Abstract
Modification of cerebral perfusion pressure and cerebral blood flow (CBF) are crucial components of the therapies designed to reduce secondary damage after traumatic brain injury (TBI). Previously we documented a robust decrease in CBF after rapid sagittal head rotation in our well-validated animal model of diffuse TBI. Mechanisms responsible for this immediate (<10 min) and sustained (∼24 h) reduction in CBF have not been explored. Because the carotid arteries are a major source of CBF, we hypothesized that blood flow through the carotid arteries (Q) and vessel diameter (D) would decrease after rapid nonimpact head rotation without cervical spine injury. Four-week-old (toddler) female piglets underwent rapid (<20 msec) sagittal head rotation without impact, previously shown to produce diffuse TBI with reductions in CBF. Ultrasonographic images of the bilateral carotid arteries were recorded at baseline (pre-injury), as well as immediately after head rotation and 15, 30, 45, and 60 min after injury. Diameter (D) and waveform velocity (V) were used to calculate blood flow (Q) through the carotid arteries using the equation Q=(0.25)πD2V. D, V, and Q were normalized to the pre-injury baseline values to obtain a relative change after injury in right and left carotid arteries. Three-way analysis of variance and post-hoc Tukey-Kramer analyses were used to assess statistical significance of injury, time, and side. The relative change in carotid artery diameter and flow was significantly decreased in injured animals in comparison with uninjured sham controls (p<0.0001 and p=0.0093, respectively) and did not vary with side (p>0.39). The average carotid blood velocity did not differ between sham and injured animals (p=0.91). These data suggest that a reduction in global CBF after rapid sagittal head rotation may be partially mediated by a reduction in carotid artery flow, via vasoconstriction.
Key words: : brain, carotid, flow, traumatic
Introduction
Traumatic brain injury (TBI) is a leading cause of death and disability in the pediatric population in the United States, with nonfatal injuries resulting in more than 173,000 emergency department visits annually between the years 2001 to 2009.1 Etiology, severity, time to presentation, and underlying neurodevelopmental baseline are widely variable, leading to increased complexity in the care of children after TBI. In spite of significant research efforts to better understand the physiological and pathophysiological processes occurring after TBI,2–6 further work is necessary to improve our understanding of TBI in children.
Maintenance of cerebral blood flow (CBF) has been demonstrated to be an important component of neurocognitive outcome in TBI7,8 and is either measured directly or calculated by dividing cerebral perfusion pressure (CPP) by vascular resistance (R) using Ohm's law. Left untreated, CPP decreases after severe TBI via an increase in intracranial pressure and a decrease in mean arterial pressure.9–16 Inadequate CPP has been demonstrated as a reasonable indicator of poor long-term outcome,17–19 and clinical management that focuses on raising CPP has been shown to improve neurocognitive outcomes. For these reasons, a critical component of our current clinical guidelines for severe TBI is maintenance of age-appropriate CPP.20,21
Less well-studied is the role of vascular resistance on CBF after TBI, and most studies analyzing changes to vascular resistance as a component of changing CBF have focused on the intracranial vasculature.22–24 Middle cerebral artery Doppler studies, for example, have demonstrated that impaired cerebral autoregulation is associated with poor neurologic outcome after TBI.22 Other studies using transcranial Doppler have demonstrated vasospasm after moderate to severe TBI in the pediatric population.25 Vascular resistance, however, is inversely proportional to the vessel radius raised to the fourth power, and intracranial vessel diameter is difficult to standardize using current transcranial Doppler techniques.26 In the absence of independent assessment of middle cerebral artery flow, it is unclear whether changes to the middle cerebral artery result in changes in blood flow. It is also unclear whether these changes are representative of global changes throughout the intracranial vasculature.
If a global decrease in CBF after TBI were partially mediated by an alteration in vascular resistance, changes in flow through the major extracranial vessels could be another mechanism mediating this process. CBF is supplied by the bilateral carotid arteries and the bilateral vertebral arteries. Neck position alters blood flow parameters through the neck vasculature,27,28 and there is evidence to suggest that processes as simple as altering neck position could also affect CBF in certain circumstances.29,30 In premature infants without intracranial pathology, for example, CBF velocity was significantly higher in the supine position compared with the prone position.29 In addition, there is evidence in adults to suggest that rapid head rotation may alter flow through the neck vasculature: during the first month after isolated whiplash injury, blood flow velocities through the vertebral arteries were significantly reduced.31
Although the carotid and vertebral arteries supply the entire cranium, no studies to date have analyzed flow through the neck vasculature after TBI. Based on our previous studies that show CBF decreases after rapid sagittal head rotation, we hypothesized that blood flow through the carotid arteries (Q) would decrease, primarily because of a decrease in carotid artery diameter (D). To evaluate this hypothesis, we used our piglet model for pediatric diffuse TBI caused by a rapid (10–20 msec) head rotation without impact or cervical spine injury,32–40 which is associated with global reductions in CBF, as well as intracranial hypertension, elevated lactate/pyruvate ratios, and brain tissue pathology.32
Methods
Piglets
All protocols were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Four-week-old female piglets (n=20) were housed in groups and were maintained in a 12:12 h light:dark cycle before and concurrent with testing. They were allowed food and water ad libitum until 2 h before the experiment, at which point they were only allowed access to water.
