Abstract
Traumatic brain injury (TBI) is associated with cerebral edema, blood brain barrier breakdown, and neuroinflammation that contribute to the degree of injury severity and functional recovery. Unfortunately, there are no effective proactive treatments for limiting immediate or long-term consequences of TBI. Therefore, the objective of this study was to determine the efficacy of methylene blue (MB), an antioxidant agent, in reducing inflammation and behavioral complications associated with a diffuse brain injury. Here we show that immediate MB infusion (intravenous; 15–30 minutes after TBI) reduced cerebral edema, attenuated microglial activation and reduced neuroinflammation, and improved behavioral recovery after midline fluid percussion injury in mice. Specifically, TBI-associated edema and inflammatory gene expression in the hippocampus were significantly reduced by MB at 1 d post injury. Moreover, MB intervention attenuated TBI-induced inflammatory gene expression (interleukin [IL]-1β, tumor necrosis factor α) in enriched microglia/macrophages 1 d post injury. Cell culture experiments with lipopolysaccharide-activated BV2 microglia confirmed that MB treatment directly reduced IL-1β and increased IL-10 messenger ribonucleic acid in microglia. Last, functional recovery and depressive-like behavior were assessed up to one week after TBI. MB intervention did not prevent TBI-induced reductions in body weight or motor coordination 1–7 d post injury. Nonetheless, MB attenuated the development of acute depressive-like behavior at 7 d post injury. Taken together, immediate intervention with MB was effective in reducing neuroinflammation and improving behavioral recovery after diffuse brain injury. Thus, MB intervention may reduce life-threatening complications of TBI, including edema and neuroinflammation, and protect against the development of neuropsychiatric complications.
Key words: : cytokines, fluid percussion injury, intervention, microglia, recovery
Introduction
It is estimated that over 2.5 million individuals experience a traumatic brain injury (TBI) each year in the United States, costing the U.S. economy more than $75 billion annually.1 These head injuries range from mild to severe, with over 90% of head injuries classified as mild or moderate. Although survival following head injury has significantly improved in the last few decades, there are no effective proactive treatment options to augment functional recovery or prevent long-term neuropsychiatric complications after TBI.
A TBI has two injury phases—a primary injury consisting of damage done directly by the trauma, and a prolonged secondary injury characterized by progressive tissue loss or cell death. Neuroinflammation, cerebral edema, and blood brain barrier breakdown are components of the prolonged secondary injury that contribute to the timing and level of recovery, and also may increase the risk for long-term cognitive deficits or depression. Although the initial inflammatory response may be beneficial for repair,2–4 prolonged and elevated brain inflammation is associated with increased excitotoxicity and cellular damage5–7 and reduced neuronal plasticity.8–11 This is clinically relevant because TBI patients often present with a loss of concentration, retrograde amnesia, and reduced short-term memory acutely after injury.12,13
Development of cognitive deficits after TBI is paralleled in rodent models. For example, after controlled cortical impact, rodents have impaired performance in a range of cognitive tasks including Morris water maze, Barnes maze, and novel object recognition.14–16 Increased neuroinflammation also may play a role in the development of depression after TBI.17–20 We have reported that increased brain inflammation after a fluid percussion injury in mice was associated with the development of depressive-like behavior 7 d post-injury.21 Increased depressive-like behavior has also been observed in rats up to six weeks after injury22,23 and up to 90 d post-injury in a mouse model of mild TBI.21,24 Approximately 30% of patients that suffer from a mild or moderate TBI develop symptoms of depression within one year after injury, and this percentage rises to approximately 60% within 18 years after injury.25–27 Thus, limiting neuroinflammation after injury may attenuate acute complications including cerebral edema, and also help to prevent the development of long-term cognitive and depressive symptoms.
Current options for TBI treatment are limited and aimed to mitigate complications as they present, but do not treat the underlying cause (e.g., edema, brain inflammation).28–30 Corticosteroid treatments were once used clinically to suppress inflammation after TBI but are no longer provided because they can reduce survival.31 Other anti-inflammatory treatments, including minocycline, are only mildly effective in rodent models and need to be combined with other treatments to lower interleukin (IL)-1β.32–34 Therefore, there is a critical need for interventions that provide immediate benefit after brain injury by targeting the physiological components of secondary injury but also help prevent the development of long lasting neuropsychiatric problems.
The ideal intervention for clinical application would demonstrate both safety and efficacy, be easily administered, be stable at room temperature, have a known therapeutic dose, and result in minimal adverse effects. Methylene blue (MB) was developed as a dye for the textile industry but also has potent antibiotic and antioxidant properties.35,36 For instance, it is used clinically to treat nitric oxide-induced hypotensive complications that arise from septic shock, ischemia, and cardiopulmonary bypass.35,37,38 Moreover, in a piglet model of cardiac arrest and resuscitation, MB infusion ameliorated blood brain barrier permeability during reperfusion by limiting nitric oxide metabolites.39 MB also may provide anti-inflammatory and neuroprotective functions distinct from its antioxidant effects. In primary microglia treated with a high dose of lipopolysaccharide (LPS), addition of MB to the cell culture media reduced inflammatory gene expression including IL-1β, IL-6, RANTES, and IL-12.
Moreover, low dose MB treatment in vivo (3 mg/kg) delayed disease onset in a SOD1 mutant mouse model of amyotrophic lateral sclerosis corresponding to increased neuroprotection and neuronal survival.40 Recently, MB was reported to have neuroprotective properties and reduced lesion size in a model of mild controlled cortical impact injury.41 The effectiveness of MB, however, has not been reported in the clinics after TBI, or in a model of fluid percussion injury. MB is particularly attractive as a TBI intervention because kinetic analysis indicates that MB rapidly crosses the blood brain barrier and is 20 times more concentrated in the central nervous system (CNS) than the plasma within 1 h of intravenous injection.42 Moreover, the ability of MB to target multiple physiological pathways within the brain makes it a plausible intervention after TBI because it represents a polypharmaceutical approach.43,44
Therefore, the objective of this study was to determine the efficacy of MB to reduce neuroinflammation and prevent the onset of behavioral complications associated with a diffuse brain injury in mice. To induce a diffuse TBI, midline fluid percussion injury (FPI) was used. FPI recapitulates several relevant aspects of a human concussion including diffuse inflammatory responses, blood brain barrier breakdown, loss of consciousness, and mild parenchymal hemorrhage without significant neuronal loss or tissue cavitation.21,45–48 Here we show that intravenous (i.v.) injection of a low dose of MB (2 mg/kg) provided within 15–30 min after TBI significantly improved physiological and behavioral recovery after TBI. Specifically, MB treatment lowered TBI-induced edema, neuroinflammation, and microglial activation (1 d post-injury), and prevented the development of TBI-associated depressive-like behavior (7 d post-injury).
Methods
Mice
Adult male BALB/c mice (three months old) were obtained from a breeding colony kept in barrier-reared conditions in a specific-pathogen-free facility at The Ohio State University. Mice were individually housed in polypropylene cages for the duration of the study and maintained at 25°C under a 12 h light/12 h dark cycle with ad libitum access to water and rodent chow. All procedures were in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and were approved by The Ohio State University Institutional Laboratory Animal Care and Use Committee.
Midline fluid percussion injury
Mice received a midline diffuse TBI using an FPI apparatus (Custom Design & Fabrication, Richmond, VA) as previously described.49 This diffuse injury occurs in the absence of a contusion, does not induce tissue cavitation or gross neuronal loss, and causes diffuse axonal injury in the neocortex, hippocampus, and dorsolateral thalamus.21,45–47 In brief, a midline craniectomy was performed with a 3 mm outer diameter trephine and a rigid Luer-loc needle hub was secured over the craniectomy and capped. After 4–6 h, injury was induced by imposing a 10 ms pulse of saline (1.2 atmospheres; 670–720 mV) onto the dura through the injury hub.21,50,51 All sham controls received the same procedure without the fluid pulse. Immediately after injury, the injury hub was removed, dural integrity was confirmed, and mice were evaluated for injury severity using the self-righting test.50 Any mice with a confirmed dura breach were euthanized immediately after injury and excluded from the study. Self-righting inclusion criteria was based on our previous work with BALB/c mice21: Only mice with a moderate TBI were used: sham≤60 sec; 60 sec<mild<200 sec; 200 sec<moderate<540 sec; severe>540 sec.
Methylene blue injection
Fifteen to 30 min after sham or TBI, mice were administered an i.v. tail vein injection of sterile water (control) or a low dose of MB (2 mg/kg body weight, ∼80 μM). Dosage above 7 mg/kg MB is considered high dose and cytotoxic.35,52 Mice were awake and restrained during i.v. injection (<2 min) and immediately returned to their home cage after injection. Methylene blue (1% solution, 10 mg/mL) was diluted and injected at a volume of 100 μL.37 MB (Anmol Chemicals [Commercial Grade], Mumbai, India) was acquired from by the Ohio State University Wexner Medical Center.
