Abstract
In patients with acute myocardial infarction undergoing reperfusion therapy to restore blood flow through blocked arteries, simultaneous inhibition of platelet P2Y12 receptors with the current standard of care neither completely prevents recurrent thrombosis nor provides satisfactory protection against reperfusion injury. Additionally, these antiplatelet drugs increase the risk of bleeding. To devise a different strategy, we engineered and optimized the apyrase activity of human nucleoside triphosphate diphosphohydrolase-3 (CD39L3) to enhance scavenging of extracellular adenosine diphosphate, a predominant ligand of P2Y12 receptors. The resulting recombinant protein, APT102, exhibited greater than four times higher adenosine diphosphatase activity and a 50 times longer plasma half-life than did native apyrase. Treatment with APT102 before coronary fibrinolysis with intravenous recombinant human tissue-type plasminogen activator in conscious dogs completely prevented thrombotic reocclusion and significantly decreased infarction size by 81% without increasing bleeding time. In contrast, clopidogrel did not prevent coronary reocclusion and increased bleeding time. In a murine model of myocardial reperfusion injury caused by transient coronary artery occlusion, APT102 also decreased infarct size by 51%, whereas clopidogrel was not effective. These preclinical data suggest that APT102 should be tested for its ability to safely and effectively maximize the benefits of myocardial reperfusion therapy in patients with arterial thrombosis.
INTRODUCTION
Acute myocardial infarction (AMI), ischemia resulting from occlusion of coronary arteries with platelet-rich thrombus (blood clot), is the leading cause of death in the industrialized world (1). The primary goal of therapy in AMI is to expedite restoration of normal coronary blood flow with the intent of decreasing heart muscle damage (2). Current American Heart Association and American College of Cardiology guidelines for patients with AMI include percutaneous coronary intervention (PCI) (balloon angioplasty and stenting) or fibrinolysis with intravenous recombinant human tissue-type plasminogen activator (rt-PA) to restore blood flow and adjunctive administration of aspirin and clopidogrel (Plavix) to reduce peri- and post-procedural platelet-rich thrombosis (1–3). Clopidogrel works by potently inhibiting P2Y12, one of two platelet receptors for adenosine diphosphate (ADP). Clopidogrel works slowly to inhibit platelet function, however, taking 2 to 6 hours for full effect, during which the drug is metabolized to its active form in the liver. Furthermore, the efficacy of platelet inhibition with clopidogrel is variable, and deficiencies in or genetic variants of liver cytochrome P450 enzymes appear responsible for decreased efficacy in as many as 40% of patients (4). These shortcomings, coupled with the irreversible inhibition of platelet function and increased bleeding risk, all detract from the usefulness of clopidogrel as an adjunctive agent for PCI or fibrinolysis.
Currently, net adverse composite end points of death, coronary reocclusion, or stroke remain as high as 7 to 12% for PCI and 10 to 12% for fibrinolysis, and the rate of bleeding is 5 to 11% (5, 6). Most of these adverse events occur within the first 6 to 9 hours of intervention (7), so it is vital that therapeutic agents act quickly and safely. Although recently approved P2Y12 antagonists, including prasugrel and ticagrelor, improve the onset of action and efficacy of platelet inhibition in patients with acute coronary syndrome, these agents carry the same risk of bleeding as clopidogrel (5, 6). Major bleeding within 48 hours of PCI is associated with a 1-year mortality of 7.2% compared to 2.1% in patients who do not have periprocedural major bleeding (7, 8). Moreover, none of the current antiplatelet therapeutics protect against reperfusion injury, defined as myocardial injury caused by reoxygenation of previously ischemic myocardium (9). Reperfusion injury accounts for up to 50% of the final size of a myocardial infarct and is characterized by impaired microvascular perfusion (9). Beyond the acute phase, adverse ventricular remodeling, heart failure, and mortality are directly related to infarct size and left ventricular dysfunction (5–7, 10). Consequently, the search for more effective and safer adjunctive antithrombotic agents that also attenuate reperfusion injury has become the holy grail of drug development for patients with AMI (9, 11).
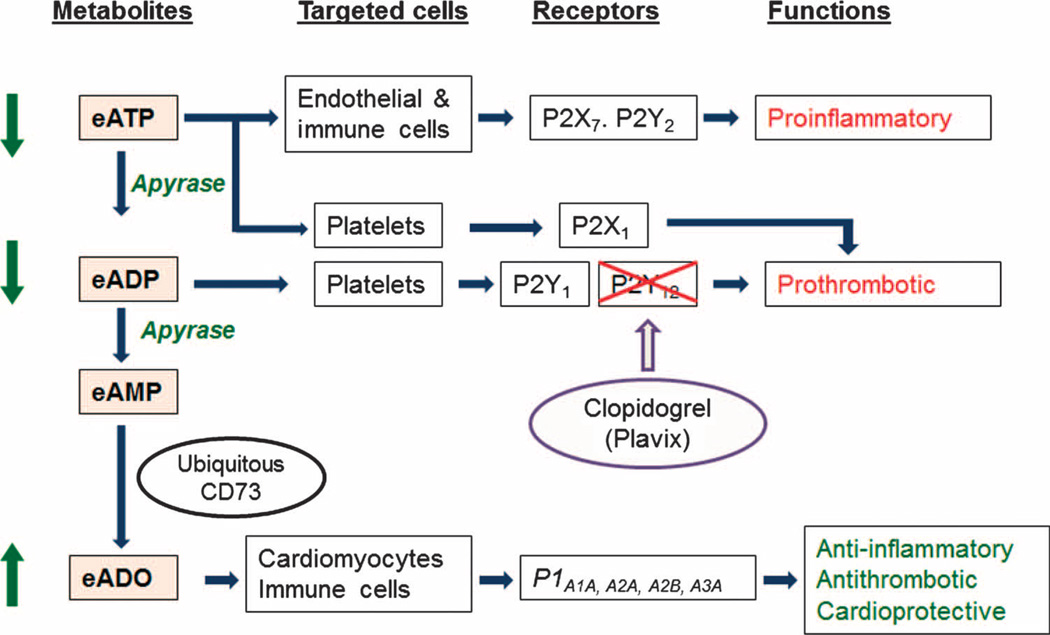
Human apyrases [ecto–nucleoside triphosphate diphosphohydrolases (E-NTPDases) of the CD39 family] constitute a family of ectoenzymes or ectonucleotidases that could address these unmet needs (12–14). Extracellular adenosine triphosphate (eATP) is proinflammatory because it binds to P2X and P2Y receptors on platelets, endothelial cells, monocytes, and lymphocytes, causing the activation and secretion of proinflammatory cytokines (15–17). Extracellular ADP (eADP) plays a central role in activating P2Y1 and P2Y12 receptors on platelets (18). Apyrase efficiently catalyzes hydrolysis of eATP to eADP, and then eADP to eAMP (extracellular adenosine monophosphate), which is converted by the ubiquitously expressed extracellular CD73/ecto-5′-nucleotidase to extracellular adenosine (eADO; Fig. 1) (14–17). Thus, apyrase-induced hydrolysis of eATP and eADP is beneficial for maintaining vascular integrity and physiologically inhibiting inflammation and thrombosis (15). Moreover, apyrase blocks eADP and eATP interaction at all three platelet P2 receptors (P2X1, P2Y1, and P2Y12), thereby producing more complete inhibition of platelet activation and recruitment than currently available antagonists that act only at the P2Y12 receptor (Fig. 1). In addition, eADO generated by the action of CD73 on eAMP is anti-inflammatory and also deaggregates platelets, thereby counteracting thrombosis and reperfusion injury (17, 19, 20). Unlike inhibitors of P2Y12 receptors that increase bleeding, apyrase preserves vascular integrity and prevents platelet desensitization because of P2Y1 internalization, resulting in a continuous level of hemostasis (21, 22).
Fig. 1. Mechanisms of action of endogenous apyrase.
eATP and eADP are scavenged by apyrase, leading to the generation of cardioprotective adenosine (eADO). Thus, administration of exogenous apyrase (APT102) boosts the endogenous control mechanism that converts a proinflammatory and prothrombotic environment, characterized by dominant P2 purinoceptor signaling, into an eADO P1 receptor signaling that is largely characterized by prevention of inflammatory responses and thrombosis, and cardioprotective mechanisms. Apyrase attenuates platelet activation mediated by P2Y1, P2Y12, and P2X1 receptors, whereas clopidogrel blocks only P2Y12 receptors. Thus, apyrase may be more effective for inhibiting platelet-rich thrombosis than P2Y12 receptor antagonists.
CD39 is the first discovered human apyrase (13–15). CD39 deficiency in mice results in disordered hemostasis and prolonged bleeding time, as well as larger infarcts, than in wild-type mice in a model of myocardial ischemia-reperfusion (21). Administration of the recombinant human soluble domain of CD39 (solCD39) to deficient mice increases eADO and decreases infarct size as compared to wild-type mice, mimicking the cardioprotective effect of ischemic preconditioning (23). Administration of solCD39 also reduces platelet activation and leukocyte accumulation in the arterial vasculature. Furthermore, overexpression of human CD39 protects against myocardial injury in both transgenic mice and swine (24, 25). Together, these results indicate that increased levels of apyrase are associated with antithrombotic, anti-inflammatory, and cardioprotection activity. Hence, apyrase-based therapeutics may provide clinical advantages over existing platelet inhibitors (14, 15).
