Abstract
Structural, biochemical and biophysical studies of eukaryotic membrane proteins are often hampered by difficulties in over-expression of the candidate molecule. Baculovirus transduction of mammalian cells (BacMam), although a powerful method to heterologously express membrane proteins, can be cumbersome for screening and expression of multiple constructs. We therefore developed plasmid Eric Gouaux (pEG) BacMam, a vector optimized for use in screening assays, as well as for efficient production of baculovirus and robust expression of the target protein. In this protocol we show how to use small-scale transient transfection and fluorescence-detection, size-exclusion chromatography (FSEC) experiments using a GFP-His8 tagged candidate protein to screen for monodispersity and expression level. Once promising candidates are identified, we describe how to generate baculovirus, transduce HEK293S GnTI− (N-acetylglucosaminyltransferase I-negative) cells in suspension culture, and over-express the candidate protein. We have used these methods to prepare pure samples of chicken acid-sensing ion channel 1a (cASIC1) and Caenorhabditis elegans glutamate-gated chloride channel (GluCl), for X-ray crystallography, demonstrating how to rapidly and efficiently screen hundreds of constructs and accomplish large-scale expression in 4-6 weeks.
INTRODUCTION
Since the initial observation that insertion of a human cytomegalovirus (CMV) promoter or a Rous sarcoma virus (RSV) promoter into an Autographa californica multiple nucleopolyhedrosis virus (AcMNPV; from here on referred to as baculovirus) transfer vector allowed for expression of foreign genes in hepatocytes and other mammalian cell lines 1,2, baculovirus -mediated gene transfer into mammalian cells (BacMam) has been employed for a growing number of applications. These applications include drug discovery (identification and development of new therapeutic agents) through recombinant protein expression for cell-based functional assays using G-protein-coupled receptors3,4, nuclear receptors5, ion channels6,7 and ATP-binding cassette drug transporters8. More recently, BacMam has been used for large-scale protein production for crystallography9–20. The success of these applications, however, depends in part on the efficient production and amplification of baculovirus and on subsequent large-scale transduction and heterologous protein expression. In addition to these challenges, obtaining sufficient quantities of membrane protein for crystallography is frequently compounded by low levels of expression and instability of the candidate membrane protein, thus requiring screening of many constructs. Furthermore, some mammalian membrane proteins require specific post translational modifications and a near native lipid environment, thus rendering expression in insect cells or in yeast untenable. Taken together, these complexities can result in a high cost for heterologous membrane protein expression in mammalian cells and thus improving the efficiency of the process is important.
Here we describe methods to screen constructs and to optimize heterologous expression of membrane proteins from BacMam transduced HEK293S GnTI− (N-acetylglucosaminyltransferase I-negative) cells for purification and crystallization (Fig. 1). We have constructed a vector (pEG BacMam) for high level expression in mammalian cells using elements derived from a previously described vector, pVLAD10. Once genes of candidate membrane proteins are fused in frame with a GFP tag and cloned into pEG BacMam, they can be rapidly screened for expression and monodispersity using transient transfection in adherent cells coupled with fluorescence-detection size-exclusion chromatography (FSEC)21,22. We also optimized virus amplification and protein expression protocols such that cost and time for expressing most membrane proteins in HEK293 GnTI− cells is similar to or better than expression in Sf9 cells.
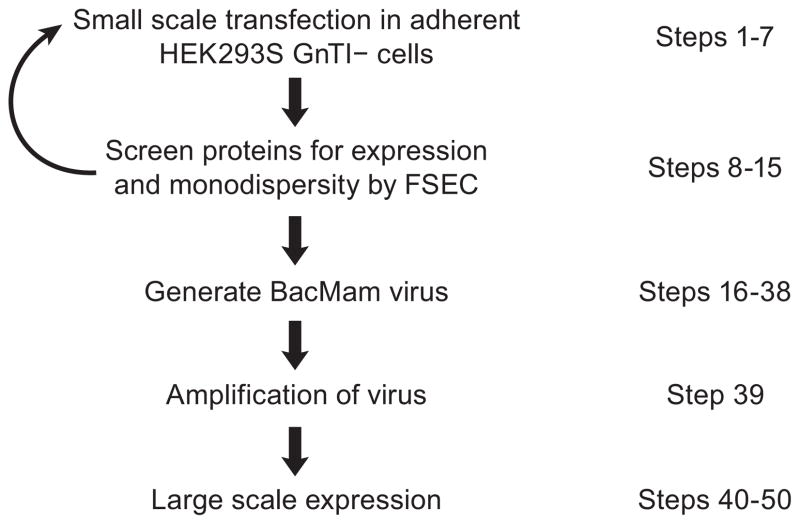
Figure 1.
Flow chart of the overexpression of membrane proteins in HEK293S GnTI− cells. After one or more rounds of screening, a few potential candidates are chosen for large-scale protein expression.
In the current protocol we provide two examples in which we express Gallus gallus Acid-sensing ion channel 1a16,23(cASIC1) and Caenorhabditis elegans glutamate-gated chloride channel (GluCl) in mammalian cells24,25. After optimizing protein expression, we compared the expression of cASIC1 and GluCl in mammalian cells and insect cells. We show that five-fold more GluCl pentamer can be obtained in mammalian cells. In the case of cASIC1, not only can two-fold more trimer be obtained in mammalian cells, but also the protein is more monodisperse and experiences less spontaneous cleavage of the GFP-His8 tag. This protocol is now in standard use in our laboratory for mammalian-expressed membrane proteins15–17,20.
Development of the protocol
To increase the heterologous expression of challenging membrane proteins we first constructed pEG BacMam for high-level protein expression in mammalian cells with the ability to express multiprotein complexes from a single vector (Fig. 2). To do this we chemically synthesized genetic elements derived from the previously described BacMam vector, pVLAD10, which include a strong CMV promoter for robust transcription, a synthetic intron for efficient RNA splicing and mRNA processing and a WPRE motif for efficient mRNA processing, stability and export. These chemically synthesized elements were combined with the pFBDM26, a bicistronic vector with a restriction enzyme module that allows the assembly of multiple expression cassettes, to generate pEG BacMam. After the gene of interest is cloned into pEG BacMam, we screen constructs by small scale transfection/FSEC before moving to the time consuming process of virus amplification21.
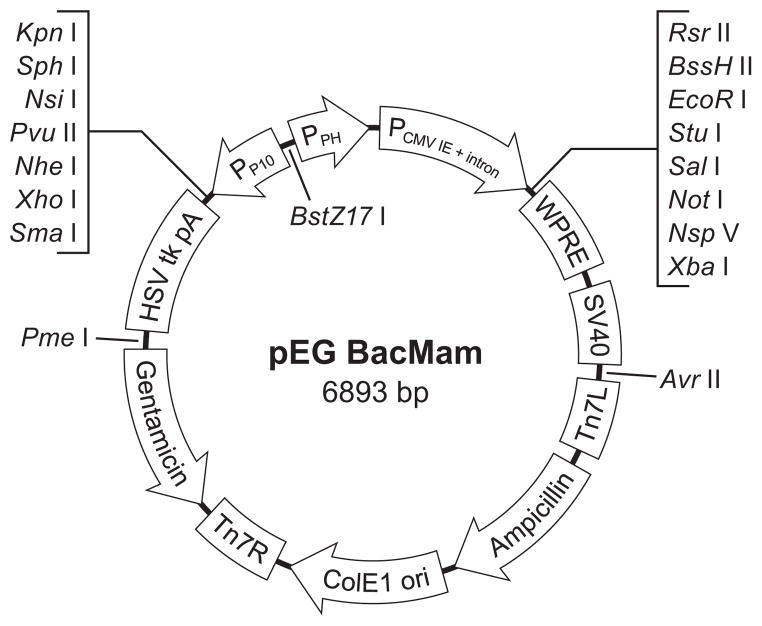
Figure 2.
Map of BacMam Expression Vector. For expression in mammalian cells, genes of interest are cloned into the multiple cloning site behind the CMV promoter using unique restriction sites. Elements that are important for high level expression are shown, including those that are important for transcription initiation (CMV promoter), transcription termination (SV40 poly A late signal), mRNA processing (intron and WPRE motif). Also indicated are the elements important for insect cell expression and baculovirus amplification including promoters (polh and p10), terminators (SV40 and HSVtk), transposon elements (Tn7L and Tn7R), and resistance markers (ampicillin and gentamicin).
