Abstract
We developed a prototype for a closed apparatus for assembling tissue-engineered vascular grafts (TEVGs) with the goal of creating a simple operator-independent method for making TEVGs to optimize safety and enable widespread application of this technology. The TEVG is made by seeding autologous bone marrow-derived mononuclear cells onto a biodegradable tubular scaffold and is the first man-made vascular graft to be successfully used in humans. A critical barrier, which has prevented the widespread clinical adoption of the TEVG, is that cell isolation, scaffold seeding, and incubation are performed using an open method. To reduce the risk of contamination, the TEVG is assembled in a clean room. Clean rooms are expensive to build, complex to operate, and are not available in most hospitals. In this investigation, we used an ovine model to compare the safety and efficacy of TEVGs created using either a standard density centrifugation-based open method or the new filter-based closed system. We demonstrated no graft-related complications and maintenance of growth capacity in TEVGs created using the closed apparatus. In addition, the use of the closed system reduced the amount of time needed to assemble the TEVG by ∼50%. Adaptation of similar methodologies may facilitate the safe translation and the widespread use of other tissue engineering technologies.
Introduction
We demonstrated the feasibility of using tissue-engineered vascular grafts (TEVGs) in the surgical repair of congenital cardiac anomalies in children undergoing open heart surgery1,2 and continue to target the pediatric patient population to take advantage of its growth potential.3–5 To construct the TEVG, autologous bone marrow-derived mononuclear cells (BM-MNCs) are isolated and seeded onto (glycolic acid) fibers knitted into a tube and coated with a 50:50 copolymer of ɛ-caprolactone and L-lactic acid.1,2 The seeded construct is then incubated for 2 h before implantation as a vascular conduit.1,2 Cell harvest, isolation, seeding, and incubation are performed during a single surgical procedure followed by surgical implantation of the TEVG. Thus, there is a significant benefit associated with minimizing the amount of time needed to assemble the TEVG.
The clinical utility of the TEVG is limited by the need to use a clean room, specifically an International Organization for Standardization (ISO) class 7 laboratory, to assemble the TEVG, which involves using an open method for cell isolation, scaffold seeding, and incubation.6 The use of an open technique increases the risk of contamination and introduces operator variability to the process of making the TEVG. Clean rooms are expensive to build and maintain and require significant manpower to operate in compliance with good manufacturing process standards. We have previously demonstrated that a simpler, alternative filtration-based method can be used to isolate BM-MNCs instead of the standard density centrifugation method that we currently use to isolate these cells.7 The filter-based cell isolation method has the added advantage of being performed more rapidly and can serve as the basis for a closed system processing.7 The use of a closed system would minimize the risk of contamination and improve the safety profile for the TEVG. We demonstrated the feasibility of using this alternative filter-based cell isolation method to successfully create neovessels using a murine model.7 Herein, we discuss the results of an investigation designed to compare the safety and efficacy of TEVGs assembled using the standard open method versus TEVGs assembled using the closed apparatus. Our goal is to create a simple, safe, operator-independent apparatus for assembling TEVGs that would eliminate the need for the use of a clean room.
Materials and Methods
Scaffolds
Scaffolds measuring 13 cm length and 12 mm inner diameter with wall thickness measuring ∼700 μm were fabricated from poly(glycolic acid) fibers, which were knitted into a tube, and coated with a 50:50 copolymer of ɛ-caprolactone and L-lactic acid (Gunze Limited).1,2 Scaffolds had 80% porosity with pore sizes from 100 to 200 μm. The scaffolds were packaged and gas sterilized using ethylene oxide before being shipped from the manufacturer for use in the study.
Bone marrow harvest
Twelve juvenile, female, Dover lambs (weight 20–30 kg) were used in this study (Morris Farms). All animals received humane care in compliance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee at the Yale University approved the use of animals and all procedures described in this study. Under general anesthesia, 50 mL of bone marrow was harvested from each animal as previously described.8 A sample of the bone marrow was obtained, and the cell viability was measured using trypan blue exclusion.