We report here the design and production of APT102, an optimized form of solCD39L3, a member of the human CD39 family, together with the in vitro characteristics and in vivo pharmacology in canine and murine models of myocardial infarction and reperfusion.
RESULTS
Protein optimization
Studies in experimental animal models of arterial injury show that solCD39 is an effective antithrombotic agent that induces minimal increases in bleeding time and intracerebral hemorrhage (13, 26–28). However, high doses are required for efficacy, and a substantial portion of recombinant human CD39, expressed in COS-1 cells, consists of misfolded and aggregated molecules (29). Moreover, solCD39 activity appears to be inhibited by clopidogrel (30). Accordingly, we focused our efforts on developing a more potent apyrase that would fold properly, have slow plasma clearance kinetics, and be insensitive to inhibition by clopidogrel.
Human CD39L3/NTPDase 3 is a member of the CD39 family, sharing 33% sequence identity with CD39 (31). The aberrant folding of recombinant human CD39 is not observed for recombinant human CD39L3 (29). We first generated the soluble domain of CD39L3 (solCD39L3, APT101, or wild type) by deleting the transmembrane domains from both the N and C termini. The protein engineering that followed was designed to construct variants that (i) had enhanced adenosine diphosphatase (ADPase) activity (antithrombotic), (ii) retained adenosine triphosphatase (ATPase) activity (anti-inflammatory), and (iii) preserved structural integrity (protein stability). Although crystal structures were not available for APT101, solCD39, or other known E-NTPDases, critical residues determining binding and specificity for ADP and ATP were identified by structural protein informatics (31, 32), as well as by biochemical analyses (33, 34). This analysis suggested that E-NTPDases are distantly related to other ATP hydrolyzing proteins, including actin and HSP-70, which are well characterized (fig. S1).
On the basis of structural insights from the related protein rabbit actin (35), the following double mutations were introduced into APT101: (i) mutation of Thr69 to Arg to enhance interaction with the α-and β-phosphates of ADP/ATP and (ii) mutation of Arg67 to Ala or Gly to increase the main chain flexibility, which could optimize the interaction between the introduced Arg69 and the α- and β-phosphates of ADP. Kinetic parameters for solCD39, APT101, and R67G/T69R and R67A/T69R (table S1) were determined with a spectrophotometric assay and a radio-TLC (thin-layer chromatography) assay. As measured by the ratio of kcat over Km, APT101 and solCD39 had comparable catalytic performances (1.13 and 1.02 µM−1 s−1, respectively) and similar ratios of ADPase-to-ATPase activity (0.34 and 0.45, respectively). Replacement of Arg67 by Ala or Gly decreased by 50% of the original enzyme activity for hydrolysis of ADP and ATP, without a significant change in substrate specificity, a result that differed from a previous report showing that the mutation of Arg67 to Gly in the full-length CD39L3 increased hydrolysis of ADP and ATP (36).
The first double mutation, R67A/T69R, increased ADPase catalysis fivefold and ATPase activity fourfold (table S1). The ADPase-to-ATPase activity ratio measured by spectrophotometry was comparable to that of the wild-type enzyme (table S1). The R67G/T69R double mutation (APT102) caused ADPase activity to increase about fourfold, whereas ATPase activity was increased only slightly. These mutations improved the Km for ADP from 134 to 46 µM and the Km for ATP from 136 to 29 µM, indicating that APT102 had enhanced binding to the α- and β-phosphates of both ADP and ATP. APT102 demonstrated about twofold higher ADPase-to-ATPase activity in the spectrophotometric assay compared to APT101 or the R67A/T69R variant (table S1), so it was selected for further development to minimize ATP-derived ADP accumulation. A more quantitative radio-TLC assay procedure for ADPase and ATPase activities confirmed that APT102 was twice as active for ADP than ATP and was significantly more potent for hydrolysis of both ATP and ADP than solCD39 (table S1 and fig. S2).
In vitro inhibition of ADP-induced platelet aggregation by APT102
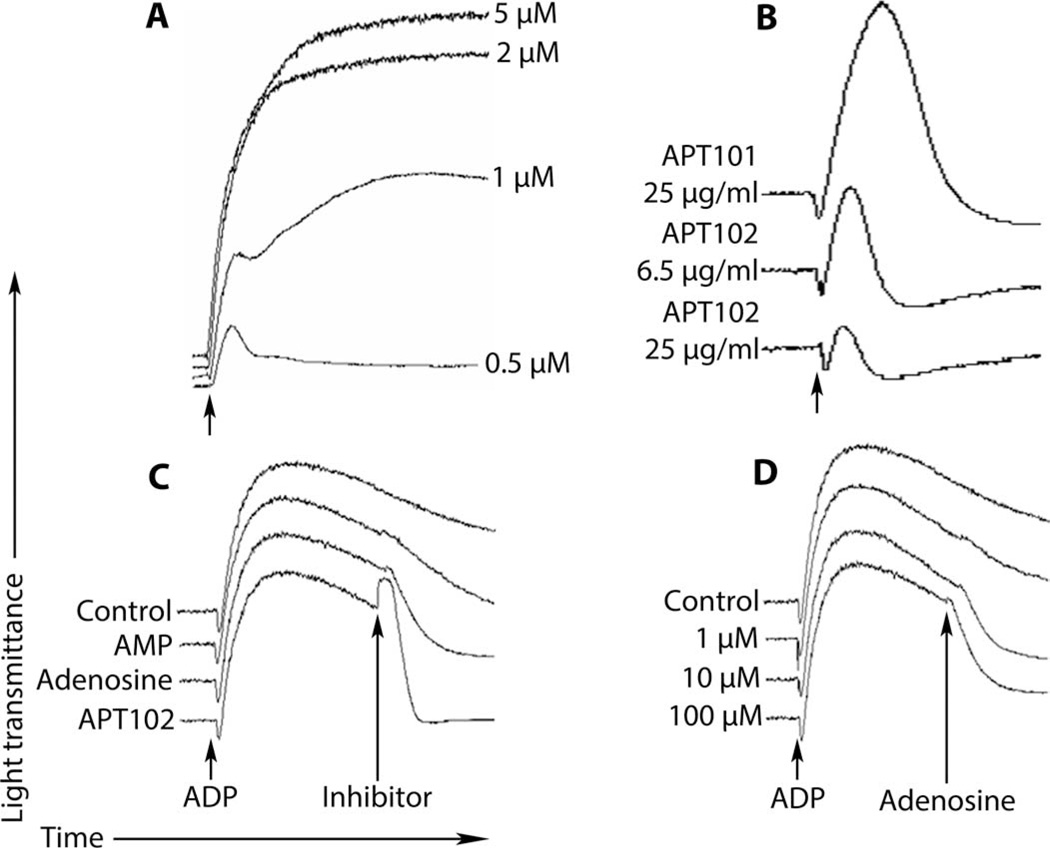
APT101 and APT102 were further compared to determine whether the greater enzymatic activity of APT102 translated into increased potency of platelet inhibition. In the absence of apyrase, ADP caused dose-dependent aggregation of human platelets in platelet-rich plasma in vitro (Fig. 2A and fig. S3). APT102 was about four times more potent than APT101 in inhibiting ADP-induced (5 µM) aggregation of human platelets (Fig. 2B). Notably, addition of APT102 to platelet-rich plasma 6 min after maximal, stable platelet aggregation caused immediate disaggregation of the platelets, as evidenced by full return of the light transmittance to the pre-aggregation levels (Fig. 2C). Reversal of stable platelet aggregation was partially achieved in dose-dependent fashion by adding adenosine (Fig. 2C and D), suggesting that disaggregation occurred as APT102 and CD73 formed adenosine from eADP. Addition of AMP was much less effective (Fig. 2C).
Fig. 2. In vitro inhibition of ADP-induced platelet aggregation and disaggregation by APT102 in platelet-rich plasma from a healthy human volunteer.
(A) Dose response of ADP added to platelet-rich plasma (at arrow). (B) Inhibition of 5 µM ADP–induced platelet aggregation by APT101 (25 µg/ml) and its optimized derivative, APT102 (6.5 and 25 µg/ml), added before ADP. (C) APT102 (2.5 µg/ml), adenosine (20 µM), or AMP (20 µM) was added at the “inhibitor” arrow 6 min after maximal aggregation. Only APT102 completely reversed aggregation. (D) Adenosine dose dependently (1, 10, and 100 µM) but only partially reversed platelet aggregation. Blood was collected in tubes containing heparin to maintain physiologic calcium concentration. Results shown are representative of triplicate assays. Similar results were obtained in two other healthy volunteers.
