Abstract
Foxp3+ Tregs are central regulators of immune tolerance. As dysregulated Treg responses contribute to disease pathogenesis, novel approaches to target the immunomodulatory functions of Tregs are currently under investigation. mTORC1 and mTORC2 are therapeutic targets of interest. Recent studies revealed that mTOR signaling impacts conventional T-cell homeostasis, activation and differentiation. Moreover, mTOR controls the differentiation and functions of Tregs, suggesting that its activity could be targeted to modulate Treg responses. Here, we summarize how Tregs suppress immune responses, their roles in disease development and methods used to alter their functions therapeutically. We also discuss the diverse effects exerted by mTOR inhibition on the development, homeostasis, and functions of conventional T cells and Tregs. We conclude with a discussion of how modulation of mTOR activity in Tregs may be therapeutically beneficial or detrimental in different disease settings.
Keywords: immunotherapy, mTORC1, rapamycin, regulatory T cells, T cells
CD4+ and CD8+ T cells are central regulators of the adaptive immune response. T cells develop in the thymus and populate the peripheral tissues in a quiescent state. Antigen-specific, naive T cells are rapidly activated when their T-cell antigen receptors (TCRs) recognize their cognate peptide-MHC ligands, which are expressed by professional APCs. Additional signals from costimulatory and cytokine receptors ultimately drive proliferation, survival and differentiation into specific effector T-cell populations [1]. For CD4+ T cells, these effector populations include T helper (TH)1, TH2, TH17 and T follicular helper (TFH) cells [2]. Signaling from these receptors also plays a critical role in driving memory T-cell formation to protect against secondary infections [1]. Because dysfunctional T-cell responses can be detrimental to the host [1,3,4], cell-intrinsic and cell-extrinsic mechanisms exist to suppress their function [1,5].
Through multiple mechanisms, CD4+CD25+ Tregs suppress T-cell responses to maintain immune homeostasis and limit immunopathology following infections [5,6]. Dysregulated Treg functions contribute to a number of disorders including autoimmune diseases, prolonged infections and cancer [4,6–8]. Given their potent immunosuppressive functions, Tregs are viable clinical targets for the treatment of human diseases [7,9]. However, the efficacies of current therapies vary in individual patients and diseases. It is therefore crucial to identify new pathways that enhance the effectiveness of Treg-based immunotherapies to treat immune-mediated diseases.
In addition to immunological cues received from molecules expressed by APCs and cytokines, nutrients control cell fate commitments and functions of different T-cell populations [4,10 –12]. As such, molecules and pathways that regulate metabolic programs are candidate therapeutic targets to modulate T-cell functions. mTOR regulates important and diverse functions in all T-cell lineages and is therefore an important therapeutic target of interest to treat many human disorders [4,10,11,13]. In this review, we first briefly describe Treg biology, including the suppressive mechanisms used by Tregs to limit immune responses, how their dysfunction contributes to disease development and the current clinical methods to modulate their functions. Next, we summarize how mTOR-mediated signaling controls conventional T-cell development, homeostasis and functional activation. Third, we highlight the different effects of pharmacological versus genetic inhibition of mTORC1 on the differentiation and functions of Tregs. Finally, we discuss the implications of targeting mTORC1 in Tregs in different clinical settings.
Mechanisms of Treg suppression & their roles in homeostasis & disease
CD4+ and CD8+ T cells are critical mediators of immune responses that promote pathogen clearance and prevent secondary infections. T-cell development in the thymus is shaped by exposure to host-derived antigens, and these T cells retain the ability to recognize these self-antigens in the periphery. Because of this feature, T cells also play deleterious roles in autoimmune disorders and solid organ transplants, among other diseases [1,3]. Tregs are a specialized subset of CD4+ T cells that suppress T-cell responses. Like conventional T cells, Treg functions are regulated by environmental stimuli that either enhance or inhibit their suppressive activities. Below, we describe the phenotypic and functional attributes of Tregs and discuss how this cell population can prevent or promote human disease development. We also briefly summarize current strategies used to modify Treg functions in different disease settings.
Overview of Treg populations
Like conventional T cells, CD4+ Tregs are activated in response to TCR and CD28 costimulatory signals. The expression of certain molecules in Tregs is shared with conventional T cells, but Tregs also express unique proteins that distinguish them from other T-cell populations. Tregs are identified by their expression of the transcription factor Foxp3, which is essential for their development, stability and suppressive functions. Foxp3+ Tregs that develop in the thymus are termed thymus-derived Tregs (tTregs; formerly referred to as natural Tregs [nTregs]) [14]. Naive T cells acquire Foxp3 expression following antigen and CD28 costimulation in the presence of the cytokines, IL-2 and TGF-β [5,8,15]. These cells are termed peripherally derived Tregs (pTregs) if generated in vivo and in vitro-derived Tregs (iTregs) when differentiated in vitro [14]. Additional suppressive CD4+Foxp3- T cells have also been identified. These subsets include Tr1 cells, iTR35 cells and TH3 cells that secrete IL-10, IL-35 and TGF-β, respectively [16,17]. CD8+ suppressive T-cell populations are also found to inhibit immune cell function under certain conditions [18]. Here, we limit our discussion to the Foxp3+ tTregs and iTregs/pTregs.
Although they develop in distinct anatomical locations, tTregs and pTregs express common surface receptors associated with their functions, including CTLA-4 (also known as CD152), GITR, CD103 and ICOS, and these receptors are also expressed on iTregs [5,6,17]. However, tTregs are distinguishable from pTregs/iTregs in that they express higher levels of PD-1 [17], CD73 [17], Helios [19 – 21] and Nrp1 [22,23]. It is noteworthy that Helios may not be exclusively expressed in tTreg, as other groups have demonstrated that Helios is expressed in iTreg and other effector T-cell populations [24–27]. Epigenetic differences are also observed in different Treg populations, with tTregs displaying more stable demethylation of the Foxp3 locus than iTregs [17,28–30]. Thus, there are multiple parameters to distinguish between different Treg populations.
Mechanisms of Treg-mediated suppression
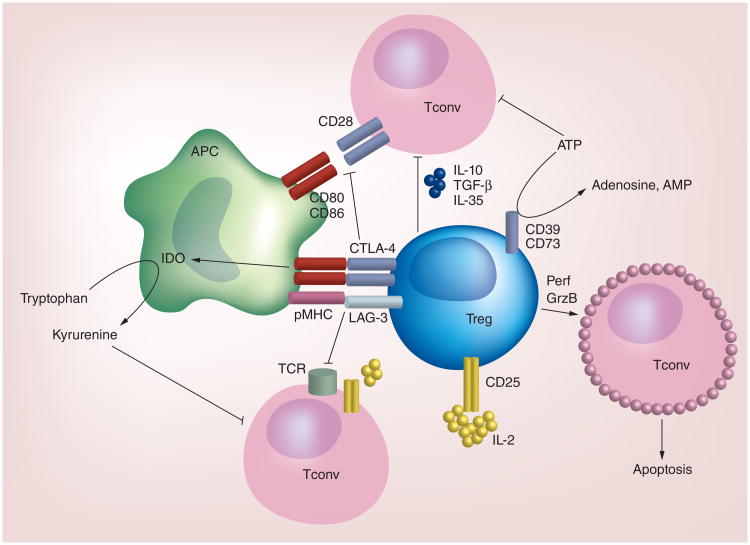
Tregs utilize multiple mechanisms to suppress conventional T-cell responses. These include cell-contact-dependent mechanisms mediated by surface receptors, such as CTLA-4, ICOS, CD103, GITR, LAG-3 and Nrp1, which can modulate the functions of T cells or other immune cells, such as APCs, to suppress T-cell responses. Additionally, Tregs suppress T-cell responses by secreting anti-inflammatory cytokines and disrupting metabolic responses such that conventional T-cell proliferation and activation are impaired. Below, we highlight some of these mechanisms, with a particular emphasis on those pathways that are current clinical targets. A summary of some of these suppressive mechanisms is shown in Figure 1.
Figure 1. The major cell-contact-dependent and -independent mechanisms utilized by Tregs to suppress conventional T-cell responses.
Tregs express surface receptors, including LAG-3 and CTLA-4, which mediate the cell-contact-dependent suppression of Tconv. These molecules bind pMHC and CD80/CD86, respectively. Subsequently, TCR-pMHC and CD28-CD80/CD86 interactions are disrupted, leading to impaired T-cell activation. CTLA-4-CD80/CD86 interactions also induce APCs to express IDO, which catabolizes tryptophan and therefore reduces the availability of this amino acid needed for T-cell activation. Tregs also produce or respond to soluble factors to suppress Tconv activation. For instance, given their high expression of CD25 relative to Tconv, IL-2 signaling is more robust in Tregs. As a result, there is less IL-2 available to Tconv to promote their activation. Tregs secrete anti-inflammatory cytokines, including IL-10, TGF-β and IL-35 to limit Tconv activation. Tregs that express CD39 and CD73 can deplete a microenvironment of ATP by generating adenosine and AMP, which have immunosuppressive effects on Tconv. Under certain conditions, Tregs may also elaborate Perf and GrzB to induce apoptosis of Tconv. Other surface receptors, including Nrp1, CD103 and ICOS, play vital roles in mediating Treg suppression, but are not depicted here.
