Abstract
The application of cell-derived extracellular matrix (ECM) in tissue engineering has gained increasing interest because it can provide a naturally occurring, complex set of physiologically functional signals for cell growth. The ECM scaffolds produced from decellularized fibroblast cell sheets contain high amounts of ECM substances, such as collagen, elastin, and glycosaminoglycans. They can serve as cell adhesion sites and mechanically strong supports for tissue-engineered constructs. An efficient method that can largely remove cellular materials while maintaining minimal disruption of ECM ultrastructure and content during the decellularization process is critical. In this study, three decellularization methods were investigated: high concentration (0.5 wt%) of sodium dodecyl sulfate (SDS), low concentration (0.05 wt%) of SDS, and freeze-thaw cycling method. They were compared by characterization of ECM preservation, mechanical properties, in vitro immune response, and cell repopulation ability of the resulted ECM scaffolds. The results demonstrated that the high SDS treatment could efficiently remove around 90% of DNA from the cell sheet, but significantly compromised their ECM content and mechanical strength. The elastic and viscous modulus of the ECM decreased around 80% and 62%, respectively, after the high SDS treatment. The freeze-thaw cycling method maintained the ECM structure as well as the mechanical strength, but also preserved a large amount of cellular components in the ECM scaffold. Around 88% of DNA was left in the ECM after the freeze-thaw treatment. In vitro inflammatory tests suggested that the amount of DNA fragments in ECM scaffolds does not cause a significantly different immune response. All three ECM scaffolds showed comparable ability to support in vitro cell repopulation. The ECM scaffolds possess great potential to be selectively used in different tissue engineering applications according to the practical requirement.
Introduction
The extracellular matrix (ECM) is the extracellular part of animal tissues, which is primarily composed of (1) fibrous structural proteins, such as collagen, fibronectin, vitronectin, and elastin; (2) proteoglycans, such as glycosaminoglycans (GAGs), that covalently attached to some core proteins; and (3) specialized proteins, such as growth factors and small matricellular proteins.1 Cellular interactions with the ECM are known to play a critical role in providing structural support to cells, directing cell function, and regulating development, homeostasis, and repair of a variety of tissues.2 Thus, tissue engineering strives to regenerate or replace malfunctioning tissues with cells seeded on supporting scaffolds designed to mimic the natural ECM in order to restore the functions of cells reseeded in the scaffolds. Besides the widely studied ECM scaffolds derived from animal tissues or organs, the ECM scaffolds fabricated from cultured cells have gained increased interest.3–6 Compared with animal tissues, cell-derived ECM avoids the risk of pathogen transfer caused by allogenic ECM and adverse host immune response caused by xenogenic ECM.7 In addition, different cell types could be used to fabricate different types of ECM that provide various sets of functional biological signals. Furthermore, the microarchitecture of the cell-derived ECM scaffolds can be modulated to achieve different space organization in order to enhance biomimicry.8,9 Several studies have shown that there is superiority for the application of cell-derived ECM as a scaffold.4,5,10 The cell-derived ECM can significantly enhance cell adhesion, migration, proliferation, and acquisition of in vivo-like morphology compared with reconstituted ECM.11 Decellularized ECM synthesized by undifferentiated mesenchymal stem cells (MSCs) in vitro has been shown to facilitate cell proliferation, prevent spontaneous differentiation, and enhance the chondrogenic and osteogenic potential of freshly reseeded MSCs.6
Fibroblasts are able to continuously grow for 14 weeks, forming a thick and robust multilayer cell sheet and secrete abundant ECM proteins and proteoglycans.12 ECM derived from fibroblast cell sheets is mainly composed of super-molecularly organized collagen fibers and more closely resembles native tissue than conventional biopolymers used for tissue engineering scaffolds.13,14 The ECM produced by fibroblasts is also mechanically stronger than reconstituted ECM, such as collagen gel and fibrin gel.15 The mechanical strength tests of tubes or strands based on fibroblast-derived ECM provide a preliminary indication that the ECM may be stronger compared with collagen gels and approach native tissue physical properties.16,17 Tissue-engineered blood vessels were successfully fabricated from human fibroblast-derived ECM seeded with smooth muscle cells.3,18,19 The vascular constructs demonstrated vasoactivity, improved mechanical properties, and reduced fabrication time. The ECM scaffolds are stable enough to be stored in phosphate-buffered saline (PBS) for future use; thus, the production time of tissue-engineered constructs can be significantly reduced. An ECM scaffold derived from fibroblast cell sheets holds great potential in serving as a tissue-engineering scaffold to construct strong and complex biological tissues.
Decellularization is a necessary process to prepare ECM from animal tissues or cultured cells. The goal of decellularization is to maximize the removal of cellular components while minimizing the ECM loss and damage. Several articles have systematically reviewed different decellularization methods for tissues or organs.20,21 The conventional decellularization protocols usually involve the combination of thermal shock, chemical treatment, osmotic shock, and mechanical disruption. Detergent is one of the most common chemical treatments for tissue decellularization. The ionic detergent sodium dodecyl sulfate (SDS) appears to be more effective than other detergents in removing cell nuclei from tissues, but more disruptive to ECM.22 Osmotic shock can readily cause cell lysis with minimal change to the matrix molecules and architecture.23 Thermal shock, or freeze-thaw cycling, does not significantly reduce the ECM proteins nor the mechanical strength of the tissues.24,25 It was reported that 0.5 wt% SDS can remove more than 99% DNA from equine carotid artery.26 Some reported that only 0.05 wt% SDS was efficient in decellularization of rat lung.27 Successful decellularization is highly dependent on tissue types due to their difference in cellular density and ECM composition. However, animal tissues or organs are quite different from cultured human cells. Very few studies have attempted a comprehensive and quantitative analysis of cell-derived ECM focusing on decellularization efficacy, mechanical strength, and cell repopulation. Therefore, it is critical to compare different decellularization procedures to optimize the production of cell-derived ECM.
In this work, we performed three decellularization methods on fibroblast cell sheets, including SDS treatment with high and low concentrations and freeze-thaw cycling. The effects of the decellularization process on removal of cellular components, ECM preservation and morphology, mechanical strength, in vitro immune response, and cell repopulation ability were compared.
Materials and Methods
Fibroblast cell sheet culture
Polydimethylsiloxane (PDMS) substrate was coated with bovine collagen I to facilitate cell adhesion, following our previous publication.28 Human dermal fibroblasts (ATCC) between passage 3 and 5 were seeded on the PDMS at a density of 10,000 cells/cm2. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 20% fetal bovine serum (FBS), 20% Ham F12, 500 μM sodium ascorbate, and 1% penicillin/streptomycin (Life Technologies). The culture was maintained by changing medium twice per week and cells were allowed to proliferate for 6 weeks. The cell sheet was harvested by gently pulling the cell layers off the PDMS substrate.
