Abstract
Heterotopic ossification (HO) is a common and debilitating complication of burns, traumatic brain injuries, and musculoskeletal trauma and surgery. Although the exact mechanism of ectopic bone formation is unknown, mesenchymal stem cells (MSCs) capable of osteogenic differentiation are known to play an essential role. Interestingly, the prevalence of HO in the elderly population is low despite the high overall occurrence of musculoskeletal injury and orthopedic procedures. We hypothesized that a lower osteogenicity of MSCs would be associated with blunted HO formation in old compared with young mice. In vitro osteogenic differentiation of adipose-derived MSCs from old (18–20 months) and young (6–8 weeks) C57/BL6 mice was assessed, with or without preceding burn injury. In vivo studies were then performed using an Achilles tenotomy with concurrent burn injury HO model. HO formation was quantified using μCT scans, Raman spectroscopy, and histology. MSCs from young mice had more in vitro bone formation, upregulation of bone formation pathways, and higher activation of Smad and nuclear factor kappa B (NF-κB) signaling following burn injury. This effect was absent or blunted in cells from old mice. In young mice, burn injury significantly increased HO formation, NF-κB activation, and osteoclast activity at the tenotomy site. This blunted, reactive osteogenic response in old mice follows trends seen clinically and may be related to differences in the ability to mount acute inflammatory responses. This unique characterization of HO and MSC osteogenic differentiation following inflammatory insult establishes differences between age populations and suggests potential pathways that could be targeted in the future with therapeutics.
Introduction
Heterotopic ossification (HO) is a clinically devastating complication of trauma, including automobile accidents, traumatic brain injury, major burns, fractures, pressure ulcers, and orthopedic surgery; HO often results in joint contractures, nerve entrapment, and persistent pain. Over 65% of military personnel sustaining major combat wounds develop HO as concomitant injuries with major inflammatory insults such as blast injuries and fractures [1,2]. In civilian populations, HO complicates over 60% of major burn wounds and 20% of orthopedic procedures and traumatic brain injuries [3]. The tendency to develop HO following musculoskeletal trauma is apparently age-dependent. In 2010, the National Inpatient Sample (NIS) showed that more than 41% of patients suffering from HO as a principal diagnosis were between the ages of 18–44, whereas less than 18% of patients were over the age of 65. This is in stark contrast to the total number of upper and lower extremity fractures and joint replacements, with over 54% of these injuries sustained by patients over the age of 65 (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd) [4]. Some possible explanations of this phenomenon could include a diminished response to inflammatory insult with age, differences in the cells responsible for HO formation [mesenchymal stem cells (MSCs)], slowed bone remodeling and general loss of bone quality, or differences in the severity of musculoskeletal trauma sustained between older and younger patient groups.
Whereas several interesting reports describe reduced ectopic bone formation following exogenous implantation of osteogenic materials, the effect of age on trauma-induced HO has yet to be examined [5]. An accepted paradigm of HO formation includes three key components: multipotent progenitor cells capable of forming bone, a permissive niche, and an inciting inflammatory incident [6–8]. The majority of current literature supports a role for MSCs in modulating HO [9–12]. This is further confirmed by the fact that we observe trauma-induced HO to occur through an endochondral ossification process. With this paradigm of MSC involvement in mind, and the discrepancy between young and old patients seen clinically, questions arise about the effect of age on MSC differentiation. Recent studies have compared the in vitro differentiation capability of MSCs with varying donor age, but report some conflicting results [13,14]. Of particular interest regarding the role of MSCs in HO formation is their osteogenic response to global inflammatory insult. In patients with burn injury, this inflammatory response is characterized by elevated levels of interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) [15]. At the cellular level, the response to acute inflammation is characterized by the activation of nuclear factor kappa B (NF-κB), which affects diverse cellular processes, including proliferation, apoptosis, and differentiation [16]. Thus the response to inflammation delivered by TNF-α signaling may have an important effect on ectopic bone development by regulating NF-κB action on stem cell differentiation.
Recent studies have found that bone morphogenetic protein (BMP) signaling through the Smad pathway plays an essential role in coordinating both osteogenic and chondrogenic differentiation of MSCs to form HO [11,12]. BMP signaling is normally initiated by the binding of ligands to signaling complexes composed of BMP type II and type I receptors, which phosphorylate BMP responsive Smads 1/5/8 which subsequently regulate osteogenic and/or chondrogenic gene transcription. Mammalian BMP ligands (BMP-2/4/6/7) are known to recruit preosteoblasts and induce MSC osteogenic differentiation [17]. In brief, endochondral ossification occurs through the condensation of MSCs into chondrocytes, which form a cartilage precursor to bone. This cartilage precursor is then replaced by mature osteoid. Thus, we expect both that our young and old mice will develop HO through an endochondral process, however, we would expect more canonical Smad1/5/8 signaling and final HO formation in young mice.
In this present study, we examine differences both in vitro and in vivo in the inflammatory response and tendency to form ectopic bone in young and old mice. Critically significant to this study is the use of an animal model that mimics the conditions responsible for HO in human patients, namely musculoskeletal trauma and burn injury. This model provides functionality to examine osteogenic signaling following trauma without the exogenous implantation of external osteogenic signals or a genetic mutation. In this study, we demonstrate that burn injury results in increased osteogenic capacity of MSCs in young mice and that this effect is blunted in old mice. Furthermore, in an Achilles tenotomy burn model, young mice developed more HO and suffered from greater restriction in their range of motion (ROM) than old mice.