Cell isolation, scaffold seeding, and incubation (open method)
Under a biosafety hood, using sterile technique, BM-MNCs were isolated from 50 mL of heparinized bone marrow using density centrifugation in the Histopaque 1077 (Sigma-Aldrich).8 BM-MNCs were then seeded onto the scaffold using the vacuum (−50 mm Hg) seeding method as previously described.6 The seeded scaffold was then placed in a sterile container and bathed in autologous serum and incubated (37°C, 5% CO2, 95% relative humidity, 760 Torr) for a minimum of 2 h before implantation.1,2 A 1×1 cm2 section of the seeded incubated scaffold was excised, fixed, and embedded using glycol methacrylate.9 The sections were stained with the Lee's methylene blue, and the number of attached cells was determined. The total time for cell isolation, scaffold seeding, and incubation was recorded.
Cell isolation, scaffold seeding, and incubation (closed method)
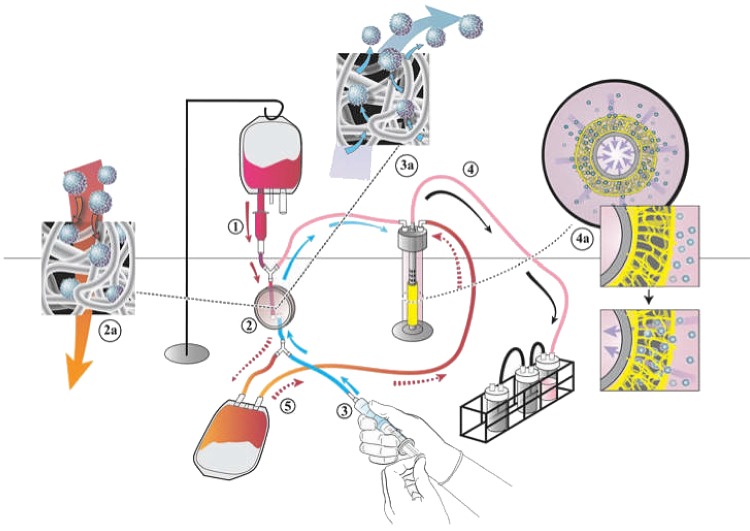
We developed a prototype for a closed disposable system designed to (1) isolate BM-MNCs from autologous bone marrow using a filtration method, (2) seed the scaffold with the BM-MNCs using a vacuum seeding technique, and (3) incubate the seeded scaffold before surgical implantation (Fig. 1). The system uses a nonwoven polyester fiber filter that works, in part, by interception to trap the BM-MNCs (Pall Corporation). The trapped cells are eluted off the filter using 60 cc of elution solution (10% Dextran 40; Pall Corporation). The cells were seeded onto the scaffold using vacuum seeding (−50 mm Hg) and incubated in an autologous effluent for a minimum of 2 h before implantation. A 1×1 cm2 section of the seeded incubated scaffold was excised, fixed, and embedded using glycol methacrylate.8 The sections were stained with the Lee's methylene blue, and the number of attached cells was determined. The total time for cell isolation, scaffold seeding, and incubation was recorded.
FIG. 1.
Schematic representation of the closed disposable seeding system for assembling tissue-engineered vascular grafts (TEVGs). Heparinized bone marrow is added to the bone marrow bag. The bone marrow bag is then suspended at a head-height of 18 inches and the bone marrow is transferred into the drip chamber, which acts to filter out large particles (1). The bone marrow then flows out through the drip chamber filter and through the cell harvest filter (2), which entraps bone marrow-derived mononuclear cells (BM-MNC) (2a). The effluent is collected in the effluent bag. Sixty milliliters of harvest solution (10% Dextran 40) is then back-flushed through the filter (3), releasing entrapped BM-MNC (3a) and collecting them in the seeding chamber. The BM-MNCs are then seeded onto the scaffold using vacuum seeding (4, 4a). The effluent is then transferred to the seeding chamber thus bathing the seeded scaffold (5). At that time, the tubing is heat sealed and the TEVG is placed in the incubation chamber for 2 h, at which point it is ready for surgical implantation. Color images available online at www.liebertpub.com/tec
Surgical implantation
The TEVGs were implanted as intrathoracic inferior vena cava (IVC) interposition grafts as previously described.8 Each anastomosis was marked with a titanium ring to facilitate their identification during computed tomography (CT). The IVC interposition graft model is a high-flow low-pressure model that we have developed for evaluating vascular grafts designed for use in congenital heart surgery, where most vascular grafts are used in high-flow low-pressure circuits, such as the pulmonary or Fontan circulation.8,10 Animals were divided into two groups (open method versus closed system) for the study, and a total of 12 autologous grafts (n=6/group) were implanted as end-to-end IVC interposition grafts as part of this study. All lamb surgeries were performed under general endotracheal anesthesia as previously described.8 Animals were anticoagulated with heparin (100 U/kg) at implantation. No postoperative antiplatelet or anticoagulant agents were used postoperatively.
