Abstract
Background: Current classification of pulmonary hypertension (PH) is based on a relatively simple combination of patient characteristics and hemodynamics. This limits customization of treatment, and lacks the clarity of a more granular identification based on individual patient phenotypes. Rapid advances in mechanistic understanding of the disease, improved imaging methods, and innovative biomarkers now provide an opportunity to define PH phenotypes on the basis of biomarkers, advanced imaging, and pathobiology. This document organizes our current understanding of PH phenotypes and identifies gaps in our knowledge.
Methods: A multidisciplinary committee with expertise in clinical care (pulmonary, cardiology, pediatrics, and pathology), clinical research, and/or basic science in the areas of PH identified important questions and reviewed and synthesized the literature.
Results: This document describes selected PH phenotypes and serves as an initial platform to define additional relevant phenotypes as new knowledge is generated. The biggest gaps in our knowledge stem from the fact that our present understanding of PH phenotypes has not come from any particularly organized effort to identify such phenotypes, but rather from reinterpreting studies and reports that were designed and performed for other purposes.
Conclusions: Accurate phenotyping of PH can be used in research studies to increase the homogeneity of study cohorts. Once the ability of the phenotypes to predict outcomes has been validated, phenotyping may also be useful for determining prognosis and guiding treatment. This important next step in PH patient care can optimally be addressed through a consortium of study sites with well-defined goals, tasks, and structure. Planning and support for this could include the National Institutes of Health and the U.S. Food and Drug Administration, with industry and foundation partnerships.
Keywords: biomarkers, consortium, metabolism, pathobiology, pulmonary circulation
Contents
Executive Summary
Introduction and Rationale
Methods
Committee Composition and Meetings
Document Preparation and Structure
Definitions and Approaches to Phenotyping
Phenotypes
Mixed Pre- and Postcapillary PH
“Severe” PH in Respiratory Disease
Maladaptive Right Ventricular Hypertrophy
Connective Tissue Disease–associated PH
Portopulmonary Hypertension
HIV-associated PAH
PH in Elderly Individuals
PAH in Children
Metabolic Syndrome
Long-Term Survivors
Summary and Conclusions
Overview
Current pulmonary hypertension (PH) classification is based on clinical presentation and hemodynamic subsets and does not support customization of treatment for the individual patient phenotype. For clinical applications, phenotypes can play a complementary role to existing PH classification schemes. For research (both clinical and basic) and therapeutic development purposes, however, phenotyping can provide new insights and allow us to see the disease through a new set of lenses, so that we can approach it in ways that are not limited by our existing methods. This document lays the framework for addressing an urgent need to develop accurate phenotypes of PH.
-
•
Rapid advances in mechanistic understanding of the disease, improved imaging methods, and innovative biomarkers now provide an opportunity to define PH phenotypes on the basis of pathobiology.
-
•
Among the phenotypes that we propose are mixed pre- and postcapillary PH, severe PH in respiratory disease, maladaptive right ventricular (RV) hypertrophy, connective tissue disease–associated PH, portopulmonary hypertension, HIV-associated pulmonary arterial hypertension (PAH), PH in elderly individuals, PAH in children, metabolic syndrome, and long-term survivors. This is certainly not intended to be an exhaustively complete list of phenotypes, but rather to provide examples.
-
•
With new knowledge, there will undoubtedly be new phenotypes discovered and described.
-
•
The largest gaps in knowledge stem from the fact that, up to this point, our understanding of phenotypes in PH has not come from any particularly organized effort to identify and/or validate such phenotypes, but rather from reinterpretations of studies and reports that were designed and performed for other purposes.
-
•
Accurate phenotyping will advance understanding of mechanisms, which can be used to guide targeted management strategies.
-
•
A prospective standardization of approach is essential to establishing valid phenotypes. This important next step in PH patient care can optimally be addressed through a consortium of study sites with well-defined goals, tasks, and structure. Planning and support for such a consortium could include the National Institutes of Health and the U.S. Food and Drug Administration, with industry and foundation partnerships.
Introduction and Rationale
The initial classification of PH in 1973 was based on clinical features (such as the presence of chronic lung disease, thromboembolism, left heart disease, etc.) and hemodynamic characteristics (i.e., mean pulmonary artery pressure [mPAP] and pulmonary vascular resistance [PVR]). The current approach to classifying patients with PH similarly relies on clinical features (1, 2). Such classification schemes have not led to the customization of treatment according to patient characteristics and responsiveness.
Rapid advances in mechanistic understanding of PH, improved imaging methods and new modalities, and the emergence of innovative biomarkers (3–6) offer an opportunity to define PH phenotypes more precisely on the basis of pathobiology, which is crucial in such a heterogeneous syndrome. This Statement gives examples of PH phenotypes, identifies gaps in our knowledge regarding phenotypes, and describes the need for research to delineate phenotypes and to detect the association of phenotypes with prognosis and/or response to management. The current clinical classification of PH includes only five major categories (PAH, PH due to left heart disease, PH due to lung disease, PH due to chronic thromboemboli, and a miscellaneous category). It is clear that, despite the usefulness of this system, far more than five phenotypes exist among patients with PH. Understanding and describing genetic, pathobiologic, hemodynamic, and clinical heterogeneity within each category of PH are critical if we are to individualize care and further advance treatment.
Methods
Committee Composition and Meetings
The project chair (R.A.D.) in consultation with executive committee members (J.N., S.C.E., S.R.) assembled a group of international experts in PH. The experts were identified by the chair and the executive committee on the basis of their publication record and expertise in clinical care (pulmonary, cardiology, pediatrics, and pathology), clinical research, and/or basic science in the areas of PH and phenotyping. Two face-to-face meetings and eight teleconferences were held. The committee members disclosed potential conflicts of interest, which were vetted in accordance with the policies and procedures of the American Thoracic Society. Furthermore, members were reminded to consider their own and other members’ potential conflicts of interest during the meetings.
Document Preparation and Structure
The outline of the Statement proposed by the chair was modified and agreed on with input from all committee members. Each member of the committee was assigned a topic and preliminary drafts were developed. Although we did not perform systematic reviews of the literature for each of the sections, each committee member was asked to individually (no librarian support was provided) assess the literature relevant to his/her section by independently completing literature searches, using PubMed and OVID to search Medline. Search terms were determined by each committee member for his/her section. The literature search covered relevant studies including randomized controlled trials, cohort studies, case–control studies, registries, and cross-sectional studies published in the English language between 2002 and 2012. Earlier studies were also included if they were believed to be relevant (Table 1). The drafts of the individual sections were collated by the Chair, the full draft document was circulated to the committee for review and comments, and this was followed by multiple cycles of revisions, review, and comments until a version was approved by the full committee.
Table 1:
Methods Checklist
| Yes | No | |
|---|---|---|
| Panel assembly | ||
| • Included experts for relevant clinical and nonclinical disciplines | X | |
| • Included individual who represents the views of patients and society at large | X | |
| • Included a methodologist with appropriate expertise (documented expertise in conducting systematic reviews to identify the evidence base and the development of evidence-based recommendations) | X | |
| Literature review | ||
| • Performed in collaboration with librarian | X | |
| • Searched multiple electronic databases | X | |
| • Reviewed reference lists of retrieved articles | X | |
| Evidence synthesis | ||
| • Applied prespecified inclusion and exclusion criteria | X | |
| • Evaluated included studies for sources of bias | X | |
| • Explicitly summarized benefits and harms | X | |
| • Used PRISMA1 to report systematic review | N/A | |
| • Used GRADE to describe quality of evidence | N/A | |
| Generation of recommendations | ||
| • Used GRADE to rate the strength of recommendations | N/A |
Definition of abbreviations: GRADE = Grading of Recommendations Assessment, Development, and Evaluation; N/A = not applicable; PRISMA1 = Preferred Items for Systematic Reviews and Meta-analyses, version 1.
Note: Official ATS Statements are not required to report their literature search in accordance with the PRISMA statement or to report their evidence appraisal using the GRADE approach.