APT102 inhibited ADP-induced in vitro aggregation of human platelets in a concentration-dependent manner, with nearly complete inhibition achieved at 2.5 µg/ml and an EC50 (median effective concentration) of 0.09 ± 0.02 µg/ml (table S2 and fig. S3B). Similarly, APT102 inhibited ADP-induced in vitro aggregation of platelets from rat, rabbit, pig, and dog in a concentration-dependent manner, with EC50s (0.29 ± 0.02, 0.11 ± 0.03, 0.65 ± 0.02, and 0.37 ± 0.01 µg/ml, respectively) that were 1.2 to 7.2 times higher than for human platelets (table S2 and fig. S4). Regardless of the concentration of ADP used to induce aggregation, APT102 at a concentration of 2.5 µg/ml yielded a consistent level of platelet inhibition (fig. S4B). This feature differs markedly from the behavior of competitive antagonists of P2Y12 receptors, because higher concentrations of ADP displace these antagonists from P2Y12 receptors, thereby reducing the antiplatelet effect of these agents (37).
Effect of clopidogrel and prasugrel on apyrase inhibition of platelet aggregation in vitro
By simulating the loading doses recommended for clopidogrel (40 µM) and prasugrel (10 µM) in AMI patients with an elimination half-life of about 6 and 7 hours, respectively (5, 6, 30), we assessed the effects of these prodrugs on ADP-induced platelet aggregation in canine platelet-rich plasma with added solCD39 or APT102. As reported previously for native apyrase on vascular endothelium (30), clopidogrel reduced the ability of solCD39 to block platelet aggregation induced by ADP (fig. S5). A similar effect was observed for prasugrel. Although ADPase activities of solCD39 and APT102 were similar even at thienopyridine concentrations above the clinical range (clopidogrel, 20 to 200 µM; prasugrel, 10 to 40 µM), the different effects of solCD39 and APT102 on ADP-induced platelet aggregation may have resulted from the fact that solCD39 and APT102 share only 40% sequence identity, and this difference may affect an as yet unknown chemical or biochemical interaction. Nevertheless, patients already being treated with clopidogrel or prasugrel at the time of an AMI may be given APT102 without compromising the added platelet inhibitory benefit.
Pharmacodynamics of APT102
Substitution of the first four amino acid residues of APT102 with EVPP (glutamic acid, valine, proline, proline), coupled with use of a stably transfected Chinese hamster ovary (CHO) cell line technology (38), resulted in the highly glycosylated APT102 with homogeneous N-terminal sequence (fig. S6). We determined the ex vivo ADPase activity in serial plasma samples from dogs given an intravenous bolus of APT102. Nonlinear regression analysis showed a biphasic exponential decay curve with a calculated distribution half-life of 2.5 to 3.5 hours and elimination half-life of 40 to 48 hours for APT102 (fig. S7), which is a 50-fold increase in pharmacodynamic half-life compared to the nonoptimized enzyme. Ex vivo platelet aggregation after intravenous APT102 (1 mg/kg) was inhibited about 90% within 10 min, compared to 4 to 6 hours for 60 to 80% inhibition after oral administration of clopidogrel (fig. S8). Inhibition of platelet aggregation with APT102 was persistent, maintaining >80% inhibition 24 hours after the single intravenous dose (fig. S8). Moreover, template bleeding times (measured with a spring-loaded blade as used clinically) were the same at baseline (111 ± 13 s) and 1 hour (94 ± 5 s, mean ± SEM, n = 4) after the injection. Thus, a single intravenous bolus of APT102 may be effective clinically for rapid, safe, and persistent inhibition of platelet activation, making it potentially appropriate for emergency room treatment as well as pre-hospital use at any time after making the diagnosis of AMI.
Efficacy and safety of APT102 and clopidogrel in canine AMI and coronary fibrinolysis
Using a clinically relevant model of thrombotic occlusion of a coronary artery and rt-PA–induced fibrinolysis in conscious dogs (39), we compared coronary patency, template bleeding time, and the extent of myocardial infarction in animals given either intravenous APT102 or oral clopidogrel adjunctively with standard clinical doses of aspirin and heparin beginning 55 min after occlusion and 5 min before administration of rt-PA (fig. S9). Because the results with APT102 (0.25 and 0.5 mg/kg) did not differ appreciably, we combined these data in a low-dose group to increase the statistical power. Reperfusion after administration of rt-PA was achieved in 75% of clopidogrel-treated dogs and 100% of APT102-treated dogs (Table 1). The time to reperfusion in dogs given high-dose APT102 (1.0 mg/kg) was shorter than in dogs given either clopidogrel or low-dose APT102, but the differences were not statistically significant.
Table 1. Comparison of event rates and times in dogs given rt-PA plus either clopidogrel or APT102.
Results are means ± SEM. Reperfusion times and incidence and times of reocclusion were determined by continuous monitoring of coronary blood flow using implanted electromagnetic flow probes.
| Treatment | Reperfusion rate (%) | Reperfusion time (min) | Reocclusion rate (%) | Reocclusion time (min) |
|---|---|---|---|---|
| Clopidogrel (4 mg/kg) | 6/8 (75) | 34.0 ± 8.9 | 100 | 99.7 ± 23.6 |
| Low-dose APT102 (0.25 or 0.5 mg/kg) | 7/7 (100) | 36.0 ± 20.1 | 100 | 160 ± 114.8 |
| High-dose APT102 (1.0 mg/kg) | 6/6 (100) | 28.2 ± 10.9 | 0* | NA |
P < 0.05 compared with clopidogrel by ANOVA.
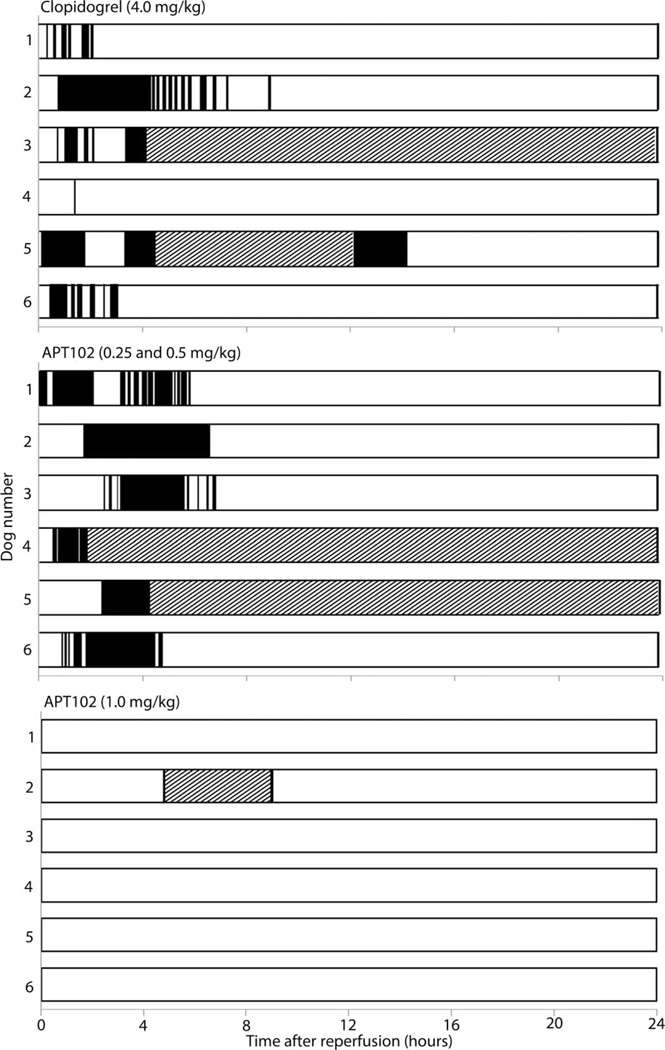
Coronary thrombotic reocclusion, monitored by continuous measurement of coronary blood flow, occurred in all clopidogrel-treated and all low-dose APT102–treated dogs within 2 hours of fibrinolysis (Fig. 3 and Table 1). In contrast, high-dose APT102 administration precluded reocclusion through the end of the study at 24 hours in all of the dogs. In other groups in which animals had reocclusions, most exhibited spontaneous coronary reperfusion between 12 and 16 hours after fibrinolysis, likely resulting from up-regulation of intrinsic vascular fibrinolytic activity, which is well developed in dogs (39). Nevertheless, coronary blood flow remained below baseline levels after 24 hours in both the clopidogrel-treated and low-dose APT102–treated groups (fig. S10). In contrast, coronary flow was higher than baseline throughout the post-fibrinolytic interval in the high-dose APT102 group (fig. S10).
Fig. 3. Coronary patency after reperfusion assessed by electromagnetic flow probe profiles in individual dogs.
Open bars, patent arteries; black bars, occluded arteries; crosshatched bars, an interval of no recorded flow because of computer or power shutdown.