Although other HEK cell lines can be used, for screening and expression we typically use HEK293S GnTI− cells, a mammalian cell line that expresses proteins that are more mannose-rich and as a result are easily removed with endoglycosidases such as EndoH27. Although the use of these cells and EndoH can reduce heterogeneity caused by complex glycans that can create problems in crystallographic studies, it may not beneficial for every protein. Therefore, it is advantageous to test protein expression in other mammalian cell lines as well as determine if the use of EndoH affects the solubility and the heterogeneity of the glycoprotein.
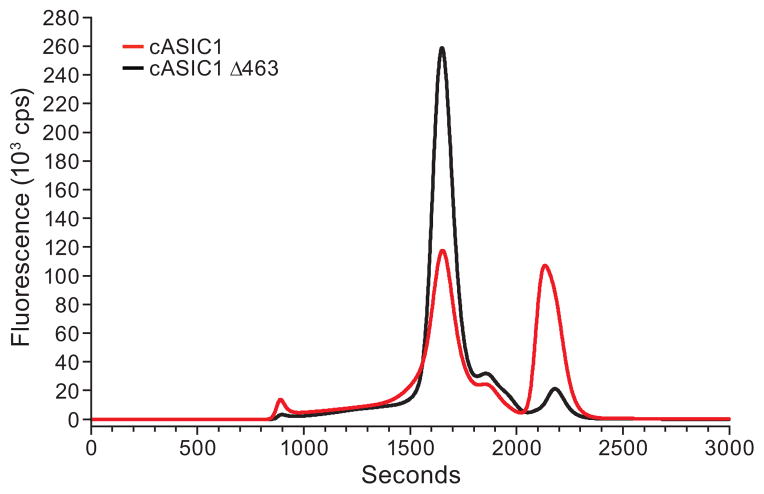
To determine the expression level and monodispersity of the candidate membrane protein, HEK293S GnTI− cells are transfected with the pEG BacMam plasmid containing the gene of interest; harvested after 48 hours; and then solubilized in a buffer containing n-dodecyl-β-D-maltoside (C12M), maltose-neopentyl glycol28 (MNG-3), or other detergent. The resulting supernatant is analyzed by FSEC (Fig. 3). As shown in Figure 3, removing the 64 residues from the carboxy terminus of cASIC1 (cASIC463) increases monodispersity and reduces cleavage of the GFP-His8 tag. In addition to removing flexible termini, there are many methods that can be used to optimize expression and stability of proteins including codon optimization, surface entropy reduction, and thermostability mutations29–31. Small scale transfection followed by whole cell solubilization and FSEC allows the screening of hundreds of candidates in ≤1 month.
Figure 3.
Screening constructs by small scale transient transfection in HEK293S GnTI− cells and FSEC. 1 μg of N-terminal eGFP tagged full length cASIC1 or cASIC463 (cASIC1 truncated 64 residues from the carboxy termini) subcloned into pEG BacMam was transfected separately into HEK293S GnTI− cells, as described in the text. Cells were harvested 48 h after transfection and solubilized extracts were analyzed by FSEC to determine the behavior of the fusion proteins. c.p.s., counts per second.
Once a promising candidate is identified, the plasmid is transformed into the DH10Bac E. coli strain to generate the recombinant bacmid DNA which is then used to transfect insect cells to generate BacMam virus. We have detailed our methods for isolation of bacmid DNA, transfection of Sf9 cells and baculovirus amplification that we use to reduce costs and ensure good quality BacMam virus. We have found for some constructs that the multiplicity of infection (MOI) during virus amplification is 10 to 100 fold below the range recommended by the Bac-to-Bac system protocol (http://www.lifetechnologies.com/us/en/home/life-science/protein-expression-and-analysis/protein-expression/insect-expression/bac-to-bac-baculovirus-expression-system.html). We have also found that a low MOI (MOI of 2 or less) is sufficient for mammalian cell transduction and that too much virus results in low cell numbers, possibly due to too much Sf9 medium or virus added to the culture. Therefore, before virus amplification or transduction of mammalian cells for protein expression, virus titer should be determined using the end point dilution assay32, flow cytometric assay33,34, or the viral plaque assay35.
In addition to MOI, we also explored different growth and expression conditions for BacMam transduced HEK293S GnTI− cells to boost protein expression. After testing several types of media for growth of suspension cells, we found that the use of Gibco FreeStyle 293 Expression Medium (Invitrogen) allowed for increased growth rates and reduced cell clumping of HEK293S GnTI− cells in suspension. To further minimize cell clumping, we also assessed the growth of suspension cells in different vessels, including square bottles, flat-bottom flasks and baffled Erlenmeyer flasks. We found that baffled flasks minimized cell clumping and promoted cell growth. To reduce costs, the polycarbonate Erlenmeyer flasks can be washed, autoclaved and used again up to 20 times. If after autoclaving the filter in the cap deteriorates, a replacement cap can be purchased.
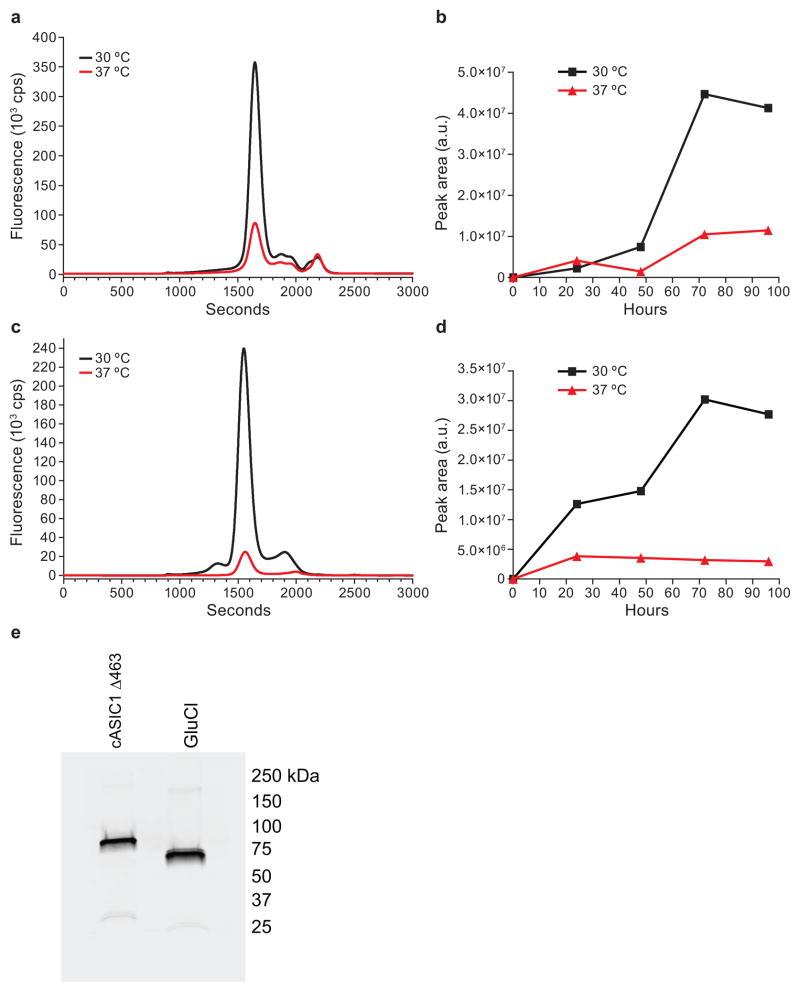
Previously it has been shown that lowering the temperature post-transduction or transfection enhances protein expression in mammalian cells36–43. In a time course experiment for cASIC1, we found that at the optimal harvest time of 72h post transduction there was a 4.3 fold increase in expression at 30 °C compared to 37 °C (Fig. 4a). Gaussian fitting, as described previously21,22, was performed on the FSEC profiles to determine the peak area of cASIC1 trimer. A graph of the trimer peak area shows that the expression of cASIC1 is higher at 30 °C than at 37 °C for most time points (Fig. 4b). To monitor the expression of GluCl in HEK293S GnTI− cells, an EGFP GluCl gene fusion25,44 was cloned into pEG BacMam and used to generate BacMam virus. At the optimal harvest time of 72 h post transduction for cells expressing GluCl, there was a 9.5-fold increase in expression at 30 °C with higher expression of GluCl at all time points at 30 °C (Fig. 4c and 4d). To show that the protein present in the major peaks for cASIC and GluCl are the expected molecular weight, peak fractions were collected and analyzed by SDS-PAGE followed by Western blot analysis using an antibody against GFP (Fig. 4e). In fact, for the expression of most proteins in HEK293S GnTI− cells, we have found that lowering the temperature during expression increases protein yields at least two-fold. In some cases, lowering the temperature is essential to obtain monodisperse, well-folded protein.