Decellularization of fibroblast cell sheet
The following three decellularization methods were performed:
(1) High SDS: The fibroblast cell sheet was placed into the first decellularization solution, which contained 1 M NaCl, 10 mM Tris, and 5 mM EDTA (Sigma). The cell sheet was shaken for 1 h at room temperature and rinsed thoroughly with PBS. The cell sheet was then placed in a second decellularization solution containing 0.5 wt% SDS, 10 mM Tris, and 25 mM EDTA (Sigma), and shaken for 0.5 h at room temperature. After a PBS wash, the sample was rinsed in DMEM with 20% FBS for 48 h at room temperature and rinsed again with PBS.
(2) Low SDS: The procedure was same as the aforementioned high SDS treatment except for using 0.05 wt% SDS.
(3) Freeze-thaw: The fibroblast cell sheet was placed in a −80°C freezer for 30 min and thawed in 37°C PBS and rinsed with PBS. This cycle was repeated three times. The sample was then rinsed in DMEM with 20% FBS for 48 h at room temperature and rinsed again with PBS.
DNA staining and quantification
Samples were fixed and incubated with diamidino-2-phenylindole (DAPI) solution to counterstain the cell nuclei, and then viewed by using an Olympus BX-51 fluorescent microscope (Olympus America). The DNA content in the samples was determined fluorometrically using PicoGreen assay kit (Life Technologies). Briefly, cells were lysed using proteinase K solution at 37°C for 2 h. Samples of 100 μL were placed in triplicate in a 96-well plate and mixed with 100 μL of Picogreen. The plate was incubated at room temperature for 10 min in the dark and then read on Fluoroskan Ascent FL fluorescent plate reader (Thermo Fisher Scientific).
β-Actin analysis using western blot
Proteins were extracted from fibroblast cell sheets and decellularized ECM scaffolds in lysis buffer, following our previous publications.28 The total protein concentration was determined using BCA Protein Assay Reagent (Thermo Fisher Scientific). The lysate samples were separated by SDS gel polyacrylamide electrophoresis, and transferred electrophoretically onto poly(vinylidene fluoride) membrane. The membrane was immunoblotted with rabbit polyclonal antibody to β-actin, followed by horseradish peroxidase–conjugated goat anti-rabbit secondary antibody. Blots were developed using enhanced chemiluminescence (Bio-Rad).
ECM staining and morphology observation
Samples were fixed, blocked, and incubated with the primary antibody against fibronectin/collagen I (Abcam). Samples were then washed and incubated with a mixture of secondary antibodies conjugated to Alexa Fluor 488 (Life Technologies). Finally the samples were mounted and viewed using an Olympus BX-51 fluorescent microscope. For scanning electron microscopy (SEM) imaging, the samples were fixed with 4% paraformaldehyde, washed with PBS, and then dehydrated through a graded series of ethanol. Finally the samples were dried in hexamethyldisilazane (Sigma), sputter-coated with a 10-nm platinum coating, and viewed using a Hitachi S-4700 field emission scanning electron microscope.
ECM in situ enzyme-linked immunosorbent assay
ECM proteins collagen I and fibronectin were quantified by in situ enzyme-linked immunosorbent assay (ELISA) following a published method.29 Briefly, samples were fixed in 4% PFA for 30 min and washed with PBS. Then, samples were permeabilized with 0.2% Triton X-100 and blocked in 1% BSA in 0.2% Triton X-100 for 30 min. Samples were incubated in primary antibodies overnight at 4°C and then incubated with an alkaline-phosphatase-conjugated secondary antibody for 1 h at room temperature. After washing, samples were incubated in the para-nitrophenolphosphate substrate at 37°C for 15 min before a 0.5 N NaOH stop solution was added. The absorbance was measured at wavelength of 405 nm and background absorbance was measured at 655 nm.
Collagen content quantification
Collagen was quantified by a colorimetric analysis using a hydroxyproline assay kit (Sigma). Fibroblast cell sheets and decellularized ECM scaffolds were homogenized in centrifuge tubes with deionized H2O and hydrolyzed in 12 M HCl at 120°C for 3 h. Twenty-five microliters of supernatant was then transferred into a 96-well plate in duplicate and the manufacturer's instruction was followed. The collagen-to-hydroxyproline ratio of 10:1 w/w was used to calculate the total collagen content in the tissue.
Elastin content quantification
Elastin was quantified using the Fasting Elastin Assay Kit (Biocolor Ltd.). Samples were treated with 0.25 M oxalic acid at 100°C for 1 h and then centrifuged at 12,000 rpm. The extraction was repeated three times, and the supernatant was pooled and analyzed following the manufacturer's protocol.
GAG content quantification
The GAG content of fibroblast cell sheet and various acellular samples was determined using the Blyscan Sulfated Glycosaminoglycan Assay Kit (Biocolor Ltd.). Samples were prepared by digestion of each construct using papain extraction reagent (0.1 mg/mL papain) and heated at 65°C for 3 h. After centrifuge at 10,000 g for 10 min, the supernatant was collected and assayed following the manufacturer's protocol. Sulfated GAG (sGAG) is tested here to represent the content of total GAG because most GAGs are sulfated.
Growth factor content quantification
The concentration of matrix-bound basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) was analyzed following a method described previously.30 The samples were incubated in 1 mL of urea-heparin extraction buffer containing 2 M urea and 5 mg/mL heparin in 50 mM Tris (pH 7.4) for 24 h at 4°C. After centrifuge at 12,000 rpm for 10 min, the supernatant was collected for analysis using human bFGF and human VEGF ELISA kits (R&D Systems).
Mechanical property evaluation
The mechanical strength measurement was performed in Bohlin CVOR rheometer (Malvern Instruments) using a parallel plate of 25 mm in diameter. The measurement went through frequency scanning at room temperature in the range of 0.1–2 Hz. All measurements were performed in the linear viscoelastic regime with a fixed strain of 0.5. The elastic modulus and viscous modulus were recorded as a function of frequency. Each measurement was performed at least three times on three different samples.
In vitro inflammatory response test
The human acute monocytic leukemia THP1 cells were obtained from ATCC, and maintained in RPMI 1640 medium supplemented with 10% FBS, 0.1% beta-mercaptoethanol, and 1% penicillin/streptomycin. To induce the differentiation of THP1 cells into monocyte-derived macrophages, THP1 cells (6.8×105/mL) were incubated in the cell culture media with 200 nM phorbol 12-myristate 13-acetate (Sigma) for 3 days. The differentiated cells were collected and seeded on decellularized cell sheets at the seeding density of 1.1×104/cm2. After 3 days, cell culture medium was changed to serum-free medium containing 1 μg/mL lipopolysaccharide (Sigma). The medium was collected at 4 and 24 h to quantify the secretion of tumor necrosis factor (TNF)-α and interleukin (IL)-10 using an ELISA kit (Abcam).