Definitions and Approaches to Phenotyping
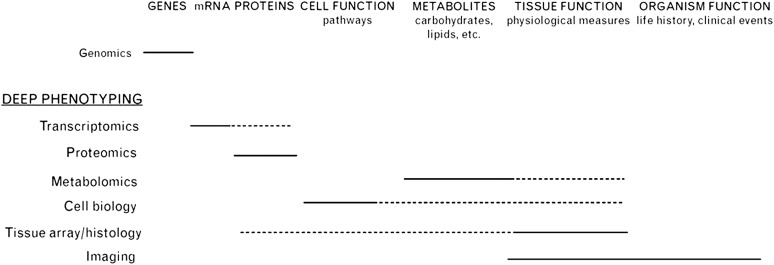
Phenotype refers to the morphological, biochemical, physiological, and/or behavioral characteristics of an organism and is a consequence of genetic and environmental interactions. In parallel with advances in genetic analyses, leveraging high-throughput technologies for phenomics has been proposed (7). Deep phenotyping calls for measuring and integrating genomics, transcriptomics, proteomics, metabolomics, cell biology and tissue functioning, and imaging (8) (Figure 1). Intermediate phenotypes (or “endophenotypes”) are clinical entities associated with the disease but are closer to the pathobiological underpinnings of the disease. Endophenotypes may be more objectively defined than the disease diagnosis and may be shared by a wide spectrum of diseases, potentially linking apparently dissimilar conditions together. The ideal endophenotype is reliably assessed, is stable over time, is associated with the disease of interest, and is at least as heritable as the disease itself (9). Levels of oxidative stress, endothelial dysfunction, and mitochondrial dysfunction are potential endophenotypes that may be shared among PH, cancer, and systemic vascular disease (10–17). Although it is traditional to establish the phenotype by its observable traits and then to search for genetic associations, the genetic or molecular markers can be used to identify the phenotype, that is, reverse phenotyping. In this approach, individuals are distinguished by the genetic marker, and then the distribution of certain traits is assessed (9). Such analyses in PH have been undertaken, specifically in reference to BMPR2 and ACVRL1 mutations (18, 19).
Figure 1.
Deep phenotyping calls for measuring and integrating genomics, transcriptomics, proteomics, metabolomics, cell biology and tissue functioning, and imaging. High-throughput and large-scale measurements are emerging as epidemiological tools, with tools for genetic measurements leading the way. Genomics is currently being used in the form of genome-wide association studies, and large-scale candidate genotyping is starting to be done. Transcriptomics measures gene expression at the mRNA level, and by extrapolation it estimates expression at the protein level (dotted line). Technically, this method is relatively easy to use, but uncertainty about translational efficiencies and posttranslational modifications limits its effectiveness in the long run. Proteomics directly addresses these issues, but is technically challenging at present. Metabolomics has emerged as a potential deep phenotyping tool but, as for proteomics, technical issues limit its current use. Integrated metabolomics holds potential for allowing deep phenotyping at the tissue level (dotted line). Cell biological approaches essentially consider small-scale physiological phenotypes, and may provide critical intermediate phenotypes that may elucidate genotype–phenotype associations (phenomics). The use of tissue arrays provides information on the tissue distribution of proteins, and high-throughput methods are emerging. Both high-resolution anatomical imaging and functional imaging are currently being used in epidemiological research to provide more detailed intermediate, and in some cases subclinical (preclinical), phenotypes. Reproduced by permission from Reference 7.
Phenotypes
PH is a heterogeneous disorder that may be present with many phenotypes. Here, we organize existing phenotype knowledge and provide information on evaluative methods to identify these and potentially other, new phenotypes. This is certainly not intended to be a complete list of phenotypes but rather to provide examples. It is our hope that this initial Statement will inspire the identification of many other phenotypes as well as refinements of the proposed phenotypes.
Mixed Pre- and Postcapillary PH
As many as 25% of patients with mitral stenosis or left heart dysfunction can develop severe PH. This phenomenon of pulmonary vasoconstriction in response to downstream pressure or pulmonary overflow was identified and called “reactive PH” by Paul Wood in 1952, shortly after the introduction of right heart catheterization. These are patients in whom the pulmonary artery (PA) diastolic pressure is elevated out of proportion to the pulmonary capillary wedge pressure (PCWP), suggesting that vasoconstriction or pulmonary arterial remodeling is contributing to the observed increase in PA pressures and vascular resistance. In contrast, group 2 PH, which is not disproportionate, has a minimal gradient between the PA diastolic and wedge pressures.
Although the term “out of proportion” is not clearly defined, based on the Dana Point classification, pulmonary arterial pressure is considered out of proportion (to the left atrial pressure) when the transpulmonary gradient (mean PA pressure – the PCWP) is more than 12 mm Hg or the (PA diastolic – PCWP) is more than 5 mm Hg. These patients also have a PVR greater than 3 Wood units. In contrast to its effectiveness in PAH, epoprostenol increased morbidity and decreased survival in patients with severe left ventricular (LV) systolic failure in the Flolan International Randomized Survival Trial (FIRST). In this trial, the mean LV ejection fraction was less than 17%, the 6-minute walk distance was less than 200 m, and PVR was modestly elevated at about 5 Wood units. Thus, it is not known what role epoprostenol might have in “out of proportion” PH related to left heart disease (20). At the world conference on PH in Nice, France, the recommendation was to use the term “combined pulmonary hypertension” for those conditions in which the PA pressure was higher than expected in systolic or diastolic heart failure or parenchymal lung disease.
The mechanism of reactive PH and the relative efficacy of PH drugs in the out-of-proportion phenotype of PH are unknown. It is also not clear whether this represents an exaggerated response to left heart disease or reflects concomitant PAH. Evaluation of left heart function (by invasive left and right heart catheterization) and careful assessment of ventricular mass/hypertrophy, valve function, ejection fraction, and most importantly, LV end diastolic pressures before and after volume infusion are needed to identify this phenotype (21–27). The value of identifying an out-of-proportion PH phenotype is unclear at present, but future studies may provide insights into how phenotyping will dictate specific beneficial therapies such as optimal use of diuretics and PAH-specific therapies.
“Severe” PH in Respiratory Disease
PH complicating idiopathic pulmonary fibrosis (28), obstructive sleep apnea (29), and chronic obstructive pulmonary disease (30) is usually associated with an mPAP below 35–40 mm Hg and a normal or high cardiac output. However, some patients are outliers and have PH with mPAP greater than 35–40 mm Hg. The term “out of proportion” is not clearly defined and it is not always clear whether this represents an exaggerated response to the lung disease or reflects concomitant pulmonary vascular disease. The more severe PH may be caused by a heritable predisposition to PH, environmental factors, concomitant comorbidities, and/or the underlying lung pathology, as seen in combined pulmonary fibrosis and emphysema syndrome (31, 32). In the case of PH associated with idiopathic pulmonary fibrosis, it has been suggested that mechanisms other than hypoxia or accumulated scarring may be important in the pathobiology (33). However, PH associated with residence at high altitude manifests differently among different ethnic groups and geographic areas, supporting the importance of genetic variability in the response to chronic hypoxia (34, 35).
Importantly, PH in association with underlying lung disease(s) has worse survival than the lung disease alone (30, 36, 37). The phosphodiesterase-5 inhibitor sildenafil has been studied in small groups of patients with chronic obstructive pulmonary disease without (38) and with (39) PH. Ventilation–perfusion mismatch has been reported, in addition to decreased PVR (39). However, a controlled trial of sildenafil revealed improved oxygenation in patients with severe idiopathic pulmonary fibrosis (40). Thus, studies are needed to discover biomarkers to identify which individuals with lung disease or exposure to chronic hypoxia are susceptible to the development of out-of-proportion PH, and to determine effective therapies for this phenotype (41).
Maladaptive RV Hypertrophy
RV failure is the leading cause of hospitalization in PH (42). Some patients rapidly develop RV failure whereas others remain stable, despite similar RV hypertrophy and similar RV pressures (43). Men with PAH have worse RV function at initial presentation compared with women, even after accounting for severity of PAH (44). Moreover, certain PH subtypes have worse outcomes associated with RV deterioration (e.g., scleroderma) (45, 46) than idiopathic PAH, whereas other forms with preserved RV function have a better prognosis, for example, Eisenmenger’s syndrome (47). These prognostic differences likely relate to phenotypic differences in the response of the RV to pressure–volume overload, which can broadly be classified as adaptive or maladaptive RVH.
The adaptive phenotype is manifest as a concentric, RV hypertrophy with minimal dilation or fibrosis and relatively preserved ejection fraction. Emerging pathophysiological concepts for the basis of the maladaptive phenotype include quantitative and qualitative differences in reactivation of the fetal gene package, RV ischemia (macro- and/or microvascular), chamber-specific activation of the adrenergic system (48), RV-specific dysregulation of key enzymes (e.g., phosphodiesterase-5 [49] and pyruvate dehydrogenase [13]), metabolic changes that favor glycolysis over glucose oxidation (50), and RV fibrosis.
Emerging rodent and human data suggest the maladaptive RVH phenotype can be identified by fluorodeoxyglucose positron emission tomography (18F-FDG PET, to quantify ischemia and glycolytic metabolism) and cardiac magnetic resonance imaging (MRI) (to quantify volumes, function, and fibrosis) (see Table E3 in the online supplement). 18F-FDG PET images the increased glucose uptake via the glucose transporter that supports glycolysis-based metabolism in maladaptive RVH. 18F-FDG PET signals are increased in the RV in PH and decreased with effective therapy (12, 17, 50, 51). These RV metabolic changes and systolic dysfunction precede overt failure (52). MRI can determine RV volume and function and noninvasively estimate PA pressure (53). MRI demonstration of late gadolinium hyperenhancement of the right ventricle–left ventricle septal hinge points identifies group 1 patients with PH with severe PH (54) and poor survival (55). Because of its shape, RV volumetric assessment is best accomplished using three-dimensional echocardiography (56) or MRI.