Figure 4.
Time course of expression of cASIC and GluCl in HEK293S GnTI− cells. HEK293S GnTI− cells (3×106 cells/ml) were transduced with BacMam virus for cASIC463 (a and b) or GluCl-EGFP (c and d) at a MOI of 1 and incubated at 37 °C. After 8 h, 10 mM sodium butyrate was added and cultures were left at 37 °C or shifted to 30 °C. Time points were taken at the indicated times, samples were frozen and later solubilized with 40 mM C12M in TBS pH 8 and analyzed by FSEC. (a and c) Representative FSEC profiles from cASIC463 (a) and GluCl-EGFP (c) detected by GFP fluorescence. (b and d) Comparison of expression at 37 °C and 30 °C from cASIC463 and (b) GluCl-EGFP (d) based on the peak area estimated by Gaussian fitting. (e) Western blot analysis using an anti-GFP polyclonal antibody of peak fractions isolated from FSEC profiles from (a) cASIC463 and (c) GluCl-EGFP.
Finally, the use of histone deacetylase inhibitors has been shown to enhance protein expression in HEK293S cells10,45. We found that for most membrane proteins histone deacetylase inhibitors boost expression. For cASIC1 there is a 7-fold increase in expression at 72 h post-transduction as well as higher expression of cASIC1 at all time points when HEK293S GnTI− cells are treated with 10 mM sodium butyrate (Fig. 5a and 5b). Previously published data suggests that valproic acid is more efficient than sodium butyrate at enhancing recombinant protein production from mammalian cells45. However, we find that both sodium butyrate and valproic acid enhance protein expression from BacMam transduced HEK293S GnTI− cells (Fig. 5c). Typically sodium butyrate is added to cultures between 8–24 hours post-transduction; however the amount and time of sodium butyrate addition should be optimized for each protein. Overall optimization of conditions (construct, MOI, cell density, temperature and harvest time of BacMam transduced HEK293S GnTI− cells) could either increase expression or decrease aggregation leading to more properly folded protein and therefore the most favorable conditions for each protein should be determined before attempting a large scale expression.
Figure 5.
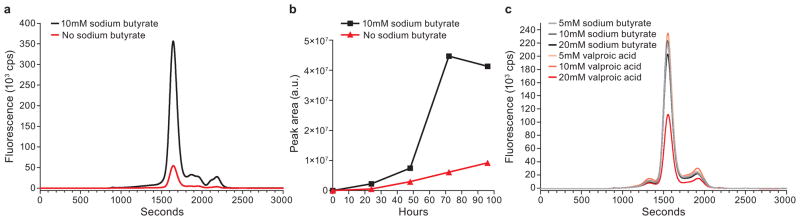
Effect of histone deacetylase inhibitors on the expression of cASIC and GluCl in HEK293S GnTI− cells. (a and b) Comparison of cASIC expression with and without sodium butyrate. Representative FSEC profiles from cASIC463 detected by GFP fluorescence (a) and comparison of expression from cASIC463 based on the peak area estimated by Gaussian fitting (b) in the presence or absence of sodium butyrate. (c) Representative FSEC profiles from GluCl-EGFP in the presence of sodium butyrate or valproic acid.
Other applications of the method
The protocol described here could also be used to optimize the expression of heteromers or protein complexes in mammalian cells. One option to simultaneously express multiple proteins is to co-infect with multiple BacMam viruses (with an optimized MOI for each virus). Alternatively, pEG BacMam could be used to express multiprotein complexes by combining two pEG BacMam plasmids using unique restriction enzyme sites. As a result, multiple genes could be transduced by a single BacMam virus allowing for the simultaneous expression of two or more genes.
Some variants of pEG BacMam also contains vesicular stomatitis Indiana virus glycoprotein (VSIV-G) under control of the P10 promoter (a baculovirus specific promoter). VSIV-G is a viral capsule protein important for mediating viral entry and has been shown to increase the transduction efficiency of baculovirus for some mammalian cells46. The P10 promoter could be used to drive the expression of VSIV-G in insect cells allowing incorporation of VSIV-G into the baculovirus to enhance transduction from other mammalian cell lines, such as the human lung carcinoma line A549 and the human hepatoma lines HepG2 and HuH746.
Comparison to other methods
Many methods have been utilized for overexpression of mammalian proteins including plasmid transfection, stable cell lines and viral expression systems such as Sindbis virus, vaccinia virus, and Semliki Forest virus, and AcMNPV47. Compared to BacMam, each of these methods has advantages and disadvantages in terms of cost, time, efficiency, safety and reproducibility.
One such transient expression method involves transfection of plasmid DNA into adherent cells or cultures and overexpression is either immediate or induced, depending on the promoter. Plasmid transfection is fast, safe and easy to use for high throughput screening48–50. If commercial transfection reagents and plasmid isolation kits are used, however, plasmid transfection can be expensive for large scale expression. Furthermore, the level of expression using plasmid transfection can be limited by the plasmid size, the number of plasmids transfected, and cytotoxic effects that have been observed with many transfection reagents. The use of BacMam can be cheaper than plasmid transfection for large scale expression and multiple rounds of expression. In addition, BacMam is not limited by the gene number or size. In our hands the level of protein expression, especially multi-subunit proteins, is higher using BacMam than plasmid transfection.
Stable expression in mammalian cells requires the integration of a transfected transgene into the cell’s genome using Geneticin or other selection methods. Once clonal cell lines are generated and sorted for high-level producers, long-term overexpression from stably transfected cells can be robust, easy and consistent51,52. Furthermore, stable cell lines can be generated using regulated expression, such as the tetracycline-inducible expression system, thus allowing for large scale expression of proteins cytotoxic to the cell51,53. Although stable expression enables the production of large quantities of protein, when compared to BacMam generating a stable cell line is time consuming.
Although other viral expression systems for protein expression such as lentiviruses54, adenoviruses55, Sindbis virus56,57, vaccinia virus58, and Semliki Forest virus59,60 (SFV) exist, SFV has been used to express a large number of membrane proteins consisting of mostly GPCRs47. To make virus, candidate genes are cloned into SFV plasmid and used as template for RNA synthesis. The RNA is then co-transfected (either using electroporation or liposome reagents) with helper RNA and packaged into SFV particles that can then be used to infect cells in culture. While SFV can be easily used for small scale studies by transfecting synthesized RNA into cells and analyzing expression, using SFV for large cultures is more challenging than BacMam due to the amount of RNA that is needed to make virus for large scale studies.
An effective method using BacMam was recently described to produce milligram quantities of proteins sufficient for crystallization10. However, the methods outlined therein to produce recombinant BacMam virus using the BD BaculoGold system are not as cost efficient as the Bac-to-Bac method if multiple constructs are expected to be generated. In addition, we optimized growth and expression conditions using BacMam in order to express sufficient amounts of our desired membrane proteins.
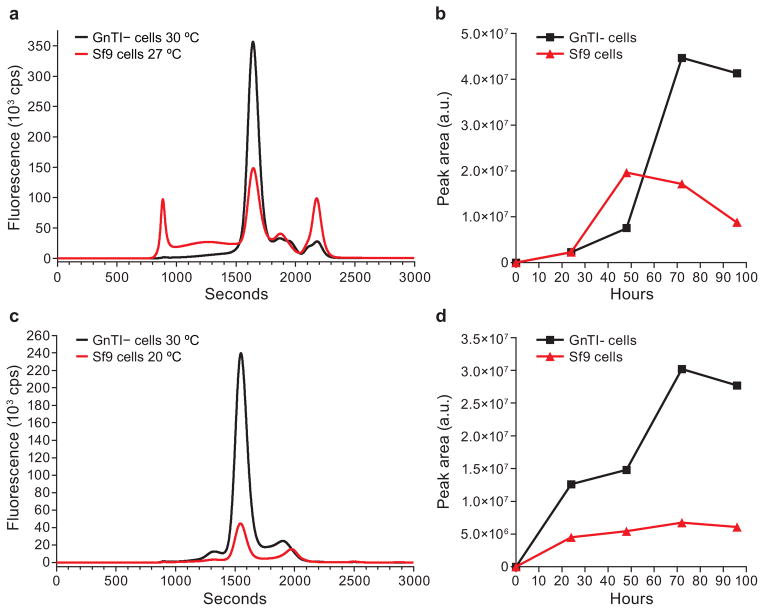
Another commonly used method for overexpression of proteins is AcMNPV baculovirus infection of insect cells. Although insect cells such as Sf9 cells have been used in our lab previously to provide sufficient protein for crystallization studies25,61–64, some membrane proteins require a near-native environment to help ensure functional expression. The advantages for expression of eukaryotic membrane proteins in mammalian cells over Sf9 include improved post-translational modifications such as N-linked glycosylation65,66 and a different lipid environment that contains higher amounts of cholesterol. We performed a side-by-side comparison of protein expression from insect cells and mammalian cells. We found a two-fold increase in cASIC1 trimer and an increase in homogeneity (Fig. 6a-b) and that five-fold more GluCl pentamer can be obtained in mammalian cells (Fig. 6c-d).
