Abstract
The effect of blood alcohol concentration (BAC) on outcome after traumatic brain injury (TBI) is controversial. We sought to assess the independent effect of positive BAC on long-term outcome in patients with TBI treated in the intensive care unit (ICU). We performed a retrospective analysis of 405 patients with TBI, admitted to the ICU of a large urban Level 1 trauma center between January 2009 and December 2012. Outcome was six-month mortality and unfavorable neurological outcome (defined as a Glasgow Outcome Scale score of 1 [death], 2, [vegetative state], or 3 [severe disability]). Patients were categorized by admission BAC into: no BAC (0.0‰; n=99), low BAC (<2.3‰; n=140) and high BAC (≥2.3‰; n=166). Logistic regression analysis, adjusting for baseline risk and severity of illness, was used to assess the independent effect of BAC on outcome (using the no BAC group as the reference). Overall six-month mortality was 25% and unfavorable outcome was 46%. Multivariate analysis showed low BAC to independently reduce risk of six-month mortality compared with no BAC (low BAC adjusted odds ratio [AOR] 0.41, 95% confidence interval [CI] 0.19–0.88, p=0.021) and high BAC (AOR 0.58, 95% CI 0.29–1.15, p=0.120). Furthermore, a trend towards reduced risk of six-month unfavorable neurological outcome for patients with positive BAC, compared to patients with negative BAC, was noted, although this did not reach statistical significance (low BAC AOR 0.65, 95% CI 0.34–1.22, p=0.178, and high BAC AOR 0.59, 95% CI 0.32–1.09, p=0.089). In conclusion, low admission BAC (<2.3‰) was found to independently reduce risk of six-month mortality for patients with TBI, and a trend towards improved long-term neurological outcome was found for BAC-positive patients. The role of alcohol as a neuroprotective agent warrants further studies.
Key words: : alcohol, mortality, outcome, prognosis, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is the leading cause of death and disability among the young.1 Alcohol intoxication is a well-known risk factor for TBI and up to half of all TBI patients are under the influence of alcohol at the time of injury.2,3
Experimental data have shown that positive blood alcohol concentrations (BAC) may have neuroprotective effects, which in theory could improve patient prognosis, after TBI.4 Supporting this, numerous clinical studies have shown decreased hospital mortality rates for alcohol intoxicated TBI patients.5–8 However, hospital mortality is a poor outcome measure in TBI, as a majority of TBI patients die following hospital discharge.9 Thus, previous results may be biased.10 Furthermore, there have been concerns that the neuroprotective effects of alcohol may be attributed to confounding factors and inadequate case-mix adjustment.11 To minimize such bias and to improve study quality, the International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT) investigators have suggested standardized covariate adjustment by baseline risk stratification.12,13
Accordingly, we sought to investigate the independent effect of BAC on long-term outcome in patients with TBI treated in the intensive care unit (ICU). We hypothesized that after appropriate case-mix adjustment, using the IMPACT model13 and early computerized tomography (CT) findings for baseline risk stratification and the Acute Physiology and Chronic Health Evaluation II (APACHE II)14 score as an marker of general severity of illness, positive BAC would not be an independent predictor of long-term outcome.
Methods
We conducted a retrospective study including patients with TBI treated in the ICU of a large urban Level 1 trauma center (Töölö Hospital, Helsinki University Hospital; catchment area population approximately two million inhabitants) during a four-year period (January 2009 to December 2012) who had BAC measured on admission. Definition of TBI was a S06.1-S06.9 International Statistical Classification of Diseases and Related Health Problems, Tenth Revision diagnosis, caused by an external force.15 Patients with a history of head trauma but no intra-cranial pathological findings (by CT imaging) during the hospital stay and those with subacute head injuries (>24 h) were not considered. Penetrating head injuries and patients younger than 14 years were excluded.
Patient admission characteristics were assessed by an emergency department physician (neurosurgeon, anesthesiologist, or trauma surgeon) and extracted from subsequent electronic records. Admission BAC was measured by venous blood sampling, and categorized to: no BAC (0.0‰), low BAC (<2.3‰), and high BAC (≥2.3‰).8,16
Primary outcome was six-month mortality, which was retrieved from the Finnish population center (available for all patients). A secondary outcome was six-month neurological outcome (by Glasgow Outcome Scale [GOS]), which was dichotomized to favorable outcome (GOS: 4 [moderate disability], 5 [good outcome]) and unfavorable outcome (GOS 1 [dead], 2 [vegetative state], 3 [severe disability]). Two authors (RR, JS) independently and retrospectively assessed GOS based on outpatient medical charts. GOS assessment agreement was good between the two authors (kappa=0.90, 95% confidence interval [CI] 0.86–0.95). Discrepancies were resolved by verbal discussion.