GrzB: Granzyme B; Perf: Perforin; pMHC: Peptide-MHC; Tconv: Conventional T cell; TCR: T-cell antigen receptor.
CTLA-4, a critical regulatory molecule expressed by Tregs [31], antagonizes CD28 costimulation needed for naive T-cell activation by competing with CD28 for binding to CD80 and CD86, and by inducing CD80/CD86 endocytosis [32 –34]. Reduced costimulation in these T cells also impairs T cell-APC crosstalk that promotes APC maturation. Moreover, CTLA-4-CD80/CD86 interactions can further alter APC function by increasing the expression of the IDO in these cells [5,32,35,36]. IDO expression by APCs facilitates tryptophan catabolism, which impairs conventional T-cell proliferation while enhancing the ability of naive T cells to become iTreg/pTreg [5,32,37]. Thus, CTLA-4 is an important molecule for Treg function.
In addition to CTLA-4, expression of ICOS and CD103 is also associated with enhanced suppressive functions of Tregs [27,38–40], although these molecules are necessary for Treg-mediated suppression only in selective settings [41,42]. Interestingly, ICOS expression is found abundantly on Tregs that localize to the B cell follicles during germinal center (GC) reactions and have been termed T follicular regulatory (TFR) cells. These cells can suppress GC reactions and are thus thought to be important inhibitors of auto-antibody production that can drive autoimmune disease pathogenesis [3]. LAG-3 and GITR expression on Tregs contribute to the direct and indirect suppression of T-cell responses by altering APC function or promoting Treg expansion [5]. Nrp1 is also important for Treg suppressive function under certain conditions [43]. In some instances, Tregs use perforin and granzyme B-dependent cytolysis to directly kill effector T cells [5]. Thus, Tregs utilize multiple cell-contact-dependent mechanisms to suppress immune responses.
Soluble and secreted chemical messengers also mediate Treg function. First, Tregs are responsive to cytokines that modulate their suppressive functions. For instance, IL-2 signaling via IL-2Rα/CD25 serves to maintain Foxp3 expression, thus facilitating Treg effector functions [5,44]. Given that Tregs express higher levels of CD25 than naive or effector T cells, it has been suggested that Tregs deplete the microenvironment of IL-2 such that conventional T cells cannot proliferate and/or are more susceptible to undergoing apoptosis [5,45]. Second, Tregs have the capacity to secrete anti-inflammatory cytokines, including IL-10, IL-35 and TGF-β, to suppress inflammation [5]. Finally, Tregs may also disrupt metabolic responses to dampen immune cell activation. The generation of IDO-expressing APCs by Tregs promotes tryptophan catabolism [36], which, in combination with the depletion of tryptophan, produces catabolites that suppress T-cell activation and augment naive T-cell differentiation into iTregs [46,47]. Moreover, Tregs induce the expression of other essential amino acid-consuming enzymes in APCs, including arginase, to promote tolerance [37]. Tregs that express the ectonucleotidases, CD39 and CD73, generate high, localized concentrations of AMP and adenosine from ATP, which suppress immune cell functions [5,48–50]. These data indicate that soluble factors, including cytokines and metabolites, are also important mediators of Treg suppressive function.
Tregs in disease: perspectives from autoimmunity & cancer
Tregs are important to maintain tolerance to endogenous antigens, but their suppressive function can also be deleterious in certain diseases. Here, we focus on how Tregs maintain immune homeostasis and prevent autoimmunity, and promote cancer development and progression. However, dysregulated Treg responses also contribute to aberrant clearance of infections and metabolic diseases, which are discussed elsewhere [4,6].
Tregs maintain immune tolerance to prevent autoimmunity
The rampant, systemic autoimmunity observed in the scurfy mouse model and in immune dysregulation polyendocrinopathy, enteropathy, X-linked syndrome patients revealed the important role of Tregs in immune homeostasis [5]. More recent studies have uncovered dysfunctional Treg responses in multiple autoimmune disorders, which contribute to disease pathologies [5,8,51]. In many murine models of autoimmune diseases, including Type 1 diabetes, experimental autoimmune encephalomyelitis and inflammatory bowel disease, disease pathogenesis is driven by inflammatory CD4+ TH1 and TH17 cells and CD8+ T cells. The presence of Tregs prevents the development of these diseases, and the administration of exogenous Tregs reduces disease severity. Disrupted Treg responses, as a consequence of their depletion, mislocalization or impaired function, have also been observed in multiple human autoimmune conditions, including Type 1 diabetes and system lupus erythematous [5,8,51]. These data indicate that Tregs play a central role in maintaining tolerance to endogenous antigens and demonstrate their protective effects in preventing and eliminating autoimmune conditions.
The suppressive functions of Tregs contribute to cancer development
The suppressive functions of Tregs have detrimental effects in solid tumors and hematological cancers. Inflammation is believed to be a driving force underlying malignant transformation [52]. As Tregs suppress inflammation, it has been hypothesized that Tregs prevent or delay inflammation that promotes tumor development. After an established tumor has developed, however, Tregs oftentimes play a detrimental role in the disease [7,53,54]. This conclusion is supported by evidence in animal models demonstrating that Treg depletion promotes tumor regression or rejection [7,53,55]. It has also been revealed that increased frequencies of Tregs in the blood and within the tumor itself are correlated with poor disease prognosis and response to therapies [7,9,53,54]. Together, these results suggest that Treg suppressive function is permissive to tumor progression and is a hindrance to antitumor therapies.
The presence of Tregs also influences how patients respond to anticancer treatment regimens. High-dose IL-2 therapy is approved for the treatment of metastatic melanoma and renal cell carcinoma. However, one complication of IL-2 therapy is the expansion of Tregs, including ICOS+ Tregs with an activated phenotype [56]. Melanoma patients with increased frequencies of ICOS+ Tregs in their blood have a poorer prognosis than those with fewer of these cells [56]. Thus, in addition to promoting tumor development, the immunosuppressive capacity of Tregs can hinder cancer therapies. Below, we discuss strategies used to attenuate or enhance Treg functions in autoimmunity or cancers.
Therapeutic strategies targeting Tregs
Immunotherapies targeting Tregs are emerging as treatments for autoimmune disorders and cancers [7,9]. Many of these strategies employ antibodies or recombinant proteins that influence the function of Tregs, including CTLA-4-Ig, anti-CD25 antibody, recombinant IL-2 and recombinant IL-2/anti-IL-2 antibody immune complexes [7,9]. A succinct summary of some of these therapies is shown in Table 1 and also reviewed elsewhere [9,10]. While such treatments have a profound effect on Treg functions or numbers, the expression of these target molecules is not limited to Tregs. As a consequence, these therapies also impact activated T-cell responses.
Table 1.
Selective description of preclinical and clinical therapies, their predicted effects on Tregs and conventional T cells, and their potential disease applicability†.
| Preclinical or clinical therapy | Effect on Tregs | Impact on conventional T cells | Disease applicability |
|---|---|---|---|
| Anti-CD25 mAb | Depletion | Depletion of effector/memory T cells | Cancer |
| Anti-CTLA-4 mAb | Depletion | Increased function?, activated cell depletion? | Cancer |
| Anti-GITR mAb | Depletion | Increased function, resistance to suppression | Cancer |
| Anti-OX40 mAb | Decreased suppression | Increased activation | Cancer |
| Rapamycin | In vitro expansion | Reduced activation | Autoimmunity, transplant tolerance |
| IL-2 | Expansion | Expansion | Cancer |
| IL-2/anti-IL-2 mAb complexes | Expansion | Depletion, impaired function | Cancer, autoimmunity, transplant tolerance |
| CTLA-4-Ig | Expansion, increased suppression? | Reduced function | Autoimmunity, transplant tolerance |
| Adoptive transfer of Tregs | Enhanced suppression | Decreased function | Autoimmunity, transplant tolerance |
Cell-based therapies utilizing purified Tregs limit chronic solid organ or hematopoietic stem cell transplant rejection and ameliorate autoimmune disorders [9]. This immunosuppressive strategy is hindered by a limited capacity to generate sufficient numbers of these cells to transfer into recipients. As such, various strategies are utilized to expand Tregs ex vivo to gain sufficient numbers, including employing mTORC1 inhibitors [9]. For the remainder of this review, we focus our discussion on pathways that regulate mTOR activation and the roles of mTOR in conventional T cells and Tregs. We also discuss therapeutic implications of targeting mTORC1 in Tregs.
mTOR is a critical regulator of T-cell homeostasis & function
Overview of mTOR activation & signaling
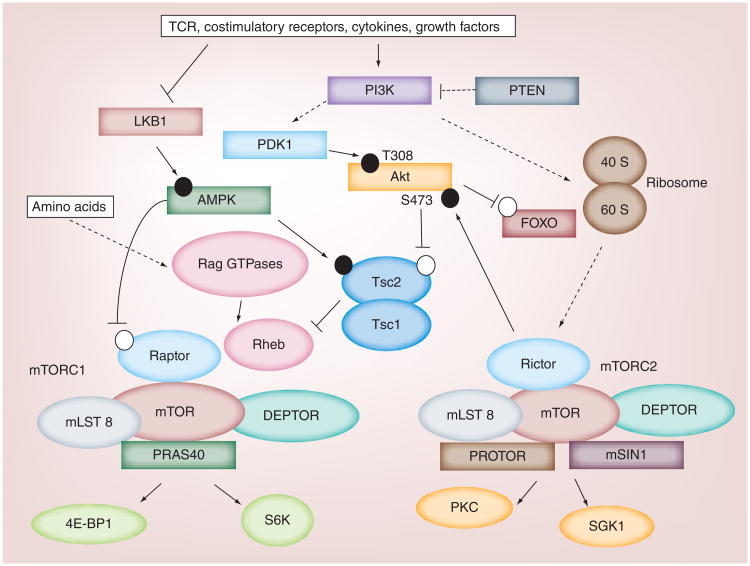
mTOR is an evolutionarily conserved serine/threonine kinase and is a critical regulator of cellular growth, proliferation and differentiation [13]. Two distinct multi-protein mTOR complexes, termed mTORC1 and mTORC2, are found in mammalian cells. These complexes share the catalytic mTOR subunit and other associated proteins, including Deptor and mLST8 (also called GbL), but also contain distinct proteins that regulate the specific functions of these complexes. PRAS40 and Raptor are unique components of mTORC1, while mTORC2 is characterized by the expression of mSIN1 and Rictor. Raptor and Rictor are the obligate adaptor proteins for mTORC1 and mTORC2, respectively [10,11]. We will discuss the roles of these proteins in T-cell homeostasis and function later in this review.