Edema
Edema was classified as increased water content in the brain and was determined as previously described.53 In brief, 24 h after sham or TBI, mice were euthanized and the rostral and caudal cortex was dissected. Tissue was weighed and oven dried overnight at 73°C before reweighing. Water content was determined by calculating the ratio of weight loss to original wet weight (% water loss=[(wet weight – dry weight)/wet weight]*100).
Isolation of brain microglia/macrophages
Brain CD11b+ cells (microglia/macrophages) were isolated and enriched as previously described.21,54,55 In brief, whole brain homogenates were centrifuged and layered in a discontinuous Percoll gradient (70/50/35/0% isotonic Percoll; GE-healthcare, Uppsala, Sweden). Enriched CD11b+ cells were collected from the interphase between the 70 and 50% Percoll layers. We have previously characterized these cells as 80–90% CD11b+.55 These cells were characterized as microglia (CD11b+/CD45lo) or macrophages (CD11b+/CD45hi) based on flow cytometric gating using CD11b/CD45 labeling. In sham mice, the Percoll gradient isolation yielded ∼84% microglia and ∼1.5% macrophages and these percentages shifted to ∼82% microglia and ∼3% macrophages after TBI (data not shown).
Isolation of blood leukocytes
Whole blood was collected by cardiac puncture with EDTA lined syringes 24 h after sham or TBI. A 30 μL aliquot of blood was used, red blood cells were lysed, samples were washed, and remaining leukocytes were stained for protein expression by flow cytometry.
BV-2 cell culture
BV-2 microglial cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) growth medium (Lonza, Walkersvill, MD) supplemented with 10% fetal bovine serum (FBS) (GE-healthcare Hyclone, Little Chalfont, UK) (3.7 g/L sodium bicarbonate, 200 mM glutamine, 100 U/mL penicillin G, 100 μg/mL streptomycin, 0.25 μg/mL fungizone) as previously described.56 Cultures were maintained and incubated at 37°C with 95% humidity and 5% CO2. Growth medium was replenished every third day until confluence. Prior to experimentation, cells were plated at 1×105 cells per well in 24 well plates and allowed to adhere overnight. Immediately before treatment, cells were washed three times with serum-free DMEM medium and supplemented with warm serum-free DMEM medium containing experimental treatments. Cells were treated with saline or LPS (100 ng/mL) for 1 h. After 1 h, water or 4.5 μM 1% MB was added to the culture media. Twenty-four hours after saline/LPS, cells were homogenized and ribonucleic acid (RNA) was isolated.
Flow cytometry
Enriched CD11b+ cells from the brain and leukocytes from the blood were assayed for surface protein expression as previously described.21,55 In brief, Fc receptors were blocked with anti-CD16/CD32 antibody (eBioscience, San Diego, CA). Cells were incubated with rat anti-mouse GR-1-FITC and CD11b-APC, or CD45-PE, Ly6C-PerCP-Cy5.5, and CD11b-APC antibodies (eBioscience, San Diego, CA). Surface protein expression was determined using a Becton-Dickinson FACSCaliber four color cytometer (BD, Franklin Lakes, NJ). Ten thousand events characterized as blood leukocytes or microglia/macrophages were recorded. For each antibody, gating was determined based on appropriate negative isotype stained controls from the representative cell population. Flow data were analyzed using FlowJo software (Tree Star, San Carlos, CA).
RNA isolation and RT-PCR
RNA was isolated from the dissected hippocampus and BV-2 microglia using the Tri-Reagent protocol according to manufacturer instructions (Sigma-Aldrich, St. Louis, MO). Messenger RNA (mRNA) was isolated from enriched brain CD11b+ cells using the PrepEase kit (Affymetrix, Santa Clara, CA) according to manufacturer instructions. Real-time polymerase chain reaction (RT-PCR) was performed using the Applied Biosystems Taqman® Assays-on-Demand™ Gene Expression protocol (Applied Biosystems, Carlsbad, CA) as previously described.56,57 In brief, experimental complementary deoxyribonucleic acid (cDNA) was amplified by RT-PCR where a target cDNA (e.g., IL-1β, Arginase, CCL2) and a reference cDNA (glyceraldehyde-3-phosphate dehydrogenase; GAPDH) were amplified simultaneously using an oligonucleotide probe with a 5′ fluorescent reporter dye (6-FAM). Gene accession numbers are listed for each primer in Table 1. Fluorescence was determined on an ABI PRISM 7300-sequence detection system (Applied Biosystems, Calsbad, CA). Data were analyzed using the comparative threshold cycle (Ct) method and results are expressed as fold difference from GAPDH.
Table 1.
Gene Accession Numbers for TaqMan RT-PCR Probes
| RefSeq number | |
|---|---|
| CD14 | NM_009841.3 |
| IL-1β | NM_008361.3 |
| TNF-α |
NM_001278601.1 NM_013693.3 |
| iNOS | NM_010927.3 |
| Arg1 | NM_007482.3 |
| IL-10 | NM_010548.2 |
IL, interleukin; TNF-α, tumor necrosis factor α; iNOS, inducible nitric oxide synthase; Arg1, arginase 1.
Behavioral assays
Rotarod
Motor coordination was assessed using the rotarod apparatus (Rotamex, Columbus, OH) as previously described.21 In brief, mice were acclimated to the apparatus 4 d before testing, and were trained on the apparatus each day for 3 d before testing. Testing consisted of three trials in which mice were placed on a stationary rod that began to rotate at 10 rpm with 0.2 rpm/sec acceleration. Trials 1 and 2 were done back-to-back to prevent associations between falling and returning to the home cage, and mice were given 10 min between Trials 2 and 3. The average time spent on the rod before falling for the two longest trials is reported. Mice were tested 1 h and 1 d, 4 d, and 7 d post injury.
Tail suspension test
Depressive-like behavior was measured using the tail suspension test (TST) as previously described.21,58 In brief, 7 d after sham or TBI, mice were suspended by their tail and video recorded for 10 min. The amount of time spent immobile was assessed by a trained observer blind to experimental conditions. Increased time spent immobile designates an increase in depressive-like behavior. We have previously determined that TBI mice have recovered to baseline motor performance (rotarod) by 7 d post-injury and do not show increased exhaustion.21
Statistical analysis
Data were subjected to the Shapiro-Wilk test using Statistical Analysis Systems (SAS, Cary, NC) statistical software. To determine significant main effects and interactions between main factors, data were analyzed using one-way (i.e., injury and treatment), two-way (i.e. injury×treatment), or three-way (i.e., injury×treatment×time) analysis of variance (ANOVA) using the general linear model procedures of SAS. When appropriate, differences between treatment means were evaluated by an F-protected t-test using the least significant difference procedure of SAS. Edema was evaluated using a one-tailed t-test after ANOVA. Body mass and rotarod data were further subjected to repeated measures ANOVA. All data are expressed as treatment means±standard error of the mean (SEM).
Results
Injury severity and righting reflex prior to control or methylene blue intervention
We previously reported that diffuse brain injury caused transient neuroinflammation, functional recovery deficits, and acute depressive-like behavior in mice.21 Therefore, the objective of this study was to determine the efficacy of methylene blue (MB) in reducing acute inflammation and behavioral complications associated with moderate TBI (1–7 d post-injury). Figure 1A shows the experimental design in which adult male (3 months old) BALB/c mice received a sham injury or TBI. Intervention with an i.v. injection of sterile water (Con) or MB (2 mg/kg) was provided 15–30 min later. Several immune and behavioral parameters were determined within hours to 7 d post-injury.
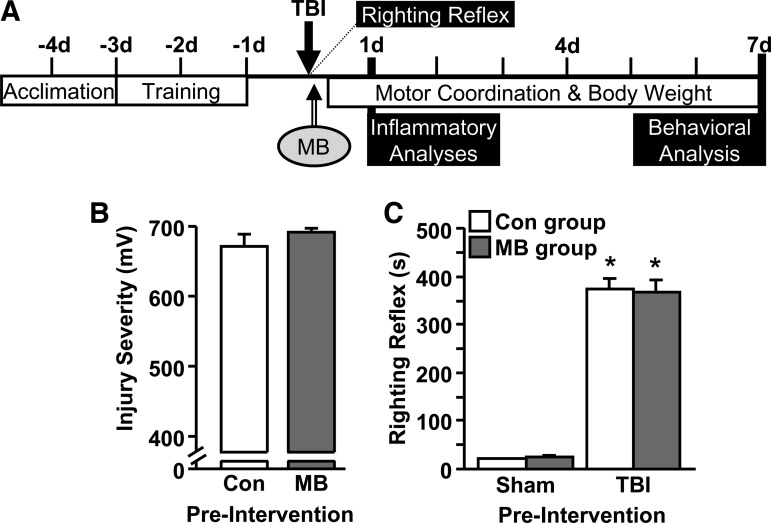
FIG. 1.