Recellularization and cell proliferation assay
Bone-marrow-derived human MSCs (hMSCs) were provided by Texas A&M University Health Sciences Center. The decellularized ECM scaffolds were sterilized with 70% ethanol and rinsed with PBS. Passage-5 hMSCs were seeded at the density of 5000 cells/cm2 and cultured in α-MEM supplemented with 20% FBS, 1% L-glutamine, and 1% penicillin/streptomycin (Life Technologies). At 6 h after seeding, the medium was removed and the samples were washed with PBS three times. The medium and the washing PBS were collected to calculate seeding efficiency by DNA assay. At days 1, 3, and 7, samples were fixed and stained with Ki67, F-actin, and DAPI. The immunofluorescence staining was viewed by Olympus Fluoview FV-1000 confocal fluorescence microscopy (Olympus America). The percentage of Ki67-positive cells was calculated as the number of Ki67-positive cells divided by the total number of cells obtained from DAPI staining, and the data were pooled for statistical analysis (n=3 for each sample).
Statistics/data analysis
Experiment results were expressed as means±standard deviations of the means of the samples. Student's t-test (Microsoft Excel) was used for comparisons and statistical significance was accepted at p<0.05.
Results
DNA staining and quantification
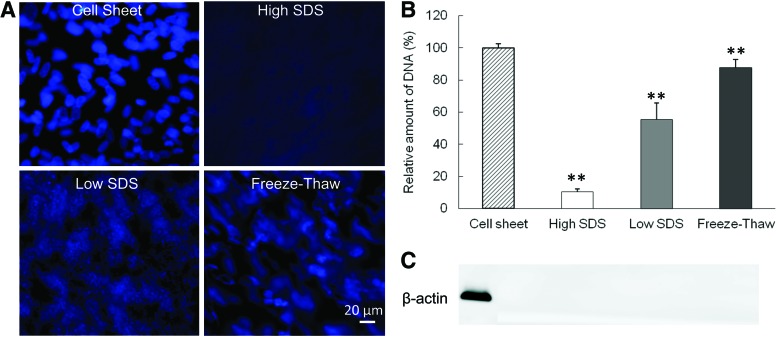
The decellularization efficiency was examined by DNA immunofluorescence staining and quantification, as well as immunoblotting for β-actin. Cell nuclei in fibroblast cell sheet exhibited normal round shape and bright blue DAPI staining (Fig. 1A). The three decellularization protocols resulted in three distinct levels of DNA content. The freeze-thaw treatment damaged most of the cell nuclear shape, but the DAPI staining could be clearly observed. The low SDS treatment caused the DAPI staining to appear as a diffused smear in the ECM, while after the high SDS treatment very little DAPI staining could be seen in the ECM. The DAPI staining was consistent with the DNA quantification (Fig. 1B). The amount of DNA in the ECM scaffolds after decellularization was 10.4%±0.7%, 55.4%±10.4%, and 87.7%±5.0% for the high SDS, low SDS, and freeze-thaw treatments, respectively. The cytoplasmic protein β-actin was absent from all treated samples (Fig. 1C).
FIG. 1.
Comparison of cellular components before and after decellularization. (A) Fluorescence diamidino-2-phenylindole (DAPI) staining of DNA. Scale bar=20 μm. (B) Quantification of relative DNA amount. **p<0.01 compared with cell sheet. (C) β-Actin staining from western blot. SDS, sodium dodecyl sulfate. Color images available online at www.liebertpub.com/tec
Characterization of ECM components
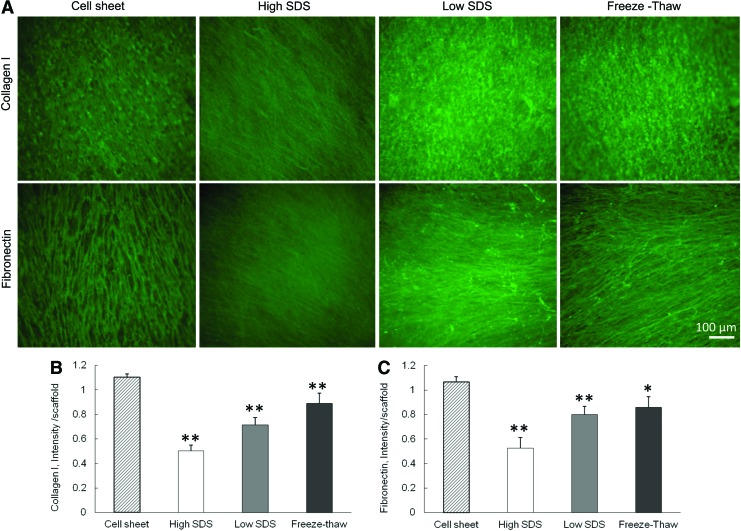
The immunofluorescent staining of collagen I and fibronectin showed that these two ECM components were preserved in the scaffolds after all the three decellularization treatments (Fig. 2A). However, the ultrastructure of the ECM fibers became different. The collagen I and fibronectin staining of cell sheet demonstrated a fibrous structure with pores that were caused by the presence of cell nuclei. The high SDS treatment removed almost all of the cell nuclei and the ECM fibers were more closely stacked with each other. The freeze-thaw treatment maintained the original ECM fibrous structure with pores because of the minimal damage to the cell nuclei. The quantification of collagen I and fibronectin revealed the difference among the three decellularization processes. After decellularization, both collagen I and fibronectin expression in the three ECM scaffolds was significantly decreased compared with cell sheets. The collagen I retained in ECM was 45.4%±4.3% for high SDS, 64.8%±5.4% for low SDS, and 80.6%±7.7% for freeze-thaw treatments. The fibronectin retained in ECM was 49.3%±8.1% for high SDS, 75.0%±6.3% for low SDS, and 80.5%±8.2% for freeze-thaw treatments.
FIG. 2.