Figure 6.
Comparison of expression time course in HEK293S GnTI− cells and Sf9 cells. HEK293S GnTI− cells were transduced with BacMam virus for cASIC463 (a and b) or GluCl-EGFP (c and d) as described above. Sf9 cells (3×106 cells/ml) were infected with baculovirus cASIC463 (a and b) or GluCl-EGFP (c and d) and placed at 27 °C. After 18 h, Sf9 cultures were left at 27 °C or shifted to 20 °C. Time points were taken, frozen and solubilized as described above. (a and c) Representative FSEC profiles from cASIC463 (a) and GluCl-EGFP (c) detected by GFP fluorescence. (b and d) Comparison of expression from cASIC463 (b) GluCl-EGFP (d) based on the peak area estimated by Gaussian fitting.
MATERIALS
REAGENTS
pEG BacMam plasmid containing the gene of interest in frame with a GFP tag and a tag appropriate for affinity chromatography (such as a His8 tag) cloned downstream of the CMV promoter (Fig. 2)
HEK293S GnTI− cells (ATCC, cat. no. CRL-3022)
Gibco Freestyle 293 Expression medium (Life Technologies, cat. no. 12338-018)
US Certified Gamma Irradiated FBS (Life Technologies, cat. no. 0984018DJ)
DMEM (with 4.5 g/l glucose, L-glutamine, and sodium pyruvate; Corning|Cellgro, cat. no. 10-013)
Opti-MEM I Reduced Serum Medium (Life Technologies, cat. no. 31985-088)
Lipofectamine 2000 (Life Technologies, cat. no. 11668-027)
PBS (without calcium and magnesium; Corning|Cellgro, cat. no. 21-040-CM)
Trypsin/EDTA (Corning|Cellgro, cat. no. 25-052-CV)
Tris base (Fisher, cat. no. BP152)
NaCl (Sigma, cat. no. 59888)
C12M (Affymetrix, cat. no. D310)
PMSF (Sigma, cat. no. 78830)
Leupeptin (Sigma, cat. no. L0649)
Aprotinin (Sigma, cat. no. A1153)
Pepstatin (Sigma, cat. no. P4265)
Sodium butyrate (Sigma, cat. no. 303410)
Valproic Acid (Sigma, cat. no. P4543)
DH10Bac competent cells (Life Technologies, cat no. 10361-012)
Sf9 Easy Titer cell line32
Cellfectin II reagent (Life Technologies, cat. no. 10362-100)
Sf9 cells (Life Technologies, cat. no. 12659017)
Sf-900 III SFM media (Life Technologies, cat. no. 12658-027)
QIAprep Spin Miniprep Kit (QIAGEN, cat. no. 27104)
HyClone SFX-Insect Media (GE/Hyclone, cat. no. SH30278.02)
G 418 disulfate salt (Sigma, cat. no. A1720-1g)
Trypan blue solution (Corning|Cellgro, cat. no. 25-900-CL)
EQUIPMENT
Incubator (Thermo Scientific, cat. no. 3950)
Tissue culture plate (100 mm; BD Falcon, cat. no. 35300)
Tissue culture plate (6 well; BD Falcon, cat. no. 353046)
Erlenmeyer baffled Flasks (2000 ml; BioExpress, cat. no. F-5909-2000B)
Nunc EasYFlask (75 cm2, Filter Cap; Thermo Scientific, cat. no. 156499)
Syringe filters (PES 13 mm Diameter, 0.22 um, PP housing; Argos, cat. no. FE12S)
Filter systems (250 ml, 0.22 μm; Corning, cat. no. 430767)
Virus counter 2100 (ViroCyt)
Optima TL Ultracentrifuge (Beckman)
Fluorescence-detection size-exclusion chromatography (FSEC)21
Tissue culture plate (Costar 96-Well Black Clear-Bottom Plates; Costar /Corning, cat. no. 3603)
Sterile Disposable Reagent Reservoirs (50ml white; Costar /Corning, cat. no. 4870)
Cluster Tube System 8-tube strip (Costar /Corning product, cat. no. 4413)
Cell Scrapers (Handle 18cm blade 1.8cm; Corning, cat. no. 353085)
Hemocytometer (Hausser Scientific, cat. no. 1492)
REAGENT SETUP
DMEM medium
To 500 ml of DMEM medium, add 50 ml of FBS. Store at 4 °C for a month.
Suspension medium
To 1 liter of Freestyle 293 Expression medium, add 20 ml of FBS. Store at 4 °C for a month.
Sf9 easy medium
To 1 liter of HyClone SFX-Insect Media, add 50 ml of FBS and 150 μg/ml G418. Store at 4 °C for a month.
Sodium butyrate (2M)
Dissolve 11 g of sodium butyrate with water to a final volume of 50 ml and filter-sterilize using a 0.2 μm filter inside the biological safety cabinet. Store at −20 °C for at least 1 month.
Tris-buffered saline (TBS)
Mix 20 mM Tris HCl (pH 8), 150 mM NaCl. Store at RT; 25 °C for at least a month.
Solubilization buffer
Mix 20 mM Tris HCl (pH8), 150 mM NaCl, and 40 mM C12M. Chill the buffer to 4 °C. Immediately before use add 1 mM PMSF, 200 μM Aprotinin, 2 μg/ml Leupeptin, and 2 μM Pepstatin A. Discard any unused buffer.
FSEC buffer
Mix 20 mM Tris HCl (pH 8), 150 mM NaCl, and 1 mM C12M. Filter using a 0.2 μm filter. Store at 4 °C for up to 1 week.
Purification of plasmid DNA
Purify plasmid DNA using the QIAprep Spin Miniprep Kit (QIAGEN) or another suitable method.
Growth and maintenance of adherent HEK293S GnTI− cells
Cells are cultured as previously described51.
Growth and maintenance of suspension HEK293S GnTI− cells
HEK293S GnTI− cells are maintained as described in Box 1.
Box 1. Growth and maintenance of suspension HEK293S GnTI− cells ● TIMING 15 min.
-
1|
Slough off HEK293S GnTI− cells from a T-75 flask at 80% confluency using 25 ml of FreeStyle293 Expression Medium (Invitrogen) supplemented with 2% FBS.
-
2|
Transfer the cell suspension to a 125 ml baffled flask and place it on an orbital shaker within a 37 °C incubator in the presence of 8% CO2.
CRITICAL The cell suspension should not exceed more than 40% of the baffled flask size.
-
3|
After 24 h dilute the cells to 0.5×106 cells/ml and maintain the suspension adapted cells between 0.2–3 × 106 cells/ml at 40% of vessel size for baffled flasks.
? TROUBLESHOOTING
Growth and maintenance of Sf9 cells
Sf9 cells are maintained as suspension cultures at 27 °C in Sf-900 III SFM medium. Isolation of bacmid DNA, transfection of Sf9 cells and amplification of virus are modified methods from the Bac-to-Bac system (Invitrogen) that we use to reduce costs and ensure production of good quality BacMam virus.
Growth and maintenance of Sf9 Easy Titer cell line
Sf9 Easy Titer cell line are maintained as adherent cells at 27 °C in Hyclone SFX-Insect media as described in Box 2.
Box 2. End point dilution assay ● TIMING 1 hr.
The following protocol is for one 96-well black plate that can be used to titer 4 viruses and is based ref. 32. Adjust volumes as necessary.
Growth and maintenance of adherent Sf9 Easy Titer cell line
-
1|
When Sf9 Easy Titer cell line reach 90% confluency, aspirate off the medium and wash cells with 5 ml PBS.
-
2|
Aspirate off the PBS and add 2 ml of trypsin for 30 sec.
-
3|
Add 10 ml of Hyclone SFX-Insect media supplemented with 5 % FBS and 150 μg/ml G418.
-
4|
Scrape the cells gently with a sterile cell scraper.
