Abstract
Background
Age at diagnosis is incorporated into all relevant staging systems for differentiated thyroid carcinoma (DTC). There is growing evidence that a specific age cutoff may not be ideal for accurate risk stratification. We sought to evaluate the interplay between age and oncologic variables in patients with DTC using the largest cohort to date.
Methods
The Surveillance, Epidemiology, and End Results (SEER) database was queried to identify patients with DTC as their only malignancy for the period 1973 to 2009. Multivariate analyses using a range of age cutoffs and age subgroupings were utilized in order to search for an optimal age that would provide the most significant risk stratification between young and old patients. The primary outcome was disease-specific survival (DSS) and covariates included: age, race, sex, tumor/nodal/metastasis (TNM) stage, decade of diagnosis, and radioactive iodine therapy.
Results
A total of 85,740 patients were identified. Seventy-six percent of patients were American Joint Committee on Cancer (AJCC) stage I, 8% were stage II, 7% were stage III, and 8% were stage IV. Age over 45 years (hazard ratio [HR] 19.2, p<0.001) and metastatic disease (HR 13.1, p<0.001) were the strongest predictors of DSS. Other factors that significantly predicted DSS included: not receiving radioactive iodine (RAI; HR 1.3, p=0.002), T3 (HR 2.6, p<0.001), and T4 disease (HR 3.3, p<0.001), and nodal spread (HR 2.6 to 3.3, p<0.001). Female sex showed a significant protective effect (HR 0.7, p=0.001). Adjusting the age-group cutoff from 25 to 55 years showed consistently high HRs for advanced age, without a distinct change at any point. Comparing HRs for T, N, and M stage between young and old patient subgroups showed that advanced disease increased the risk for DSS regardless of age, and was oftentimes a worse prognosticator in young patient groups.
Conclusions
The contribution of age at diagnosis to a patient's DSS is considerable, but there is no age cutoff that affords any unique risk-stratification in patients with DTC.
Introduction
Papillary and follicular carcinomas are differentiated thyroid cancers (DTC) that account for more than 90% of the nearly 50,000 cases of thyroid cancer diagnosed in the United States every year (1). Due in large part to improved early detection, the number of newly diagnosed cases of DTC has been consistently increasing. Notably, this disease carries a high 5-year survival rate of around 97% (2). Despite an overall favorable prognosis, DTC can be aggressive, with considerable mortality in advanced cases (3). In order to counsel patients appropriately on their prognosis and to guide treatment decisions, accurate clinical staging is crucial.
Along with parameters considered relevant for staging in most cancers (primary tumor size, nodal status, and distant metastasis), all relevant DTC staging systems incorporate age at diagnosis (4). The European Organization for the Treatment of Cancer (EORTC) developed a system that adds a point to the patient's score for every year of age (5). Most other staging systems chose an age cutoff, above which patient survival is thought to be worse. The Mayo Clinic's Metastasis, Age, Completeness of resection, Invasion and Size (MACIS) classification drew the line at age 40 (6). The Lahey Clinic's Age, Metastases, Extent and Size (AMES) system has different cutoffs for men (age 40) and women (age 50) (7). The Grade, Age, Metastases, Extent and Size (GAMES) staging, developed at Memorial Sloan-Kettering, assigned patients above age 45 a higher score (8).
The most relevant clinical staging system today, the American Joint Committee on Cancer (AJCC) staging protocol for DTC (Table 1) assigns patient age such strong prognostic value that it limits classification for patients under 45 to stages I (absence of metastatic disease) and II (presence of metastatic disease); while patients age 45 and above are divided into stages I through IVc (9). This age cutoff of 45 years has been a component since the second edition of the AJCC Cancer Staging Manual (10). It was recently reported by Oyer et al. (11), as they analyzed the prognostic significance of age in DTC, that none of the sources cited in the first staging manual actually evaluated an age cutoff of 45 years. Furthermore, these early studies were based on cohorts of several hundred patients (12,13), and thus do not approach the statistical power of today's national databases.
Table 1.