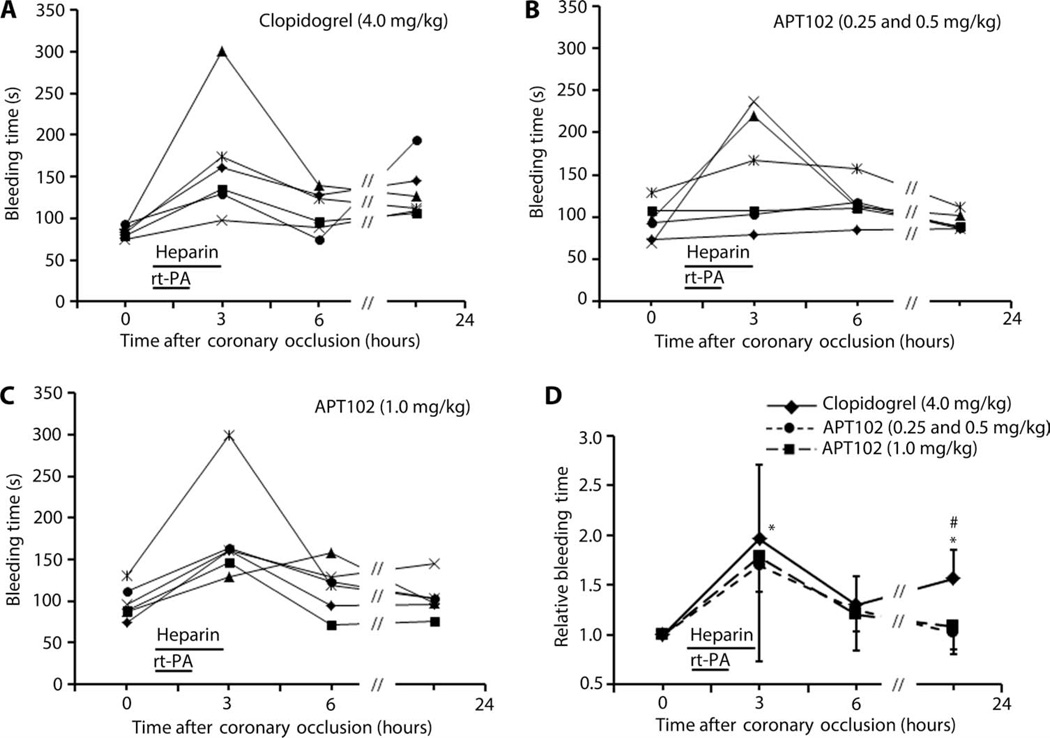
As expected, bleeding times at 3 hours (end of heparin infusion) were elevated and variable compared to other intervals because of the known variable effects of heparin on bleeding in dogs (Fig. 4), documented previously (39). However, bleeding times promptly returned toward baseline levels in APT102-treated dogs after clearance of heparin and rt-PA from the circulation. In contrast, bleeding time in clopidogrel-treated dogs plateaued at 1.5-fold baseline until 6 hours and then continued to increase, reflecting prolonged release into the circulation of the active metabolite produced in the liver that irreversibly inhibits platelet P2Y12 receptors. Furthermore, there was absence of bleeding from surgery sites on the neck and chest in any dogs given APT102. However, dogs given clopidogrel showed significant oozing and frequent hematomas at surgery sites by the end of the infusions of rt-PA and heparin, some of which required placement of pressure bandages around the neck and/or chest. Consistent with these increases in bleeding time attributable to heparin, activated partial thromboplastin time (aPTT) was also increased significantly at 3 and 6 hours in all groups, with no differences observed between groups (table S3). By 24 hours, aPTT was generally returning to, or back at, baseline values. Noincreases in prothrombin time (PT) were observed in any of the groups (table S3). There were also no differences in hematology or chemistry values in blood between any of the groups (tables S4 and S5).
Fig. 4. Bleeding times after coronary occlusion in individual dogs.
Data were averaged (n = 6) relative to baseline values before administration of either clopidogrel or APT102 followed by rt-PA and heparin (horizontal bars). (A to C) Each data point represents an average of duplicate bleeding time measurements for an individual animal. (D) Data are presented as the means ± SD. *P < 0.05 versus baseline; #P < 0.05 versus APT102 by one-way analysis of variance (ANOVA) and a two-tailed t test.
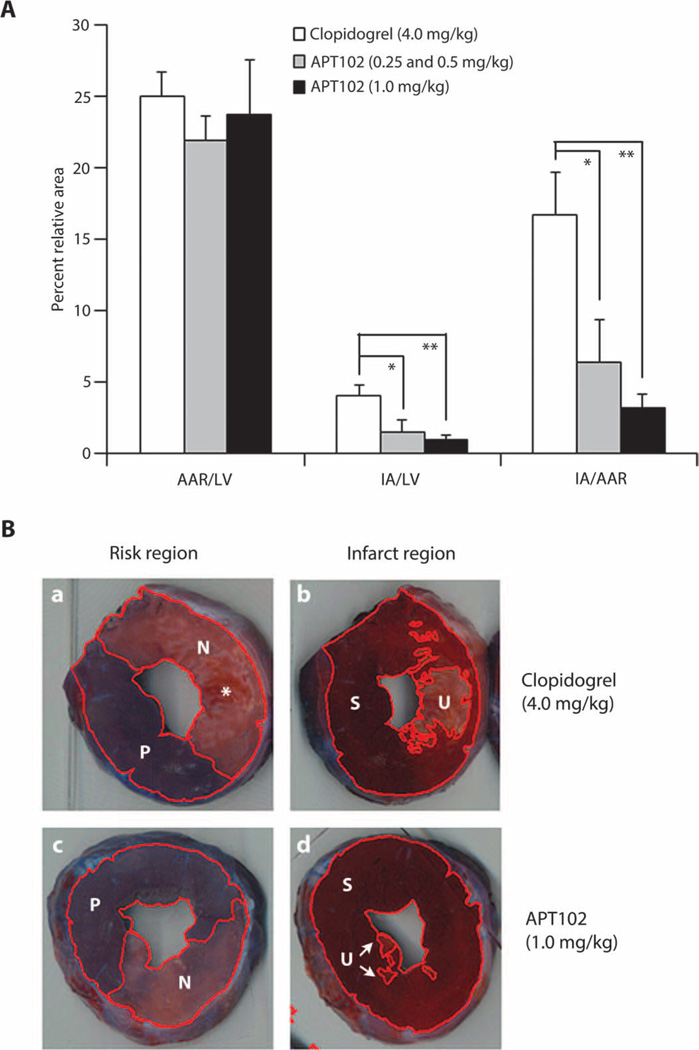
A unique attribute of APT102 compared with other platelet inhibitors is its ability to attenuate myocardial infarction after coronary reperfusion. In this canine study, APT102 decreased myocardial infarction expressed as a percentage of the ischemic area (area at risk) by 62% (P < 0.05) in the low-dose group and by 81% (P < 0.01) in the high-dose group compared to clopidogrel-treated dogs (Fig. 5A). Strikingly smaller regions of infarction, visible as areas deficient in dehydrogenase enzymes, were readily apparent on the transverse sections of the left ventricle in dogs given high-dose APT102 (Fig. 5B, panel d) compared to those given clopidogrel (Fig. 5B, panel b).
Fig. 5. Effect of clopidogrel and APT102 on myocardial ischemic damage in dogs.
(A) Area at risk (AAR) and infarcted area (IA; nonviable) after coronary artery occlusion of left ventricular (LV) heart muscle of dogs (n = 6) expressed as a percent of the total LV and as a ratio (29). (B) Photographs of representative LV slices from two hearts at the same level showing (left) similar AAR after clopidogrel (a) or high-dose APT102 (c) defined by the area that was nonperfused (N) by Evans blue dye compared to the total surface area including the area perfused (P) with dye. IA, defined as unstained (U) tissue after incubation with triphenyltetrazolium chloride (TTC), was compared to the total area including still viable TTC-stained (S) myocardium for the dog given APT102 (d, arrows) compared to the area in the dog given clopidogrel (b). Infarction in clopidogrel-treated hearts was sometimes marked by more abundant areas of hemorrhage seen in the pre-TTC–stained tissue (*, panel a). Bars represent means ± SEM. *P < 0.02, **P < 0.002 by t test.