Anesthesia and euthanasia
At the beginning of the experiment, animals were induced with an intramuscular injection of ketamine (20 mg/kg) and xylazine (2 mg/kg), followed by 4% isoflurane inhaled via snout mask until deep anesthesia was achieved. They were intubated and mechanically ventilated. Animals received femoral arterial and venous catheters using standard cutdown technique. After catheter placement, they received a physiologic saline bolus of 20 mL/kg, which could be repeated up to two times during the baseline period (before injury) as needed to maintain normotension. Bolus doses of fentanyl and midazolam (50 μg/kg and 1 mg/kg intravenously [IV], respectively) were given, followed by cessation of isoflurane and transition to total IV anesthetic with a combination of infusions, including fentanyl (100 μg/kg/h), midazolam (1 mg/kg/h), and dexmedetomidine (0.2 μg/kg/h). For ongoing hydration, animals received a physiologic saline infusion (4 mL/kg/h). After completion of the experiment, animals were euthanized with phenobarbital 150 mg/kg IV bolus.
Maintenance and monitoring
After intubation, mechanical ventilation parameters were a tidal volume of 10 mL/kg, a positive end-expiratory pressure (PEEP) of 5, a respiratory rate of 35, and an FiO2 of 28–30%. Animals were connected to standard cardiorespiratory monitors (Surgivet V9204, Smiths Medical, Dublin, OH), and a rectal probe was placed for continuous temperature monitoring (Gaymar Industries, Inc, Stryker, Kalamazoo, MI). Vital signs, including heart rate (HR), mean arterial blood pressure (MAP), respiratory rate (RR), end tidal CO2 (ETCO2), pulse oximetry (SpO2), and temperature were monitored continuously and recorded at least every 15 min throughout the experiment. Response to a reflexive toe pinch was also assessed and recorded every 15 min to monitor anesthetic depth. An arterial blood gas (ABG) was collected every 30 min. PEEP and FiO2 were not adjusted during the experiment. Minute ventilation was occasionally adjusted to maintain a PaCO2 of ∼40–45 mm Hg. Temperature was maintained from 37–38°C using a warming pad.
Experimental groups and injury
Piglets were designated as injured (n=10) or shams (n=10). Sham animals were anesthetized and instrumented with the previously described protocol for the length of the experiment. For injury, piglet heads were rotated rapidly 60 degrees in the sagittal plane without impact using a pneumatic device previously described,33–35 which results in higher levels of intracranial pressure and unconscious time than other planes of head rotation.38 Velocity, measured using an angular rate sensor (ATA, Albuquerque, NM) attached to the side arm of the pneumatic device, averaged 149.3±3.1 rad/sec. Subsequently, all animals were positioned prone for the remainder of the experiment.
Doppler ultrasonography
Ultrasonographic assessments were taken at time points held consistent between animals: immediately before injury (baseline), immediately after injury (denoted as time 0 in graphs but was typically 1–3 min post-injury), and at post-injury minutes 15, 30, 45, and 60. All measurements were taken by the same person to provide consistent technique, and the left side was always imaged before the right to minimize small time-related differences. Using a Sequoia Acuson C256 (Siemans Medical Solutions USA, Inc, Malvern, PA) with a 15L8 linear transducer, ultrasound gel, and a consistent technique for localization, the bilateral common carotid arteries were identified in the longitudinal plane using grayscale scanning. This resulted in the end of the probe approximately 1 inch from the humeral head. Positioning was further confirmed with color Doppler to ensure imaging of arterial rather than venous flow, and imaging parameters were then manually optimized (color gain, pulse repetition frequency (PRF)).
Following consistent identification of the left or right common carotid artery, five grayscale images were recorded digitally for repeated diameter measurements. Spectral Doppler was then used to digitally record three different 5-sec intervals of waveforms for repeated velocity measurements. Imaging of the left and right sides took approximately 3 min total to complete for each time point.
Image processing
All ultrasonography images were processed using Showcase Clinical Case Presentation Tools (version 5.3.0.0, Trillium Technologies, Inc, Ann Arbor, MI), a medical image analysis software program frequently used for ultrasonography analyses. From each of the five grayscale diameter images recorded, a typical diameter was obtained along the length of the vessel. The five diameter measurements from that time point from that vessel were then averaged into a single diameter measurement for a given side and time point.