AJCC Cancer Staging Manual, 7th Edition: Protocol for Differentiated Thyroid Carcinoma
| AJCC Staging Protocol for DTC, 7th Edition | |
|---|---|
| Primary tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | ≤2cm, limited to the thyroid |
| T1a | ≤1cm |
| T1b | >1 cm and ≤2 cm |
| T2 | >2 cm and ≤4 cm, limited to the thyroid |
| T3 | >4 cm, limited to the thyroid or any tumor with minimal extra-thyroid extension (e.g., to the sternothyriod muscle or perithyroid soft tissues) |
| T4a | Tumor of any size extending beyond the thyroid capsule to invade subcutaneous soft tissues, larynx, trachea, esophagus, or recurrent laryngeal nerve |
| T4b | Tumor invades prevertebral fascia or encases carotid artery or mediastinal vessels |
| Regional nodes (N) | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional node metastasis |
| N1 | Regional node involvement |
| N1a | Nodal metastasis to level VI (pretracheal, paratracheal, and prelaryngeal/Delphian lymph nodes) |
| N1b | Nodal metastasis to unilateral, bilateral, or contralateral cervical (Levels I, II, III, IV, or V) or retropharyngeal or cervical or superior mediastinal lymph nodes (Level VII) |
| Distant metastasis (M) | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| AJCC Staging grouping | |||
|---|---|---|---|
| For patients <45 years | |||
| Stage I | Any T | Any N | M0 |
| Stage II | Any T | Any N | M1 |
| For patients >45 years | |||
| Stage I | T1 | N0 | M0 |
| Stage II | T2 | N0 | M0 |
| Stage III | T3 | N0 | M0 |
| T1–3 | N1a | M0 | |
| Stage IVa | T4a | N0–1a | M0 |
| T1–4a | N1b | M0 | |
| Stage IVb | T4b | Any N | M0 |
| Stage IVc | Any T | Any N | M1 |
While age is an undeniably important predictor of outcome for patients with DTC, Lang et al. (14) showed that none of the above-mentioned staging systems were able to account for a small number of cancer-related deaths in the younger patient groups. Tran Cao et al. (15) found that the current AJCC staging might overestimate the protective effect of young age. Furthermore, there has been evidence that age incrementally adds to the risk of mortality starting at age 35 (11). Work by Jonklaas et al. (16) reported that women younger than 55 years had better outcomes than men, but in patients older than 55 years there were equal outcomes in men and women.
Because of the lack of consensus on the contribution of age in patients with DTC, we conducted a detailed analysis of patient age and disease status as predictors of disease-specific survival (DSS) using the Surveillance, Epidemiology, and End Results (SEER) database.
Materials and Methods
Patients with the diagnosis of DTC as their only known malignancy were identified using the SEER database for the period 1973 to 2009 (ICD-O site code of C73.9). The diagnosis codes used to capture all patients with DTC included: papillary carcinoma, papillary adenocarcinoma, oxyphilic adenocarcinoma, follicular adenocarcinoma, papillary and follicular adenocarcinoma, and papillary cystadenocarcinoma. Age was recorded at the time of diagnosis. Race/ethnicity categories as defined by SEER were grouped as white, black, Asian, Hispanic, and other/unknown. Tumor, nodal, and metastasis characteristics were compiled from the SEER dataset, and patients were staged using the seventh edition of the AJCC staging criteria for DTC (Table 1). Regional metastatic disease was captured in the nodal staging variable. Metastatic disease was defined as distant metastasis. Univariate statistics were generated for patient demographics and oncologic variables.
The SEER variable encoding surgery type was found to be unreliable to be used as a covariate. We found that 40.4% of patients in the dataset had information regarding surgery type, whereas 93.4% of patients had data encoded regarding radiation therapy usage. Radioactive iodine therapy was designated for patients coded as receiving a radioactive isotope.
We performed multivariate analyses using Cox proportional hazard models with the outcome of DSS. Covariates for all models included: race, sex, tumor (T), nodal (N), metastasis (M) stage, decade of diagnosis, and radioactive iodine (RAI) therapy. Use of RAI, white race, male sex, T2 tumor, N0 nodal disease, and absence of metastasis served as reference groups. Age was also a covariate, and the specific age cutoff was varied depending on the model. In order to systematically analyze the contribution of age, the age group reference was incrementally stepped from younger than 19 years to older than 99 years, in 1-year increments, and the hazard ratio (HR) for older age was compared across models.
Using various age cutoffs (30 to 75 in 5-year increments), patients were divided for subgroup analyses: younger patients below the cutoff and older patients above the cutoff. To analyze the contributors toward DSS in these subgroups, multivariate analyses were performed using the same covariates as described above. For example, one analysis was run for patients younger than 30 years, and a separate analysis for patients over 30 years. HRs for various T, N, and M stages were compared for each of the subgroups. The HRs for each TNM variable (T1a, T1b, T2, T3, T4, N1a, N1b, M1) were plotted separately, with a connecting line to demonstrate the difference in the HR between younger and older patient subgroups.
