Abstract
Mesenchymal stem cells (MSCs) and neural progenitor cells (NPCs) have been regarded for their clinical therapeutic potential for central nervous system (CNS) pathologies. Their potential utility is a result of their intrinsic ability to repair damaged tissues, deliver therapeutic proteins, and migrate to sites of pathology within the brain. However, it remains unclear whether the CNS promotes any changes in these potential therapeutic cells, which would be critical to understand before clinical application. A major component of the CNS is cerebrospinal fluid (CSF). Therefore, the aim of this study was to evaluate the influence that human CSF has on the function of human adipose-derived MSCs (hAMSCs) and human fetal-derived NPCs (hfNPCs) in regard to cell proliferation, survival, and migration. This study demonstrated that human noncancerous CSF promoted proliferation and inhibited apoptosis of hAMSCs and hfNPCs. Preculturing these stem cells in human CSF also increased their migratory speed and distance traveled. Furthermore, insulin-like growth factor-1 (IGF-1) in human CSF enhanced the migration capacity and increased the expression of C-X-C chemokine receptor type 4 (CXCR4) in both stem cell types. These current findings highlight a simple and natural way in which human CSF can enhance the proliferation, migration, and viability of human exogenous primary hAMSCs and hfNPCs. This study may provide insight into improving the clinical efficacy of stem cells for the treatment of CNS pathologies.
Introduction
Stem cells have been regarded for their therapeutic potential for the treatment of a wide variety of diseases, especially neurological diseases [1,2]. This notion can be attributed to their intrinsic ability, unlike their more mature cell types, to migrate to sites of pathology including stroke, brain trauma, neoplasms, and neurodegenerative diseases, among others [3–6]. Previous studies have demonstrated that mesenchymal stem cells (MSCs) and neural progenitor cells (NPCs) could help repair damaged tissue and deliver specific therapeutic proteins in the central nervous system (CNS) [7–11]. However, whether the CNS affects the function of these cells has not been fully explored.
Cerebrospinal fluid (CSF) is an important component of the CNS that has a multitude of functions including providing shock absorption for the brain, removing metabolic byproducts from the parenchyma, and distributing proteins. It has also been shown to be critical in guiding migration and neuronal development [12]. Moreover, it contains large quantities of proteins and trophic factors including insulin-like growth factors (IGFs), bone morphogenetic proteins (BMPs), transforming growth factor-β (TGF-β), and wingless-type MMTV integration site family members (Wnts), which are known to affect the function of stem cells [12–14]. Recent experiments showed that human CSF and its contents influence the differentiation and proliferation of NPCs [15–17], but the effects of CSF on the viability and mobility of exogenous MSCs and NPCs have yet to be explored. Since stem cell therapy is being investigated for a variety of neurological disorders, it is critical to understand the impact that the CSF can have on exogenous stem cell populations.
Previous studies have demonstrated that the insulin-like growth factor-1 (IGF-1) secreted by neural cells and circulated by the CSF plays a significant role in development, survival, and plasticity of the CNS [12,18,19]. In addition, IGF-1 is known to be one of the most potent growth factors in promoting migration of MSCs when compared with other growth factors in vitro [20]. We hypothesized that human CSF can stimulate the migration capacity of both MSCs and NPCs, and promote proliferation and viability. Moreover, this effect is partly attributable to IGF-1 in CSF. This is the first study assessing the potential effects of human noncancerous CSF on the function of human exogenous adipose-derived MSCs and NPCs. The aim of our study was to demonstrate a proof-of-principle that CSF has an effect on hAMSCs and hfNPCs in regard to cell proliferation, survival, and migration speed and distance. Our main goal was to demonstrate that CSF has different effects on these cells. This is especially important because, if these cells are to be used to treat a variety of neurological pathologies including stroke, brain tumors, and degenerative diseases, understanding the effects of CSF on these cells is critical.
Materials and Methods
Cell expansion
Following approval by the Johns Hopkins University Institutional Review Board Early passaged primary (up to passage 7) human adipose-derived mesenchymal stem cells (JHH hAMSCs 738), human fetal-derived neural progenitor cells (JHH hfNPCs 61), and human glioblastoma (GBM) cells (JHH GBM 276) were obtained from our patients undergoing neurosurgical procedures, which were established as previously described [21–24]. The hAMSCs were cultured in MSC complete media [MesenPRO RS basal media (12747-010; Gibco) with one vial of MesenPRO RS growth supplement (12748-018; Gibco), 1% Antibiotic/Antimycotic (15240-062; Invitrogen), and 1% Glutamax (35050-061; Gibco)]. The hfNPCs were cultured on laminin (L2020; Sigma)-coated flasks at a concentration of 1 μg/cm2 in media composed of 65% Dulbecco's modified Eagle's medium (DMEM, 11965-092; Gibco) with 32% F12 (10080148; Corning), 1% Antibiotic/Antimycotic, 2% B27 (17504-044; Invitrogen), 20 ng/mL human fibroblast growth factor-b (hFGF-b) (100-18B; PeproTech), and 20 ng/mL human epithelial growth factor (hEGF) (AF-100-15; PeproTech). To evaluate the stem cell features of our cell cultures, the MSCs were tested for the expression of CD31, CD34, CD45, CD73, CD90, and CD105 via flow cytometry. NPCs were stained with Nestin, GFAP, and Tuj-1. GBM cells were cultured in DMEM/F12 (11330-032; Gibco) with 2% Antibiotic/Antimycotic, 2% B27, 20 ng/mL hFGF-b, and 20 ng/mL hEGF in laminin (1 μg/cm2)-coated flasks. These cells were maintained in an incubator at 37°C and an atmosphere of 5% CO2, 20% O2.
CSF collection
The human CSF samples were collected from noncancer hydrocephalic patients. All patients were undergoing ventriculo-peritoneal shunts; CSF was obtained from their ventricular space, and none had cancers, infections or evidence of meningitis, multiple sclerosis or any other diseases known to humans. These were mainly patients with either obstructive or communicating hydrocephalus. Moreover, these samples were only used if all standard parameters were normal and had not been contaminated with blood. The pooled CSF was centrifuged at 1,000 g at 4°C for 15 min to remove any cells or debris from the fluid. The sample was then frozen on dry ice and stored at −80°C until use. The artificial CSF was prepared as follows: H2O, NaCl (124 mM), KCl (2.5 mM), NaH2PO4 (1.25 mM), CaCl2·2H2O (2.5 mM), MgSO4·7H2O (1.5 mM), and glucose (10 mM). Then, NaHCO3 was used to adjust the pH during 7.35–7.45 [25,26].
Glioblastoma (GBM) conditioned media collection
For collection of GBM media for the transwell migration experiments, 2×106 GBM cells (JHH GBM 276) were seeded in 25 mL flasks and cultured in complete media for 48 h. The conditioned media containing the soluble factors secreted by the GBM cells was passed through a 0.45 μm filter (Corning) and stored at −80°C.
Cell treatment
To determine the effects of CSF on hAMSCs and hfNPCs, hAMSCs were cultured in 100% MSC complete media (Ctrl), 25% artificial CSF plus 75% MSC complete media (A-CSF), or 25% human CSF plus 75% MSC complete media (H-CSF) respectively. Meanwhile, hfNPCs were cultured in 100% NPC complete media (Ctrl), 25% artificial CSF plus 75% NPC complete media (A-CSF), or 25% human CSF plus 75% NPC complete media (H-CSF). These culture media were replaced every 3 days, and the passaging time was recorded. Analysis of proliferation, apoptosis, and migration were subsequently performed.
MTT assay
The proliferation capacity of hAMSCs and hfNPCs were determined using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (M2128-1G; Sigma). The cells were seeded in 96-well plates (1,500 cells/well) and cultured in different media for 10 days (Ctrl, A-CSF, or H-CSF). The hAMSCs and hfNPCs were treated with 5 mg/mL MTT for 3 h at 37°C. Then, 2-Propanol was used to dissolve the formazan crystals. Absorbance of each well was read at a wavelength of 570 nm. The cell proliferation was analyzed every 2 days for each experimental condition.