From the three waveform images, a total of five waveforms for each side for each time point were analyzed. This was completed using the first full waveform of each image and the third full waveform from the first and second recorded image. Waveforms were manually traced in Showcase, which then computed output waveform data. These data included the velocity time integral (VTI, the area underneath the entire waveform curve) and the peak systolic velocity (Vmax). The time over which each waveform occurred (dT) was also measured, and the average velocity for the waveform was calculated by dividing the VTI by the dT. This was completed for each of the five velocity waveforms for that time point and vessel and averaged to obtain a single average velocity measurement for a given side and time point.
Common carotid artery flow was calculated using the equation Flow=(0.25)πD2V, using the average diameter and average velocity for a given side and time point.
Data analysis
Primary outcomes were measured carotid artery diameter and velocity, and calculated flow. Unless otherwise specified in the results section, all post-injury measurements were normalized to baseline values. Three-way analyses of variance (ANOVA) were completed for group, time, and side. Post-hoc Tukey-Kramer analyses were used to confirm statistical differences. All statistical analyses were completed using JMP (version 10.0.0, SAS Institute, Inc., Cary, NC).
Results
Baseline parameters were equivalent between injured and sham groups
To evaluate for inherent differences between groups of piglets, baseline data were compared and are shown in Table 1. There was no statistically significant difference between groups for HR, MAP, temperature, and ETCO2 (p=0.94, 0.21, 0.9, and 0.16, respectively). There was a statistically higher oxygen saturation in the sham animals in comparison with the injured animals (98.2%±0.3% vs. 95.6%±0.4%, p<0.0001), but this was not thought to be clinically significant. In evaluation of baseline ultrasonography parameters, there was no difference between sham and injured animals for diameter, velocity, or flow (p=0.99, 0.46, and 0.4, respectively).
Table 1.
Baseline data
| Group | Sham | Injured | p value |
|---|---|---|---|
| HR | 107±10 | 108±10 | 0.94 |
| MAP | 72±3 | 66±3 | 0.21 |
| SpO2 | 98.2±0.3 | 95.6±0.4 | <0.0001* |
| Temp | 36.2±0.4 | 36.3±0.4 | 0.9 |
| ETCO2 | 45±2 | 49±2 | 0.16 |
| Diameter | 0.32±0.01 | 0.32±0.01 | 0.99 |
| Velocity | 10.2±0.8 | 11.0±0.8 | 0.46 |
| Flow | 0.78±0.1 | 0.87±0.1 | 0.4 |
HR, heart rate; MAP, mean arterial blood pressure; SpO2, pulse oximetry; ETCO2, end tidal CO2.
Comparison of baseline parameters between sham and injured animals, Student t test. Values are mean±standard error. Asterisk indicates statistical significance with a p value of ≤0.05.
Differences between sides
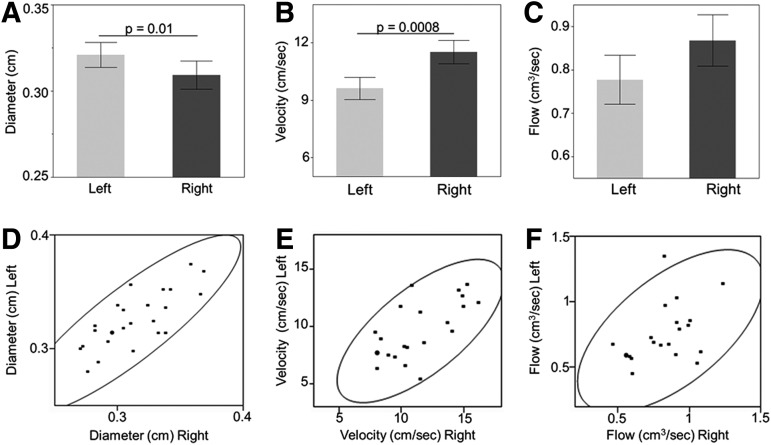
We analyzed flow parameters in the carotid arteries during the baseline period to assess asymmetry between the left and right sides. These analyses were completed using two-tailed t tests, and the results are demonstrated in Figure 1. By comparing the diameter between the left and right carotid arteries at baseline for all animals, we found that the average diameter on the left (0.32 cm±0.003) was greater than the average diameter on the right (0.31 cm±0.003) (Fig. 1A, p=0.01). Average velocity, in contrast, was faster on the right than on the left (11.5 cm/sec vs. 9.6 cm/sec±0.6, respectively, Fig. 1B, p=0.0008). When these values were combined to calculate flow, we found no asymmetry between the left and the right sides, with an average flow of 0.78 cm3/sec±0.06 on the left and 0.87 cm3/sec±0.06 on the right, p=0.1 (Fig. 1C). Each parameter was highly correlated between the right and left sides (Fig. 1D–F), such that when one side had a larger than average velocity, the other side did also.