Materials and Methods
Animals
Old (age 18–20 months) or young (age 6–8 weeks) C57/BL6 male mice from the Jackson Laboratory were used for all experiments. The span of 18–24 months has been defined as old and corresponds with humans age 56–69 [18]. All animal procedures were carried out in accordance with the guidelines provided in the Guide for the Use and Care of Laboratory Animals: Eighth Edition from the Institute for Laboratory Animal Research (ILAR, 2011) and were approved by the Institutional Animal Care and Use Committee of the University of Michigan (PRO0001553).
Burn injury and isolation and culture of primary adipose-derived mesenchymal cells
Thirty percent total body surface area (TBSA) partial thickness scald burn injury on the dorsum was performed as previously described [19,20]. Mouse MSCs were harvested from the inguinal fat pads of mice 2 h after burn injury or nonburn control (n=3 per group) as previously described [21]. This MSC source has previously been shown to be capable of bone formation both in vivo and in vitro [22]. Adipose-derived MSCs were chosen as a proxy for the still unidentified mesenchymal cell responsible for HO formation based on the availability and ease of use. Furthermore, we have found the effect of burn injury on MSCs from adipose tissue to mimic that of local MSCs and bone marrow-derived mesenchymal cells. Cells were passaged three times before being used for osteogenic assays.
In vitro osteogenic differentiation of MSCs
MSCs were seeded in triplicate onto six-well plates at a density of 100,000 cells/well and onto a 12-well plate at a density of 35,000 cells/well as previously described [21,23]. Growth media were substituted for osteogenic differentiation media (ODM: Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, 10 mM β-glycerophosphate, 100 μg/mL ascorbic acid; Invitrogen). Early osteogenic differentiation was assessed by alkaline phosphatase (ALP) stain and quantification of ALP enzymatic activity after 7 days as previously described [21,24]. Alizarin red staining for bone mineral deposition and colorimetric quantification was completed at 2 weeks as previously described [21,25].
Quantitative polymerase chain reaction
RNA was harvested from cells after 7 days in ODM using the RNeasy Mini Kit (Qiagen) according to the manufacturer's specifications. Reverse transcription was performed with 1 μg RNA using the Taqman Reverse Transcription Reagents (Applied Biosystems). Quantitative real-time polymerase chain reaction (PCR) was carried out using the Applied Biosystems Prism 7900HT Sequence Detection System and Sybr Green PCR Master Mix (Applied Biosystems) as previously described [21,26]. Specific primers for these genes were chosen based on their PrimerBank sequence and can be found in Supplementary Table S1.
Western blot analysis
Cells were lysed and protein collected after 7 days differentiation in ODM, separated on polyacrylamide gels, transferred to polyvinylidene fluoride membranes, and assayed with standard immunoblotting technique using the following primary antibodies: anti-phospho-Smad1/5/8, anti-Smad5, anti-phospho-NF-κB, anti-NF-κB (Cell Signaling Technologies) as previously described [26].
Serum collection and enzyme-linked immunoabsorbant assay
Blood samples were collected from mice 2 h following burn injury or nonburn control and serum was isolated with clot activator tubes (n=3 per group; BD). enzyme-linked immunoabsorbant assay (ELISA) was performed for IL-6 and TNF-α.
Achilles tenotomy
All mice used for in vivo analysis of HO formation received an Achilles tenotomy (n=4 per group) with sharp dissection at the midpoint in the left leg as previously described [27]. Both old and young mice were further divided into burn and nonburn groups with the burn group receiving a 30% TBSA partial-thickness burn injury on the dorsum immediately following tenotomy procedure.
μCT analysis
In vivo development of HO was assessed with longitudinal μCT scans at 5 days, 3 weeks, 5 weeks, 7 weeks, 9 weeks, and 15 weeks posttenotomy with or without concurrent burn injury (μCT; GE Healthcare Biosciences, using 80 kVp, 80 mA, and 1,100 ms exposure). Images were reconstructed and HO volume formation was analyzed using a calibrated imaging protocol as previously described [27].
ROM analysis
Fifteen weeks posttenotomy, mice were briefly anesthetized and assessed for ROM at the tenotomy site by extending the ankle with 75 g weight to full extension. Photographs from a fixed distance were taken with the extended ankle centered on a disk of fixed size. The angle of extension was assessed by three independent blinded observers using the ruler tool in the Adobe Photoshop (n=4 per group).
Raman spectroscopy
Cross sections of the tenotomy site were analyzed on a Raman microscopy system using macroscopic visualization of anatomic landmarks and the corresponding μCT scan slices to define areas of interest for ectopic bone formation as previously described [12]. Reduced crystallinity and mineral to matrix band ratio (MTMR) are indicative of immature mineralized tissue and are markers of HO [27].
Histology
At 15 weeks posttenotomy, animals were euthanized and the tenotomized leg was fixed, decalcified in a 19% EDTA solution, paraffin embedded, and sectioned at 5 μm for staining with Aniline Blue, H&E, and pentachrome for analysis of ectopic bone development and tartrate-resistant acid phosphatase (TRAP) staining for osteoclast activity as previously described [26]. Histomorphometric quantification of Aniline Blue staining was completed by summing the area of HO development on every 15th slide on cross sections through the tenotomy site. Immunohistochemical staining with the following primary antibodies: anti-phospho-NF-κB and anti-NF-κB was completed with 1:1,000 dilution of an appropriate biotinylated secondary antibody (Vector Laboratories) and visualized with diaminobenzidine (Zymed Laboratories) [28,29].