Immunofluorescence staining
Nuclear Ki67 expression was used to detect cells in active proliferative phases. 1×104 hAMSCs or hfNPCs, which were cultured in different culture media, were seeded on a 24-well plate for 3 days (with glass slides precoated with poly-1-ornithine and laminin). The cells were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 30 min, and preincubated in PBS containing 0.1% Triton X-100 with 10% goat serum prior to incubation with anti-Ki67 (RM-9106-s1; Thermo). Alexa 594-labeled secondary antibody (A11080; Gibco) was used for visualization, and DAPI was used to counterstain cell nuclei. The number of Ki67+/DAPI were visualized and recorded with an inverted fluorescence microscope (Axio Observer Z1; Zeiss).
Flow cytometry assay
An EdU assay was performed to evaluate cell proliferation in response to human CSF. hAMSCs and hfNPCs were cultured in pediatric CSF (younger than 18) or adult CSF (older than 18) for 14 days. These cells were then incubated with EdU reagent for 4 h (Click-iT® EdU Flow Cytometry Assay Kits; Invitrogen) and tested via flow cytometry. All measurements were performed according to the recommended protocol. EdU incorporation was detected via flow cytometry. Three replicate experiments were performed for each sample.
To determine the effect of human CSF on the viability of hAMSCs and hfNPCs, annexin V and propidium iodide (PI) were used to assess apoptosis. The hAMSCs and hfNPCs were cultured in the different media for 3 days (Ctrl, A-CSF, or H-CSF), and then flow cytometry was performed. The stem cells were diluted to 1×106/mL in 100 μL of binding buffer, then incubated with 5 μL annexin V for 15 min at room temperature, and counterstained with PI for 5 min. Both hAMSCs and hfNPCs were subsequently tested three times by flow cytometry. Each sample contained at least 1×105 cells. The cells then underwent detection via flow cytometry [Becton Dickinson (BD)].
CXCR4 is a major cytokine and chemokine receptor on the cell surface [27,28]. Here, APC-conjugated monoclonal anti-CXCR4 (555976; BD) was used to evaluate the changes in CXCR4 expression of hAMSCs and hfNPCs. The cells were pretreated in different culture media (Ctrl, A-CSF, or H-CSF) for 14 days. Then, 1×105 stem cells in 100 μL of binding buffer were incubated with 20 μL APC-conjugated CXCR4 or isotype-matched antibodies (555576; BD) for 45 min at 4°C. Flow cytometry data were analyzed with Flowjo 7.6.1.
Cell migration on nanopattern surface
To determine changes in the mobility of hAMSCs and hfNPCs in response to human CSF, nano-ridges, and grooves, constructed of transparent polyurethane acrylate, were used to evaluate cell migration distance and speed as established in our laboratory [29,30]. The advantage of using the nanopattern is we can analyze single cell migration without the confounding variable of proliferation, because we exclude any proliferating cells from our nanopattern migration analysis [30]. These nanopattern surfaces were precoated with poly-1-ornithine (for 30 min) and laminin (for 2 h) consecutively. 2×104 hAMSCs or hfNPCs were pretreated with different culture media (Ctrl, A-CSF, or H-CSF) and were seeded on nanopattern surfaces. Cell mobility was monitored by time-lapse microscopy at 10 min intervals for 16 h. Cell speed and distance were calculated with MATLAB by a single blinded observer (50 cells in each group).
Transwell assay
Boyden transwell chambers (3422; Corning) were used to evaluate the migration capacity of hAMSCs and hfNPCs toward GBM-conditioned media. To analyze the influence of human CSF on migration, hAMSCs or hfNPCs were precultured in the different culture media (Ctrl, A-CSF, or H-CSF) for 1, 7, or 14 days before a transwell assay was performed. hAMSCs or hfNPCs were added to the upper chambers (2×104 cells), while 600 μL of GBM-conditioned media with 1% fetal bovine serum was placed in the bottom wells. The Boyden transwell chambers were placed in an incubator overnight (16 h) at 37°C and 5% CO2. The cells that failed to migrate to the bottom were removed with cotton wool swabs. The stem cells on the bottom of the filters were stained using a Diff-Quik staining kit. The total number of migrated cells was counted using light microscopy at 100× magnification from nine random fields.
Stemness characteristics tests
To test whether both cell types retained their stemness characteristics, the hAMSCs and hfNPCs were cultured in H-CSF (25% CSF+75% complete media) or Ctrl (100% complete media) for 14 days. Then, the hAMSCs were tested for CD31, CD34, CD45, CD73, CD90, and CD105 expression via flow cytometry. Meanwhile, hfNPCs were stained and quantified with Nestin and DAPI.
ELISA of IGF-1
IGF-1 was measured in human CSF samples using Abcam's IGF-1 Human ELISA Kit (ab100545; Abcam). The samples and standards were placed in triplicate wells and incubated overnight at 4°C. Then, biotinylated IGF-1, HRP-Streptavidin solution, TMB substrate reagent, and a stop solution were added sequentially. All measurements were performed according to the recommended protocol. Absorbance of these samples was read at a wavelength of 450 nm.
Neutralizing IGF-1
An IGF-1 antibody (ab9572; Abcam) was used as an inhibitor (1.0 μg/mL) to test whether neutralization of the IGF-1 in CSF could affect the functional characteristics of hAMSCs and hfNPCs. These cells were cultured in 100% complete media (Ctrl), 100% complete media plus IGF-1 inhibitor (Ctrl+IGF-1 inhibitor), 25% human CSF plus 75% complete media (H-CSF), or 25% human CSF plus 75% complete media and the IGF-1 inhibitor (H-CSF+IGF-1 inhibitor). Moreover, the complete media of hAMSC and hfNPC does not contain insulin or IGF-1. The flow cytometry of apoptosis and CXCR4 expression, MTT assay, and transwell migration assay were performed to quantify changes in viability, proliferation, and migration capacities of hAMSCs and hfNPCs.
Statistical analysis
The results are presented as mean±standard error of the mean. Data within the two groups were analyzed with the Student's t-test. One-way ANOVA with Dunnett post hoc analysis was used to evaluate multiple groups. Statistical significance was defined as P<0.05.
Results
Human CSF promotes proliferation and inhibits apoptosis of hAMSCs and hfNPCs
Since these experiments used noncancerous human CSF including pediatric and adult (Table 1), we tested whether these two types of CSF have a different effect on hAMSCs and hfNPCs. EdU assays and Boyden chamber transwell assays were performed to evaluate their effects on proliferation and migration capacities of the stem cells. We found that hAMSCs and hfNPCs cultured with pediatric CSF displayed no significant difference in proliferation (hAMSCs: P=0.37; hfNPCs: P=0.31) and migration (hAMSCs: P=0.71; hfNPCs: P=0.45) compared with those cultured with adult CSF (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd).
Table 1.