It appears that RV ischemia contributes to the maladaptive phenotype. With extreme RV pressure overload, right coronary flow reserve is reduced and ischemia/hypokinesis results (57). Alternatively, RV ischemia in PH may result from reduced perfusion pressure due to RV hypertension (57, 58) and/or rarefaction of the microvasculature (59). Various biomarkers are associated with the maladaptive phenotype including prolongation of the QTc interval on ECG (60) and elevation of brain natriuretic peptide (61). RV ischemia, suggested by elevations of plasma troponin, also portends a worse prognosis (62, 63). Studies are needed to understand the maladaptive phenotype and to determine the value of therapeutic interventions on blood flow, O2 consumption, and/or metabolism. In a rare disease (such as group 1 PH) such studies of the RV phenotype are possible only if the study groups are precisely defined, allowing recruitment of a homogeneous cohort. In general, there is a need to expand hemodynamic phenotyping for PH. See Table E3 for a more detailed discussion of RV phenotyping.
Connective Tissue Disease–associated PH
The prevalence of PH in connective tissue diseases has been estimated on the basis essentially of echocardiographic studies except for systemic sclerosis (SSc), in which PH was assessed, in more recent studies, on the basis of strict hemodynamic criteria (64, 65). PH can be associated with systemic lupus erythematosus (prevalence, 0.5–14%), mixed connective tissue disease (prevalence, ∼50% as assessed by echocardiographic criteria [64]), rheumatoid arthritis, primary Sjögren’s disease, and SSc (prevalence, 8–12%) (65). Pulmonary manifestations of SSc include pulmonary vascular diseases such as PH and pulmonary veno-occlusive disease, as well as interstitial lung disease. PH in patients with SSc worsens survival (66, 67), and after interstitial lung disease is the leading cause of mortality in SSc (68–70). Patients with SSc–PH are predominantly women, have limited but also diffuse SSc, are older, and have seemingly less severe hemodynamic impairment compared with idiopathic PH (71) but tend to have depressed RV function (72, 73) and diastolic dysfunction (74), and pulmonary venous involvement (75). They have more severe hormonal and metabolic dysfunction (e.g., elevated N-terminal brain natriuretic peptide [76, 77] and hyponatremia [78]), which correlates with survival.
Pulmonary veno-occlusive disease in SSc–PAH is characterized by intimal proliferation and fibrosis of the intrapulmonary veins and venules in addition to involvement of the arteriolar bed (75, 79). Typically, patients with isolated pulmonary vascular disease (PAH) are more likely to present with limited SSc disease and develop PH after a duration of 10–15 years (80). In contrast, patients with diffuse SSc are at greater risk of developing interstitial lung disease and PH in the context of chronic pulmonary disease and/or hypoxia (81). However, patients with limited or diffuse disease may present at any stage in the course of their disease with PH, whether associated or not with interstitial lung disease (82).
From a phenotypic standpoint, patients with isolated PAH belong to group 1 PAH, whereas patients with PH and interstitial lung disease fall into group 3 PH (83). Median survival is less than 4 years for patients with SSc–PAH, which is significantly lower than for patients with idiopathic PAH (71). Patients with SSc with interstitial lung disease alone have a median survival of 5–8 years (84); however, survival is significantly worse in patients with SSc with interstitial lung disease and PH (1-, 2-, and 3-yr survival of 82, 46, and 39%, respectively [85]). Considering the overall impact on survival (70), studies are needed to phenotype patients with connective tissue diseases and to evaluate therapies on the basis of phenotypes.
Portopulmonary Hypertension
Prospective screening transthoracic echocardiography has documented that approximately 10% of liver transplant candidates with liver cirrhosis and portal hypertension have RV systolic pressure greater than 50 mm Hg (86). Right heart catheterization in such patients further distinguishes portopulmonary hypertension (POPH) from hyperdynamic (high flow) circulatory states commonly seen with portal hypertension, as well as pulmonary venous hypertension due to excess volume and heart failure (systolic and/or diastolic) that may complicate liver disorders such as alcoholic liver disease and hemochromatosis. Within the POPH group, liver transplantation can result in normalization of pulmonary hemodynamics and liberation from PH medications in highly selected patients that fulfill standardized hemodynamic response criteria (mPAPs < 35 mm Hg; PVR < 400 dyn·s·cm–5) (86).
Studies have addressed the frequency and outcomes of POPH and selected patients with POPH who underwent liver transplantation (87–90). Further phenotyping is needed to identify factors that influence and distinguish various outcomes, for example, mortality, hospitalization, treatment change, and transplant effect (88, 91, 92) (Tables E1 and E2). Studies are needed to determine the specific circulating mediator(s) and/or genetic predisposition interactions that predispose to POPH, the correlates of pulmonary hemodynamic resolution after liver transplantation, whether resolution of pathology is associated with hemodynamic normalization, and whether such normalization is sustained over time.
HIV-associated PAH
HIV infection increases the risk of developing PAH regardless of the route of infection, the stage of HIV infection, and the degree of immunodeficiency (93–100). Importantly, coexisting risk factors, such as illicit drug use and chronic liver disease, add to the complexity of this phenotype. The prevalence of PAH is approximately 0.5% in HIV-infected individuals (>1,000-fold higher than the prevalence of idiopathic PH in the general population) (93, 99). Modern HIV management with highly active antiretroviral therapy (HAART) has presumably resulted in improved survival and decreased incidence of HIV–PAH. Taken together, these effects on survival and incidence have resulted in stable PAH prevalence in HIV-infected patients (97, 100, 101). However, when PAH is present, patients with HIV are more likely to die of PAH rather than of other HIV complications, emphasizing the clinical relevance of pulmonary vascular involvement in that setting (92).
In the absence of specific recommendations, treatment of HIV–PAH follows guidelines for treatment of idiopathic PAH together with HAART (97, 100, 101). On multivariate analysis, a cardiac index greater than 2.8 L/minute/m2 and a CD4+ lymphocyte count greater than 200 cells/ml were independent predictors of survival (99, 100). Interestingly, cases of reversible disease have been identified in patients with HIV–PAH treated with HAART and PAH-specific therapies (100). This finding together with the decreased incidence of HIV–PAH in the modern management era may indicate that aggressive management improves outcomes in this patient population. Further studies are needed to understand the underlying reasons of improved outcomes in these patients, which may reveal treatments applicable to other PH phenotypes.
PH in Elderly Individuals
PH occurs at both extremes of life, in the pediatric population (102) and in the elderly (103), with distinctive clinical presentations and associations in the elderly age group (see the online supplement). In a retrospective series (104) of elderly with PH, PCWP was more frequently greater than 15 mm Hg than in those more than 65 years of age. The higher wedge pressure in the elderly may reflect a higher incidence of diastolic LV dysfunction, pulmonary venous hypertension, or age-related LV diastolic stiffness. In support of age-related pulmonary vascular stiffening, age-related increases in PA pressures were reported in a large cohort study of the general population concomitant with age-related increases in systemic systolic blood pressure (105). Thus, there is a need to better understand the role of pulmonary vascular elastance in the development of PH in the elderly and whether this is invariably associated with systemic vascular stiffening and LV diastolic dysfunction. Studies are needed to develop noninvasive testing to assess pulmonary vascular impedance and elastance and to determine the effect(s) of therapies on both pulmonary vascular elastance and resistance.
PAH in Children
Task forces have emphasized the importance of distinct phenotyping of PH in childhood because of specific causes, the influence of growth and development, and concern regarding long-term outcomes with therapy. This topic is covered in detail in the online supplement.
Metabolic Syndrome
Metabolic syndrome is a cluster of risk factors for atherosclerosis including increased fasting glucose, elevated blood pressure and triglyceride levels, reduced high-density lipoprotein cholesterol, and increased waist circumference. Adults with PH may also present with characteristics of the metabolic syndrome. Features of metabolic syndrome were more common in patients with pulmonary venous hypertension in one study (21). The Multi-Ethnic Study of Atherosclerosis (MESA)-RV study found a greater RV mass, end-diastolic volume and RV stroke volume, and lower RV ejection fraction in obese, as compared with lean, participants (106). Although this suggests that obese individuals might have increased RV afterload, it is also possible that increased blood volume, hormonal effects, or other effects of obesity have adverse effects on the RV. In addition, a few studies have found that features of metabolic syndrome in patients with group 1 PAH were associated with worse survival (107, 108) and worse functional status (108) independent of other cardiovascular risk factors, insulin resistance, and severity of PH.
Because patients with PH have a high incidence of unrecognized glucose intolerance (109), it is possible that metabolic syndrome is not limited to patients with pulmonary venous hypertension, but is also a factor in the phenotype of group 1 PH. Whether the poorer prognosis in patients with PH with metabolic syndrome is just an effect of the comorbidity or a direct effect of the pathobiology of PH is not currently clear and needs further study.