-
5|
Dilute cells 1:4 in a 100mm tissue culture dish and incubate at 27 °C.
End point dilution assay
-
6|
Using a 100 mm tissue culture dish of Sf9 Easy Titer cells that is 90% confluent, follow Steps 1–4 above.
-
7|
Make a 10 ml stock of Sf9 Easy Titer cells at a density of 0.75 × 106 cells/ml in a sterile culture reservoir.
-
8|
Using the multi-channel pipet, seed 100 μl/well of 96-well black plate (75,000 cells/well) and let attach for ~15 min at 25 °C.
-
9|
Using the sterile, deep-well 8-strip clusters, make 10−1 to 10−8 stock of virus in Hyclone SFX-Insect media supplemented with 5 % FBS and 150 μg/ml G418 (360 μl medium + 40 μl virus).
360 μl medium + 40 μl P1 virus = 10−1
360 μl medium + 40 μl 10−1 dilution = 10−2
360 μl medium + 40 μl 10−2 dilution = 10−3
360 μl medium + 40 μl 10−3 dilution = 10−4
360 μl medium + 40 μl 10−4 dilution = 10−5
360 μl medium + 40 μl 10−5 dilution = 10−6
360 μl medium + 40 μl 10−6 dilution = 10−7
360 μl medium + 40 μl 10−7 dilution = 10−8
-
10|
Once cells have attached to the 96-well plate (~ 20 min incubation at 27 °C), remove medium and replace with the 100 μl diluted virus using a multi-channel pipet.
-
11|
Infect each virus in triplicate.
-
12|
Incubate the 96-well plate at 27 °C.
-
12|At 72 hr post-infection, count the number of green foci in the dilution that gives <10 foci/well. To calculate viral titer, use the following equation:
? TROUBLESHOOTING
BacMam virus titer determination
Determine the titer of the BacMam virus using one of the several methods for virus titer determination. We prefer the Sf9 Easy Titer cell line and the endpoint dilution assay32 (see Box 2) or using the flow cytometric assay33,34.
FSEC
In our laboratory, this is performed as described by Kawate and Gouaux21. The analyte is loaded onto a Superose 6 column (10/30, Amersham Biosciences) that has been pre-equilibrated with FSEC buffer. Separation is performed at a flow rate of 0.5 ml/min. The eluent from the SEC column is passed through a Shimadzu fluorometer (RF-20A) fluorometer fitted with a flow cell as described by manufactures instructions. The fluorometer settings are as follows: band-pass, 3 nm/3 nm; excitation, 488 nm; emission, 507 nm; time increment, 1 s; integration time, 1 s; and recording time, 3,000–3,600 s. Calibration with known quantities of GFP have demonstrated that 1–10 ng of GFP can readily be detected.
PROCEDURE
Cell seeding (day 1) ● TIMING 15 min
-
1|
Add 1×106 HEK293S GnTI− cells in 2 ml of DMEM, supplemented with FBS to each well of each six-well culture plate. Incubate at 37 °C with 5% CO2 for 16–24 h.
! CAUTION Cell cultures are a potential biohazard. Work in an approved laminar flow hood using aseptic techniques and check institutional and governmental guidelines for recommended protective clothing and proper disposal of waste prior to performing experiments.
? TROUBLESHOOTING
Small scale transient transfection to screen constructs (day 2) ● TIMING 45 min
-
2|
for each well, prepare an autoclaved 1.5 ml centrifuge tube. Using a pipette, add 4 μl of Lipofectamine 2000 into 50 μl of Opti-MEM I.
-
3|
Add 1 μg of Qiagen MiniPrep-purified DNA into 50 μl of Opti-MEM I in a separate 1.5 ml centrifuge tube.
-
4|
Add DNA/Opti-MEM I mixture to the Opti-MEM/Lipofectamine mixture, gently mix and incubate for 20 min at RT.
-
5|
Pipette the Opti-MEM I-DNA mixture drop wise onto 70–80% confluent HEK293S GnTI− cells. Ensure even dispersal.
-
6|
After 8–24 hours, replace the medium with DMEM plus 10 mM sodium butyrate.
-
7|
Incubate the cells at 37 °C with 5% CO2 for 2 days.
Screen constructs by FSEC for monodispersity and expression level (day 4) ● TIMING 3 h
-
8|
Aspirate off the medium and wash the transfected adherent cells carefully with 2 ml TBS.
-
9|
Add 1 ml TBS to each well, collect the cells and transfer them to a 1.5 ml centrifuge tube.
-
10|
Centrifuge the cells at 1,500xg for 5 min, 4 °C.
-
11|
Remove the supernatant and resuspend the cell pellet in 200 μl solubilization buffer.
-
12|
Nutate samples for 1 h at 4 °C.
-
13|
Centrifuge the solubilized sample at 70,000xg in a TL100 ultracentrifuge for 40 min, 4 °C.
-
14|
Collect supernatant and analyze 50 μl by FSEC21. Allow 1hr for each sample to be analyzed by FSEC. Samples should be stored at 4 °C until analysis.
-
15|
Identify the best expressed and monodisperse candidate via FSEC (Fig. 3 and Kawate and Gouaux21).
Transformation of DH10Bac E. coli (day 5) ● TIMING 1 h
-
16|
Transform purified plasmid DNA into DH10Bac E. coli for transposition into the bacmid as described in the Bac-to-Bac system (Invitrogen; http://www.lifetechnologies.com/us/en/home/life-science/protein-expression-and-analysis/protein-expression/insect-expression/bac-to-bac-baculovirus-expression-system.html).
Inoculation of bacmid containing cultures (day 7) ● TIMING 15 min
-
17|
Inoculate 5 ml of LB media containing 50 μg/ml kanamycin, 7 μg/ml gentamicin, 10 μg/ml tetracycline with a white colony and grow cells overnight at 37 °C.
Isolation of bacmid (day 8) ● TIMING 1 h
-
18|
Centrifuge the cells for 10 min at 1,500xg, RT.
-
19|
Optional: Make a glycerol stock of the DH10Bac E. coli containing the bacmid DNA for future bacmid DNA isolation. In an autoclaved 1.5 ml centrifuge tube, take 250 μl of cell suspension (from Step 17) and add 250 μl of sterile 50 % glycerol, mix and store for years at −80 °C.
-
20|
Discard supernatant and resuspend the pellet in 200 μl of P1 (Qiagen kits). Transfer suspension into a 1.5 ml centrifuge tube.
-
21|
Add 200 μl of P2 (Qiagen kits) and mix by inverting the centrifuge tube a few times.
! CAUTION Do not vortex samples as this could shear bacmid DNA.
-
22|
Add 200 μl of N3 (Qiagen kits) and mix by inverting the centrifuge tube a few times; centrifuge the tube for 10 min at 1,500xg, RT.
-
23|
Transfer the supernatant to a 2 ml centrifuge tube and add 1ml isopropanol and gently invert.
-
24|
Place the tube for 10 min into −20 °C freezer.
-
25|
Centrifuge the tube at 1,500xg for 15 min, RT.
-
26|
Remove supernatant, preserve the pellet. Add 1 ml of 70% EtOH and wash the pellet by gently inverting the centrifuge tube.
-
27|
Centrifuge the tube at 1,500xg for 15 min, RT.
-
28|
Remove supernatant and dry the pellet 5 min.
-
29|
Resuspend the pellet in 50 μl of autoclaved MilliQ water. Determine the concentration of the bacmid DNA.
! CAUTION Do not pipet samples more than 1–2 times as this could shear bacmid DNA.
PAUSE POINT Store the bacmid DNA at 4 °C until ready to proceed with Step 30 (up to 3 days).
Transfection of Sf9 cells with bacmid ● TIMING 2 h
-
30|
Seed 9×105 of Sf9 cells in 2 ml of Sf-900 media per well of a 6-well plate.
? TROUBLESHOOTING
-
31|
Incubate cells at 27 °C until they attach (about 20 min).
-
32|
while waiting for the cells to attach, add 8 μl of Cellfectin II to 100 μl of Sf-900 III SFM media in centrifuge tubes for each transfection.
-
33|
In a different centrifuge tube, add 1 μg of bacmid DNA to 100 μl of Sf-900 III SFM.
-
34|
Mix the Cellfectin II/Sf-900 III SFM media mixture and the bacmid DNA/Sf-900 III SFM mixture and incubate for 30 min at RT.
-
35|
Change media in each well with 2 ml of Sf-900 III SFM media and add the Cellfectin II/DNA mixture drop wise onto the Sf9 cells. Ensure even dispersal.