Characterization of collagen I and fibronectin before and after different decellularization methods. (A) Fluorescence staining of collagen I and fibronectin. Scale bar=100 μm. (B) Quantification of collagen I. (C) Quantification of fibronectin. *p<0.05, **p<0.01 compared with cell sheet. Color images available online at www.liebertpub.com/tec
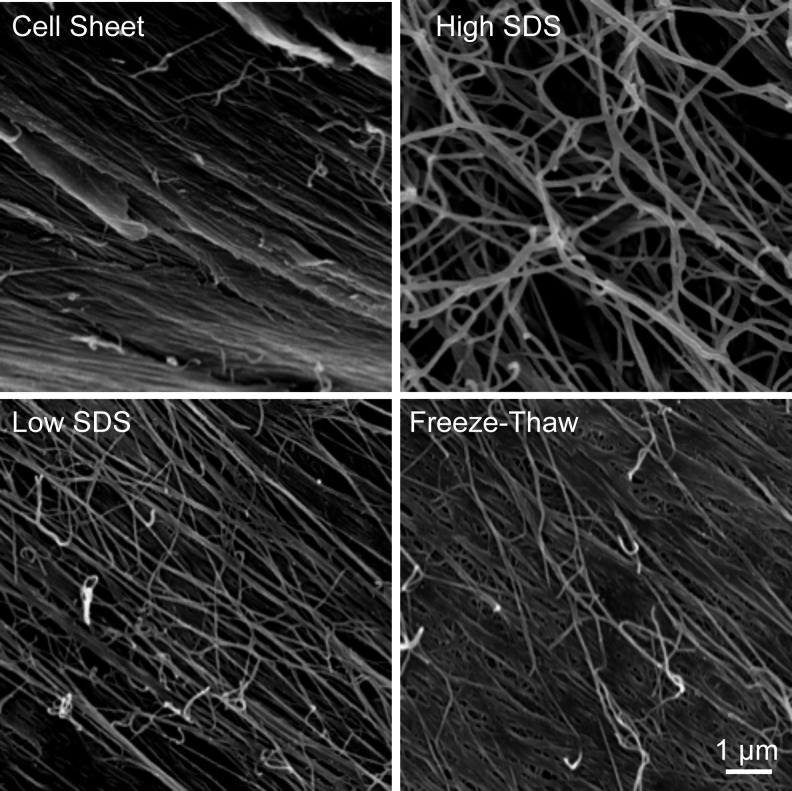
SEM images revealed a continuous three-dimensional (3D) nanofibrous ECM microstructure in both cell sheets and decellularized scaffolds (Fig. 3). The protein fibers embedded in the cell sheets were packed together with adhesive molecules, which possess multiple binding domains capable of binding collagen and proteoglycans. The decellularization processes removed some adhesive molecules and proteins; thus, the protein fibers were clearly exposed. Compared with low SDS and freeze-thaw treatments, high SDS treatment removed the proteins more efficiently.
FIG. 3.
Scanning electron microscopy images of fibroblast cell sheet before and after decellularization. Scale bar=1 μm.
Collagen, elastin, and GAG content analyses
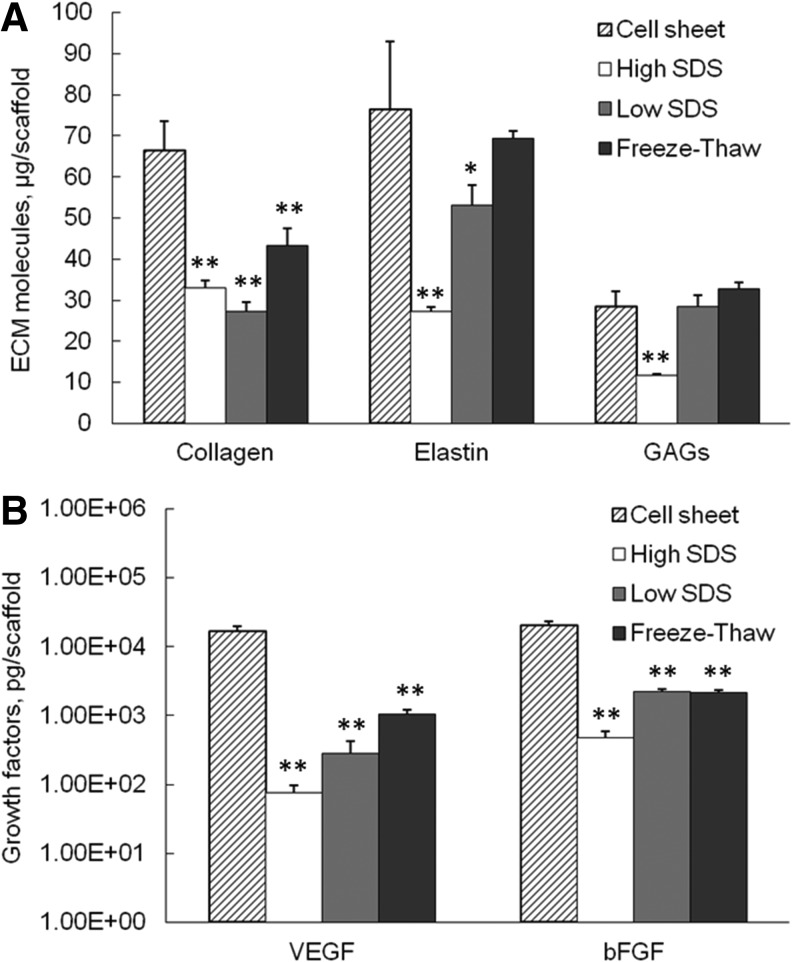
As shown in Figure 4A, the average collagen content in each scaffold significantly decreased after all decellularization processes compared with cell sheet, with 33.1±1.8 μg collagen remained per scaffold in high SDS (p<0.01)–treated samples, 27.2±2.5 μg for low SDS (p<0.01), and 43.2±4.3 μg for freeze-thaw treatments (p<0.01). The elastin content also decreased significantly in high-SDS- (27.3±0.9 μg/scaffold, p<0.01) and low-SDS- (53.0±5.1 μg/scaffold, p<0.05) treated samples compared with cell sheet (76.6±16.4 μg/scaffold, p<0.05). The freeze-thaw treatment preserved comparable elastin amount (p>0.05) with the cell sheet. Only high-SDS-treated samples had significant reduction in GAG content compared with cell sheet, with 11.7±0.4 μg GAG per scaffold verses 28.5±3.6 μg GAG per cell sheet (p<0.01). The GAG amount in low-SDS- and freeze-thaw-treated samples was 28.5±2.8 μg and 32.8±1.6 μg per scaffold, respectively, which were not statistically different from the GAG amount in the fibroblast cell sheet (p>0.05).
FIG. 4.
Extracellular matrix (ECM) molecules (A) and growth factor (B) content in fibroblast cell sheet and decellularized tissues. *p<0.05, **p<0.01 compared with cell sheet.
