Abstract
Macrophage subtypes are characterized as proinflammatory (M1) or immunomodulatory and tissue remodeling (M2). Since macrophages play a pivotal role in controlling Herpes simplex virus type-1 (HSV-1) replication, effects of HSV-1 by 24 h of infection were determined in murine J774A.1 macrophages unpolarized (M0) or polarized to either an M1 or M2 phenotype. Morphology, cell viability, and expression of CD14 (co-receptor for lipopolysaccharide), CD86 (B7.2-immune co-stimulatory molecule), and suppressors of cytokine signaling (SOCS1 and SOCS3) were determined. M1 macrophages were flattened and vacuolated, while M2 cells appeared elongated with a few vacuoles. Compared with unpolarized M0 cells, M1 cells showed a 31% decrease in viability, a 2-fold increase in the number of CD14+-CD86+ cells, no change in SOCS1 expression, and an 11-fold decrease in SOCS3 expression. M2 cells exhibited a 9% decrease in viability, a 26.0% decrease in the number of CD14+-CD86+ cells, and no change in SOCS1/SOCS3 expression levels compared with M0 cells. After HSV-1 infection, all phenotypes appeared rounded, cell viabilities decreased as did numbers of M1 cells expressing CD14 and CD86. At 24 h after infection, M0 control and M2 cells showed greater virus yield than did the M1 cells, presumably reflecting the loss of viable M1 cells. SOCS1 expression was predominant in uninfected M1-polarized cells and in virus-infected control (M0) cells. SOCS1/SOCS3 expression ratio was 7:1 in uninfected M1 macrophages and approached 1:1 in M1 cells at 24 h after infection with HSV-1. In contrast, little differences were seen in SOCS1/SOCS3 expression ratios in uninfected M2-polarized cells or virus-infected M2 cells. These observations suggest that SOCS1/SOCS3 expression ratios can be used to characterize HSV-1-infected and uninfected macrophages.
Introduction
Herpes simplex virus type-1 (HSV-1) is a double-stranded DNA virus that affects ∼70%–80% of adults within the United States (Dakvist and others 1995; Miller and others 1998; Stock and others 2001; Roizman and others 2007). Under normal conditions, a latent infection is established and maintained within the host. If the host immune system is compromised, the virus can be reactivated, resulting in a lytic infection (Cunningham and others 2006; Roizman and others 2007; Diefenbach and others 2008; Koelle and Corey 2008). Lytic infections clinically manifest as mild cutaneous disease. Less frequently, HSV-1 reactivation results in infection of the corneal epithelium, which can lead to blindness (Jones C. 2003). The host immune response to HSV-1 infection involves cells of both the innate and adaptive immune system. The innate immune response to HSV-1 infection comprises natural killer cells, macrophages, and γ/δ T cells. These cells are recruited to the site of infection and activated when infected keratinocytes release high levels of cytokines. This release of cytokines activates innate immune cells that attempt to control the infection by killing infected cells and inhibiting virus replication (Mikloska and others 1998; Cunningham and others 2006). Macrophages play a pivotal role in controlling HSV-1 replication. Macrophages are capable of inhibiting virus replication and possess the ability to target and destroy virus-infected cells, slowing virus replication in infected neighboring cells (Wu and Morahan 1992; Mosser and Edwards 2008).
Macrophages are considered “professional” phagocytic cells and express a wide variety of cell surface receptors, enabling them to recognize signals not usually found within the host. Signals present within the microenvironment can alter macrophage function and lead to multiple effector subpopulations (Martinez and others 2008; Murray and Wynn 2011). This ability to alter function is known as macrophage “polarization.” The 2 polarized macrophage subpopulations we examined in this study of HSV-1 infection of the murine J774A.1 macrophages are known as M1 and M2 macrophages. Depending on the environmental stimuli, one M1 phenotype or several M2 macrophage phenotypes can form. M1 macrophages are a proinflammatory, “classically” activated, population that secrete high amounts of proinflammatory cytokines, such as inducible nitric oxide synthases (iNOS) and tumor necrosis factor-α (TNF-α), after activation by interferon-gamma (IFN-γ) and lipopolysaccharide (LPS). Depending on the activation signal, there are multiple M2-like subtypes. M2 macrophages are activated by interleukin-4 (IL-4) or interleukin-13 (IL-13), and they are considered anti-inflammatory due to the molecules they release, such as interleukin-10 (IL-10), that lead to tissue remodeling and angiogenesis (Kigerl and others 2009; Ma and others 2010; Wang and others 2010).
Suppressor of cytokine signaling (SOCS) proteins are frequently manipulated by viruses to maintain an infection within the host (Akhtar and Benveniste 2011). The different SOCS proteins inhibit the cytokine-signaling pathway, thereby influencing the inflammatory response (Akhtar and Benveniste 2011). SOCS proteins can be quickly upregulated in macrophages (Whyte and others 2011), making SOCS protein expression levels a target of observation in our study. SOCS1 is critical in the signaling pathway programs involved in M1 and M2 polarization of mouse peritoneal macrophages and rat bone marrow-derived macrophages (Whyte and others 2011). SOCS3 represses the M1 proinflammatory murine macrophage phenotype, dampening macrophage inflammatory responses (Qin and others 2012a, 2012b). We previously noted that murine fibroblast and keratinocyte cell lines respond differentially to IFN-γ induction of an antiviral state against HSV-1 (Frey and others 2009). Hyperinduction of SOCS1 in keratinocytes led to their resistance to IFN-γ induction of an anti-viral state.