Statistical analysis
Data were analyzed using the SPSS software (v21; IBM). Means and standard deviations were calculated from numerical data and statistical analysis was performed using an appropriate ANOVA. Equivariance was assessed with Levene's test and a Welch correction was applied when indicated. Post hoc analysis was completed with Tukey's test with equivariant data or Games-Howell if Levene's test failed. In figures, bar graphs represent means, whereas error bars represent one standard deviation. For all assays, significance was defined as a P<0.05.
Results
The osteogenic capacity of MSCs is increased following burn injury, but this effect is blunted in old mice
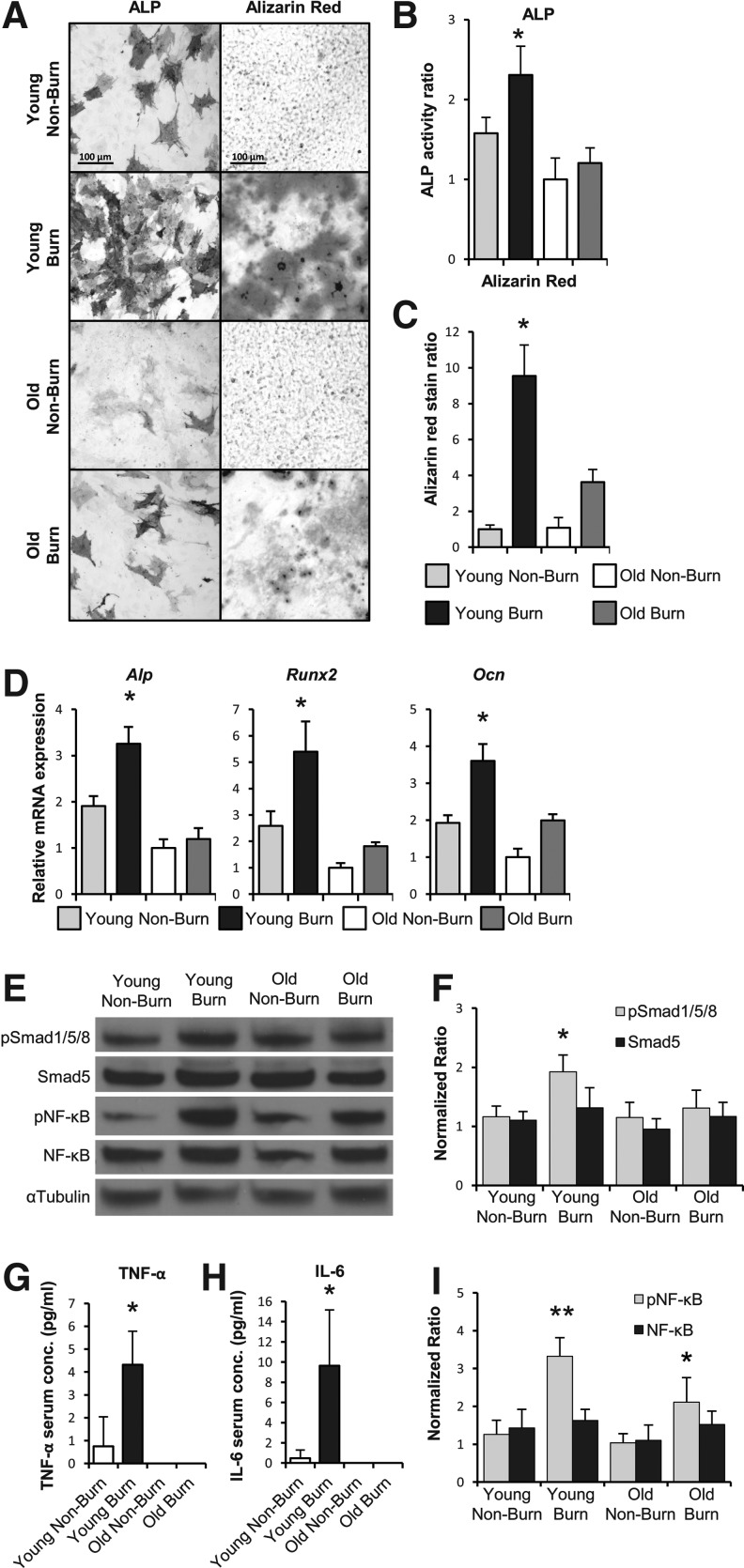
MSCs were harvested from the adipose compartment of either young (6–8 weeks) or old (18–20 months) mice 2 h following burn injury, or nonburn control (n=4 per group). Significantly more ALP staining and enzymatic activity was seen in MSCs from young mice that received burn injury than their nonburn counterparts (Fig. 1A). However, this trend was not seen in MSCs from old mice after burn injury. Instead, quantification of ALP activity in the old burn cells was similar to nonburn controls; ALP activity of MSCs from old mice with or without burn injury was similar to MSCs from young nonburn mice (Fig. 1B). In vitro bone mineral deposition followed a similar pattern, as assessed by Alizarin red staining (Fig. 1A). Young mouse-derived MSCs developed over ninefold increase in osteoid formation, whereas old mouse-derived MSCs only had a threefold increase (Fig. 1C).
FIG. 1.