Injury severity and righting reflex prior to control or methylene blue (MB) intervention. (A) Diagram of the experimental study design showing that mice were subjected to a sham injury (Sham) or traumatic brain injury (TBI) at day 0 and immediately (15–30 min later) provided with control of MB interventions. Rotarod acclimation and training were performed prior to injury. Several biochemical and behavioral analyses were performed 1 h – 7 d post-injury. (B) Injury severity was determined by the electromotive force induced by the fluid pulse onto the brain (mV; n=28–29). (C) Time for mice to self-right after sham injury or TBI. Bars represent the mean±standard error of the mean. Means with (*) are significantly different (p<0.05) from Sham-vehicle control (Con).
First, early indices (i.e., prior to intervention with Con or MB) of injury severity were determined to confirm each group started with the same injury. Figure 1B shows that all TBI mice received the same electromotive force induced by the pressure wave (millivolt [mV]) during fluid percussion. In addition, righting reflex time was significantly increased in TBI mice, compared with sham mice (p<0.0001), but this was not different between mice that would go on to receive Con or MB injection (Fig. 1C). The average righting reflex time prior to Con and MB intervention was 374.4 sec±20.3 sec (6 min, 14 sec) and 366.6 sec±25.5 sec (6 min, 7 sec), respectively. Sham mice had an average righting reflex time of ∼25 sec (Fig. 1C). These data indicate that both TBI groups received the same midline fluid percussion injury prior to Con or MB intervention.
MB intervention attenuated TBI-induced edema and neuroinflammation
In the first set of studies, mice were subjected to sham or TBI and then administered Con or MB interventions within 15–30 min after injury. The brain was collected and edema was determined in the cortex 1 d post-injury. Because of the craniectomy, edema in the midline FPI model is often difficult to detect.48 Figure 2A shows that water accumulation was increased in the brain 1 d after TBI, though this value was not significantly different from sham mice. Nonetheless, this slight increase in edema after TBI was not detected in the cortex of mice that received MB intervention. In the hippocampus of the same mice, mRNA of several inflammatory (CD14, IL-1β, tumor necrosis factor [TNF]-α, and CCL2) and anti-inflammatory (arginase) mediators was determined. Figure 2B through 2E show that CD14, IL-1β, TNF-α, and CCL2 mRNA levels were increased in the hippocampus 1 d after TBI (p<0.02, for each) and expression of these markers was attenuated by MB intervention after TBI (CD14 (F1,35=4.48; p<0.05), IL-1β (F1,38=2.32; p=0.1), TNF-α (F1,36=7.87; p<0.001), and CCL2 (F1, 36=2.89; p=0.1).
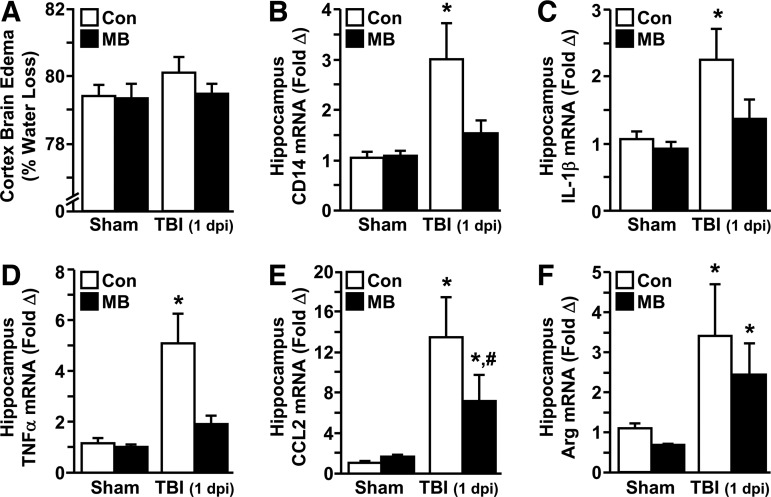
FIG. 2.
Methylene blue (MB) intervention attenuated traumatic brain injury (TBI)-induced edema and neuroinflammation. Adult male (3 months old) BALB/c mice were subjected to sham injury (Sham) or TBI and within 15–30 min received an intravenous injection of vehicle control (Con) or MB (2 mg/kg). The cortex and hippocampus were collected from the brain 1 d post-injury. (A) Relative brain edema was determined in the cortex (n=8–12). In the hippocampus, messenger ribonucleic acid (mRNA) expression of (B) CD14, (C) interleukin (IL)-1β, (D) tumor necrosis factor (TNF)-α, (E) CCL2, and (F) arginase (Arg) were determined (n=4–7). Bars represent the mean±standard error of the mean. Means with (*) are significantly different (p<0.05) from Sham-Con. Means with (#) are significantly different (p<0.05) from TBI-Con.
Post hoc analysis confirmed that mRNA expression of these inflammatory mediators was significantly attenuated in the TBI-MB group, compared with the TBI-Con group (p<0.05 for each). The expression of these inflammatory mediators was also determined 3 d post-injury but had returned to baseline levels (data not shown). Figure 2F shows that arginase mRNA expression also was increased in the hippocampus 1 d post-injury (p<0.002) and this increase was unaffected by MB. IL-10 mRNA was also examined in the hippocampus but was unaffected by either injury or MB treatment 1 d post-injury (data not shown). Collectively, these results indicate that immediate intervention with MB reduced the TBI-associated increase in pro-inflammatory mediators at 1 d post-injury.
MB intervention reduced the percentage of myeloid (CD11b+/GR1+) cells in circulation after TBI but had no effect on the percent of brain-associated macrophages
Because the TBI-associated increase in inflammatory gene expression was reduced by MB, myeloid cell distribution (i.e., monocytes and granulocytes) was determined in circulation and brain 1 d post-injury.21,54 Mice were subjected to TBI and MB intervention as described above and blood and brain were collected 1 d later. Figure 3A shows representative bivariate dot plots of CD11b/GR1 labeling in the blood. Figure 3A and Figure 3B show that the percentage of CD11b+/GR1+ cells in circulation (neutrophils and activated monocytes) was increased after TBI (p<0.008) and this was significantly attenuated by MB intervention (F1,34=6.88; p<0.02).
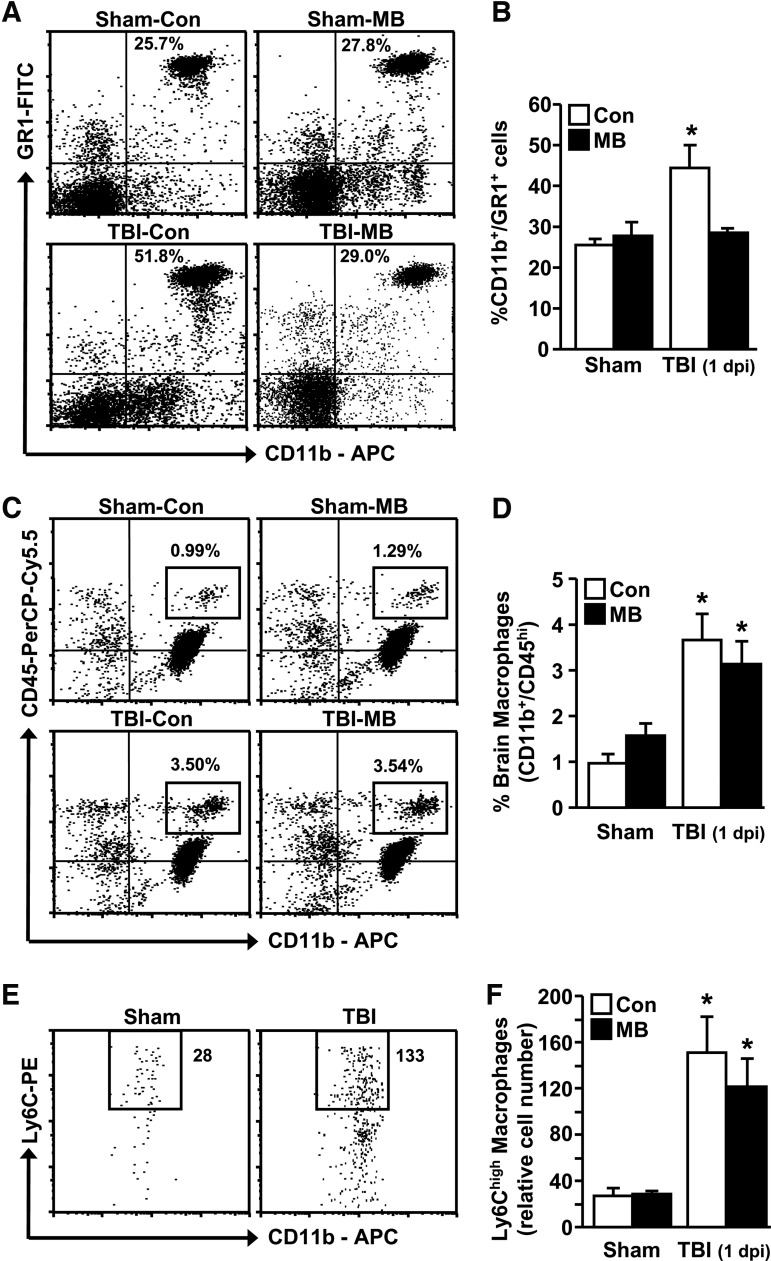
FIG. 3.