Efficacy and safety of APT102 and clopidogrel in murine myocardial ischemia-reperfusion
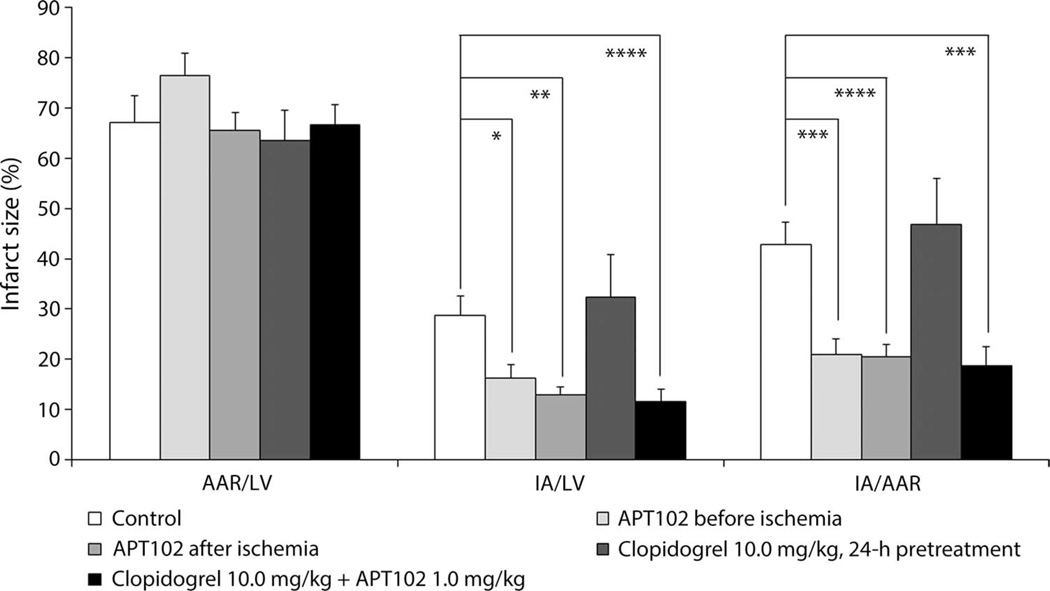
To discern the protective effects of APT102 on reperfusion injury in another species, we also conducted experiments in C57BL/6J mice with left anterior descending (LAD) coronary artery occlusion for 60 min followed by release and observation for 24 hours. When compared to controls, APT102 administered either 5 min before ischemia or 10 min before reperfusion reduced significantly the infarcted area expressed as a percentage of the area at risk (51 and 52%, respectively; P < 0.05) measured 24 hours later (Figs. 6 and 7). In contrast, 24-hour pretreatment with clopidogrel was not protective in this model. Combining APT102 and clopidogrel pretreatment did not change the protective efficacy (56%, P < 0.05) seen with APT102 alone (Fig. 6). Meanwhile, pretreatment with clopidogrel in mice prolonged bleeding time more than twofold over baseline levels by 24 hours (fig. S11). When APT102 was administered to clopidogrel-treated mice, bleeding times actually decreased significantly within 10 min compared to those given clopidogrel alone (fig. S11). These data confirm that APT102 is safe and effective in attenuating reperfusion injury, whereas clopidogrel increases bleeding risk without preventing reperfusion injury.
Fig. 6. Effect of APT102 and clopidogrel on myocardial tissue after ischemia-reperfusion injury in mice.
C57BL/6J mice were given equivalent volumes of saline (intravenously as a control) or APT102 (1.0 mg/kg, intravenously) 5 min before or 50 min after the onset of 60 min of ischemia (via LAD coronary occlusion), clopidogrel (10.0 mg/kg, orally) pretreatment 24 hours before ischemia, or a combination of clopidogrel pretreatment and APT102 5 min before ischemia. Bars represent means ± SEM. Control, n = 12; APT102 before ischemia, n = 12; APT102 after ischemia, n = 30; clopidogrel, n = 15; clopidogrel plus APT102, n = 33. *P < 0.05, **P < 0.0005, ***P < 0.0001, ****P < 0.00001 by t tests.
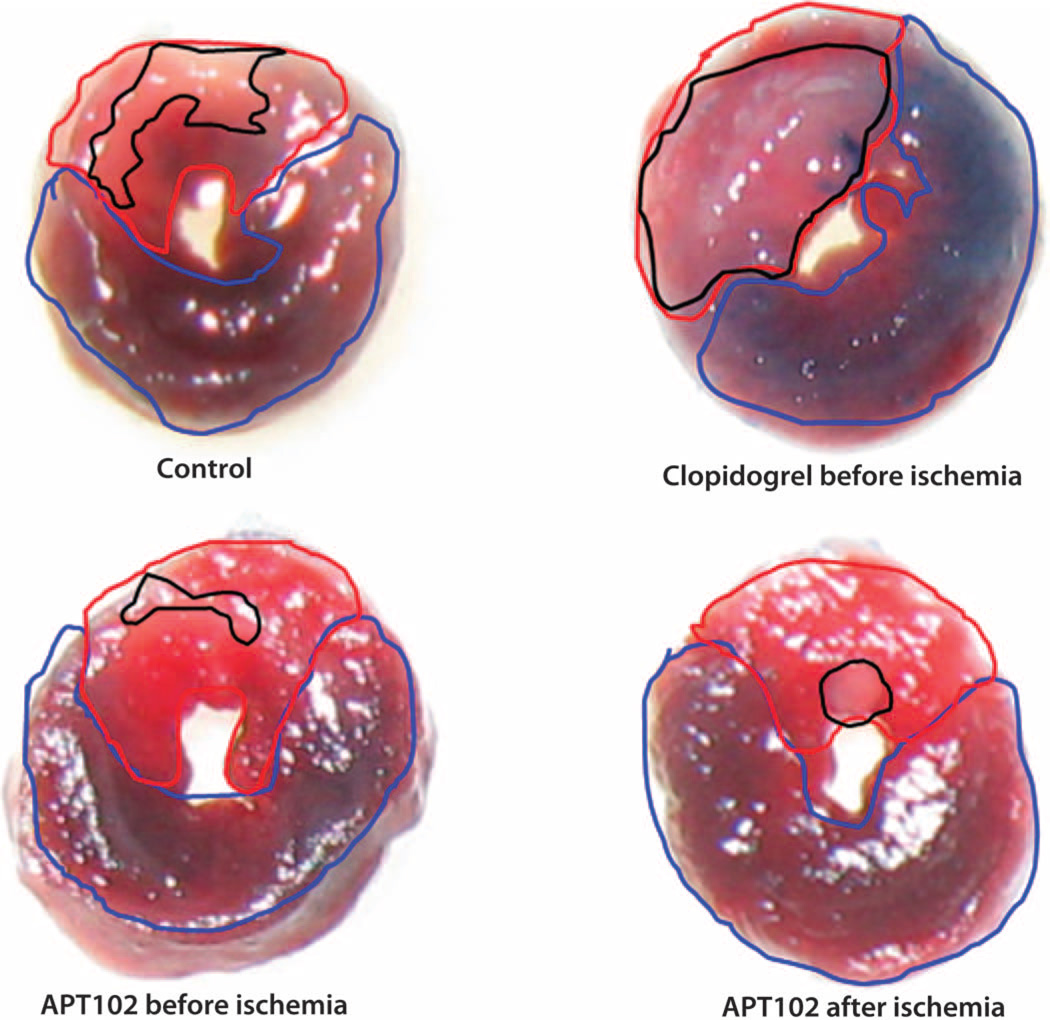
Fig. 7. Representative transverse slices taken at a similar level of the left ventricle from mice undergoing 60 min of ischemia (via LAD coronary occlusion).
The slices were obtained after 24 hours and show a similar area at risk (outlined in red) for the four treatments defined by the area that was nonperfused by Evans blue dye compared to the total surface area including the area perfused with Evans blue dye (outlined in blue). Shown in black outline are the same heart slices after incubation with TTC to identify the area of unstained and therefore infarcted myocardium relative to the total area, including the TTC-stained myocardium that was still viable.
DISCUSSION
Here, we have tested adjunctive use of APT102 in both dogs and mice with complete occlusion of an epicardial coronary artery, mimicking the most serious type of human AMI (ST-segment elevation AMI), followed by coronary reperfusion. This treatment resulted in decreased extent of the myocardial infarction area (Figs. 5 to 7) 24 hours later without increasing bleeding (Fig. 4 and fig. S11) compared to the effects of the standard of care clopidogrel. We also used an AMI model in dogs with clinically relevant pharmacologic fibrinolysis of coronary thrombi, which generates a shower of platelet-rich microemboli that lead to microvascular obstruction and “no reflow,” similar to the events in patients (40). In this model, APT102 administered before fibrinolysis completely inhibited reocclusive platelet-rich thrombosis (Table 1) and improved coronary blood flow during reperfusion (fig. S10), possibly by inhibiting platelet aggregates that cause microvascular obstruction.
Our data suggest a new paradigm for AMI adjunctive therapy. Rather than direct inhibition of platelet activation at a single receptor, as with clopidogrel acting at P2Y12, our results point to the use of multimodal, indirect inhibition of platelet activation and inflammation. In our experiments, APT102 scavenged the key agonists (eADP and eATP) for multiple purinergic receptors responsible for platelet aggregation and inflammation, ultimately yielding cardioprotective eADO. Compared to P2Y12 antagonists such as clopidogrel, this approach may prove to be both more effective and safer.
Platelet activation and rethrombosis are obstacles for all treatments for AMI because the ruptured plaque releases the same substrates that originally initiated the thrombosis (collagen and tissue factor) (41). Moreover, with rt-PA–induced fibrinolysis, free thrombin is generated that directly activates platelets and the coagulation system(42, 43), whereas PCI stretches the vessel walls, causing additional trauma and exposure of more tissue factor, which accelerates platelet activation and thrombosis (41, 42). The benefits of circulating APT102 that we have seen in our animal models may derive from the continuous metabolic elimination of eATP released from the vessel and adherent neutrophils and of eADP released from the vessel and platelets, which would otherwise increase local platelet aggregation. APT102 fully reversed stable aggregated platelets (Fig. 2). Partial disaggregation was achieved with physiologically relevant concentrations of adenosine. Meanwhile, others have shown that platelet aggregation stability is inversely proportional to platelet ADP receptor occupancy (44), and partial disaggregation can be caused by the P2Y12 antagonist 2-MeSAMP (45). Hence, the immediate and complete reversal of human platelet aggregation by APT102 (Fig. 2C) is likely a result of a combination of eADP hydrolysis and eADO generation from eAMP by CD73 located in the platelet membrane (46).