Treatment guidelines in the trauma center follows the Brain Trauma Foundation guidelines.17 The Helsinki University Hospital ethics committee and The Finnish National Institute for Health and Welfare approved the study and waived the need for informed consent.
Statistical analysis
Differences in univariate variables between the BAC groups were tested using the χ2 test (two-tailed) test for categorical data, the Student's t-test for parametric data, and the Mann-Whitney U test for non-parametric data. Categorical data is presented as number (%), parametric data as mean (standard deviation), and non-parametric as median (interquartile range). To assess the correlation between admission BAC and Glasgow Coma Scale (GCS) score, the Spearman's rho was used.
To assess the independent effect of BAC on outcome, we created a multivariate binary logistic regression model (multivariate analysis). To adjust for case-mix and injury severity differences between the BAC groups, we used the International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT) prognostic model together with the APACHE II scoring system by using the newly developed IMPACT-APACHE II prediction model.18 The IMPACT-APACHE II prediction model has shown superior performance in predicting six-month outcome in patients with TBI compared to the individual IMPACT and APACHE II models.18
We used the IMPACTlab-APACHE II model to achieve best possible case-mix adjustment. The IMPACT lab portion of the IMPACTlab-APACHE II prediction model includes several admission characteristics strongly associated with long-term outcome after TBI: age, motor score, pupillary light reaction, Marshall CT score, presence of traumatic subarachnoid hemorrhage, presence of epidural hematoma, hypotensive or hypoxic insults, glucose and hemoglobin concentrations.13 The APACHE II portion of the IMPACTlab-APACHE II model, on the other hand accounts for 12 physiological variables measured in the first 24 h in the ICU (most abnormal physiological value), and a chronic health evaluation.14 Thus, the IMPACTlab-APACHE II prediction model serves as a marker of both baseline TBI risk and general severity of illness in the ICU. Furthermore, due to the possible risk of BAC-positive patients having a lower GCS score on arrival due to alcohol intoxication and not TBI severity, we also adjusted for the Rotterdam Computerized Tomography (CT) score.19 The final multivariate model included: IMPACTlab-APACHE II Rotterdam CT score, and BAC groups (with no BAC as the reference).
For the statistical analysis, the 2012 IBM SPSS Statistics for Windows, Version, 21.0. (IBM Corp., Armonk, NY) was used.
Results
Baseline characteristics
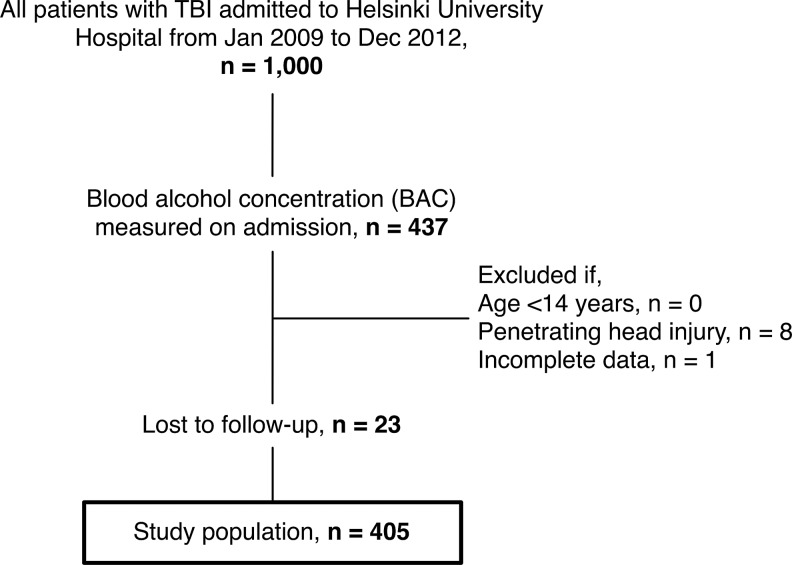
A total of 405 patients, with a median age of 53 years (41–62), were included (Fig. 1). Of included patients, 99 patients (25%) were categorized to the no BAC group, 140 patients (35%) to the low BAC group, and 166 patients (40%) to the high BAC group. Admission BAC showed no statistically significant relationship with admission GCS score (Spearman's rho, 0.056; p=0.264). Accordingly, no significant differences in admission GCS score (p=0.312) or motor score (p=0.272) between the BAC groups were noted. Patient baseline characteristics by BAC group are shown in Table 1. There were no significant differences in age (p=0.052), injury energy (p=0.057), pupillary light reactivity (p=0.112), number of hypoxic (p=0.831) or hypotensive insults (p=0.128), rate of acute mass lesion evacuation (p=0.240), Rotterdam CT score (p=0.416), or length of ICU (p=0.194) or hospital (p=0.788) stay between the three BAC groups. In contrast, traumatic subarachnoid hemorrhage was more frequently seen among the high BAC patients (no BAC, 48%; low BAC, 62%; high BAC, 69%; p=0.003). Furthermore, patients in the low BAC and high BAC groups had lower admission glucose concentrations (p<0.001) and higher hemoglobin concentrations (p<0.001) compared to the BAC-negative patients.