The activation of mTOR is tightly regulated and is induced by multiple stimuli, including the TCR, costimulatory molecules, cytokines, chemokines, adipocytokines, growth factors and nutrients [10,11]. In the absence of stimulation, the complex comprised of Tsc1/2 inhibits mTORC1 activity. Tsc1/2 are GTPase-activating proteins that inactivate the small GTPase Rheb, a protein that directly activates mTORC1. Upon T-cell activation, the PI3K-Akt signaling pathway is induced. This event leads to the direct phosphorylation of Tsc1/2 by Akt, which inactivates Tsc1/2. PTEN antagonizes the catalytic actions of PI3K [10]. The LKB1-AMPK signaling axis is another suppressor of mTORC1 catalytic function, in that AMPK positively modulates Tsc2 activity and impairs Raptor function. Less is known about the mechanisms that induce mTORC2 activation, although ribosomal assembly appears to induce mTORC2 function in non-T-cell lineages [10,11].
Essential and non-essential amino acids also activate mTORC1; however, the mechanism appears to be distinct from growth factors that drive PI3K-Akt activation. Amino acids activate mTORC1 via the RAG GTPases (RAGA, RAGB, RAGC and RAGD). The active RAG complex is a heterodimer comprised of GTP-bound RAGA or RAGB and GDP-bound RAGC or RAGD [57]. This active complex binds to Raptor and is recruited to the lysosome by binding Ragulator (p18, p14 and MP1), where it activates Rheb to induce mTORC1 activation [57,58]. Later in this review, we discuss how sensing of amino acids controls T-cell responses.
Signaling downstream of mTOR activation is linked to diverse cellular processes (Figure 2). The mTORC2 complex activates AGC kinases, including Akt, SGK1 and PKCα, and this links mTORC2 activity to cell survival, metabolism and cytoskeletal rearrangement. S6K and 4E-BP1 are well-characterized substrates for mTORC1. As with mTORC2, S6K activity is induced by mTORC1, and S6K-mediated phosphorylation of the ribosomal S6 protein is important to induce protein translation, which maintains cellular survival and proliferation. By contrast, 4E-BP1 activity is inhibited by mTORC1-mediated phosphorylation, allowing for the synthesis of proteins to occur more efficiently. Thus, mTORC1 and mTORC2 serve similar and unique cellular functions governed by their ability to modulate the activities of shared and distinct signaling targets. To gain further insight into mTOR signaling and biological functions, the reader is referred to other reviews [10,11,13,59].
Figure 2. Overview of mTOR signaling.
Antigen stimulation via the TCR, in combination with costimulatory receptors, cytokines and growth factors, inhibit LKB1-AMPK signaling and drive PI3K-PDK1-Akt signaling. These events subsequently inactivate the Tsc1/Tsc2 complex, which inhibits mTORC1 activity. When activated, mTORC1 phosphorylates downstream proteins, including S6K and 4E-BP1, to enhance protein synthesis and alter metabolic programs. Ultimately, these changes direct cell fate decisions and promote cell growth, proliferation or survival. Although the mechanisms are not clear, mTORC2 is also catalytically activated upon T-cell activation, which leads to the phosphorylation of Akt S473, PKC and SGK1. mTORC2 signaling influences metabolic programs and protein synthesis, but can also influence cytoskeletal rearrangements. In this diagram, the black and white circles represent activating and inhibitory phosphorylation events, respectively.
TCR: T-cell antigen receptor.
mTOR & its regulators control conventional T-cell development, homeostasis & function
In this subsection, we discuss how the mTOR complexes regulate T-cell development, peripheral homeostasis and functional activation. We summarize these functions in Figure 3.
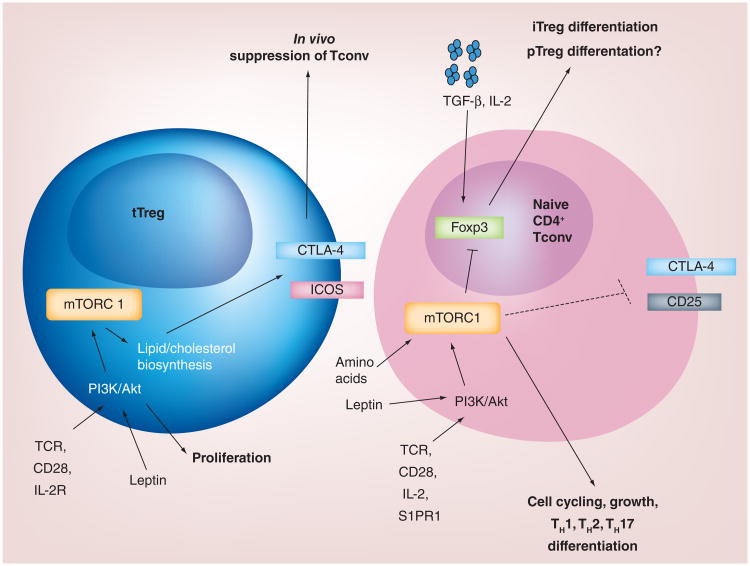
Figure 3. Proposed model for mTORC1-mediated regulation of T-cell responses.
In Foxp3+ Tregs, mTORC1 activates metabolic pathways to induce lipid biosynthesis and cholesterol metabolism, which trigger Treg proliferation. Additionally, the activation of these pathways induces the expression of CTLA-4 and ICOS to mediate Treg suppression. In naive T cells, mTOR signaling induced by various surface receptors inhibits the expression of Foxp3 and instead favors the generation of TH1, TH2 and/or TH17 cells.
iTreg: In vitro induced Treg; pTreg: Peripherally derived Treg; Tconv: Conventional T cell; tTreg: Thymus-derived Treg.
T-cell development
T-cell development occurs in the thymus and is shaped by environmental cues that induce mTOR activation [4,10,11]. The deletion of Rictor using Vav-Cre or systemically impairs thymocyte development [60,61]. If mTORC1 is inhibited during the earliest stages of thymocyte development, thymic atrophy is observed in vivo. This phenotype is partially explained by defects in cell cycling, which results in less proliferation and more apoptosis in Raptor-deficient thymocytes [60]. Interestingly, abrogation of mTORC1 function does not appear to regulate later stages of thymocyte development, as no major developmental defects are observed when mTOR is deleted using Cd4-Cre [62] or when Raptor is removed using Lck-Cre or Cd4-Cre [60,63]. These results demonstrate that mTORC1 and mTORC2 serve different functional roles in thymocyte development, and mTORC1 activation is differentially required at distinct stages of thymopoiesis.
The functions of negative regulators of mTOR signaling in T-cell development have also been investigated. Akt and mTOR activation are elevated in Pten−/−T cells, which contributes to malignant transformation of these cells [64,65]. However, although Akt regulates thymocyte development [66,67], PTEN deficiency does not affect conventional T-cell development prior to lymphomagenesis [68]. Studies have demonstrated that the T-cell-specific deletion of Tsc1 does not impair thymocyte development [69 –71]. By contrast, Lkb1−/− thymocytes have a severe developmental block linked to defects in proliferation and survival [72,73]. However, the established substrates for LKB1 [73], AMPK1α or the related protein, MARK2, are dispensable for thymocyte development [74,75]. These observations suggest that LKB1 may mediate its effects on thymocyte development via AMPK-independent pathways or that AMPK family members are functionally redundant in thymocyte development.
Maintenance of naive T-cell quiescence & homeostasis
Conventional T-cell activation is coupled to metabolic and energy changes. Resting T cells utilize oxidative phosphorylation to generate energy, whereas activated T cells rely upon oxidative glycolysis, a phenomenon known as the Warburg effect [11,12]. Several studies have demonstrated the important role of mTOR in regulating metabolic pathways in naive, activated and memory T cells. Below we summarize these findings, but more detailed discussion may be found in other reviews [4,10].