Burn injury enhances the osteogenic and inflammatory signaling as well as the osteogenic capacity of mesenchymal stem cells (MSCs) to a greater degree in young mice compared with old mice. (A) Alkaline phosphatase (ALP) and Alizarin red stain of mouse MSCs harvested 2 h after burn injury or nonburn control. Positively staining cells indicate expression of ALP, Alizarin red deposits indicate in vitro bone formation. (B) Assay based quantification of ALP activity normalized to overall total protein content. (C) Colorimetric quantification of Alizarin red staining. (D) Quantitative real-time PCR for Alp, runt-related transcription factor 2 (Runx2), and oseteocalcin (Ocn) mRNA expression in MSCs after 7 days exposure to osteogenic differentiation media (ODM). (E) Immunoblot of protein extracted from MSCs after 7 days in ODM for pSmad1/5/8, Smad5, phosphor-nuclear factor kappa B (pNF-κB), NF-κB, and αTubulin as loading control. (F) Densitometry analysis of Smad signaling showing significantly increased ratio of phosphorylation (pSmad1/5/8) in the cells from young mice that received burn injury. (G) Enzyme-linked immunoabsorbant assay (ELISA) analysis for levels of inflammatory cytokine tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) (H) in mice following burn injury or nonburn control. (I) Densitometry analysis of NF-κB signaling showing phosphorylation of NF-κB was significantly increased in cells from old and young mice following burn injury, but was more pronounced in young mice. Data are means±SD, n=3 for all assays. *P<0.05, **P<0.01.
Osteogenic and inflammatory signaling is increased to a greater degree in MSCs derived from young mice following burn injury compared with old mice
Quantitative real-time PCR was performed on RNA from these MSCs following burn injury or nonburn control for key mediators in the bone formation pathway. Transcription factors osteocalcin (Ocn), runt-related transcription factor 2 (Runx2), and Alp were all significantly increased in the MSCs from young mice following burn injury (Fig. 1D). This trend, however, did not occur in old mice as there was only a minor, nonsignificant increase in osteogenic gene expression.
We next interrogated the BMP signaling pathway by determining the proportion of activated (phosphorylated) Smad5 (pSmad1/5/8) protein in these cells after exposure to ODM for 7 days. A significantly greater ratio of pSmad1/5/8 to Smad5 was observed by immunoblot in MSCs from young mice following burn injury than in their nonburn counterparts (Fig. 1E, F). No significant difference was seen between burn and nonburn cells from old mice, or in the overall amount of Smad5 protein levels between all groups.
To assess the systemic inflammatory response following burn injury, serum was collected at the time of MSC harvest and analyzed by ELISA. Serum levels of key inflammatory markers TNF-α and IL-6 were nine times and three times higher, respectively in young mice that sustained burn injury than nonburn mice (Fig. 1G, H). However, TNF-α and IL-6 levels were nearly undetectable in serum from old mice even after burn injury. Young mice clearly respond more vigorously with regard to the level of their inflammatory cytokines than old mice. In addition to an increase in TNF-α ligand in young mice, we also noted an increase in signaling through the NF-κB pathway. As would be expected following inflammatory insult, levels of phosphorylated (active) NF-κB were higher in MSCs from burn mice (Fig. 1E, I).
We next set out to assess if the differences we saw in MSCs were due to simply higher levels of cytokines such as TNF-α or if the MSCs from the younger mice differed in their osteogenic potential. To help differentiate, we treated MSCs from young and old burn mice to the same level of recombinant TNF-α and found no difference in osteogenicity (Supplementary Fig. S2). Thus, the differences observed are likely due to higher levels of inflammatory cytokines in the young mice rather than cells that were more responsive to TNF-α. Furthermore, inflammation following burn injury has a pro-osteogenic effect on MSCs from young mice, but this effect, and overall inflammatory response, is blunted with age.
Burn injury enhances HO formation in an Achilles tenotomy model in young mice only
Using our trauma/burn model, we analyzed young and old mice weekly by μCT. Concurrent burn injury resulted in significantly increased HO development at the tenotomy site in young mice starting as early as 5 weeks and reaching statistical significance at 9 and 15 weeks. Old mice, however, formed little tenotomy site HO with or without a burn. Also of note, the burn injury did not seem to have a pro-osteogenic effect as it did on the young mice. (Fig. 2A, B). Very little HO formation was observed in the old mice even at late time points. This trend of HO development increasing with inflammation caused by burn injury correlated well with ROM studies. As was found for the quantification of HO growth, only the young burned mice had a significantly reduced ROM, resulting in a significantly restricted angle of ankle extension (Fig. 2C, D).
FIG. 2.
Young mice developed more heterotopic ossification (HO) following burn injury and suffered more pronounced joint contracture. (A) μCT scan image reconstruction showing advancement of HO longitudinally over 15 weeks. Gray areas represent HO formation. A large nidus of HO developed in the young burn mice, but was absent in old burn mice, indicated by the circle. (B). Quantification of ectopic bone at each μCT scan time point. (C) Representative images of range of motion (ROM) testing by maximum extension at the ankle 15 weeks following tenotomy with or without concurrent burn injury. (D) Measurement of maximum extension angle at the ankle, which was most significantly reduced in young burn mice. Data are means±SD, n=4 for all assays. *P<0.05.