In order to graphically demonstrate the gradual worsening of prognosis as patient age increases, several Kaplan-Meier plots were created for patients with metastatic disease. Three plots were created with different age stratification points (35, 45, and 55 years).
Analyses were performed using STATA SE, version 11.1 (StataCorp, College Station, TX). Statistical significance was defined as a p value less than 0.05. All confidence intervals and error bars are reported as 95% confidence intervals.
This study did not meet criteria for review by the Institutional Review Board at the University of California San Diego because it utilized deidentified patient information from a publically available database.
Results
General findings
Using the SEER database, 85,740 patients with DTC as their only malignancy were identified. Patients were predominantly female (77.8%) and white (68.4%). The mean age at diagnosis was 45.6 years (mean age at diagnosis was 44.8±15.3 (±standard deviation) years for females and 48.5±17.9 years for males). Overall, most cases of DTC were diagnosed in stage I (76.4%), and the remaining cases were stage II (8.1%), III (7.4%), and IV (8.2%). Among patients under the age of 45, the vast majority (99.3%) were stage I. Thyroid cancer-specific mortality for all patients identified was 2.1%, with a median follow-up of 85 months. Patient characteristics are provided in Table 2.
Table 2.
Characteristics of Patients with Differentiated Thyroid Cancer from the Surveillance, Epidemiology, and End Results Database
| Summary of SEER data on DTC | ||
|---|---|---|
| Patient characteristics | n | % |
| Sex | ||
| Female | 66,705 | 77.8 |
| Male | 19,035 | 22.2 |
| Ethnicity | ||
| White | 59,105 | 68.4 |
| Other | 26,635 | 31.6 |
| AJCC stage at diagnosis | ||
| I | 65,530 | 76.4 |
| II | 6,901 | 8.1 |
| III | 6,300 | 7.4 |
| IV | 7,009 | 8.2 |
| AJCC stage at diagnosis for patients <45 | ||
| I | 44,103 | 99.3 |
| II | 316 | 0.7 |
DTC, differentiated thyroid cancer; AJCC, American Joint Committee on Cancer.
Predictors of mortality in DTC
Using 45 years as the age stratification point, multivariate analysis showed that age younger than 45 years (HR 19.2, p<0.001) and metastatic disease (HR 13.1, p<0.001) were the strongest predictors of DSS (Table 3). The HR for patients older than 45 years can also be seen as one data point in Figure 1 (45 years, HR 19.2). Other factors that significantly predicted DSS included: not receiving radioactive iodine (RAI; HR 1.3, p=0.002), T3 (HR 2.6, p<0.001) and T4 disease (HR 3.3, p<0.001), and nodal spread (HR 2.6 for N1a disease, and 3.3 for N1b disease, p<0.001 for both). Female sex showed a significant protective effect (HR 0.7, p=0.001). There was adequate information for 61,049 of all identified DTC patients to be included in the multivariate analysis. When a similar analysis was computed with the outcome of all-cause mortality, the covariates demonstrated the same relative impact and trends, but were found to have smaller effect sizes (data not shown).
Table 3.
Multivariate Analysis Thyroid Cancer-Specific Mortality (Age Comparison with Cutoff of 45 Years)
| Covariate | HR | p value | 95% confidence interval | |
|---|---|---|---|---|
| Age at diagnosis | ||||
| Age <45 | reference | |||
| Age >45 | 19.2 | <0.001 | 13.8 | 26.8 |
| Sex | ||||
| Male | reference | |||
| Female | 0.7 | 0.001 | 0.6 | 0.9 |
| Race | ||||
| White | reference | |||
| Black | 1.0 | 0.807 | 0.7 | 1.5 |
| Asian | 0.8 | 0.427 | 0.4 | 1.4 |
| Hispanic | 1.0 | 0.716 | 0.8 | 1.2 |
| Other | 0.9 | 0.246 | 0.7 | 1.1 |
| T stage | ||||
| 1a | 0.2 | <0.001 | 0.1 | 0.3 |
| 1b | 0.3 | <0.001 | 0.2 | 0.4 |
| 2 | reference | |||
| 3 | 2.6 | <0.001 | 1.9 | 3.5 |
| 4 | 3.3 | <0.001 | 2.7 | 4.0 |
| N stage | ||||
| 0 | reference | |||
| 1a | 2.6 | <0.001 | 1.9 | 3.5 |
| 1b | 3.3 | <0.001 | 2.7 | 4.0 |
| M stage | ||||
| M0 | reference | |||
| M1 | 13.1 | <0.001 | 10.7 | 16.1 |
| RAI | ||||
| RAI use | reference | |||
| No RAI | 1.3 | 0.002 | 1.1 | 1.5 |
| Decade of diagnosis | ||||
| 1973–1979 | reference | |||
| 1980–1989 | 2.9 | <0.001 | 2.0 | 4.2 |
| 1990–1999 | 1.6 | <0.001 | 1.3 | 1.9 |
| 2000–2009 | omitted | |||
Reference groups are: RAI, age <45, male, white, T2, N0, M0. Number of observations: 61,049. Bold indicates statistical significance.