FIG. 1.
Study population flowchart.
Table 1.
Patient Baseline Characteristics
| Variables | No BAC(n=99) | Low BAC(n=140) | High BAC(n=166) | p Value |
|---|---|---|---|---|
| Age | 55 (44–66) | 51 (40–61) | 54 (41–60) | 0.052 |
| Injury mechanism | ||||
| Ground level fall | 53 (53) | 68 (49) | 107 (65) | 0.005 |
| Fall from height | 3 (3) | 13 (9) | 9 (5) | |
| Road traffic accident | 16 (16) | 28 (20) | 13 (8) | |
| Assault or suicide | 11 (11) | 21 (15) | 23 (14) | |
| Other/Unknown | 16 (16) | 10 (7) | 14 (8) | |
| High-injury energy* | 19 (19) | 31 (22) | 20 (12) | 0.057 |
| Glasgow Coma Scale score | ||||
| 3–8 | 56 (57) | 73 (52) | 98 (59) | 0.312 |
| 9–12 | 24 (24) | 27 (19) | 27 (16) | |
| 13–15 | 19 (19) | 40 (29) | 41 (25) | |
| Motor score | ||||
| Obeys/localizes | 56 (57) | 77 (55) | 84 (50) | 0.272 |
| Normal/abnormal flexion | 18 (18) | 28 (20) | 24 (15) | |
| Extension/none | 25 (25) | 35 (25) | 58 (35) | |
| Pupils | ||||
| Both reacts | 19 (19) | 197 (76) | 111 (67) | 0.112 |
| One reacts | 9 (9) | 11 (8) | 28 (17) | |
| None reacts | 71 (72) | 22 (16) | 27 (16) | |
| Hypoxia | 18 (18) | 22 (16) | 30 (18) | 0.831 |
| Hypotension | 5 (5) | 16 (11) | 21 (13) | 0.128 |
| Traumatic SAH | 47 (48) | 87 (62) | 114 (69) | 0.003 |
| Epidural hematoma | 10 (10) | 19 (14) | 16 (10) | 0.516 |
| Acute mass lesion evacuation | 42 (42) | 45 (32) | 57 (34) | 0.240 |
| Marshall CT classification | ||||
| I | 0 (0) | 7 (5) | 2 (1) | 0.034 |
| II | 23 (23) | 30 (21) | 40 (24) | |
| III-IV | 7 (7) | 24 (17) | 23 (14) | |
| EML/NEML | 69 (70) | 79 (57) | 101 (61) | |
| Rotterdam CT score | ||||
| 1–2 | 18 (18) | 31 (22) | 30 (18) | 0.416 |
| 3–4 | 54 (55) | 74 (53) | 79 (48) | |
| 5–6 | 27 (27) | 35 (25) | 57 (34) | |
| Glucose (mmol/L) | 7.8 (6.5–9.7) | 6.7 (5.9–7.8) | 6.7 (6.0–8.3) | <0.001 |
| Hemoglobin (g/L) | 119 (107–132) | 131 (116–144) | 127 (113–142) | <0.001 |
| INR | 1.1 (1.0–1.2) | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | 0.004 |
| Platelet count (109/L) | 183 (115–224) | 196 (146–245) | 191 (140–236) | 0.175 |
| Predicted outcome | ||||
| IMPACTlab sum score | 9 (6–13) | 8 (5–12) | 8 (6–15) | 0.111 |
| APACHE II score | 19 (14–26) | 16 (11–21) | 17 (13–22) | 0.003 |
| IMPACTlab-APACHE II risk | ||||
| Six-month mortality (%) | 10.4 (5.6–44.7) | 6.3 (2.5–21.7) | 10.1 (3.3–31.7) | 0.012 |
| Six-month unfavorable outcome (%) | 37.7 (25.4–72.1) | 29.4 (16.0–55.2) | 36.5 (19.7–64.9) | 0.018 |
| Observed outcome | ||||
| Length of stay (days) | ||||
| ICU | 3 (1–6) | 2 (1–7) | 4 (1–7) | 0.194 |
| Hospital | 10 (4–16) | 9 (4–16) | 9 (5–16) | 0.788 |
| Six-month outcome | ||||
| Median GOS | 3 (1–4) | 4 (3–5) | 4 (1–5) | 0.020 |
| Unfavorable outcome† | 55 (56) | 56 (40) | 75 (45) | 0.057 |
| Mortality | 24 (34) | 25 (18) | 43 (26) | 0.015 |
Continuous variables are presented as median (interquartile range), categorical presented as n (%).
