Abstract
CONTEXT
NRG1 is a schizophrenia candidate gene and plays an important role in brain development and neural function. Schizophrenia is a complex disorder, with etiology likely due to epistasis.
OBJECTIVE
We sought to examine epistasis between NRG1 and selected NMDA-glutamate pathway partners implicated in its effects, including ERBB4, AKT1, DLG4, NOS1, NOS1AP.
DESIGN
Schizophrenia case-control sample analyzed using machine learning algorithms and logistic regression with follow-up using neuroimaging on an independent sample of healthy controls.
PARTICIPANTS
A referred sample of schizophrenic patients (N = 296) meeting DSM-IV criteria for schizophrenia-spectrum disorder and a volunteer sample of controls for case-control comparison (N = 365) and a separate volunteer sample of controls for neuroimaging (N = 172).
MAIN OUTCOME MEASURES
Epistatic association between SNPs and case-control status; epistatic association between SNPs and the BOLD physiological response during working memory measured by functional magnetic resonance imaging (fMRI).
RESULTS
We observed interaction between NRG1 5’ and 3’ SNPs: rs4560751-rs3802160 (likelihood ratio test (LRT) p=0.00020) and schizophrenia which was validated using fMRI of working memory in healthy controls; carriers of risk-associated genotypes showed inefficient processing in dorsolateral prefrontal cortex (DLPFC) (p=0.015, FWE corrected). We observed epistasis between NRG1 (rs10503929; Val1066Ile) and its receptor ERBB4 (rs1026882; LRT p=0.035); a three-way interaction with these two SNPs and AKT1 (rs2494734) was also observed (OR=27.13; 95% confidence interval 3.30, 223.03; LRT p=0.042). These same two- and three-way interactions were further biologically validated via fMRI: healthy individuals carrying risk genotypes for NRG1 and ERBB4, or these two together with AKT1, were disproportionately less efficient in DLPFC processing. Lower-level interactions were not observed between NRG1/ERBB4-AKT1 in association or neuroimaging, consistent with biological evidence that NRG1-ERBB4 interaction modulates downstream AKT1 signaling.
CONCLUSIONS
Our data suggest complex epistatic effects implicating a NRG1 molecular pathway in cognitive brain function and the pathogenesis of schizophrenia.
Neuregulin 1 (NRG1) is considered a schizophrenia candidate gene because of its chromosomal position within a linkage peak, direct evidence supporting genetic association to various polymorphisms1-12 and its biological role in brain development and neural function mediated partially via the N-methyl D-aspartate (NMDA)-glutamate pathway. The first association between NRG1 and schizophrenia described a risk-associated haplotype in an Icelandic sample (HAPICE) at the 5′ end1, which was replicated in a Scottish population2. Association has been confirmed at both the 5’ and 3’ ends3-12, although null studies have appeared13-20. A meta-analysis21 of HAPICE examined 13 independent studies and revealed a small effect size (OR = 1.22) but strong statistical association (p-value = 8.0e−10), though a nonconservative approach of combining p-values showed a significant, but weaker, association (p-value = 0.036)22. However, these meta-analyses do not take into account studies from the past two years nor do they consider recently-published GWAS data, which have provided less robust evidence. NRG1 spans nearly 1,125 kb, with multiple functional domains, raising the possibility of intragenic heterogeneity.
Although schizophrenia is thought to be a polygenic disorder, few studies of NRG1 epistasis have been conducted. One study reported interaction between HAPICE and a SNP in its receptor, v-erb-a erythroblastic leukemia viral oncogene homolog 4 (avian) (ERBB4)23. Another study also found evidence for interaction between NRG1-ERBB424 although at different ends of both genes. An additional study assessed 2-way interactions between SNPs in 8 genes related to the ErbB pathway, including NRG1 and ERBB425; the authors report significant interactions between SNPs in most gene pairs, intragenic interactions in NRG1 and suggestive evidence for interaction between ERBB4 and NRG1. However, none of these interactions were independently validated and recent data suggest that epistasis is likely the product of higher-order (i.e., more than two-SNP) interactions which this study25 was not designed to detect26. The search for higher order interactions places potentially insurmountable statistical hurdles on traditional approaches for genetic association even in very large datasets in the absence of specific hypotheses.
Machine learning algorithms (MLAs), developed for high-dimensional data, may be a viable approach to detecting higher order epistasis that avoid some of the obstacles of more traditional statistical approaches. We applied three algorithms based on classification trees which simultaneously model both main effects and interactions to an analysis of interactions of selected genes in the NRG1/NMDA network and risk for schizophrenia. An example of the creation of a forest of classification trees is shown in Figure 1. Essentially, with a case/control outcome, trees use the predictor variables (here SNPs) to partition the data, creating more homogeneous subsets containing only cases or controls. One MLA is the random forest (RF) algorithm27, which creates a forest of classification trees using recursive partitioning. An extension of RF is the conditional inference forest (CIF) algorithm28-29. CIF bases tree-building on the p-value after correction for multiplicity30. Estimates of association (called variable importance measures (VIs)) are obtained with these techniques using the observations not used in creation of each tree and thus are a measure of predictive ability. VIs are derived by comparing the observed accuracy of classification to permuted values under no association (H0). A third approach, Monte Carlo logic regression (MCLR)31, differs in a few ways: the algorithm used is reversible jump Markov chain Monte Carlo (rjMCMC)) and VI in MCLR is a count of the number of times a variable is selected into the model across the length of the Markov chain. Algorithms are described in more detail in Methods.
Figure 1.
Diagram showing random forest algorithm. Before growing a classification tree, steps 1 and 2 are performed, selecting a subset of cases/controls and a subset of SNPs from the full set of observations and predictors. In step 3, while the tree is being grown on the same subset of observations, different subsets of SNPs are selected at each node, allowing SNPs without strong main effects to participate in interactions. The goal of the recursive partitioning is to provide purer (i.e., only cases or only controls) daughter nodes. Because this can lead to overfitting of chance fluctuations in the data, the out-of-bag sample, which is independent of the observations used to grow each tree, is used to calculate the measures of variable importance, as shown in step 4 and the decrease in misclassification of cases and controls is calculated. After permuting case status in this same out-of-bag set (e.g., under H0) these observations are again run through the tree, and the difference in the misclassification rate for the observed versus permuted data is calculated. In step 5, we create a forest of these classification trees by repeating steps 1-4 5,000 times. Finally, the measures of variable importance are calculated for each SNP by taking the average of the decrease in classification performance calculated in step 4 across all the trees in the forest. Figure based on58.
We examined association with schizophrenia and markers in NRG1 and related genes in an ErbB4 signaling pathway and validated functional effects of associated SNP interactions by testing these interactions on brain function in normal subjects carrying risk-associated alleles using functional neuroimaging. We previously demonstrated that putative intermediate phenotypes at the level of brain information processing are robust reflections of biological mechanisms related to potential schizophrenia susceptibility genes32. Our primary emphasis was to examine epistasis, focusing on genes with prior evidence for association and conditional on established biological interaction between protein products encoded by each gene. Because power to show higher-order interactions is dependent on sample size and effect size, we restricted this study to a few genes in a specific NRG1-related biological network that has been implicated in schizophrenia33.
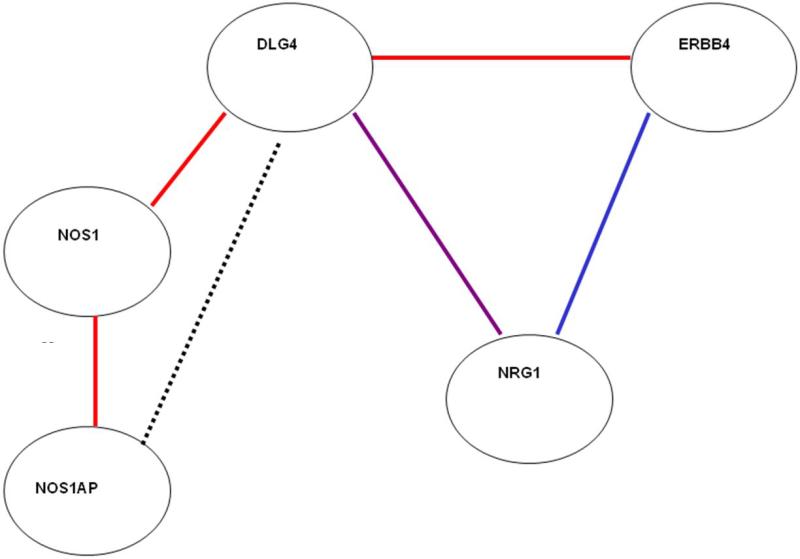
NRG1 is a complex gene with many biological effects. Its role as a potential susceptibility factor in schizophrenia may be related to a number of these functions, but its role in NMDA signaling has been of particular interest34-35. NRG1 pathway partners implicated in NMDA signaling were selected because they either directly interacted with neuregulin1: erbB436 (gene symbol: ERBB4, an NRG1 tyrosine kinase receptor), PSD-9537 (discs, large homolog 4 (Drosophila) ; DLG4); or they interacted directly with genes in the extended ERBB4-NMDA signaling pathway: PSD-95- nNOS138 (DLG4, nitric oxide synthase 1, (neuronal); NOS1), nNOS1-neuroneal nitric oxide synthase39 (NOS1, nitric oxide synthase 1 (neuronal) adaptor protein; NOS1AP), AKT33(v-akt murine thymoma viral proto-oncogene homolog 1; AKT1) (Figure 2). Only one of the partner genes selected has not shown significant independent evidence for association with schizophrenia in at least some prior studies (DLG433; see, for example: ERBB423, NOS1AP40, NOS141, AKT142) but was included because of the central role of PSD95 in NMDA function and also because of evidence of altered coupling of ErbB4 and DLG4 in schizophrenic brain33.