Clinical Presentation of Cerebrospinal Fluid from Noncancerous Patients
| Patient | Age | Gender | Volume used (mL) | Diagnosis |
|---|---|---|---|---|
| 1 | 19 | F | 3 | Communicating hydrocephalus |
| 2 | 32 | M | 5 | Communicating hydrocephalus |
| 3 | 19 | M | 5 | Congenital hydrocephalus |
| 4 | 76 | M | 2 | Normal pressure hydrocephalus |
| 5 | 16 | F | 5 | Communicating hydrocephalus |
| 6 | 3 | F | 3 | Obstructive hydrocephalus |
| 7 | 17 | M | 8 | Congenital hydrocephalus (Chiari I) |
| 8 | 39 | F | 5 | Congenital hydrocephalus |
| 9 | 49 | F | 3 | Congenital hydrocephalus (Chiari II) |
| 10 | 2 | M | 10 | Congenital hydrocephalus |
| 11 | 25 | F | 5 | Communicating hydrocephalus |
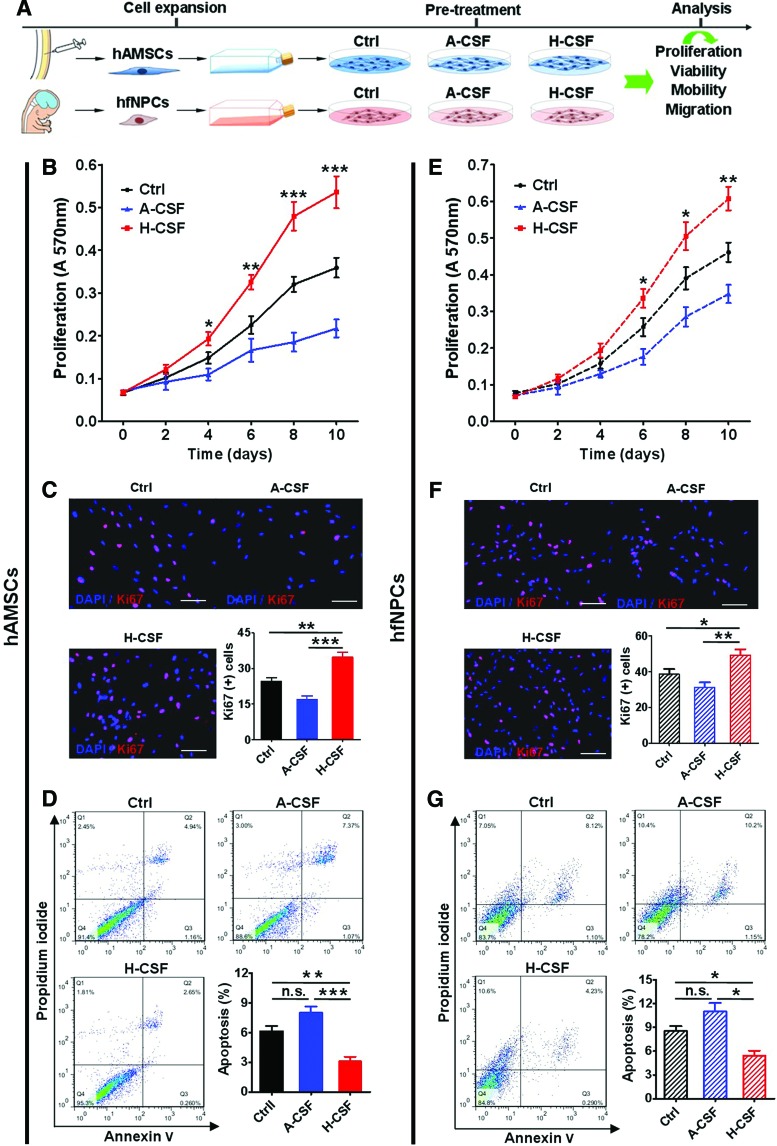
Based on these findings, the pooled CSF was used throughout the study (Fig. 1A). First, we tested whether human CSF could affect the proliferation and viability of hAMSCs and hfNPCs [31–33]. H-CSF induced an increase in the proliferation of hAMSCs when compared with cells cultured in Ctrl media or A-CSF, as demonstrated by MTT assays and immunostaining for Ki67 (MTT assay, days 4, 6, 8, and 10: H-CSF vs. Ctrl, P<0.05, 0.01, 0.001, and 0.001; H-CSF vs. A-CSF, P<0.05, 0.001, 0.001, and 0.001; Ki67 assay: H-CSF vs. Ctrl, P<0.01; H-CSF vs. A-CSF, P<0.001) (Fig. 1B, C). Moreover, hAMSCs cultured in H-CSF showed a lower percentage of apoptosis when compared with the Ctrl and A-CSF group, as evidenced by a lower level of annexin V and PI staining (P<0.05) (Fig. 1D). Human CSF had a similar effect on the proliferation of hfNPCs as it increased their proliferation and decreased their apoptosis rate (MTT assay, days 6, 8, and 10: H-CSF vs. Ctrl, P<0.05, 0.05 and 0.01; H-CSF vs. A-CSF, P<0.01, 0.001, and 0.001; Ki67 assay: H-CSF vs. Ctrl, P<0.05; H-CSF vs. A-CSF, P<0.01) (Fig. 1E–G). These results indicate that human CSF can promote proliferation and decrease the rate of apoptosis for both hAMSCs and hfNPCs.
FIG. 1.
Human CSF promotes the proliferation and inhibits apoptosis of hAMSCs and hfNPCs. (A) The hAMSCs and hfNPCs were cultured in their corresponding 100% complete media (Ctrl), 25% artificial CSF plus 75% complete media (A-CSF), or 25% human CSF plus 75% complete media (H-CSF) respectively. (B) MTT assay quantification of hAMSCs exhibited that hAMSCs cultured in H-CSF displayed an increased rate of proliferation at the time points of 4, 6, 8, and 10 days when compared with those cultured in the Ctrl and A-CSF conditions. (C) Representative pictures of Ki67 (red) and DAPI (blue) staining of hAMSCs. The hAMSCs cultured in H-CSF had higher number of Ki67+ cells (C) and a lower percentage of apoptosis (D) when compared with those cultured in Ctrl and A-CSF conditions. (E) Human CSF also had a positive effect on the proliferation of hfNPCs at days 6, 8, and 10 when compared with Ctrl and A-CSF groups. (F) The hfNPCs cultured in H-CSF expressed a higher number of Ki67+ cells compared with the other groups. (G) The hfNPCs cultured in H-CSF also exhibited a higher survival rate compared with the Ctrl and A-CSF groups. Scale bars=100 μm (C, F). Error bars represent SEM. *P<0.05, **P<0.01, ***P<0.001. CSF, cerebrospinal fluid; hAMSCs, human adipose-derived mesenchymal stem cells; hfNPCs, human fetal-derived neural progenitor cells; SEM, standard error of the mean. Color images available online at www.liebertpub.com/scd
Moreover, we tested the passaging time of hAMSCs and hfNPCs. These cells cultured in H-CSF exhibited a shorter passaging time when compared with Ctrl groups (Supplementary Fig. S2A, B). These findings share a commonality with the MTT and Ki67 staining results. Furthermore, we used different concentrations of artificial CSF and human CSF to culture both cell types (Supplementary Fig. S3). We found that the hAMSCs cultured in 50% human CSF had the highest A570 value and percentage of Ki67+ cells when compared with other groups (P<0.05) (Ctrl 25%, 50%, 75%, and 100% human CSF) (Supplementary Fig. S3A, B). The hfNPCs cultured in 25% human CSF exhibited the highest A570 value and number of Ki67+ cells compared with the other groups (P<0.05) (Ctrl 25%, 50%, 75%, and 100% human CSF) (Supplementary Fig. S3C, D). These results are likely due to the wide variety of growth factors in human CSF, and other growth factors in the commercial complete media. With an increase in the amount and types of growth factors in the culture media, the effect on proliferation of the stem cells was more apparent.