T-cell homeostasis is maintained in the periphery by TCR stimulation via host-derived peptide-MHC molecules and cytokines, including IL-7 [76]. Deletion of mTOR, Raptor or Rictor in T cells does not overtly alter peripheral T-cell homeostasis [62,63]; however, active suppression of mTORC1 activity maintains CD4+ and CD8+ T-cell quiescence. Consistent with this idea, excessive mTORC1 function in Tsc1−/− T cells promotes aberrant cell cycling and hyperactivation upon TCR stimulation, which leads to peripheral CD4+ and CD8+ T-cell apoptosis [69–71,77].
PTEN and LKB1 are also regulators of peripheral T-cell homeostasis. Mature PTEN-deficient T cells are hyperproliferative, resistant to apoptosis and drive autoimmunity [78]. Similar to Tsc1 −/− T cells, peripheral Lkb1−/− T cells are hyperactivated and are more sensitive to TCR-induced apoptosis [79]. After initial IL-7 stimulation, the viability of proliferating Lkb1−/− T cells is similar to controls when these cells are re-stimulated with IL-7 in vitro, but their survival in response to anti-CD3 and anti-CD28 antibody stimulation is impaired [72]. Interestingly, multiple metabolic pathways including mitochondrial functions are dysregulated in Tsc1−/− T cells [69,70], while glycolysis is enhanced in the absence of LKB1 [79]. These observations indicate that, in addition to suppressing mTORC1 activity, Tsc1 and LKB1 may instruct additional molecular programs to regulate peripheral T-cell homeostasis.
Regulation of metabolic programs that drive functional activation of conventional T cells
The activation of mTOR also regulates various effector functions of CD4+ and CD8+ T cells. It has been demonstrated that mTOR, Rheb and Raptor-deficient T cells have defects in proliferation [62,63], and in particular, Raptor-deficient T cells have markedly reduced capacity for cell cycle entry from quiescence [63]. The cell cycling defect is likely a result of decreased glycolysis, oxidative phosphorylation and/or lipogenesis in the absence of Raptor [63], suggesting that mTORC1 regulates metabolic programs to facilitate the switch from quiescent to activated T cells. Rapamycin-mediated inhibition of mTORC1 in murine or human T cells has also yielded similar results [63,80].
In addition to driving T-cell proliferation, mTORC1 and mTORC2 also serve different roles in priming effector CD4+ T-cell differentiation. In the absence of mTOR function, TH1, TH2 and TH17 polarizations are all impaired [62,63,80 –83]. However, it is contentious as to the effects of mTORC2 inactivation on TH1 generation [81,82]. Furthermore, associated with complete loss of mTORC1 activity, TH2 polarization and function is severely impaired in the absence of Raptor [63], but is retained in Rheb-deficient T cells that exhibit a partial loss of mTORC1 activity [63,82]. Importantly, rapamycin treatment of Rictor−/− cells is able to diminish TH2 polarization [63,81], thereby highlighting an indispensable role of mTORC1 in TH2 generation. In CD8+ T cells, mTORC1 inhibition or deletion increases memory CD8+ T-cell formation or maintenance [84–87]. Thus, mTORC1 and mTORC2 regulate diverse processes that control T-cell development, functional activation and differentiation. The roles of mTOR complexes in Tregs are discussed in a later section.
T-cell trafficking is coupled to mTOR activity
To become activated or fight infections, T cells must reside in the correct anatomical location. Chemokine and adhesion receptors direct T-cell trafficking throughout the blood, lymphatic tissues and into sites of inflammation. For instance, CD62L, CCR7 and S1PR1 promote trafficking to peripheral lymph nodes, while receptors including CXCR3 mediate recruitment into inflammatory sites [88]. Several studies have shown a role for mTOR in directing T-cell trafficking. In activated CD8+ T cells, PI3K inhibition and rapamycin treatment inhibit CCR7, CD62L and S1PR1 downregulation induced by IL-2 signaling [89]. The opposite effect is observed when mTOR activity is elevated in the absence of PTEN or Tsc1 [70,89,90]. The aberrant expression of these molecules causes CD8+ T cells to traffic to peripheral lymph nodes [89,91], which is correlated with enhanced memory CD8+ T-cell differentiation [91]. Mechanistically, mTORC1 regulates the expression of these receptors by modulating KLF2 and HIF1 expression [89,92]. Furthermore, by regulating Akt activity, mTORC2 may inhibit FoxO1 function, which has been shown to regulate the expression of lymph node homing receptors [90]. Finally, mTORC1 activity induces T-bet expression [91], which up regulates CXCR3 expression to localize T cells to sites of infection [93,94]. Thus, mTOR activity regulates T-cell trafficking via multiple mechanisms.
Amino acids & leptin modulate T-cell responses
In addition to signals received from antigens and cytokines, amino acids have also been demonstrated to modulate mTOR activation in T cells. Studies conducted in the absence of select amino acids have revealed an important role for these nutrients in T-cell responses. Depletion of arginine, leucine and tryptophan impairs T-cell responses [37,47,95]. Moreover, glutamine is rapidly imported into T cells and is required for efficient T-cell responses [96,97]. Recent studies have linked amino acid uptake to mTOR activation in T cells. CD98 senses neutral and branched amino acids, including leucine, and forms a heterodimer with Slc7a5, Slc7a8, Slc7a7 or Slc7a6 [98]. Deletion of CD98 compromises T-cell proliferation, and Slc7a5-deficient T cells have reduced mTOR signaling and functional activation [99]. ASCT2 is a sodium-coupled transporter for neutral amino acids, including glutamine, alanine, serine, threonine and cysteine [100]. A recent report showed that glutamine and glucose uptake are reduced in ASCT2-deficient T cells [97,101]; the latter observation is due to the fact that the glucose receptor, Glut1, is expressed at lower levels in these cells. As a consequence, Asct2−/− T cells have reduced mTORC1 activation and metabolic defects that specifically attenuate TH1 and TH17 differentiation and function [97,101]. Further investigation is needed to determine if different amino acids share common transporters to induce functional T-cell responses.
Leptin is a hormone derived from adipocytes that regulates multiple aspects of T-cell biology. Leptin signaling modulates T-cell proliferation and the preferential differentiation of TH1 cells over TH2 cells. [102,103]. Moreover, autoreactive CD4+ T cells are activated and survive in a leptin-dependent manner [104]. Thus, various nutritional factors modulate effector T-cell responses.
Control of Treg responses by mTOR
Tregs are also activated by upstream signals that drive mTOR activation, but these cells can influence the availability of mTOR-activating nutrients that induce effector T-cell responses. Therefore, the same upstream stimuli that induce mTOR function in effector T cells have different effects on the differentiation and functions of Tregs. Recent reports link hyperactivation of mTOR to dysregulated T-cell responses in autoimmunity [105,106], underscoring the importance of addressing the role mTOR signaling serves in Treg biology. Below, we discuss the pharmacological and genetic studies that have interrogated the functions mTOR complexes have in Tregs (Figure 3). We also describe how different upstream stimuli can tune mTOR activity to modulate Treg proliferation and suppressive responses.
Effects of rapamycin treatment on Treg differentiation
Several groups have employed rapamycin to elucidate how mTOR inhibition modulates Treg function. In this regard, rapamycin treatment induces the de novo expression of Foxp3 and thus functional Foxp3+ T-cell expansion from naive T cells in vitro [63,107–113]. Pre-existing CD4+CD25+ T cells also expand in the presence of rapamycin and have enhanced suppressive function relative to non-rapamycin treated cells [109 –111,114]. This enhanced suppressive capacity is likely due, in part, to their increased expression of CD25 and CTLA-4 [114]. Despite its ability to promote Treg proliferation upon acute treatment, chronic rapamycin stimulation does not induce Treg proliferation in the absence of exogenous IL-2 [115]. This observation may be due to the fact that rapamycin also suppresses mTORC2 function or enhances Akt activation after long-term treatment [116,117]. Furthermore, rapamycin treatment ablates some, but not all, of mTORC1 function [11,118]. For instance, rapamycin treatment can suppress S6 phosphorylation, while 4E-BP1 phosphorylation remains largely intact [11,118]. Finally, Tregs were found to be more resistant to rapamycin treatment than conventional T cells [119], potentially because ex vivo and in vitro activated Tregs have higher levels of mTORC1 activation than naive T cells [115,120]. These limitations highlighted the need for direct genetic models that assess the role of mTORC1 in Treg functions.
Effects of genetic inhibition of mTORC1 on Treg differentiation & function
More recent work has utilized genetic models to ascertain the role mTORC1 serves in Treg differentiation and functions. Delgoffe et al. were the first to demonstrate that mTor −/− T cells spontaneously develop into iTregs in the absence of IL-2 and TGF-β [62]. This spontaneous iTreg differentiation requires ablation of both mTORC1 and mTORC2, as Rheb or Rictor deletion alone is not sufficient to drive iTreg formation [82]. IL-2 and TGF-β induced iTreg differentiation is also retained in Rheb−/− T cells [62].
A recent study from our group has demonstrated that mTORC1 is a positive regulator of Treg functions in vivo [120]. Mice bearing the Raptor-deficient Tregs develop lymphadenopathy and multi-organ auto-immunity, associated with the hyperactivation of CD4+ and CD8+ T cells in these mice. Mechanistically, Raptor regulates the expression of CTLA-4, and to a lesser extent ICOS, and further links cholesterol biogenesis and metabolic pathways to mediate the proliferation and expression of these immunosuppressive molecules in Tregs [120]. It was recently demonstrated that, after CD4 T-cell depletion, Tregs that reemerge are less suppressive if mTOR function is inhibited in vivo [121], further supporting the notion that mTORC1 is a critical positive regulator of Treg functions in vivo. Moreover, the observation that Rictor deletion could delay lethality in the Raptor−/− Treg-bearing mice suggests that the positive effects of rapamycin and mTOR deletion on iTreg differentiation may be attributed to concomitant reductions in mTORC1 and mTORC2 activity.