Figure 2.
NRG1 interaction partners. Line color denotes method of interaction detection: red = in vivo, in vitro, and yeast two-hybrid; blue = in vivo and in vitro, violet = in vitro, green = in vitro and yeast two-hybrid; black = competition for mutual protein binding partner.
Methods
The CBDB/NIMH Sibling Study
This study was conducted according to the principles expressed in the Declaration of Helsinki. The study was approved by the Institutional Review Board of the National Institute of Mental Health. All patients provided written informed consent for the collection of samples for genotyping and phenotyping information. Subjects were ascertained as part of the Clinical Brain Disorders Branch/National Institute of Mental Health Sibling Study. Potential probands met DSM-IV criteria for a broad diagnosis category (N = 296)43. Control individuals (N = 365) were ascertained from the NIH Normal Volunteer Office and were screened for psychiatric disorders as described elsewhere43. All subjects from this study who had available genotypes for the genes of interest were included. Controls and probands were between 18 and 60 years old, without significant medical problems or history of head trauma or recent history of drug and/or alcohol abuse, and had measured IQ (for affected individuals, premorbid IQ) of above 70. A research psychiatrist screened and interviewed all participants using the Structured Clinical Interview for the DSM-IV. All individuals self-identified as Caucasian.
The German Sample
Individuals with schizophrenia (N = 905) and unrelated controls (N = 1323) were ascertained from Munich, Germany and self-identified as Caucasian. The details of ascertainment, screening and diagnosis are described elsewhere44.
Genetic Marker Selection and Genotyping
We selected 49 markers in NRG1 based on reports of association with schizophrenia in previously published studies1-12 or using tagSNPs from Phase I of the International HapMap project (http://www.hapmap.org). SNPs in NOS1AP (N = 31), DLG4 (N = 14) and NOS1 (N = 4) were based on HapMap tagSNPs; SNPs in ERBB4 (N = 11) were selected on previously published associated SNPs22, likewise for AKT1 SNPs42 (N = 12), and HapMap tagSNPs within the associated region. TagSNPs were selected using two marker tagging from HAPLOVIEW, based on the minimal number of possible SNPs with an r2 cutoff value of 0.8 and minor allele frequency > 5%, and extended 10 kb 5’ and 5 kb 3’ of the gene, and were selected using the May 2004 build. In both datasets, blood was collected and DNA was extracted using standard methods. Genotypes for all SNPs were obtained using the Taqman 5’-exonuclease allelic discrimination assay (see Supplementary Data). Supplementary Table 1 lists all markers tested.
Statistical Methodologies
Single Gene Association Analyses
Testing for Hardy-Weinberg Equilibrium was performed using Fisher's Exact test. Case-control analyses were conducted to assess evidence for association between single SNPs in each gene and the NRG1 HAPICE haplotype and included 296 unrelated cases and 365 unrelated normal controls. Unconditional logistic regression was used to test for genotypic association between single SNPs, the R package haplo.stats was used to perform HAPICE haplotype analyses and plotting of –log10 (p-values) against an r2 linkage disequilibrium heatmap was performed using the R package snp.plotter. Reported single marker p-values were not adjusted for multiple testing.
Complex Epistasis – Machine Learning Approaches
We used 3 MLAs to assess complex interactions to find consensus across methods. We considered a SNP for follow-up if the median VI was in the top 10% of the distribution of all VI measures using > 1 algorithm. Missing genotypes were inferred using haplo.stats for individuals who had 80% or greater genotype data (N = 415; N cases = 220, N controls = 195). Cases were more likely to have genotype data that passed this threshold (75% of cases versus 53% of controls) due to using historical data. Among cases, the proportion of male and female cases passing the threshold did not differ (Fisher's exact p-value = 0.42) although the proportion of male controls was higher (59.8%) than the proportion of female controls (47.1%; Fisher's exact test p-value = 0.016). However, given the excess of males in the case sample passing the genotyping threshold (77.7%) we do not expect this excess of male controls to impact our results. Because permutation-based VIs may be slightly unstable45-46 we repeated each analysis 500 times to obtain medians. Further, analyses were performed on 500 null replicates where case status had been randomly permuted to obtain empirical p-values. Following MLA analysis, we performed two-way interaction modeling via logistic regression among the subset of SNPs considered influential by MLAs. Significance of the logistic regression models was obtained using a likelihood ratio test (LRT) comparing nested models; a reduced model containing main effect terms and a full model containing main effect and interaction terms.
Forest-Based Algorithms
Both RF27 (R package randomForest) and CIF29-30 (R package party) rely on multiple classification trees. To begin, a subset of predictors (here, SNPs, N = 10) is randomly sampled. In addition, a subsample (63.2%) of the observations (here, cases and controls) is selected for tree-building30. A single tree is created using recursive partitioning of the subset of predictors on the subsampled observations. RF terminates when no additional variables produce significant decreases in impurity or when the terminal node size is less than 10 observations; the stopping rule in CF uses the multiplicity-corrected p-value of the χ2 test. This process is repeated to create a forest of classification trees (N = 5,000). VI measures are calculated using the 36.8% left-aside independent observations; the observed mean decrease in accuracy of prediction of these independent observations per predictor versus that observed when case-control status has been permuted is averaged across all the trees in the forest. Large decreases in accuracy should be observed for associated predictors.
Monte Carlo Logic Regression
Monte Carlo logic regression31, (R package LogicReg) uses a classification tree design and, starting with a single particular tree, allows several moves during modeling which is conducted via an rjMCMC algorithm to find the best-fitting tree. Because MCLR accepts binary predictors, we recoded each SNP into two variables: one with the minor allele coded as dominant and one as recessive. MCLR used a burn-in interval of 10,000 and a Markov chain length (number of moves in finding the best tree) of 1,000,000. For consistency with RF/CIF the maximum model size was set to 2 trees/4 leaves (8 predictors). The VI measure is a count of the number of times a predictor is selected into a tree; associated predictors should be selected with greater frequency than unassociated predictors.
Functional magnetic resonance imaging
Subjects
Two samples of controls participated in 2 fMRI studies designed to explicitly test the effect of NRG1 intragenic epistasis and NRG1-ERBB4-AKT1 epistasis on the physiological BOLD response in brain during an N back working memory task. All available subjects who had completed the fMRI paradigm and had available genotypes were included. The two imaging samples were largely independent, overlapping to a small extent (28 subjects out of a total of 172). Within imaging samples, all SNPs were in Hardy-Weinberg equilibrium. For the NRG1 intragenic epistasis study (rs4560751- rs3802160), 87 subjects were included (Supplementary Table 2). The subgroups by rs4560751-rs3802160 (6 cells) did not differ by age, IQ, or 2-back accuracy or reaction time. This allows for a comparison of the genetic effect on brain physiology per se, i.e. how the brain handles the information without confounding by task performance. The genotype groups differed by gender: the subgroup rs4560751 1/2- rs3802160 2/2 (n=4) consisted of males only. Consequently, we added sex as a covariate in the fMRI design to adjust for any potential gender effect. For the NRG1-ERBB4-AKT1 epistasis study (rs10503929-rs1026882-rs2494734) 114 subjects were included. In this sample, there were no main effects or interactions of these 3 SNPs on age, gender, IQ and during behavioral performance on-task.