Studies are also needed to define the incidence of metabolic syndrome in PH, the effects on survival, response to PAH-specific therapy, or PVR and elastance.
Long-Term Survivors
In the current therapeutic era, PAH remains a progressive, fatal disease (110–112). In a cohort of 674 patients with incident and prevalent PAH recruited in France in 2002–2003, 1-, 2-, and 3-year survival rates were 87% (95% confidence interval, 84–90%), 76%, and 67% (112), respectively. Registries consistently show better survival among females, younger patients, and in patients in New York Heart Association functional class I or II (110–112). Survival is better in prevalent cohorts, as compared with incident, emphasizing the fact that a survivor bias exists in prevalent populations (112, 113). It is assumed that better outcomes in mixed incident–prevalent populations relate to the enrichment of prevalent cohorts with patients who have preserved RV function and/or patients with a stronger response to PAH management (112). Better 3-year survival rates are observed in PAH associated with congenital heart diseases, whereas SSc-associated PAH cohorts have worse survival, highlighting the influence of associated conditions on PAH outcomes (111–113). In idiopathic, heritable, and anorexigen-induced PH, mortality is most closely associated with male sex, RV function, and exercise limitation (110, 112). Other characteristics such as response to therapies are associated with long-term outcomes. This is particularly true in patients responding to acute vasodilator challenge (114, 115).
Besides vasodilator responders, better long-term survival rates are consistently observed in patients reaching near normal hemodynamic parameters with management, including patients with HIV and systemic lupus erythematosus or mixed connective tissue diseases (100, 114–117). Improved outcome after successful endarterectomy for chronic thromboembolic PH supports the notion that correcting PVR allows marked improvements in survival (118). On the other hand, better survival rates are observed in patients undergoing therapy and who do not achieve hemodynamic normalization but maintain a normal cardiac output and preserved exercise capacity, which emphasizes the relevance of adaptive RV phenotypes to PH outcomes (111, 116). Studies are needed to identify phenotypes with long-term survival and the underlying mechanisms so that therapies might be strategically developed for those patients with more aggressive disease.
Summary and Conclusions
This document lays the framework to address a pressing need to develop accurate phenotyping in PH. As our understanding of the disease advances and as innovative evaluative tools are developed for imaging, hemodynamics, and biomarkers of disease, phenotypes will be developed, expanded, and refined (Table 2). Prospective standardization of approach, even for simple and straightforward evaluative measures, will be essential to establishing valid phenotypes. Strategies such as written protocols and standard operating procedures, standardization across study sites against reference methods, and quality assurance and quality control are critical elements for phenotyping studies, which can minimize technical and interpretive variability, so that the resulting variance is mostly biological and informative. Accurate phenotyping of PH can be used in research studies to increase the homogeneity of study cohorts. Once the ability of the phenotypes to predict outcomes has been validated, phenotyping may also be useful for determining prognosis and guiding treatment. This important next step in PH patient care can optimally be addressed through a consortium of study sites with well-defined goals, tasks, and structure. Planning and support for such a consortium could include the National Institutes of Health and the U.S. Food and Drug Administration, with industry and foundation partnerships. The tremendously rapid pace of development of new therapies has led to better patient outcomes, and the future opportunity to apply the wide array of medications so that patients can live longer and more satisfying lives. Definition of phenotypes will enable us to test whether selective targeting of care can achieve this goal.
Table 2:
Phenotyping Tools and Applications in Pulmonary Hypertension: Current Practice and Future Directions
| Component(s)/Tool(s) | Examples of Current Practice | Examples of Future Directions |
|---|---|---|
| Medical history | High index of suspicion | Diet |
| Physician history, questionnaires | Family history | Environmental exposures |
| Physical examination | Height, weight, BP | Waist-to-hip ratio, waist circumference |
| Anthropometrics | Capillaroscopy: Retinal, nailfold, sublingual | |
| Medical devices | ||
| Hemodynamic | PAP, PAOP | TP (PAM to PAOP) gradient |
| Right heart catheterization, hemodynamics | CO | LVEDP before and after volume infusion |
| PVR | PA stiffness | |
| Vasodilator testing | Pulmonary vascular impedance | |
| Portal venous pressure | ||
| Molecular phenotyping | HIV | Glucose, CRP, IL-1, IL-6, vWF, coagulation factors, T-cell responsiveness, SNPs, PBMC gene expression, proteomics, autoimmune antibodies, estrogen levels, circulating endothelial cells |
| Biochemical, serology, immunologic, tissue, genetics | ANA (and other connective tissue serologies) | |
| LFT | ||
| Functional and morphological phenotyping | Walk distance | Desaturation index/burden |
| 6-min walk, Echo, CXR, CT, MRI, PET, angiogram, ECG, lung function, PSG, pathology | RV function, RVSP, RVE, RAE, LVEF | RV mass |
| FEV1, FEV1/FVC, DlCO | Lung perfusion | |
| AHI | RV glucose uptake | |
| CT pulmonary vascular volume | ||
| 3D echo | ||
| QTc intervals | ||
| Biopsy (lung, heart, peripheral muscle), tissue/cell morphology | ||
| Metabolic and ischemia phenotyping | Coronary flow in RVH | |
| Nuclear perfusion imaging, or 18F-fluorodeoxyglucose PET | Phosphocreatine-to-ATP ratio | |
| Fatty acid oxidation | ||
| Extracellular matrix products | ||
| Iron metabolism | ||
| Biomarkers for phenotyping | Troponin | |
| Fasting glucose, triglycerides, HDL cholesterol | ||
| Circulating endothelial progenitor cells | ||
| Circulating microvesicles | ||
| Use of existing population data | Predictive algorithms from registries | |
| Local, national, and international registries |
Definition of abbreviations: 3D = three-dimensional; AHI = apnea–hypopnea index; ANA = anti-nuclear antibody; BP = blood pressure; CO = cardiac output; CRP = C-reactive protein; CT = computed tomography; CXR = chest X-ray; DlCO = diffusion lung capacity for carbon monoxide; echo = echocardiography; HDL = high-density lipoprotein; LAE = left atrial enlargement; LFT = liver function tests; LVEDP = left ventricular end-diastolic pressure; LVEF = left ventricular ejection fraction; MRI = magnetic resonance imaging; PA = pulmonary artery; PAM = pulmonary artery mean; PAP = pulmonary artery pressure; PAOP = pulmonary artery occlusion pressure; PAP = pulmonary artery pressure; PBMC = peripheral blood mononuclear cell; PET = positron emission tomography; PSG = polysomnogram; PVR = pulmonary vascular resistance; RV = right ventricular; RVE = right ventricular enlargement; RVH = right ventricular hypertrophy; RVSP = right ventricular systolic pressure; SNPs = single-nucleotide polymorphisms; TP = transpulmonary; vWF = von Willebrand factor.
Note: Table E3 provides an example of how this phenotyping approach can be used to identify and characterize a specific (in this case maladaptive RV) phenotype. Table E4 provides examples of data used to identify the phenotype examples discussed in this document.
Acknowledgments
This official Statement was prepared by an ad hoc committee of the Pulmonary Circulation (PC) Assembly of the American Thoracic Society.
Members of the committee:
Raed A. Dweik, M.D.* (Chair)
Sharon Rounds, M.D.*
Serpil C. Erzurum, M.D.*
Stephen Archer, M.D.
Kenneth Bloch, M.D.†
Karen Fagan, M.D.
Benjamin Gaston, M.D.†
Paul M. Hassoun, M.D.
Nicholas S. Hill, M.D.
Marc Humbert, M.D., Ph.D.
Steven M. Kawut, M.D., M.S.
Michael Krowka, M.D.
Evangelos Michelakis, M.D.
Nicholas W. Morrell, M.D.
Kurt Stenmark, M.D.
Rubin M. Tuder, M.D.
John Newman, M.D.*
Footnotes
This official Statement of the American Thoracic Society (ATS) was approved by the ATS Board of Directors, September 2013
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
ATS Executive Committee members.
Drs. Bloch and Gaston served only as members of the committee.