FIG. 1.
Baseline diameter, velocity, and flow differences between sides. Comparison of carotid artery diameter, velocity, and flow between the left and the right sides during pre-injury baseline. Bars in A-C indicate average values, with error bars denoting standard error. Horizontal line indicates significance with a p value of ≤0.05. (A) Diameter (cm). (B) Velocity (cm/sec). (C) Flow (cm3/sec). (D–F) demonstrate correlation between the left and right sides for diameter (D, correlation coefficient 0.81), velocity (E, correlation coefficient 0.95), and flow (F, correlation coefficient 0.88). The black circle is a density ellipse indicating correlation with a 95% confidence interval.
Carotid flow and geometry after injury
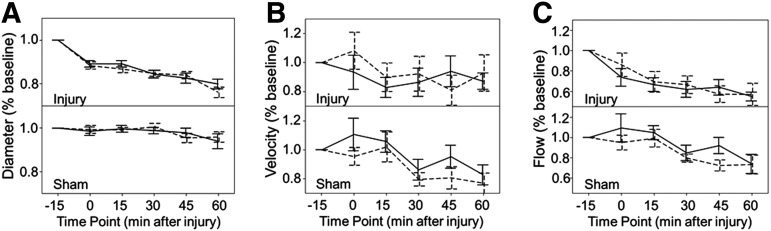
All subsequent time points were normalized by baseline values for that animal and side. A three-way ANOVA for group, time, and side after injury revealed that normalized Dave, Vave, and Qave at each time point were independent of side (p=0.64, 0.51, and 0.39, Fig, 2A–C, respectively). Consequently, the right and left normalized values were averaged together for a single value of Dave and Vave for each animal at a given time point. Dave and Vave were then used to calculate the average flow for that animal at that time point.
FIG. 2.
Normalized diameter, velocity, and flow differences between sides. Comparison of carotid artery diameter, velocity, and flow between the left and the right sides over time after normalization to baseline values. Values at time 0, 15, 30, 45, and 60 min were normalized to the −15 baseline value. Left side shown by solid line, right side shown by dashed line. For each parameter: diameter (A, p=0.64), velocity (B, p=0.51), and flow (C, p=0.39), values for injured animals are shown above values for sham animals. Error bars indicate standard error of the mean.
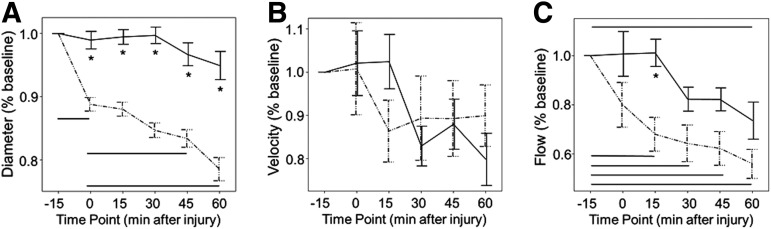
Two-way ANOVAs followed by post-hoc Tukey-Kramer analyses showed that carotid artery diameter decreased after injury in comparison with sham animals (p<0.0001) and did so at all time points (Fig. 3A). Within the first 3 min after injury, diameter had decreased to 89%±1% of baseline values versus 99%±1% in sham animals (p<0.05). Diameter further decreased over time after injury, reaching 79%±1% at 60 min (p<0.05). Sham animals had no significant difference in diameter at any time point evaluated. The difference in diameter between sham and injured animals was significant at every time point (p<0.05).
FIG. 3.
Normalized diameter, velocity, and flow differences between groups. Carotid artery diameter (A), velocity (B), and flow (C) over time. Values at time 0, 15, 30, 45, and 60 min were normalized to the −15 baseline value. Injured animals are shown by dashed line, sham animals by solid line. Asterisks indicate statistical significance between groups with a p value of≤0.05. Horizontal lines indicate statistical significance between time points for each group with a p value≤0.05. Error bars indicate standard error of the mean.
Despite significant reductions in vessel diameter, there was no compensatory increase in blood velocity (Fig. 3B). In comparison with sham animals, there was no change in velocity in injured animals (average velocity=92% baseline±5 in injured vs. 93% baseline±5 in shams, p=0.91). Although the two-way ANOVA identified a small overall effect of time on velocity (p=0.01), post-hoc Tukey-Kramer analysis by group did not reveal statistically significant changes over time in either sham or injured animals. At the 60 min time point, for example, the average velocity in injured animals was 92%±7% of baseline.