High-energy injury is defined as fall from over 2 m height or speed >20 km/h.
†Defined as a Glasgow Outcome Scale score of 1–3.
BAC, blood alcohol concentration; SAH, subarachnoid hemorrhage; CT, computed tomography; EML, evacuated mass lesions; NEML, non-evacuated mass lesions; INR, International Normalized Ratio; IMPACT, International Mission for Prognosis and Analysis of Clinical Trials in TBI (traumatic brain injury); APACHE II, Acute Physiology and Chronic Health Evaluation II; ICU, intensive care unit; GOS, Glasgow Outcome Scale.
There was no significant difference in baseline TBI severity (by the IMPACT model) between the BAC groups (p=0.111). However, patients in the low BAC group had a significantly lower APACHE II score (indicating better physiology in the first 24 h in the ICU), compared with patients in the no BAC and high BAC groups (p=0.003). Thus, after accounting for both baseline TBI risk and early ICU physiology (by the IMPACTlab-APACHE II), patients in the low BAC group had a significantly lower baseline risk, compared with the no BAC and high BAC groups (p=0.012 for six-month mortality; p=0.018 for six-month unfavorable neurological outcome).
Differences in baseline characteristics between BAC measured (included patients, n=405) and non-measured patients (excluded patients, n=480) are shown in Supplementary Table 1 (see online supplementary material at http://www.liebertpub.com). There were no significant differences in baseline risk or in six-month outcome between included patients and those excluded due to non-measured BAC on admission (p>0.05).
Unadjusted outcome
Overall six-month mortality was 25% (n=102/405) and unfavorable neurological outcome was 46% (n=186/405). After combining the low BAC and the high BAC groups into one positive BAC group and comparing it to the BAC-negative group, we found a significantly lower six-month mortality rate (22% vs. 34%, p=0.026) and unfavorable neurological outcome rate (43% vs. 56%, p=0.027) for the BAC-positive patients. Furthermore, univariate analysis showed a significantly lower six-month mortality rate for patients with low BAC, compared with no BAC and high BAC (no BAC 34%; low BAC 18%; high BAC 26%; p=0.015).
Adjusted outcome
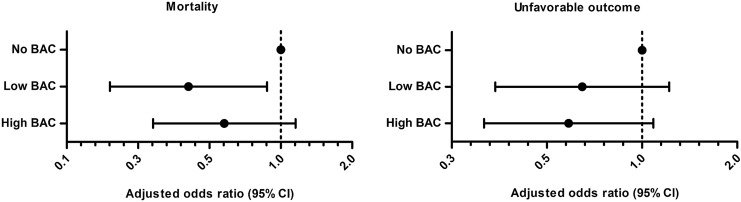
Comparison of all BAC-positive patients with the BAC-negative patients in multivariate logistic regression analysis revealed a significantly reduced risk of six-month mortality (adjusted odds ratio [AOR], 0.48; 95% CI 0.25–0.92; p=0.026) and a trend towards reduced risk of six-month unfavorable neurological outcome (AOR, 0.62; 95% CI 0.36–1.09; p=0.099) for the BAC-positive patients. Further analysis showed low BAC, but not high BAC, to be an independent predictor of reduced risk of six-month mortality, compared to BAC (low BAC AOR 0.41, 95% CI 0.19–0.88, p=0.021, and high BAC AOR 0.58, 95% CI 0.29–1.15, p=0.120; Fig. 2). Furthermore, a trend towards improved six-month neurological outcome was noted for both low BAC and high BAC patients, as compared to BAC-negative patients (low BAC AOR 0.65, 95% CI 0.34–1.22; p=0.178, and high BAC AOR 0.59, 95% CI 0.32–1.09; p=0.089).