Because APT102 indirectly inhibits platelet function by removing agonists, the platelets remain fully functional and capable of aggregation once APT102 is cleared from the circulation. This differs from the active clopidogrel metabolite that irreversibly binds to the receptor and permanently inhibits platelet function, placing the patient at risk for spontaneous bleeding and increasing the risk of bleeding if emergency surgery is needed to reestablish coronary blood flow.
The anti-inflammatory effects of APT102 may be largely responsible for the marked reduction in reperfusion injury and decreased infarct size we observed in both dogs and mice in comparison to the effects of clopidogrel (Figs. 5 to 7). Reduced infarction area with APT102 did not appear to be secondary to differences in the duration of ischemia, because these differences were insignificant for dogs (Table 1) and held constant for mice (Fig. 6). Although low doses of APT102 did not prevent transient coronary reocclusion in dogs, these doses did decrease myocardial infarction area by 62% compared with clopidogrel treatment (Fig. 5A). These effects may have resulted from generation of cardioprotective eADO during reperfusion because APT102 increases the concentrations of eAMP for reduction to eADO by CD73 (Fig. 1), but the exact mechanism remains to be elucidated.
In rabbit and mouse models, increased eADO generated from endogenous CD39 action mediates the beneficial effects of cardiac ischemic preconditioning (23, 47). Similarly, when we gave APT102 (1.0 mg/kg, intravenously) 5 min before the onset of myocardial ischemia in mice, infarct size was reduced by about 51% compared to saline controls (Fig. 6). Notably, APT102 was nearly equally effective for reducing infarct size (52%) when administered 50 min after the onset of ischemia (Fig. 6), consistent with upstream metabolism of adenine nucleotides releasing adenosine rather than adenosine production by ischemic cells (23). In contrast, 24-hour pretreatment with clopidogrel did not reduce infracted area in mice. The combination of APT102 and clopidogrel reduced infarcted area to the same extent as APT102 alone, suggesting that any additional inhibition of platelet activation did not, by itself, increase the therapeutic benefit.
Ischemia-reperfusion injury can be decreased by recombinant human solCD39 administration before or 3 hours after induction of stroke in rodents (26, 27). APT102 also prevents reperfusion injury after ischemia of lung isografts after transplantation (48). Thus, the combined antiplatelet/anti-inflammatory properties of APT102 not only increase the efficacy of reperfusion therapy but also mitigate the potentially harmful effects of reoxygenation of ischemic tissue. This salutary effect may provide lasting benefit for the functional recovery of the heart and improve the prognosis of patients after AMI.
Unlike clopidogrel, APT102 induced minimal bleeding risk, as assessed with an assay designed to mimic minor surgery. Bleeding times increased about 1.5-fold above baseline in APT102-treated dogs only during the time of coadministration of heparin and rt-PA (Fig. 4). Then, bleeding time returned toward baseline by 6 hours. In contrast, clopidogrel-treated dogs showed an increase in bleeding times with heparin and rt-PA and then a greater increase to 1.5-to 2-fold baseline as clopidogrel entered the circulation between 4 and 6 hours (Fig. 4). After 24 hours, the differences in bleeding were significant for clopidogrel compared with baseline and APT102 (Fig. 4). Similar results were observed in mice. How APT102 can decrease clopidogrel-induced bleeding is unclear (fig. S11), but it will be important to examine potential interactions of these agents in preclinical safety studies and clinical trials because many patients are taking clopidogrel at the time of their AMI. Nonetheless, our data demonstrate that, compared to clopidogrel, APT102 provides additional efficacy without adding to the bleeding risk.
Given its rapid onset, optimal pharmacodynamic half-life, potent antiplatelet effect, and cardioprotection without an increased bleeding risk, APT102 could potentially change the adjunctive treatment of AMI. This treatment regimen may substantially preserve left ventricular myocardial function and improve long-term outcomes for patients.
MATERIALS AND METHODS
Study design
Predefined study components
Previous data indicated that five animals were required in each group of dogs to detect a 50% difference in infarcted area with 80% power and type I error probability of 0.05. Dogs that failed to achieve reperfusion with rt-PA were excluded from analysis.
Rationale and design of study
The overall objective of the study was to determine whether APT102 more effectively decreases early reocclusion and improves 24-hour patency after coronary fibrinolysis and reduces myocardial reperfusion injury in dogs and reperfusion injury in mice with reduced risk of bleeding compared with clopidogrel. The primary end points were coronary patency (dogs) and myocardial infarction area (dogs and mice).
Randomization and blinding
Assignment to treatment groups was done in a randomized fashion. Investigators were blinded to the treatment group during analysis of flow and infarction data.
Replication
All groups are sufficiently powered to meet the desired objectives. Assays of platelet function and blood analytes were done in duplicate or triplicate as indicated.
In vivo methods
All procedures involving animals were approved by the Institutional Animal Care and Use Committees at Washington University or Harvard.
Pharmacodynamics of APT102 in dogs
Time courses (0 to 24 hours) of ADPase activity and ex vivo ADP-induced platelet aggregation in platelet-rich plasma were generated in dogs given APT102 (n = 2) or clopidogrel. APT102 was given as a single intravenous bolus (0.25, 0.5, 0.75, or 1.0 mg/kg), and a clinically relevant dose of clopidogrel (Plavix, 4.0 mg/kg, Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership) was given by orogastric gavage (tablets were ground to a fine powder and administered as a slurry in 20 to 30 ml of 0.9% saline). Blood samples were collected serially in heparinized tubes. Pharmacodynamics was assessed by measurement of ADPase activity and ADP-induced platelet aggregation in platelet-rich plasma after intravenous APT102 or oral clopidogrel.
AMI and coronary fibrinolysis in dogs
Coronary plaque rupture leading to AMI in a patient was simulated by electrolytic injury to the LAD coronary artery in conscious dogs instrumented 1 week previously as described (39). The experimental protocol is shown in fig. S9. Briefly, APT102 was administered intravenously 55 min after thrombotic occlusion of the coronary artery as assessed by continuous measurement of coronary flow. Blood pressure was measured in the first 10 min to verify stability. Aspirin (5.0 mg/kg) and clopidogrel (Plavix, 4.0 mg/kg) were administered orally, and unfractionated heparin was given intravenously as a bolus (100 IU/kg) at 55 min and then infused continuously (50 IU/kg per hour) for 125 min. Fibrinolysis was induced with intravenous rt-PA [Activase (1.0 mg/kg)] infused over 60 min, and blood flow monitoring was continued for 24 hours (Fig. 3 and fig. S10). Buccal mucosa bleeding times were measured serially with a spring-loaded Simplate blade device applied to the inner lip as an index of bleeding risk (39). The time from the cut to the end of bleeding from the wound was taken as the bleeding time. PT and aPTT were measured serially to assess coagulation. After 24 hours of reperfusion, infarct size was determined by Evans blue and TTC double staining.
Coronary occlusion and release in mice (ischemia-reperfusion injury)
Myocardial ischemia was induced in anesthetized C57BL/6J mice by temporary ligation of the LAD coronary artery (23). The mice were separated into the following treatment groups (n = 12 to 33 per group): saline, 5 min before a 60-min interval of ischemia; APT102 (1.0 mg/kg, intravenously), 5 min before 60-min interval of ischemia; APT102 (1.0 mg/kg, intravenously), 50 min after ischemia and 10 min before reperfusion; clopidogrel (10 mg/kg, orally), 24 hours before the 60-min interval of ischemia; and clopidogrel (10 mg/kg, orally), 24 hours before the 60-min interval of ischemia followed by APT102 (1.0 mg/kg, intravenously) 5 min before 60-min interval of ischemia. Reperfusion was induced by removal of the ligature, the chest was closed, and the animals recovered from anesthesia. After 24 hours of reperfusion, bleeding time was measured while the mice were anesthetized with isoflurane. A razor was used to transect 0.5 cm of the distal tip of the tail, and the tail was immediately placed into a tube of prewarmed saline (37°C). Hearts were then excised, and the area at risk and infarcted area were determined by Evans blue perfusion and triphenyltetrazolium staining.
Statistical analysis
All data are presented as means ± SEM or SD as indicated. Gaussian distribution was assessed by the D’Agostino-Pearson normality test. Two-group comparisons were performed with Student’s two-tailed t test. One-way ANOVA with Tukey’s post hoc test was used to perform multiple-group comparisons. Serial data were analyzed using a general linear model for repeated-measures ANOVA (SigmaStat v.3.11, Systat Software Inc.). Statistical differences with two-tailed probability values of P < 0.05 were considered significant. All data were analyzed using Excel (Microsoft).