The goal of this study was to determine the effects of HSV-1 challenge on morphology, CD14-CD86 expression, cell viability, and SOCS protein levels in J774A.1 murine macrophages unpolarized and polarized to M1 and M2 phenotypes.
Materials and Methods
Cells and virus
The J774A.1 murine macrophage cell line (ATCC, Manassas, VA) is a reticulum cell sarcoma, adherent macrophage cell line derived from an adult female BALB/cN mouse. J774A.1 cells were cultured in a Dulbecco's modified Eagle's medium (MEM) containing 10% heat-inactivated fetal bovine calf serum, propagated in 25 cm2 vented cap cell culture flasks, and incubated at 37°C in 95% air/5% CO2 in a humidified incubator. J774A.1 cells were subcultured at a dilution of 1:6, twice to thrice weekly. In 1 experiment, RAW264.7 (TIB-71; ATCC) macrophages were used. Vero cells (CCL-81; ATCC) were plated into 75 cm2 tissue culture flasks and incubated at 37°C, 95% air/5% CO2 in a humidified incubator. HSV-1 (syn 17+) (provided initially by Dr. Nancy Sawtell, Children's Hospital Medical Center, Cincinnati, OH) was routinely passaged and titrated in Vero cells.
HSV-1 titers in macrophage subsets
At 24 h after infection, supernatant fluids from the HSV-1-infect macrophage cultures were collected, and various dilutions of each macrophage subset were added to confluent monolayers of Vero cells. After 2 h, the culture supernatant fluids were aspirated and the cultures were overlaid with 1% methylcellulose in MEM. Triplicates of each sample were examined microscopically for plaque-forming units after 48–72 h of incubation. When plaques were visible, the culture plates were stained with 0.5% crystal violet, air dried, and plaques were counted. The results were reported as plaque-forming units (pfus)/mL of sample.
Polarization treatments
J774A.1 macrophages were grown to ∼50% confluency, at which time the polarization treatment was administered. To induce the M1 phenotype, J774A.1 macrophages were treated with 20 ng/mL IFN-γ (Peprotech, Inc., Rocky Hill, NJ) and 100 ng/mL LPS (Chondrex, Inc., Redmond, WA) for 24 h; 20 ng/mL IL-4 (Peprotech, Inc.) was used to induce the M2 phenotype. After 24 h, cells were removed from the cell culture flasks using a cell scraper and analyzed.
Cell viability
J774A.1 macrophages were grown to ∼50% confluency, at which time either IFN-γ/LPS (M1) or IL-4 (M2) was added with or without virus. Untreated cells were used as a control. After 24 h, cells were removed from the cell culture flasks using a cell scraper. Cells were pelleted by centrifugation at 4°C for 5 min. The supernatant fluid was aspirated after centrifugation, and the cell pellet was resuspended in 1 mL of complete growth medium. Trypan blue (Fisher Scientific, Hampton, NH) was used to determine viability, and a hemocytometer was used to obtain cell counts.
Immunofluorescent staining
Cells were grown in slide chambers (Fisher Scientific) to ∼50% confluency, at which time either IFN-γ and LPS (M1) or IL-4 (M2) were added for 24 h with and without virus. Untreated cells were used as an experimental control. After polarization treatment, cells were rinsed with 1% bovine serum albumin (BSA) and suspended in phosphate-buffered saline (PBS). Cells were then fixed using 4% formaldehyde (15 min at room temperature) and permeabilized using ice-cold acetone (10 min at −20°C). Blocking buffer (5% normal serum from the same species as the primary or secondary antibody, 3% BSA, suspended in PBS) was added for 1 h at room temperature. After blocking, antibodies diluted in blocking buffer were added per recommended concentration from the distributor. Slide chambers were then incubated overnight at 4°C. Cells stained with fluorochrome-conjugated primary antibodies (anti-CD14 and anti-CD86; BioLegend, San Diego, CA) and Texas Red-Phalloidin X (Life Technologies Corp, Carlsbad, CA) were rinsed after incubation, and a cover slip was added using a hard set mounting medium (VectaShield from Vector Laboratories, Burlingame, CA). Cells incubated with rabbit polyclonal anti-SOCS1 and anti-SOCS3 (provided by H.M. Johnson, University of Florida at Gainesville) were rinsed thrice for 3 min using 1% BSA/PBS after incubation, at which time a fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit secondary antibody (Invitrogen, Life Technologies, Carlsbad, CA) was added for 1 h (1:50 dilution) at room temperature, rinsed thrice for 3 min using 1% BSA/PBS. A coverslip was then added using a hard set mounting medium. The slides were analyzed in the Wright State University Microscopy Core Facility using an Olympus fluorescence microscope, Olympus (USA) BX51 Epi Fluorescence Spot Scope with SPOT RT (RT230) color camera (Sterling Heights, MI).
Flow cytometry
Cells were grown to ∼50% confluency in cell culture flasks, at which point M1 or M2 treatment was administered with or without virus. After 24 h of treatment, cells were removed using a cell scraper and centrifuged at 1,500 rpm (4°C) for 5 min. After centrifugation, the supernatant was aspirated and the cell pellet was resuspended using 1 mL of complete growth medium. A hemocytometer was used to obtain cell counts; 1 million viable cells were used for each sample.