FIG. 2.
Independent effect of blood alcohol concentration (blood alcohol concentration) on six-month mortality and unfavorable neurological outcome. Adjusted odds ratios (AORs) for the International Mission for Prognosis and Analysis of Clinical Trials in TBI lab/Acute Physiology and Chronic Health Evaluation II model and the Rotterdam CT (computed tomography) score. To the left, AORs for six-month mortality and to the right, AORs for six-month unfavorable neurological outcome. AORs greater than one indicate an increased risk of poor outcome and vice versa. Low BAC was significantly associated with a reduced risk of six-month mortality (AOR, 0.41; 95% confidence interval 0.19–0.88; p=0.021), compared with no BAC. A trend towards a lower risk of unfavorable neurological outcome was noted for the BAC-positive groups with adjusted odds ratio notably under one, although not reaching statistical significance (p=0.178 for low BAC and p=0.089 for high BAC).
To further validate our results, and minimize the effect of selection bias, we built an additional logistic regression model including patients who were excluded due to non-measured BAC on admission (n=480) as a separate group. Following this, low BAC was still found to independently decrease risk of six-month mortality (AOR, 0.42; 95% CI 0.21–0.87; p=0.020), as compared to no BAC.
Post hoc power analysis
Our study had a 94% power of detecting the 12% absolute difference in mortality between BAC-positive and BAC-negative patients and an 88% power of detecting the 13% absolute difference in unfavorable neurological outcome (power, 80%; type I error, 0.05). We found an absolute difference of 16% in rate of six-month mortality between patients with no BAC and low BAC and an absolute difference of 8% between patients with no BAC and high BAC. Thus, we had a 104% power of detecting the differences in six-month mortality between the no BAC and low BAC patients and a 26% power of detecting the difference between the no BAC and high BAC patients. With regard to neurological outcome, our study had an 80% power of detecting the difference in unfavorable neurological outcome between the no BAC and low BAC groups and a 48% power of detecting the difference in unfavorable neurological outcome between the no BAC and high BAC groups.
Thus, our study was adequately powered for finding the difference in mortality between BAC-positive and BAC-negative patients, as well as mortality differences between the low BAC and no BAC groups. However, for the neurological outcome analysis and the remaining BAC subgroup analyses, our study was underpowered.
Discussion
We conducted a single-center retrospective study investigating the relationship between alcohol intoxication at the time of injury and long-term outcome in patients with TBI treated in the ICU. Initially in univariate analysis, positive admission BAC was significantly associated with a decreased risk of six-month mortality and a trend towards lower rates of unfavorable neurological outcome also was noted. Following case-mix adjustment, low BAC was found to independently decrease risk of six-month mortality, and a trend towards improved six-month neurological outcome was noted for the BAC-positive patients, although the latter did not reach statistical significance. Post hoc power analysis revealed our study to be underpowered to detect the decrease in unfavorable outcome, and adequately powered to detect the difference in mortality. Thus, the reason why neurological outcome was found to be insignificant may simply be due to lack of power.
Several studies have shown reduced hospital mortality rates for alcohol intoxicated TBI patients.5–8,16,20–22 However, most of these studies are deficient in baseline risk adjustment and concerns about the validity of these results have been raised.11,23,24 Furthermore, most previous studies have used hospital mortality as primary end point, which may be a cause of significant result bias, especially in TBI patients, where a majority die following hospital discharge.9,10 Few studies have investigated the effect of BAC on long-term outcome after TBI. Alexander and colleagues found no relationship between BAC on admission and GOS at 3, 6, or 12-months following injury.23 Likewise, Shandro and colleagues found no significant association between BAC on admission and 3 and 12 months after injury.24 In contrast to these findings, we found low BAC to significantly favor survival, with an odds ratio of approximately 2.5.
The effect of high BAC on hospital mortality is controversial, some studies have found decreased mortality rates with increasing BAC levels and some studies have found increasing mortality rates with increasing BAC levels.8,16 It has been suggested that the neurotoxic effects of high BAC cancels out the neuroprotective effects of low BAC.4 In the present study, a trend towards a reduced risk of six-month mortality and unfavorable neurological outcome was noted for patients in the high BAC group, compared with the no BAC group. However, these analyses were largely underpowered (26% for mortality and 48% for unfavorable outcome) and may explain why they remained statistically insignificant.