Human CSF increases the migration speed and distance of hAMSCs and hfNPCs
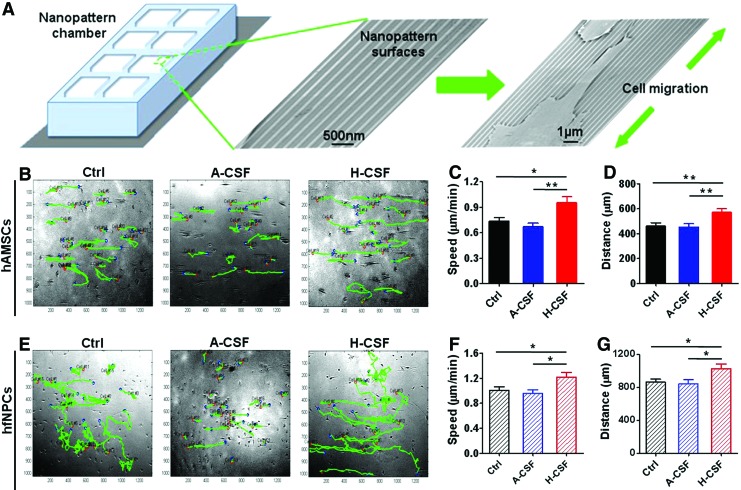
Migration of hAMSCs and hfNPCs were evaluated by plating cells on nanopatterned surfaces and recording them using time-lapse microscopy for a period of 16 h. Cell speed and distance traveled was determined for cells cultured in Ctrl, A-CSF, and H-CSF for 14 days (Fig. 2A and Supplementary Online Videos SV1 and SV2). hAMSCs displayed an increased migratory speed and distance migrated in H-CSF when compared to Ctrl and A-CSF (speed: H-CSF vs. Ctrl, P<0.05; H-CSF vs. A-CSF, P<0.01; distance: H-CSF vs. Ctrl, P<0.01; H-CSF vs. A-CSF, P<0.01) (Fig. 2B–D). The hfNPCs in H-CSF also displayed significant differences in both speed and distance migrated when compared with other groups (speed: H-CSF vs. Ctrl, P<0.05; H-CSF vs. A-CSF, P<0.05; distance: H-CSF vs. Ctrl, P<0.05; H-CSF vs. A-CSF, P<0.05) (Fig. 2E–G). These results indicate that human CSF can promote the migration speed and distance of hAMSCs and hfNPCs.
FIG. 2.
Human CSF promotes the mobility and migration of hAMSCs and hfNPCs. Nanopatterned surfaces were used to quantify the speed and distance of the hAMSCs and hfNPCs after they were cultured in Ctrl, A-CSF, or H-CSF for 14 days. A schematic of the nanopatterned surface, along with electron microscopy images can be seen in (A). (B–D) The hAMSCs cultured in H-CSF displayed a higher speed and longer distance traveled when compared with those cultured in Ctrl and A-CSF conditions. Green tracks represent the migratory paths taken by hAMSCs on the nanopatterned surface. (E–G) The hfNPCs cultured in H-CSF had a significant difference in speed and distance compared with the other groups. Error bars represent SEM. *P<0.05, **P<0.01. Color images available online at www.liebertpub.com/scd
Human CSF enhances the migration capacity of hAMSCs and hfNPCs to GBM-conditioned media
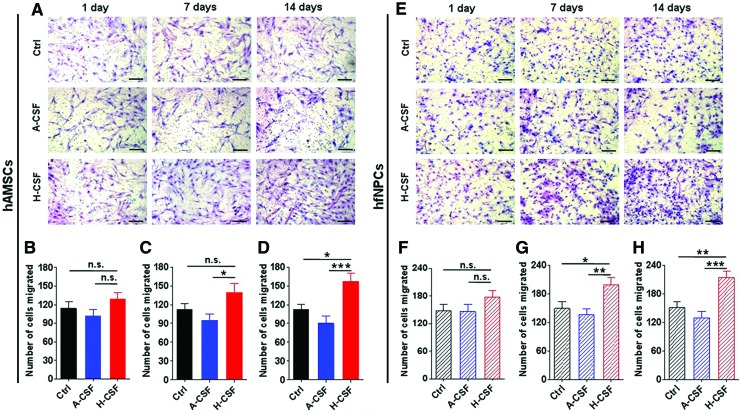
Since hAMSCs and hfNPCs have been considered for the treatment of glioblastoma, we subsequently explored whether human CSF could increase the migration capacities of both stem cell types to culture media conditioned by glioblastoma cells. The effect of pretreating hAMSCs and hfNPCs with CSF on migration to GBM-conditioned media was subsequently evaluated. hAMSCs and hfNPCs were pretreated with Ctrl, A-CSF, and H-CSF for 1, 7, or 14 days. Transwell migration assays were used to assess their migratory capacity. hAMSCs precultured with H-CSF displayed a higher number of migrated cells compared with A-CSF and Ctrl groups at 14 days (P<0.05) (Fig. 3A–D). Meanwhile, hfNPCs precultured in H-CSF also displayed a higher number of migrated cells when compared with Ctrl and A-CSF groups treated for 7 and 14 days (P<0.05) (Fig. 3E–H). Furthermore, we also tested the stemness characteristics of both cell types when they were pretreated in H-CSF for 14 days. As shown in Supplementary Figure S4, the hAMSCs, which were cultured in H-CSF, exhibited the same percentage of stemness markers (CD31, CD34, CD45, CD73, CD90, and CD105) when compared with the Ctrl group. Meanwhile, the hfNPCs displayed no significant difference in the number of Nestin-positive cells between the Ctrl and H-CSF group.
FIG. 3.
Human CSF helps to enhance the migration capacity of hAMSCs and hfNPCs. The hAMSCs and hfNPCs were precultured in Ctrl, A-CSF, or H-CSF for 1, 7, or 14 days. Next, Boyden chamber transwell assays were used to assess changes in migration. (A) Representative pictures of Boyden chamber transwells using hAMSCs and (E) hfNPCs are shown. (B–D) The hAMSCs precultured in H-CSF had a significantly higher number of migrated cells when compared with hAMSCs precultured in Ctrl and A-CSF conditions. (F–H) At days 7 and 14, H-CSF significantly increased the migration of hfNPCs when compared with the other conditions. Scale bars=100 μm (A, E). Error bars represent SEM. *P<0.05, **P<0.01, ***P<0.001. Color images available online at www.liebertpub.com/scd
To test whether the observed effects of human CSF on hAMSCs and hfNPCs migration were dose dependent, these cells were cultured in different concentrations of human CSF (Ctrl 25%, 50%, 75%, and 100% human CSF) for 2 days. Both hAMSCs and hfNPCs pretreated in higher concentrations of human CSF (100% and 75%) showed an enhanced transwell migration capacity when compared with Ctrl groups (P<0.05) (Supplementary Fig. S5). Moreover, the percentage increase in migration capacity of hAMSCs and hfNPCs were similar (hAMSCs, number of cell migrated in 100% CSF/number of cell migrated in Ctrl=156%; hfNPC, number of cell migrated in 100% CSF/number of cell migrated in Ctrl=162%). These results demonstrate that H-CSF can enhance the migratory capabilities of both hAMSCs and hfNPCs.
IGF-1 in human CSF affects the apoptosis and proliferation of hAMSCs and hfNPCs
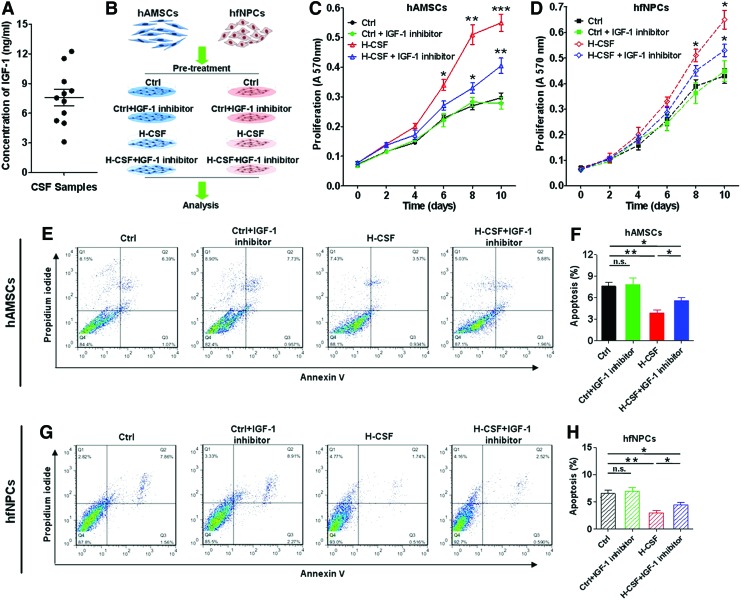
Previous studies have shown that IGF-1 is present in human CSF, and that IGF-1 plays a pivotal role in regulating a number of pathways important for maintaining cell viability [12,34]. We hypothesized that IGF-1 in human CSF may influence the proliferation and viability of hAMSCs and hfNPCs. First, we confirmed the presence of IGF-1 in our intraoperative noncancerous CSF samples (7.58±0.827 ng/mL) (Fig. 4A, Supplementary Table S1). Then, an IGF-1 inhibitor was added to Ctrl or H-CSF media to assess the effects on proliferation and viability of hAMSCs and hfNPCs (Fig. 4B).