The comparison of flow between injured and sham animals is shown in Figure 3C. Flow through the carotid artery decreased significantly in the injured animals in comparison with the anesthetized sham controls (average flow=72% baseline±4% in the injured vs. 89%±4% in the shams, p=0.0093). Both groups demonstrated decreased flow over time, with a p value of<0.0001. The group-time interaction was not significant (p=0.08). Evaluating the differences more closely using Tukey-Kramer post-hoc analyses, flow decreased significantly from baseline by 15 min after injury, with 68%±6% of baseline (p<0.05), and continued to trend down over time. Sham animals, while they did demonstrate a mild decreased flow over time, did not reach significance until the 60 min time point, with an average of 73%±6% of baseline flow at that time (p<0.05).
General monitoring parameters post-injury were similar between groups
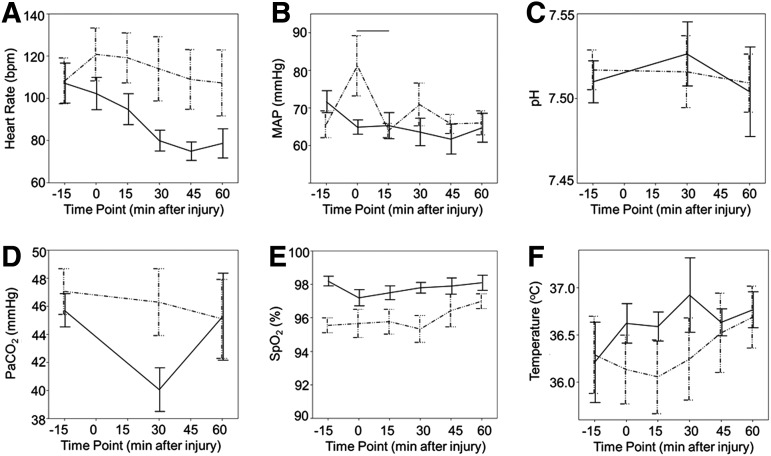
Because the decrease in carotid artery blood flow after injury is mediated by a decrease in diameter, we analyzed several putative etiologies as to why this might occur. Two-way ANOVAs for group and time point were completed to assess for general systemic differences between groups. Evaluation of average HR, pH, paCO2, and temperature revealed no significant differences between sham and injured animals (Fig. 4A, C, D, F, respectively). Evaluation of MAP revealed that in the injury group, MAP was higher in the immediate post-injury period (time 0) than 15 min after injury (Fig. 4B), with average values of 81±4 at time point 0 and 64±4 at time point 15. This was thought to be because of a catecholamine surge immediately after injury, which subsequently resolved. Across all time points, SpO2 continued to differ between the groups, with a mean sham SpO2 of 98%±0.4 and a mean injury SpO2 of 96%±0.4 (Fig. 4E, p=0.005). As with the baseline SpO2 values, this was not thought to be clinically significant. The pO2 did not differ between sham and injured animals.
FIG. 4.
Vital sign differences between groups. Comparison of mean vital signs and arterial blood gases between groups and across time. (A) Heart rate. (B) MAP, mean arterial blood pressure. (C) pH. (D) PaCO2, partial pressure of CO2. (E) SpO2, pulse oximetry. (F) Temperature. Injured animals are shown by dashed line, sham animals by solid line. Horizontal lines indicate statistical significance between time points for each group with a p value of ≤0.05. Error bars indicate standard error of the mean.
Discussion
Carotid artery blood flow versus CBF
With an estimated 1.7 million people with TBI each year,41 combined with a wide variety of mechanisms for secondary injury, TBI remains an area in need of increased investigation. Although we know that decreased CBF is an important predictor of poor neurologic outcome after TBI, the exact mechanisms by which global CBF decreases in the TBI patient population remain less clear. In our high-fidelity model of diffuse pediatric TBI, flow through the common carotid arteries decreases after TBI in comparison with sham controls. This decreased flow is immediate, persists during the first hour after injury, and is mediated by decreased diameter. Although further mechanistic investigation remains, this study provides the first evidence that changes to blood flow through the neck could be an important mediator of changes to global CBF.
There are several correlations between decreased blood flow through the carotid arteries and decreased global CBF after TBI. The decrease in carotid artery diameter resulting in decreased carotid artery blood flow occurs within 3 min of injury, the timing of which is consistent with the rapid (<10 min) decrease in global CBF that we have previously reported for this rapid sagittal head rotation model.37 Decreased carotid artery blood flow over the first hour is also associated with the timing seen for decreased brain tissue oxygenation and increased lactate to pyruvate ratio seen in similar injuries in this animal model.32 Using microsphere and diffuse correlation spectroscopy, we have demonstrated an approximately 50% reduction in CBF that occurs within 10 min of injury and approximately 50–60% reduction in CBF by 60 min.37,38 The approximately 30% reduction in carotid artery blood flow by 15 min and approximately 50% reduction by 60 min could be significant mediators of these effects.