The neuroprotective mechanisms of alcohol have been attributed to inhibition of N-methyl-D-aspartate receptor–mediated excitotoxicity, blunting of adrenergic response and catecholamine surge, improved cerebral metabolic coupling, inhibition of pro-inflammatory cytokines, attenuation of TBI induced hyperthermia, and lower incidence of coagulopathy.21,25–28 As a surrogate marker of inhibited adrenergic response, patients with positive BAC were found to have significantly lower admission glucose concentrations, compared with patients with negative BAC. Furthermore, alcohol has been shown to lower cerebral blood flow (and consequently intracranial pressure), and reduce risk of post-admission pneumonia, which might partly explain the improved outcome.23,29
However, it also has been shown that alcohol in high levels disturbs the body's ability to compensate for shock, causing significant hemodynamic and respiratory dysregulation.16,30 Another aspect that should to be considered is the effect of alcohol on GCS score. The level of consciousness-lowering effects of alcohol has been known for a long time.31 However, the relationship between alcohol and GCS score remains controversial, with some studies suggesting a significant reduction in GCS score due to alcohol intoxication and some suggesting no relationship.32–34 This is of importance as alcohol intoxicated patients may be classified as having a more severe TBI due to the potential GCS score–lowering effects of alcohol.34
Accordingly, when assessing the role of BAC on outcome, it may misleadingly appear that alcohol intoxicated patients have a better outcome, compared with sober patients (at the time of injury), as they drastically recover from low GCS score. Such case-mix confounders have been a major concern in previous studies. However, in the present study we adjusted for both admission characteristics (by the IMPACT model) and early ICU care predictors (by the APACHE II) by using a novel prediction model, the IMPACT-APACHE II prediction model.18 The IMPACT-APACHE II model diminishes the GCS score–lowering effects of alcohol as it includes both admission GCS score (motor score component) and a GCS score from the first 24 h in the ICU. Furthermore, there were no differences in TBI severity as measured by the Rotterdam CT score between the BAC groups. Thus, case-mix differences between the BAC-positive and negative groups did probably not skew our results.
There are limitations to the present study that have to be acknowledged. First, due to the retrospective nature of this study, all patients did not undergo routine BAC screening upon admission to the hospital. This leaves a significant amount of patients in the study population who were not tested for BAC and thus potentially causing selection bias. However, we found no differences in baseline characteristics or outcome measures between included patients and patients excluded (Supplementary Table 1). Thus, we do not consider selection bias to be major limitation of the present study. Furthermore, our results remained robust after we validated them by including the missing BAC patients as a separate control group in multivariate analysis. Second, our findings come from one single trauma center and our results should be replicated in different settings. Third, we were not able to assess the actual BAC at the time of injury but instead used BAC measured upon hospital admission. Although all patients were acutely admitted (<24 h post-injury), some patients may have had higher BAC at the time of injury than measured in the hospital. Fourth, we were not able to accurately differentiate between patients with a history of chronic alcohol abuse and the occasional drinker. Further studies should aim at distinguishing between these two patient groups as the chronic alcohol abuse may be an important confounding factor.35
Conclusion
Low admission BAC (<2.3‰) independently reduced risk of long-term mortality in patients with TBI treated in the ICU. Furthermore, a trend towards improved long-term neurological outcome for BAC-positive patients was noted, although not reaching statistical significance. The role of alcohol as a neuroprotective agent warrants further studies.
Supplementary Material
Author Disclosure Statement
The study was funded by a Helsinki University Hospital EVO grant (TYH2012142), Medicinska Understödsföreningen Liv och Hälsa and the Maire Taponen foundation. No competing financial interests exist.