RAI, radioactive iodine.
FIG. 1.
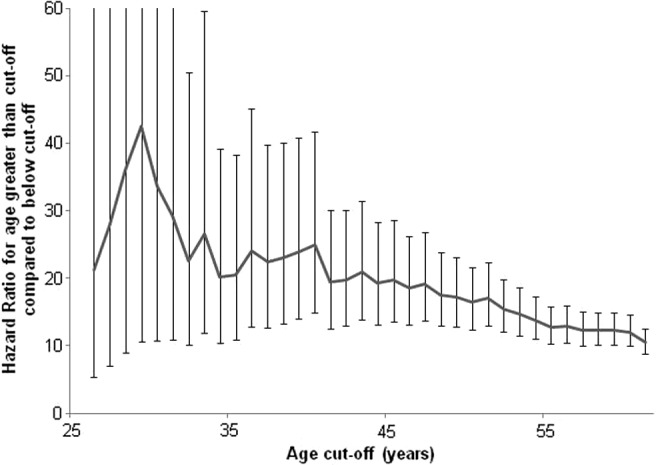
Contribution of age toward thyroid cancer-specific mortality. Covariates that were identical to those listed in Table 3 included: race, sex, tumor (T), nodal (N), metastasis (M) stage, and radioactive iodine therapy. Age was also a covariate, and this was incrementally stepped from 19–99 years. The hazard ratios (HRs) for age cutoffs from 25–59 are shown. The vertical axis represents the HR for the older age group, compared to the younger group. For example, the HR for an age cutoff of 45 years is 19.2. Regardless of the age cutoff chosen, the HR remains similar, with slight downsloping trend with increasing age. Ninety-five percent confidence intervals are shown as vertical whisker bars.
Age as a risk factor for mortality
In order to determine an optimal age that would provide the most significant risk stratification between young and old patients, we performed multivariate analyses selecting age-group cutoffs in 1-year increments from 19 to 99 years. HR data for advanced age, from age cutoffs 25 to 55 years, are plotted in Figure 1. The HR for advanced age was statistically significant (p<0.001) in every model that was generated. HRs were mostly between 15 and 20, until an age division above 95 years where an exponential increase was demonstrated (data not shown).
Interaction between age, T, N, and M status
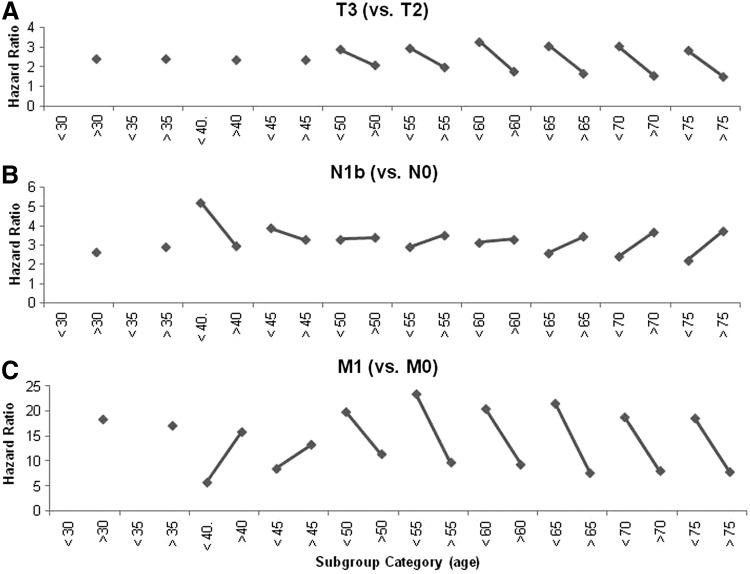
Trends in the contribution of TNM factors in various age subgroups (age cutoffs from 30 to 75 in 5-year increments) are shown in Figure 2. HRs for T, N, and M stage for patients below or above various age cutoffs were compared. The contribution of each oncologic characteristic toward DSS demonstrated varied behavior across subgroups. Data points are only shown for statistically significant HRs.