-
36|
Incubate the cells for 72 hours in 27 °C incubator (make sure to have water inside the incubator to prevent strong evaporation of media).
-
37|
Collect supernatant containing P1 virus (~2 ml from each well) and filter the media containing P1 virus into 2 ml centrifuge tube using 3 ml syringe fitted with a small 0.2 μm filter. This is a stock of P1 virus that should be stored at 4 °C light protected for up to a month. Add 2% FBS to stabilize virus stock. It might also be helpful to use the TIPS method67 to preserve Sf9 cells infected with P1 virus.
-
38|
Determine the titer of the P1 BacMam virus using the Sf9 Easy Titer cell line and the endpoint dilution assay or by using the Virus counter 2100.
? TROUBLESHOOTING
Infection of Sf9 cells with P1 virus to produce P2 virus ● TIMING 2 h
-
39|
Based on the desired volume of P2 virus, add P1 virus to a MOI of 0.1 to 0.0001 to Sf9 cells that are 1.0–1.5×106 cells/ml in an Erlenmeyer flask of the corresponding size. CRITICAL STEP For the amplification of some viruses we have found that it is essential to infect at a lower MOI than recommended by the Bac-to-Bac system (Invitrogen). Therefore it may be important to determine the optimal MOI for the virus amplification prior to making a large amount of P2 virus.
? TROUBLESHOOTING
-
40|
Incubate the Sf9 cells infected with the P1 virus for 96 hours in 27 °C orbital shaker at 115 rpm. CRITICAL STEP For the amplification of some viruses the harvest time of the P2 BacMam virus may need to be optimized. We advise initially trying 72 h and 96 h.
-
41|
Centrifuge the cells at 8,000xg for 15 min, 4 °C and collect supernatant containing P2 virus.
-
42|
Filter the supernatant using disposable 0.2 μm filters (50 ml Steriflip filters from Millipore for small amounts or 250 ml, 0.5 liter, or 1 liter Corning filter systems for large amounts). Add 2% FBS to stabilize virus stock. This is a stock of P2 virus that should be stored at 4 °C light protected (we use aluminum foil) for up to a month.
-
43|
Determine the titer of the P2 BacMam virus using the Sf9 Easy Titer cell line and the endpoint dilution assay or by using the Virus counter 2100.
? TROUBLESHOOTING
Transduction of suspension HEK293S GnTI− cells with BacMam Virus (day 15) ● TIMING 2 h
-
44|
Expansion of HEK293S GnTI- cells should be prepared in advance (~ 10 days in advance) so that a sufficient amount of cells are available on day 15. To expand HEK293S GnTI- cells, determine the total number of cells and percent viability using a hemocytometer and Trypan Blue exclusion and make sure the density of the cells are 2.5 – 3 × 106 cells/ml (from Box 1).
-
45|
When a 25 ml culture of HEK293S GnTI− cells are 2.5 – 3 × 106 cells/ml, dilute the culture to 0.2 × 106 cells/ml in 200 ml and incubate the cells on an orbital shaker within a 37 °C incubator in the presence of 8% CO2 for ~ 5 days until the density is 3 × 106 cells/ml.
-
46|
Based on the volume of cells needed (2.4 to 6.4 liters) calculate the volume of media that you need to add to dilute the culture to a seeding density of 0.2 × 106 cells/ml. We prefer to have a starting density of 0.2 × 106 cells/ml. For 2.4 liters one will need 4.8 × 108 cells, approximately 2.2 liters of medium and three 2 liter flasks.
-
47|
Aseptically add the appropriate volume of pre-warmed growth medium into the culture flask (the total volume should be 800 ml in a 2L flask). Split the culture to multiple flasks as needed and incubate the cells on an orbital shaker within a 37 °C incubator in the presence of 8% CO2 for ~ 5–6 days until the cells reach a density of 2–3.5 × 106 cells/ml.
-
48|
Add BacMam P2 virus at a MOI of 1 to infect 2.4 liters of HEK293S GnTI− cells at a density of 2–3.5 × 106 cells/ml and incubate the cells on an orbital shaker within a 37 °C incubator in the presence of 8% CO2.
CRITICAL STEP The amount of virus added should not exceed more than 10% of the culture volume.
-
49|
After 8–24 h at 37 °C add 10 mM sodium butyrate and incubate the cells on an orbital shaker within a 30 °C incubator in the presence of 8% CO2.
CRITICAL STEP The incubation temperature for the BacMam transduced HEK293S GnTI− cells should be determined before attempting a large scale expression (Fig. 4). Also the amount and time of sodium butyrate addition should be optimized for each protein (Fig. 5).
-
50|
Harvest the cells 60–90 h post transduction by centrifugation for 20 min at 6,200xg, 4 °C.
CRITICAL STEP The harvest time of BacMam transduced HEK293S GnTI− cells should be determined before attempting a large scale expression (Fig. 4).
? TROUBLESHOOTING
PAUSE POINT The cell pellets can be stored at −80 °C for weeks until ready to proceed with protein purification 15–17,20.
TROUBLESHOOTING
Troubleshooting advice can be found in Table 1.
TABLE 1.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 1, 30, 39, 43, Boxes 1 and 2 | Change in media color and/or presence of foul smell | Contamination of cell culture due to improper sterile technique | Ensure proper sterile technique is used. Clean and disinfect hood work surfaces before and after every manipulation. Before working in the hood disinfect gloves with 70% alcohol. Spray all items to be placed in the hood (media bottles, pipetting devices, outside of the plastic wrap the sterile containers, etc) with 70% ethanol and wipe them with paper towels. |
| Contaminated virus | Ensure virus has been filter sterilized. | ||
| Contaminated reagent | Filter sterilize histone deacetylase inhibitors (i.e. sodium butyrate) in the hood. | ||
| 38 | Low virus titer | Sf9 cells infected at the wrong MOI | Generate P2 virus using lower MOI (0.05–0.0001). |
| Remake the bacmid ensuring that the bacmid culture is inoculated from a white colony and that the bacmid DNA is not sheared during purification. | |||
| 45, 50 | Low cell harvest density | Poor-quality cells | It is important not to let cells overgrow (i.e. to subculture them on a regular basis) and to avoid using cells that have undergone more than 30 continuous passages since being raised from liquid nitrogen. Check health and viability of cells prior to each experiment. |
| Low expression | Infection at the wrong MOI (i.e. too little or too much virus), non-optimal expression conditions, toxicity of membrane protein when overexpressed | Always determine the titer of the BacMam virus to caculate a MOI. Screen for optimal temperature, harvest time and concentration of histone deacetylase inhibitors. | |
| Virus is not stable when stored for extended periods | Remake the P2 virus starting from either the P1 virus or a glycerol stock of DH10Bac E. coli containing bacmid DNA. Use TIPS method66 to preserve Sf9 cells infected with P1 virus. |
● TIMING
The entire protocol, starting from transfection (Step 1) to the harvest of BacMam virus transduced suspension HEK293S GnTI− cells (Step 50), takes approximately 3 weeks to complete if a promising candidate is identified (Step 14). The hands-on timing for each stage of the procedure is summarized below.
Step 1, Cell seeding: 15 min
Steps 2–7, Small scale transient transfection to screen constructs: 45 min
Steps 8–15, Screen constructs by FSEC for monodispersity and expression level: 3 h
Step 16, Transformation of DH10Bac E. coli: 1 h
Step 17, Inoculation of bacmid containing cultures: 15 min
Steps 18–29, Isolation of bacmid: 1 h
Steps 30–38, Transfection of Sf9 cells with bacmid (producing P1 virus): 2 h (45 min transfection + 15 min P1 virus harvest + 1 h virus titer determination using the Virus Counter 2100)
Steps 39–43, Infection of Sf9 cells with P1 virus to produce P2 virus: 2 h (15 min infection of Sf9 cells with P1 virus+ 45 min harvest P2 virus + 1 h virus titer determination using the Virus Counter 2100)
Steps 44–50, Expansion of HEK293S GnTI− cells and transduction of suspension HEK293S GnTI− cells with BacMam Virus: 2 h
Box 1, Growth and maintenance of suspension HEK293S GnTI− cells: 15 min
Box 2, End point dilution assay: 1 h
ANTICIPATED RESULTS
This protocol (as outlined in Fig. 1) has been used in our laboratory to successfully express cASIC1, Drosophila melanogaster dopamine transporter (DAT), N-methyl-D-aspartate (NMDA) receptors and many other membrane proteins in HEK293S GnTI− cells15,16,20,61. The time it takes to identify a promising candidate (Fig. 3), is likely to vary significantly depending on (for example) the number of flexible regions to be removed, surface entropy reduction mutations and thermostability mutations. Although few changes made for cASIC, several construct changes were needed for DAT and NMDA to obtain the best expressed and monodisperse candidate via FSEC15–17,20. Once a promising candidate is identified, the most favorable conditions for MOI, cell density, expression time, temperature (Fig. 4), and the presence of histone deacetylase inhibitors (i.e. sodium butyrate; Fig. 5) should be determined for each protein before attempting a large scale expression. The protocol for optimized expression can be completed in approximately 3 weeks. Purification of membrane proteins from transduced HEK293S GnTI− cell pellets (which may include Affinity Chromatography, tag cleavage, removal of N-linked glycosylation, and size exclusion chromatography), depending on the candidate protein, can produce 0.25–1.5 milligrams of protein per liter of media sufficient for crystallization. The protein expression and yield can vary depending on factors such as the titer of the virus, the toxicity of the protein when expressed, and the stability of the protein.