Task and Functional Image Processing
During the fMRI acquisition the participants performed the N-back task as previously described47. Whole brain BOLD fMRI data were acquired on a GE Signa 3T scanner (GE Systems; Milwaukee, WI) with a GE-EPI pulse sequence acquisition (24 contiguous axial slices of dimensions 3.75 × 3.75 × 6 mm; flip angle 90°; TR/TE 2000/ 30 ms; FOV- 24 cm; matrix 64 × 64 voxels). Images were processed with SPM5 software (http://www.fil.ion.ucl.ac.uk/) with realignment and correction for movement artifacts, spatial normalization in a standard stereotactic space (MNI template), smoothing with a 10 mm full width half maximum Gaussian filter. First level images for each subject were created by modeling the two experimental conditions (2B and 0B) as boxcars convolved with a canonical hemodynamic response. A contrast image for the 2B>0B contrast was estimated for each subject. These contrast images were used for a second- level random effect analysis. It was not possible to partition each genotype group across these epistatic analyses based on gender, thus we included gender as a covariate in all subsequent analyses. Additionally, we and other groups have shown that ‘risk’ alleles generate DLPFC inefficiency; thus we utilized a DLPFC ROI and one-sided t-tests where this directionality could reasonably be predicted for the interactions as indicated below (also, see Supplementary Methods)
Second-level image analysis
To study the epistatic effect of rs4560751 × rs3802160, a full-factorial model in SPM5 (2-way ANOVA) with two factors: rs4560751 (2 levels) and rs3802160 (3 levels), was employed. Correspondingly, 6 cells were created: rs4560751 (1/1) - rs3802160 (1/1; 1/2; 2/2) and rs4560751 (1/2) - rs3802160 (1/1; 1/2; 2/2). For rs450751 there were no minor allele homozygotes in our sample. Since we were specifically interested in the directionality of BOLD response intensity corresponding to the pattern of inefficiency on the background of risk alleles in the two SNPs, we tested this hypothesis by one-tailed t contrasts. To examine the effect of NRG1 (rs10503929) and ERBB4 (rs1026882) interaction, a 2-way ANOVA was constructed based on the risk architecture suggested by the clinical association, with individuals carrying homozygous major alleles considered at risk, in contrast to the minor allele carriers of each SNP. Significant brain activation was defined surviving p<0.05 corrected for number of voxels in this independently selected ROI. Finally, to examine the 3-SNP interaction of NRG1 and ERBB4 SNPs with AKT1 rs2494734, parameter estimates were extracted from the ROI peak showing the 2-way NRG1 by ERBB4 effect and conditioned on the AKT1 effect predicted by the clinical association in a 3-way ANOVA model, with significance set at p<0.05 in this higher-order analysis. We employed a nested ANOVA design with three factors: ‘gene’ (NRG1, ERBB4, AKT1), ‘genotype’ (1/1=homozygous for the major allele, 1/2 =heterozygous, 2/2= homozygous for the minor allele), and ‘risk’ nested within ‘genotype’ and ‘gene’ (1/1=risk genotype for NRG1 and ERBB4, 1/2-2/2=risk genotype for AKT1; 1/2-2/2=non-risk genotype for NRG1, ERBB4, 1/1=non-risk genotype for AKT1).
This secondary analysis was conducted in three steps: 1. We compared the non-risk group (no risk genotype) with each of the groups carrying the risk genotype in only one NRG1, ERBB4 or AKT1 gene; the non-risk group versus groups carrying the risk genotypes in 2 and 3 risk genotypes; the group with 3 risk genotypes versus all the other subjects collapsed in one group. We used one-tailed t contrasts for these comparisons and we report results surviving a statistical threshold of p<0.05 corrected within the predefined DLPFC ROIs according to the principles of Gaussian random fields48.
Results
Single Gene Analyses
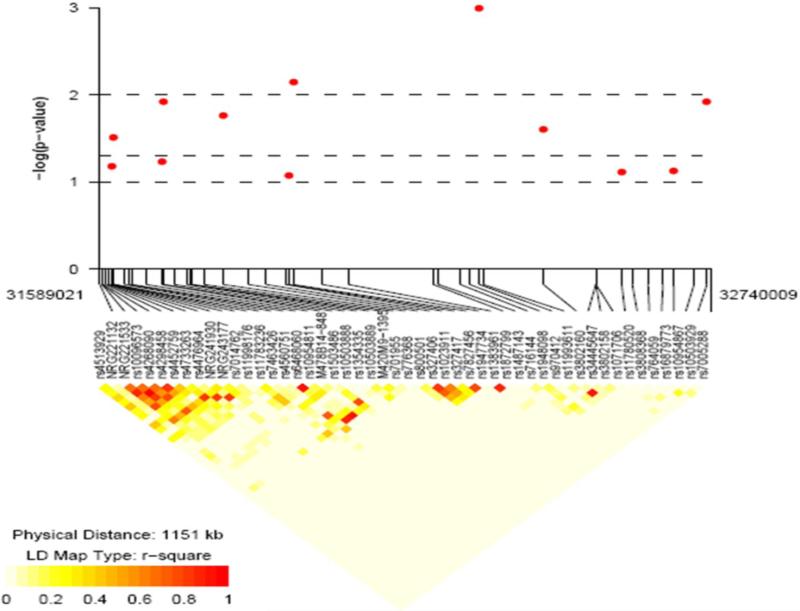
In NRG1, SNP8NRG221132, rs4560751 and rs327417 showed a trend toward deviation from HWE in controls (exact p-value = 0.043, 0.036 and 0.0085, respectively) and in cases rs3802158, rs3802160 and rs1023911 were out of HWE (exact p-value = 0.027, 0.0075 and 0.046, respectively); although both cases (exact p-value = 0.018) and controls (exact p-value = 0.035) showed deviation from HWE for rs1948098, this is likely due to strong association between this SNP and case status, so it was retained in all analyses. Further, recent studies have shown that although SNPs out of HWE may be less powerful, they do not appear to increase false positive findings49. Neither HapICE nor any single SNP within HapICE was significantly associated with schizophrenia (Supplementary Table 1). However, positive association was observed between the +2.0 allele at the 478B14-848 NRG1 microsatellite and schizophrenia (OR = 1.72 (1.16, 2.56), p-value 0.007) and for several SNPs 3’ to HapICE (Supplementary Table 1; Figure 3). Four SNPs in the 5’ end of NOS1AP showed evidence for association with schizophrenia (Supplementary Table 1) as did AKT1 SNP rs1130233. No significant association was observed in ERBB4, NOS1 or DLG4. We note that none of the single SNPs or microsatellite markers showed significant association with schizophrenia after correction for multiple testing.
Figure 3.
Association results for NRG1. Y-axis = -log10 p-values, X-axis = SNPs genotyped with lines showing physical location. Below association plot = linkage disequilibrium (r2) heatmap showing correlation between genotyped SNP.
Machine Learning Algorithm Results
In the first MLA analyses, 7 SNPs had median VI rankings in the top 10% by all 3 MLAs, including one SNP in the HAPICE region of NRG1 (rs4560751) and 4 SNPs located 3’ to the HAPICE region (rs1948098, rs3802160, rs3802158 and rs10503929); one SNP in ERBB4 (rs1026882) and one SNP in NOS1AP (rs7538490) (Table 1). Empirical p-values based on 500 null replicates revealed that only rs1026882 in ERBB4 was not significantly associated at the single SNP-only 0.05 level, although p-values estimated using MCLR and RF were suggestive (p-values = 0.094 and 0.052 for RF and MCLR, respectively). Because AKT1 is regulated by the upstream effects of the genes in the first analysis, a secondary analysis was performed adding AKT1, which produced virtually identical results as in Table 1 and also highlighted a SNP in AKT1, rs2494734 (p-values = 0.018 and < 0.002 for RF and CIF, respectively).
Table 1.
Median variable importance values and associated empirical p-values from 3 machine learning algorithms.
| Algorithm | Random Forest | Conditional Inference Forest | Monte Carlo Logic Regression | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | SNP | observed range |
median | permute range |
median empirical p-value |
observed range |
median | permute range |
median empirical p-value |
range | median | permute range |
median empirical p-value |
| NRG1 | rs4560751 | 0.59-1.07 | 0.835 | −0.45-1.17 | 0.01 | −2.4E-4 - 2.6E-3 | 0.0012 | −1.2E-3 - 3.0E-3 | 0.024 | 4.0-13.2 | 7.4 | 0.34-12.0 | 0.008 |
| NRG1 | rs1948098 | 0.98-1.45 | 1.23 | −0.49-0.95 | < 0.002 | 4.8E-4 - 3.4E-3 | 0.0021 | −1.2E-3 - 3.7E-3 | 0.004 | 54.2-65.1 | 59.3 | 1.1-1.3 | < 0.002 |
| NRG1 | rs3802160 | 0.34-0.83 | 0.56 | −0.49-0.81 | 0.012 | −2.3E-4 - 3.1E-3 | 0.0012 | −1.2E-3 - 2.8E-3 | 0.028 | 2.3-11.9 | 5.8 | 0.89-2.9 | < 0.002 |
| NRG1 | rs3802158 | 0.19-0.71 | 0.49 | −0.48-0.76 | 0.024 | −2.8E-4 - 2.5E-3 | 0.001 | −1.3E-3 - 2.9E-3 | 0.04 | 2.8-15.4 | 7.8 | 0.38-46.4 | 0.02 |
| NRG1 | rs10503929 | 0.22-0.69 | 0.5 | −0.48-1.26 | 0.018 | −1.6E-4 - 2.6E-3 | 0.0011 | −9.2E-4 - 2.9E-3 | 0.03 | 4.0-7.1 | 5 | 0.30-20.0 | 0.026 |
| ERBB4 | rs1026882 | 0.01-0.55 | 0.28 | −0.49-0.83 | 0.094 | −1.1E-3 - 2.1E-3 | 0.00054 | −1.3E-3 - 2.2E-3 | 0.13 | 2.6-4.2 | 3.2 | 0.38-20.4 | 0.052 |
| NOS1AP | rs7538490 | 0.20-0.73 | 0.45 | −0.43-1.15 | 0.018 | −4.4E-4 - 2.3E-3 | 0.00092 | −1.2E-2 - 2.3E-3 | 0.036 | 5.8-8.8 | 7.3 | 0.36-14.0 | 0.014 |
| AKT1 | rs2494734 | 0.27-0.77 | 0.52 | −0.49 - 2.04 | 0.018 | −4.8E-4 - 2.3E-3 | 0.00081 | −9.9E-4 2.7E-3 | < 0.002 | 0.6 - 1.9 | 0.95 | 0.46 - 90.4 | 0.70 |
We confirmed epistasis and estimated the effect size of 2-SNP interactions using logistic regression. Of the total 20 (7C2 = 21; one model could not be tested because of sparse cells) interactions performed, 4 were significant at the 0.05 level (expected = 1), and two of the LRT p-values testing intragenic interaction in NRG1 (rs4560751-rs3802160 and rs4560751-rs3802158) passed Bonferroni correction for 20 tests. In all, interactions were observed between rs4560751 and three 3’ SNPs in NRG1, and between the NRG1 3’ nonsynonymous SNP rs10503929 and the ERBB4 SNP rs1026882 (Table 2). Because AKT1 is downstream of and regulated by NRG1-ERRB4 interactions, we specifically tested whether a 3-way interaction between NRG1 SNP rs10503929, ERBB4 rs1026882 and AKT1 rs2494734 was present using logistic regression. In comparison to the reference group (N controls = 25, N cases = 14) who carried no risk genotypes at any of the 3 loci, we found 3.87-fold (95% CI; 1.20, 12.44; p-value = 0.023; N controls = 6, N cases = 13) increased risk for individuals carrying only the risk genotype at AKT1 rs2494734, a 2.38-fold (95% CI; 1.11, 5.09; p-value = 0.025; N controls = 45, N cases = 60) increased risk for those carrying only the risk genotype at NRG1 rs10503929, the same NRG1-ERBB4 interaction as above (OR = 3.27; 95% CI; 1.37-7.81, p-value = 0.0080; N controls = 18, N cases = 33) and a 27.13-fold (95% CI; 3.30, 223.03; p-value = 0.0020; N controls = 5, N cases = 17) increased risk for schizophrenia among individuals carrying all 3 risk genotypes versus those carrying no risk genotypes (LRT p-value = 0.042; p-value for model = 0.0082), suggesting the AKT1 interaction may be dependent on the NRG1-ERBB4 interaction. AKT1 did not statistically interact directly with ERBB4 or NRG1, which is interesting in relation to what is known about its biology33. No other genotype combination was statistically different from the reference group. Note that although cell sizes were all greater than 5, the wide confidence intervals indicate imprecision in estimation and warrant cautious interpretation. In the German case-control sample, we observed a marginally significant interaction (LRT p-value = 0.054) between NRG1 SNPs rs4560751 and rs3802158; however, the risk allele at rs3802158 was the opposite of the Sibling Study sample.