In vivo CT angiography
In vivo 64-slice x-ray CT angiography (Discovery NM-CT 570c; GE Healthcare) with iodinated contrast (350 mgI/mL, Omnipaque; GE Healthcare) was used to assess graft dilatation, narrowing, and luminal and longitudinal growth at 2 and 6 months following the TEVG implantation. Animals were mechanically ventilated with 35% oxygen, 65% nitrous oxide, and 1–3% isoflurane (Venturi; Cardiopulmonary Incorporated) while hemodynamics were continuously monitored (IntelliVue MP50; Philips). A 5F catheter was placed in a hind limb vein for the administration of fluids and CT contrast agent. Intravenous contrast injections were performed using a power injector (Stellant D; MEDRAD). Lambs were kept NPO on the night before CT imaging and were given an intravenous 20 cc/kg bolus of normal saline after induction of anesthesia to standardize their hydrational status. Images were acquired at a slice thickness of 0.625 mm, at 300 mA, and 120 kVp. TEVG luminal diameter, wall thickness, luminal volume, and length were quantified at 2 and 6 months using commercially available software (Advanced Workstation v4.4; GE Healthcare).
Histology
The animals were euthanized and the vascular grafts were pressure fixed with formalin and harvested after the 6-month implantation as previously described.8 Tissue were embedded in paraffin, sectioned (5 μm sections), and stained with hematoxylin and eosin (H&E), Masson's trichrome, Elastica van Gieson, Hart's, Alcian blue, and Von Kossa.
Statistical analyses
Statistical analyses were performed with the Student's t-test for continuous variables with normal distribution and the chi-square test for dichotomous variables. One-way analysis of variance was used to determine significant differences between three or more groups. p-Values less than 0.05 indicated statistical significance. Numeric values are listed as mean±SD.
Results
We used either an open (density centrifugation) or a closed (filter-based) system to isolate BM-MNCs, seed the scaffold, and incubate the seeded construct before TEVG implantation as an intrathoracic IVC interposition graft in a juvenile in lamb model. Cells were seeded using a vacuum-seeding (−50 mm Hg) technique. Results of the trypan blue exclusion of the bone marrow cells demonstrated cell viability of >90% for both the groups. Results of the cell attachment studies demonstrated that a similar number of cells were seeded onto scaffolds in both the groups as measured by the Lee's methylene blue staining on glycol methacrylate-fixed tissue (density centrifugation: 2900±2050 cells/mm2; filtration: 2630±1590 cells/mm2, p=0.77). Total procedure time was significantly decreased using the closed system (average 2 h, 17 min) compared with the open method (average 4 h, 28 min). The time saving was primarily the result of using the filter-based method instead of the density centrifugation method for isolating BM-MNCs.