Despite differences in the amount of HO formed between young and old mice, the mineral maturity (crystallinity) of ectopic bone was not significantly different, as measured by Raman spectroscopy
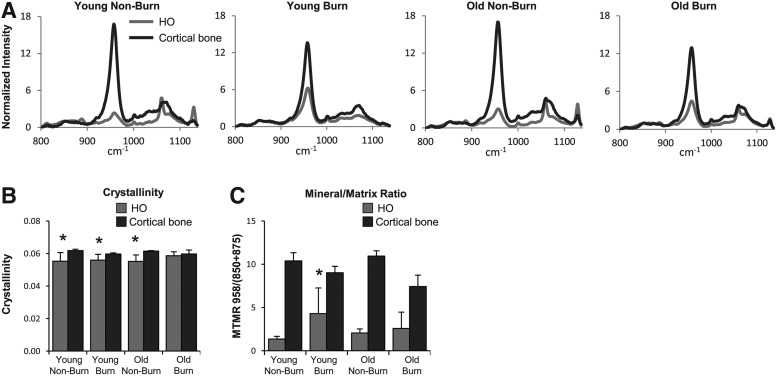
Characteristic Raman spectra for bone hydroxyapatite mineral with bands near 958 cm−1 were seen in all regions that corresponded to areas of ectopic bone as identified by μCT (Fig. 3A). However, overall crystallinity (inverse of the width of bone mineral band near 958 cm−1) was significantly lower for regions of ectopic bone than for cortical bone in all groups, except old burn (Fig. 3B). The bone MTMR was significantly lower for regions of ectopic bone than for cortical bone in all groups (Fig. 3C). In young mice with burn injury, the MTMR was significantly higher for all other HO groups. However, it remained significantly lower than that of cortical bone (Fig. 3C). Of note, spatial heterogeneity and a wider range of MTMRs were observed for specimens receiving burn injury. Young burn specimens exhibited the highest heterogeneity in MTMR, whereas more uniform distributions were observed for old burn mice.
FIG. 3.
Raman spectroscopic analysis of the tenotomy site demonstrates increased mineral to matrix ratio of HO tissue in young mice after burn injury. (A) Representative spectra from each treatment group with the key peak measurement for bone mineral occurring around 958 cm−1. Raman microscopy using 10 frames per 100 μm2 area, 10 μm step size, were collected for cortical bone and for the region where HO was seen through μCT (B). Crystallinity (1/full width at half maximum intensity at 958 cm-1) was seen to be lower in HO versus cortical bone in all groups, except old burn. (C) Mineral to matrix ratio (MTMR) was significantly lower for HO than cortical bone across all measurements (not indicated on chart), however compared across HO groups, MTMR for HO in young burn mice was significantly higher. Data are means±SD. *P<0.05.
Histologic analysis of tenotomy site confirms differences in ectopic bone development between old and young groups
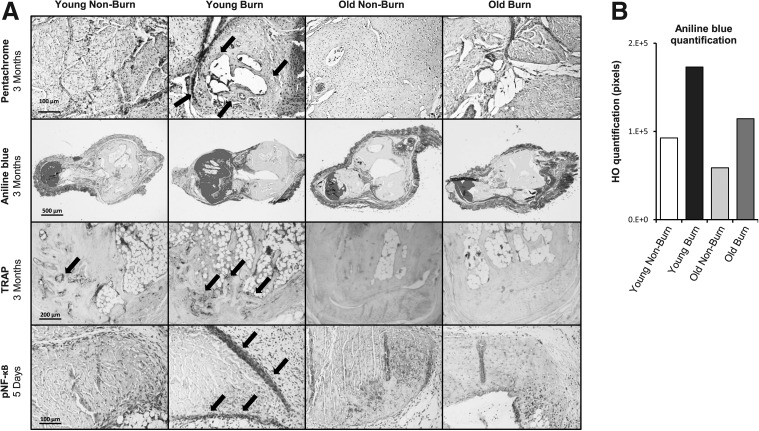
Following the volumetric and qualitative assessments by μCT and Raman spectroscopy respectively, we turned our inquiry to the structure and composition of ectopic bone growth. Characteristic bone structure was identified by pentachrome and Aniline Blue staining on cross sections of the tenotomy sites at locations, which corresponded to those identified as HO on μCT scans (Fig. 4A). The amount of HO apparent in Aniline Blue stain confirmed the significantly more robust reactive bone formation in young burn mice over all other groups (Fig. 4B).
FIG. 4.
Histological analysis of HO formation. (A) Sections through the tenotomy site at 3 months or 5 days posttenotomy with or without concurrent burn injury. Top row: pentachrome stains show organized ectopic bone and cartilage most notable in young burn samples (indicated by arrows). Second row: serial Aniline Blue stains on cross sections through the tenotomy site were quantified by histomorphometric analysis after recoloring to include HO and exclude native bone structure and normal tissue. Third row: tartrate-resistant acid phosphatase (TRAP) staining for osteoclast activity (indicated by arrows) was more robust in young mice. Fourth row: immunohistochemistry for pNF-κB showed a band of pNF-κB-positive cells surround the tenotomy site in young burn mice (arrows) at 5 days posttenotomy and burn injury. This corresponded to the location where HO developed at later time points. (B) Quantification of HO across serial Aniline Blue sections.
Extraskeletal heterotopic bone formation occurs by an endochondral differentiation in both old and young mice
We next set out to demonstrate the endochondral nature of trauma-induced HO in old mice similar to that seen in young mice. We noted that after a less robust fibroproliferative response, pentachrome stain, which includes Safranin O, demonstrated both cartilage and mature bone in the region of HO separate from the native cortical bone (Fig. 4A). This cartilage, which is enveloped by mature bone, occurs in regions with corresponding formation of bone marrow (Supplementary Fig. SF3). Thus, both old and young mice develop HO through endochondral ossification.