Methylene blue (MB) intervention reduced the percentage of myeloid (CD11b+/GR1+) cells in circulation after traumatic brain injury (TBI) but had no effect on the percent of brain-associated macrophages. Adult male (3 months old) BALB/c mice were subjected to sham injury (Sham) or TBI and within 15–30 min received an intravenous injection of vehicle control (Con) or MB (2mg/kg). At 1 d post-injury, brain and blood were collected for flow cytometry analysis. (A) Representative bivariate dot plots of CD11b/GR1 labeling of blood leukocytes. (B) Percent CD11b+/GR1+ myeloid cells in circulation (n=8–12). (C) Representative bivariate dot plots of CD11b/CD45 labeling of brain-associated macrophages. (D) Percent CD11b+/CD45hi macrophages associated with the brain (n=8–12). (E) Representative bivariate dot plots from Sham and TBI of CD11b/Ly6C labeling of brain-associated macrophages. (F) Total relative number of Ly6Chi macrophages associated with the brain (out of 10,000 live cells; n=8–12). Bars represent the mean±standard error of the mean. Means with (*) are significantly different (p<0.05) than Sham-Con.
Next, the percentage of macrophages associated with the brain after TBI was determined. Figure 3C and Figure 3E show representative bivariate dot plots of CD11b/CD45 and CD11b/Ly6C labeling in the brain, respectively. Figure 3D and Figure 3F show that the percentage of macrophages (CD11b+/CD45hi/Ly6Chi) associated with the brain was increased at 1 d post-injury (p<0.0006). These data are consistent with our previous study.21 This increase in brain macrophages after TBI, however, was unaffected by MB intervention. Overall, MB reduced the percentage of myeloid cells in circulation but did not influence the percentage of myeloid cells associated with the brain after TBI.
MB reduced IL-1β and enhanced IL-10 expression in microglia after TBI
MB attenuated the mRNA expression of inflammatory mediators in the hippocampus after TBI but did not affect the increase in brain-associated macrophages. Therefore, we next sought to determine the influence of MB intervention on the inflammatory profile of microglia and macrophages after TBI. In this experiment, enriched microglia/macrophages were isolated from the whole brain by Percoll gradient separation 1 d post-injury and mRNA levels of several inflammatory (CD14, IL-1β, and inducible nitric oxide synthase [iNOS]) and anti-inflammatory (arginase and IL-10) mediators were determined. Figure 4A through Figure 4C show that CD14 (p=0.06), IL-1β (p<0.05), and iNOS (p<0.05) mRNA levels were increased in enriched microglia/macrophages after TBI. Moreover, MB intervention attenuated this TBI-associated increase in CD14 (F1,35=6.51; p<0.02) and IL-1β (F1,36=8.82; p<0.006) but had no effect on the mRNA expression of iNOS. Figure 4D and Figure 4E show that TBI and MB also influenced the mRNA expression of arginase and IL-10 in microglia/macrophages.
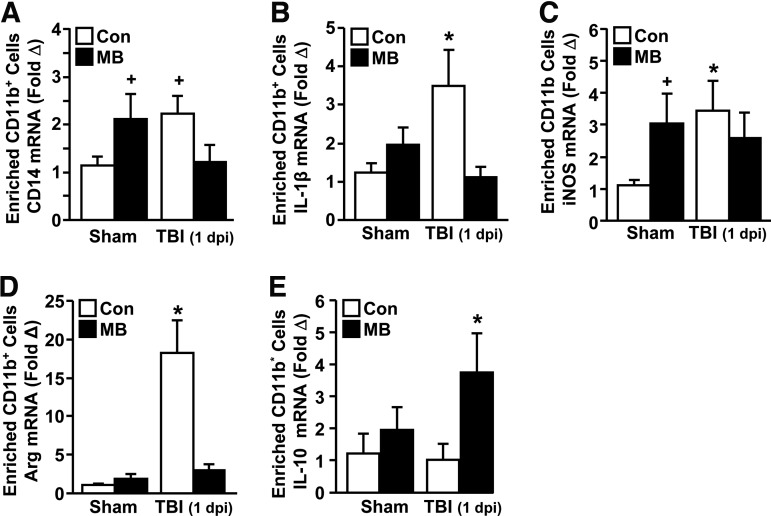
FIG. 4.
Methylene blue (MB) reduced IL-1β and enhanced IL-10 expression in microglia/macrophages after traumatic brain injury (TBI). Adult male (3 months old) BALB/c mice were subjected to sham injury (Sham) or TBI and within 15–30 min received an intravenous injection of vehicle control (Con) or MB (2 mg/kg). At 1 d post-injury, enriched brain CD11b+ cells (microglia/macrophages) were isolated from whole brain homogenates. The levels of messenger ribonucleic acid (mRNA) expression for (A) CD14, (B) interleukin (IL)-1β, (C) inducible nitric oxide synthase (iNOS), (D) arginase (Arg), and (E) IL-10 were determined (n=7–10). Bars represent the mean±standard error of the means. Means with (*) are significantly different (p<0.05) from Sham-Con. Means with (+) tend to be different (p=0.1) from Sham-Con.
For example, arginase mRNA expression was significantly increased with TBI (p<0.0001) but was restored to Sham-Con levels with MB treatment (F1,31=17.12; p<0.0003). Moreover, IL-10 expression was not induced in microglia/macrophages 1 day after TBI but was significantly increased in microglia/macrophages from TBI-MB mice (p<0.05). Collectively, immediate MB intervention reduced TBI-associated increases in CD14, IL-1β, and arginase mRNA expression and enhanced expression of the anti-inflammatory mediator IL-10 in microglia/macrophages.
MB altered the immune profile of LPS-activated BV2 microglia
Immediate intervention with MB reduced the inflammatory profile of microglia/macrophages after TBI. Therefore, we next sought to determine if this was a direct effect of MB on microglia activation. To begin to address this question, BV-2 microglia were activated with LPS in vitro, then 1 h later cultures were supplemented with vehicle (Con) or a low dose of 1% MB (4.5 μM). Although in vivo a dose of approximately 80 μM was used, it is expected that as MB diffuses through the tissues, tissue-resident cells, including microglia, would be exposed to a lower dose. Thus, 4.5 μM was selected as this dose has shown benefits in previous cell culture models.40,59,60 As expected, mRNA expression of inflammatory-associated genes (CD14, IL-1β, TNF-α, and iNOS) were increased in BV-2 microglia 1 d (24 h) after LPS (p<0.001 for each; Table 2).
Table 2.
MB Altered the Immune Profile of LPS-Activated BV2 Microglia
| Saline-Con | Saline-MB | LPS-Con | LPS-MB | |
|---|---|---|---|---|
| CD14 | 1.07±0.16a | 0.97±0.06a | 1.69±0.12b | 2.97±0.35c |
| IL-1β | 1.02±0.10a | 0.52±0.16b | 113.7±35.3c | 74.8±6.5d |
| TNF-α | 1.00±0.04a | 0.54±0.20b | 6.10±1.17c | 4.84±0.59c |
| iNOS | 1.02±0.10a | 1.03±0.20a | 518.4±139.1b | 551.7±91.6b |
| Arg1 | 1.01±0.10a | 0.91±0.04a | 0.32±0.01b | 0.54±0.08c |
| IL-10 | 1.03±0.11a | 2.14±0.65a | 7.28±0.46b | 17.85±4.05c |
BV-2 microglia were cultured in vitro and treated with saline or LPS (100 ng/mL) for 1 h. After 1 h, control (double deionized water) or methylene blue (MB; 4.5 μM) was added to the culture media. After 24 h, messenger ribonucleic acid (mRNA) was isolated and gene expression of CD14, interleukin (IL)-1β, tumor necrosis factor (TNF)-α, inducible nitric oxide synthase (iNOS), arginase (Arg1), and IL-10 was determined (two independent experiments; n=3 per experiment). Values represent the mean±standard error of the mean. Means with different letters (a,b,c) are significantly different (p<0.05) from each other. Means with underlined letters (d) tend to be different (p=0.1) from lipopolysaccharide (LPS)-vehicle control (Con).
MB treatment enhanced LPS-induced CD14 mRNA expression, compared with LPS alone (F1,23=11.55, p<0.003) but tended to reduce IL-1β mRNA expression (p=0.1). Although MB alone reduced TNF-α expression, compared with saline controls (p<0.05), there was no effect of MB after LPS on either TNF-α or iNOS mRNA expression. MB treatment did, however, promote an increase in anti-inflammatory gene expression after LPS. For example, LPS significantly reduced arginase mRNA expression, compared with saline controls (p<0.0001) but arginase levels were partially restored by MB (F1,11=5.60; p<0.05). Moreover, the LPS-induced increase in IL-10 mRNA expression (p<0.0001) was significantly enhanced with MB treatment (F1,23=5.24; p<0.04). Collectively, MB directly attenuated IL-1β and augmented IL-10 mRNA expression in active BV-2 microglia.