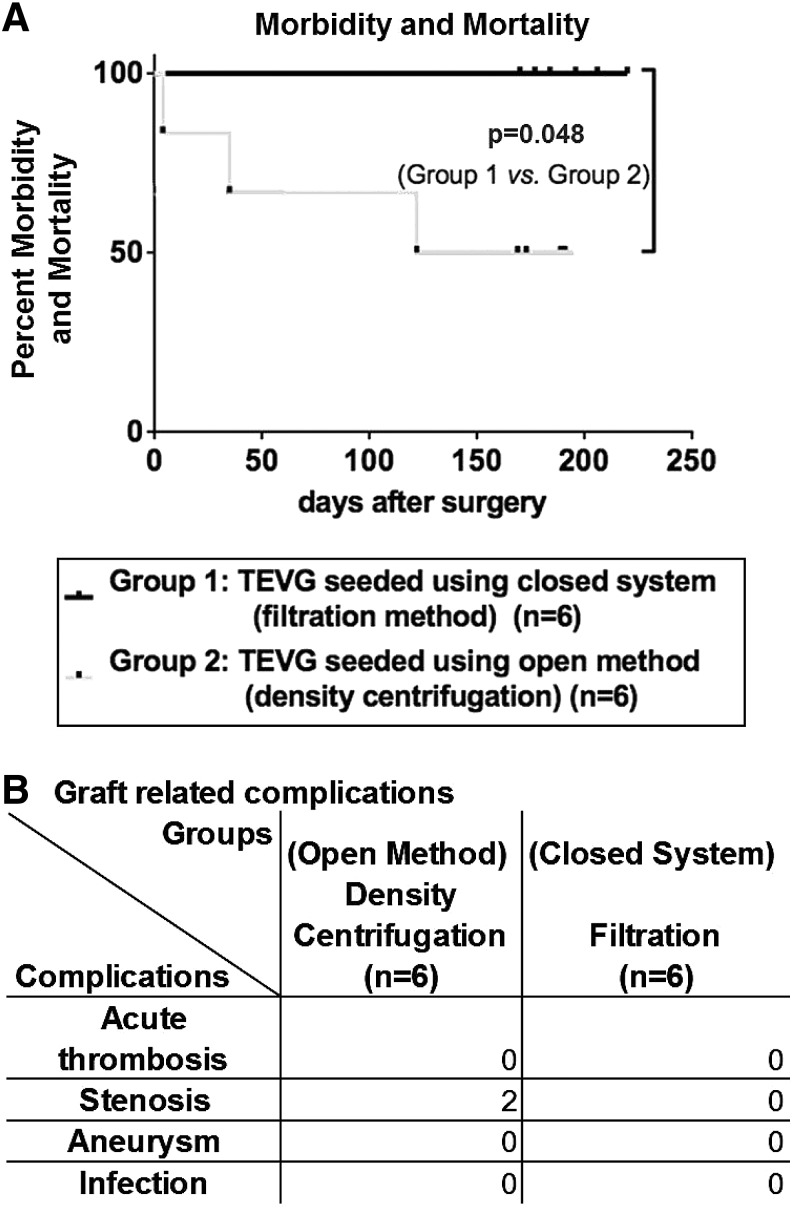
There was one surgical complication resulting from a prolonged clamp time that caused a neurological injury, which necessitated early sacrifice of one of the animals from the open method group. Two additional animals in the open method group developed critical stenoses, which caused portal hypertension, hepatic dysfunction, and ascites requiring early sacrifice. All six lambs implanted with TEVG assembled using the closed disposable seeding apparatus survived surgery and demonstrated no evidence of any graft-related complications throughout the 6-month time course of the study (Fig. 2).
FIG. 2.
Summary of morbidity and mortality data for TEVG implanted in the lamb model. TEVG were implanted as intrathoracic IVC interposition grafts and monitored over a 6-month time course. A total of 12 TEVG were implanted and divided into two equal groups (n=6/group). Group 1consisted of TEVG seeded using a closed filter-based system and group 2 consisted of TEVG seeded using an open density centrifugation-based method. (A) Survival curves: There was one surgical complication, which occurred in the open method group. It consisted of a neurological injury that resulted from a prolonged clamp time. Due to the severity of the neurological deficit, the animal required euthanasia. (B) Graft-related complications: There were two graft-related complications (critical stenosis), which developed in the open method group and also required euthanasia. No deaths or graft-related complications occurred in the TEVG assembled using the closed system. IVC, inferior vena cava.