FIG. 2.
Contribution of oncologic characteristics toward thyroid cancer-specific mortality at different age cutoffs. Covariates were identical to those listed in Table 3. There are variable trends in hazard ratios across age subgroups. (A) T3 tumor status carries a uniformly worse prognosis (compared to T2 tumors) in younger versus older patient subgroups, regardless of the subgroup age cutoff. (B) N1b nodal disease (compared to N0 disease) carries a worse prognosis in younger patients than in older patients. This trend holds up until age 55 where we see opposite behavior: younger patients have better prognosis than their older counterparts. (C) Demonstrates change in prognostic implication for M1 disease (versus M0 disease) as patients are compared across various age cutoffs. For example, M1 disease carries a higher HR in patients under age 30 than in patients over age 30. At age cutoffs 40 and 45, M1 disease carries a higher HR in older patients. Above these cutoffs, younger patients' prognosis is worse again.
HRs for T1a, T1b, and T3 disease each had similar behavior: older patients (above a given age cut-off) tended to have lower HRs compared to their younger counterparts. Therefore, for every T stage, younger patients had a higher risk of cancer-specific mortality than in the older patient subgroup. This is shown in Figure 2A, which depicts the HRs for T3 disease (with T2 disease as a reference), lines downslope from left to right across all pairings. Overall, these HRs were in the 1.5 to 3.3 range.
HRs for nodal disease (N1a and N1b compared to N0 disease) were higher in younger patients up to a cutoff age of 50 years. As the cutoff age was further increased beyond 50 years, the HRs for older patients were higher than for their younger counterparts. HRs for N1b disease were in the 2.2 to 5.2 range and are shown in Figure 2B.
For metastatic disease (M1 compared to M0 disease), younger patients had higher HRs than their older counterparts for most analyzed cutoff ages except for those at age 40 and 45, where HRs for younger patients were slightly lower. HRs for metastatic disease were in the range of approximately 5.8 to 23.5 (shown in Fig. 2C), which represented the highest level of contribution toward DSS among all oncologic characteristics.
Across multiple age subgroupings, female sex showed a protective effect (HRs from 0.4 to 0.8), with younger females having a slightly larger survival benefit than their older counterparts (data not shown).
Survival in patients with metastatic disease
Figure 3 shows Kaplan-Meier plots for patients with metastatic (M1) disease when stratified at different age cutoffs (35, 45, and 55 years). Older patients uniformly showed worse survival. The difference between the young/old groups decreased as the age cutoff was advanced. The behavior of patients older than 45 years with M1 disease (Fig. 3B) showed a similar pattern as when looking at patients older than 35 years, and older than 55 years (Figs. 3A and 3C, respectively).
FIG. 3.
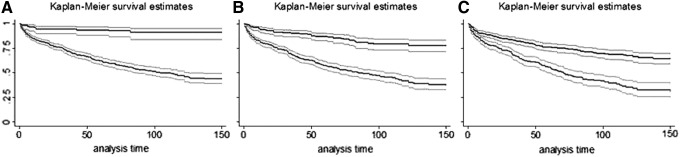
Kaplan-Meier plots comparing thyroid cancer-specific mortality in patients with metastatic disease. Patients are stratified at various age cutoffs (A, 35; B, 45, and C, 55 years). Top set of lines represent young patients (A: under age 35, B: under age 45, C: under age 55). Bottom set of lines represent the older patients (A: >35, B: >45, C: >55). 95% confidence intervals are denoted by the shaded regions. Vertical axis is proportion of survivors. Analysis time is in months.
Discussion
The findings in our univariate analysis show patient demographics and staging consistent with previously reported figures. Patients with DTC are predominately female, white, and the majority are diagnosed with early-stage disease.
Institutional case series and other small cohort studies may offer more detailed clinical information, but cannot always be generalized on the national scale. The SEER dataset is a United States population-based cancer registry that is supported by the National Cancer Institute and Centers for Disease Control and Prevention. SEER is comprised of data across multiple geographic regions, and contains demographic, staging, and treatment information.