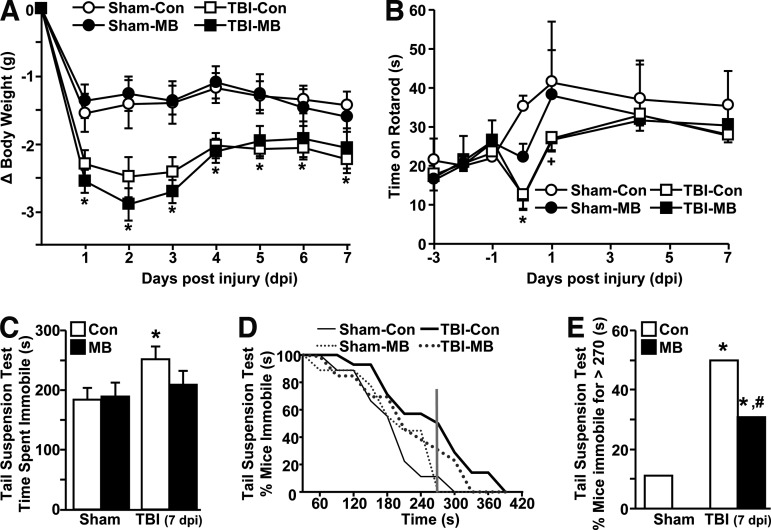
MB intervention did not improve deficits in motor coordination after TBI but attenuated acute depressive-like behavior
We have previously reported that diffuse TBI resulted in transient functional deficits (body weight loss, motor coordination deficits) that resolved within 7 d post-injury.21 Because MB intervention reduced the neuroinflammatory profile of the hippocampus and in microglia/macrophages at 1 d post-injury, the ability of MB to improve functional recovery after TBI was assessed. Figure 5A shows that body weight was reduced after TBI (F1,35=22.79; p<0.0001) but this reduction was unaffected by MB intervention. Similarly, motor coordination, determined by time spent on the accelerating rotarod task, was reduced by TBI in a time-dependent manner (F6,126=3.17; p<0.007). For example, mice had initial deficits in motor coordination after TBI but returned to baseline within 4 d post-injury (Fig. 5B). Immediate MB intervention, however, did not prevent loss of motor function at 1 hour after injury or facilitate any early recovery to baseline motor coordination (Fig. 5B). Thus, body weight loss and transient motor coordination impairments associated with TBI were not influenced by a single MB intervention.
FIG. 5.
Methylene blue (MB) intervention attenuated acute depressive-like behavior 7 d post-injury. Adult male (3 months old) BALB/c mice were subjected to sham injury (Sham) or traumatic brain injury (TBI) and within 15–30 min received an intravenous injection of vehicle control (Con) or MB (2 mg/kg). (A) Body weight was assessed every day for 7 d post-injury (n=9–14). (B) Motor coordination was determined 3 d before injury and again at 1 h, and 1, 4, and 7 d post-injury (n=4–8). (C) At 7 d post-injury, depressive-like behavior was determined by tail suspension test (TST). Total average time spent immobile is shown for all groups (n=9–14). (D) Total time spent immobile was plotted versus the percentage of mice that were immobile. The vertical line at 270 sec denotes that less than 10% of sham mice were immobile for longer than 270 sec. (E) Percentage of mice from each group that were immobile for longer than 270 sec (4.5 min) in the TST. Points and bars represent the mean±standard error of the mean. Means with (*) are significantly different (p<0.05) than Sham-Con. Means with (#) are significantly different (p<0.05) from TBI-Con. Means with (+) tend to be different (p=0.1) from Sham-Con.
We also have shown that following the resolution of body weight loss and motor coordination deficits at 7 d post-injury, depressive-like behavior is still evident in TBI mice.21 To examine behavioral recovery with MB intervention, depressive-like behavior was determined using the TST. Consistent with our previous study, depressive-like behavior (i.e., increased immobility) was evident in TBI mice at 7 d post-injury compared to sham controls (Fig. 5C; p<0.05). After MB intervention, however, depressive-like behavior in the TBI mice was attenuated to levels similar to the control groups (Sham-Con and Sham-MB) and these levels tended to be different from TBI-Con mice (p=0.1).
To better assess the differences in depressive-like behavior between TBI-Con and TBI-MB mice, total time spent immobile was plotted against percentage of mice (Fig. 5D). This graph illustrates that fewer than 10% of sham mice (Sham-Con+Sham-MB) were immobile for longer than 270 sec in the 600 sec test. Therefore, the percentage of mice immobile for greater than 270 sec was determined for the TBI groups. Figure 5E shows that ∼50% of TBI-Con mice were immobile in the TST longer than 270 sec but this was significantly attenuated by MB treatment down to ∼30% (p<0.001; Fig. 5E). Taken together, acute depressive-like behavior was increased by TBI (7 d post-injury) but this increase was attenuated in mice receiving MB infusion within 30 min after TBI.
Discussion
More than 2.5 million people in the United States experience a TBI each year. Despite the frequency of occurrence, there are no proactive treatments for limiting either the acute physiological or long-term neuropsychological complications associated with TBI. Here, we show that in a mouse model, immediate (15–30 min after injury) intervention with MB attenuated TBI-induced edema and neuroinflammation, and improved behavioral recovery. Specifically, a single i.v. injection of 2 mg/kg MB attenuated the TBI-induced increase in IL-1β, TNF-α, and CCL2 in the hippocampus. Moreover, MB treatment reduced the percentage of inflammatory myeloid cells (CD11b+/GR1+) cells in circulation at 1 d post-injury. Although MB did not reduce the percentage of brain-associated macrophages after TBI, MB shifted the inflammatory profile of microglia/macrophages towards less inflammatory state with reduced IL-1β and increased IL-10 mRNA expression. Last, immediate MB intervention protected mice from the development of TBI-associated depressive-like behavior at 7 d post-injury.
One important aspect of this study is that a single injection of a low dose of MB reduced TBI-associated edema 1 d after injury. It is important to highlight that in the midline FPI model, there is less edema than other models of TBI and this difference is related to the craniectomy required for FPI.48 Nonetheless, water accumulation was slightly increased in the cortices of TBI mice. These data are consistent with a recent study showing that MB significantly attenuated hyperintense lesions by MRI (associated with edema) in a controlled cortical impact model of TBI.41 Edema increases intracranial pressure (ICP), which is a primary endpoint for patients with a TBI at a neurotrauma center.28–30 This is critical issue because higher ICP corresponds with a worse neurological outcome.33
For example, increased ICP over 40 mm Hg is associated with a six-fold increase in mortality compared to individuals with normal ICP.28 Despite this fact, careful ICP monitoring and reactive treatments to mitigate aberrant ICP/cerebral perfusion pressure levels have not significantly improved neurological outcome after TBI.29 Our data indicate that immediate intervention with MB prevented edema associated with diffuse brain injury. These data are interpreted to indicate that MB intervention may help to improve survival and neurological outcome after moderate to severe TBI.
Another major finding was that MB intervention significantly reduced neuroinflammation after TBI. This is important because in mild to moderate concussions in humans, loss of function is not related to gross neuronal loss in one particular area but rather results from a progressive and prolonged secondary injury process including edema and neuroinflammation, along with excitotoxicity, production of oxygen free radicals, and blood brain barrier breakdown.61 Here, immediate MB intervention reduced mRNA levels of pro-inflammatory mediators including IL-1β, TNF-α, and CCL2 in the hippocampus at 1 d post-injury. Moreover, MB reduced the activation profile of enriched CD11b+microglia/macrophages after TBI skewing cells toward an anti-inflammatory, IL-1β-/IL-10+ phenotype. Cell culture experiments with BV-2 microglia confirmed that MB had a direct effect on microglia and the promotion of the IL-1β-/IL-10+ phenotype.
Although a previous in vitro study reporting anti-inflammatory effects of MB showed efficacy only at high doses (100 μM), that study used a high dose of LPS and primary microglia, whereas here the dose of LPS was ten-fold lower and BV-2 microglia were used.40 Reducing immediate neuroinflammation also could promote long-lasting benefits for TBI patients. We previously published that the secondary injury after a TBI promotes the development of a primed (major histocompatibility complex [MHC] II+) microglial profile at one month post-injury.21 This is a key alteration in microglia because transient activation of the innate immune system 30 d post-injury caused amplified microglia activation and the re-establishment of depressive-like behavior.21 Therefore, immediate inflammatory events after TBI may lead to profound alterations in microglia that persist well after the initial inflammation has resolved. Because TBI-induced inflammatory damage is likely the event that induces microglial priming, it is plausible that MB intervention reduces microglial priming and immune reactivity protecting patients from recurring depressive symptoms. Further experimentation, however, is required to test this hypothesis.