The greater HO volume in young mice was not due to decreased bone resorption compared with older mice and correlated with regions in which inflammatory pathways were active before bone formed
In an effort to examine bone turnover, we completed TRAP staining for osteoclast activity. The site in which osteoclasts were the most prevalent corresponded with the sites in young burned mice where there was the most robust HO response (Fig. 4A). In contrast to young mice, osteoclast activity was notably diminished in old mice, regardless of treatment. Thus, the differences observed were not due to older mice having an increase in bone breakdown. Immunohistochemistry staining for NF-κB completed on tenotomy sites 5 days postsurgery with or without burn injury showed similar staining intensity across all four groups (Supplementary Fig. SF4). Upon examining phosphorylated NF-κB signaling, however, a more robust response was seen in young burned mice compared with nonburn or old mice (Fig. 4A). This finding included an apparent confluence of cells surrounding the cut Achilles tendon that were strongly positive for activation of NF-κB at a location correspondent to future ectopic bone formation.
Discussion
HO is a clinically frustrating condition that often affects adolescent and young adult patients following musculoskeletal trauma as well as older patients. It is an especially pressing concern for trauma, burn, and orthopedic surgery patients [2]. Our investigation of HO in young and old mice was inspired by our observation that although the majority of musculoskeletal trauma and joint reconstruction cases occur in the elderly population, the majority of HO cases actually occur in patients less than 65 years of age [4].
Our first course of action in exploring this phenomenon was to examine the osteogenic potential of MSCs in old compared with young mice. A key factor in the formation of ectopic bone is a cell population capable of osteogenic differentiation. Sufficient evidence suggests that this trauma-induced HO forms through an endochondral ossification process which begins with the condensation of MSCs [12,30–33]. The reduction in osteogenic potential of MSCs from aged sources has already been reported [13,14]. However, the specific relationships between the inflammatory insult of a burn injury, age, and their respective contributions to MSC osteogenic differentiation have not been adequately explored. Our findings that burn injury increases the in vitro osteogenic potential of MSCs from young mice to a significantly greater degree than in cells from old mice suggest that further study of the role of MSCs in HO formation is warranted.
Although many cytokines are dysregulated after burn injuries, TNF-α has been shown to play a significant role in burn inflammation in adults and humans. This difference between young and old mice may contribute to the differences we see in MSC osteogenesis as MSCs in young mice are exposed to higher levels of TNF-α, which has been shown to increase osteogenic differentiation in an acute setting [34]. Having observed an increase in TNF-α after burn injury in young mice, we next interrogated the role of aging in NF-κB signaling, a master regulatory transcription factor linking bone formation and inflammatory signaling [35]. Phosphorylation of NF-κB permits translocation to the nucleus where gene transcription is activated affecting diverse cellular processes, including proliferation, apoptosis, angiogenesis, and differentiation [16,36]. We found that activation of NF-κB was increased in both old and young cell populations, as evidenced by increases in the ratio of phosphorylation (pNF-κB), suggesting that burn injury increased inflammatory response in both age groups. Although increases in both groups were statistically significant, the difference in activation ratios was larger in young cells. Additionally, TNF-α serum levels were significantly higher in younger mice. NF-κB has been shown to have an antiapoptotic effect on osteoclasts through the transforming growth factor-β signaling pathway [37]. Thus, an important role of inflammation in HO development early on may include enhanced osteoclast activity. Indeed our TRAP staining results corresponded as those younger mice had increased bony turnover at the site of HO.
Of particular importance in this study is the agreement of in vitro and in vivo results on age and bone formation capacity. While several models exist to study HO formation in vivo, the advantage of our Achilles tenotomy model is its physiologic basis that mirrors causes of HO seen clinically [38]. The resulting formation of ectopic bone is caused solely by musculoskeletal and burn trauma rather than by the introduction of foreign scaffolds, cells, osteoinductive substances, or genetic mutations. The differences seen in HO volume and timing may be clinically relevant because the causes of HO formation (motor vehicle accident, major burn injury, combat wounds) are similar in nature.
Several unanswered questions remain with regard to the effect of aging and HO. Signaling through BMP receptor 1 has been shown to play a crucial role in MSC condensation and endochondral ossification [39]. Additionally Acvr1/Alk2 signaling has been shown to play a key role in mouse models of congenital HO [40]. Although we show differences in Smad1/5/8 signaling, it is unknown if this is due to differences in Acvr1/Alk2 signaling or whether these differences are due to Alk3 or Alk6. Additionally, the exact BMP ligand responsible for HO and the differences in HO based on age is unknown. Recent studies implicate BMP-4, however, clinical reports have shown unequivocally that the use of recombinant BMP-2 for indications such as spinal fusion in humans causes HO [40]. Finally, although we know that TNF-α and IL-6 are upregulated following burn injury, a large number of other cytokines are also aberrantly activated and suppressed and deserve further evaluation [41].
In conclusion, our study indicates that osteogenic and inflammatory changes associated with aging affect the tendency to form ectopic bone. This effect is seen in vitro at the level of mesenchymal cells, which tend to increase in osteogenic capacity, following burn injury in young mice. Aging also reduced the in vivo HO response to burn injury and Achilles tenotomy. These results mirror clinical trends, but more studies are warranted to further elucidate the mechanism of HO formation. Key factors leading to these effects may include inflammatory regulation through NF-κB and osteogenic regulation through canonical Smad signaling. This study is clinically relevant because it mimics the clinical observation that younger individuals are at increased risk of developing HO. This increased incidence in young patients might be due to increased inflammation. Thus, future studies that aim to treat patients at high risk of HO development might want to focus treatments on younger patients. For example, current trials of drugs such as celecoxib, which have significant side effects, might have an improved risk–benefit ratio if they are used in a younger patient population.