This analysis relies upon the accuracy of data coded within SEER. In addition to weaknesses related to data accuracy, specific clinical details that would be available in smaller databases are commonly unavailable in population datasets. For example, the timing between diagnosis and treatment, patient comorbidities, and RAI dosage are not captured in SEER. Additionally, there was no reliable information regarding the extent of surgery. By using RAI as a covariate, and not accounting for surgical variables, the assumption is that RAI was not used on patients treated unless they underwent total or subtotal thyroidectomy. This cannot be determined for certain, and should be understood when examining this study. These and other unmeasured variables are potential confounders that can have unknown effects.
Patients with DTC can have long-term survival with indolent disease, making cancer recurrence an interesting outcome in this population. Data regarding recurrence are not captured in SEER, so DSS was chosen as the outcome. Similar to all other variables in the database, DSS is subject to the same considerations with regard to data accuracy and completeness. Importantly, the designation of disease-specific death is susceptible to overestimation bias, particularly for diseases such as DTC. Despite these limitations, we feel that disease-specific mortality is a clinically meaningful outcome for this analysis.
Although there are several inherent weaknesses with national retrospective databases, their strengths are derived from their population data gathered across varied regions and hospital settings. SEER provides large patient numbers that are vital in multivariate analyses capable of controlling for demographic and clinical variables that confound the results in smaller studies. The study period spans several decades and the workup and management of regional and distant metastasis undoubtedly changed over this time. Recognizing these secular trends as potential confounders, the decade of diagnosis was incorporated into the multivariate model to account for practice patterns over time.
The strongest predictors of DSS in all patients with DTC were age and metastatic disease. Poor survival in patients with DTC that present with metastatic disease has been demonstrated previously (17), and is not disputed. Advanced age and stage have also been associated with an increased risk of thyroid cancer-related mortality (3,11). The initial age division for our multivariate analysis was chosen according to the current AJCC staging protocol (45 years). The HR of 19.2 for age older than 45 supports the belief that patients above the age of 45 have a worse prognosis than their younger counterparts.
Based on our analysis, it is evident that higher age considerably increases the likelihood of thyroid cancer-specific mortality. However, age as a covariate does not display a major change in predicting survival at 35, 45, 55, or any other age cutoff (Fig. 1). While age is an important factor to assess an individual patient's prognosis, this suggests that there is no age cutoff that stratifies patients into unique risk categories.
Other recent studies have indicated that there may not be a sudden increase in mortality risk at one age point as many staging systems imply, but that the relationship between increasing age and risk might be more complex (18,19). Oyer et al. (11) suggested that survival disadvantages for DTC patients may come into effect as early as age 35, supporting the findings of Tran Cao et al. (15) that younger patients may be under-staged in the current AJCC protocol. The data proposed herein represent an important expansion on this prior work because it contains almost twice as many patients as each of these two studies, controls for RAI treatment, and uses DSS.
A recent study demonstrated that men are more likely to present with more advanced disease, but this study did not identify sex as an independent prognostic factor for DSS (20). Other work by Jonklaas et al. (16) provided an in-depth analysis of the impact of age and sex on survival in papillary thyroid cancer and noted that sex was a significant factor in younger patients (younger than 55 years). After controlling for multiple confounders not accounted for in these prior studies, our analysis showed that female sex was associated with a statistically significant decrease in cancer-specific mortality.
Advanced T stage and nodal involvement were negative prognosticators, but their hazard ratios are about 3 to 6 times smaller than those of age and metastatic disease (Table 3). In the past, there has been debate about whether N1 disease increases the mortality risk. Several previous studies demonstrated that nodal disease does not negatively affect patient prognosis (21,22). In fact, Cady et al. (23) described a paradoxical positive protective effect of positive lymph node status in an early study. Our analysis supports more recent findings that lymph node disease is associated with worse survival (24).
The average age of patients diagnosed with DTC in our analysis was 45.6 years and nearly 25% of all patients in our study were between 40 and 50 years old at the time of diagnosis. Hence, a substantial number of patients were within a few years of the 45-year-old cutoff, which determined their allocation to either of the two different AJCC staging categories.
Even if age alone does not stratify patients into significantly different risk-groups, the argument could be made that perhaps mortality in patients under 45 is less influenced by advanced disease (i.e., N1, T3+, and M1). Previous groups have concluded that many DTC staging algorithms with specific age cutoffs may not adequately account for mortality risks in younger patients with advanced disease (15,25). In order to evaluate youth's potential protective effect, we compared the factors most predictive of mortality in patients below and above various age points (Fig. 2). T3 or M1 disease increases the risk of cancer-specific mortality in patients younger than 30 to a greater degree than in patients over 30. This is seen by the first trend line that slopes downward from left to right in Figure 2A and 2C. This downsloping trend line is seen for all age-pairings with T3 disease as a covariate (with T2 disease as the reference). Similarly, Figure 2C shows that having M1 disease increases the relative likelihood of cancer-specific mortality more in younger patients (even those younger than 30 and 35 years old) than it does in the older patient subgroups.