Cell surface staining (CD14 and CD86)
Cells were rinsed thrice with 1% BSA/PBS. For cell surface markers, no permeabilization step was required. Blocking buffer (5% mouse serum/3% BSA/PBS) was added to cells for 45 min at room temperature. After blocking, antibodies were diluted in blocking buffer and added to cells using concentrations suggested by the distributor for 45 min at 4°C. After antibody incubation, cells were fixed using 4% paraformaldehyde for 15 min at room temperature. Cells were then rinsed thrice and resuspended in 250 μL of ice cold PBS with 0.5% sodium azide. Samples were then analyzed using a BD Accuri C6 flow cytometer (BD Bioscience, San Jose, CA).
Intracellular staining (SOCS1 and SOCS3)
Cells were rinsed thrice with 1% BSA/PBS. After rinsing, cells were fixed with 4% formaldehyde for 15 min at room temperature. Cells were then rinsed thrice. They were permeabilized using 0.2% Triton-X/PBS for 15 min at room temperature and then rinsed thrice with 1% BSA/PBS. Blocking buffer (5% goat serum/3% BSA/PBS) was added to cells for 1 h at room temperature. After blocking, SOCS1 and SOCS3 antibodies were suspended in blocking buffer at a concentration of 10 μg/million cells and added to cells for 45 min at 4°C. Cells were then rinsed thrice. After rinsing, FITC-conjugated secondary antibody was diluted in blocking buffer (1:50 dilution) and added to cells for 45 min at 4°C. Cells were then rinsed thrice and suspended in ice cold PBS with 0.5% sodium azide. Samples were analyzed using an Accuri C6 flow cytometer.
Virus challenge
Cells were grown to ∼50% confluency in cell culture flasks. Cells were then removed using a cell scraper and pelleted by centrifugation for 5 min at 4°C. After centrifugation, the supernatant fluid was aspirated and the cell pellet was resuspended using 1 mL of complete growth medium. Packed cell volume cell counting tubes (MidSci, St. Louis, MO) were used to obtain cells counts so that multiplicity of infection (MOI) could be calculated. Cells were added to cell culture flasks; M1 and M2 treatment was then administered simultaneously with 0.1 MOI of HSV-1. Twenty-four hours later, cells were collected and analyzed.
Statistical significance
Statistical significance was calculated using a paired t-test and or ANOVA (SigmaPlot 12.0); all experiments were completed 2–3 times.
Results
Polarized macrophages show distinct morphological changes, while virus challenge induces the same morphology in each of the macrophage subpopulations
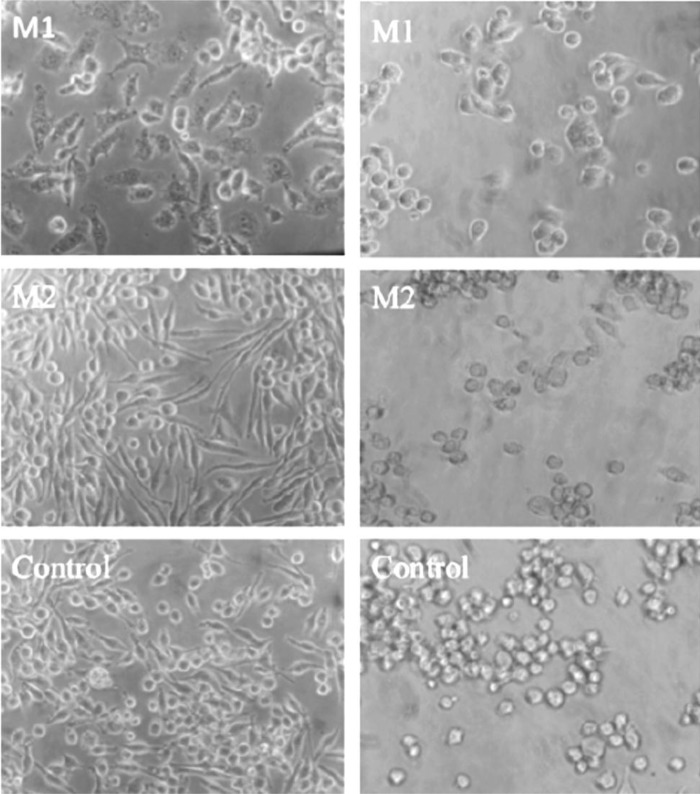
We noted that polarization treatments of murine J774A.1 macrophages caused morphological changes in the cells. After IFN-γ and LPS treatment for 24 h, M1 macrophages appeared flattened, were irregularly shaped, and contained many visible intracellular vacuoles (Fig. 1, left panel). M2 treatment induced cells with an elongated morphology, similar to that seen in control macrophages cultures. Untreated control macrophages appeared as round or elongated in culture (Fig. 1). The morphological changes exhibited by M1 and M2 macrophages made it possible to differentiate between the 2 phenotypes based on morphology alone. After challenge with 0.1 MOI of HSV-1, M1, M2, and M0 control macrophages appeared rounded (Fig. 1, right panel). Consequently, virus-induced morphological changes made it difficult to differentiate between phenotypes based on morphology alone.
FIG. 1.