FIG. 4.
IGF-1 in human CSF affects the proliferation and apoptosis of hAMSCs and hfNPCs. (A) The concentration of IGF-1 in human CSF samples was measured using an ELISA Kit (7.58±0.827 ng/mL). (B) The IGF-1 inhibitor was added in Ctrl and H-CSF groups to detect changes in viability and proliferation of hAMSCs and hfNPCs. (C) MTT assay quantification showed that hAMSCs cultured in H-CSF+IGF-1 inhibitor had a higher proliferation rate than hAMSCs cultured in Ctrl and Ctrl+IGF-1 inhibitor, but lower than the H-CSF group. (D) hfNPCs displayed a decrease in proliferation when the IGF-1 inhibitor was added to H-CSF. Flow cytometry of annexin V and PI for both hAMSCs and hfNPCs is shown in (E, G). (F, H) Both hAMSCs and hfNPCs cultured in H-CSF+IGF-1 inhibitor exhibited an increased rate of apoptosis when compared with those cultured in H-CSF without inhibition of IGF-1. Error bars represent SEM. *P<0.05, **P<0.01, ***P<0.001. IGF-1, insulin-like growth factor-1; PI, propidium iodide. Color images available online at www.liebertpub.com/scd
The IGF-1 inhibitor was able to partially counteract the proliferative response of hAMSCs to H-CSF, as evidenced by MTT assay analysis performed at 6, 8, and 10 days upon exposure to H-CSF (H-CSF+IGF-1 inhibitor vs. H-CSF, P<0.05, 0.01, and 0.001) (Fig. 4C). A similar effect was observed when the IGF-1 inhibitor was added to hfNPCs in H-CSF (H-CSF+IGF-1 inhibitor vs. H-CSF, P<0.05, and 0.05) (Fig. 4D). The IGF-1 inhibitor alone did not have an effect on cell proliferation.
Similarly, the protective effects on apoptosis, exerted by H-CSF on hAMSCs and hfNPCs were partially blocked when IGF-1 was inhibited. Both hAMSCs and hfNPCs in H-CSF+IGF-1 inhibitor groups displayed an increase in the rate of apoptosis when compared with the groups cultured without the IGF-1 inhibitor (P<0.05) (Fig. 4E–H). However, these levels were still lower when compared with control cells cultured without H-CSF. This result suggests that IGF-1 in human CSF is one of the major factors able to enhance the proliferation and maintain the viability of hAMSCs and hfNPCs.
IGF-1 in human CSF can enhance the migration capacity and affect the expression of CXCR4 in hAMSCs and hfNPCs
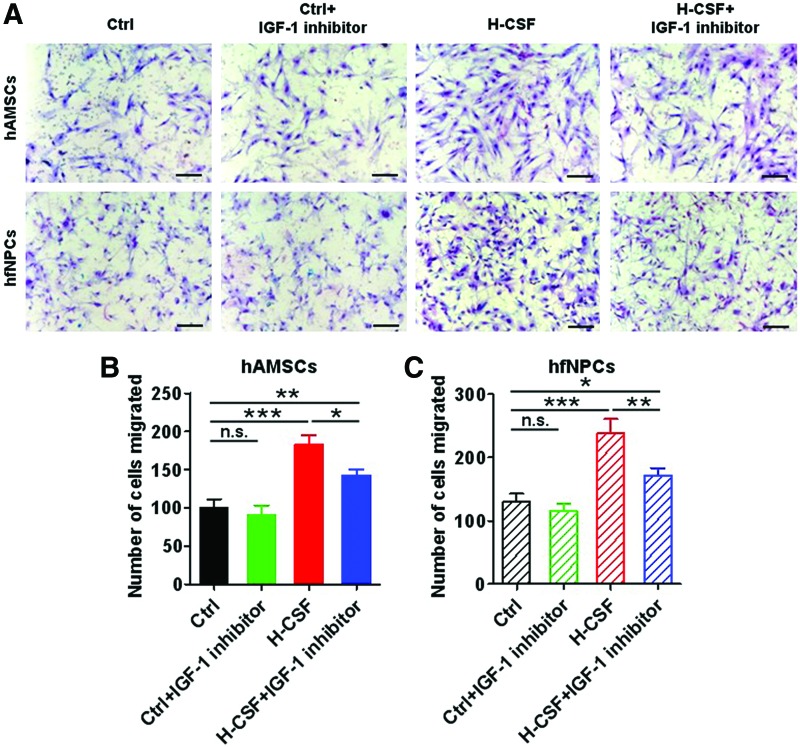
Consequently, IGF-1 inhibitor was able to partially decrease the transwell migration response of hAMSCs and hfNPCs pretreated in H-CSF (P<0.05). However, hAMSCs and hfNPCs cultured in H-CSF+IGF-1 inhibitor still displayed a greater migration capacity when compared with the Ctrl groups (P<0.05) (Fig. 5A–C). These results suggest that H-CSF contains factors in addition to IGF-1 responsible for enhancing migration.
FIG. 5.
IGF-1 in human CSF enhances the migration capacity of hAMSCs and hfNPCs. The hAMSCs and hfNPCs were pretreated in Ctrl, Ctrl+IGF-1 inhibitor, H-CSF, or H-CSF+IGF-1 inhibitor, for 14 days. Boyden transwell chamber assay were conducted to measure the migration capacity. (A) Representative pictures of Boyden chamber transwell assays of hAMSCs and hfNPCs can be seen. (B, C) The hAMSCs and hfNPCs cultured in H-CSF+IGF-1 inhibitor displayed a decrease in migration compared with the H-CSF groups, but contained a greater number of migrated cells when compared with the Ctrl and Ctrl+IGF-1 inhibitor groups. Scale bars=100 μm (A). Error bars represent the SEM. *P<0.05, **P<0.01, ***P<0.001. Color images available online at www.liebertpub.com/scd
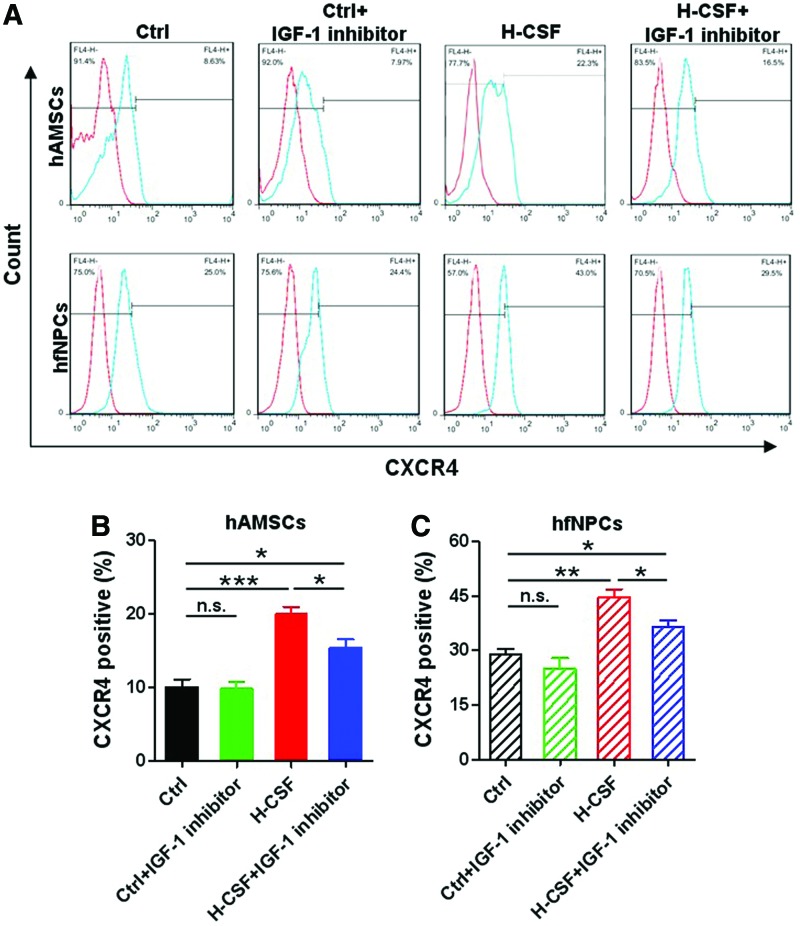
Then, we tested the expression of CXCR4 in hAMSCs and hfNPCs. Both hAMSCs and hfNPCs cultured in H-CSF expressed a higher level of CXCR4 when compared with other groups (P<0.05) (Fig. 6A–C). When the IGF-1 inhibitor was added to the human CSF, the expression of CXCR4 in hAMSCs and hfNPCs displayed a statistically significant decrease (P<0.05). These results suggest that human CSF can promote the migration capacity of hAMSCs and hfNPCs; and is suggestive that this may be partly due to the presence of IGF-1. Moreover, human CSF can stimulate the expression of CXCR4 in both stem cell types.