Increased edema and neuroinflammation after TBI involves the recruitment of peripherally-derived myeloid cells to the CNS.47,62–64 Here we show that the percentage of inflammatory, CD11b+/GR1+ myeloid cells in circulation was significantly increased after TBI. These data are consistent with several studies demonstrating a robust humoral response to head trauma and trafficking of peripheral cells to the brain.3,64,65 A potential benefit of MB intervention after TBI was a decreased concentration of inflammatory myeloid cells in circulation by 1 d post-injury. This reduction in circulating inflammatory cells may be partially responsible for reduced edema and brain inflammation with MB treatment. It is unclear if MB limits the release of these cells from the bone marrow, promotes selected apoptosis of activated myeloid cells, or restricts the activation of monocytes limiting their expression of GR1. Although not graphically represented, the total percentage of CD11b+ cells was increased with TBI and returned to baseline with MB (data not shown). These data are interpreted to indicate that MB reduced myeloid egress from the bone marrow after TBI or induced cellular apoptosis. Notably, the restriction in circulating CD11b+/GR1+ cells after MB had no effect on the percentage of brain associated CD11b+/CD45hi/Ly6Chi macrophages after TBI.
Nonetheless, there was a shift in the inflammatory profile of these microglia/macrophages from an inflammatory IL-1β+phenotype to a more anti-inflammatory IL-1β-/IL-10+ phenotype with MB intervention. In contrast, minocycline treatment after a closed head injury in mice reduced cortical gene expression of IL-1β, but had no effect on IL-10 expression or any other inflammatory mediator.32 Therefore, MB treatment uniquely drove anti-inflammatory gene expression and significantly reduced multiple inflammatory cytokines. Moreover, minocycline treatment was ineffective in improving neurological outcome or reducing cellular apoptosis or lesion volume past 1 d post-injury in a closed head injury model.32 Effective minocycline treatment required multiple doses at a high concentration to reduce inflammation and edema at 1 d post-injury.33 Here, a single dose of MB at a low dosage was effective in reducing inflammatory myeloid cells in circulation, limiting edema, and reducing the activation state of brain microglia and macrophages.
A key finding in this study was that immediate MB intervention after TBI reduced acute depressive-like behavior at 7 d post-injury. It is important to highlight that MB intervention did not prevent TBI-induced weight loss or transient reductions in motor coordination during the first week after injury. Thus, one interpretation is that the antioxidant and anti-inflammatory mechanisms do not underlie these physiological deficits. Nonetheless, the effectiveness of MB in attenuating TBI-associated depressive-like behavior has relevant implications for the long-term mental health outcome of TBI patients. Mental health illness, including depression, is the leading cause of healthy years lost in the United States.66 Moreover, rates of depression double in individuals after TBI, compared with the general population, and risk of depression appears to increase with time after injury.67–69 Further, TBI patients are typically refractory to classical anti-depressant therapies, making treatment more difficult.70,71 Thus, depressive-like behavior after TBI may have a unique etiology that is more in line with the “inflammatory hypothesis” of depression.18,20
In support of this idea, several basic and clinical studies have provided evidence that increased levels of circulating and central inflammatory cytokines cause symptoms of depression.19,25,26,72,73 Moreover, here we show that reduced brain inflammation after MB treatment was associated with reduced depressive-like behavior. We also have previously reported that activation of the innate immune response and exaggerated brain inflammation promotes the re-establishment of depressive behavior in TBI mice.21 Considering MB is currently in clinical trial for neuropsychiatric use,35 MB provides a unique treatment option that could reduce the rate of psychiatric complications after TBI and extend the number of healthy years lived.68,69,74,75
One unexpected result was that MB treatment alone increased some inflammatory immune markers in CD11b+ cells. For example, MB alone increased CD14 and iNOS gene expression in microglia/macrophages. Corresponding with these data, sham mice that received MB had reduced rotarod performance, compared with Sham-Con mice at 1 h post-sham injury, though the values were not different from their baseline. It is unclear why MB alone induced these effects but perhaps the nitric oxide-inhibiting properties created a hypertensive environment inducing a low level of oxidative stress.35,38 Alternatively, the blockade of endothelial NOS under conditions of normal homeostasis could have resulted in a low level of inflammatory responses and neuronal distress as constitutive nitric oxide has multiple anti-inflammatory and neuroprotective properties.76,77 Nonetheless, these inflammatory markers were not grossly upregulated in whole brain tissue and MB alone had no effect on brain edema or depressive-like behavior. Thus, these studies support the idea that MB is a safe drug at low doses (<5 mg/kg) with limited adverse effects.35,37,39
It is important to discuss the relevance of the timing and mode of MB intervention used in the current study. MB was administered i.v. within 30 min after injury. This is a relevant time point clinically because individuals who suffer from a TBI typically receive treatment within this time span. For example, after a life-threatening car accident, it takes an average of 5–10 min for the ambulance to reach an accident victim.78 Because MB is stored at room temperature and is administered i.v., it can be provided within minutes of head injury in the locker room, ambulance, or battlefield. In addition, MB can be provided multiple times over a 24 h period with minimal adverse effects (i.e., green discoloration of urine). Indeed, a recent study demonstrated that two acute, low doses of MB (1 mg/kg and 0.5 mg/kg) given 1 h and 3 h after a mild controlled cortical impact injury were effective in reducing neuronal cell death and lesion size.41 We understand that more basic research is required to determine the timing, dosage, and efficacy of MB treatment after TBI. Based on the data presented here, however, immediate MB intervention provides a plausible therapy for head injuries in humans.
In summary, the findings presented here demonstrate a unique role for MB in the proactive treatment of TBI with no adverse effects. MB is safe at low doses (<5 mg/kg) and already used clinically for other ailments, stable at room temperature, can be injected i.v., will rapidly cross the blood brain barrier, and is inexpensive—making this the ideal drug for the treatment of TBI. Our data in mice indicate that MB intervention is a promising therapy that may reduce life-threatening complications of TBI including cerebral edema and neuroinflammation, and also protect against the development of secondary and long-lasting neuropsychiatric complications.
Acknowledgments
This work was supported by Ohio State University funding by the Center for Brain and Spinal Cord Repair (to JPG), Department of Surgery (to DSE), and a College of Medicine Dean's Discovery Grant (to JPG). AMF was supported by the OSU Presidential Fellowship. The authors thank Christopher Burnsides for his technical assistance, and Dr. Joseph Travers and Dr. Jason Nasse for use of their isoflurane setup and their technical assistance in developing an isoflurane system.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.National Hospital Discharge Survey (NHDS), 2010. National Hospital Ambulatory Medical Care Survey (NHAMCS), 2010. National Vital Statistics System (NVSS), 2010. Injury prevention and control: Traumatic brain injury. In Vol. C.F.D.C.A.P. (CDC), (eds). CDC National Center for Health Statistics [Google Scholar]
- 2.Lenzlinger P., Morganti-Kossmann M., Laurer H., and Mcintosh T. (2001). The duality of the inflammatory response to traumatic brain injury. Mol. Neurobiol. 24, 169–181 [DOI] [PubMed] [Google Scholar]
- 3.Woodcock T. and Morganti-Kossmann M.C. (2013). The role of markers of inflammation in traumatic brain injury. Front. Neurol. 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.David S. and Kroner A. (2011). Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 12, 388–399 [DOI] [PubMed] [Google Scholar]
- 5.Guillemin G.J., Smythe G., Takikawa O., and Brew B.J. (2005). Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia 49, 15–23 [DOI] [PubMed] [Google Scholar]