Table 2.
Significant 2-SNP interactions between SNPs selected via machine learning algorithms.
| Interaction | SNP1 | SNP2 | SNP1 risk genotype | SNP2 risk genotype | interaction OR | interaction 95% CI | LRT p-value |
|---|---|---|---|---|---|---|---|
| NRG1-NRG1 | rs4560751 | rs1948098 | G/G | A/A | 1.25 | 0.66, 2.39 | 0.016 |
| NRG1-NRG1 | rs4560751 | rs3802160 | G/G | T carrier | 4.56 | 1.97, 10.58 | 0.00020 |
| NRG1-NRG1 | rs4560751 | rs3802158 | T carrier | G carrier | 0.29 | 0.13, 0.61 | 0.00010 |
| NRG1-ERBB4 | rs10503929 | rs1026882 | C/C | A/A | 2.25 | 1.11, 4.54 | 0.035 |
Neuroimaging Results
To extend our genetic findings and test their validity at the level of human brain function, we tested whether epistatic effects on risk would show similar effects on aspects of brain function associated with increased risk for illness. In other words, if interactions between specific SNPs in these genes increase risk for schizophrenia, this happens presumably because the interaction increases the expression of biologic abnormalities in brain that also are associated with increased risk of manifest illness. We focused this level of analysis on a brain-based intermediate phenotype relevant for schizophrenia and human cognitive function47 - a pattern of inefficient activation in DLPFC revealed with an fMRI working memory paradigm (the N-back task) in normal subjects carrying risk-associated alleles. The fMRI efficiency response during this task has been shown to be heritable50, and increased activation for a fixed level of performance, i.e. “physiological inefficiency,” has been associated with increased genetic risk for schizophrenia47 and with genes implicated as schizophrenia susceptibility genes32. We tested two specific biologic hypotheses from the clinical data on the Blood Oxygenation Level-Dependent (BOLD) physiologic response in DLFPC during the N-back task47: epistasis between rs4560751 and rs3802160 within NRG1; (we tested only one of the three NRG1 intragenic models since three SNPs in the 3’ end of NRG1 were in LD) and 2- and 3-SNP epistasis between rs10503929-rs1026882-rs2494734 (NRG1-ERBB4-AKT1). For image presentation, a Statistical Parametric Map was overlaid onto ‘Single subject T1’ canonical image within SPM5 and the image is thresholded at p<0.05 uncorrected for display purposes only.
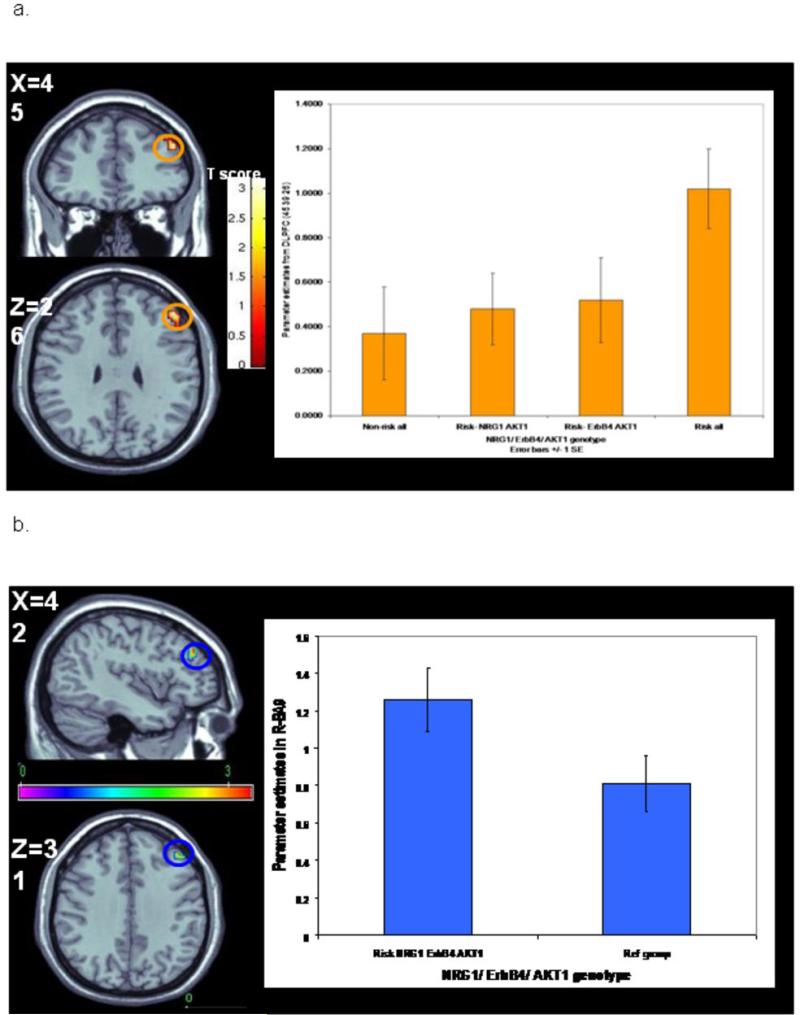
Epistatic effect of NRG1 rs4560751 x rs3802160 on DLPFC function
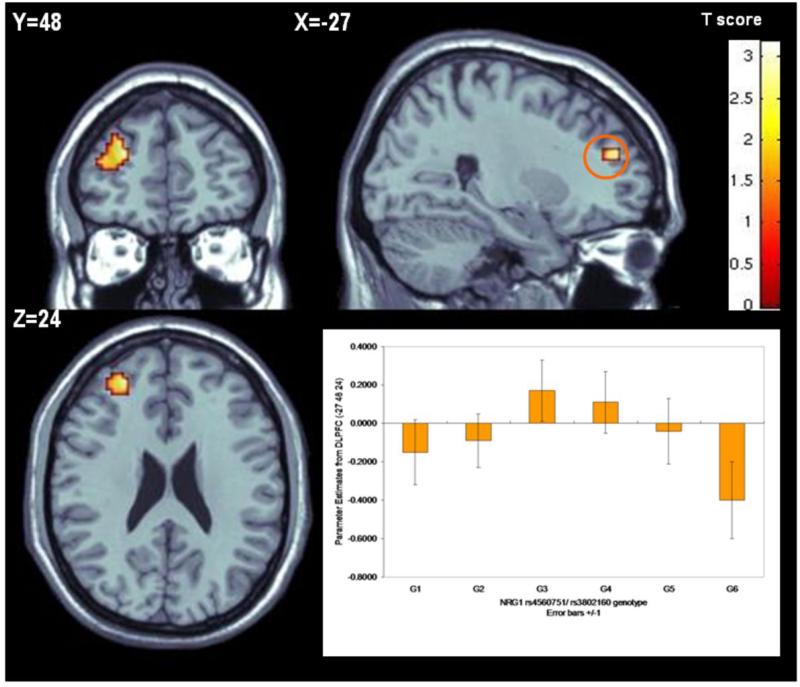
Consistent with the epistasis observed in the case-control sample, an interaction between rs4560751 and rs3802160 was observed in DLPFC (BA10) (Talairach coordinates: -27 48 20; Z score 3.07, p=0.025 FWE corrected) (Figure 4). Carriers of risk alleles at both genotypes revealed the most inefficient activation. On the background of rs4560751 major allele homozygosity, the mean BOLD response increased in allele dose-dependent fashion for rs38021160: 1/1<1/2<2/2 (Figure 4), while on the non-risk rs4560751 background, the opposite effect was observed.