Growth factor quantification
As shown in Figure 4B, the matrix-bound growth factors, including VEGF and bFGF, dramatically decreased after all three decellularization procedures compared with cell sheet (p<0.01 for all samples). However, the ECM scaffolds still contained considerable amount of growth factors. The VEGF content in high-SDS-, low-SDS-, and freeze-thaw-treated samples was 74.8±21.8 pg, 285.2±133.6 pg, and 1043.8±184.9 pg per scaffold, respectively. The bFGF content in high-SDS-, low-SDS-, and freeze-thaw-treated samples was 471.0±119.6 pg, 2237.7±59.9 pg, and 2148.0±82.3 pg per scaffold, respectively. The high-SDS-treated samples contained significantly lower amount of VEGF and bFGF compared with the other two treatments.
Mechanical strength
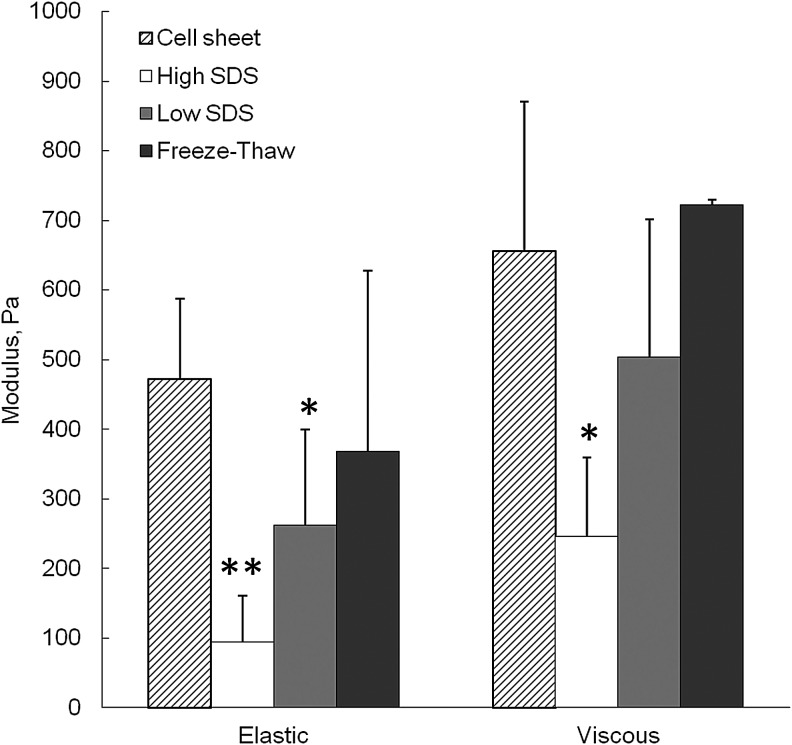
Mechanical properties of the cell sheet and ECM scaffolds after decellularization were shown in Figure 5. Compared with the fresh cell sheet, the elastic modulus significantly decreased after both high SDS and low SDS treatments (p<0.01 and p<0.05, respectively), and the viscous modulus only significantly decreased after high SDS treatment (p<0.05). The freeze-thaw method did not show significant loss of mechanical strength compared with the cell sheet.
FIG. 5.
Average elastic and viscous moduli of fibroblast cell sheet before and after decellularization. *p<0.05, **p<0.01 comparing with cell sheet.
In vitro evaluation of inflammatory response to ECM scaffolds
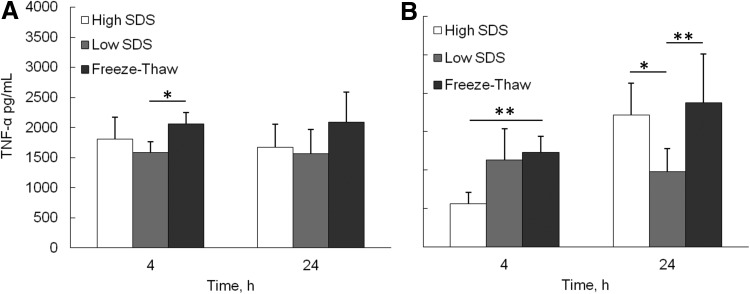
The inflammatory response of the decellularized ECM was investigated by analyzing cytokine secretion from differentiated macrophages cultured on ECM scaffolds (Fig. 6). At 4 and 24 h, the average proinflammatory cytokine, TNF-α, secretion level from freeze-thaw samples was consistently higher than high SDS and low SDS samples. However, the significant difference was only found between freeze-thaw and low SDS samples at 4 h (p<0.05). The anti-inflammatory cytokine IL-10 secretion from the freeze-thaw samples was significantly higher than high SDS samples at 4 h (p<0.01), and higher than low SDS samples at 24 h (p<0.01). The significant difference was also found between high SDS and low SDS samples at 24 h (p<0.05).
FIG. 6.
Cytokine secretion by differentiated macrophages seeded on the top of the fibroblast cell sheet after decellularization. (A) Tumor necrosis factor (TNF)-α, (B) IL-10. *p<0.05, **p<0.01.
Recellularization
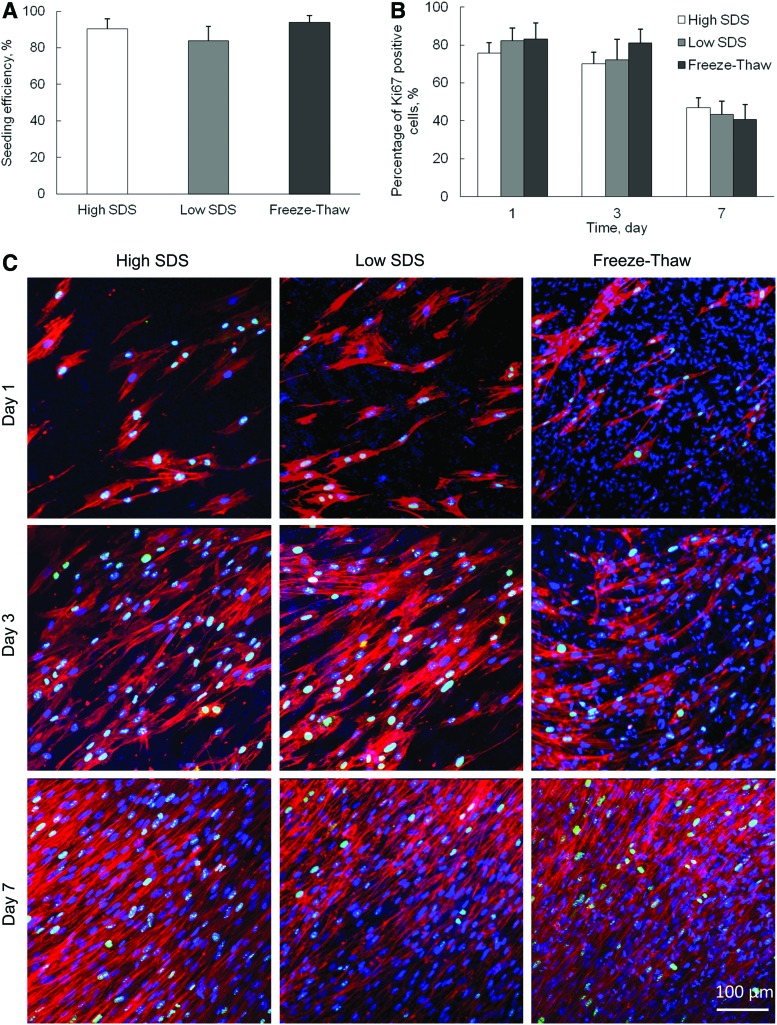
To evaluate the ability of decellularized scaffolds to support the in vitro cell growth, hMSCs were seeded on the surface of decellularized scaffolds and grown up to 7 days. The seeding efficiency was 90.4%±5.4%, 84.0%±7.8%, and 94.0%±3.6% for high SDS, low SDS, and freeze-thaw treatments, respectively (Fig. 7A). There was no significant difference between each other (p>0.05). The proliferation of hMSCs was analyzed by Ki67 expression. The Ki67-positive cell nuclei were stained, counted, and compared with the total number of cell nuclei that were positively stained with DAPI. The average percentage of Ki67-positive cells at day-1, -3, and -7 culture on different decellularized scaffolds was calculated and demonstrated in Figure 7B and C. It was observed that the hMSCs continuously proliferated during the 7-day culture. F-actin staining showed that the cells became more than 90% confluent on all three decellularized scaffolds by day 7. The percentage of proliferating cells decreased with time for all samples, but there was no significant difference among the three samples at all time points.