Expression of CD14 and CD86 by J774A.1 macrophages treated with interferon-gamma (IFN-γ) and lipopolysaccharide (LPS) (top panel) or interleukin-4 (IL-4) for 24 h (middle panel). Macrophages exposed to culture medium alone for 24 h (bottom panel). (Images captured at 400× magnification, scale bar=50 μm.)
Virus-infected macrophages experience a significant decrease in cell viability
M1 induction also led to a marked decrease in cell viability, possibly due to IFN-γ/TNF-α toxicity (Kyoungho and others 2001). After IFN-γ and LPS treatment, virus-infected M1 macrophages exhibited a 31% decrease in the number of viable cells when compared with virus-infected control cells (Fig. 2). M2 polarized cells did not show a decrease in cell viability compared with the control (M0) population.
FIG. 2.
After polarization with LPS and IFN-γ or IL-4, percentage of viable cells at 24 h after infection with 0.1 multiplicity of infection (MOI) Herpes simplex virus type-1 (HSV-1). Virus-treated M1 macrophages experienced a decrease (31.0%, P-value=0.003) in cell viability after IFN-γ/LPS treatment. M2 macrophages had cell viability values that were not statistically significant from virus-treated control (M0) cells (*P-value<0.05, **P-value<0.001).
Virus-infected macrophages show differences in capacity to replicate HSV-1
In comparison with unpolarized J774A.1 macrophages, a 4-fold reduction in virus pfu in M1-polarized macrophages and a 1.4-fold reduction in M2 cells occurred by 24 h after infection with 0.1 MOI of HSV-1. It should be noted that a 2.5-fold decrease in pfu was seen in infected M1-polarized cells compared with infected M2 macrophages (Table 1). To confirm this observation, similar experiments were carried out using polarized and unpolarized RAW264.7 murine macrophages that showed comparable differences in cell viabilities at 24 h after infection with 0.1 MOI of HSV-1 (data not shown). A similar trend in capacity to replicate HSV-1 was seen. A 3-fold reduction in virus pfu was seen in the infected M1 cells in comparison with the M2-polarized or -unpolarized cells. In both types of M1 macrophages, the viability dropped by 30% in J774A.1 (Fig. 2) and 45% RAW264.7 (data not shown) cells, after infection, which presumably led to a concomitant decrease in the virus pfu observed.
Table 1.
HSV-1 Titers in HSV-Infected Unpolarized and Polarized Macrophage Cell Lines
| Cell line/treatment | pfu/mL | Fold decrease from M0 |
|---|---|---|
| J774A.1 | ||
| M0 | 10.2×102 | — |
| M1 | 2.5×102 | 4 |
| M2 | 6.3×102 | 1.4a |
| RAW264.7 | ||
| M0 | 30×102 | — |
| M1 | 10×102 | 3 |
| M2 | 35×102 | — |
M1 cells demonstrated a 2.5-fold reduction in virus pfu by comparison with infected M2 cells.
HSV, Herpes simplex virus; pfu, plaque forming unit.
CD86 is upregulated in M1 macrophages and downregulated in M2 macrophages
To identify the M1 and M2 phenotype after treatment, expression of cell surface markers that discriminated between the 2 phenotypes was characterized by flow cytometry. CD86 was upregulated in macrophages treated with IFN-γ and LPS, making it an accurate identifier of the M1 phenotype (Fig. 3A) when used in conjunction with CD14 expression. In addition, CD14-CD86 expression was downregulated after IL-4 treatment, enabling identification of the M2 phenotype (Fig. 3A). In comparisons with unpolarized cells, the M1 phenotype expressed an increase in the number of CD14+-CD86+ cells (41%), while the M2 phenotype expressed a decrease in the number of CD14+-CD86+ cells (27%). Unpolarized (M0) cells were used as an experimental control for CD14+-CD86+ expression (Fig. 3A).
FIG. 3.
Flow cytometry analysis of CD14-CD86 co-expression levels in M1, M2, and unpolarized M0 control macrophages. (A) Left, uninfected cells; right, cells infected with 01 MOI HSV-1 for 24 h. (B) M1 macrophages showed a significant increase (41.2%) in the number of CD14+/CD86+ cells when compared with control (M0) cells, while M2 macrophages had an insignificant decrease (26.0%) in the number of CD14+/CD86+ cells. In HSV-1-infected cultures, a 29.6% increase in virus-treated M1 macrophages was observed in comparison with virus-infected control (M0) cells, and an 11.4% decrease was observed in virus-infected M2 macrophages; these differences were not significant. In comparisons with uninfected control cultures, uninfected M1 cultures exhibited a significant increase (41.2%) in the percentage of CD14+-CD86+ cells, while percentages of M2-polarized cells were decreased (26.0%). *P<0.05.
Immunofluorescent images of virus-infected M1 macrophages stained with anti-CD86 antibodies showed an increase in CD86 expression in comparison to virus-infected M0 control cells (not shown). Immunostaining of M2 macrophages with anti-CD86 antibodies did not show any observable differences when compared with virus-infected M0 control cells, but differences were detectable by flow cytometry (Fig. 3). When compared with their uninfected counterparts, CD14-CD86 expression in virus-infected M1, M2, and M0 control cells was decreased (Fig. 3B); HSV-1-infected M1 macrophages showed a 40% decrease (P<0.001), and virus-infected M2 macrophages showed a 13% decrease (P<0.05). Virus-infected control (M0) macrophages also showed a significant decrease of 28% (P<0.05) in the expression of CD14+-CD86 cells.