Figure 4.
NRG1-NRG1 neuroimaging epistasis. On the background of the risk genotype for rs4560751 (1/1), the activation within left Brodmann area 10 (Talairach coordinates -27 48 24) increased in a dose dependent manner for risk allele carriers with the most inefficient processing (highest activation) for the rs3802160 homozygotes (G1-G3 groups: rs450751- 1/1; rs3802160- 1/1, 1/2, and 2/2 respectively; G4-G6 groups: rs4560751- 1/2; rs3802160- 1/1, 1/2, and 2/2 respectively).
Epistatic effects of NRG1-ERBB4-AKT1 SNPs on DLPFC function
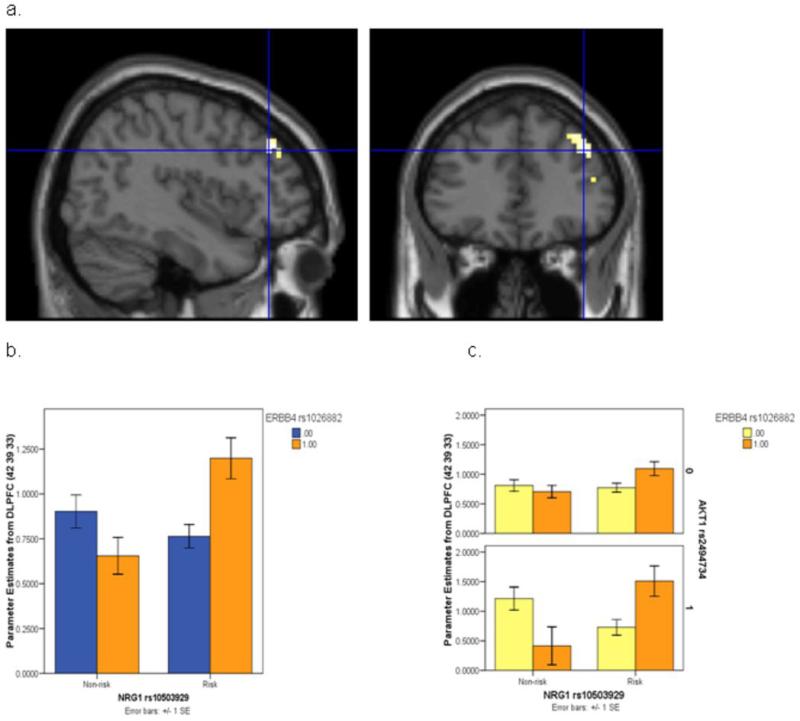
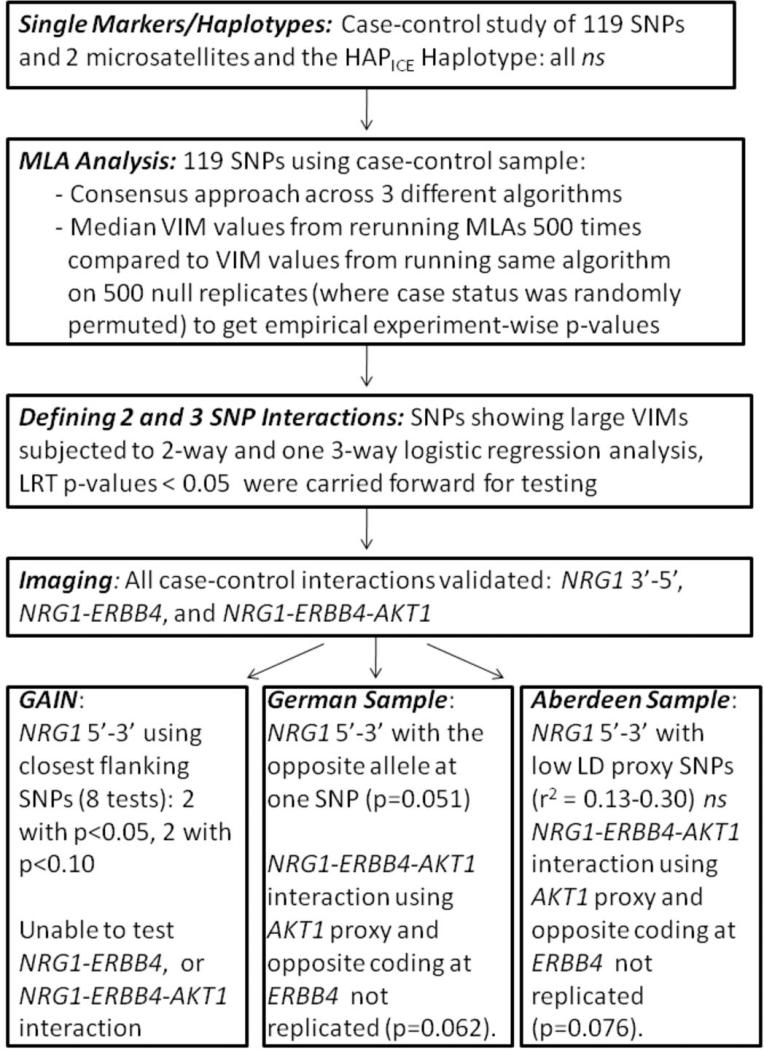
We investigated the combined physiologic effects of NRG1, ERBB4 and AKT1. In this sample (n=114), there were no main effects, 2-way or 3-way interaction effects of these 3 SNPs on age, IQ and behavioral performance on the task. A significant interaction of NRG1 rs10503929 and ERBB4 rs1026882 was observed at the DLPFC, where individuals who were homozygous for risk SNPs in both NRG1 and ERBB4 were disproportionately inefficient in cortical processing (peak 42 39 33, F(1,110)=11.308, z=3.20, p<0.001 uncorrected, p<0.05 FWE corrected; Figure 5). To explicitly test the prediction that conditioning on the AKT1 SNP would further impact the risk architecture of the NRG1 and ERBB4 SNPs on DLPFC function, as suggested by the 3-way interaction of association with schizophrenia, we performed a 3-way ANOVA on extracted parameter estimates from this same peak, which showed that the NRG1-ERBB4 epistasis was accentuated in the background of homozygous AKT1 risk alleles. Individuals who carried all 3 NRG1-ERBB4-AKT1 risk genotypes were disproportionately inefficient in DLPFC function at this location (F(1,106)=4.663, p=0.033; Figure 6). A subsequent analysis first compared DLPFC function in groups carrying one risk genotype at any of the three genes with the non-risk genotype carriers in all genes; none showed significant DLPFC inefficiency. We then compared groups carrying risk genotypes at NRG1-AKT1, ERBB4-AKT1 and all three risk genotypes with the non-risk genotype carriers. While none of the groups with 2 risk genotypes significantly differed from the non-risk group, the carriers of risk genotypes in all three genes (N = 4) combined demonstrated significantly greater activation compared with non-risk carriers (N = 17) in Right Middle Frontal Gyrus (BA46, Talairach coordinates 45 39 26; T score= 3.33; Z score=3.19, p=0.019 FWE corrected) (Figure 6a). The group with all 3 risk genotypes was also more inefficient compared to the group with 2 risk genotypes in NRG1 and AKT1 (BA9, Talairach coordinates 45 38 28; Z score=4.53; p=0.00002 FWE corrected, N = 12), to the group with 2 risk genotypes in ERBB4 and AKT1 (BA46, Talairach coordinates 45 39 26; Z score=3.01; p=0.03 FWE corrected, N = 3), and to all other subjects at right DLPFC (BA9, Talairach coordinates 42 39 31; Z score=3.23; p=0.015 FWE corrected N = 95) (Figure 6b). These findings dramatically support the clinical results which showed association with case status for the risk carriers in all the three genes but not for the risk carriers in NRG1-AKT1 or ERBB4-AKT1. A post hoc statistical analysis of this contrast (carriers of all 3 risk genotypes versus those carrying any other combination of genotypes) in the schizophrenia case-control sample revealed a 3.04-fold increase in risk (95% CI; 1.09, 8.45; p-value = 0.033) for those carrying all 3 risk-associated genotypes. Thus, each of the SNP interactions observed in the clinical datasets impacting on risk for schizophrenia showed consistent effects on cortical function in an independent sample of normal individuals carrying combinations of the same SNP genotypes.
Figure 5.
NRG1-ERBB4 neuroimaging epistasis. a. Impact of NRG1 rs10503929 by ERBB4 rs1026882 epistatic effect on DLPFC neural processing. b. Extracted parameter estimates from the DLPFC peak where individuals who were homozygous for the risk SNPs in both NRG1 and ERBB4 were disproportionately inefficient in cortical processing. c. Individuals who carried all 3 NRG1- ERBB4-AKT1 risk genotypes appeared disproportionately inefficient in DLPFC function at this same DLFPC location.
Figure 6.