References
- 1.Lu J.J., Marmarou A.A., Choi S.S., Maas A.A., Murray G.G., and Steyerberg E.W.E. (2004). Mortality from traumatic brain injury. Acta Neurochir. Suppl. 95, 281–285 [DOI] [PubMed] [Google Scholar]
- 2.Tagliaferri F., Compagnone C., Korsic M., Servadei F., and Kraus J. (2006). A systematic review of brain injury epidemiology in Europe. Acta Neurochir (Wien) 148, 255–68 [DOI] [PubMed] [Google Scholar]
- 3.Savola O., Niemelä O., and Hillbom M. (2005). Alcohol intake and the pattern of trauma in young adults and working aged people admitted after trauma. Alcohol Alcohol. 40, 269–273 [DOI] [PubMed] [Google Scholar]
- 4.Kelly D.F. (1995). Alcohol and head injury: an issue revisited. J. Neurotrauma 12, 883–890 [DOI] [PubMed] [Google Scholar]
- 5.Salim A., Ley E.J., Cryer H.G., Margulies D.R., Ramicone E., and Tillou A. (2009). Positive serum ethanol level and mortality in moderate to severe traumatic brain injury. Arch. Surg. 144, 865–871 [DOI] [PubMed] [Google Scholar]
- 6.Salim A., Teixeira P., Ley E.J., DuBose J., Inaba K., and Margulies D.R. (2009). Serum ethanol levels: predictor of survival after severe traumatic brain injury. J. Trauma 67, 697–703 [DOI] [PubMed] [Google Scholar]
- 7.Berry C., Salim A., Alban R., Mirocha J., Margulies D.R., and Ley E.J. (2010). Serum ethanol levels in patients with moderate to severe traumatic brain injury influence outcomes: a surprising finding. Am. Surg. 76, 1067–1070 [PubMed] [Google Scholar]
- 8.Berry C., Ley E.J., Margulies D.R., Mirocha J., Bukur M., Malinoski D., and Salim A. (2011). Correlating the blood alcohol concentration with outcome after traumatic brain injury: too much is not a bad thing. Am. Surg. 77, 1416–1419 [PubMed] [Google Scholar]
- 9.Myburgh J.A., Cooper D.J., Finfer S.R., Venkatesh B., Jones D., Higgins A., Bishop N., Higlett T., Australasian Traumatic Brain Injury Study (ATBIS) Investigators for the Australian, New Zealand Intensive Care Society Clinical Trials Group. (2008). Epidemiology and 12-month outcomes from traumatic brain injury in Australia and New Zealand. J. Trauma 64, 854–862 [DOI] [PubMed] [Google Scholar]
- 10.Pouw M.E., Peelen L.M., Moons K.G.M., Kalkman C.J., and Lingsma H.F. (2013). Including post-discharge mortality in calculation of hospital standardised mortality ratios: retrospective analysis of hospital episode statistics. BMJ 347, f5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C.M., Yi H.-Y., Yoon Y.-H., and Dong C. (2012). Alcohol use at time of injury and survival following traumatic brain injury: results from the National Trauma Data Bank. J. Stud. Alcohol. Drugs 73, 531–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maas A.I.R., Murray G.D., Roozenbeek B., Lingsma H.F., Butcher I., McHugh G.S., Weir J., Lu J., Steyerberg E.W., International Mission on Prognosis Analysis of Clinical Trials in Traumatic Brain Injury (IMPACT) Study Group. (2013). Advancing care for traumatic brain injury: findings from the IMPACT studies and perspectives on future research. Lancet Neurol. 12, 1200–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steyerberg E.W., Mushkudiani N., Perel P., Butcher I., Lu J., McHugh G.S., Murray G.D., Marmarou A., Roberts I., Habbema J.D.F., and Maas A.I.R. (2008). Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 5, e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knaus W.A., Draper E.A., Wagner D.P., and Zimmerman J.E. (1985). APACHE II: a severity of disease classification system. Crit. Care Med. 13, 818–829 [PubMed] [Google Scholar]
- 15.Menon D.K., Schwab K., Wright D.W., Maas A.I., Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health. (2010). Position statement: definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 91, 1637–164021044706 [Google Scholar]
- 16.Tien H.C.N., Tremblay L.N., Rizoli S.B., Gelberg J., Chughtai T., Tikuisis P., Shek P., and Brenneman F.D. (2006). Association between alcohol and mortality in patients with severe traumatic head injury. Arch. Surg. 141, 1185–1191 [DOI] [PubMed] [Google Scholar]
- 17.Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons. (2007). Guidelines for the management of severe traumatic brain injury. J. Neurotrauma 24Suppl 1, S1–S106 [DOI] [PubMed] [Google Scholar]