Virus challenge upregulates SOCS3 expression in M1 macrophages, and SOCS1 expression in unpolarized (M0) control cells
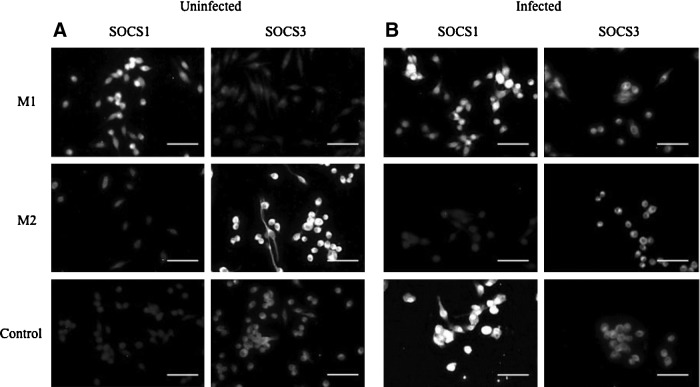
Immunofluorescent images showed that virus-infected M1 macrophages expressed higher levels of SOCS1 compared with SOCS3, while virus-infected M2 macrophages expressed higher levels of SOCS3 compared with SOCS1 (Fig. 4A). Virus-infected control cells showed an increase in SOCS1 expression compared with uninfected control cells, while SOCS3 was decreased in control cells after virus infection (Fig. 4B).
FIG. 4.
Suppressor of cytokine signaling (SOCS)1 and SOCS3 expression levels in polarized and unpolarized macrophages. (A) SOCS1 and SOC3 expression in uninfected M1, M2, and control (M0) macrophages. (B) SOCS1 and SOC3 expression in HSV-1-infected M1, M2, and control (M0) macrophages. (Images captured at 400× magnification, scale bar=50 μm.)
Flow cytometric analysis of virus-infected M1 macrophages revealed that virus challenge led to upregulation of SOCS3 expression (Fig. 5). After virus challenge, M1 macrophages exhibited a SOCS1/SOCS3 ratio of 1:1 (Fig. 5, bottom left panel), compared with a SOCS1/SOCS3 ratio of ∼7:1 in uninfected polarized M1 macrophages (Fig. 5, bottom panel). SOCS3 expression levels appeared relatively unchanged in virus-infected M2 macrophages (85%) when compared with uninfected M2 macrophages (83%). Both samples expressed markedly higher levels of SOCS3 compared with SOCS1. Both uninfected and virus-infected M2 macrophages exhibited SOCS1:SOCS3 ratios of ∼1:2. Virus-infected control cells (M0) showed higher expression levels of SOCS1 (Fig. 5B) when compared with SOCS3; the SOCS1:SOCS3 ratio in virus-infected control cells was 4:1 (Fig. 5, bottom right panel), while uninfected control cells exhibited a ratio of 1:2 (Fig. 5, bottom left panel).
FIG. 5.
Flow cytometry analysis of SOCS1 and SOCS3 expression by uninfected and infected macrophage subpopulations. Top Panel (A) Uninfected cells at 24 h after polarization. M1 cells expressed higher levels of SOCS1 than SOCS2 with a SOC/SOCS3 ratio of 6.1:1. M2-polarized cells and -unpolarized cells expressed higher levels of SOCS3 with SOCS/SOCS3 ratios of 1:2.2 and 1:1.9, respectively. (B) Polarized cells at 24 h after infection after polarization and concomitant infection with 0.1 MOI of HSV-1. Virus-infected M1 cells expressed a SOCS1/SOCS3 ratio of 1:1.1, while M2-infected cells exhibited a SOCS1/SOCS3 ratio of 1:1.8. Interestingly, infected unpolarized controls expressed a 3.8:1 SOCS1/SOCS3 ratio. These differences are summarized in the bar graphs shown in the bottom panel.
Discussion
In this study, we found that polarization treatment led to distinct morphological changes by 24 h in cell culture (Fig. 1). The M1 macrophages appeared flattened, irregularly shaped, and contained many visible intracellular vacuoles. The majority of IL-4-treated M2 cells exhibited an elongated morphology, similar to that seen in M0 control cultures. Virus infection induced all of these populations of macrophages to appear rounded and aggregated in culture and led to decreases in cell viability. At the 24 h observation time, the M1 phenotype showed the greatest decrease in viability, whether uninfected or infected with HSV-1 (Fig. 2).
On the assumption that the major factors contributing to the decrease in viability of the M1 cell populations were due to IFN-γ/TNF-α toxicity, we attempted to neutralize the effect of TNF-α by adding anti-TNF-α neutralizing antibodies to culture at the same time point as M1 polarization treatment and virus infection. Only a slight, statistically insignificant, increase in cell viability of the M1 cells resulted (data not shown). M1 polarization can occur within 12 h, leading to TNF-α production (Kyoungho and others 2001). The continuous production of TNF-α by M1 macrophages may not allow sufficient time for the neutralizing antibodies to bind and inhibit the function of newly synthesized TNF-α in culture within the 24-h observation period used in this study. Other cytotoxic factors produced by M1 macrophages may contribute to this cytotoxicity, for example, iNOS and reactive oxygen species (ROS). Microglial cells of the nervous system are macrophages or macrophage-like in behavior and function. Using the same HSV-1 strain 17 syn+ strain that we used in the present study, Schachtele and others (2010) observed that HSV-1 infection induced oxidative damage in co-cultures of purified murine microglial cells and neuronal cells; microglial cell Toll-like receptor-2-induced production of ROS. Intranasal infection of mice with HSV-1 strain 17 syn+ resulted in early macrophage and neutrophil infiltration into the brain, leading to prolonged activation of microglial cell-driven proinflammatory chemokine production and T-lymphocyte retention (Marques and others 2008). The greater decrease in cell viability of the M1 polarized cells may also have contributed to a decrease in the production of HSV-1.