FIG. 6.
IGF-1 in human CSF affects the expression of CXCR4 in hAMSCs and hfNPCs. (A) Flow cytometry results to detect CXCR4 expression in hAMSCs and hfNPCs are shown. (B, C) The hAMSCs and hfNPCs cultured in H-CSF expressed a higher level of CXCR4 when compared with the other groups. CXCR4 expression was decreased in both hAMSCs and hfNPCs when the IGF-inhibitor was added to H-CSF. Error bars represent the SEM. *P<0.05, **P<0.01, ***P<0.001. CXCR4, C-X-C chemokine receptor type 4. Color images available online at www.liebertpub.com/scd
Discussion
The intrinsic ability of hAMCSs and hfNPCs to home to sites of disease makes them attractive therapeutic candidates for cell-based therapies [8,10,11,35]. However, whether the factors in the CNS and, more specifically, the CSF affect the viability and the mobility of these stem cells have yet to be explored. Our study was aimed at exploring the effects of CSF on NSCs and MSCs exogenously before they are incorporated into a patient with a neurological disease. In this study, we show that human CSF may be able to enhance the therapeutic potential of both human early-passage hAMSCs and hfNPCs by promoting their growth, decreasing their passaging time, inhibiting their apoptosis, and enhancing their migration speed and distance. These findings may suggest an effective way to enhance stem cells for cell-based therapies in the treatment of CNS pathologies.
Recent studies have highlighted the clinical therapeutic potential of using hAMSCs and hfNPCs due to their homing and immunosuppressive capabilities [4,36]. The benefits of these stem cells may involve migrating to brain tumors, repairing damaged cells, and excreting neurotrophic factors for a wide variety of diseases in the CNS. Amariglio et al. reported a child with ataxia telanglectasia was diagnosed with a multifocal brain tumor after being treated with transplanted neural stem cells [37]. However, just like the complications of radiotherapy and chemotherapy, lots of clinical applications have their demerits and merits. Previous studies used hAMSCs and hfNPCs as vehicles to deliver therapeutic agents to brain tumors without oncogenisis occurring [8,38,39]. Moreover, these cell resources have also been reported to attenuate brain injury after neonatal stroke and have been used in the treatment of neurodegenerative diseases [11,40–42]. However, attempts to deliver these stem cells to specific sites including the CNS, typically face low efficiencies [4,43]. To receive a better clinical effect, previous studies have genetically manipulated these stem cells to maximize their migratory and regenerative capabilities [44–46]. Although genetic engineering could enhance the function of these stem cells, the complications of these techniques have yet to be determined, which hinders their probability of clinical application [47,48].
For these stem cell therapies to be applied to treat neurological disorders, there must be an understanding of what the CNS, namely CSF, does to these cells. CSF is a key component of the CNS and has a multitude of functions; including protecting the brain and spinal cord from trauma, chemical stability by removing metabolic byproducts, distributing proteins and growth factors, and guiding migration and neuronal development [12,14]. The effect of CSF on the clinical therapeutic potential of human exogenous MSCs and NSPCs is not well known. In this study, we found that human noncancerous CSF increased the proliferation, decreased apoptosis, and stimulated the migration speed and distance of human exogenous hAMSCs and hfNPCs. Therefore, pretreating these stem cells with CSF may augment their ability to not only home to sites of pathologies but also increase their therapeutic effectiveness, which is currently a limitation of stem cell therapy for neurological diseases.
IGF-1 is a neurotrophic factor found in human CSF that has been shown to play an important role in cellular metabolism during embryonic and postnatal development [18,34]. IGF-1 has also been shown to promote proliferation and inhibit apoptosis of neuronal precursors [19,49]. As previously published, IGF-1 can mediate its effects through the IGF-1 receptor (IGF-1R), which possesses intrinsic tyrosine kinase activity and activates a number of downstream mediators, including PI3K and RAS-ERK [50–52]. Both pathways converge on downstream molecules, such as FOXO proteins, which are important for cell survival [53,54]. Maucksch et al. and Ponte et al. found that IGF-1 can stimulate the migration capacity of NPCs of adult rat and bone marrow MSCs of human commercial cell lines [20,55]. Additionally, IGF-1 has been shown to accelerate cellular mobilization in heart cells via paracrine activation of CXCR4 [56]. In this study, human primary NPCs and MSCs were grown in the presence of IGF-1 in human noncancerous CSF. Interestingly, we found that the IGF-1 in noncancerous human CSF plays an important role in the viability and mobility of MSCs and NPCs. Moreover, noncancerous human CSF also may affect the expression of CXCR4 in both cell types. When the IGF-1 inhibitor was added to the human CSF media, both stem cell types exhibited a decrease in CXCR4 expression.
In this study, we evaluated the effects of IGF-1 in human CSF; the effects of other trophic factors and proteins in human CSF on hAMSCs and hfNPCs remain to be explored. Other factors including BMPs, TGF-β, and WNTs may also have effects on hAMSCs and hfNPCs. Buddensiek et al. proved that human leptomeningeal CSF promotes survival, but inhibits proliferation of adult human NPCs [17]. This conclusion seems to partially conflict with ours. However, a part of our findings share a commonality. In our experiments, we also found the hfNPCs that were cultured in 100% CSF had a lower proliferation capacity compared with the 100% NPCs complete media group, while the NPCs cultured in 25% CSF+75% NPC complete media displayed the most significant effects on proliferation. These results are probably due to the high concentration of hEGF and hFGF in the commercial complete media. With an increase in the amount and types of growth factors in the culture media, the positive effects on these stem cells were more apparent. Moreover, even though these MSCs and NPCs were pretreated in pure H-CSF (100% CSF), they still exhibited the greatest number of migrated cells. Therefore, this suggests that the proliferation capacity is not significantly influencing the migration results.