Author Disclosures: S.C.E. was principal investigator for an Asthmatx clinical trial on bronchial thermoplasty (payments made to institution, amounts not reported). K.F. was a consultant to Gilead (PAH Research Scholars Program grant reviewer), Up-to-Date, Inc. (no payments reported), and Novartis (data safety managing board for imatinib in PAH; $5,000–24,999); she received research support from Actelion ($25,000–49,999), Bayer Schering Pharma ($25,000–49,999), Gilead ($25,000–49,999), and United Therapeutics ($5,000–24,999); she was a speaker in nonpromotional activities of Actelion (no payments reported), Bayer Schering Pharma ($5,000–24,999), and Gilead ($5,000–24,999). N.S.H. was on advisory committees of Gilead ($1–4,999) and Aerogen ($1–4,999). M.H. was a consultant and/or speaker for Actelion ($5,000–24,999), Bayer Schering Pharma ($1–4,999), GlaxoSmithKline ($1–4,999), Eli Lilly ($1–4,999), Novartis ($1–4,999), Pfizer ($1–4,999), and United Therapeutics ($1–4,999); he received research support from Actelion ($5,000–24,999) and GlaxoSmithKline ($5,000–24,999). S.M.K. was on advisory committees of Gilead, Ikaria, and Insamed ($1–4,999); he was a speaker in nonpromotional activities of Actelion ($25,000–49,999), Gilead ($5,000–24,999), Ikaria ($5,000–24,999), Lung Rx ($5,000–24,999), Merck ($5,000–24,999), Pfizer ($5,000–24,999), and United Therapeutics ($5,000–24,999); he received research support from Actelion ($25,000–49,999) and Gilead ($25,000–49,999). M.K. was on an advisory committee of Gilead ($1–4,999). E.M. was a consultant to Bayer, Merck, and United Therapeutics (amounts not reported). N.W.M. was on an advisory committee of Novartis ($1–4,999) and received research support from Novartis (more than $100,000). R.A.D., S.R., S.A., P.M.H., K.S., R.M.T., and J.N. reported no relevant commercial interests.
References
- 1.Simonneau G, Galiè N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43(12 Suppl S):5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 2.Proceedings of the 4th World Symposium on Pulmonary Hypertension, February 2008, Dana Point, CA. J Am Coll Cardiol. 2009;54(1 Suppl):S1–S117. [PubMed] [Google Scholar]
- 3.Wang L, Zhang Y, Yan C, He J, Xiong C, Zhao S, Fang W. Evaluation of right ventricular volume and ejection fraction by gated 18F-FDG PET in patients with pulmonary hypertension: comparison with cardiac MRI and CT. J Nucl Cardiol. 2013;20:242–252. doi: 10.1007/s12350-013-9672-8. [DOI] [PubMed] [Google Scholar]
- 4.Aytekin M, Aulak KS, Haserodt S, Chakravarti R, Cody J, Minai OA, Dweik RA. Abnormal platelet aggregation in idiopathic pulmonary arterial hypertension: role of nitric oxide. Am J Physiol Lung Cell Mol Physiol. 2012;302:L512–L520. doi: 10.1152/ajplung.00289.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lundgrin EL, Park MM, Sharp J, Tang WH, Thomas JD, Asosingh K, Comhair SA, Difilippo FP, Neumann DR, Davis L, et al. Fasting 2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography to detect metabolic changes in pulmonary arterial hypertension hearts over 1 year. Ann Am Thorac Soc. 2013;10:1–9. doi: 10.1513/AnnalsATS.201206-029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aytekin M, Comhair SA, de la Motte C, Bandyopadhyay SK, Farver CF, Hascall VC, Erzurum SC, Dweik RA. High levels of hyaluronan in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295:L789–L799. doi: 10.1152/ajplung.90306.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tracy RP. “Deep phenotyping”: characterizing populations in the era of genomics and systems biology. Curr Opin Lipidol. 2008;19:151–157. doi: 10.1097/MOL.0b013e3282f73893. [DOI] [PubMed] [Google Scholar]
- 8.Loscalzo J, Kohane I, Barabasi AL. Human disease classification in the postgenomic era: a complex systems approach to human pathobiology. Mol Syst Biol. 2007;3:124. doi: 10.1038/msb4100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulze TG, McMahon FJ. Defining the phenotype in human genetic studies: forward genetics and reverse phenotyping. Hum Hered. 2004;58:131–138. doi: 10.1159/000083539. [DOI] [PubMed] [Google Scholar]
- 10.Masri FA, Comhair SAA, Dostanic-Larson I, Kaneko FT, Dweik RA, Arroliga AC, Erzurum SC. Deficiency of lung antioxidants in idiopathic pulmonary arterial hypertension. Clin Transl Sci. 2008;1:99–106. doi: 10.1111/j.1752-8062.2008.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masri FA, Comhair SA, Koeck T, Xu W, Janocha A, Ghosh S, Dweik RA, Golish J, Kinter M, Stuehr DJ, et al. Abnormalities in nitric oxide and its derivatives in lung cancer. Am J Respir Crit Care Med. 2005;172:597–605. doi: 10.1164/rccm.200411-1523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu W, Koeck T, Lara AR, Neumann D, DiFilippo FP, Koo M, Janocha AJ, Masri FA, Arroliga AC, Jennings C, et al. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci USA. 2007;104:1342–1347. doi: 10.1073/pnas.0605080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piao L, Marsboom G, Archer SL. Mitochondrial metabolic adaptation in right ventricular hypertrophy and failure. J Mol Med (Berl) 2010;88:1011–1020. doi: 10.1007/s00109-010-0679-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piao L, Fang YH, Cadete VJ, Wietholt C, Urboniene D, Toth PT, Marsboom G, Zhang HJ, Haber I, Rehman J, et al. The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: resuscitating the hibernating right ventricle. J Mol Med (Berl) 2010;88:47–60. doi: 10.1007/s00109-009-0524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria–ROS–HIF-1α–Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294:H570–H578. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 16.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L548–L554. doi: 10.1152/ajplung.00428.2006. [DOI] [PubMed] [Google Scholar]
- 17.Fijalkowska I, Xu W, Comhair SA, Janocha AJ, Mavrakis LA, Krishnamachary B, Zhen L, Mao T, Richter A, Erzurum SC, et al. Hypoxia inducible-factor 1α regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am J Pathol. 2010;176:1130–1138. doi: 10.2353/ajpath.2010.090832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elliott CG, Glissmeyer EW, Havlena GT, Carlquist J, McKinney JT, Rich S, McGoon MD, Scholand MB, Kim M, Jensen RL, et al. Relationship of BMPR2 mutations to vasoreactivity in pulmonary arterial hypertension. Circulation. 2006;113:2509–2515. doi: 10.1161/CIRCULATIONAHA.105.601930. [DOI] [PubMed] [Google Scholar]
- 19.Girerd B, Montani D, Eyries M, Yaici A, Sztrymf B, Coulet F, Sitbon O, Simonneau G, Soubrier F, Humbert M. Absence of influence of gender and BMPR2 mutation type on clinical phenotypes of pulmonary arterial hypertension. Respir Res. 2010;11:73. doi: 10.1186/1465-9921-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Califf RM, Adams KF, McKenna WJ, Gheorghiade M, Uretsky BF, McNulty SE, Darius H, Schulman K, Zannad F, Handberg-Thurmond E, et al. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: the Flolan International Randomized Survival Trial (FIRST) Am Heart J. 1997;134:44–54. doi: 10.1016/s0002-8703(97)70105-4. [DOI] [PubMed] [Google Scholar]
- 21.Robbins IM, Newman JH, Johnson RF, Hemnes AR, Fremont RD, Piana RN, Zhao DX, Byrne DW. Association of the metabolic syndrome with pulmonary venous hypertension. Chest. 2009;136:31–36. doi: 10.1378/chest.08-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oudiz RJ.Pulmonary hypertension associated with left-sided heart disease Clin Chest Med 200728233–241.x [DOI] [PubMed] [Google Scholar]
- 23.Costard-Jäckle A, Fowler MB. Influence of preoperative pulmonary artery pressure on mortality after heart transplantation: testing of potential reversibility of pulmonary hypertension with nitroprusside is useful in defining a high risk group. J Am Coll Cardiol. 1992;19:48–54. doi: 10.1016/0735-1097(92)90050-w. [DOI] [PubMed] [Google Scholar]
- 24.Abramson SV, Burke JF, Kelly JJ, Jr, Kitchen JG, III, Dougherty MJ, Yih DF, McGeehin FC, III, Shuck JW, Phiambolis TP. Pulmonary hypertension predicts mortality and morbidity in patients with dilated cardiomyopathy. Ann Intern Med. 1992;116:888–895. doi: 10.7326/0003-4819-116-11-888. [DOI] [PubMed] [Google Scholar]