FIG. 7.
Repopulation of human mesenchymal stem cells (hMSCs) on ECM after different decellularization treatments. (A) Cell seeding efficiency. (B) Percentage of Ki67-positive cells, obtained from arbitrary fields at days 1, 3, and 7 in culture. (C) Confocal images of hMSCs stained with DAPI, Ki67, and F-actin. Scale bar=100 μm. Color images available online at www.liebertpub.com/tec
Discussion
The ECM production and remodeling from fibroblasts has important functions during tissue homeostasis and repair. The fibroblast cell sheets can synthesize collagen fibril assemblies that form a fully functional ECM network, while fibroblasts cultured in isolated biopolymer do not form collagen fibril bundles.13 Compared with other ECM derived from smooth muscle cells and hMSCs, fibroblast-derived ECM is stronger and denser and contains more elastin that makes the ECM more elastic.31 Therefore, the ECM scaffolds derived from fibroblast cell sheets could serve as a stronger and more effective scaffold for tissue regeneration. The purpose of decellularization is to reduce cellular components that potentially hinder tissue remodeling and induce immune responses.32 By comparing different decellularization protocols, we may be able to find an effective method to produce nonimmunogenic ECM scaffolds.
The current study used DNA content as a quantitative marker of cellular remnants. It has been implicated that the DNA remaining in the scaffolds may cause inflammation responses after implantation.33 The osmotic shock treatment alone is not ideal to successfully remove cell nuclei from 3D cell matrix. Since DNA tends to stick to the ECM after being released from the cell nuclei, a second step is necessary to remove the DNA fragments in the ECM. The ionic detergent SDS is able to solubilize the nuclear and cytoplasmic cell membranes, as well as dissociate DNA from proteins.34 A high concentration of SDS is more efficient to remove DNA content than a lower concentration of SDS,35 which is also proved by our results (Fig. 1). Freeze-thaw cycling can effectively lyse the cells within tissues and organs36; however, we found that the resulting intracellular components such as DNA remained largely in the decellularized cell sheet (Fig. 1).
The ideal decellularization process should minimally disrupt the ECM ultrastructure and content. Collagen I and fibronectin are two of the most important ECM components. Collagen I is the most abundant collagen in human body, which strengthens and supports many tissues.37 Fibronectin plays a major role in cell adhesion, growth, migration, and differentiation.38 All three decellularization processes were able to largely preserve collagen I and fibronectin, but the freeze-thaw method better maintained the ECM ultrastructure (Fig. 2A). The quantitative comparison of collagen I and fibronectin expression in samples treated with different decellularization processes further confirmed that the freeze-thaw treatment preserved these two ECM proteins best. This was consistent with the previous finding that freeze-thaw cycling does not significantly increase the loss of ECM proteins from tissue.24 Although SDS is a very effective chemical to make a difference between complete and incomplete cell nuclei removal, it is more disruptive to ECM structure compared with other detergents.
Collagen and elastin are two of the major structural proteins in the ECM that impart mechanical properties to the natural tissues. High SDS treatment removed around 50 wt% collagen and 65 wt% elastin per scaffold, which was closely associated with the significant mechanical strength reduction of high-SDS-treated ECM scaffolds. The freeze-thaw treatment preserved the highest amount of collagen and elastin per construct (Fig. 4), which is one of the reasons that freeze-thaw-treated ECM scaffolds had the best elastic and viscous moduli than the other two samples (Fig. 5). GAGs are known to play important roles in tissues such as cartilage where GAGs provide time-dependent compressive properties. Here we found that high SDS treatment significantly removed the sGAG biomolecules, which typically represents the content of GAG, and only around 41% left in the scaffold. The mechanical strength loss may be also contributed by the removal of GAGs. The GAGs form large complexes between fibrous matrix proteins such as collagen. It was anticipated that the GAGs bridging between collagen fibrils played a role in transmitting and resisting tensile stresses and contributed to the strength of the tissues.39 Removal of GAGs had a negative effect on the viscoelastic properties of the scaffold.40
ECM-bound growth factors, including bFGF, VEGF, transforming growth factor-β, and platelet-derived growth factor, can synergistically regulate cell migration, proliferation, and differentiation.41 Although the majority of VEGF and bFGF were removed from the cell sheets, there were still considerable amount of growth factors present in the matrix after the decellularization process. There was an FBS incubation step included right before the final PBS washing step. Therefore, the growth factors in the ECM arose from two possible sources: (1) growth factors preserved in the fibroblast cell sheet and (2) growth factors adsorbed from FBS. Our results indicated that high SDS treatment can remove much more growth factors than the low SDS and freeze-thaw treatments or the high-SDS-treated ECM had much lower growth factor binding capacity than the low-SDS- and freeze-thaw-treated ECM.