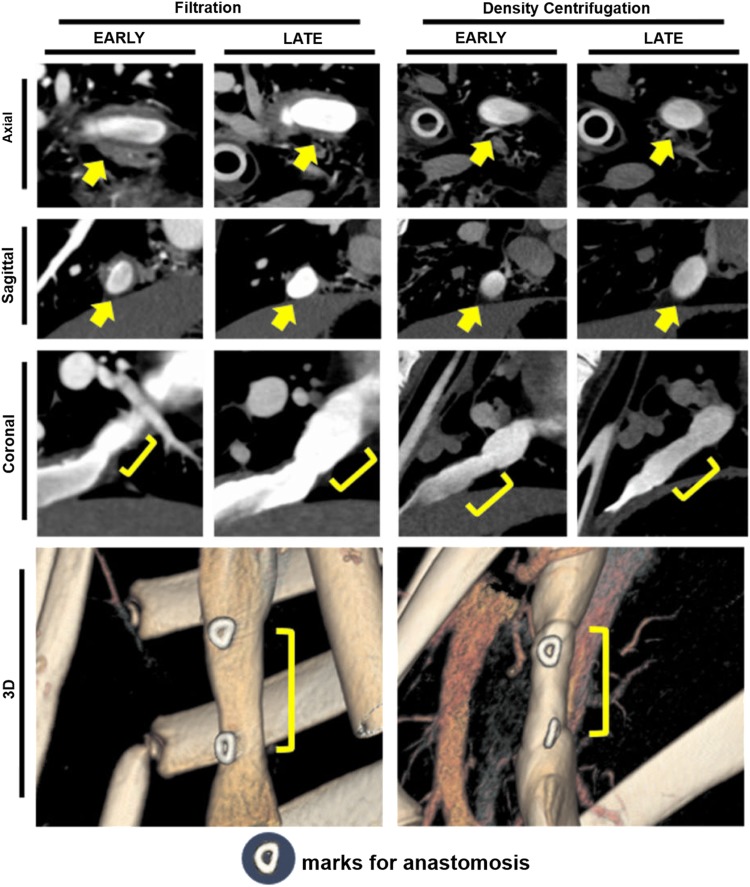
CT angiography was performed at early (2 months) (n=11) and late (6 months) (n=9) time points. All TEVGs that underwent CT angiography at the two separate time points (n=9) demonstrated both luminal growth and increase in graft length without radiological evidence of thrombosis or aneurismal dilation. The mean graft diameter increased 19% from an average diameter of 10.7±2.3 to 12.7±1.6 mm. The graft length increased 16% from a mean length of 20.9±1.3 to 24.2±2.3 mm. The graft volume increase of 48%, from 2128±927 to 3145±739 mm3, was similar to the 36% increase in volume of the native superior vena cava over the same time period. The graft wall thickness decreased from a mean of 4.4±1.0 to 2.7±1.0 mm, which approached the thickness of the native IVC (Fig. 3).
FIG. 3.
Computed tomography (CT) analysis of TEVG implanted in lamb model. Serial CT imaging was used to assess the change in size and shape of the TEVG implanted in juvenile lambs over a 6-month time course. All surviving animals were serially imaged at early (2 months) and late (6 months) time points. At the early time point, the TEVG showed thickening of the graft wall accompanied in some instances by graft luminal narrowing (n=11). There was no radiographic evidence of thrombosis in any graft. The graft luminal volume increased over the course of the experiment in all (n=9) animals that survived the 6-month time course. There was no radiographic evidence of aneurismal dilation in any grafts. Arrows show implanted graft by horizontal CT slice. “J” shows parts of implanted grafts by long-axis CT slice. Color images available online at www.liebertpub.com/tec
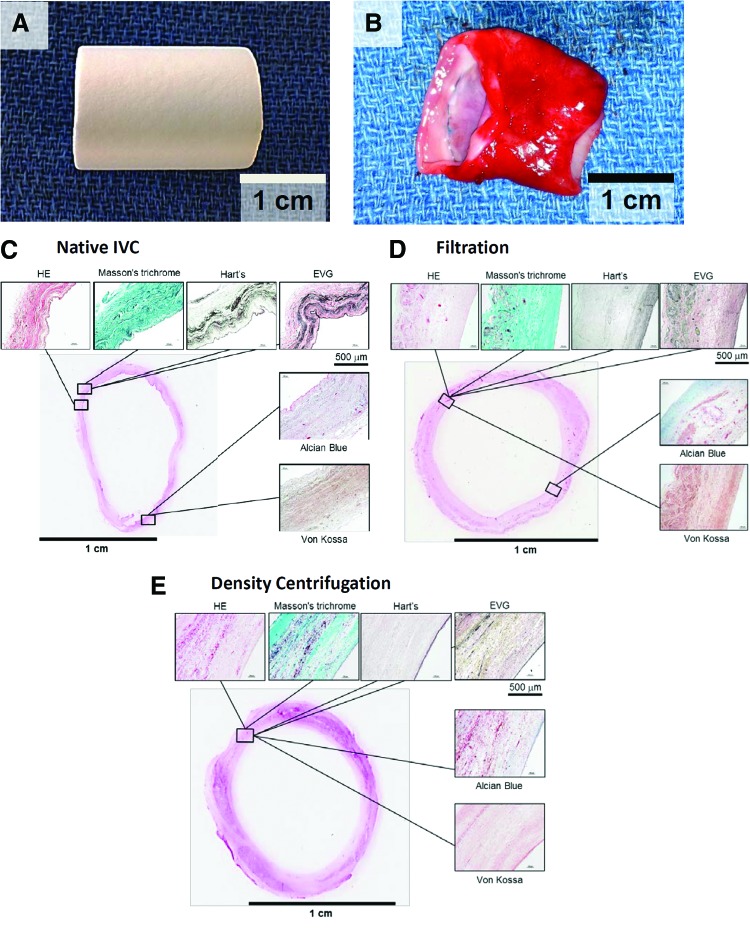
Qualitative histological assessment performed on tissue explanted 6 months after implantation demonstrated a TEVG wall that resembled native tissue given the presence of three distinct layers, including a neointima, neomedia, and neoadventitia. H&E staining revealed cellular architecture in the grafts resembling that of a native vessel. Masson's trichrome showed robust collagen formation in both the groups. There was no evidence of ectopic calcification in any specimens (Von Kossa). Elastin staining was present in both the groups but not as well developed or organized as in the native IVC (Elastica Van Gieson and Hart's). Glycosaminoglycan staining was seen in both the groups (Alcian blue) (Fig. 4).
FIG. 4.