- 25.Zener JC, Hancock EW, Shumway NE, Harrison DC. Regression of extreme pulmonary hypertension after mitral valve surgery. Am J Cardiol. 1972;30:820–826. doi: 10.1016/0002-9149(72)90005-7. [DOI] [PubMed] [Google Scholar]
- 26.Braunwald E, Braunwald NS, Ross J, Jr, Morrow AG. Effects of mitral-valve replacement on the pulmonary vascular dynamics of patients with pulmonary hypertension. N Engl J Med. 1965;273:509–514. doi: 10.1056/NEJM196509022731001. [DOI] [PubMed] [Google Scholar]
- 27.Delgado JF, Conde E, Sánchez V, López-Ríos F, Gómez-Sánchez MA, Escribano P, Sotelo T, Gómez de la Cámara A, Cortina J, de la Calzada CS. Pulmonary vascular remodeling in pulmonary hypertension due to chronic heart failure. Eur J Heart Fail. 2005;7:1011–1016. doi: 10.1016/j.ejheart.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 28.Lettieri CJ, Nathan SD, Barnett SD, Ahmad S, Shorr AF. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2006;129:746–752. doi: 10.1378/chest.129.3.746. [DOI] [PubMed] [Google Scholar]
- 29.Chaouat A, Weitzenblum E, Krieger J, Oswald M, Kessler R. Pulmonary hemodynamics in the obstructive sleep apnea syndrome: results in 220 consecutive patients. Chest. 1996;109:380–386. doi: 10.1378/chest.109.2.380. [DOI] [PubMed] [Google Scholar]
- 30.Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J. 2008;32:1371–1385. doi: 10.1183/09031936.00015608. [DOI] [PubMed] [Google Scholar]
- 31.Jankowich MD, Rounds SI. Combined pulmonary fibrosis and emphysema syndrome: a review. Chest. 2012;141:222–231. doi: 10.1378/chest.11-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cottin V, Le Pavec J, Prévot G, Mal H, Humbert M, Simonneau G, Cordier JF Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM“O”P) Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur Respir J. 2010;35:105–111. doi: 10.1183/09031936.00038709. [DOI] [PubMed] [Google Scholar]
- 33.Farkas L, Gauldie J, Voelkel NF, Kolb M. Pulmonary hypertension and idiopathic pulmonary fibrosis: a tale of angiogenesis, apoptosis, and growth factors. Am J Respir Cell Mol Biol. 2011;45:1–15. doi: 10.1165/rcmb.2010-0365TR. [DOI] [PubMed] [Google Scholar]
- 34.Pasha MA, Newman JH. High-altitude disorders: pulmonary hypertension: pulmonary vascular disease: the global perspective. Chest. 2010;137(6 Suppl):13S–19S. doi: 10.1378/chest.09-2445. [DOI] [PubMed] [Google Scholar]
- 35.Beall CM, Laskowski D, Erzurum SC. Nitric oxide in adaptation to altitude. Free Radic Biol Med. 2012;52:1123–1134. doi: 10.1016/j.freeradbiomed.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mejía M, Carrillo G, Rojas-Serrano J, Estrada A, Suárez T, Alonso D, Barrientos E, Gaxiola M, Navarro C, Selman M. Idiopathic pulmonary fibrosis and emphysema: decreased survival associated with severe pulmonary arterial hypertension. Chest. 2009;136:10–15. doi: 10.1378/chest.08-2306. [DOI] [PubMed] [Google Scholar]
- 37.Patel NM, Lederer DJ, Borczuk AC, Kawut SM. Pulmonary hypertension in idiopathic pulmonary fibrosis. Chest. 2007;132:998–1006. doi: 10.1378/chest.06-3087. [DOI] [PubMed] [Google Scholar]
- 38.Lederer DJ, Bartels MN, Schluger NW, Brogan F, Jellen P, Thomashow BM, Kawut SM. Sildenafil for chronic obstructive pulmonary disease: a randomized crossover trial. COPD. 2012;9:268–275. doi: 10.3109/15412555.2011.651180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blanco I, Gimeno E, Munoz PA, Pizarro S, Gistau C, Rodriguez-Roisin R, Roca J, Barberà JA. Hemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Am J Respir Crit Care Med. 2010;181:270–278. doi: 10.1164/rccm.200907-0988OC. [DOI] [PubMed] [Google Scholar]
- 40.Zisman DA, Schwarz M, Anstrom KJ, Collard HR, Flaherty KR, Hunninghake GW Idiopathic Pulmonary Fibrosis Clinical Research Network. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010;363:620–628. doi: 10.1056/NEJMoa1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dweik RA. Pulmonary hypertension and the search for the selective pulmonary vasodilator. Lancet. 2002;360:886–887. doi: 10.1016/S0140-6736(02)11067-1. [DOI] [PubMed] [Google Scholar]
- 42.Campo A, Mathai SC, Le Pavec J, Zaiman AL, Hummers LK, Boyce D, Housten T, Lechtzin N, Chami H, Girgis RE, et al. Outcomes of hospitalisation for right heart failure in pulmonary arterial hypertension. Eur Respir J. 2011;38:359–367. doi: 10.1183/09031936.00148310. [DOI] [PubMed] [Google Scholar]
- 43.Rich S, Pogoriler J, Husain AN, Toth PT, Gomberg-Maitland M, Archer SL. Long-term effects of epoprostenol on the pulmonary vasculature in idiopathic pulmonary arterial hypertension. Chest. 2010;138:1234–1239. doi: 10.1378/chest.09-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawut SM, Al-Naamani N, Agerstrand C, Rosenzweig EB, Rowan C, Barst RJ, Bergmann S, Horn EM. Determinants of right ventricular ejection fraction in pulmonary arterial hypertension. Chest. 2009;135:752–759. doi: 10.1378/chest.08-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawut SM, Taichman DB, Archer-Chicko CL, Palevsky HI, Kimmel SE. Hemodynamics and survival in patients with pulmonary arterial hypertension related to systemic sclerosis. Chest. 2003;123:344–350. doi: 10.1378/chest.123.2.344. [DOI] [PubMed] [Google Scholar]
- 46.Kuhn KP, Byrne DW, Arbogast PG, Doyle TP, Loyd JE, Robbins IM. Outcome in 91 consecutive patients with pulmonary arterial hypertension receiving epoprostenol. Am J Respir Crit Care Med. 2003;167:580–586. doi: 10.1164/rccm.200204-333OC. [DOI] [PubMed] [Google Scholar]
- 47.Hopkins WE, Ochoa LL, Richardson GW, Trulock EP. Comparison of the hemodynamics and survival of adults with severe primary pulmonary hypertension or Eisenmenger syndrome. J Heart Lung Transplant. 1996;15:100–105. [PubMed] [Google Scholar]
- 48.Piao L, Fang YH, Parikh KS, Ryan JJ, D’Souza KM, Theccanat T, Toth PT, Pogoriler J, Paul J, Blaxall BC, et al. GRK2-mediated inhibition of adrenergic and dopaminergic signaling in right ventricular hypertrophy: therapeutic implications in pulmonary hypertension. Circulation. 2012;126:2859–2869. doi: 10.1161/CIRCULATIONAHA.112.109868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Archer SL, Michelakis ED. Phosphodiesterase type 5 inhibitors for pulmonary arterial hypertension. N Engl J Med. 2009;361:1864–1871. doi: 10.1056/NEJMct0904473. [DOI] [PubMed] [Google Scholar]
- 50.Lundgrin E, Park MA, Sharp J, Tang WH, Thomas J, Asosingh K, Comhair S, DiFilippo FP, Neumann DR, Davis L, et al. Fasting FDG-PET to detect metabolic changes in pulmonary arterial hypertension hearts over one year. Ann Am Thorac Soc. 2013;10:1–9. doi: 10.1513/AnnalsATS.201206-029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oikawa M, Kagaya Y, Otani H, Sakuma M, Demachi J, Suzuki J, Takahashi T, Nawata J, Ido T, Watanabe J, et al. Increased [18F]fluorodeoxyglucose accumulation in right ventricular free wall in patients with pulmonary hypertension and the effect of epoprostenol. J Am Coll Cardiol. 2005;45:1849–1855. doi: 10.1016/j.jacc.2005.02.065. [DOI] [PubMed] [Google Scholar]
- 52.Fernandes F, Ramires FJ, Arteaga E, Ianni BM, Bonfá ES, Mady C. Cardiac remodeling in patients with systemic sclerosis with no signs or symptoms of heart failure: an endomyocardial biopsy study. J Card Fail. 2003;9:311–317. doi: 10.1054/jcaf.2003.51. [DOI] [PubMed] [Google Scholar]
- 53.Urboniene D, Haber I, Fang YH, Thenappan T, Archer SL. Validation of high-resolution echocardiography and magnetic resonance imaging vs. high-fidelity catheterization in experimental pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2010;299:L401–L412. doi: 10.1152/ajplung.00114.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanz J, Dellegrottaglie S, Kariisa M, Sulica R, Poon M, O’Donnell TP, Mehta D, Fuster V, Rajagopalan S. Prevalence and correlates of septal delayed contrast enhancement in patients with pulmonary hypertension. Am J Cardiol. 2007;100:731–735. doi: 10.1016/j.amjcard.2007.03.094. [DOI] [PubMed] [Google Scholar]
