the tear film may be modified by various factors including retinoic acid, androgens, innervation, polyunsaturated fatty acids (PUFA). Aging and/or stress alter PPARγ response gene expression, leading to acinar atrophy and gland dropout, reduced meibum quantity, reduced meibum on the tear film, and evaporative dry eye.
In age-related MGD, we hypothesize that meibomian gland aging leads to altered PPARγ response gene regulation that directly effects meibocyte differentiation, resulting in acinar atrophy, gland dropout, and a hyposecretory MGD. This mechanism is consistent with our finding that MG dropout significantly correlates with age and MG expression grade, which has been shown to correlate with increased ocular surface water evaporation.15,16,51 Importantly, this mechanism suggests that the quantity, not the quality, of the meibomian gland secretions may play an initial role in the development of age-related MGD and evaporative dry eye that may occur alone or in conjunction with obstructive MGD. It also suggests that the inflammatory changes that develop during evaporative dry eye may be secondary downstream changes, as indicated by the stronger correlation between acinar infiltration and MGD expression grade in favor of age and MG dropout. This stronger association may also be explained by the pleiotropic effects of PPARγ on cytokine expression within the gland.34 Clearly, additional study into the role of PPARγ in regulating meibomian gland function may provide important insights into the mechanism underlying age-related MGD as well as suggest novel therapeutic approaches to the treatment of evaporative dry eye.
Acknowledgments
Funding/Support: This study was supported by National Institutes of Health infrastructure grant EY016663; Research to Prevent Blindness, Inc; The Skirball Program in Molecular Ophthalmology; and a Research Gift from Alcon.
Footnotes
Author Contributions: Dr Nien and Ms Massei contributed equally to the article.
Financial Disclosure: None reported.
Contributor Information
Dr. Chyong Jy Nien, Gavin Herbert Eye Institute, University of California, Irvine.
Ms. Salina Massei, Gavin Herbert Eye Institute, University of California, Irvine.
Ms. Gloria Lin, Gavin Herbert Eye Institute, University of California, Irvine.
Dr. Cameron Nabavi, Gavin Herbert Eye Institute, University of California, Irvine.
Dr. Jeremiah Tao, Gavin Herbert Eye Institute, University of California, Irvine.
Dr. Donald J. Brown, Gavin Herbert Eye Institute, University of California, Irvine.
Mr. Jerry R. Paugh, Southern California College of Optometry, Fullerton.
Dr. James V. Jester, Gavin Herbert Eye Institute, University of California, Irvine.
References
- 1.Lemp MA, Nichols KK. Blepharitis in the United States 2009: a survey-based perspective on prevalence and treatment. Ocul Surf. 2009;7(2 suppl):S1–S14. doi: 10.1016/s1542-0124(12)70620-1. [DOI] [PubMed] [Google Scholar]
- 2.Hom MM, Martinson JR, Knapp LL, Paugh JR. Prevalence of Meibomian gland dysfunction. Optom Vis Sci. 1990;67(9):710–712. doi: 10.1097/00006324-199009000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Ong BL. Relation between contact lens wear and Meibomian gland dysfunction. Optom Vis Sci. 1996;73(3):208–210. doi: 10.1097/00006324-199603000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Ong BL, Larke JR. Meibomian gland dysfunction: some clinical, biochemical and physical observations. Ophthalmic Physiol Opt. 1990;10(2):144–148. doi: 10.1111/j.1475-1313.1990.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 5.Mishima S, Maurice DM. The oily layer of the tear film and evaporation from the corneal surface. Exp Eye Res. 1961;1:39–45. doi: 10.1016/s0014-4835(61)80006-7. [DOI] [PubMed] [Google Scholar]
- 6.Gilbard JP, Rossi SR, Heyda KG. Tear film and ocular surface changes after closure of the meibomian gland orifices in the rabbit. Ophthalmology. 1989;96 (8):1180–1186. doi: 10.1016/s0161-6420(89)32753-9. [DOI] [PubMed] [Google Scholar]
- 7.McCulley JP, Shine WE. Meibomian gland function and the tear lipid layer. Ocul Surf. 2003;1(3):97–106. doi: 10.1016/s1542-0124(12)70138-6. [DOI] [PubMed] [Google Scholar]
- 8.Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol. 1995;113(10):1266–1270. doi: 10.1001/archopht.1995.01100100054027. [DOI] [PubMed] [Google Scholar]