It has been shown that the in vitro culture of macrophages on decellularized scaffolds with different DNA contents exhibited distinct phenotypic polarization profiles.42 Our in vitro inflammation evaluation of ECM scaffolds also showed some difference (Fig. 6). At 4 h, low-SDS-treated samples had the lowest TNF-α secretion. The proinflammatory cytokine TNF-α is typically associated with inflammation, tumor resistance, and graft rejection.43 Freeze-thaw-treated samples contained large amount of DNA, so it may increase the inflammatory response. High-SDS-treated samples maintained lower amount of proteins, including GAG and growth factors bFGF and VEGF, which may have negative effect on the immune response suppression.30 However, the TNF-α secretion difference at 24 h was not prominent. The anti-inflammatory cytokine IL-10 is involved in immunoregulation, matrix deposition, and remodeling.44 Freeze-thaw-treated samples had higher IL-10 secretion at both 4 and 24 h. It has been suggested that the presence of xenogeneic DNA within biologic scaffold materials is a possible cause of an “inflammatory response.”33 Our results suggested that DNA content alone is not the sole determinant of the host immune response. The other study also showed that more M2 macrophage polarization was seen on ECM scaffolds that contained a greater amount of DNA remnant.42 M2 macrophages possess the ability to facilitate tissue repair and constructive remodeling.45 Further in vivo study is required to investigate the role of DNA in host immune responses.
The hMSCs are often employed as a model cell line to evaluate the feasibility of using decellularized scaffolds for tissue engineering,46 because hMSCs have wide applications in bone, tendon, skin, vascular, and neural tissue engineering. It was reported that when SDS was added to the decellularization protocol, the ability of porcine dermis to support in vitro cell growth significantly decreased due to the harsh effect of SDS.30 The retention of different amount of growth factors in hMSC-derived ECM can significantly affect the cell viability and cytotoxicity.29 However, our recellularization study demonstrated that hMSCs attached, spread, and proliferated well on all decellularized scaffolds, even on high-SDS-treated samples (Fig. 7). Other research also showed that MSCs reseeded on 2% SDS-treated equine tendon ECM were successful in terms of plating efficiency, cell proliferation, and viability.46 Therefore, the recellularization ability of ECM scaffolds derived from fibroblast cell sheets is independent of decellularization methods and the amount of cellular remnants in our study.
Besides providing structural support and regulating cell function, the ECM scaffolds are also a native modulator of cell activity in immune response and tissue repair.47 Several ECM components, such as collagen, fibronectin, and GAGs, have shown the ability to control inflammatory responses.48–50 The ECM scaffolds in our study maintained considerable amount of natural collagen I and fibronectin, which may exert immunomodulating effects in vivo. Different decellularization methods resulted in ECM scaffolds with different components and mechanical properties, which could be applied in different tissue regeneration applications. For example, the ECM scaffolds with high SDS treatment had relatively low DNA content, which could offer an oral mucosa reconstruction. The mechanically stronger ECM scaffolds obtained through freeze-thaw cycling could be potentially used in the skin tissue engineering.
Conclusions
The present work represents the first study on the effects of different decellularization methods on fibroblast-cell-sheet derived ECM scaffold composition, mechanical properties, in vitro inflammation response, and recellularization ability. The high SDS treatment significantly reduced the mechanical strength of the scaffolds, while the DNA fragments were more efficiently removed. ECM proteins, such as collagen, elastin, and GAGs, as well as growth factors, were preserved and the decellularized matrix showed excellent recellularization ability in vitro. Although the three decellularization procedures did not result in a completely decellularized construct with maintenance of biochemical and biomechanical properties, the ECM scaffolds provide good support for in vitro cell attachment and proliferation. The natural and nanofibrous ECM scaffolds derived from fibroblast cell sheets hold great potential in offering a biomimetic cell delivery platform for various tissue engineering applications.
Acknowledgments
This study was supported by the National Institutes of Health (1R15HL115521-01A1), Young Clinical Scientist Award from Flight Attendance Medical Research Institute (062518-YCSA), and the Research Excellence Fund-Research Seed Grant (REF-RS) from Michigan Technological University.
Disclosure Statement
The authors declare no competing financial interests.
References
- 1.Daley W.P., Peters S.B., and Larsen M.Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci 121,255, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Mostafavi-Pour Z., Askari J.A., Parkinson S.J., Parker P.J., Ng T.T.C., and Humphries M.J.Integrin-specific signaling pathways controlling focal adhesion formation and cell migration. J Cell Biol 161,155, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourget J.-M., Gauvin R., Larouche D., Lavoie A., Labbe R., Auger F.A., et al. Human fibroblast-derived ECM as a scaffold for vascular tissue engineering. Biomaterials 33,9205, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Lu H.X., Hoshiba T., Kawazoe N., Koda I., Song M.H., and Chen G.P.Cultured cell-derived extracellular matrix scaffolds for tissue engineering. Biomaterials 32,9658, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Sadr N., Pippenger B.E., Scherberich A., Wendt D., Mantero S., Martin I., et al. Enhancing the biological performance of synthetic polymeric materials by decoration with engineered, decellularized extracellular matrix. Biomaterials 33,5085, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Pei M., He F., and Kish V.L.Expansion on extracellular matrix deposited by human bone marrow stromal cells facilitates stem cell proliferation and tissue-specific lineage potential. Tissue Eng Part A 17,3067, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scobie L., Padler-Karavani V., Le Bas-Bernardet S., Crossan C., Blaha J., Matouskova M., et al. Long-term IgG response to porcine Neu5Gc antigens without transmission of PERV in burn patients treated with porcine skin xenografts. J Immunol 191,2907, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grenier G., Remy-Zolghadri M., Larouche D., Gauvin R., Baker K., Bergeron F., et al. Tissue reorganization in response to mechanical load increases functionality. Tissue Eng 11,90, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Xing Q., Vogt C., Leong K.W., and Zhao F.Highly aligned nanofibrous scaffold derived from decellularized human fibroblasts. Adv Funct Mater 24,3027, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Datta N., Pham Q.P., Sharma U., Sikavitsas V.I., Jansen J.A., and Mikos A.G.In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc Natl Acad Sci U S A 103,2488, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cukierman E., Pankov R., Stevens D.R., and Yamada K.M.Taking cell-matrix adhesions to the third dimension. Science 294,1708, 2001 [DOI] [PubMed] [Google Scholar]