- 55.Freed BH, Gomberg-Maitland M, Chandra S, Mor-Avi V, Rich S, Archer SL, Jamison EB, Jr, Lang RM, Patel AR. Late gadolinium enhancement cardiovascular magnetic resonance predicts clinical worsening in patients with pulmonary hypertension. J Cardiovasc Magn Reson. 2012;14:11. doi: 10.1186/1532-429X-14-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sugeng L, Mor-Avi V, Weinert L, Niel J, Ebner C, Steringer-Mascherbauer R, Bartolles R, Baumann R, Schummers G, Lang RM, et al. Multimodality comparison of quantitative volumetric analysis of the right ventricle. JACC Cardiovasc Imaging. 2010;3:10–18. doi: 10.1016/j.jcmg.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 57.Vlahakes GJ, Turley K, Hoffman JI. The pathophysiology of failure in acute right ventricular hypertension: hemodynamic and biochemical correlations. Circulation. 1981;63:87–95. doi: 10.1161/01.cir.63.1.87. [DOI] [PubMed] [Google Scholar]
- 58.van Wolferen SA, Marcus JT, Westerhof N, Spreeuwenberg MD, Marques KM, Bronzwaer JG, Henkens IR, Gan CT, Boonstra A, Postmus PE, et al. Right coronary artery flow impairment in patients with pulmonary hypertension. Eur Heart J. 2008;29:120–127. doi: 10.1093/eurheartj/ehm567. [DOI] [PubMed] [Google Scholar]
- 59.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120:1951–1960. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]
- 60.Rich JD, Thenappan T, Freed B, Patel AR, Thisted RA, Childers R, Archer SL. QTc prolongation is associated with impaired right ventricular function and predicts mortality in pulmonary hypertension. Int J Cardiol. 2013;167:669–676. doi: 10.1016/j.ijcard.2012.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagaya N, Nishikimi T, Uematsu M, Satoh T, Kyotani S, Sakamaki F, Kakishita M, Fukushima K, Okano Y, Nakanishi N, et al. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation. 2000;102:865–870. doi: 10.1161/01.cir.102.8.865. [DOI] [PubMed] [Google Scholar]
- 62.Filusch A, Giannitsis E, Katus HA, Meyer FJ. High-sensitive troponin T: a novel biomarker for prognosis and disease severity in patients with pulmonary arterial hypertension. Clin Sci (Lond) 2010;119:207–213. doi: 10.1042/CS20100014. [DOI] [PubMed] [Google Scholar]
- 63.Torbicki A, Kurzyna M, Kuca P, Fijałkowska A, Sikora J, Florczyk M, Pruszczyk P, Burakowski J, Wawrzyńska L. Detectable serum cardiac troponin T as a marker of poor prognosis among patients with chronic precapillary pulmonary hypertension. Circulation. 2003;108:844–848. doi: 10.1161/01.CIR.0000084544.54513.E2. [DOI] [PubMed] [Google Scholar]
- 64.Sullivan WD, Hurst DJ, Harmon CE, Esther JH, Agia GA, Maltby JD, Lillard SB, Held CN, Wolfe JF, Sunderrajan EV, et al. A prospective evaluation emphasizing pulmonary involvement in patients with mixed connective tissue disease. Medicine (Baltimore) 1984;63:92–107. doi: 10.1097/00005792-198403000-00003. [DOI] [PubMed] [Google Scholar]
- 65.Jimenez SA, Derk CT. Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis. Ann Intern Med. 2004;140:37–50. [PubMed] [Google Scholar]
- 66.Mukerjee D, St George D, Coleiro B, Knight C, Denton CP, Davar J, Black CM, Coghlan JG. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: application of a registry approach. Ann Rheum Dis. 2003;62:1088–1093. doi: 10.1136/ard.62.11.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hachulla E, Gressin V, Guillevin L, Carpentier P, Diot E, Sibilia J, Kahan A, Cabane J, Francès C, Launay D, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum. 2005;52:3792–3800. doi: 10.1002/art.21433. [DOI] [PubMed] [Google Scholar]
- 68.Mayes MD. Scleroderma epidemiology. Rheum Dis Clin North Am. 2003;29:239–254. doi: 10.1016/s0889-857x(03)00022-x. [DOI] [PubMed] [Google Scholar]
- 69.Koh ET, Lee P, Gladman DD, Abu-Shakra M. Pulmonary hypertension in systemic sclerosis: an analysis of 17 patients. Br J Rheumatol. 1996;35:989–993. doi: 10.1093/rheumatology/35.10.989. [DOI] [PubMed] [Google Scholar]
- 70.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis. 2007;66:940–944. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fisher MR, Mathai SC, Champion HC, Girgis RE, Housten-Harris T, Hummers L, Krishnan JA, Wigley F, Hassoun PM. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis Rheum. 2006;54:3043–3050. doi: 10.1002/art.22069. [DOI] [PubMed] [Google Scholar]
- 72.Hsiao SH, Lee CY, Chang SM, Lin SK, Liu CP. Right heart function in scleroderma: insights from myocardial Doppler tissue imaging. J Am Soc Echocardiogr. 2006;19:507–514. doi: 10.1016/j.echo.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 73.Lee CY, Chang SM, Hsiao SH, Tseng JC, Lin SK, Liu CP. Right heart function and scleroderma: insights from tricuspid annular plane systolic excursion. Echocardiography. 2007;24:118–125. doi: 10.1111/j.1540-8175.2007.00365.x. [DOI] [PubMed] [Google Scholar]
- 74.Meune C, Avouac J, Wahbi K, Cabanes L, Wipff J, Mouthon L, Guillevin L, Kahan A, Allanore Y. Cardiac involvement in systemic sclerosis assessed by tissue-Doppler echocardiography during routine care: a controlled study of 100 consecutive patients. Arthritis Rheum. 2008;58:1803–1809. doi: 10.1002/art.23463. [DOI] [PubMed] [Google Scholar]
- 75.Günther S, Jaïs X, Maitre S, Bérezné A, Dorfmüller P, Seferian A, Savale L, Mercier O, Fadel E, Sitbon O, et al. Computed tomography findings of pulmonary venoocclusive disease in scleroderma patients presenting with precapillary pulmonary hypertension. Arthritis Rheum. 2012;64:2995–3005. doi: 10.1002/art.34501. [DOI] [PubMed] [Google Scholar]
- 76.Williams MH, Handler CE, Akram R, Smith CJ, Das C, Smee J, Nair D, Denton CP, Black CM, Coghlan JG. Role of N-terminal brain natriuretic peptide (N-TproBNP) in scleroderma-associated pulmonary arterial hypertension. Eur Heart J. 2006;27:1485–1494. doi: 10.1093/eurheartj/ehi891. [DOI] [PubMed] [Google Scholar]
- 77.Mathai SC, Bueso M, Hummers LK, Boyce D, Lechtzin N, Le Pavec J, Campo A, Champion HC, Housten T, Forfia PR, et al. Disproportionate elevation of N-terminal pro-brain natriuretic peptide in scleroderma-related pulmonary hypertension. Eur Respir J. 2010;35:95–104. doi: 10.1183/09031936.00074309. [DOI] [PubMed] [Google Scholar]
- 78.Forfia PR, Mathai SC, Fisher MR, Housten-Harris T, Hemnes AR, Champion HC, Girgis RE, Hassoun PM. Hyponatremia predicts right heart failure and poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;177:1364–1369. doi: 10.1164/rccm.200712-1876OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dorfmüller P, Humbert M, Perros F, Sanchez O, Simonneau G, Müller KM, Capron F. Fibrous remodeling of the pulmonary venous system in pulmonary arterial hypertension associated with connective tissue diseases. Hum Pathol. 2007;38:893–902. doi: 10.1016/j.humpath.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 80.Steen V, Medsger TA., Jr Predictors of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Arthritis Rheum. 2003;48:516–522. doi: 10.1002/art.10775. [DOI] [PubMed] [Google Scholar]
- 81.Steen V. Predictors of end stage lung disease in systemic sclerosis. Ann Rheum Dis. 2003;62:97–99. doi: 10.1136/ard.62.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang B, Wigley FM, White B, Wise RA. Scleroderma patients with combined pulmonary hypertension and interstitial lung disease. J Rheumatol. 2003;30:2398–2405. [PubMed] [Google Scholar]
- 83.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54(1 Suppl):S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 84.Altman RD, Medsger TA, Jr, Bloch DA, Michel BA. Predictors of survival in systemic sclerosis (scleroderma) Arthritis Rheum. 1991;34:403–413. doi: 10.1002/art.1780340405. [DOI] [PubMed] [Google Scholar]
- 85.Mathai SC, Hummers LK, Champion HC, Wigley FM, Zaiman A, Hassoun PM, Girgis RE. Survival in pulmonary hypertension associated with the scleroderma spectrum of diseases: impact of interstitial lung disease. Arthritis Rheum. 2009;60:569–577. doi: 10.1002/art.24267. [DOI] [PubMed] [Google Scholar]