Acknowledgments
We thank members of the Gouaux lab for helpful discussions. We are grateful to D. Goodman and G. Westbrook for encouragement and L. Vaskalis for assistance with figures. This work was supported by an OHSU Brain Institute Graduate Student Fellowship for Research on the Neurobiology of Disease (C.H. L.), by a F32 Postdoctoral NRSA from NIMH (K.W.), by a postdoc fellowship (Forschungsstipendium AL 1725-1/1) from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), by a F32 Postdoctoral NRSA from NIGMS (D.C.), by the NIH (E.G.) and by the Vollum Institute. E.G. is an investigator with the Howard Hughes Medical Institute.
Footnotes
AUTHOR CONTRIBUTIONS
A.G. screened and optimized expression conditions for cASIC and GluCl. A.G., C.L, K.W. J.C.M. and D.C. optimized the cell growth and virus amplification conditions. C.L. designed the BacMam construct and performed initial characterization of the BacMam construct. I.B. and T.A. cloned and optimized the cASIC and GluCl pEG BacMam constructs, respectively. K. C. G. and S. F. provided the pVLAD construct, incubator configuration, and consultations to optimize cell growth during the early stages of the project. All authors wrote the manuscript and edited the manuscript.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.Boyce FM, Bucher NL. Baculovirus-mediated gene transfer into mammalian cells. Proc Natl Acad Sci U S A. 1996;93:2348–2352. doi: 10.1073/pnas.93.6.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hofmann C, et al. Efficient gene transfer into human hepatocytes by baculovirus vectors. Proc Natl Acad Sci U S A. 1995;92:10099–10103. doi: 10.1073/pnas.92.22.10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kost TA, Condreay JP, Ames RS, Rees S, Romanos MA. Implementation of BacMam virus gene delivery technology in a drug discovery setting. Drug Discov Today. 2007;12:396–403. doi: 10.1016/j.drudis.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Ames R, et al. BacMam recombinant baculoviruses in G protein-coupled receptor drug discovery. Receptors Channels. 2004;10:99–107. doi: 10.1080/10606820490514969. [DOI] [PubMed] [Google Scholar]
- 5.Boudjelal M, et al. The application of BacMam technology in nuclear receptor drug discovery. Biotechnol Annu Rev. 2005;11:101–125. doi: 10.1016/S1387-2656(05)11003-5. [DOI] [PubMed] [Google Scholar]
- 6.Pfohl JL, et al. Titration of KATP channel expression in mammalian cells utilizing recombinant baculovirus transduction. Receptors Channels. 2002;8:99–111. [PubMed] [Google Scholar]
- 7.Fonfria E, et al. Cloning and pharmacological characterization of the guinea pig P2X7 receptor orthologue. Br J Pharmacol. 2008;153:544–556. doi: 10.1038/sj.bjp.0707596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shukla S, Schwartz C, Kapoor K, Kouanda A, Ambudkar SV. Use of baculovirus BacMam vectors for expression of ABC drug transporters in mammalian cells. Drug metabolism and disposition: the biological fate of chemicals. 2012;40:304–312. doi: 10.1124/dmd.111.042721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott MJ, et al. Efficient expression of secreted proteases via recombinant BacMam virus. Protein Expr Purif. 2007;52:104–116. doi: 10.1016/j.pep.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Dukkipati A, Park HH, Waghray D, Fischer S, Garcia KC. BacMam system for high-level expression of recombinant soluble and membrane glycoproteins for structural studies. Protein Expr Purif. 2008;62:160–170. doi: 10.1016/j.pep.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ely LK, Fischer S, Garcia KC. Structural basis of receptor sharing by interleukin 17 cytokines. Nat Immunol. 2009;10:1245–1251. doi: 10.1038/ni.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu H, et al. The mechanism of shared but distinct CSF-1R signaling by the non-homologous cytokines IL-34 and CSF-1. Biochim Biophys Acta. 2012;1824:938–945. doi: 10.1016/j.bbapap.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lupardus PJ, et al. Structural snapshots of full-length Jak1, a transmembrane gp130/IL-6/IL-6Ralpha cytokine receptor complex, and the receptor-Jak1 holocomplex. Structure. 2011;19:45–55. doi: 10.1016/j.str.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deupi X, et al. Stabilized G protein binding site in the structure of constitutively active metarhodopsin-II. Proc Natl Acad Sci U S A. 2012;109:119–124. doi: 10.1073/pnas.1114089108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baconguis I, Bohlen CJ, Goehring A, Julius D, Gouaux E. X-ray structure of Acid-sensing ion channel 1-snake toxin complex reveals open state of a na(+)-selective channel. Cell. 2014;156:717–729. doi: 10.1016/j.cell.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baconguis I, Gouaux E. Structural plasticity and dynamic selectivity of acid-sensing ion channel-spider toxin complexes. Nature. 2012;489:400–405. doi: 10.1038/nature11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penmatsa A, Wang KH, Gouaux E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature. 2013;503:85–90. doi: 10.1038/nature12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen PH, Chen X, Lin Z, Fang D, He X. The structural basis of R-spondin recognition by LGR5 and RNF43. Genes Dev. 2013;27:1345–1350. doi: 10.1101/gad.219915.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504:107–112. doi: 10.1038/nature12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CH, et al. NMDA receptor structures reveal subunit arrangement and pore architecture. Nature. 2014;511:191–197. doi: 10.1038/nature13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawate T, Gouaux E. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure. 2006;14:673–681. doi: 10.1016/j.str.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Hattori M, Hibbs RE, Gouaux E. A fluorescence-detection size-exclusion chromatography-based thermostability assay for membrane protein precrystallization screening. Structure. 2012;20:1293–1299. doi: 10.1016/j.str.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coric T, Zheng D, Gerstein M, Canessa CM. Proton sensitivity of ASIC1 appeared with the rise of fishes by changes of residues in the region that follows TM1 in the ectodomain of the channel. J Physiol. 2005;568:725–735. doi: 10.1113/jphysiol.2005.087734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cully DF, et al. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature. 1994;371:707–711. doi: 10.1038/371707a0. [DOI] [PubMed] [Google Scholar]
- 25.Hibbs RE, Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011;474:54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berger I, Fitzgerald DJ, Richmond TJ. Baculovirus expression system for heterologous multiprotein complexes. Nat Biotechnol. 2004;22:1583–1587. doi: 10.1038/nbt1036. [DOI] [PubMed] [Google Scholar]
- 27.Reeves PJ, Callewaert N, Contreras R, Khorana HG. Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc Natl Acad Sci U S A. 2002;99:13419–13424. doi: 10.1073/pnas.212519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chae PS, et al. Maltose-neopentyl glycol (MNG) amphiphiles for solubilization, stabilization and crystallization of membrane proteins. Nat Methods. 2010;7:1003–1008. doi: 10.1038/nmeth.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper DR, et al. Protein crystallization by surface entropy reduction: optimization of the SER strategy. Acta Crystallogr D Biol Crystallogr. 2007;63:636–645. doi: 10.1107/S0907444907010931. [DOI] [PubMed] [Google Scholar]
- 30.Magnani F, Shibata Y, Serrano-Vega MJ, Tate CG. Co-evolving stability and conformational homogeneity of the human adenosine A2a receptor. Proc Natl Acad Sci U S A. 2008;105:10744–10749. doi: 10.1073/pnas.0804396105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serrano-Vega MJ, Magnani F, Shibata Y, Tate CG. Conformational thermostabilization of the beta1-adrenergic receptor in a detergent-resistant form. Proc Natl Acad Sci U S A. 2008;105:877–882. doi: 10.1073/pnas.0711253105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hopkins R, Esposito D. A rapid method for titrating baculovirus stocks using the Sf-9 Easy Titer cell line. Biotechniques. 2009;47:785–788. doi: 10.2144/000113238. [DOI] [PubMed] [Google Scholar]