NRG1-ERBB4-AKT1 neuroimaging epistasis. a. Risk genotype carriers in NRG1 (rs10503929; 1/1), ERBB4 (rs1026882; 1/1), AKT1 (rs2494734; 1/2-2/2) had significantly higher activity within right Brodmann area 46 (Talairach coordinates 45 39 26), compared with non-risk genotype carriers in all the three genes. b. Subjects with risk genotypes for NRG1, ERBB4, AKT1 have a significantly higher activity in DLPFC (right Brodmann area 9, Talairach coordinates: 42 39 31) during N back task.
Confirmatory Analyses in Public GWA Datasets
We searched a publically-available schizophrenia genome-wide association study dataset (GAIN) (http://www.ncbi.nlm.nih.gov/projects/gap/cgibin/study.cgi?study_id=phs000021.v2.p1)51 and the dataset described by Need et al. (2009)44 for replication of epistasis. The GAIN dataset consists of 1,172 cases with schizophrenia or schizoaffective disorder and 1,378 controls of European Ancestry and was genotyped on the Affymetrix 6.0 platform containing nearly 1M SNPs. The Need et al.44 dataset was comprised of two separately-collected datasets: one from Germany (N cases = 630, N controls = 547) and one from Aberdeen (N cases = 669, N controls = 597); cases in both studies had to qualify for diagnosis of schizophrenia under both DSM-IV and ICD-10. The German sample was genotyped using the Illumina HumanHap300 chip (with over 300,000 SNPs) and the Aberdeen sample was genotyped on the Illumina HumanHap550 chip (containing over 555,000 SNPs). None of our VI SNPs from MLA analyses were genotyped in the GAIN sample. Imputation of these SNPs in GAIN could not be performed as only one SNP participating in epistasis was found in HapMap. Therefore, we tested the closest flanking SNPs in the GAIN sample, assuming these would be among the most likely to show LD with our SNPs or with an unknown causal variant, although we cannot confirm this because our MLA SNPs and the flanking SNPs in the GAIN sample have not been genotyped on the same sample, so we cannot estimate LD between them. Using the GAIN data and flanking SNPs for our 5’-3’ epistasis SNPs within NRG1 (a total of 6 SNPs – no GAIN SNPs were found between rs3802160 and rs3802158), after performing 8 possible 2-SNP interaction tests, we observed two interactions with LRT p-values < 0.05 (rs7812662-rs7830691 (0.024) and rs10098401-rs17631978 (0.042)), uncorrected, and two interaction models with suggestive evidence for interaction (p-values < 0.1; rs7812662-rs6468119 (0.079) and rs7812662-rs17631978 (0.094)); none of the 6 SNPs were individually associated (p-values > 0.10). The expected number of tests showing p-values < 0.05 for 8 independent tests is 0.4 and the number expected to show p-values < 0.10 is 0.8; we observed 2 and 4, respectively. Thus, in 4 independent samples reported to date (the present study, German, and GAIN samples and the sample reported in Benzel et al.25) modest evidence for epistasis between SNPs in the 5’ and 3’ regions of NRG1 has been observed (see Supplementary Data). Because of low MAF at the closest flanking ERBB4 SNPs in this dataset, we were unable to test the two- and three-way interaction between NRG1-ERBB4-AKT1 (for details, please see Supplementary Data).
In the data described by Need et al.44, consisting of an independent sample from Aberdeen (AS) and an overlapping sample from Germany (GS) to our German samples, the NRG1 and ERBB4 SNPs participating in the three-way interaction were genotyped; however, additional SNPs were not, and, as above, were not genotyped in HapMap. The SNPs genotyped in the Need et al.44 data overlapped with SNPs genotyped in our data, thus proxies were selected using LD from our control sample for the intragenic NRG1 interacting SNPs and the AKT1 SNP. While there were trends for association with schizophrenia at 4 of the 5 single SNPs tested, we did not replicate our interactions in this pooled dataset due to allelic heterogeneity of the SNPs selected and very modest LD between the SNPs selected as proxies and our SNPs (see Supplementary Data). However, in the separate datasets, individuals carrying all 3 risk genotypes (using the Aberdeen coding of risk genotypes at ERBB4) did show marginally increased risk for schizophrenia (OR = 1.60, 95% CI 0.98, 2.61, p-value = 0.062) versus those carrying no risk genotypes in the GS; the same pattern of results was found in the AS (LRT p-value = 0.076) with individuals carrying all three risk genotypes showing increased risk (OR = 1.91, 95% CI 0.98, 3.69, p-value = 0.056). The NRG1 5’-3’ interaction was not replicated in the AS, possibly due to the low LD between the best proxy SNPs and our epistasis SNPs (r2 = 0.13 and 0.30).
Discussion
Epistasis between SNPs within NRG1 and between NRG1 and selected protein interaction partners influenced risk for schizophrenia in this study, which we biologically validated using fMRI in healthy controls (see Figure 7 for a summary of the convergence of our findings). Individuals carrying the same combinations of schizophrenia risk genotypes showed significantly less efficient cognitive processing, similar to findings in patients with schizophrenia and in their healthy siblings. A 3-way interaction between a nonsynonymous SNP in NRG1 and SNPs in ERBB4 and AKT1 was associated with substantial increased risk for schizophrenia and inefficient physiological processing of working memory in normal subjects. Markers in NRG1 showing epistasis were found at the 5’ and 3’ ends of the gene, in regions that have been individually associated in earlier studies1-12, and we also observed interaction between one marker in the 5’ end and three markers at the 3’ end confirming previous reports24-25. SNPs in AKT1/ERBB4 interacted with the nonsynonymous NRG1 SNP Thr286/289/294Met (rs10503929) which has been reported to be marginally associated with schizophrenia8,19, and a recent meta-analysis of this SNP showed statistically significant association52. It is interesting to note that epistatic interactions were stronger than single SNP effects and in some cases occurred between SNPs not showing individual main effects, a classic characteristic of epistasis described in other genetic contexts53.
Figure 7.
Convergence of evidence for epistasis. ns = not significant at 0.05, uncorrected.
MLA p-values were generated using experiment-wise permutation testing and neuroimaging analyses utilized conservative FWE correction54. However, we have performed a number of statistical tests, and we report uncorrected p-values for analyses using unconditional logistic regression. We recognize that few of our p-values for these regression models would survive naïve correction for all tests. Nevertheless, in searching the public databases of GWA studies of schizophrenia, we have identified evidence within the GAIN dataset of interactions with 5’ and 3’ SNPs in NRG1. Higher order interactions were not testable in this dataset and replication in another sample was complicated by allelic heterogeneity. We also note that 4 of 5 SNPs in this latter sample showed single-SNP level association in one or both of the datasets and we showed a trend toward interaction for the three-way interaction.
We have taken an alternative strategy to validate our statistical associations at the level of diagnosis by hypothesis testing predetermined interactions at the level of brain function, arguing that for interactions of SNPs to exaggerate clinical risk, they should exaggerate the biologic underpinnings of risk55. Schizophrenia is associated with abnormal frontal lobe function, which has been extensively documented from many research directions over many years56 and qualitatively similar abnormalities are found in healthy family members of patients with schizophrenia (including unaffected co-MZ twins) implicating frontal cortex functional abnormalities as intermediate biological phenotypes related to genetic risk for schizophrenia55-56. We used a measure of frontal lobe physiologic function – the efficiency or “tuning” of prefrontal cortical engagement during working memory – as an intermediate phenotype to biologically validate statistical associations to clinical illness. Our fMRI results establish brain-functional interactions between the SNPs tested, both the intra-NRG1 interaction and the NRG1-ERBB4-AKT1 interactions, implicating a neural systems mechanism for the clinical epistasis.
Schizophrenia is highly heritable, but thus far many genome-wide association studies have not been “successful” in the sense of finding genome-wide significant results that are conclusively replicated in independent studies. However, nearly all of the genome-wide studies of schizophrenia have focused on the analysis of single SNPs, which seem to fall short of explaining the heritability - with the exception of a recent study that proposed a polygenic model of schizophrenia57. Genetic variation exists within in a biologic context and this context is likely epistatic and/or polygenic. Studies employing the current GWA approach, which look for individual variant effects, may be compromised in finding association without taking these contextual issues into effect. Future studies will determine whether the epistatic and/or polygenic model is the rule, rather than the exception, in other samples involving NRG1 or other gene networks of interest. We believe our approach of independent biologic validation provides strong support that this is fertile territory for further research related to genetic risk for schizophrenia.
In conclusion, we report evidence for statistical epistasis between SNPs in the 3’ end of NRG1 with 5’ NRG1 SNPs, between NRG1 and ERBB4, and a three-way interaction between a functional SNP in NRG1 with ERBB4 and AKT1. The 3’ end of NRG1 is exon-rich, providing biological support for 5’ promoter-3’ interactions observed in the present study and by Benzel et al.25, and may play a role in increasing risk for schizophrenia. The NRG1-ERBB4-AKT1 interaction is consistent with the work of Hahn et al.33, showing AKT1 function is modulated by NRG1-ERBB4 interactions. We have validated each of these genetic interactions in terms of functional influence on cognitive processing in the PFC of healthy controls.
Acknowledgments
The authors declare the following competing interest: Pierandrea Muglia is a full-time employee of the pharmaceutical company GlaxoSmithKline who have filed patent applications for SNPs associated with schizophrenia (United States Patent Applications 20080176239 and 20080176240 and International Application Number PCT/EP2008/050477). The funders (NIMH Intramural Program) had no role in the design or conduct of the study; collection, management, analysis or interpretation of the data; and preparation, review or approval of the manuscript. This study utilized the high-performance computational capabilities of the Biowulf Linux cluster at the National Institutes of Health, Bethesda, MD (http://biowulf.nih.gov).