- 9.Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf. 2003;1(3):107–126. doi: 10.1016/s1542-0124(12)70139-8. [DOI] [PubMed] [Google Scholar]
- 10.Gutgesell VJ, Stern GA, Hood CI. Histopathology of meibomian gland dysfunction. Am J Ophthalmol. 1982;94(3):383–387. doi: 10.1016/0002-9394(82)90365-8. [DOI] [PubMed] [Google Scholar]
- 11.Jester JV, Nicolaides N, Kiss-Palvolgyi I, Smith RE. Meibomian gland dysfunction II: the role of keratinization in a rabbit model of MGD. Invest Ophthalmol Vis Sci. 1989;30(5):936–945. [PubMed] [Google Scholar]
- 12.Jester JV, Rajagopalan S, Rodrigues M. Meibomian gland changes in the rhino (hrrhhrrh) mouse. Invest Ophthalmol Vis Sci. 1988;29(7):1190–1194. [PubMed] [Google Scholar]
- 13.Jester JV, Rife L, Nii D, Luttrull JK, Wilson L, Smith RE. In vivo biomicroscopy and photography of meibomian glands in a rabbit model of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 1982;22(5):660–667. [PubMed] [Google Scholar]
- 14.Ohnishi Y, Kohno T. Polychlorinated biphenyls poisoning in monkey eye. Invest Ophthalmol Vis Sci. 1979;18(9):981–984. [PubMed] [Google Scholar]
- 15.Guillon M, Maïssa C. Tear film evaporation: effect of age and gender. Cont Lens Anterior Eye. 2010;33(4):171–175. doi: 10.1016/j.clae.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Maïssa C, Guillon M. Tear film dynamics and lipid layer characteristics: effect of age and gender. Cont Lens Anterior Eye. 2010;33(4):176–182. doi: 10.1016/j.clae.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Hykin PG, Bron AJ. Age-related morphological changes in lid margin and meibomian gland anatomy. Cornea. 1992;11(4):334–342. doi: 10.1097/00003226-199207000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Obata H. Anatomy and histopathology of human meibomian gland. Cornea. 2002;21(7 suppl):S70–S74. doi: 10.1097/01.ico.0000263122.45898.09. [DOI] [PubMed] [Google Scholar]
- 19.Nien CJ, Paugh JR, Massei S, Wahlert AJ, Kao WW, Jester JV. Age-related changes in the meibomian gland. Exp Eye Res. 2009;89(6):1021–1027. doi: 10.1016/j.exer.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen ED, Spiegelman BM. PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276(41):37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 21.Nien CJ, Massei SR, Lin G, et al. The development of meibomian glands in mice. Mol Vis. 2010;16:1132–1140. [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen ED, Sarraf P, Troy AE, et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4(4):611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 23.Mathers WD, Shields WJ, Sachdev MS, Petroll WM, Jester JV. Meibomian gland dysfunction in chronic blepharitis. Cornea. 1991;10(4):277–285. doi: 10.1097/00003226-199107000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Nichols JJ, Berntsen DA, Mitchell GL, Nichols KK. An assessment of grading scales for meibography images. Cornea. 2005;24(4):382–388. doi: 10.1097/01.ico.0000148291.38076.59. [DOI] [PubMed] [Google Scholar]
- 25.Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14(11):1293–1307. [PubMed] [Google Scholar]
- 26.Willson TM, Lambert MH, Kliewer SA. Peroxisome proliferator-activated receptor gamma and metabolic disease. Annu Rev Biochem. 2001;70:341–367. doi: 10.1146/annurev.biochem.70.1.341. [DOI] [PubMed] [Google Scholar]
- 27.Hammarstedt A, Andersson CX, Rotter Sopasakis V, Smith U. The effect of PPAR-gamma ligands on the adipose tissue in insulin resistance. Prostaglandins Leukot Essent Fatty Acids. 2005;73(1):65–75. doi: 10.1016/j.plefa.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 29.Fan W, Yanase T, Nomura M, et al. Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes. 2005;54(4):1000–1008. doi: 10.2337/diabetes.54.4.1000. [DOI] [PubMed] [Google Scholar]
- 30.Ciana P, Biserni A, Tatangelo L, et al. A novel peroxisome proliferator-activated receptor responsive element-luciferase reporter mouse reveals gender specificity of peroxisome proliferator-activated receptor activity in liver. Mol Endocrinol. 2009;21:388–400. doi: 10.1210/me.2006-0152. [DOI] [PubMed] [Google Scholar]
- 31.Makrantonaki E, Zouboulis CC. Testosterone metabolism to 5alpha-dihydrotestosterone and synthesis of sebaceous lipids is regulated by the peroxisome proliferator-activated receptor ligand linoleic acid in human sebocytes. Br J Dermatol. 2007;156(3):428–432. doi: 10.1111/j.1365-2133.2006.07671.x. [DOI] [PubMed] [Google Scholar]