Mechanism of decreased carotid blood flow
While the mechanism of decreased carotid artery blood flow will need to be elucidated in future experiments (see below), several important potential confounds were evaluated in the study and were not found to be significant potential etiologies. Although cardiac output was not measured directly, this study used several surrogates of cardiac output (including HR, MAP, and systemic pH), and found no difference between the groups. Nor did the differences measured appear to be related to respiratory factors, because ETCO2/PaCO2 did not differ between the groups and SpO2/PaO2 did not differ in a clinically significant manner between groups. There were no temperature differences between groups to suggest a temperature-mediated vasodilation or vasoconstriction in process. The lack of difference between groups for these parameters suggests a more complex mechanism worthy of further evaluation.
Carotid artery blood flow and anesthetic effect
Interestingly, by the 60 min time point, there was a decrease in carotid artery blood flow seen in sham animals, although this reduction was less than that seen in injured animals. While this was not an anticipated result, it is not entirely surprising. Previous studies have demonstrated differences in CBF and CBF autoregulation based on anesthetic administration,42–45 and previous studies in our laboratory have demonstrated a trend toward decreased CBF in sham animals who were anesthetized for prolonged periods.38 Although anesthetic effects were not tested specifically during this study, the reduction in carotid artery blood flow after 60 min in sham animals was thought likely to be an anesthetic effect. Further work will be needed to evaluate this hypothesis.
Limitations
During ultrasonographic measurements, the ultrasound technician was not masked to group. Blinding was not feasible in this study because of the short interval between the baseline measurement, the injury/sham event, and the first ultrasonographic measurement after the injury/sham event. The investigator completing the calculations of diameter and velocity using the ultrasonography images was blind to group (animals were analyzed in batches and without unique identifiers). It was thought that this would limit investigator bias to the extent possible.
To maintain consistent positioning for repeated ultrasonography measurements, it was not anatomically possible to obtain ultrasonography measurements from both the carotid arteries and the vertebral arteries. Because the carotid arteries supply the majority of the blood flow to the anterior circulation, measurements were therefore limited to the carotid arteries. Future experiments will be necessary to characterize changes to the posterior circulation. Similarly, given the desire for rapid determination of carotid artery blood flow after injury, the common carotid artery was imaged rather than the internal carotid. Decreased blood flow to the face could therefore account for some proportion of the decreased flow seen in the common carotid arteries in this study.
To definitively answer whether decreased cardiac output resulted in decreased carotid artery flow in our study, one would need to measure cardiac output directly. This was not assessed directly in this study to maintain consistent animal positioning. As discussed above, however, surrogates of cardiac output did not differ between the groups.
Future directions
One important goal of future studies is to better elucidate the relationship between blood flow through the neck vasculature and CBF after TBI. In this initial study, we chose to focus on time points immediately after injury. We thought comorbid mechanisms by which CBF is reduced, such as tissue swelling, to be limited at earlier time points. Studying later time points, however, could provide important additional information. For example, CBF remains decreased for at least 24 h after injury in our animal model.37,46 Future analysis of carotid artery flow parameters at these later time points could indicate whether carotid artery blood flow contributes to altered CBF only immediately after injury (e.g., immediate injury-induced vasospasm) or also contributes to sustained alterations in CBF. Also, direct measurement of carotid artery blood flow, vertebral artery blood flow, and CBF in the same animals would elucidate causal relationships and the relative contributions of vertebral and carotid flow to CBF.
Another important goal of future studies is to elucidate the local chemical and physical mechanisms by which carotid artery blood flow decreases after TBI. For example, if the vasospasm is a local stretch-mediated phenomenon, then an asymmetric head rotation (e.g., axial) should produce an asymmetric reduction in carotid artery blood flow, with larger decreases seen on the stretched side than the compressed side. If vasospasm is found to be stretch-mediated, then future studies should investigate local changes in proteins such as endothelin or small molecules such as nitric oxide as potential chemical signaling mechanisms.47–50 If axial rotation reveals bilateral carotid artery vasospasm, in contrast, then systemic chemical signaling pathways should be considered, such as circulating cytokines or general activation of the sympathetic nervous system.51–53
Conclusion
Carotid artery blood flow decreases after sagittal, nonimpact rotational injury without cervical spine injury using our well-validated animal model of pediatric TBI. This decrease is rapid, symmetric, and persistent over at least the first hour after injury. Timing and the degree of change suggest this could be one mechanism by which global CBF reductions occur. This project is a promising first step in better understanding the mechanisms which contribute to global CBF changes, but much more work remains, including direct correlation of carotid artery blood flow to CBF and investigating responses to other head rotation directions.