Expression of the LPS co-receptor CD14 (Dobrovolskaia and Vogel 2002; Kitchens RL 2000) and the B7.2 co-stimulatory molecule CD86 differed between the M1 and M2 subpopulations (Fig. 3) with M1 macrophages showing a significantly higher number of CD14+-CD86+ cells compared with M0 control cells. In contrast, the M2-polarized macrophages showed a significant decrease in the number of CD14+-CD86+ cells. In the M1 polarization process, the LPS inducer interacts with CD14 and TLR 4 on the macrophage surface and the IFN-γ component of the inducer cocktail upregulates expression of CD86 for the antigen-presenting function of the macrophage.
These results suggest that HSV-1 infection decreases the ability of unpolarized and polarized macrophages to express both CD14 and CD86. While CD14 may play a role in the innate response to HSV-1 and CD86, a direct role in the adaptive response to HSV-1 infection, CD14-CD86 co-expression is an indicator of a macrophage's ability to mount an effective proinflammatory immune response. CD86 stimulates naive T cells and leads to their maturation and activation (Chen and others 1994). T cells play an important role in the adaptive immune response to HSV-1 infection, signifying the importance of CD86 expression levels on the surface of macrophages found at the infection site.
Since most studies characterizing macrophage phenotypes have been conducted using cultured murine cells (Gordon and Martinez 2010), Jaguin and others (2013) recently used monocytes purified from the buffy coats of human peripheral blood cells to characterize phenotypic and genomic markers. They generated macrophages from these primary human cells by treatment with macrophage colony-stimulating factor (M-CSF) and polarized them using the same inducers as used in the present study, LPS and IFN-γ to induce M1 phenotype and IL-4 to induce the M2 phenotype (Jaguin and others 2013). They found that the cell membrane marker unique to M1 cells was CD80 (B7.1). We had not looked for expression of this marker on murine M1 cell cells but did not find B7.2 (CD86) expression unique to M1-polarized cells. However, we observed a significant increase in the percentage of infected and uninfected M1-polarized cells I expression of CD14/CD86. Jaguin and others (2013) found that CD200R expression was unique to the M2-polarized human macrophages. As we did using M1- and M2-polarized murine macrophage cell lines (data not shown), they found that the mannose receptor CD206 did not distinguish between M1 and M2 phenotypes of human macrophages.
SOCS protein expression is rapidly upregulated in macrophages (Whyte and others 2011) and is frequently manipulated by viruses to maintain an infection within the host (Akhtar and Benveniste 2011). SOCS proteins modulate cytokine-signaling pathways, thereby influencing the inflammatory response (Akhtar and Benveniste 2011). We previously noted that murine fibroblasts and keratinocyte cell lines (Frey and others 2009) respond differentially to IFN-γ induction of an antiviral state against HSV-1. The keratinocyte cell lines produced large amounts of SOCS1 mRNA and protein, while fibroblasts exhibited a minimal increase in SOCS1 levels when treated with IFN-γ after infection with HSV-1. An antiviral state was induced in fibroblasts but not in keratinocytes. This resistance of keratinocytes to IFN-γ corresponded to the hyperinduction of SOCS1 in these cells.
As summarized in Figure 6, HSV-1-infected M1 macrophages expressed comparable levels of SOCS1 and SOCS3, while infected M2 macrophages expressed higher levels of SOCS3 compared with SOCS1. Upregulation of SOCS3 expression in HSV-1-infected M1 macrophages over that seen in uninfected M1 cells (SOCS1/SCOS3 ratio of 7:1 decreasing to 1:1 after infection Fig. 5) suggests the cell's attempt to counteract effects of proinflammatory molecules. Qasimi and others (2006) showed that different domains of SOCS3 protein are used to mediate IL-10 inhibition of TNF-α and nitric oxide production by this same macrophage cell line. In this same macrophage cell line, Il-10 was responsible for the anti-inflammatory response to Borrelia burgdorferi (Dennis and others 2006). SOCS1/SOCS3 expression levels appeared relatively unchanged in virus-infected M2 macrophages when compared with their uninfected counterparts, suggesting that microenvironment signals such as IL-4 play a greater role in SOCS expression levels than does HSV-1 infection.
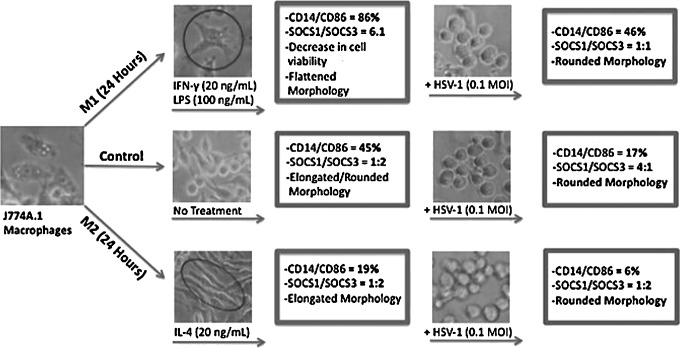
FIG. 6.