Role of mTORC2 in Treg differentiation & function
Several studies have also investigated the role of mTORC2 in Treg development, differentiation and function. We recently found that mTORC1 antagonizes mTORC2 function to partially regulate Treg functions in vivo [120]. However, Rictor deletion in Tregs alone results in no gross abnormalities, unlike those observed in mice bearing Raptor-deficient Tregs [120]. Thus, the contribution of mTORC2 to Treg functions in vivo is less dominant than that of mTORC1. Similarly, Rictor−/− T cells retain their capacity to become iTregs [81,82]. Thus, mTORC2 does not appear to have a dominant role in maintaining Treg functions in vivo or in promoting iTreg generation, although the function of mTORC2 in pTreg differentiation in various inflammatory settings remains undefined. Collectively, these data highlight the unique biological properties of the mTOR complexes in tTreg and iTreg cells.
Multiple cellular inputs tune mTOR activation in Treg to modulate their function
As we have discussed earlier in this review, multiple upstream stimuli activate and tune mTOR activation. Therefore, quantitative differences in mTOR activation also contribute to differences in Treg responses. As we noted above, mTORC1 activity is high in human and murine Tregs [115,120], which restrains TCR and/or IL-2 stimulation-induced proliferation in vitro [115]. Because Tregs robustly proliferate in vivo [122], several studies have addressed what signals influence mTOR activation to promote efficient Treg proliferation. Leptin is a key factor in this process. Tregs express high levels of leptin receptor, and leptin restrains Treg proliferation, as neutralization of leptin enhances TCR and IL-2-induced Treg proliferation [123]. Leptin receptor-deficient Tregs also have increased proliferative responses, linked to reduced mTOR activation [115]. Thus, leptin sensing in Tregs is critical to dampen excessive mTOR activation and drives Treg proliferation. Both mTOR inhibition and amino acid deprivation can synergize with TGF-β to augment Foxp3 expression in vitro [37,47]. Thus, by modulating the function of APCs, Treg-dependent amino acid deprivation also appears to mitigate mTORC1 activation in these cells to subsequently drive Treg differentiation and/or proliferation in vitro and in vivo.
The ability to tune mTOR activity is also critical to support Treg suppressive functions in different inflammatory settings, which requires the maintenance of Foxp3 expression [8]. Transient TCR stimulation drives PI3K-Akt-mTOR signaling that antagonizes Foxp3 expression [124]. Consistent with this observation, Tsc1 deficiency in T cells leads to a loss of Foxp3+ T cells in the periphery, and Tsc1-deficient Tregs lose suppressive function in vivo [125]. Moreover, Tregs from relapsing-remitting multiple sclerosis patients have altered IL-2 signaling, which drives excessive mTOR signaling in these cells [105]. This defect is correlated with reduced Treg proliferation and Foxp3 expression [105], further supporting the notion that excessive mTOR signaling dampens Treg responses.
The S1PR1-mTORC1 signaling axis also regulates Treg differentiation and functional fates. S1PR1 signaling to mTORC1 restrains Treg differentiation in the thymus and periphery, and limits their suppressive functions in vitro and in vivo during homeostasis and inflammation [126,127]. Chronic TCR stimulation or TGF-β signaling stabilizes Foxp3 expression to promote iTreg induction and regulate their suppressive functions [62,82,124]. The activation of mTORC2 may also influence Foxp3 stability to maintain Treg suppressive functions, as Rictor-deficiency restores Treg functions in vitro and partially reverses the fatal autoimmune disease observed in mice bearing Raptor-deficient Tregs [120]. Future studies will investigate how currently unknown activators of mTORC2 and other mTORC1-inducing agents, including leptin and amino acids, influence Foxp3 stability and Treg suppressive function.
Clinical perspectives for the targeting of mTOR in Tregs
Rapamycin is approved for use in solid organ transplants, and mTOR inhibitors are potential therapeutics for solid tumors and hematological cancers, metabolic diseases and autoimmune disorders [60]. Rapamycin promotes the generation of memory CD8+ T cells [84–86], which are protective in infectious and tumor models and enhance vaccine efficacy [87,128–130]. However, rapamycin also has profound effects on CD4+ T-cell differentiation and function, as noted above. Therefore, rapamycin-induced immunosuppression has the potential to impair the ability to fight infections. Long-term immunosuppression upon rapamycin treatment may also predispose individuals to developing cancers, as immune surveillance mechanisms are likely to be disrupted. Thus, more targeted immunosuppressive regimens are still needed to limit these potentially negative effects.
Several ATP-competitive mTOR inhibitors have recently been developed [131]. These inhibitors, including Torin1 and PP242, completely attenuate mTORC1 activation as evident by suppression of 4E-BP1 phosphorylation, and are also better at targeting mTORC2 activity that can feedback enhance Akt function [132–134]. These compounds are currently entering into clinical trials and have shown enhanced promise as anticancer therapies compared with rapamycin [131,135,136]. As loss of mTORC2 in combination with mTORC1 exerts different effects on Treg differentiation and in vivo functions than repression of mTORC1 alone [82,120], future studies should explore the effects these new inhibitors have on Treg responses in different clinical settings.
Although Treg-targeted therapies are rapidly emerging as a means to regulate adaptive immune responses in different diseases, there are clear limitations to Treg-mediated therapies, thereby highlighting the need for more Treg-specific targeting strategies. What potential effects would targeting mTORC1 in Tregs have in different diseases? Our work suggests that mTORC1 inhibition in Tregs would exacerbate immune responses that could contribute to autoimmunity or excessive inflammation [1,3,8,120]. However, it may be possible to suppress inflammatory diseases or conditions by targeting mTORC1 in naive T cells, predisposing them to becoming Tregs instead of effector CD4+ T- cell populations. Furthermore, mTORC1 inhibition in combination with other methods (e.g., CTLA-4 engagement or IL-2 treatment) may provide a means to generate a stable pool of Tregs to suppress inflammation in the context of infections, transplants or autoimmunity.
mTORC1 inhibition in Tregs present in the solid tumor microenvironment, which contains Tregs and other immunosuppressive cell populations [54], may enhance the effectiveness of different antitumor treatment regimens. For instance, inhibiting mTORC1 may suppress Treg functions such that high-dose IL-2 therapy would be more beneficial to treat melanoma and renal cell carcinoma [56]. Other cancer therapies, including CTLA-4 antagonism or cytotoxic T-cell therapy to certain tumor antigens, may also be enhanced by mTORC1 inhibition in Tregs [28,137]. However, we argue that mTORC1 inhibition in this setting would need to be very specific to Tregs, as the effector T-cell populations in this system would also be impacted by mTORC1 inhibition.
The ability to generate sufficient numbers of Tregs ex vivo for adoptive therapies is one major obstacle for using Treg-mediated transfer therapy to treat autoimmune conditions. Previous works using transient rapamycin treatment or deletion of mTOR or Rheb have demonstrated that naive T cells retain or have an enhanced capacity to become iTregs [62,63,82,107–115]. Given that rapamycin mediates the expansion of functional Tregs from non-human primate and human T cells [107,108,114], this may be a viable option to generate sufficient numbers of Tregs for adoptive transfers in vitro. It was recently demonstrated that rapamycin-expanded human Tregs maintain their suppressive function in the presence of TH17-polarizing conditions in vitro and in a xenograft transfer model into immunodeficient hosts [138]. However, more long-term clinical studies are needed to determine if the progeny of these iTregs retain suppressive capacity or instead revert to other T-cell effector lineages in autoimmune or inflammatory environments. These studies will be vital toward evaluating the long-term efficacy and safety of therapeutics using iTreg that are generated in the presence of mTORC1 inhibition.
Conclusion & future perspective
Despite the overwhelming immunotherapeutic potential of targeting Tregs to modulate different diseases, the limitations with current Treg-targeted therapies highlight the need to study the precise molecular mechanisms underlying Treg functions. Recent work has revealed the important roles of mTOR in immune responses mediated by Tregs and conventional T cells. We argue that mTORC1 targeting in Tregs may be harmful or beneficial in different disease settings. Although we have demonstrated that Tregs deficient in both Rictor and Raptor are modestly more suppressive than Raptor−/− Tregs [120], further studies are needed to address if loss of mTORC1 and mTORC2, alone or in combination, in Tregs differentially alters their functions in different disease settings. These experiments will guide clinicians as they develop new Treg-mediated therapies to treat different conditions.
Both Treg- and mTOR-targeted therapies will benefit from an improved ability to target specific cell lineages. Aptamer-mediated delivery of therapeutic agents, such as drug compounds or RNA interference sequences, is an attractive means by which Treg functions may be specifically modulated. In support of this idea, mTORC1 function was recently inhibited in vivo using an aptamer that specifically targeted activated CD8+ T cells [86]. Before this strategy may be used clinically, however, the further classification of surface receptors that distinguish tTreg and pTreg/iTreg cells from naive and activated conventional T-cell populations is needed. These studies would ameliorate some of the off-target effects that are currently observed with treatments that target both Tregs and activated conventional T cells, such as anti-CD25 antibody or CTLA-4-Ig administration.