- 12.El Ghalbzouri A., Commandeur S., Rietveld M.H., Mulder A.A., and Willemze R.Replacement of animal-derived collagen matrix by human fibroblast-derived dermal matrix for human skin equivalent products. Biomaterials 30,71, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Grinnell F., Fukamizu H., Pawelek P., and Nakagawa S.Collagen processing, crosslinking and fibril bundle assembly in matrix produced by fibroblasts in long-term cultures supplemented with ascorbic-acid. Exp Cell Res 181,483, 1989 [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa O., Kondo A., Okada K., Miyachi Y., and Furumura M.Morphological and biochemical analyses on fibroblasts and self-produced collagens in a novel three-dimensional culture. Br J Dermatol 136,6, 1997 [PubMed] [Google Scholar]
- 15.Ahlfors J.-E.W., and Billiar K.L.Biomechanical and biochemical characteristics of a human fibroblast-produced and remodeled matrix. Biomaterials 28,2183, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Calve S., Dennis R.G., Kosnik P.E., Baar K., Grosh K., and Arruda E.M.Engineering of functional tendon. Tissue Eng 10,755, 2004 [DOI] [PubMed] [Google Scholar]
- 17.L'Heureux N., Paquet S., Labbe R., Germain L., and Auger F.A.A completely biological tissue-engineered human blood vessel. FASEB J 12,47, 1998 [DOI] [PubMed] [Google Scholar]
- 18.L'Heureux N., Dusserre N., Konig G., Victor B., Keire P., Wight T.N., et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med 12,361, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konig G., McAllister T.N., Dusserre N., Garrido S.A., Iyican C., Marini A., et al. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials 30,1542, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crapo P.M., Gilbert T.W., and Badylak S.F.An overview of tissue and whole organ decellularization processes. Biomaterials 32,3233, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilbert T.W.Strategies for tissue and organ decellularization. J Cell Biochem 113,2217, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Du L.Q., Wu X.Y., Pang K.P., and Yang Y.M.Histological evaluation and biomechanical characterisation of an acellular porcine cornea scaffold. Br J Ophthalmol 95,410, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Xu C.C., Chan R.W., and Tirunagari N.A biodegradable, acellular xenogeneic scaffold for regeneration of the vocal fold lamina propria. Tissue Eng 13,551, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Patel N., Solanki E., Picciani R., Cavett V., Caldwell-Busby J.A., and Bhattacharya S.K.Strategies to recover proteins from ocular tissues for proteomics. Proteomics 8,1055, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Elder B.D., Kim D.H., and Athanasiou K.A.Developing an articular cartilage decellularization process toward facet joint cartilage replacement. Neurosurgery 66,722, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boer U., Lohrenz A., Klingenberg M., Pich A., Haverich A., and Wilhelmi M.The effect of detergent-based decellularization procedures on cellular proteins and immunogenicity in equine carotid artery grafts. Biomaterials 32,9730, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Petersen T.H., Calle E.A., Colehour M.B., and Niklason L.E.Matrix composition and mechanics of decellularized lung scaffolds. Cells Tissues Organs 195,222, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao F., Veldhuis J.J., Duan Y.J., Yang Y., Christoforou N., Ma T., et al. Low oxygen tension and synthetic nanogratings improve the uniformity and stemness of human mesenchymal stem cell layer. Mol Ther 18,1010, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J., and Ma T.Autocrine fibroblast growth factor 2-mediated interactions between human mesenchymal stem cells and the extracellular matrix under varying oxygen tension. J Cell Biochem 114,716, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Reing J.E., Brown B.N., Daly K.A., Freund J.M., Gilbert T.W., Hsiong S.X., et al. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials 31,8626, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gauvin R., Ahsan T., Larouche D., Levesque P., Dube J., Auger F.A., et al. A novel single-step self-assembly approach for the fabrication of tissue-engineered vascular constructs. Tissue Eng Part A 16,1737, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Portmann-Lanz C.B., Ochsenbein-Kolble N., Marquardt K., Luthi U., Zisch A., and Zimmermann R.Manufacture of a cell-free amnion matrix scaffold that supports amnion cell outgrowth in vitro. Placenta 28,6, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Zheng M.H., Chen J., Kirilak Y., Willers C., Xu J., and Wood D.Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J Biomed Mater Res Part B 73B,61, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Giusti S., Bogetti M.E., Bonafina A., and de Plazas S.F.An improved method to obtain a soluble nuclear fraction from embryonic brain tissue. Neurochem Res 34,2022, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Elder B.D., Eleswarapu S.V., and Athanasiou K.A.Extraction techniques for the decellularization of tissue engineered articular cartilage constructs. Biomaterials 30,3749, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cortiella J., Niles J., Cantu A., Brettler A., Pham A., Vargas G., et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A 16,2565, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Stamov D.R., and Pompe T.Structure and function of ECM-inspired composite collagen type I scaffolds. Soft Matter 8,10200, 2012 [Google Scholar]
- 38.Nuttelman C.R., Mortisen D.J., Henry S.M., and Anseth K.S.Attachment of fibronectin to poly(vinyl alcohol) hydrogels promotes NIH3T3 cell adhesion, proliferation, and migration. J Biomed Mater Res 57,217, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Cribb A.M., and Scott J.E.Tendon response to tensile-stress—an ultrastructural investigation of collagen-proteoglycan interactions in stressed tendon. J Anat 187,423, 1995 [PMC free article] [PubMed] [Google Scholar]
- 40.Lovekamp J.J., Simionescu D.T., Mercuri J.J., Zubiate B., Sacks M.S., and Vyavahare N.R.Stability and function of glycosaminoglycans in porcine bioprosthetic heart valves. Biomaterials 27,1507, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Badylak S.F., Freytes D.O., and Gilbert T.W.Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater 5,1, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Keane T.J., Londono R., Turner N.J., and Badylak S.F.Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 33,1771, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Esposito E., and Cuzzocrea S.TNF-alpha as a therapeutic target in inflammatory diseases, ischemia-reperfusion injury and trauma. Curr Med Chem 16,3152, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Saraiva M., and O'Garra A.The regulation of IL-10 production by immune cells. Nat Rev Immunol 10,170, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Martinez F.O., Sica A., Mantovani A., and Locati M.Macrophage activation and polarization. Front Biosci (Landmark Ed) 13,453, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Youngstrom D.W., Barrett J.G., Jose R.R., and Kaplan D.L.Functional characterization of detergent-decellularized equine tendon extracellular matrix for tissue engineering applications. PLoS One 8,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franz S., Rammelt S., Scharnweber D., and Simon J.C.Immune responses to implants—a review of the implications for the design of immunomodulatory biomaterials. Biomaterials 32,6692, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Salek-Ardakani S., Arrand J.R., Shaw D., and Mackett M.Heparin and heparan sulfate bind interleukin-10 and modulate its activity. Blood 96,1879, 2000 [PubMed] [Google Scholar]
- 49.Rammelt S., Schulze E., Bernhardt R., Hanisch U., Scharnweber D., Worch H., et al. Coating of titanium implants with type-I collagen. J Orthop Res 22,1025, 2004 [DOI] [PubMed] [Google Scholar]
- 50.de Fougerolles A.R., and Koteliansky V.E.Regulation of monocyte gene expression by the extracellular matrix and its functional implications. Immunol Rev 186,208, 2002 [DOI] [PubMed] [Google Scholar]