Younger patients tended to have higher HRs for T, N, and M factors (Fig. 2), but the overall mortality was also lower in these younger subgroups. While some retrospective studies show better 3- and 10-year survival rates for patients younger than 45, compared to older ones (26,27), others indicate that there is no statistical difference in survival between younger and older patients with metastatic disease (28), except in patients over 70 (29). Even though the HR for M1 disease may be higher in the under-35 subgroup than in the over-35 subgroup, the Kaplan-Meier plot in Figure 3A clearly shows that younger patients with metastatic disease have better survival than older patients. This trend holds, regardless of the age used to stratify patients with M1 disease (35, 45, or 55 years, Fig. 3).
It should be noted that of 44,419 patients under age 45 in our analysis, only 0.7% had metastatic disease. Therefore, although this factor had a strong negative correlation with DSS, it only helps explain mortality in a small percentage of patients with DTC. The statistically significant findings in this analysis emphasize the value of large outcomes datasets in evaluating low-frequency events with numerous potential confounders.
To date, this study of 85,740 patients is the largest of its kind addressing DSS in DTC. To summarize our findings, age and advanced disease were the strongest predictors of DSS. Based on our analyses, we conclude that patients under 45 with advanced disease may be understaged by current AJCC guidelines. The contribution of age at diagnosis to a patient's DSS is considerable, but there is no age cutoff that affords any unique risk-stratification in patients with DTC. A cumulative staging score may be able to account for negative effect of age more accurately than a specific cutoff. Revisiting DTC staging criteria may provide an improved risk-stratification schema. Better prognostication would improve patient counseling and guide therapeutic considerations more realistically.
Acknowledgments
This research was supported by a National Institutes of Health Ruth Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant (T32 DC000128) to RKO.
This work was accepted as a poster presentation at the American Thyroid Association (ATA), 84th Annual Meeting, Coronado, California, October 29 to November 2, 2014.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A.2011Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 61:212–236 [DOI] [PubMed] [Google Scholar]
- 2.Davies L, Welch HG.2006Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167 [DOI] [PubMed] [Google Scholar]
- 3.Eustatia-Rutten CF, Corssmit EP, Biermasz NR, Pereira AM, Romijn JA, Smit JW.2006Survival and death causes in differentiated thyroid carcinoma. J Clin Endocrinol Metab 91:313–319 [DOI] [PubMed] [Google Scholar]
- 4.Wong RM, Bresee C, Braunstein GD.2013Comparison with published systems of a new staging system for papillary and follicular thyroid carcinoma. Thyroid 23:566–574 [DOI] [PubMed] [Google Scholar]
- 5.Byar DP, Green SB, Dor P, Williams ED, Colon J, van Gilse HA, Mayer M, Sylvester RJ, van Glabbeke M.1979. A prognostic index for thyroid carcinoma. A study of the E.O.R.T.C. Thyroid Cancer Cooperative Group. Eur J Cancer 15:1033–1041 [DOI] [PubMed] [Google Scholar]
- 6.Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS.1993Predicting outcome in papillary thyroid carcinoma: Development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 114:1050–1057; discussion 1057–1058. [PubMed] [Google Scholar]
- 7.Cady B, Rossi R.1988An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery 104:947–953 [PubMed] [Google Scholar]
- 8.Shaha AR, Loree TR, Shah JP.1994Intermediate-risk group for differentiated carcinoma of thyroid. Surgery 116:1036–1040; discussion 1040–1031. [PubMed] [Google Scholar]
- 9.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. (eds) 2010AJCC Cancer Staging Manual. Seventh edition. Springer, New York [Google Scholar]
- 10.Beahrs O, Myers M.1983American Joint Committee on Cancer Manual for Staging of Cancer. Second edition. Lippincott, Philadelphia, PA [Google Scholar]
- 11.Oyer SL, Smith VA, Lentsch EJ.2012Reevaluating the prognostic significance of age in differentiated thyroid cancer. Otolaryngol Head Neck Surg 147:221–226 [DOI] [PubMed] [Google Scholar]