It will also be important to further characterize how mTORC1 activity regulates Treg development or functions in different disease conditions. For instance, how does mTORC1 modulate Treg functions in different inflammatory environments (e.g., TH1, TH2, TH17-associated inflammation)? Furthermore, how does the interplay between Tregs and other suppressive T cell and/or myeloid populations influence mTORC1-mediated functions in Tregs? Addressing this question will be especially useful for determining how mTORC1 targeting may influence the antitumor response, as various suppressive immune cell populations are found within tumors [54]. Finally, what role does mTOR serve in the generation of humoral responses mediated by TFH and TFR? This concept would be interesting to address, as it is possible that dysregulated mTOR function in these cells may contribute to autoantibody production that is pathogenic in autoimmune disorders [139]. Gaining further insight into how mTOR regulates immune responses will be crucial for designing safe and effective methods to tune mTOR activation as a means to modulate Treg activity in various diseases.
Executive summary.
Background
T cells are central regulators of the adaptive immune response to infections, but dysfunctional T-cell responses contribute to disease development or progression.
APCs that express antigens and costimulatory molecules activate T cells expressing specific T-cell antigen receptors (TCRs). Additional factors, including cytokines, growth factors and nutrients, also tune how T cells respond to different pathogenic antigens.
CD4+CD25+Foxp3+ Tregs are critical suppressors of T-cell responses and thus are central regulators of immune homeostasis.
mTOR is a critical integrator of environmental stimuli and regulates signaling in T cells that influence their homeostasis, differentiation and activation.
Mechanisms of Treg suppression & their roles in homeostasis & disease
Tregs are phenotypically distinct from other CD4+ T-cell populations, but also share common surface receptors with activated and memory T cells. This is a challenge for the select targeting of Tregs in clinical settings.
- Tregs utilize multiple suppressive mechanisms to inhibit T-cell responses which are broadly classified into the following:
- Cell contact-dependent modulation of APC function (e.g., CTLA-4 downmodulation of costimulatory signals or induction of IDO expression).
- Secretion of various anti-inflammatory cytokines (e.g., IL-10, TGF-β, IL-35).
- Disruption of metabolic processes (e.g., localized depletion of IL-2, ATP).
- Induction of apoptosis in conventional T cells (via perforin and granzyme B).
Dysfunctional Treg responses contribute to autoimmunity or cancer development.
Different therapies that modulate Treg functions are in development or under clinical investigation to treat diseases, including autoimmunity and cancer.
mTOR is a critical regulator of T-cell homeostasis & function
Elevated mTORC1 signaling disrupts conventional T-cell homeostasis.
- Genetic inhibition of mTOR function has profound effects on CD4+ T-cell differentiation and functional activation:
- Reduced induction of glycolytic, oxidative phosphorylation and lipolysis metabolism, which contributes to reduced T-cell proliferation.
- Impaired generation of TH1, TH17, TH2 responses.
Memory CD8+ T-cell differentiation and function is negatively regulated by mTORC1.
Control of Treg responses by mTOR
Short-term inhibition of mTORC1 induces Foxp3 expression in naive T cells and drives functional Treg expansion in vitro.
Long-term suppression of mTORC1 does not promote Treg proliferation under select conditions.
Genetic inhibition of mTORC1, but not mTORC2, ablates Treg suppressive functions and drives fatal autoimmune disease development in vivo.
mTORC2 inhibition partially restores the in vivo suppressive functions of Tregs after mTORC1 function is impaired and may increase Treg differentiation in vitro.
Clinical perspectives for the targeting of mTORC1 in Tregs
mTOR inhibitors are emerging as treatments for different human diseases, but the global immunosuppressive effects of these inhibitors demonstrate that more targeted strategies are needed to harness their function in immune-mediated diseases.
Recent studies indicate that mTORC1 inhibition in Tregs may disrupt immune homeostasis and drive autoimmunity or hyperinflammation. However, targeted inhibition of mTORC1 in a tumor microenvironment may enhance antitumor immune responses by overcoming the suppressive environment mediated by Tregs.
Naive T-cell targeting of mTORC1 inhibitors may provide dual therapeutic effects in autoimmune conditions that are driven by pathogenic CD4+ T-cell responses, in that Tregs would be induced and effector CD4+ T-cell differentiation and function would be inhibited.
Short-term mTORC1 inhibition may be therapeutically beneficial to expand Tregs in vitro, which could then be used in Treg-adoptive transfer therapies in autoimmune diseases.
Conclusion & future perspective
Targeting Tregs for immune modulation holds strong therapeutic promise, but more specific methods of modulating Treg differentiation or functions without compromising conventional T-cell activation are still needed.
mTORC1 inhibition offers an attractive strategy to tune Treg responses under select conditions, but future work should investigate how mTORC1 inhibition in these cells influences their functions in different inflammatory settings.
Acknowledgments
The authors thank H Zeng for critically reading this manuscript.
This work is supported by US NIH (AI101407, AI105887, CA176624 and NS064599), American Cancer Society, National Multiple Sclerosis Society, Crohn's & Colitis Foundation of America, the American Lebanese Syrian Associated Charities (H Chi) and by The Hartwell Foundation Biomedical Research Fellowship (N Chapman).
Footnotes
Financial & competing interests disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tangye SG, Ma CS, Brink R, Deenick EK. The good, the bad and the ugly – TFH cells in human health and disease. Nat Rev Immunol. 2013;13(6):412–426. doi: 10.1038/nri3447. [DOI] [PubMed] [Google Scholar]
- 4.Zeng H, Chi H. mTOR and lymphocyte metabolism. Curr Opin Immunol. 2013;25(3):347–355. doi: 10.1016/j.coi.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5•.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. Review that summarizes the major discoveries in the development, maintenance and function of Tregs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchez AM, Yang Y. The role of natural regulatory T cells in infection. Immunol Res. 2011;49(1–3):124–134. doi: 10.1007/s12026-010-8176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol. 2014;27C:1–7. doi: 10.1016/j.coi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3 + regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 9•.Singer BD, King LS, DAlessio FR. Regulatory T Cells as Immunotherapy. Front Immunol. 2014;5:46. doi: 10.3389/fimmu.2014.00046. Review of Treg therapies in different human diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang K, Chi H. mTOR and metabolic pathways in T cell quiescence and functional activation. Semin Immunol. 2012;24(6):421–428. doi: 10.1016/j.smim.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12(5):325–338. doi: 10.1038/nri3198. Review that discusses the functions of mTOR signaling in T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang R, Green DR. Metabolic checkpoints in activated T cells. Nat Immunol. 2012;13(10):907–915. doi: 10.1038/ni.2386. [DOI] [PubMed] [Google Scholar]
- 13.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbas AK, Benoist C, Bluestone JA, et al. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol. 2013;14(4):307–308. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- 15.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collison LW, Chaturvedi V, Henderson AL, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11(12):1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin X, Chen M, Liu Y, et al. Advances in distinguishing natural from induced Foxp3(+) regulatory T cells. Int J Clin Exp Pathol. 2013;6(2):116–123. [PMC free article] [PubMed] [Google Scholar]
- 18.Gravano DM, Hoyer KK. Promotion and prevention of autoimmune disease by CD8+ T cells. J Autoimmun. 2013;45:68–79. doi: 10.1016/j.jaut.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Sugimoto N, Oida T, Hirota K, et al. Foxp3-dependent and -independent molecules specific for CD25+CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int Immunol. 2006;18(8):1197–1209. doi: 10.1093/intimm/dxl060. [DOI] [PubMed] [Google Scholar]
- 20.Getnet D, Grosso JF, Goldberg MV, et al. A role for the transcription factor Helios in human CD4(+)CD25(+) regulatory T cells. Mol Immunol. 2010;47(7–8):1595–1600. doi: 10.1016/j.molimm.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thornton AM, Korty PE, Tran DQ, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184(7):3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss JM, Bilate AM, Gobert M, et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med. 2012;209(10):S1721. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yadav M, Louvet C, Davini D, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209(10):S1711–S1719. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akimova T, Beier UH, Wang L, Levine MH, Hancock WW. Helios expression is a marker of T cell activation and proliferation. PLoS ONE. 2011;6(8):e24226. doi: 10.1371/journal.pone.0024226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottschalk RA, Corse E, Allison JP. Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J Immunol. 2012;188(3):976–980. doi: 10.4049/jimmunol.1102964. [DOI] [PubMed] [Google Scholar]
- 26.Serre K, Benezech C, Desanti G, et al. Helios is associated with CD4 T cells differentiating to T helper 2 and follicular helper T cells in vivo independently of Foxp3 expression. PLoS ONE. 2011;6(6):e20731. doi: 10.1371/journal.pone.0020731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zabransky DJ, Nirschl CJ, Durham NM, et al. Phenotypic and functional properties of Helios+ regulatory T cells. PLoS ONE. 2012;7(3):e34547. doi: 10.1371/journal.pone.0034547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitagawa Y, Ohkura N, Sakaguchi S. Molecular determinants of regulatory T cell development: the essential roles of epigenetic changes. Front Immunol. 2013;4:106. doi: 10.3389/fimmu.2013.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baron U, Floess S, Wieczorek G, et al. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3(+) conventional T cells. Eur J Immunol. 2007;37(9):2378–2389. doi: 10.1002/eji.200737594. [DOI] [PubMed] [Google Scholar]