- 6.Sinz E.H., Kochanek P.M., Heyes M.P., Wisniewski S.R., Bell M.J., Clark R.S.B., Dekosky S.T., Blight A.R., and Marion D.W. (1998). Quinolinic acid is increased in CSF and associated with mortality after traumatic brain injury in humans. J. Cereb. Blood Flow Metab. 18, 610–615 [DOI] [PubMed] [Google Scholar]
- 7.Tavazzi B., Signoretti S., Lazzarino G., Amorini A.M., Delfini R., Cimatti M., Marmarou A., and Vagnozzi R. (2005). Cerebral oxidative stress and depression of energy metabolism correlate with severity of diffuse brain injury in rats. Neurosurgery 56, 582–589 [DOI] [PubMed] [Google Scholar]
- 8.Barrientos R., Frank M., Hein A., Higgins E., Watkins L., Rudy J., and Maier S. (2009). Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav. Immun. 23, 46–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrientos R.M., Higgins E.A., Biedenkapp J.C., Sprunger D.B., Wright-Hardesty K.J., Watkins L.R., Rudy J.W., and Maier S.F. (2006). Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol. Aging 27, 723–732 [DOI] [PubMed] [Google Scholar]
- 10.Chen J., Buchanan J., Sparkman N., Godbout J., Freund G., and Johnson R. (2008). Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav. Immun. 22, 301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richwine A.F., Parkin A.O., Buchanan J.B., Chen J., Markham J.A., Juraska J.M., and Johnson R.W. (2008). Architectural changes to ca1 pyramidal neurons in adult and aged mice after peripheral immune stimulation. Psychoneuroendocrinology 33, 1369–1377 [DOI] [PubMed] [Google Scholar]
- 12.Mckee A.C., Stern R.A., Nowinski C.J., Stein T.D., Alvarez V.E., Daneshvar D.H., Lee H.S., Wojtowicz S.M., Hall G., Baugh C.M., Riley D.O., Kubilus C.A., Cormier K.A., Jacobs M.A., Martin B.R., Abraham C.R., Ikezu T., Reichard R.R., Wolozin B.L., Budson A.E., Goldstein L.E., Kowall N.W., and Cantu R.C. (2013). The spectrum of disease in chronic traumatic encephalopathy. Brain 136, 43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mcallister T.W., Saykin A.J., Flashman L.A., Sparling M.B., Johnson S.C., Guerin S.J., Mamourian A.C., Weaver J.B., and Yanofsky N. (1999). Brain activation during working memory 1 month after mild traumatic brain injury: a functional MRI study. Neurology 53, 1300–1308 [DOI] [PubMed] [Google Scholar]
- 14.Tang Y., Noda Y., Hasegawa, T. and Nabeshima T. (1997). A concussive-like brain injury model in mice (i): Impairment in learning and memory. J. Neurotrauma 14, 851–862 [DOI] [PubMed] [Google Scholar]
- 15.Zohar O., Schreiber S., Getslev V., Schwartz J., Mullins P., and Pick C. (2003). Closed-head minimal traumatic brain injury produces long-term cognitive deficits in mice. Neuroscience 118, 949. [DOI] [PubMed] [Google Scholar]
- 16.Siopi E., Llufriu-Daben G., Fanucchi F., Plotkine M., Marchand-Leroux C., and Jafarian-Tehrani M. (2012). Evaluation of late cognitive impairment and anxiety states following traumatic brain injury in mice: The effect of minocycline. Neurosci. Lett. 511, 110–115 [DOI] [PubMed] [Google Scholar]
- 17.Steiner J., Walter M., Gos T., Guillemin G., Bernstein H.-G., Sarnyai Z., Mawrin C., Brisch R., Bielau H., Zu Schwabedissen L., Bogerts B., and Myint A.-M. (2011). Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: Evidence for an immune-modulated glutamatergic neurotransmission? J. Neuroinflamm 8, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dantzer R., O'connor J.C., Lawson M.A., and Kelley K.W. (2011). Inflammation-associated depression: From serotonin to kynurenine. Psychoneuroendocrinology 36, 426–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haroon E., Raison C.L., and Miller A.H. (2012). Psychoneuroimmunology meets neuropsychopharmacology: Translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 37, 137–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raison C.L., Capuron L., and Miller A.H. (2006). Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 27, 24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fenn A.M., Gensel J.C., Huang Y., Popovich P.G., Lifshitz J., and Godbout J.P. (2013). Immune activation promotes depression 1 month after diffuse brain injury: A role for primed microglia. Biol. Psychiat. 2013October25; [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 22.Fromm L., Heath D.L., Vink R., and Nimmo A.J. (2004). Magnesium attenuates post-traumatic depression/anxiety following diffuse traumatic brain injury in rats. J. Am. Coll. Nutr. 23, 529S–533S [DOI] [PubMed] [Google Scholar]
- 23.Pandey D.K., Yadav S.K., Mahesh R., and Rajkumar R. (2009). Depression-like and anxiety-like behavioural aftermaths of impact accelerated traumatic brain injury in rats: A model of comorbid depression and anxiety? Behav. Brain Res. 205, 436–442 [DOI] [PubMed] [Google Scholar]
- 24.Milman A., Rosenberg A., Weizman R., and Pick C. (2005). Mild traumatic brain injury induces persistent cognitive deficits and behavioral disturbances in mice. J. Neurotrauma 22, 1003–1010 [DOI] [PubMed] [Google Scholar]
- 25.Miller A.H. and Raison C.L. (2008). Immune system contributions to the pathophysiology of depression. Focus 6, 36–45 [Google Scholar]
- 26.Dantzer R., O'connor J., Freund G., Johnson R., and Kelley K. (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godbout J., Moreau M., Lestage J., Chen J., Sparkman N., O'connor J., Castanon N., Kelley K., Dantzer R., and Johnson R. (2008). Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacol 33, 2341–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Treggiari M.M., Schutz N., Yanez N.D., and Romand J.A. (2007). Role of intracranial pressure values and patterns in predicting outcome in traumatic brain injury: a systematic review. Neurocrit. Care 6, 104–112 [DOI] [PubMed] [Google Scholar]
- 29.Cremer O.L., Van Dijk G.W., Van Wensen E., Brekelmans G.J., Moons K.G., Leenen L.P., and Kalkman C.J. (2005). Effect of intracranial pressure monitoring and targeted intensive care on functional outcome after severe head injury. Crit. Care Med. 33, 2207–2213 [DOI] [PubMed] [Google Scholar]
- 30.Bratton S.L., Chestnut R.M., Ghajar J., Mcconnell Hammond F.F., Harris O.A., Hartl R., Manley G.T., Nemecek A., Newell D.W., Rosenthal G., Schouten J., Shutter L., Timmons S.D., Ullman J.S., Videtta W., Wilberger J.E., and Wright D.W. (2007). Guidelines for the management of severe traumatic brain injury. Viii. Intracranial pressure thresholds. J. Neurotrauma 24Suppl 1, S55–S58 [DOI] [PubMed] [Google Scholar]
- 31.Roberts I., Yates D., Sandercock P., Farrell B., Wasserberg J., Lomas G., Cottingham R., Svoboda P., Brayley N., Mazairac G., Laloë V., Muñoz-Sánchez A., Arango M., Hartzenberg B., Khamis H., Yutthakasemsunt S., Komolafe E., Olldashi F., Yadav Y., Murillo-Cabezas F., Shakur H., Edwards, P.; CRASH trial collaborators. (2004). Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC crash trial): Randomised placebo-controlled trial. Lancet 364, 1321–1328 [DOI] [PubMed] [Google Scholar]
- 32.Bye N., Habgood M., Callaway J., Malakooti N., Potter A., Kossmann T., and Morganti-Kossmann M. (2007). Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Exp. Neurol. 204, 220–233 [DOI] [PubMed] [Google Scholar]
- 33.Homsi S., Federico F., Croci N., Palmier B., Plotkine M., Marchand-Leroux C., and Jafarian-Tehrani M. (2009). Minocycline effects on cerebral edema: Relations with inflammatory and oxidative stress markers following traumatic brain injury in mice. Brain Res. 1291, 122–132 [DOI] [PubMed] [Google Scholar]
- 34.Abdel Baki S.G., Schwab B., Haber M., Fenton A.A., and Bergold P.J. (2010). Minocycline synergizes with N-acetylcysteine and improves cognition and memory following traumatic brain injury in rats. PLoS One 5, e12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oz M., Lorke D.E., Hasan M., and Petroianu G.A. (2011). Cellular and molecular actions of methylene blue in the nervous system. Med. Res. Rev. 31, 93–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schirmer R.H., Coulibaly B., Stich A., Scheiwein M., Merkle H., Eubel J., Becker K., Becher H., Muller O., Zich T., Schiek W., and Kouyate B. (2003). Methylene blue as an antimalarial agent. Redox Rep. 8, 272–275 [DOI] [PubMed] [Google Scholar]
- 37.Paciullo C.A., Horner D.M., Hatton K.W., and Flynn J.D. (2012). Methylene blue for the treatment of septic shock. Pharmacotherapy 30, 702–715 [DOI] [PubMed] [Google Scholar]
- 38.Maslow A.D., Stearns G., Batula P., Schwartz C.S., Gough J., and Singh A.K. (2006). The hemodynamic effects of methylene blue when administered at the onset of cardiopulmonary bypass. Anesth. Analg. 103, 2–8 [DOI] [PubMed] [Google Scholar]
- 39.Miclescu A., Sharma H.S., Martijn C., and Wiklund L. (2010). Methylene blue protects the cortical blood brain-barrier against ischemia/reperfusion-induced disruptions. Crit. Care Med. 38, 2199–2206 [DOI] [PubMed] [Google Scholar]