Acknowledgments
This research was funded by NIH R01NS039679 and the Children's Hospital of Philadelphia Critical Care Endowed Chair Fund. The authors are grateful to Jill Ralston, Sarah Sullivan, Melissa Byro, Ashley Bebee, Lorre Atlan, Stephanie Pasquesi, and Samer Jaber for their time and technical assistance. The authors also thank Joseph Coll from Siemans Medical Solutions USA, Inc (Malvern, PA) and Susan Schultz in the University of Pennsylvania Ultrasound Laboratory for their technical expertise and training for the ultrasound imaging.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Gilchrist J., M., Thomas K.E., Xu L., Lisa C.McGuire L.C., and Victor Coronado V; Centers for Disease Control and Prevention. (2011). Nonfatal traumatic brain injuries related to sports and recreation activities among persons aged ≤19 years–United States, 2001–2009. MMWR Morb. Mortal. Wkly. Rep. 60, 1337–1342 [PubMed] [Google Scholar]
- 2.Esposito E., Cordaro M., and Cuzzocrea S. (2014). Roles of fatty acid ethanolamides (FAE) in traumatic and ischemic brain injury. Pharmacol. Res. 86, 26–31 [DOI] [PubMed] [Google Scholar]
- 3.Walsh J.T., Watson N., and Kipnis J. (2014). T cells in the central nervous system: messengers of destruction or purveyors of protection? Immunology 141, 340–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tewari A., Mahendru V., Sinha A., and Bilotta F. (2014). Antioxidants: The new frontier for translational research in cerebroprotection. J. Anaesthesiol. Clin. Pharmacol. 30, 160–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagberg H., Mallard C., Rousset C. I., and Thornton C. (2014). Mitochondria: hub of injury responses in the developing brain. Lancet Neurol. 13, 217–232 [DOI] [PubMed] [Google Scholar]
- 6.Bartnik-Olson B.L., Harris N.G., Shijo K., and Sutton R.L. (2013). Insights into the metabolic response to traumatic brain injury as revealed by (13)C NMR spectroscopy. Front. Neuroenergetics 5, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adelson P.D., Srinivas R., Chang Y., Bell M., and Kochanek P.M. (2011). Cerebrovascular response in children following severe traumatic brain injury. Childs Nerv. Syst. 27, 1465–1476 [DOI] [PubMed] [Google Scholar]
- 8.Adelson P.D., Clyde B., Kochanek P.M., Wisniewski S.R., Marion D.W., and Yonas H. (1997). Cerebrovascular response in infants and young children following severe traumatic brain injury: A preliminary report. Pediatr. Neurosurg. 26, 200–207 [DOI] [PubMed] [Google Scholar]
- 9.Barzilay Z., Augarten A., Sagy M., Shahar E., Yahav Y., and Boichis H. (1988). Variables affecting outcome from severe brain injury in children. Intensive Care Med. 14, 417–421 [DOI] [PubMed] [Google Scholar]
- 10.Bruce D.A., Raphaely R.C., Goldberg A.I., Zimmerman R.A., Bilaniuk L.T., Schut L., and Kuhl D.E. (1979). Pathophysiology, treatment and outcome following severe head injury in children. Childs Brain 5, 174–191 [DOI] [PubMed] [Google Scholar]
- 11.Cruz J., Nakayama P., Imamura J.H., Rosenfeld K.G., de Souza H.S., and Giorgetti G.V. (2002). Cerebral extraction of oxygen and intracranial hypertension in severe, acute, pediatric brain trauma: Preliminary novel management strategies. Neurosurgery 50, 774–780 [DOI] [PubMed] [Google Scholar]
- 12.Esparza J., M-Portillo J, Sarabia M., Yuste J. A., Roger R., and Lamas E. (1985). Outcome in children with severe head injuries. Childs Nerv. Syst. 1, 109–114 [DOI] [PubMed] [Google Scholar]
- 13.Kasoff S.S., Lansen T.A., Holder D., and Filippo J.S. (1988). Aggressive physiologic monitoring of pediatric head trauma patients with elevated intracranial pressure. Pediatr. Neurosci. 14, 241–249 [DOI] [PubMed] [Google Scholar]
- 14.Pfenninger J., and Santi A. (2002). Severe traumatic brain injury in children—are the results improving? Swiss Med. Wkly 132, 116–120 [DOI] [PubMed] [Google Scholar]
- 15.Shapiro K., and Marmarou A. (1982). Clinical applications of the pressure-volume index in treatment of pediatric head injuries. J. Neurosurg. 56, 819–825 [DOI] [PubMed] [Google Scholar]