- 33.Ferris MM, et al. Evaluation of the Virus Counter(R) for rapid baculovirus quantitation. J Virol Methods. 2011;171:111–116. doi: 10.1016/j.jviromet.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen CF, Meghrous J, Kamen A. Quantitation of baculovirus particles by flow cytometry. J Virol Methods. 2002;105:321–330. doi: 10.1016/s0166-0934(02)00128-3. [DOI] [PubMed] [Google Scholar]
- 35.Dulbecco R, Vogt M. Some problems of animal virology as studied by the plaque technique. Cold Spring Harb Symp Quant Biol. 1953;18:273–279. doi: 10.1101/sqb.1953.018.01.039. [DOI] [PubMed] [Google Scholar]
- 36.Al-Fageeh MB, Marchant RJ, Carden MJ, Smales CM. The cold-shock response in cultured mammalian cells: harnessing the response for the improvement of recombinant protein production. Biotechnol Bioeng. 2006;93:829–835. doi: 10.1002/bit.20789. [DOI] [PubMed] [Google Scholar]
- 37.Ho YC, Chen HC, Wang KC, Hu YC. Highly efficient baculovirus-mediated gene transfer into rat chondrocytes. Biotechnol Bioeng. 2004;88:643–651. doi: 10.1002/bit.20239. [DOI] [PubMed] [Google Scholar]
- 38.Hsu CS, Ho YC, Wang KC, Hu YC. Investigation of optimal transduction conditions for baculovirus-mediated gene delivery into mammalian cells. Biotechnol Bioeng. 2004;88:42–51. doi: 10.1002/bit.20213. [DOI] [PubMed] [Google Scholar]
- 39.Kumar N, Gammell P, Clynes M. Proliferation control strategies to improve productivity and survival during CHO based production culture : A summary of recent methods employed and the effects of proliferation control in product secreting CHO cell lines. Cytotechnology. 2007;53:33–46. doi: 10.1007/s10616-007-9047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan Y, et al. Efficient gene delivery into mammalian cells by recombinant baculovirus containing a hybrid cytomegalovirus promoter/Semliki Forest virus replicon. J Gene Med. 2009;11:1030–1038. doi: 10.1002/jgm.1390. [DOI] [PubMed] [Google Scholar]
- 41.Ramos L, et al. Rapid expression of recombinant proteins in modified CHO cells using the baculovirus system. Cytotechnology. 2002;38:37–41. doi: 10.1023/A:1021189628274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sumitomo Y, et al. Identification of a novel enhancer that binds Sp1 and contributes to induction of cold-inducible RNA-binding protein (cirp) expression in mammalian cells. BMC Biotechnol. 2012;12:72. doi: 10.1186/1472-6750-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wulhfard S, et al. Mild hypothermia improves transient gene expression yields several fold in Chinese hamster ovary cells. Biotechnol Prog. 2008;24:458–465. doi: 10.1021/bp070286c. [DOI] [PubMed] [Google Scholar]
- 44.Li P, Slimko EM, Lester HA. Selective elimination of glutamate activation and introduction of fluorescent proteins into a Caenorhabditis elegans chloride channel. FEBS Lett. 2002;528:77–82. doi: 10.1016/s0014-5793(02)03245-3. [DOI] [PubMed] [Google Scholar]
- 45.Backliwal G, et al. Valproic acid: a viable alternative to sodium butyrate for enhancing protein expression in mammalian cell cultures. Biotechnol Bioeng. 2008;101:182–189. doi: 10.1002/bit.21882. [DOI] [PubMed] [Google Scholar]
- 46.Barsoum J, Brown R, McKee M, Boyce FM. Efficient transduction of mammalian cells by a recombinant baculovirus having the vesicular stomatitis virus G glycoprotein. Hum Gene Ther. 1997;8:2011–2018. doi: 10.1089/hum.1997.8.17-2011. [DOI] [PubMed] [Google Scholar]
- 47.Andrell J, Tate CG. Overexpression of membrane proteins in mammalian cells for structural studies. Mol Membr Biol. 2013;30:52–63. doi: 10.3109/09687688.2012.703703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aricescu AR, Lu W, Jones EY. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr D Biol Crystallogr. 2006;62:1243–1250. doi: 10.1107/S0907444906029799. [DOI] [PubMed] [Google Scholar]
- 49.Lee JE, Fusco ML, Saphire EO. An efficient platform for screening expression and crystallization of glycoproteins produced in human cells. Nat Protoc. 2009;4:592–604. doi: 10.1038/nprot.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wurm F, Bernard A. Large-scale transient expression in mammalian cells for recombinant protein production. Curr Opin Biotechnol. 1999;10:156–159. doi: 10.1016/s0958-1669(99)80027-5. [DOI] [PubMed] [Google Scholar]
- 51.Chaudhary S, Pak JE, Gruswitz F, Sharma V, Stroud RM. Overexpressing human membrane proteins in stably transfected and clonal human embryonic kidney 293S cells. Nat Protoc. 2012;7:453–466. doi: 10.1038/nprot.2011.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reeves PJ, Thurmond RL, Khorana HG. Structure and function in rhodopsin: high level expression of a synthetic bovine opsin gene and its mutants in stable mammalian cell lines. Proc Natl Acad Sci U S A. 1996;93:11487–11492. doi: 10.1073/pnas.93.21.11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reeves PJ, Kim JM, Khorana HG. Structure and function in rhodopsin: a tetracycline-inducible system in stable mammalian cell lines for high-level expression of opsin mutants. Proc Natl Acad Sci U S A. 2002;99:13413–13418. doi: 10.1073/pnas.212519199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cockrell AS, Kafri T. Gene delivery by lentivirus vectors. Mol Biotechnol. 2007;36:184–204. doi: 10.1007/s12033-007-0010-8. [DOI] [PubMed] [Google Scholar]
- 55.Russell WC. Update on adenovirus and its vectors. J Gen Virol. 2000;81:2573–2604. doi: 10.1099/0022-1317-81-11-2573. [DOI] [PubMed] [Google Scholar]
- 56.Dubensky TW, Jr, et al. Sindbis virus DNA-based expression vectors: utility for in vitro and in vivo gene transfer. J Virol. 1996;70:508–519. doi: 10.1128/jvi.70.1.508-519.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nivitchanyong T, Tsai YC, Betenbaugh MJ, Oyler GA. An improved in vitro and in vivo Sindbis virus expression system through host and virus engineering. Virus Res. 2009;141:1–12. doi: 10.1016/j.virusres.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bennink JR, Yewdell JW. Recombinant vaccinia viruses as vectors for studying T lymphocyte specificity and function. Curr Top Microbiol Immunol. 1990;163:153–184. doi: 10.1007/978-3-642-75605-4_6. [DOI] [PubMed] [Google Scholar]
- 59.Berglund P, et al. Semliki Forest virus expression system: production of conditionally infectious recombinant particles. Biotechnology (N Y) 1993;11:916–920. doi: 10.1038/nbt0893-916. [DOI] [PubMed] [Google Scholar]
- 60.Liljestrom P, Garoff H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology (N Y) 1991;9:1356–1361. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- 61.Gonzales EB, Kawate T, Gouaux E. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature. 2009;460:599–604. doi: 10.1038/nature08218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hattori M, Gouaux E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature. 2012;485:207–212. doi: 10.1038/nature11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- 64.Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462:745–756. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hill DR, Aumiller JJ, Shi X, Jarvis DL. Isolation and analysis of a baculovirus vector that supports recombinant glycoprotein sialylation by SfSWT-1 cells cultured in serum-free medium. Biotechnol Bioeng. 2006;95:37–47. doi: 10.1002/bit.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jarvis DL. Developing baculovirus-insect cell expression systems for humanized recombinant glycoprotein production. Virology. 2003;310:1–7. doi: 10.1016/s0042-6822(03)00120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wasilko DJ, et al. The titerless infected-cells preservation and scale-up (TIPS) method for large-scale production of NO-sensitive human soluble guanylate cyclase (sGC) from insect cells infected with recombinant baculovirus. Protein Expr Purif. 2009;65:122–132. doi: 10.1016/j.pep.2009.01.002. [DOI] [PubMed] [Google Scholar]