- 40.Dibaj P., Zschuntzsch J., Steffens H., Scheffel J., Goricke B., Weishaupt J.H., Le Meur K., Kirchhoff F., Hanisch U.K., Schomburg E.D., and Neusch C. (2012). Influence of methylene blue on microglia-induced inflammation and motor neuron degeneration in the sod1(g93a) model for ALS. PLoS One 7, e43963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Talley Watts L., Long J.A., Chemello J., Van Koughnet S., Fernandez A., Huang S., Shen Q., and Duong T.Q. (2014). Methylene blue is neuroprotective against mild traumatic brain injury. J. Neurotrauma 31, 1063–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peter C., Hongwan D., Kã¼Pfer A., and Lauterburg B.H. (2000). Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur. J. Clin. Pharmacol. 56, 247–250 [DOI] [PubMed] [Google Scholar]
- 43.Marklund N. and Hillered L. (2011). Animal modelling of traumatic brain injury in preclinical drug development: Where do we go from here? Br. J. Pharmacol. 164, 1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenfeld J.V., Maas A.I., Bragge P., Morganti-Kossmann M.C., Manley G.T., and Gruen R.L. (2012). Early management of severe traumatic brain injury. Lancet 380, 1088–1098 [DOI] [PubMed] [Google Scholar]
- 45.Bachstetter A.D., Rowe R.K., Kaneko M., Goulding D., Lifshitz J., and Van Eldik L.J. (2013). The p38α MAPK regulates microglial responsiveness to diffuse traumatic brain injury. J. Neurosci. 33, 6143–6153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelley B.J., Farkas O., Lifshitz J., and Povlishock J.T. (2006). Traumatic axonal injury in the perisomatic domain triggers ultrarapid secondary axotomy and wallerian degeneration. Exp.Neurol. 198, 350–360 [DOI] [PubMed] [Google Scholar]
- 47.Kelley B.J., Lifshitz J., and Povlishock J.T. (2007). Neuroinflammatory responses after experimental diffuse traumatic brain injury. J, Neuropathol, Exp, Neurol, 66, 989–1001 [DOI] [PubMed] [Google Scholar]
- 48.Lifshitz J. (2008). Fluid percussion injury model, in: Animal Models of Acute Neurological Injuries. Chen J., Xu X-M. and Xu ZC. (eds). Humana Press: New York City [Google Scholar]
- 49.Witgen B., Lifshitz J., Smith M., Schwarzbach E., Liang S., Grady M., and Cohen A. (2005). Regional hippocampal alteration associated with cognitive deficit following experimental brain injury: A systems, network and cellular evaluation. Neuroscience 133, 1–15 [DOI] [PubMed] [Google Scholar]
- 50.Lifshitz J., Witgen B., and Grady M. (2007). Acute cognitive impairment after lateral fluid percussion brain injury recovers by 1 month: Evaluation by conditioned fear response. Behav. Brain Res. 177, 347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Witgen B., Lifshitz J., and Grady M. (2006). Inbred mouse strains as a tool to analyze hippocampal neuronal loss after brain injury: a stereological study. J. Neurotrauma 23, 1320–1329 [DOI] [PubMed] [Google Scholar]
- 52.Vutskits L., Briner A., Klauser P., Gascon E., Dayer A.G., Kiss J.Z., Muller D., Licker M.J., and Morel D.R. (2008). Adverse effects of methylene blue on the central nervous system. Anesthesiology 108, 684–692 [DOI] [PubMed] [Google Scholar]
- 53.Shohami E., Novikov M. and Mechoulam R. (1993). A nonpsychotropic cannabinoid, HU-211, has cerebroprotective effects after closed head injury in the rat. J. Neurotrauma 10, 109–119 [DOI] [PubMed] [Google Scholar]
- 54.Wohleb E.S., Powell N.D., Godbout J.P., and Sheridan J.F. (2013). Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J. Neurosci 33, 13820–13833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henry C., Huang Y., Wynne A.M., and Godbout J.P. (2009). Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1 beta and anti-inflammatory IL-10 cytokines. Brain Behav. Immun. 23, 309–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fenn A.M., Henry C.J., Huang Y., Dugan A., and Godbout J.P. (2012). Lipopolysaccharide-induced interleukin (IL)-4 receptor-α expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain Behav. Immun. 26, 766–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wohleb E.S., Fenn A.M., Pacenta A.M., Powell N.D., Sheridan J.F., and Godbout J.P. (2012). Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology 37, 1491–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Corona A., Huang Y., O'connor J., Dantzer R., Kelley K., Popovich P., and Godbout J. (2010). Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. J. Neuroinflammation 7, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zembowicz A. and Vane J.R. (1992). Induction of nitric oxide synthase activity by toxic shock syndrome toxin 1 in a macrophage-monocyte cell line. Proc. Natl. Acad. Sci. U. S. A. 89, 2051–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boulanger C., Xfc and Scher T.F. (1990). Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. J. Clin. Invest. 85, 587–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greve M.W. and Zink B.J. (2009). Pathophysiology of traumatic brain injury. Mt. Sinai J. Med. A 76, 97–104 [DOI] [PubMed] [Google Scholar]
- 62.Schwab J., Seid K., and Schluesener H. (2001). Traumatic brain injury induces prolonged accumulation of cyclooxygenase-1 expressing microglia/brain macrophages in rats. J. Neurotrauma 18, 881–890 [DOI] [PubMed] [Google Scholar]
- 63.Utagawa A., Bramlett H.M., Daniels L., Lotocki G., Dekaban G.A., Weaver L.C., and Dietrich W.D. (2008). Transient blockage of the CD11d/CD18 integrin reduces contusion volume and macrophage infiltration after traumatic brain injury in rats. Brain Res. 1207, 155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morganti J.M., Jopson T.D., Liu S., Gupta N., and Rosi S. (2014). Cranial irradiation alters the brain's microenvironment and permits ccr2+ macrophage infiltration. PLoS One 9, e93650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beschorner R., Nguyen T.D., Gã¶Zalan F., Pedal I., Mattern R., Schluesener H.J., Meyermann R., and Schwab J.M. (2002). Cd14 expression by activated parenchymal microglia/macrophages and infiltrating monocytes following human traumatic brain injury. Acta Neuropathol. 103, 541–549 [DOI] [PubMed] [Google Scholar]
- 66.The world health report 2003 Annex table 3: Burden of disease in DALYs by cause, sex, and mortality stratum in who regions, estimates for 2002. Available at: www.who.int/whr/2003/en/Annex3-en.pdf Accessed September8, 2014
- 67.Fleminger S. (2008). Long-term psychiatric disorders after traumatic brain injury. Eur. J. Anesth. 25, 123–130 [DOI] [PubMed] [Google Scholar]
- 68.Jorge R., Robinson R., Moser D., Tateno A., Crespo-Facorro B., and Arndt S. (2004). Major depression following traumatic brain injury. Arch. Gen. Psychiatry 61, 4250. [DOI] [PubMed] [Google Scholar]
- 69.Holsinger T., Steffens D.C., Phillips C., Helms M.J., Havlik R.J., Breitner J.C., Guralnik J.M., and Plassman B.L. (2002). Head injury in early adulthood and the lifetime risk of depression. Arch. Gen. Psychiatry 59, 17–22 [DOI] [PubMed] [Google Scholar]
- 70.Ashman T.A., Cantor J.B., Gordon W.A., Spielman L., Flanagan S., Ginsberg A., Engmann C., Egan M., Ambrose F., and Greenwald B. (2009). A randomized controlled trial of sertraline for the treatment of depression in persons with traumatic brain injury. Arch. Phys. Med. Rehab. 90, 733–740 [DOI] [PubMed] [Google Scholar]
- 71.Saran A. (1988). Antidepressants not effective in headache associated with minor closed head injury. Int. J. Psychiatr. Med. 18, 75–83 [DOI] [PubMed] [Google Scholar]
- 72.Yirmiya R., Pollak Y., Morag M., Reichenberg A., Barak O., Avitsur R., Shavit Y., Ovadia H., Weidenfeld J., Morag A., Newman M.E., and Pollmächer T. (2000). Illness, cytokines, and depression. Ann. N.Y. Acad. Sci. 917, 478–487 [DOI] [PubMed] [Google Scholar]
- 73.O'Connor J.C., Lawson M.A., Andre C., Moreau M., Lestage J., Castanon N., Kelley K.W., and Dantzer R. (2008). Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry. 14, 511–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hibbard M.R., Uysal S., Kepler K., Bogdany J., and Silver J. (1998). Axis i psychopathology in individuals with traumatic brain injury. J. Head Trauma Rehab. 13, 24–39 [DOI] [PubMed] [Google Scholar]
- 75.Jorge R., Robinson R., Arndt S., Starkstein S., Forrester A., and Geisler F. (1993). Depression following traumatic brain injury: a 1 year longitudinal study. J Affect Disorders 27, 233–243 [DOI] [PubMed] [Google Scholar]
- 76.Cardenas A., Moro M.A., Hurtado O., Leza J.C., and Lizasoain I. (2005). Dual role of nitric oxide in adult neurogenesis. Brain Res. Brain Res. Rev. 50, 1–6 [DOI] [PubMed] [Google Scholar]
- 77.De Palma C., Falcone S., Panzeri C., Radice S., Bassi M.T., and Clementi E. (2008). Endothelial nitric oxide synthase overexpression by neuronal cells in neurodegeneration: a link between inflammation and neuroprotection. J. Neurochem. 106, 193–204 [DOI] [PubMed] [Google Scholar]
- 78.Wang H.E., Mann N.C., Jacobson K.E., Ms M.D., Mears G., Smyrski K. and Yealy D.M. (2013). National characteristics of emergency medical services responses in the united states. Prehosp. Emerg. Care 17, 8–14 [DOI] [PubMed] [Google Scholar]