Histological analysis of TEVG: TEVGs in lambs explanted at 6 months after implantation resemble native blood vessels. (A) Scaffold. Before surgical implantation, all grafts were measured and cut to 20 mm in length. (B) At time of explantation, seeded scaffolds had transformed into neovessels resembling native inferior vena cava. (C–E) H&E staining revealed cellular architecture in the grafts resembling that of native vessels. Masson's trichrome staining showed robust collagen formation in both the groups. There was no evidence of ectopic calcification (Von Kossa). Elastin staining was present in both the groups but not as well developed as seen in the native IVC (Elastica Van Gieson [EVG] and Hart's stain). Glycosaminoglycan staining was seen in both the groups (Alcian blue stain). H&E, hematoxylin and eosin. Color images available online at www.liebertpub.com/tec
Discussion
In this study, we evaluated a prototype for a closed disposable seeding system for constructing tissue-engineered vascular grafts and compared it with our currently used open system. We performed a study comparing the incidence of graft-related complications and growth capacity of TEVG constructed using either the closed filter-based seeding system or the open density centrifugation-based methodology. Using an ovine model, we demonstrated no graft-related complications in the TEVG constructed using the closed disposable seeding system and confirmed the growth capacity of the TEVG using serial CT angiography. Taken together, these data support the feasibility of creating a closed system for assembling TEVGs and suggest that such a system could improve both the safety and the clinical utility of this technology by creating a method that is simpler, faster, and operator-independent.
Upon initiation of our clinical trial in the United States, we switched from a manual seeding method to a vacuum seeding method in an attempt to reduce the operator variability and mitigate the risks associated with making a TEVG. We performed a series of experiments demonstrating the equivalence of both techniques before introducing this technology to the clinic.6 This sort of incremental process improvement is critical to rational design of a better safer product. In this investigation, we evaluate the next step in process improvement for TEVG production. We developed a closed disposable system based on filtration method for isolating BM-MNCs. The resulting closed disposable system is simpler, faster, and operator-independent. In our previous work, preliminary characterization of this methodology demonstrated that it was feasible. However, we noted differences in the subpopulations of the BM-MNCs, including increased numbers of red blood cells in the BM-MNCs isolated using the filter-based method.7 Results of our current study demonstrate that despite these differences in the cell populations we were able to create viable neovessels using either technique. Furthermore, the absence of any graft-related complications and the preservation of the growth potential in the group of TEVGs created using the closed disposable seeding system highlight the safety and efficacy of this methodology.