- 32.Halvorsen B, Heggen E, Ueland T, et al. Treatment with the PPARgamma agonist rosiglitazone downregulates interleukin-1 receptor antagonist in individuals with metabolic syndrome. Eur J Endocrinol. 2010;162(2):267–273. doi: 10.1530/EJE-09-0706. [DOI] [PubMed] [Google Scholar]
- 33.Moulin D, Bianchi A, Boyault S, et al. Rosiglitazone induces interleukin–1 receptor antagonist in interleukin–1 beta-stimulated rat synovial fibroblasts via a peroxisome proliferator-activated receptor beta/delta-dependent mechanism. Arthritis Rheum. 2005;52:759–769. doi: 10.1002/art.20868. [DOI] [PubMed] [Google Scholar]
- 34.Pershadsingh HA. Peroxisome proliferator-activated receptor-gamma: therapeutic target for diseases beyond diabetes: quo vadis? Expert Opin Investig Drugs. 2004;13(3):215–228. doi: 10.1517/13543784.13.3.215. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan DA, Sullivan BD, Ullman MD, et al. Androgen influence on the meibomian gland. Invest Ophthalmol Vis Sci. 2000;41(12):3732–3742. [PubMed] [Google Scholar]
- 36.Schirra F, Suzuki T, Richards SM, et al. Androgen control of gene expression in the mouse meibomian gland. Invest Ophthalmol Vis Sci. 2005;46(10):3666–3675. doi: 10.1167/iovs.05-0426. [DOI] [PubMed] [Google Scholar]
- 37.Richards SM, Yamagami H, Schirra F, Suzuki T, Jensen RV, Sullivan DA. Sex-related effect on gene expression in the mouse meibomian gland. Curr Eye Res. 2006;31(2):119–128. doi: 10.1080/02713680500514644. [DOI] [PubMed] [Google Scholar]
- 38.Sullivan DA. Tearful relationships? sex, hormones, the lacrimal gland, and aqueous-deficient dry eye. Ocul Surf. 2004;2(2):92–123. doi: 10.1016/s1542-0124(12)70147-7. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan BD, Evans JE, Cermak JM, Krenzer KL, Dana MR, Sullivan DA. Complete androgen insensitivity syndrome: effect on human meibomian gland secretions. Arch Ophthalmol. 2002;120(12):1689–1699. doi: 10.1001/archopht.120.12.1689. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan DA, Sullivan BD, Evans JE, et al. Androgen deficiency, Meibomian gland dysfunction, and evaporative dry eye. Ann N Y Acad Sci. 2002;966:211–222. doi: 10.1111/j.1749-6632.2002.tb04217.x. [DOI] [PubMed] [Google Scholar]
- 41.Cermak JM, Krenzer KL, Sullivan RM, Dana MR, Sullivan DA. Is complete androgen insensitivity syndrome associated with alterations in the meibomian gland and ocular surface? Cornea. 2003;22(6):516–521. doi: 10.1097/00003226-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg ES, Asbell PA. Essential fatty acids in the treatment of dry eye. Ocul Surf. 2010;8(1):18–28. doi: 10.1016/s1542-0124(12)70214-8. [DOI] [PubMed] [Google Scholar]
- 43.Afonso AA, Monroy D, Stern ME, Feuer WJ, Tseng SC, Pflugfelder SC. Correlation of tear fluorescein clearance and Schirmer test scores with ocular irritation symptoms. Ophthalmology. 1999;106(4):803–810. doi: 10.1016/S0161-6420(99)90170-7. [DOI] [PubMed] [Google Scholar]
- 44.Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42(10):2283–2292. [PubMed] [Google Scholar]
- 45.Pflugfelder SC, Stern ME. Symposium Participants. Immunoregulation on the ocular surface: 2nd Cullen symposium. Ocul Surf. 2009;7(2):67–77. doi: 10.1016/s1542-0124(12)70297-5. [DOI] [PubMed] [Google Scholar]
- 46.Lambert RW, Smith RE. Pathogenesis of blepharoconjunctivitis complicating 13-cis-retinoic acid (isotretinoin) therapy in a laboratory model. Invest Ophthalmol Vis Sci. 1988;29(10):1559–1564. [PubMed] [Google Scholar]
- 47.Lambert RW, Smith RE. Effects of 13-cis-retinoic acid on the hamster meibomian gland. J Invest Dermatol. 1989;92(3):321–325. doi: 10.1111/1523-1747.ep12277122. [DOI] [PubMed] [Google Scholar]
- 48.Mathers WD, Shields WJ, Sachdev MS, Petroll WM, Jester JV. Meibomian gland morphology and tear osmolarity: changes with Accutane therapy. Cornea. 1991;10(4):286–290. doi: 10.1097/00003226-199107000-00002. [DOI] [PubMed] [Google Scholar]
- 49.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 50.Robin JB, Jester JV, Nobe J, Nicolaides N, Smith RE. In vivo transillumination biomicroscopy and photography of meibomian gland dysfunction: a clinical study. Ophthalmology. 1985;92(10):1423–1426. doi: 10.1016/s0161-6420(85)33848-4. [DOI] [PubMed] [Google Scholar]
- 51.Mathers WD. Ocular evaporation in meibomian gland dysfunction and dry eye. Ophthalmology. 1993;100(3):347–351. doi: 10.1016/s0161-6420(93)31643-x. [DOI] [PubMed] [Google Scholar]