- 12.Halan K.1966Influence of age and sex on incidence and prognosis of thyroid cancer: Three hundred forty-four cases followed for ten years. Cancer 19:1534–1536 [DOI] [PubMed] [Google Scholar]
- 13.Cady B, Sedgwick C, Meissner W, Bookwalter J, Romagosa V, Werber J.1976Changing clinical, pathologic, therapeutic, and survival patterns in differentiated thyroid carcinoma. Ann Surg 184:541–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang BH, Lo CY, Chan WF, Lam KY, Wan KY.2007Staging systems for papillary thyroid carcinoma: A review and comparison. Ann Surg 245:366–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran Cao HS, Johnston LE, Chang DC, Bouvet M.2012. A critical analysis of the American Joint Committee on Cancer (AJCC) staging system for differentiated thyroid carcinoma in young patients on the basis of the Surveillance, Epidemiology, and End Results (SEER) registry. Surgery 152:145–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonklaas J, Nogueras-Gonzalez G, Munsell M, Litofsky D, Ain KB, Bigos ST, Brierley JD, Cooper DS, Haugen BR, Ladenson PW, Magner J, Robbins J, Ross DS, Skarulis MC, Steward DL, Maxon HR, Sherman SI.2012The impact of age and gender on papillary thyroid cancer survival. J Clin Endocrinol Metab 97:E878–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nixon IJ, Whitcher M, Palmer FL, Shaha ARM, Shah JP, Patel S, Ganly I.2012The impact of distant metastases at presentation on prognosis in patients differentiated carcinoma of the thyroid gland. Thyroid 22:884–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston LE, Tran Cao HS, Chang DC, Bouvet M.2012Sociodemographic Predictors of survival in differentiated thyroid cancer: Results from the SEER database. ISRN Endocrinol 2012:384707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilliland FD, Hunt WC, Morris DM, Key CR.1997Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–1991. Cancer 79:564–573 [DOI] [PubMed] [Google Scholar]
- 20.Nilubol N, Zhang L, Kebebew E.2013Multivariate analysis of the relationship between male sex, disease-specific survival, and features of tumor aggressiveness in thyroid cancer of follicular cell origin. Thyroid 23:695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes CJ, Shaha AR, Shah JP, Loree TR.1996Impact of lymph node metastasis in differentiated carcinoma of the thyroid: A matched-pair analysis. Head Neck 18:127–132 [DOI] [PubMed] [Google Scholar]
- 22.Cunningham MP, Duda RB, Recant W, Chmiel JS, Sylvester JA, Fremgen A.1990Survival discriminants for differentiated thyroid cancer. Am J Surg 160:344–347 [DOI] [PubMed] [Google Scholar]
- 23.Cady B, Sedgwick CE, Meissner WA, Bookwalter JR, Romagosa V, Werber J.1976Changing clinical, pathologic, therapeutic, and survival patterns in differentiated thyroid carcinoma. Ann Surg 184:541–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Podnos YD, Smith D, Wagman LD, Ellenhorn JD.2005The implication of lymph node metastasis on survival in patients with well-differentiated thyroid cancer. Am Surg 71:731–734 [DOI] [PubMed] [Google Scholar]
- 25.Lang BH, Lo CY, Chan WF, Lam KY, Wan KY.2007Staging systems for follicular thyroid carcinoma: application to 171 consecutive patients treated in a tertiary referral centre. Endocr Relat Cancer 14:29–42 [DOI] [PubMed] [Google Scholar]
- 26.Showalter TN, Siegel BA, Moley JF, Baranski TJ, Grigsby PW.2008Prognostic factors in patients with well-differentiated thyroid cancer presenting with pulmonary metastasis. Cancer Biother Radiopharm 23:655–659 [DOI] [PubMed] [Google Scholar]
- 27.Sampson E, Brierley JD, Le LW, Rotstein L, Tsang RW.2007Clinical management and outcome of papillary and follicular (differentiated) thyroid cancer presenting with distant metastasis at diagnosis. Cancer 110:1451–1456 [DOI] [PubMed] [Google Scholar]
- 28.Shaha AR, Shah JP, Loree TR.1997Differentiated thyroid cancer presenting initially with distant metastasis. Am J Surg 174:474–476 [DOI] [PubMed] [Google Scholar]
- 29.Haq M, Harmer C.2005Differentiated thyroid carcinoma with distant metastases at presentation: Prognostic factors and outcome. Clin Endocrinol (Oxf) 63:87–93 [DOI] [PubMed] [Google Scholar]