The TEVG described herein is the first man-made vascular graft with growth potential. Results of our pilot study evaluating the use of the TEVG in congenital heart surgery confirmed the growth capacity of the TEVG in humans.1,2 This has significant implications for children, particularly those with congenital heart disease.3–5 Before the potential of the TEVG can be fully realized, simpler, safer, and more rapid methods for assembling TEVGs (i.e., cell isolation, cell seeding, and incubation) need to be developed. Development of a closed disposable seeding system would overcome a critical barrier and would make this technology available to many more patients. Adoption of a similar approach for creating other tissue-engineered products could serve as a paradigm for process improvement, which would improve both the safety and the clinical utility of other tissue-engineered products, thereby facilitating the translation of these technologies from the bench to the clinic.
Acknowledgments
We thank Chris Hawley for her technical assistance with animal care and experiments. We acknowledge Martin Smith from the Pall Corporation for his assistance in the design of the prototype for the closed disposable seeding system. Funding for this study was also provided by the OHSE Committee (Department of Surgery, Yale University School of Medicine) and by a gift from the Pall Corporation.
Disclosure Statement
Dr. Breuer and Dr. Shinoka received grant support from the Pall Corporation and Gunze Corporation. Dr. Snyder receives consulting fees from the Pall Corporation.
References
- 1.Shinoka T., Matsumura G., Hibino N., Naito Y., Watanabe M., Konuma T., Sakamoto T., Nagatsu M., and Kurosawa H.Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg 129,1330, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Hibino N., McGillicuddy E., Matsumura G., Ichihara Y., Naito Y., Breuer C., and Shinoka T.Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg 139,431, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Mirensky T.L., and Breuer C.K.The development of tissue-engineered grafts for reconstructive cardiothoracic surgical applications. Pediatr Res 63,559, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Duncan D., and Breuer C.Challenges in translating vascular tissue engineering to the pediatric clinic. Vasc Cell 14,23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patterson J., Gilliland T., Maxfield M., Church S., Naito Y., Shinoka T., and Breuer C.Tissue-engineered vascular grafts for use in the treatment of congenital heart disease: from the bench to the clinic and back again. Regen Med 7,409, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Udelsman B., Hibino N., Villalona G., McGillicuddy E., Nieponice A., Sakamoto Y., Matsuda S., Vorp D., Shinoka T., and Breuer C.Development of an operator-independent method for seeding tissue-engineered vascular grafts. Tissue Eng Part C Methods 17,731, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hibino N., Nalbandian A., Devine L., Sawh-Martinez R.F., McGillicuddy E., Yi T., Karandish S., Ortolano G., Shin'oka T., Snyder E., and Breuer C.K.Comparison of human bone marrow mononuclear cell isolation methods for creating tissue-engineered vascular grafts: novel filter system versus traditional density centrifugation method. Tissue Eng Part C Methods 17,993, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan M.P., Dardik A., Hibino N., Roh J.D., Nelson G.N., Papademitris X., Shinoka T., and Breuer C.K.Tissue engineered vascular grafts demonstrate evidence of growth and development when implanted in a juvenile animal model. Ann Surg 248,370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roh J.D., Kacena M.A., Lopez-Soler R.I., Coady C.E., Troiano N.W., and Breuer C.K.Glycolmethacrylate is superior to methylmethacrylate for histologic evaluation of biodegradable polymer scaffolds used for vascular tissue engineering. J Histotechnol 29,245, 2006 [Google Scholar]
- 10.Hibino N., Shin'oka T., Matsumura G., Ikada Y., and Kurosawa H.The tissue-engineered vascular graft using bone marrow without culture. J Thorac Cardiovasc Surg 129,1064, 2005 [DOI] [PubMed] [Google Scholar]