- 86.Krowka MJ, Swanson KL, Frantz RP, McGoon MD, Wiesner RH. Portopulmonary hypertension: results from a 10-year screening algorithm. Hepatology. 2006;44:1502–1510. doi: 10.1002/hep.21431. [DOI] [PubMed] [Google Scholar]
- 87.Krowka MJ, Miller DP, Barst RJ, Taichman D, Dweik RA, Badesch DB, McGoon MD. Portopulmonary hypertension: a report from the US-based REVEAL Registry. Chest. 2012;141:906–915. doi: 10.1378/chest.11-0160. [DOI] [PubMed] [Google Scholar]
- 88.Le Pavec J, Souza R, Herve P, Lebrec D, Savale L, Tcherakian C, Jaïs X, Yaïci A, Humbert M, Simonneau G, et al. Portopulmonary hypertension: survival and prognostic factors. Am J Respir Crit Care Med. 2008;178:637–643. doi: 10.1164/rccm.200804-613OC. [DOI] [PubMed] [Google Scholar]
- 89.Kawut SM, Krowka MJ, Trotter JF, Roberts KE, Benza RL, Badesch DB, Taichman DB, Horn EM, Zacks S, Kaplowitz N, et al. Pulmonary Vascular Complications of Liver Disease Study Group. Clinical risk factors for portopulmonary hypertension. Hepatology. 2008;48:196–203. doi: 10.1002/hep.22275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murray KF, Carithers RL, Jr American Association for the Study of Liver Disease (AASLD) AASLD practice guidelines: evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407–1432. doi: 10.1002/hep.20704. [DOI] [PubMed] [Google Scholar]
- 91.Kawut SM, Taichman DB, Ahya VN, Kaplan S, Archer-Chicko CL, Kimmel SE, Palevsky HI. Hemodynamics and survival of patients with portopulmonary hypertension. Liver Transpl. 2005;11:1107–1111. doi: 10.1002/lt.20459. [DOI] [PubMed] [Google Scholar]
- 92.Swanson KL, Wiesner RH, Nyberg SL, Rosen CB, Krowka MJ. Survival in portopulmonary hypertension: Mayo Clinic experience categorized by treatment subgroups. Am J Transplant. 2008;8:2445–2453. doi: 10.1111/j.1600-6143.2008.02384.x. [DOI] [PubMed] [Google Scholar]
- 93.Speich R, Jenni R, Opravil M, Pfab M, Russi EW. Primary pulmonary hypertension in HIV infection. Chest. 1991;100:1268–1271. doi: 10.1378/chest.100.5.1268. [DOI] [PubMed] [Google Scholar]
- 94.Opravil M, Pechère M, Speich R, Joller-Jemelka HI, Jenni R, Russi EW, Hirschel B, Lüthy R. HIV-associated primary pulmonary hypertension: a case control study. Swiss HIV Cohort Study. Am J Respir Crit Care Med. 1997;155:990–995. doi: 10.1164/ajrccm.155.3.9117037. [DOI] [PubMed] [Google Scholar]
- 95.Nunes H, Humbert M, Sitbon O, Morse JH, Deng Z, Knowles JA, Le Gall C, Parent F, Garcia G, Hervé P, et al. Prognostic factors for survival in human immunodeficiency virus–associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2003;167:1433–1439. doi: 10.1164/rccm.200204-330OC. [DOI] [PubMed] [Google Scholar]
- 96.Mehta NJ, Khan IA, Mehta RN, Sepkowitz DA. HIV-related pulmonary hypertension: analytic review of 131 cases. Chest. 2000;118:1133–1141. doi: 10.1378/chest.118.4.1133. [DOI] [PubMed] [Google Scholar]
- 97.Zuber JP, Calmy A, Evison JM, Hasse B, Schiffer V, Wagels T, Nuesch R, Magenta L, Ledergerber B, Jenni R, et al. Swiss HIV Cohort Study Group. Pulmonary arterial hypertension related to HIV infection: improved hemodynamics and survival associated with antiretroviral therapy. Clin Infect Dis. 2004;38:1178–1185. doi: 10.1086/383037. [DOI] [PubMed] [Google Scholar]
- 98.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 99.Sitbon O, Lascoux-Combe C, Delfraissy JF, Yeni PG, Raffi F, De Zuttere D, Gressin V, Clerson P, Sereni D, Simonneau G. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177:108–113. doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- 100.Degano B, Guillaume M, Savale L, Montani D, Jaïs X, Yaici A, Le Pavec J, Humbert M, Simonneau G, Sitbon O. HIV-associated pulmonary arterial hypertension: survival and prognostic factors in the modern therapeutic era. AIDS. 2010;24:67–75. doi: 10.1097/QAD.0b013e328331c65e. [DOI] [PubMed] [Google Scholar]
- 101.Pellicelli AM, Palmieri F, Cicalini S, Petrosillo N. Pathogenesis of HIV-related pulmonary hypertension. Ann N Y Acad Sci. 2001;946:82–94. doi: 10.1111/j.1749-6632.2001.tb03904.x. [DOI] [PubMed] [Google Scholar]
- 102.Berger RM, Beghetti M, Humpl T, Raskob GE, Ivy DD, Jing ZC, Bonnet D, Schulze-Neick I, Barst RJ. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012;379:537–546. doi: 10.1016/S0140-6736(11)61621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Braman SS, Eby E, Kuhn C, Rounds S. Primary pulmonary hypertension in the elderly. Arch Intern Med. 1991;151:2433–2438. [PubMed] [Google Scholar]
- 104.Shapiro BP, McGoon MD, Redfield MM. Unexplained pulmonary hypertension in elderly patients. Chest. 2007;131:94–100. doi: 10.1378/chest.06-1571. [DOI] [PubMed] [Google Scholar]
- 105.Lam CS, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation. 2009;119:2663–2670. doi: 10.1161/CIRCULATIONAHA.108.838698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chahal H, McClelland RL, Tandri H, Jain A, Turkbey EB, Hundley WG, Barr RG, Kizer J, Lima JA, Bluemke DA, et al. Obesity and right ventricular structure and function: the MESA-Right Ventricle Study. Chest. 2012;141:388–395. doi: 10.1378/chest.11-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zamanian RT, Hansmann G, Snook S, Lilienfeld D, Rappaport KM, Reaven GM, Rabinovitch M, Doyle RL. Insulin resistance in pulmonary arterial hypertension. Eur Respir J. 2009;33:318–324. doi: 10.1183/09031936.00000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Heresi GA, Aytekin M, Hazen SL, Dweik RA. Plasma levels of high-density lipoprotein cholesterol and outcomes in pulmonary hypertension. Am J Respir Crit Care Med. 2010;182:661–668. doi: 10.1164/rccm.201001-0007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pugh ME, Robbins IM, Rice TW, West J, Newman JH, Hemnes AR. Unrecognized glucose intolerance is common in pulmonary arterial hypertension. J Heart Lung Transplant. 2011;30:904–911. doi: 10.1016/j.healun.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaïci A, Weitzenblum E, Cordier JF, Chabot F, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156–163. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 111.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122:164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 112.Humbert M, Sitbon O, Yaïci A, Montani D, O’Callaghan DS, Jaïs X, Parent F, Savale L, Natali D, Günther S, et al. French Pulmonary Arterial Hypertension Network. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J. 2010;36:549–555. doi: 10.1183/09031936.00057010. [DOI] [PubMed] [Google Scholar]
- 113.Humbert M, Yaici A, de Groote P, Montani D, Sitbon O, Launay D, Gressin V, Guillevin L, Clerson P, Simonneau G, et al. Screening for pulmonary arterial hypertension in patients with systemic sclerosis: clinical characteristics at diagnosis and long-term survival. Arthritis Rheum. 2011;63:3522–3530. doi: 10.1002/art.30541. [DOI] [PubMed] [Google Scholar]
- 114.Sitbon O, Humbert M, Jaïs X, Ioos V, Hamid AM, Provencher S, Garcia G, Parent F, Hervé P, Simonneau G. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105–3111. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 115.Montani D, Savale L, Natali D, Jaïs X, Herve P, Garcia G, Humbert M, Simonneau G, Sitbon O. Long-term response to calcium-channel blockers in non-idiopathic pulmonary arterial hypertension. Eur Heart J. 2010;31:1898–1907. doi: 10.1093/eurheartj/ehq170. [DOI] [PubMed] [Google Scholar]
- 116.Sitbon O, Humbert M, Nunes H, Parent F, Garcia G, Hervé P, Rainisio M, Simonneau G. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40:780–788. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 117.Jais X, Launay D, Yaici A, Le Pavec J, Tchérakian C, Sitbon O, Simonneau G, Humbert M. Immunosuppressive therapy in lupus- and mixed connective tissue disease–associated pulmonary arterial hypertension: a retrospective analysis of twenty-three cases. Arthritis Rheum. 2008;58:521–531. doi: 10.1002/art.23303. [DOI] [PubMed] [Google Scholar]
- 118.Dartevelle P, Fadel E, Mussot S, Chapelier A, Hervé P, de Perrot M, Cerrina J, Ladurie FL, Lehouerou D, Humbert M, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2004;23:637–648. doi: 10.1183/09031936.04.00079704. [DOI] [PubMed] [Google Scholar]