Supplementary Material
Acknowledgments
We thank P. Baum and S. Grathwohl for veterinary technical assistance with the dog preparation, F. Katchi for help with the data analysis, and R. Leadley, Ph.D., for advice during the planning stages of the studies. Funding: This work was supported by NIH grant HL095169 (to R.C. and D.A.) and APT Therapeutics Inc. fund.
Footnotes
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/6/248/248ra105/DC1
Materials and Methods
Fig. S1. Design of human ADPase-enhanced apyrases.
Fig. S2. Comparison of the enzymatic activities of APT102 and solCD39.
Fig. S3. Inhibition by APT102 of ADP-induced aggregation in human platelet-rich plasma.
Fig. S4. In vitro inhibition by APT102 of ADP-induced aggregation of rabbit, pig, and dog platelet-rich plasma.
Fig. S5. Effects of clopidogrel and prasugrel prodrugs on inhibition of ADP-induced aggregation of canine platelet-rich plasma achieved with recombinant solCD39 and APT102.
Fig. S6. Purification of APT102 from the supernatant of CHO cells stably transfected with the APT102 gene.
Fig. S7. Pharmacokinetics of APT102 in dogs.
Fig. S8. Pharmacodynamics of APT102 and clopidogrel in dogs.
Fig. S9. Experiment protocol for dogs undergoing coronary occlusion and fibrinolysis.
Fig. S10. Coronary artery blood flow in dogs before and after occlusion and fibrinolysis.
Fig. S11. Bleeding times in mice after clopidogrel with or without added APT102.
Table S1. Kinetic parameters of wild-type and mutant solCD39L3 and solCD39 toward ADP and ATP.
Table S2. In vitro inhibition of ADP-induced platelet aggregation with APT102.
Table S3. Coagulation parameters versus time after coronary occlusion and fibrinolysis in dogs.
Table S4. Hematologic values at baseline and 24 hours after coronary occlusion and fibrinolysis in individual dogs.
Table S5. Serum chemistry values at baseline and 24 hours after coronary occlusion and fibrinolysis in individual dogs.
Author contributions: D.M. designed and performed the experiments and helped write the manuscript. S.S.J. designed and performed the experiments and helped write the manuscript. X.S. designed and performed the experiments. M.J.B. designed and performed the experiments. A.N. designed and performed the experiments. J.H.F.D. designed and performed the experiments. A.J.M. designed the experiments and helped write the manuscript. S.C.R. designed the experiments and helped write the manuscript. R.C. designed and performed the experiments and helped write the manuscript. D.A. designed and performed the experiments and helped write the manuscript.
Competing interests: R.C. is a founder of, and both R.C. and S.S.J. have equity interest in, APT Therapeutics Inc., which holds five issued patents and the commercial rights for APT102. The patents are as follows: “Therapeutic apyrase constructs, apyrase agents, and production,” US8771683 (2014); “Apyrase therapy for bleeding conditions,” US8535662 (2013); “Therapeutic use of ADPase enhanced apyrase,” US8021866 (2011); “Design and therapeutic use of ADPase-enhanced apyrase,” US7390485 (2008); “Therapeutics use of soluble CD39L3,” US7247300 (2007). The other authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. American Heart Association Statistics Committee and Stroke Statistics Subcommittee, Executive summary: Heart disease and stroke statistics—2013 update: A report from the American Heart Association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 2.Kushner FG, Hand M, Smith SC, Jr, King SB, III, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE, Green LA, Hochman JS, Jacobs AK, Krumholz HM, Morrison DA, Ornato JP, Pearle DL, Peterson ED, Sloan MA, Whitlow PL, Williams DO. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:2271–2306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 3.Mason PJ, Jacobs AK, Freedman JE. Aspirin resistance and atherothrombotic disease. J. Am. Coll. Cardiol. 2005;46:986–993. doi: 10.1016/j.jacc.2004.08.070. [DOI] [PubMed] [Google Scholar]
- 4.Wallentin L. P2Y12 inhibitors: Differences in properties and mechanisms of action and potential consequences for clinical use. Eur. Heart J. 2009;30:1964–1977. doi: 10.1093/eurheartj/ehp296. [DOI] [PubMed] [Google Scholar]
- 5.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 6.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Freij A, Thorsén M PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 7.Mehran R, Pocock SJ, Stone GW, Clayton TC, Dangas GD, Feit F, Manoukian SV, Nikolsky E, Lansky AJ, Kirtane A, White HD, Colombo A, Ware JH, Moses JW, Ohman EM. Associations of major bleeding and myocardial infarction with the incidence and timing of mortality in patients presenting with non-ST-elevation acute coronary syndromes: A risk model from the ACUITY trial. Eur. Heart J. 2009;30:1457–1466. doi: 10.1093/eurheartj/ehp110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thel MC, Califf RM, Tardiff BE, Gardner LH, Sigmon KN, Lincoff AM, Topol EJ, Kitt MM, Blankenship JC, Tcheng JE. Timing of and risk factors for myocardial ischemic events after percutaneous coronary intervention (IMPACT-II). Integrilin to minimize platelet aggregation and coronary thrombosis. Am. J. Cardiol. 2000;85:427–434. doi: 10.1016/s0002-9149(99)00767-5. [DOI] [PubMed] [Google Scholar]
- 9.Yellon DM, Hausenloy DL. Myocardial reperfusion injury. N. Engl. J. Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 10.Montalescot G, Wiviott SD, Braunwald E, Murphy SA, Gibson CM, McCabe CH, Antman EM TRITON-TIMI 38 investigators. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): Double-blind, randomised controlled trial. Lancet. 2009;373:723–731. doi: 10.1016/S0140-6736(09)60441-4. [DOI] [PubMed] [Google Scholar]
- 11.Jackson SP, Schoenwaelder SM. Antiplatelet therapy: In search of the ‘magic bullet’. Nat. Rev. Drug Discov. 2003;2:775–789. doi: 10.1038/nrd1198. [DOI] [PubMed] [Google Scholar]
- 12.Kaczmarek E, Koziak K, Sévigny J, Siegel JB, Anrather J, Beaudoin AR, Bach FH, Robson SC. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J. Biol. Chem. 1996;271:33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- 13.Marcus AJ, Broekman MJ, Drosopoulos JH, Islam N, Alyonycheva TN, Safier LB, Hajjar KA, Posnett DN, Schoenborn MA, Schooley KA, Gayle RB, Maliszewski CR. The endothelial cell ecto-ADPase responsible for inhibition of platelet function is CD39. J. Clin. Invest. 1997;99:1351–1360. doi: 10.1172/JCI119294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcus AJ, Broekman MJ, Drosopoulos JH, Olson KE, Islam N, Pinsky DJ, Levi R. Role of CD39 (NTPDase-1) in thromboregulation, cerebroprotection, and cardioprotection. Semin. Thromb. Hemost. 2005;31:234–246. doi: 10.1055/s-2005-869528. [DOI] [PubMed] [Google Scholar]
- 15.Robson SC, Wu Y, Sun X, Knosalla C, Dwyer K, Enjyoji K. Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin. Thromb. Hemost. 2005;31:217–233. doi: 10.1055/s-2005-869527. [DOI] [PubMed] [Google Scholar]
- 16.Knowles AF. The GDA1_CD39 superfamily: NTPDases with diverse functions. Purinergic Signal. 2011;7:21–45. doi: 10.1007/s11302-010-9214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonioli L, Pacher P, Vizi ES, Haskó G. CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 2013;19:355–367. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gachet C. P2 receptors, platelet function and pharmacological implications. Thromb. Haemost. 2011;99:466–472. doi: 10.1160/TH07-11-0673. [DOI] [PubMed] [Google Scholar]
- 19.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5’-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Kumar V, Sharma A. Adenosine: An endogenous modulator of innate immune system with therapeutic potential. Eur. J. Pharmacol. 2009;616:7–15. doi: 10.1016/j.ejphar.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Enjyoji K, Sévigny J, Lin Y, Frenette PS, Christie PD, Esch JS, Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat. Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 22.Guckelberger O, Sun XF, Sévigny J, Imai M, Kaczmarek E, Enjyoji K, Kruskal JB, Robson SC. Beneficial effects of CD39/ecto-nucleoside triphosphate diphosphohydrolase-1 in murine intestinal ischemia-reperfusion injury. Thromb. Haemost. 2004;91:576–586. doi: 10.1160/TH03-06-0373. [DOI] [PubMed] [Google Scholar]