- 30.Floess S, Freyer J, Siewert C, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5(2):e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 32.Wing K, Yamaguchi T, Sakaguchi S. Cell-autonomous and -non-autonomous roles of CTLA-4 in immune regulation. Trends Immunol. 2011;32(9):428–433. doi: 10.1016/j.it.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Qureshi OS, Zheng Y, Nakamura K, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332(6029):600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Der Merwe PA, Bodian DL, Daenke S, Linsley P, Davis SJ. CD80 (B7–1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med. 1997;185(3):393–403. doi: 10.1084/jem.185.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grohmann U, Orabona C, Fallarino F, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3(11):1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 36.Fallarino F, Grohmann U, Hwang KW, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4(12):1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 37.Cobbold SP, Adams E, Farquhar CA, et al. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc Natl Acad Sci USA. 2009;106(29):12055–12060. doi: 10.1073/pnas.0903919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anz D, Mueller W, Golic M, et al. CD103 is a hallmark of tumor-infiltrating regulatory T cells. Int J Cancer. 2011;129(10):2417–2426. doi: 10.1002/ijc.25902. [DOI] [PubMed] [Google Scholar]
- 39.Suffia I, Reckling SK, Salay G, Belkaid Y. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J Immunol. 2005;174(9):5444–5455. doi: 10.4049/jimmunol.174.9.5444. [DOI] [PubMed] [Google Scholar]
- 40.Simpson TR, Quezada SA, Allison JP. Regulation of CD4 T cell activation and effector function by inducible costimulator (ICOS) Curr Opin Immunol. 2010;22(3):326–332. doi: 10.1016/j.coi.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Annacker O, Coombes JL, Malmstrom V, et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202(8):1051–1061. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo F, Iclozan C, Suh WK, Anasetti C, Yu XZ. CD28 controls differentiation of regulatory T cells from naive CD4 T cells. J Immunol. 2008;181(4):2285–2291. doi: 10.4049/jimmunol.181.4.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delgoffe GM, Woo SR, Turnis ME, et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nat. 2013;501(7466):252–256. doi: 10.1038/nature12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6(11):1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 45.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8(12):1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 46.Terness P, Bauer TM, Rose L, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196(4):447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fallarino F, Grohmann U, You S, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176(11):6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 48.Bopp T, Becker C, Klein M, et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med. 2007;204(6):1303–1310. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borsellino G, Kleinewietfeld M, Di Mitri D, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110(4):1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 50.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J Immunol. 2006;177(10):6780–6786. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 51.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10(12):849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whiteside TL. Regulatory T cell subsets in human cancer: are they regulating for or against tumor progression? Cancer Immunol Immunother. 2014;63(1):67–72. doi: 10.1007/s00262-013-1490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gajewski TF, Woo SR, Zha Y, et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol. 2013;25(2):268–276. doi: 10.1016/j.coi.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 56.Sim GC, Martin-Orozco N, Jin L, et al. IL-2 therapy promotes suppressive ICOS+ Treg expansion in melanoma patients. J Clin Invest. 2014;124(1):99–110. doi: 10.1172/JCI46266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sancak Y, Peterson TR, Shaul YD, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320(5882):1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141(2):290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laplante M, Sabatini DM. mTOR Signaling. Cold Spring Harbor Perspectives Biol. 2012;4(2) doi: 10.1101/cshperspect.a011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoshii T, Kasada A, Hatakeyama T, et al. Loss of mTOR complex 1 induces developmental blockage in early T-lymphopoiesis and eradicates T-cell acute lymphoblastic leukemia cells. Proc Natl Acad Sci USA. 2014;111(10):3805–3810. doi: 10.1073/pnas.1320265111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee K, Nam KT, Cho SH, et al. Vital roles of mTOR complex 2 in Notch-driven thymocyte differentiation and leukemia. J Exp Med. 2012;209(4):713–728. doi: 10.1084/jem.20111470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delgoffe GM, Kole TP, Zheng Y, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30(6):832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang K, Shrestha S, Zeng H, et al. T cell exit from quiescence and differentiation into Th2 cells depend on Raptor-mTORC1-mediated metabolic reprogramming. Immunity. 2013;39(6):1043–1056. doi: 10.1016/j.immuni.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Finlay DK, Sinclair LV, Feijoo C, et al. Phosphoinositide-dependent kinase 1 controls migration and malignant transformation but not cell growth and proliferation in PTEN-null lymphocytes. J Exp Med. 2009;206(11):2441–2454. doi: 10.1084/jem.20090219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo W, Schubbert S, Chen JY, et al. Suppression of leukemia development caused by PTEN loss. Proc Natl Acad Sci USA. 2011;108(4):1409–1414. doi: 10.1073/pnas.1006937108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Juntilla MM, Wofford JA, Birnbaum MJ, Rathmell JC, Koretzky GA. Akt1 and Akt2 are required for alphabeta thymocyte survival and differentiation. Proc Natl Acad Sci USA. 2007;104(29):12105–12110. doi: 10.1073/pnas.0705285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mao C, Tili EG, Dose M, et al. Unequal contribution of Akt isoforms in the double-negative to double-positive thymocyte transition. J Immunol. 2007;178(9):5443–5453. doi: 10.4049/jimmunol.178.9.5443. [DOI] [PubMed] [Google Scholar]
- 68.Kishimoto H, Ohteki T, Yajima N, et al. The Pten/PI3K pathway governs the homeostasis of Valpha14iNKT cells. Blood. 2007;109(8):3316–3324. doi: 10.1182/blood-2006-07-038059. [DOI] [PubMed] [Google Scholar]
- 69.O'brien TF, Gorentla BK, Xie D, et al. Regulation of T-cell survival and mitochondrial homeostasis by TSC1. Eur J Immunol. 2011;41(11):3361–3370. doi: 10.1002/eji.201141411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang K, Neale G, Green DR, He W, Chi H. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat Immunol. 2011;12(9):888–897. doi: 10.1038/ni.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu Q, Liu Y, Chen C, et al. The tuberous sclerosis complex-mammalian target of rapamycin pathway maintains the quiescence and survival of naive T cells. J Immunol. 2011;187(3):1106–1112. doi: 10.4049/jimmunol.1003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tamas P, Macintyre A, Finlay D, et al. LKB1 is essential for the proliferation of T-cell progenitors and mature peripheral T cells. Eur J Immunol. 2010;40(1):242–253. doi: 10.1002/eji.200939677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cao Y, Li H, Liu H, Zheng C, Ji H, Liu X. The serine/threonine kinase LKB1 controls thymocyte survival through regulation of AMPK activation and Bcl-XL expression. Cell Res. 2010;20(1):99–108. doi: 10.1038/cr.2009.141. [DOI] [PubMed] [Google Scholar]
- 74.Hurov JB, Stappenbeck TS, Zmasek CM, et al. Immune system dysfunction and autoimmune disease in mice lacking Emk (Par-1) protein kinase. Mol Cell Biol. 2001;21(9):3206–3219. doi: 10.1128/MCB.21.9.3206-3219.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mayer A, Denanglaire S, Viollet B, Leo O, Andris F. AMP-activated protein kinase regulates lymphocyte responses to metabolic stress but is largely dispensable for immune cell development and function. Eur J Immunol. 2008;38(4):948–956. doi: 10.1002/eji.200738045. [DOI] [PubMed] [Google Scholar]
- 76.Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol. 2011;12(6):478–484. doi: 10.1038/ni.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang L, Zhang H, Li L, et al. TSC1/2 signaling complex is essential for peripheral naive CD8+ T cell survival and homeostasis in mice. PLoS ONE. 2012;7(2):e30592. doi: 10.1371/journal.pone.0030592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu X, Karnell JL, Yin B, et al. Distinct roles for PTEN in prevention of T cell lymphoma and autoimmunity in mice. J Clin Invest. 2010;120(7):2497–2507. doi: 10.1172/JCI42382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maciver NJ, Blagih J, Saucillo DC, et al. The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J Immunol. 2011;187(8):4187–4198. doi: 10.4049/jimmunol.1100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kopf H, De La Rosa GM, Howard OM, Chen X. Rapamycin inhibits differentiation of Th17 cells and promotes generation of FoxP3+ T regulatory cells. Int Immunopharmacol. 2007;7(13):1819–1824. doi: 10.1016/j.intimp.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81••.Lee K, Gudapati P, Dragovic S, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32(6):743–753. doi: 10.1016/j.immuni.2010.06.002. Describes the role of Rictor in CD4+ T-cell differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82••.Delgoffe GM, Pollizzi KN, Waickman AT, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12(4):295–303. doi: 10.1038/ni.2005. First genetic characterization of the distinct functions of mTOR complexes in T-cell fate decisions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kurebayashi Y, Nagai S, Ikejiri A, et al. PI3K-Akt-mTORC1-S6K1/2 axis controls Th17 differentiation by regulating Gf1 expression and nuclear translocation of RORgamma. Cell Rep. 2012;1(4):360–373. doi: 10.1016/j.celrep.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 84.Araki K, Turner AP, Shaffer VO, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460(7251):108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pearce EL, Walsh MC, Cejas PJ, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460(7251):103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berezhnoy A, Castro I, Levay A, Malek TR, Gilboa E. Aptamer-targeted inhibition of mTOR in T cells enhances antitumor immunity. J Clin Invest. 2014;124(1):188–197. doi: 10.1172/JCI69856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Turner AP, Shaffer VO, Araki K, et al. Sirolimus enhances the magnitude and quality of viral-specific CD8+ T-cell responses to vaccinia virus vaccination in rhesus macaques. Am J Transpl. 2011;11(3):613–618. doi: 10.1111/j.1600-6143.2010.03407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Finlay D, Cantrell DA. Metabolism, migration and memory in cytotoxic T cells. Nat Rev Immunol. 2011;11(2):109–117. doi: 10.1038/nri2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sinclair LV, Finlay D, Feijoo C, et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9(5):513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kerdiles YM, Beisner DR, Tinoco R, et al. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009;10(2):176–184. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32(1):67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Finlay DK, Rosenzweig E, Sinclair LV, et al. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med. 2012;209(13):2441–2453. doi: 10.1084/jem.20112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Taqueti VR, Grabie N, Colvin R, et al. T- betcontrols pathogenicity of CTLs in the heart by separable effects on migration and effector activity. J Immunol. 2006;177(9):5890–5901. doi: 10.4049/jimmunol.177.9.5890. [DOI] [PubMed] [Google Scholar]