Effects of HSV-1 on polarized J774A.1 macrophages. Viral challenge led morphological changes in M1, M2, and control macrophages. HSV-1 infection decreased the ability of the 3 subpopulations of macrophages to express CD14 and CD86. HSV-1 infection of unpolarized control cells led to increased expression of SOCS1 in contrast to expression in uninfected control cells. M1-polarized macrophages expressed higher levels of SOCS3 after virus infection. HSV-1 infection did not alter the ratio of SOCS1/SOCS3 expression in M2-polarized cells; this ratio was similar to that observed in uninfected, unpolarized control cells.
The goal of the present study was to determine the effects of HSV-1 infection on morphology, CD14-CD86 expression, cell viability, and SOCS protein levels in M1 and M2 macrophages during the first 24 h of infection (Fig. 6). The results reveal the importance of the polarizing cytokine signals in expression of SOCS1 and SOCS3 proteins in regulating these early signaling events in the HSV-1-infected macrophages. This is particularly relevant given the roles of M1/M2 macrophages (microglial cells) in neuroinflammatory disease. In a murine model of experimental autoimmune encephalitis (EAE), Qin and others (2012a, 2012b) showed that SOCS3 produced by M2 myeloid cells ameliorated inflammation. In their model, adoptive transfer of M2 macrophages into myeloid SOCS3-deficient mice caused a delayed onset and reduced severity of atypical EAE (Qin and others 2012b). Qin and others (2012a) cite the restrictive role that SOCS3 exerts in a variety of inflammatory conditions in animal models for multiple sclerosis, arthritis, allograft rejection, lung injury, atherosclerosis, and septic shock.
In the present study, HSV-1 infection revealed the different roles that these 2 SOCS proteins play in the macrophage. HSV-1-infection stimulated increases in expression levels of SOCS1 compared with those of SOCS3 in both unpolarized M0 and polarized M1 macrophages In contrast, the polarization process caused an increase in expression of the anti-inflammatory SOCS3 protein, a decrease in cell viability, and a reduction in virus yield in the M1-polarized macrophages. The shortened lifespan of the M1 polarized cell is beneficial to the infected host in that this phenotype is rapidly activated on infection, killing ingested microbes with toxic ROS, NO, and TNF-α, which also contribute to the demise of the M1 cell. IFN-γ markedly enhances this activity. These observations, coupled with the greater expression of CD14-CD86 by M1 macrophages, underscore the importance of SOCS1 inhibitors as useful adjuncts in the treatment of recurrent herpes virus infections as well as inflammatory diseases. Greater expression of CD86 by the infected M1 cells will enhance their capacity as antigen presenters. SOCS-1 peptide mimetics have already been shown to protect mice against lethal poxvirus infection (Ahmed and others 2009). Future studies will determine whether a SOCS1 peptide mimetic could block the polarization of the M0 macrophage to the M1 phenotype by 24 h after simultaneous polarization and infection and, if so, whether this would affect subsequent HSV-1 replication over a 3–4 day period. Since polarization of M0 to M1 macrophages produced increased appearance of cytoplasmic granules within 16 h after polarization, it would be of interest to determine whether the differences were also detectable in the appearance of early HSV-1 proteins; for example, ICP0 and ICP4, in infected M0, M1, and M2 macrophage populations at intervals during the first 24 h of polarization and infection. It would also be of interest to determine whether the membrane markers unique to human M1 and M2 macrophages, CD80 for M1 and CD200R for M2 (Jaguin and others 2013), were also expressed by the polarized M1 and M2 murine cells used in the present study.
Acknowledgments
This work was supported by the Wright State University Research Foundation, Cellular Immunology Fund 550527 (to N.J.B.), and Sigma Xi Grant in Aid of Research (to A.C. R.). The authors extend their sincere gratitude to Barbara E. Hull, PhD, Director of the Microbiology and Immunology Program at Wright State University, for her encouragement of this work and for her editing of this article and to Noura Shaklawoon for her assistance in performing the virus plaque assays.
Author Disclosure Statement
No competing financial interests exist.