FUNDING SOURCES: NIH intramural. Dr. Amanda J. Law is a Medical Research Council (MRC), UK Career Development Fellow. The recruitment of the German patients was partially supported by GlaxoSmithKline.
References
- 1.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H, Stefansson K. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stefansson H, Sarginson J, Kong A, Yates P, Steinthorsdottir V, Gudfinnsson E, Gunnarsdottir S, Walker N, Petursson H, Crombie C, Ingason A, Gulcher JR, Stefansson K, St Clair D. Association of neuregulin 1 with schizophrenia confirmed in a Scottish population. Am J Hum Genet. 2003;72:83–87. doi: 10.1086/345442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang JZ, Si TM, Ruan Y, Ling YS, Han YH, Wang XL, Zhou M, Zhang HY, Kong QM, Zhang DR, Yu YQ, Liu SZ, Ju GZ, Shu L, Ma DL, Zhang D. Association study of neuregulin 1 gene with schizophrenia. Mol Psychiatry. 2003;8:706–709. doi: 10.1038/sj.mp.4001377. [DOI] [PubMed] [Google Scholar]
- 4.Corvin AP, Morris DW, McGhee K, Schwaiger S, Scully P, Quinn J, Meagher D, Clair DS, Waddington JL, Gill M. Confirmation and refinement of an ‘at-risk’ haplotype for schizophrenia suggests the EST cluster, Hs. 97362, as a potential susceptibility gene at the Neuregulin- 1 locus. Mol Psychiatry. 2004;9:208–213. doi: 10.1038/sj.mp.4001412. [DOI] [PubMed] [Google Scholar]
- 5.Li T, Stefansson H, Gudfinnsson E, Cai G, Liu X, Murray RM, Steinthorsdottir V, Januel G, Gudnadottir VG, Petursson J, Ingason A, Gulcher JR, Stefansson K, Collier DA. Identification of a novel neuregulin 1 at-risk haplotype in Han schizophrenia Chinese patients, but no association with the Icelandic/Scottish risk haplotype. Mol Psychiatry. 2004;9:698–704. doi: 10.1038/sj.mp.4001485. [DOI] [PubMed] [Google Scholar]
- 6.Hall D, Gogos JA, Karayiorgou M. The contribution of three strong candidate schizophrenia susceptibility genes in demographically distinct populations. Genes, Brain Behav. 2004;3:240–248. doi: 10.1111/j.1601-183X.2004.00078.x. [DOI] [PubMed] [Google Scholar]
- 7.Petryshen TL, Middleton FA, Kirby A, Aldinger KA, Purcell S, Tahl AR, Morley CP, McGann L, Gentile KL, Rockwell GN, Medeiros HM, Carvalho C, Macedo A, Dourado A, Valente J, Ferreira CP, Patterson NJ, Azevedo MH, Daly MJ, Pato CN, Pato MT, Sklar P. Support for involvement of neuregulin 1 in schizophrenia pathophysiology. Mol Psychiatry. 2005;10:366–374. doi: 10.1038/sj.mp.4001608. [DOI] [PubMed] [Google Scholar]
- 8.Lachman HM, Pedrosa E, Nolan KA, Glass M, Ye K, Saito T. Analysis of polymorphisms in AT-rich domains of neuregulin 1 gene in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2006;141:102–109. doi: 10.1002/ajmg.b.30242. [DOI] [PubMed] [Google Scholar]
- 9.Fukui N, Muratake T, Kaneko N, Amagane H, Someya T. Supportive evidence for neuregulin 1 as a susceptibility gene for schizophrenia in a Japanese population. Neurosci Lett. 2006;396:117–120. doi: 10.1016/j.neulet.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Kim JW, Lee YS, Cho EY, Jang YL, Park DY, Choi KS, Jeun HO, Cho SH, Jang SY, Hong KS. Linkage and association of schizophrenia with genetic variations in the locus of neuregulin 1 in Korean population. Am J Med Genet B Neuropsychatr Genet. 2006;141:281–286. doi: 10.1002/ajmg.b.30209. [DOI] [PubMed] [Google Scholar]
- 11.Thomson PA, Christoforou A, Morris SW, Adie E, Pickard BS, Porteous DJ, Muir WJ, Blackwood DH, Evans KL. Association of Neuregulin 1 with schizophrenia and bipolar disorder in a second cohort from a Scottish population. Mol Psychiatry. 2007;12:94–104. doi: 10.1038/sj.mp.4001889. [DOI] [PubMed] [Google Scholar]
- 12.Turunen JA, Peltonen JO, Pietiläinen OP, Hennah W, Loukola A, Paunio T, Silander K, Ekelund J, Varilo T, Partonene T, Lönnqvist J, Peltonen L. The role of DTNBP1, NRG1, and AKT1 in the genetics of schizophrenia in Finland. Schizophr Res. 2007;91:27–36. doi: 10.1016/j.schres.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 13.Thiselton DL, Webb BT, Neale BM, Ribble RC, O'Neill FA, Walsh D, Riley BP, Kendler KS. No evidence for linkage or association of neuregulin- 1 (NRG1) with disease in the Irish study of high-density schizophrenia families (ISHDSF). Mol Psychiatry. 2004;9:777–783. doi: 10.1038/sj.mp.4001530. [DOI] [PubMed] [Google Scholar]
- 14.Hong CJ, Huo SJ, Liao DL, Lee K, Wu JY, Tsai SJ. Case-control and family-based association studies between the neuregulin 1 (Arg38Gln) polymorphism and schizophrenia. Neurosci Lett. 2004;366:158–161. doi: 10.1016/j.neulet.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 15.Duan J, Martinez M, Sanders AR, Hou C, Krasner AJ, Schwartz DB, Gejman PV. Neuregulin 1 (NRG1) and schizophrenia: analysis of a US family sample and the evidence in the balance. Psychol Med. 2005;35:599–610. doi: 10.1017/S0033291705005428. [DOI] [PubMed] [Google Scholar]
- 16.Ingason A, Søeby K, Timm S, Wang AG, Jakobsen KD, Fink-Jensen A, Hemmingsen R, Berg Rasmussen H, Werge T. No significant association of the 5’ end of neuregulin 1 and schizophrenia in a large Danish sample. Schizophr Res. 2006;83:1–5. doi: 10.1016/j.schres.2005.12.850. [DOI] [PubMed] [Google Scholar]
- 17.Walss-Bass C, Raventos H, Montero AP, Armas R, Dassori A, Contreras S, Liu W, Medina R, Levinson DF, Pereira M, Leach RJ, Almasy L, Escamilla MA. Association analyses of the neuregulin 1 gene with schizophrenia and manic psychosis in a Hispanic population. Acta Psychiatr Scand. 2006;113:314–321. doi: 10.1111/j.1600-0447.2005.00631.x. [DOI] [PubMed] [Google Scholar]
- 18.Vilella E, Costas J, Sanjuan J, Guitart M, De Diego Y, Carracedo A, Martorell L, Valero J, Labad A, De Frutos R, Nájera C, Moltó MD, Toirac I, Guillamat R, Brunet A, Vallès V, Pérez L, Leon M, de Fonseca FR, Phillips C, Torres M. Association of schizophrenia with DTNBP1 but not DAO, DAOA, NRG1 and RGS4 nor their genetic interaction. J Psychiatr Res. 2008;42:278–288. doi: 10.1016/j.jpsychires.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Rosa A, Gardner M, Cuesta MJ, Peralta V, Fatjó-Vilas M, Miret S, Navarro ME, Comas D, Fañanás L. Family-based association study of neuregulin-1 gene and psychosis in a Spanish sample. Am J Med Genet B Neuropsychiatr Genet. 2007;144:954–957. doi: 10.1002/ajmg.b.30511. [DOI] [PubMed] [Google Scholar]
- 20.Sanders AR, Duan J, Levinson DF, Shi J, He D, Hou C, Burrell GJ, Rice JP, Nertney DA, Olincy A, Rozic P, Vinogradov S, Buccola NG, Mowry BJ, Freedman R, Amin F, Black DW, Silverman JM, Byerley WF, Crowe RR, Cloninger CR, Martinez M, Gejman PV. No significant association of 14 candidate genes with schizophrenia in a large European ancestry sample: implications for psychiatric genetics. Am J Psychiatry. 2008;165:497–506. doi: 10.1176/appi.ajp.2007.07101573. [DOI] [PubMed] [Google Scholar]
- 21.Li D, Collier DA, He L. Meta-analysis shows strong positive association of the neuregulin 1 (NRG1) gene with schizophrenia. Hum Mol Genet. 2006;15:1995–2002. doi: 10.1093/hmg/ddl122. [DOI] [PubMed] [Google Scholar]
- 22.Munafò MR, Attwood AS, Flint J. Neuregulin 1 genotype and schizophrenia. Schizophr Bull. 2008;34:9–12. doi: 10.1093/schbul/sbm129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norton N, Moskvina V, Morris DW, Bray NJ, Zammit S, Williams NM, Williams HJ, Preece AC, Dwyer S, Wilkinson JC, Spurlock G, Kirov G, Buckland P, Waddington JL, Gill M, Corvin AP, Owen MJ, O'Donovan MC. Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2006;141:96–101. doi: 10.1002/ajmg.b.30236. [DOI] [PubMed] [Google Scholar]
- 24.Shiota S, Tochigi M, Shimada H, Ohashi J, Kasai K, Kato N, Tokunaga K, Sasaki T. Association and interaction analyses of NRG1 and ERBB4 genes with schizophrenia in a Japanese population. J Hum Genet. 2008;53:929–935. doi: 10.1007/s10038-008-0332-9. [DOI] [PubMed] [Google Scholar]