Supplementary Material
Acknowledgments
The authors thank Kevin Heist, Benjamin Hoff, Amanda Fair, and the staff of the Center for Molecular Imaging at UM for their assistance with animal care and μCT scanning. Source of funding: R01 AR055222, 1K08GM109105-01, and Plastic Surgery Foundation National Endowment Award.
Author Disclosure Statement
All authors confirm that they have no conflicts of interest or commercial associations in connection with this work.
References
- 1.Potter BK, Forsberg JA, Davis TA, Evans KN, Hawksworth JS, Tadaki D, Brown TS, Crane NJ, Burns TC, O'Brien FP. and Elster EA. (2010). Heterotopic ossification following combat-related trauma. J Bone Joint Surg Am 92Suppl 2:74–89 [DOI] [PubMed] [Google Scholar]
- 2.Alfieri KA, Forsberg JA. and Potter BK. (2012). Blast injuries and heterotopic ossification. Bone Joint Res 1:174–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanden Bossche L. and Vanderstraeten G. (2005). Heterotopic ossification: a review. J Rehabil Med 37:129–136 [DOI] [PubMed] [Google Scholar]
- 4.HCUP Nationwide Inpatient Sample (NIS). (2010). Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality, Rockville, MD: www.hcup-us.ahrq.gov/nisoverview.jsp [Google Scholar]
- 5.Fleet JC, Cashman K, Cox K. and Rosen V. (1996). The effects of aging on the bone inductive activity of recombinant human bone morphogenetic protein-2. Endocrinology 137:4605–4610 [DOI] [PubMed] [Google Scholar]
- 6.Leblanc E, Trensz F, Haroun S, Drouin G, Bergeron E, Penton CM, Montanaro F, Roux S, Faucheux N. and Grenier G. (2011). BMP-9-induced muscle heterotopic ossification requires changes to the skeletal muscle microenvironment. J Bone Miner Res 26:1166–1177 [DOI] [PubMed] [Google Scholar]
- 7.Shore EM. (2011). Osteoinductive signals and heterotopic ossification. J Bone Miner Res 26:1163–1165 [DOI] [PubMed] [Google Scholar]
- 8.Wosczyna MN, Biswas AA, Cogswell CA. and Goldhamer DJ. (2012). Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J Bone Miner Res 27:1004–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eguchi Y, Wakitani S, Imai Y, Naka Y, Hashimoto Y, Nakamura H. and Takaoka K. (2010). Antitumor necrotic factor agent promotes BMP-2-induced ectopic bone formation. J Bone Miner Metab 28:157–164 [DOI] [PubMed] [Google Scholar]
- 10.Janicki P, Boeuf S, Steck E, Egermann M, Kasten P. and Richter W. (2011). Prediction of in vivo bone forming potency of bone marrow-derived human mesenchymal stem cells. Eur Cell Mater 21:488–507 [DOI] [PubMed] [Google Scholar]
- 11.Medici D. and Olsen BR. (2012). The role of endothelial-mesenchymal transition in heterotopic ossification. J Bone Miner Res 27:1619–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peterson JR, De La Rosa S, Sun H, Eboda O, Cilwa KE, Donneys A, Morris M, Buchman SR, Cederna PS, et al. (2013). Burn injury enhances bone formation in heterotopic ossification model. Ann Surg 259:993–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen HT, Lee MJ, Chen CH, Chuang SC, Chang LF, Ho ML, Hung SH, Fu YC, Wang YH, et al. (2012). Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J Cell Mol Med 16:582–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gala K, Burdzinska A, Idziak M, Makula J. and Paczek L. (2011). Characterization of bone-marrow-derived rat mesenchymal stem cells depending on donor age. Cell Biol Int 35:1055–1062 [DOI] [PubMed] [Google Scholar]
- 15.Mace JE, Park MS, Mora AG, Chung KK, Martini W, White CE, Holcomb JB, Merrill GA, Dubick MA, et al. (2012). Differential expression of the immunoinflammatory response in trauma patients: burn vs. non-burn. Burns 38:599–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel SA, Heinrich AC, Reddy BY. and Rameshwar P. (2009). Inflammatory mediators: parallels between cancer biology and stem cell therapy. J Inflamm Res 2:13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song DS, Park JC, Jung IH, Choi SH, Cho KS, Kim CK. and Kim CS. (2011). Enhanced adipogenic differentiation and reduced collagen synthesis induced by human periodontal ligament stem cells might underlie the negative effect of recombinant human bone morphogenetic protein-2 on periodontal regeneration. J Periodontal Res 46:193–203 [DOI] [PubMed] [Google Scholar]
- 18.Flurkey KCJ. and Harrison DE. (2007). The mouse in biomedical research. In: The Mouse in Aging Research, 2nd ed. Fox JG, Quimby FW, Barthold SW, Newcomer CE. and Smith AL. Elsevier, Burlington, MA, pp. 637–672 [Google Scholar]