References
- Ahmed CM, Dabelic R, Waiboci LW, et al. 2009. SOCS-1 mimetics protect mice against lethal poxvirus infection: identification of a novel endogenous antiviral system. J Virol 83:1402–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar LN, Benveniste EN. 2011. Viral exploitation of host SOCS protein functions. J Virol 85:1912–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Gault A, Shen L, Nabavi N. 1994. Molecular cloning and expression of early T cell costimulatory molecule-1 and its characterization as B7-2 molecule. J Immunol 152:4929–4936 [PubMed] [Google Scholar]
- Cunningham AL, Diefenbach RJ, Miranda-Saksena M, et al. 2006. The cycle of human herpes simplex virus infection: virus transport and immune control. J Infect Dis 194:S11–S18 [DOI] [PubMed] [Google Scholar]
- Dakvist J, Wahlin TR, Bartsch E, Forsbeck M. 1995. Herpes simplex and mood: a prospective study. Psychosum Med 57:127–137 [DOI] [PubMed] [Google Scholar]
- Dennis VA, Jefferson AJ, Shree R, et al. 2006. Interleukin-10 anti-inflammatory response to Borrelia burgdorferi, the agent of Lyme disease: a possible role for suppressors of cytokine signaling 1 and 3. Infect Immun 74:5780–5789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach RJ, Miranda-Saksena M, Douglas MW, Cunningham AL. 2008. Transport and egress of herpes simplex virus in neurons. Rev Med Virol 18:35–51 [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia MA, Vogel SN. 2002. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microb Infect 4:903–914 [DOI] [PubMed] [Google Scholar]
- Frey KG, Ahmed CHI, Dabelic R, et al. 2009. HSV-1-induced SOCS-1 expression in keratinocytes: Use of a SOCS-1 antagonist to block a novel mechanism of viral immune evasion. J Immunol 183:1253–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Martinez FO. 2010. Alternative activation of macrophages: mechanism and function. Immunity 32:593–604 [DOI] [PubMed] [Google Scholar]
- Jaguin M, Houlbert N, Fardel O, et al. 2013. Polarization profiles of human M-CSF-generated macrophages and comparison of M1 markers in classically activated macrophages from GM-CSF and MS origin. Cell Immunol 281:51–61 [DOI] [PubMed] [Google Scholar]
- Jones C. 2003. Herpes simplex virus type 1 and bovine herpesvirus 1 latency. Clin Microbiol Rev 16:79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, et al. 2009. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 29:13435–13444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchens RL. 2000. Role of CD14 in cellular recognition of bacterial lipopolysaccharides. Chem Immunol 74:61–82 [DOI] [PubMed] [Google Scholar]
- Koelle DM, Corey L. 2008. Herpes simplex: insights on pathogenesis and possible vaccines. Ann Rev Med 59:381–395 [DOI] [PubMed] [Google Scholar]
- Kyoungho S, Sunshin K, Yun-Hee K, et al. 2001. IFN-γ/TNF-α synergism as the final effector in autoimmune diabetes: a key role for STAT/IFN regulatory factor-1 pathway in pancreatic β cell death. J Immunol 166:4481–4489 [DOI] [PubMed] [Google Scholar]
- Ma J, Liu L, Che G, et al. 2010. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer 10:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques CP, Cheeran MC-J, Palmquist JM, et al. 2008. Prolonged microglial cell activation and lymphocyte infiltration following experimental herpes encephalitis. J Immunol 181:6417–6426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Sica A, Mantovani A, Locati M. 2008. Macrophage activation and polarization. Front Biosci 13:453–461 [DOI] [PubMed] [Google Scholar]
- Mikloska Z, Danis VA, Adams S, et al. 1998. In vivo production of cytokines and beta (C-C) chemokines in human recurrent herpes simplex lesions: do herpes simplex virus infected keratinocytes contribute to their production? J Infect Dis 177:827–838 [DOI] [PubMed] [Google Scholar]
- Miller CS, Danaher RJ, Jacob RJ. 1998. Molecular aspects of herpes simplex virus I latency, reactivation, and recurrence. Crit Rev Oral Biol Med 9:541–562 [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. 2008. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA. 2011. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11:723–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qasimi P, Ming-Lum A, Ghanipour A, et al. 2006. Divergent mechanisms utilized by SOCS3 to mediate interleukin-10 inhibition of tumor necrosis factor α and nitric oxide production by macrophages. J Biol Chem 281:6316–6324 [DOI] [PubMed] [Google Scholar]
- Qin H, Holdbrooks AT, Liu Y, et al. 2012a. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J Immunol 189:3439–3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Yeh W-I, De Sarno P, et al. 2012b. Signal transducer and activator of transcription-3/suppressor of cytokine signaling-3 (STAT3/SOCS3) axis in myeloid cells regulates neuroinflammation. Proc Natl Acad Sci U S A 109:5004–5009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B, Knipe DM, Whitley RJ. 2007. Herpes simplex viruses. In Knipe DM.Howley PM, eds. Fields Virology, 3rd ed., Philadelphia: Lippincott Williams & Wilkins; pp. 2501–2602 [Google Scholar]
- Schachtele SJ, Hu S, Little MR, Lokensgard JR. 2010. Herpes simplex virus induces neural oxidative damage via microglial cell Toll-like receptor-2. J Neuroinflamm 7:35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock C, Guillen-Grima F, Hermosa de Mendoza J, et al. 2001. Risk factors of herpes simplex type 1 (HSV-1) infection and lifestyle factors associated with HSV-1 manifestations. Eur J Epidemiol 17:885–890 [DOI] [PubMed] [Google Scholar]
- Wang YC, He F, Feng F, et al. 2010. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res 70:4840–4849 [DOI] [PubMed] [Google Scholar]
- Whyte CS, Bishop ET, Ruckerl D, et al. 2011. Suppressor of cytokine signaling (SOCS)1 is a key determinant of differential macrophage activation and function. J Leukoc Biol 90:845–854 [DOI] [PubMed] [Google Scholar]
- Wu L, Morahan PS. 1992. Macrophages and other non-specific defenses: role of modulating resistance against herpes simplex virus. Curr Top Microbiol Immunol 79:89–110 [DOI] [PubMed] [Google Scholar]