- 25.Benzel I, Bansal A, Browning BL, Galwey NW, Maycox PR, McGinnis R, Smart D, St Clair D, Yates P, Purvis I. Interactions among genes in the ErbB-Neuregulin signaling network are associated with increased susceptibility to schizophrenia. Behav Brain Funct. 2007;3:31. doi: 10.1186/1744-9081-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao H, Burrage LC, Sinasac DS, Hill AE, Ernest SR, O'Brien W, Courtland HW, Jepsen KJ, Kirby A, Kulbokas EJ, Daly MJ, Broman KW, Lander ES, Nadeau JH. Genetic architecture of complex traits: Large phenotypic effects and pervasive epistasis. Proc Natl Acad Sci U S A. 2008;105:19910–19914. doi: 10.1073/pnas.0810388105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breiman L. Random forests. Mach Learn. 2001;45:5–32. [Google Scholar]
- 28.Hothorn T, Lausen B, Benner A, Radespiel-Troeger M. Bagging survival trees. Stat Med. 2004;23:77–91. doi: 10.1002/sim.1593. [DOI] [PubMed] [Google Scholar]
- 29.Hothorn T, Bühlmann P, Dudoit S, Molinaro A, van der Laan MJ. Survival ensembles. Biostatistics. 2006;7:355–373. doi: 10.1093/biostatistics/kxj011. [DOI] [PubMed] [Google Scholar]
- 30.Strobl C, Boulesteix AL, Zeileis A, Hothorn T. Bias in random forest variable importance measures: illustrations, sources and a solution. BMC Bioinformatics. 2007;8:25. doi: 10.1186/1471-2105-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kooperberg C, Ruczinski I. Identifying interacting SNPs using Monte Carlo logic regression. Genet Epidemiol. 2005;28:157–170. doi: 10.1002/gepi.20042. [DOI] [PubMed] [Google Scholar]
- 32.Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- 33.Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, Gallop RJ, Arnold SE. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- 34.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagata K, Kohda D, Hatanaka H, Ichikawa S, Matsuda S, Yamamoto T, Suzuki A, Inagaki F. Solution structure of the epidermal growth factor-like domain of heregulin-alpha, a ligand for p180erbB-4. EMBO J. 1994;13:3517–3523. doi: 10.1002/j.1460-2075.1994.tb06658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bao J, Lin H, Ouyang Y, Lei D, Osman A, Kim TW, Mei L, Dai P, Ohlemiller KK, Ambron RT. Activity-dependent transcription regulation of PSD-95 by neuregulin-1 and Eos. Nat Neurosci. 2004;7:1250–1258. doi: 10.1038/nn1342. [DOI] [PubMed] [Google Scholar]
- 38.Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin medianted by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 39.Jaffrey SR, Snowman AM, Eliasson MJ, Cohen NA, Snyder SH. CAPON: a protein associated with neuronal nitric oxide synthase that regulates its interactions with PSD95. Neuron. 1998;20:115–124. doi: 10.1016/s0896-6273(00)80439-0. [DOI] [PubMed] [Google Scholar]
- 40.Brzustowicz LM, Simone J, Mohseni P, Hayter JE, Hodgkinson KA, Chow EW, Bassett AS. Linkage disequilibrium mapping of schizophrenia susceptibility to the CAPON region of chromosome 1q22. Am J Hum Genet. 2004;74:1057–1063. doi: 10.1086/420774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shinkai T, Ohmori O, Hori H, Nakamura J. Allelic association of the neuronal nitric oxide synthase (NOS1) gene with schizophrenia. Mol Psychiatry. 2002;7:560–563. doi: 10.1038/sj.mp.4001041. [DOI] [PubMed] [Google Scholar]
- 42.Xu MQ, Xing QH, Zheng YL, Li S, Gao JJ, He G, Guo TW, Feng GY, Xu F, He L. Association of AKT1 gene polymorphisms with risk of schizophrenia and with response to antipsychotics in the Chinese population. J Clin Psychiatry. 2007;68:1358–1367. doi: 10.4088/jcp.v68n0906. [DOI] [PubMed] [Google Scholar]
- 43.Egan MF, Goldberg TE, Gscheidle T, Weirich M, Bigelow LB, Weinberger DR. Relative risk of attention deficits in siblings of patients with schizophrenia. Am J Psychiatry. 2000;157:1309–1316. doi: 10.1176/appi.ajp.157.8.1309. [DOI] [PubMed] [Google Scholar]
- 44.Need AC, Ge D, Weale ME, Maia J, Feng S, Heinzen EL, Shianna KV, Yoon W, Kasperaviciūte D, Gennarelli M, Strittmatter WJ, Bonvicini C, Rossi G, Jayathilake K, Cola PA, McEvoy JP, Keefe RS, Fisher EM, St Jean PL, Geigling I, Hartmann AM, Möller HJ, Ruppert A, Fraser G, Crombie C, Middleton LT, St Clair D, Roses AD, Muglia P, Francks C, Rujescu D, Meltzer HY, Goldstein DB. A genome-wide investigation of SNPs and CNVs in schizophrenia. PLoS Genet. 2009;5:e1000373. doi: 10.1371/journal.pgen.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nicodemus KK, Wang W, Shugart YY. Stability of variable importance scores and rankings using statistical learning tools on single nucleotide polymorphisms (SNPs) and risk factors involved in gene-gene and gene-environment interactions. BMC Proceedings. 2007;1(Suppl 1):S58. doi: 10.1186/1753-6561-1-s1-s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicodemus KK, Malley JD. Predictor correlation impacts machine learning algorithms: Implications for genomic studies. Bioinformatics. 2009 doi: 10.1093/bioinformatics/btp331. in press. [DOI] [PubMed] [Google Scholar]
- 47.Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Goldberg TE, Weinberger DR. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- 48.Worsley KJ, Marrett S, Neelin P, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 49.Fardo DW, Becker KD, Bertram L, Tanzi RE, Lange C. Recovering unused information in genome-wide association studies: the benefit of analyzing SNPs out of Hardy-Weinberg equilibrium. Eur J Hum Genet. 2009;17:1676–1682. doi: 10.1038/ejhg.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blokland GA, McMahon KL, Hoffman J, Zhu G, Meredith M, Martin NG, Thompson PM, de Zubicaray GI, Wright MJ. Quantifying the heritability of task-related brain activation and performance during the N-back working memory task: a twin fMRI study. Biol Psychol. 2008;79:70–79. doi: 10.1016/j.biopsycho.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suarez BK, Duan J, Sanders AR, Hinrichs AL, Jin CH, Hou C, Buccola NG, Hale N, Weilbaecher AN, Nertney DA, Olincy A, Green S, Schaffer AW, Smith CJ, Hannah DE, Rice JP, Cox NJ, Martinez M, Mowry BJ, Amin F, Silverman JM, Black DW, Byerley WF, Crowe RR, Freedman R, Clininger CR, Levinson DF, Gejman PV. Genomewide linkage scan of 409 European-ancestry and African American families with schizophrenia: suggestive evidence of linkage at 8p23.3-p21.2 and 11p13-1-q14.1 in the combined sample. Am J Hum Genet. 2006;78:315–333. doi: 10.1086/500272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allen NC, Bagade S, McQueen MB, Ioannidis JP, Kavvoura FK, Khoury MJ, Tanzi RE, Bertram L. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40:499–506. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- 53.Cordell HJ. Genome-wide association studies: Detecting gene-gene interactions that underlie human diseases. Nat Rev Genet. 2009 doi: 10.1038/nrg2579. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyer-Lindenberg A, Nicodemus KK, Egan MF, Callicott JH, Mattay V, Weinberger DR. False positives in imaging genetics. Neuroimage. 2008;40:655–661. doi: 10.1016/j.neuroimage.2007.11.058. [DOI] [PubMed] [Google Scholar]
- 55.Tan HY, Callicott JH, Weinberger DR. Intermediate phenotypes in schizophrenia genetics redux: is it a no brainer? Mol Psychiatry. 2008;13:233–238. doi: 10.1038/sj.mp.4002145. [DOI] [PubMed] [Google Scholar]
- 56.Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, Berman KF, Goldberg TE. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50:825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- 57.The International Schizophrenia Consortium Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;406:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nicodemus KK, Callicott JH, Higier RG, Luna A, Nixon DC, Lipska BK, Vakkalanka R, Giegling I, Rujescu D, St. Clair D, Muglia P, Shugart YY, Weinberger DR. Evidence of statistical epistasis between DISC1, CIT and NDEL1 impacting risk for schizophrenia: Biological validation with functional neuroimaging. Hum Genet. doi: 10.1007/s00439-009-0782-y. (in press) [DOI] [PubMed] [Google Scholar]