- 18.Raj R., Siironen J., Kivisaari R., Hernesniemi J., and Skrifvars M.B. (2014). Predicting outcome after traumatic brain injury: development of prognostic scores based on the IMPACT and the APACHE II. J. Neurotrauma 2014August29; [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maas A.I.R., Hukkelhoven C.W.P.M., Marshall L.F., and Steyerberg E.W. (2005). Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery 57, 1173–1182 [DOI] [PubMed] [Google Scholar]
- 20.Talving P., Plurad D., Barmparas G., DuBose J., Inaba K., Lam L., Chan L., and Demetriades D. (2010). Isolated severe traumatic brain injuries: association of blood alcohol levels with the severity of injuries and outcomes. J. Trauma 68, 357–362 [DOI] [PubMed] [Google Scholar]
- 21.Lustenberger T., Inaba K., Barmparas G., Talving P., Plurad D., Lam L., Konstantinidis A., and Demetriades D. (2011). Ethanol intoxication is associated with a lower incidence of admission coagulopathy in severe traumatic brain injury patients. J. Neurotrauma 28, 1699–1706 [DOI] [PubMed] [Google Scholar]
- 22.O'Phelan K., McArthur D.L., Chang C.W.J., Green D., and Hovda D.A. (2008). The impact of substance abuse on mortality in patients with severe traumatic brain injury. J. Trauma 65, 674–677 [DOI] [PubMed] [Google Scholar]
- 23.Alexander S., Kerr M.E., Yonas H., and Marion D.W. (2004). The effects of admission alcohol level on cerebral blood flow and outcomes after severe traumatic brain injury. J. Neurotrauma 21, 575–583 [DOI] [PubMed] [Google Scholar]
- 24.Shandro J.R., Rivara F.P., Wang J., Jurkovich G.J., Nathens A.B., and MacKenzie E.J. (2009). Alcohol and risk of mortality in patients with traumatic brain injury. J. Trauma 66, 1584–1590 [DOI] [PubMed] [Google Scholar]
- 25.Gottesfeld Z., Moore A.N., and Dash P.K. (2002). Acute ethanol intake attenuates inflammatory cytokines after brain injury in rats: a possible role for corticosterone. J. Neurotrauma 19, 317–326 [DOI] [PubMed] [Google Scholar]
- 26.Taylor A.N., Romeo H.E., Beylin A.V., Tio D.L., Rahman S.U., and Hovda D.A. (2002). Alcohol consumption in traumatic brain injury: attenuation of TBI-induced hyperthermia and neurocognitive deficits. J. Neurotrauma 19, 1597–1608 [DOI] [PubMed] [Google Scholar]
- 27.Opreanu R.C., Kuhn D., and Basson M.D. (2010). Influence of alcohol on mortality in traumatic brain injury. J. Am. Coll. Surg. 210, 997–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly D.F., Kozlowski D.A., Haddad E., Echiverri A., Hovda D.A., and Lee S.M. (2000). Ethanol reduces metabolic uncoupling following experimental head injury. J. Neurotrauma 17, 261–272 [DOI] [PubMed] [Google Scholar]
- 29.Hadjibashi A.A., Berry C., Ley E.J., Bukur M., Mirocha J., Stolpner D., and Salim A. (2012). Alcohol is associated with a lower pneumonia rate after traumatic brain injury. J. Surg. Res. 173, 212–215 [DOI] [PubMed] [Google Scholar]
- 30.Molina P.E., Sulzer J.K., and Whitaker A. (2013). Alcohol Abuse and The Injured Host; Dysregulation of Counteregulatory Mechanisms: Review. Shock 39, 240–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacEwen W. (1879). The diagnosis of alcoholic coma. Glasgow Med. J. 11, 1–14 [PMC free article] [PubMed] [Google Scholar]
- 32.Stuke L., Diaz-Arrastia R., Gentilello L.M., and Shafi S. (2007). Effect of alcohol on Glasgow Coma Scale in head-injured patients. Ann. Surg. 245, 651–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lange R.T., Iverson G.L., Brubacher J.R., and Franzen M.D. (2010). Effect of blood alcohol level on Glasgow Coma Scale scores following traumatic brain injury. Brain Inj. 24, 919–927 [DOI] [PubMed] [Google Scholar]
- 34.Shahin H., Gopinath S.P., and Robertson C.S. (2010). Influence of alcohol on early Glasgow Coma Scale in head-injured patients. J. Trauma 69, 1176–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jurkovich G.J.G., Rivara F.P.F., Gurney J.G.J., Fligner C.C., Ries R.R., Mueller B.A.B., and Copass M.M. (1993). The effect of acute alcohol intoxication and chronic alcohol abuse on outcome from trauma. JAMA 270, 51–56 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.