More specifically, we found that human CSF could regulate the characteristics of hAMSCs and hfNPCs. Furthermore, our study provides an alternative protocol for stem cell culturing; these findings may provide the preliminary data for studying the effects of the human CNS microenvironment on exogenous MSCs and NPCs and how it influences behaviors such as proliferation and migration. Our findings are not meant to explain what would happen to them in the nervous system, but rather how we can pretreat them outside of the human body to increase their therapeutic capabilities when placed back in the body and understand the effects that CSF may have on these cells. In addition, we performed these experiments using noncancerous human CSF. The concentrations and presence of trophic factors may differ by disease, therefore, the effects of CSF from disease subtypes remain to be explored [15,16].
French-Constant and colleagues established protocols to quantify the radial and chain migration of neural stem cells [57]. In this study, we used nanopattern and transwell assays to test the migration capacity in vitro. However, it is not clear whether the nanopatterned migration resemble the observed migration in the rostral migratory stream as radial migration. In addition, alternative methods such activated caspase-3 can be used in the future to confirm the apoptosis results. Our experiments are performed using primary cell lines and are therefore not applicable to commercial lines, which are known to be genetically and phenotypically different than primary cell lines. Moreover, our present investigation is a proof of principle to see the effects of noncancerous CSF on human primary NPCs and MSCs in vitro; and the lack of an animal model with human CSF makes it difficult to strictly assess the effects of human noncancerous CSF in in vivo experiments. The limitation of this study is that this study lacks an in-depth analysis of the effects of CSF at the molecular level, there were no in vivo experiments performed, and the safety of this treatment modality was not explored in this current study.
In conclusion, we show that human CSF can promote proliferation, viability, and migration speed and distance of human adipose-derived stem cells and human NPCs; and this effect is in part attributable to the presence of IGF-1 in CSF. Furthermore, our study establishes an alternative stem cell culturing strategy and details our in vitro findings of the effects of noncancerous human CSF on exogenous MSCs and NPCs. In addition, this investigation provides further evidence in an in vitro setting of the interaction between human CSF and human stem cells that may one day be used for personalized medicine.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health, RO1, NS070024 (A.Q.H.) and the Maryland Stem Cell Research Fund (A.Q.H. and H.G.C.). This work was supported in part by the scholarship from China Scholarship Council (CSC) under the grant CSC no. 201206160079. We thank Dr. Ulf Kahlert and Dr. Qian Li for their technical support, and Dr. Samuel T. Rodriguez and Dr. Onur Kilic for their illustrations.
Author Disclosure Statement
The authors declare no conflicts of interest and no financial disclosures.
References
- 1.Titomanlio L, Kavelaars A, Dalous J, Mani S, El Ghouzzi V, Heijnen C, Baud O. and Gressens P. (2011). Stem cell therapy for neonatal brain injury: perspectives and challenges. Ann Neurol 70:698–712 [DOI] [PubMed] [Google Scholar]
- 2.Aboody KS, Najbauer J. and Danks MK. (2008). Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther 15:739–752 [DOI] [PubMed] [Google Scholar]
- 3.Trounson A, Thakar RG, Lomax G. and Gibbons D. (2011). Clinical trials for stem cell therapies. BMC Med 9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karp JM. and Leng Teo GS. (2009). Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 4:206–216 [DOI] [PubMed] [Google Scholar]
- 5.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, et al. (2005). Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res 65:3307–3318 [DOI] [PubMed] [Google Scholar]
- 6.Bachoud-Levi AC, Gaura V, Brugieres P, Lefaucheur JP, Boisse MF, Maison P, Baudic S, Ribeiro MJ, Bourdet C, et al. (2006). Effect of fetal neural transplants in patients with Huntington's disease 6 years after surgery: a long-term follow-up study. Lancet Neurol 5:303–309 [DOI] [PubMed] [Google Scholar]
- 7.Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D, Galli R, Selleri S, Di MF, et al. (2000). Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med 6:447–450 [DOI] [PubMed] [Google Scholar]
- 8.Lee DH, Ahn Y, Kim SU, Wang KC, Cho BK, Phi JH, Park IH, Black PM, Carroll RS, Lee J. and Kim SK. (2009). Targeting rat brainstem glioma using human neural stem cells and human mesenchymal stem cells. Clin Cancer Res 15:4925–4934 [DOI] [PubMed] [Google Scholar]
- 9.Lee C. and Agoston DV. (2010). Vascular endothelial growth factor is involved in mediating increased de novo hippocampal neurogenesis in response to traumatic brain injury. J Neurotrauma 27:541–553 [DOI] [PubMed] [Google Scholar]
- 10.Guan J, Zhu Z, Zhao RC, Xiao Z, Wu C, Han Q, Chen L, Tong W, Zhang J, et al. (2013). Transplantation of human mesenchymal stem cells loaded on collagen scaffolds for the treatment of traumatic brain injury in rats. Biomaterials 34:5937–5946 [DOI] [PubMed] [Google Scholar]
- 11.van Velthoven CT, Sheldon RA, Kavelaars A, Derugin N, Vexler ZS, Willemen HL, Maas M, Heijnen CJ. and Ferriero DM. (2013). Mesenchymal stem cell transplantation attenuates brain injury after neonatal stroke. Stroke 44:1426–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zappaterra MW. and Lehtinen MK. (2012). The cerebrospinal fluid: regulator of neurogenesis, behavior, and beyond. Cell Mol Life Sci 69:2863–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johanson CE, Duncan JA, Stopa EG. and Baird A. (2005). Enhanced prospects for drug delivery and brain targeting by the choroid plexus-CSF route. Pharm Res 22:1011–1037 [DOI] [PubMed] [Google Scholar]
- 14.Lehtinen MK. and Walsh CA. (2011). Neurogenesis at the brain-cerebrospinal fluid interface. Annu Rev Cell Dev Biol 27:653–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M, Maynard T, Gonzalez D, Kim S, et al. (2011). The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 69:893–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cristofanilli M, Cymring B, Lu A, Rosenthal H. and Sadiq SA. (2013). Cerebrospinal fluid derived from progressive multiple sclerosis patients promotes neuronal and oligodendroglial differentiation of human neural precursor cells in vitro. Neuroscience 250:614–621 [DOI] [PubMed] [Google Scholar]
- 17.Buddensiek J, Dressel A, Kowalski M, Runge U, Schroeder H, Hermann A, Kirsch M, Storch A. and Sabolek M. (2010). Cerebrospinal fluid promotes survival and astroglial differentiation of adult human neural progenitor cells but inhibits proliferation and neuronal differentiation. BMC Neurosci 11:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benarroch EE. (2012). Insulin-like growth factors in the brain and their potential clinical implications. Neurology 79:2148–2153 [DOI] [PubMed] [Google Scholar]
- 19.Liu W, Ye P, O'Kusky JR. and D'Ercole AJ. (2009). Type 1 insulin-like growth factor receptor signaling is essential for the development of the hippocampal formation and dentate gyrus. J Neurosci Res 87:2821–2832 [DOI] [PubMed] [Google Scholar]
- 20.Ponte AL, Marais E, Gallay N, Langonne A, Delorme B, Herault O, Charbord P. and Domenech J. (2007). The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells 25:1737–1745 [DOI] [PubMed] [Google Scholar]