- 19.Ipaktchi K, Mattar A, Niederbichler AD, Hoesel LM, Vollmannshauser S, Hemmila MR, Minter RM, Su GL, Wang SC. and Arbabi S. (2007). Topical p38 MAPK inhibition reduces bacterial growth in an in vivo burn wound model. Surgery 142:86–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niederbichler AD, Hoesel LM, Ipaktchi K, Olivarez L, Erdmann M, Vogt PM, Su GL, Arbabi S, Westfall MV, Wang SC. and Hemmila MR. (2011). Burn-induced heart failure: lipopolysaccharide binding protein improves burn and endotoxin-induced cardiac contractility deficits. J Surg Res 165:128–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levi B, Nelson ER, Brown K, James AW, Xu D, Dunlevie R, Wu JC, Lee M, Wu B, et al. (2011). Differences in osteogenic differentiation of adipose-derived stromal cells from murine, canine, and human sources in vitro and in vivo. Plast Reconstr Surg 128:373–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levi B, James AW, Wan DC, Glotzbach JP, Commons GW. and Longaker MT. (2010). Regulation of human adipose-derived stromal cell osteogenic differentiation by insulin-like growth factor-1 and platelet-derived growth factor-alpha. Plast Reconstr Surg 126:41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levi B, James AW, Nelson ER, Li S, Peng M, Commons GW, Lee M, Wu B. and Longaker MT. (2011). Human adipose-derived stromal cells stimulate autogenous skeletal repair via paracrine Hedgehog signaling with calvarial osteoblasts. Stem Cells Dev 20:243–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.James AW, Xu Y, Wang R. and Longaker MT. (2008). Proliferation, osteogenic differentiation, and fgf-2 modulation of posterofrontal/sagittal suture-derived mesenchymal cells in vitro. Plast Reconstr Surg 122:53–63 [DOI] [PubMed] [Google Scholar]
- 25.Xu Y, James AW. and Longaker MT. (2008). Transforming growth factor-beta1 stimulates chondrogenic differentiation of posterofrontal suture-derived mesenchymal cells in vitro. Plast Reconstr Surg 122:1649–1659 [DOI] [PubMed] [Google Scholar]
- 26.Levi B, Hyun JS, Nelson ER, Li S, Montoro DT, Wan DC, Jia FJ, Glotzbach JC, James AW, et al. (2011). Nonintegrating knockdown and customized scaffold design enhances human adipose-derived stem cells in skeletal repair. Stem Cells 29:2018–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterson JR, Okagbare PI, De La Rosa S, Cilwa KE, Perosky JE, Eboda ON, Donneys A, Su GL, Buchman SR, et al. (2013). Early detection of burn induced heterotopic ossification using transcutaneous Raman spectroscopy. Bone 54:28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levi B, Nelson ER, Li S, James AW, Hyun JS, Montoro DT, Lee M, Glotzbach JP, Commons GW. and Longaker MT. (2011). Dura mater stimulates human adipose-derived stromal cells to undergo bone formation in mouse calvarial defects. Stem Cells 29:1241–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, et al. (2003). Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 174:101–109 [DOI] [PubMed] [Google Scholar]
- 30.Culbert AL, Chakkalakal SA, Theosmy EG, Brennan TA, Kaplan FS. and Shore EM. (2014). Alk2 regulates early chondrogenic fate in fibrodysplasia ossificans progressiva heterotopic endochondral ossification. Stem Cells 32:1289–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamiya N, Ye L, Kobayashi T, Lucas DJ, Mochida Y, Yamauchi M, Kronenberg HM, Feng JQ. and Mishina Y. (2008). Disruption of BMP signaling in osteoblasts through type IA receptor (BMPRIA) increases bone mass. J Bone Miner Res 23:2007–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson ER, Wong VW, Krebsbach PH, Wang SC. and Levi B. (2012). Heterotopic ossification following burn injury: the role of stem cells. J Burn Care Res 33:463–470 [DOI] [PubMed] [Google Scholar]
- 33.Kronenberg HM. (2003). Developmental regulation of the growth plate. Nature 423:332–336 [DOI] [PubMed] [Google Scholar]
- 34.Hess K, Ushmorov A, Fiedler J, Brenner RE. and Wirth T. (2009). TNFalpha promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-kappaB signaling pathway. Bone 45:367–376 [DOI] [PubMed] [Google Scholar]
- 35.Chen S, Guttridge DC, Tang E, Shi S, Guan K. and Wang CY. (2001). Suppression of tumor necrosis factor-mediated apoptosis by nuclear factor kappaB-independent bone morphogenetic protein/Smad signaling. J Biol Chem 276:39259–39263 [DOI] [PubMed] [Google Scholar]
- 36.Sun XF. and Zhang H. (2007). NFKB and NFKBI polymorphisms in relation to susceptibility of tumour and other diseases. Histol Histopathol 22:1387–1398 [DOI] [PubMed] [Google Scholar]
- 37.Gingery A, Bradley EW, Pederson L, Ruan M, Horwood NJ. and Oursler MJ. (2008). TGF-beta coordinately activates TAK1/MEK/AKT/NFkB and SMAD pathways to promote osteoclast survival. Exp Cell Res 314:2725–2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott MA, Levi B, Askarinam A, Nguyen A, Rackohn T, Ting K, Soo C. and James AW. (2012). Brief review of models of ectopic bone formation. Stem Cells Dev 21:655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Derynck R. and Zhang YE. (2003). Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425:577–584 [DOI] [PubMed] [Google Scholar]
- 40.Chakkalakal SA, Zhang D, Culbert AL, Convente MR, Caron RJ, Wright AC, Maidment AD, Kaplan FS. and Shore EM. (2012). An Acvr1 R206H knock-in mouse has fibrodysplasia ossificans progressiva. J Bone Miner Res 27:1746–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finnerty CC, Jeschke MG, Herndon DN, Gamelli R, Gibran N, Klein M, Silver G, Arnoldo B, Remick D. and Tompkins RG. (2008). Temporal cytokine profiles in severely burned patients: a comparison of adults and children. Mol Med 14:553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.