- 23.Köhler D, Eckle T, Faigle M, Grenz A, Mittelbronn M, Laucher S, Hart ML, Robson SC, Müller CE, Eltzschig HK. CD39/ectonucleoside triphosphate diphosphohydrolase-1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation. 2007;116:1784–1794. doi: 10.1161/CIRCULATIONAHA.107.690180. [DOI] [PubMed] [Google Scholar]
- 24.Cai M, Huttinger ZM, He H, Zhang W, Li F, Goodman LA, Wheeler DG, Druhan LJ, Zweier JL, Dwyer KM, He G, d’Apice AJ, Robson SC, Cowan PJ, Gumina RJ. Transgenic over expression of ectonucleotide triphosphate diphosphohydrolase-1 protects against murine myocardial ischemic injury. J. Mol. Cell. Cardiol. 2011;51:927–935. doi: 10.1016/j.yjmcc.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wheeler DG, Joseph ME, Mahamud SD, Aurand WL, Mohler PJ, Pompili VJ, Dwyer KM, Nottle MB, Harrison SJ, d’Apice AJ, Robson SC, Cowan PJ, Gumina RJ. Transgenic swine: Expression of human CD39 protects against myocardial injury. J. Mol. Cell. Cardiol. 2012;52:958–961. doi: 10.1016/j.yjmcc.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinsky DJ, Broekman MJ, Peschon JJ, Stocking KL, Fujita T, Ramasamy R, Connolly ES, Huang J, Kiss S, Zhang Y, Choudhri TF, McTaggart RA, Liao H, Drosopoulos JH, Price VL, Marcus AJ, Maliszewski CR. Elucidation of the thromboregulatory role of CD39/ectoapyrase in the ischemic brain. J. Clin. Invest. 2002;109:1031–1040. doi: 10.1172/JCI10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belayev L, Khoutorova L, Deisher TA, Belayev A, Busto R, Zhang Y, Zhao W, Ginsberg MD. Neuroprotective effect of SolCD39, a novel platelet aggregation inhibitor, on transient middle cerebral artery occlusion in rats. Stroke. 2003;34:758–763. doi: 10.1161/01.STR.0000056169.45365.15. [DOI] [PubMed] [Google Scholar]
- 28.Drosopoulos JHF, Kraemer R, Shen H, Upmacis RK, Marcus AJ, Musi E. Human solCD39 inhibits injury-induced development of neointimal hyperplasia. Thromb. Haemost. 2010;103:426–434. doi: 10.1160/TH09-05-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivanenkov VV, Sevigny J, Kirley TL. Trafficking and intracellular ATPase activity of human ecto-nucleotidase NTPDase3 and the effects of ER-targeted NTPDase3 on protein folding. Biochemistry. 2008;47:9184–9197. doi: 10.1021/bi800402q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lecka J, Rana MS, Sévigny J. Inhibition of vascular ectonucleotidase activities by the prodrugs ticlopidine and clopidogrel favours platelet aggregation. Br. J. Pharmacol. 2010;161:1150–1160. doi: 10.1111/j.1476-5381.2010.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chadwick BP, Frischauf AM. The CD39-like gene family: Identification of three new human members (CD39L2, CD39L3, and CD39L4), their murine homologues, and a member of the gene family from Drosophila melanogaster. Genomics. 1998;50:357–367. doi: 10.1006/geno.1998.5317. [DOI] [PubMed] [Google Scholar]
- 32.Chen R, Jeong SS. Functional prediction: Identification of protein orthologs and paralogs. Protein Sci. 2000;9:2344–2353. doi: 10.1110/ps.9.12.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drosopoulos JH, Broekman MJ, Islam N, Maliszewski CR, Gayle RB, III, Marcus AJ. Site-directed mutagenesis of human endothelial cell ecto-ADPase/soluble CD39. Requirement of glutamate 174 and serine 218 for enzyme activity and inhibition of platelet recruitment. Biochemistry. 2000;39:6936–6943. doi: 10.1021/bi992581e. [DOI] [PubMed] [Google Scholar]
- 34.Ivanenkov VV, Meller J, Kirley TL. Characterization of disulfide bonds in human nucleoside triphosphate diphosphohydrolase 3 (NTPDase3): Implications for NTPDase structural modeling. Biochemistry. 2005;44:8998–9012. doi: 10.1021/bi047487z. [DOI] [PubMed] [Google Scholar]
- 35.Kabsch W, Mannherz HG, Suck D, Pai EF, Holmes KC. Atomic structure of the actin: DNase I complex. Nature. 1990;347:37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- 36.Yang F, Hicks-Berger CA, Smith TM, Kirley TL. Site-directed mutagenesis of human nucleoside triphosphate diphosphohydrolase 3: The importance of residues in the apyrase conserved regions. Biochemistry. 2001;40:3943–3950. doi: 10.1021/bi002711f. [DOI] [PubMed] [Google Scholar]
- 37.Bonello R, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, Bhatt DL, Cattaneo M, Collet JP, Cuisset T, Gachet C, Montalescot G, Jennings LK, Kereiakes D, Sibbing D, Trenk D, Van Werkum JW, Paganelli F, Price MJ, Waksman R, Gurbel PA Working Group on High On-Treatment Platelet Reactivity. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J. Am. Coll. Cardiol. 2010;56:919–933. doi: 10.1016/j.jacc.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 38.Bleck GT. An alternative method for the rapid generation of stable high-expressing mammalian cell lines. BioProcess. J. 2006;5:1–7. [Google Scholar]
- 39.Abendschein DR, Baum PK, Verhallen P, Eisenberg RR, Sullivan ME, Light DR. A novel synthetic inhibitor of factor Xa decreases early reocclusion and improves 24-h patency after coronary fibrinolysis in dogs. J. Pharmacol. Exp. Ther. 2001;296:567–572. [PubMed] [Google Scholar]
- 40.Kloner RA. No-reflow phenomenon: Maintaining vascular integrity. J. Cardiovasc. Pharmacol. Ther. 2011;16:244–250. doi: 10.1177/1074248411405990. [DOI] [PubMed] [Google Scholar]
- 41.St Pierre J, Yang LY, Tamirisa K, Scherrer D, De Ciechi P, Eisenberg E, Tolunay D, Abendschein D. Tissue factor pathway inhibitor attenuates procoagulant activity and upregulation of tissue factor at the site of balloon-induced arterial injury in pigs. Arterioscler. Thromb. Vasc. Biol. 1999;19:2263–2268. doi: 10.1161/01.atv.19.9.2263. [DOI] [PubMed] [Google Scholar]
- 42.Coller BS. Platelets and thrombolytic therapy. N. Engl. J. Med. 1990;322:33–42. doi: 10.1056/NEJM199001043220107. [DOI] [PubMed] [Google Scholar]
- 43.Haskel EJ, Torr SR, Day KC, Palmier MO, Wun TC, Sobel BE, Abendschein D. Prevention of arterial reocclusion after thrombolysis with recombinant lipoprotein-associated coagulation inhibitor. Circulation. 1991;84:821–827. doi: 10.1161/01.cir.84.2.821. [DOI] [PubMed] [Google Scholar]
- 44.Maayani JS, Tagliente TM, Schwarz T, Martinelli G, Martinez R, Shore-Lesserson L. The balance of concurrent aggregation and deaggregation processes in platelets is linked to differential occupancy of ADP receptor subtypes. Platelets. 2001;12:83–93. doi: 10.1080/09537100020032846. [DOI] [PubMed] [Google Scholar]
- 45.Stephens G, He M, Wong C, Jurek M, Luedemanna HC, Shapurian G, Munnelly K, Muir C, Conley PB, Philips DR, Andre P. Development of a perfusion chamber assay to study in real time the kinetics of thrombosis and the antithrombotic characteristics of antiplatelet drugs. Thromb. J. 2012;10:11. doi: 10.1186/1477-9560-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lunkes GI, Lunkes DS, Leal D, Araujo MDC, Correa M, Becker L, Rosa CSD, Morsch VM, Schetinger MRC. Effect of high glucose levels in human platelet NTPDase and 5’-nucleotidase activities. Diabetes Res. Clin. Pract. 2008;81:351–357. doi: 10.1016/j.diabres.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Headrick JP. Ischemic preconditioning: Bioenergetic and metabolic changes and the role of endogenous adenosine. J. Mol. Cell. Cardiol. 1996;28:1227–1240. doi: 10.1006/jmcc.1996.0113. [DOI] [PubMed] [Google Scholar]
- 48.Sugimoto SS, Lin X, Lai J, Okazaki M, Das NA, Li W, Krupnick AS, Chen R, Jeong SS, Patterson GA, Kreisel D, Gelman AE. Apyrase treatment prevents ischemia–reperfusion injury in rat lung isografts. J. Thorac. Cardiovasc. Surg. 2009;138:752–759. doi: 10.1016/j.jtcvs.2009.04.049. [DOI] [PubMed] [Google Scholar]
- 49.Uluçkan Ö, Eagleton MC, Floyd DH, Morgan EA, Hirbe AC, Kramer M, Dowland N, Prior JL, Piwnica-Worms D, Jeong SS, Chen R, Weilbaecher K. APT102, a novel adpase, cooperates with aspirin to disrupt bone metastasis in mice. J. Cell. Biochem. 2008;104:1311–1323. doi: 10.1002/jcb.21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baykov AA, Evtushenko OA, Avaeva SM. A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal. Biochem. 1988;171:266–270. doi: 10.1016/0003-2697(88)90484-8. [DOI] [PubMed] [Google Scholar]
- 51.Marcus AJ, Broekman MJ, Drosopoulos JH, Islam N, Pinsky DJ, Sesti C, Levi R. Metabolic control of excessive extracellular nucleotide accumulation by CD39/ecto-nucleotidase- 1: Implications for ischemic vascular diseases. J. Pharmacol. Exp. Ther. 2003;305:9–16. doi: 10.1124/jpet.102.043729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.