- 21.Pendleton C, Li Q, Chesler DA, Yuan K, Guerrero-Cazares H. and Quinones-Hinojosa A. (2013). Mesenchymal stem cells derived from adipose tissue vs bone marrow: in vitro comparison of their tropism towards gliomas. PLoS One 8:e58198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaichana KL, Guerrero-Cazares H, Capilla-Gonzalez V, Zamora-Berridi G, Achanta P, Gonzalez-Perez O, Jallo GI, Garcia-Verdugo JM. and Quinones-Hinojosa A. (2009). Intra-operatively obtained human tissue: protocols and techniques for the study of neural stem cells. J Neurosci Methods 180:116–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guerrero-Cazares H, Chaichana KL. and Quinones-Hinojosa A. (2009). Neurosphere culture and human organotypic model to evaluate brain tumor stem cells. Methods Mol Biol 568:73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tzeng SY, Guerrero-Cazares H, Martinez EE, Sunshine JC, Quinones-Hinojosa A. and Green JJ. (2011). Non-viral gene delivery nanoparticles based on poly(beta-amino esters) for treatment of glioblastoma. Biomaterials 32:5402–5410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verkuyl JM. and Matus A. (2006). Time-lapse imaging of dendritic spines in vitro. Nat Protoc 1:2399–2405 [DOI] [PubMed] [Google Scholar]
- 26.Kiiski H, Aanismaa R, Tenhunen J, Hagman S, Yla-Outinen L, Aho A, Yli-Hankala A, Bendel S, Skottman H. and Narkilahti S. (2013). Healthy human CSF promotes glial differentiation of hESC-derived neural cells while retaining spontaneous activity in existing neuronal networks. Biol Open 2:605–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, et al. (2004). Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A 101:18117–18122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahn J, Byk T, Jansson-Sjostrand L, Petit I, Shivtiel S, Nagler A, Hardan I, Deutsch V, Gazit Z, et al. (2004). Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood 103:2942–2949 [DOI] [PubMed] [Google Scholar]
- 29.Kim DH, Provenzano PP, Smith CL. and Levchenko A. (2012). Matrix nanotopography as a regulator of cell function. J Cell Biol 197:351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garzon-Muvdi T, Schiapparelli P, ap Rhys C, Guerrero-Cazares H, Smith C, Kim DH, Kone L, Farber H, Lee DY, et al. (2012). Regulation of brain tumor dispersal by NKCC1 through a novel role in focal adhesion regulation. PLoS Biol 10:e1001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehtinen MK, Bjornsson CS, Dymecki SM, Gilbertson RJ, Holtzman DM. and Monuki ES. (2013). The choroid plexus and cerebrospinal fluid: emerging roles in development, disease, and therapy. J Neurosci 33:17553–17559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angot E, Loulier K, Nguyen-Ba-Charvet KT, Gadeau AP, Ruat M. and Traiffort E. (2008). Chemoattractive activity of sonic hedgehog in the adult subventricular zone modulates the number of neural precursors reaching the olfactory bulb. Stem Cells 26:2311–2320 [DOI] [PubMed] [Google Scholar]
- 33.Castells A, Parvas M. and Bueno D. (2012). Homeostasis of cerebrospinal fluid has a role in early brain development. Neuroreport 23:917–921 [DOI] [PubMed] [Google Scholar]
- 34.Bunn RC, King WD, Winkler MK. and Fowlkes JL. (2005). Early developmental changes in IGF-I, IGF-II, IGF binding protein-1, and IGF binding protein-3 concentration in the cerebrospinal fluid of children. Pediatr Res 58:89–93 [DOI] [PubMed] [Google Scholar]
- 35.Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, et al. (2000). Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A 97:12846–12851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim SK, Kim SU, Park IH, Bang JH, Aboody KS, Wang KC, Cho BK, Kim M, Menon LG, Black PM. and Carroll RS. (2006). Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin Cancer Res 12:5550–5556 [DOI] [PubMed] [Google Scholar]
- 37.Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M, Waldman D, et al. (2009). Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med 6:e1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aboody KS, Najbauer J, Metz MZ, D'Apuzzo M, Gutova M, Annala AJ, Synold TW, Couture LA, Blanchard S, et al. (2013). Neural stem cell-mediated enzyme/prodrug therapy for glioma: preclinical studies. Sci Transl Med 5:184ra59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Q, Wijesekera O, Salas SJ, Wang JY, Zhu M, Aprhys C, Chaichana KL, Chesler DA, Zhang H, et al. (2014). Mesenchymal stem cells from human fat engineered to secrete BMP4 are nononcogenic, suppress brain cancer, and prolong survival. Clin Cancer Res 20:2375–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu M, Shu K, Wang H, Li X, Xiao Q, Chan W, Emmanuel B, Jiang W. and Lei T. (2013). Microtransplantation of whole ganglionic eminence cells ameliorates motor deficit, enlarges the volume of grafts, and prolongs survival in a rat model of Huntington's disease. J Neurosci Res 91:1563–1571 [DOI] [PubMed] [Google Scholar]
- 41.Ma L, Hu B, Liu Y, Vermilyea SC, Liu H, Gao L, Sun Y, Zhang X. and Zhang SC. (2012). Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell Stem Cell 10:455–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang B, Xi X, Aronowski J. and Savitz SI. (2012). Ischemic stroke may activate bone marrow mononuclear cells to enhance recovery after stroke. Stem Cells Dev 21:3332–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chavakis E, Urbich C. and Dimmeler S. (2008). Homing and engraftment of progenitor cells: a prerequisite for cell therapy. J Mol Cell Cardiol 45:514–522 [DOI] [PubMed] [Google Scholar]
- 44.Zhang D, Fan GC, Zhou X, Zhao T, Pasha Z, Xu M, Zhu Y, Ashraf M. and Wang Y. (2008). Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol 44:281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen W, Li M, Cheng H, Yan Z, Cao J, Pan B, Sang W, Wu Q, Zeng L, Li Z. and Xu K. (2013). Overexpression of the mesenchymal stem cell Cxcr4 gene in irradiated mice increases the homing capacity of these cells. Cell Biochem Biophys 67:1181–1191 [DOI] [PubMed] [Google Scholar]
- 46.Kim DS, Kim JH, Lee JK, Choi SJ, Kim JS, Jeun SS, Oh W, Yang YS. and Chang JW. (2009). Overexpression of CXC chemokine receptors is required for the superior glioma-tracking property of umbilical cord blood-derived mesenchymal stem cells. Stem Cells Dev 18:511–519 [DOI] [PubMed] [Google Scholar]
- 47.Daley GQ. and Scadden DT. (2008). Prospects for stem cell-based therapy. Cell 132:544–548 [DOI] [PubMed] [Google Scholar]
- 48.Daley GQ. (2012). The promise and perils of stem cell therapeutics. Cell Stem Cell 10:740–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu JP, Baker J, Perkins AS, Robertson EJ. and Efstratiadis A. (1993). Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75:59–72 [PubMed] [Google Scholar]
- 50.Joseph DA. and Ye P. (2008). Expanding the mind: insulin-like growth factor I and brain development. Endocrinology 149:5958–5962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bibollet-Bahena O. and Almazan G. (2009). IGF-1-stimulated protein synthesis in oligodendrocyte progenitors requires PI3K/mTOR/Akt and MEK/ERK pathways. J Neurochem 109:1440–1451 [DOI] [PubMed] [Google Scholar]
- 52.Hardy KM, Yatskievych TA, Konieczka J, Bobbs AS. and Antin PB. (2011). FGF signalling through RAS/MAPK and PI3K pathways regulates cell movement and gene expression in the chicken primitive streak without affecting E-cadherin expression. BMC Dev Biol 11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rafalski VA. and Brunet A. (2011). Energy metabolism in adult neural stem cell fate. Prog Neurobiol 93:182–203 [DOI] [PubMed] [Google Scholar]
- 54.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, et al. (2007). FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128:325–339 [DOI] [PubMed] [Google Scholar]
- 55.Maucksch C, McGregor AL, Yang M, Gordon RJ, Yang M. and Connor B. (2013). IGF-I redirects doublecortin-positive cell migration in the normal adult rat brain. Neuroscience 241:106–115 [DOI] [PubMed] [Google Scholar]
- 56.Haider HK, Jiang S, Idris NM. and Ashraf M. (2008). IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res 103:1300–1308 [DOI] [PubMed] [Google Scholar]
- 57.Loulier K, Lathia JD, Marthiens V, Relucio J, Mughal MR, Tang SC, Coksaygan T, Hall PE, Chigurupati S, et al. (2009). Beta1 integrin maintains integrity of the embryonic neocortical stem cell niche. PLoS Biol 7:e1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.