- 94.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10(6):595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zheng Y, Delgoffe GM, Meyer CF, Chan W, Powell JD. Anergic T cells are metabolically anergic. J Immunol. 2009;183(10):6095–6101. doi: 10.4049/jimmunol.0803510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carr EL, Kelman A, Wu GS, et al. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol. 2010;185(2):1037–1044. doi: 10.4049/jimmunol.0903586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nakaya M, Xiao Y, Zhou X, et al. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity. 2014;40(5):692–705. doi: 10.1016/j.immuni.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fotiadis D, Kanai Y, Palacin M. The SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med. 2013;34(2–3):139–158. doi: 10.1016/j.mam.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 99.Cantor J, Slepak M, Ege N, Chang JT, Ginsberg MH. Loss of T cell CD98 H chain specifically ablates T cell clonal expansion and protects from autoimmunity. J Immunol. 2011;187(2):851–860. doi: 10.4049/jimmunol.1100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Utsunomiya-Tate N, Endou H, Kanai Y. Cloning and functional characterization of a system ASC-like Na+-dependent neutral amino acid transporter. J Biol Chem. 1996;271(25):14883–14890. doi: 10.1074/jbc.271.25.14883. [DOI] [PubMed] [Google Scholar]
- 101.Poffenberger MC, Jones RG. Amino acids fuel T cell-mediated inflammation. Immunity. 2014;40(5):635–637. doi: 10.1016/j.immuni.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 102.Procaccini C, De Rosa V, Galgani M, et al. Leptin-induced mTOR activation defines a specific molecular and transcriptional signature controlling CD4+ effector T cell responses. J Immunol. 2012;189(6):2941–2953. doi: 10.4049/jimmunol.1200935. [DOI] [PubMed] [Google Scholar]
- 103.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394(6696):897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 104.Galgani M, Procaccini C, De Rosa V, et al. Leptin modulates the survival of autoreactive CD4+ T cells through the nutrient/energy-sensing mammalian target of rapamycin signaling pathway. J Immunol. 2010;185(12):7474–7479. doi: 10.4049/jimmunol.1001674. [DOI] [PubMed] [Google Scholar]
- 105.Carbone F, De Rosa V, Carrieri PB, et al. Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat Med. 2014;20(1):69–74. doi: 10.1038/nm.3411. [DOI] [PubMed] [Google Scholar]
- 106.Daley SR, Coakley KM, Hu DY, et al. Rasgrp1 mutation increases naive T-cell CD44 expression and drives mTOR-dependent accumulation of Helios+ T cells and autoantibodies. eLife. 2013;2:e01020. doi: 10.7554/eLife.01020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transpl. 2007;7(7):1722–1732. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kang J, Huddleston SJ, Fraser JM, Khoruts A. De novo induction of antigen-specific CD4 + CD25 + Foxp3+ regulatory T cells in vivo following systemic antigen administration accompanied by blockade of mTOR. J Leukoc Biol. 2008;83(5):1230–1239. doi: 10.1189/jlb.1207851. [DOI] [PubMed] [Google Scholar]
- 109.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4 + CD25 + FOXP3+ regulatory T cells of both healthy subjects and Type 1 diabetic patients. J Immunol. 2006;177(12):8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 110.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4 + CD25 + FoxP3+ regulatory T cells. Blood. 2005;105(12):4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 111.Golovina TN, Mikheeva T, Brusko TM, Blazar BR, Bluestone JA, Riley JL. Retinoic acid and rapamycin differentially affect and synergistically promote the ex vivo expansion of natural human T regulatory cells. PLoS ONE. 2011;6(1):e15868. doi: 10.1371/journal.pone.0015868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bocian K, Borysowski J, Wierzbicki P, et al. Rapamycin, unlike cyclosporine A, enhances suppressive functions of in vitro-induced CD4 + CD25+ Tregs. Nephrol Dial Transpl. 2010;25(3):710–717. doi: 10.1093/ndt/gfp586. [DOI] [PubMed] [Google Scholar]
- 113.Valmori D, Tosello V, Souleimanian NE, et al. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4 + T cells. J Immunol. 2006;177(2):944–949. doi: 10.4049/jimmunol.177.2.944. [DOI] [PubMed] [Google Scholar]
- 114.Singh K, Kozyr N, Stempora L, et al. Regulatory T cells exhibit decreased proliferation but enhanced suppression after pulsing with sirolimus. Am J Transpl. 2012;12(6):1441–1457. doi: 10.1111/j.1600-6143.2011.03963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115•.Procaccini C, De Rosa V, Galgani M, et al. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity. 2010;33(6):929–941. doi: 10.1016/j.immuni.2010.11.024. Demonstrates that acute versus chronic inhibition of mTORC1 has different effects on CD4+ CD25hi T-cell proliferation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22(2):159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 117.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26(13):1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 118.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci USA. 2008;105(45):17414–17419. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zeiser R, Leveson-Gower DB, Zambricki EA, et al. Differential impact of mammalian target of rapamycin inhibition on CD4 + CD25 + Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111(1):453–462. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120••.Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature. 2013;499(7459):485–490. doi: 10.1038/nature12297. First paper demonstrating that mTORC1 is a critical positive regulator of metabolic programs that establish Treg functions in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Y, Sparwasser T, Figlin R, Kim HL. Foxp3 + T cells inhibit antitumor immune memory modulated by mTOR inhibition. Cancer Res. 2014;74(8):2217–2228. doi: 10.1158/0008-5472.CAN-13-2928. [DOI] [PubMed] [Google Scholar]
- 122.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198(2):249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.De Rosa V, Procaccini C, Cali G, et al. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26(2):241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 124.Sauer S, Bruno L, Hertweck A, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA. 2008;105(22):7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Park Y, Jin HS, Lopez J, et al. TSC1 regulates the balance between effector and regulatory T cells. J Clin Invest. 2013;123(12):5165–5178. doi: 10.1172/JCI69751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu G, Burns S, Huang G, et al. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol. 2009;10(7):769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat Immunol. 2010;11(11):1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ferrer IR, Wagener ME, Robertson JM, et al. Cutting edge: Rapamycin augments pathogen-specific but not graft-reactive CD8+ T cell responses. J Immunol. 2010;185(4):2004–2008. doi: 10.4049/jimmunol.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li Q, Rao RR, Araki K, et al. A central role for mTOR kinase in homeostatic proliferation induced CD8+ T cell memory and tumor immunity. Immunity. 2011;34(4):541–553. doi: 10.1016/j.immuni.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Y, Wang XY, Subjeck JR, Shrikant PA, Kim HL. Temsirolimus, an mTOR inhibitor, enhances anti-tumour effects of heat shock protein cancer vaccines. Br J Cancer. 2011;104(4):643–652. doi: 10.1038/bjc.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10(11):868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 132.Apsel B, Blair JA, Gonzalez B, et al. Targeted polypharmacology: discovery of dual inhibitors of tyrosine and phosphoinositide kinases. Nat Chem Biol. 2008;4(11):691–699. doi: 10.1038/nchembio.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Feldman ME, Apsel B, Uotila A, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7(2):e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Thoreen CC, Kang SA, Chang JW, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284(12):8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hsieh AC, Costa M, Zollo O, et al. Genetic dissection of the oncogenic mTOR pathway reveals druggable addiction to translational control via 4EBP-eIF4E. Cancer Cell. 2010;17(3):249–261. doi: 10.1016/j.ccr.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Janes MR, Limon JJ, So L, et al. Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nat Med. 2010;16(2):205–213. doi: 10.1038/nm.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013;39(1):49–60. doi: 10.1016/j.immuni.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tresoldi E, Dell'albani I, Stabilini A, et al. Stability of human rapamycin-expanded CD4+CD25+ T regulatory cells. Haematologica. 2011;96(9):1357–1365. doi: 10.3324/haematol.2011.041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ma CS, Deenick EK. Human T follicular helper (Tfh) cells and disease. Immunol Cell Biol. 2014;92(1):64–71. doi: 10.1038/icb.2013.55. [DOI] [PubMed] [Google Scholar]