- 16.White J.R., Farukhi Z., Bull C., Christensen J., Gordon T., Paidas C., and Nichols D.G. (2001). Predictors of outcome in severely head-injured children. Crit. Care Med. 29, 534–540 [DOI] [PubMed] [Google Scholar]
- 17.Catala-Temprano A., Claret Teruel G., Cambra Lasaosa F. J., Pons Odena M., Noguera Julian A., and Palomeque Rico A. (2007). Intracranial pressure and cerebral perfusion pressure as risk factors in children with traumatic brain injuries. J. Neurosurg. 106, Suppl 6, 463–466 [DOI] [PubMed] [Google Scholar]
- 18.Chambers I.R., Stobbart L., Jones P.A., Kirkham F.J., Marsh M., Mendelow A.D., Minns R.A., Struthers S., and Tasker R.C. (2005). Age-related differences in intracranial pressure and cerebral perfusion pressure in the first 6 hours of monitoring after children's head injury: association with outcome. Childs Nerv. Syst. 21, 195–199 [DOI] [PubMed] [Google Scholar]
- 19.Downard C., Hulka F., Mullins R.J., Piatt J., Chesnut R., Quint P., and Mann N.C. (2000). Relationship of cerebral perfusion pressure and survival in pediatric brain-injured patients. J. Trauma 49, 654–659 [DOI] [PubMed] [Google Scholar]
- 20.Adelson P.D., Bratton S.L., Carney N.A., Chesnut R.M., du Coudray H.E., Goldstein B., Kochanek P.M., Miller H.C., Partington M.D., Selden N.R., Warden C.R., and Wright D.W. (2003). Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 1: Introduction. Pediatr. Crit. Cre Med. 4, Suppl 3, S2–4 [DOI] [PubMed] [Google Scholar]
- 21.Kochanek P.M., Carney N., Adelson P.D., Ashwal S., Bell M.J., Bratton S., Carson S., Chesnut R.M., Ghajar J., Goldstein B., Grant G.A., Kissoon N., Peterson K., Selden N.R., Tasker R.C., Tong K.A., Vavilala M.S., Wainwright M S., and Warden C R. (2012). Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents—second edition. Pediatr. Crit. Care Med. 13, Suppl 1, S1–S82 [DOI] [PubMed] [Google Scholar]
- 22.Chaiwat O., Sharma D., Udomphorn Y., Armstead W.M., and Vavilala M.S. (2009). Cerebral hemodynamic predictors of poor 6-month Glasgow Outcome Score in severe pediatric traumatic brain injury. J. Neurotrauma 26, 657–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang E.W., Czosnyka M., and Mehdorn H.M. (2003). Tissue oxygen reactivity and cerebral autoregulation after severe traumatic brain injury. Crit. Care Med. 31, 267–271 [DOI] [PubMed] [Google Scholar]
- 24.Ter Minassian A., Dube L., Guilleux A.M., Wehrmann N., Ursino M., and Beydon L. (2002). Changes in intracranial pressure and cerebral autoregulation in patients with severe traumatic brain injury. Crit. Care Med. 30, 1616–1622 [DOI] [PubMed] [Google Scholar]
- 25.O'Brien N.F., Reuter-Rice K.E., Khanna S., Peterson B.M., and Quinto K.B. (2010). Vasospasm in children with traumatic brain injury. Intensive Care Med. 36, 680–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moppett I.K., and Mahajan R.P. (2004). Transcranial Doppler ultrasonography in anaesthesia and intensive care. Br. J. Anaesth. 93, 710–724 [DOI] [PubMed] [Google Scholar]
- 27.Aristokleous N., Seimenis I., Papaharilaou Y., Georgiou G.C., Brott B.C., Eracleous E., and Anayiotos A.S. (2011). Effect of posture change on the geometric features of the healthy carotid bifurcation. IEEE Trans. Inf. Technol. Biomed. 15, 148–154 [DOI] [PubMed] [Google Scholar]
- 28.Sakaguchi M., Kitagawa K., Hougaku H., Hashimoto H., Nagai Y., Yamagami H., Ohtsuki T., Oku N., Hashikawa K., Matsushita K., Matsumoto M., and Hori M. (2003). Mechanical compression of the extracranial vertebral artery during neck rotation. Neurology 61, 845–847 [DOI] [PubMed] [Google Scholar]
- 29.Eichler F., Ipsiroglu O., Arif T., Popow C., Heinzl H., Urschitz M., and Pollak A. (2001). Position dependent changes of cerebral blood flow velocities in premature infants. Eur. J. Pediatr. 160, 633–639 [DOI] [PubMed] [Google Scholar]
- 30.Wilson T.D., Serrador J.M., and Shoemaker J.K. (2003). Head position modifies cerebrovascular response to orthostatic stress. Brain Res. 961, 261–268 [DOI] [PubMed] [Google Scholar]
- 31.Seric V., Blazic-Cop N., and Demarin V. (2000). Haemodynamic changes in patients with whiplash injury measured by transcranial Doppler sonography (TCD). Coll. Antropol. 24, 197–204 [PubMed] [Google Scholar]
- 32.Friess S.H., Ralston J., Eucker S.A., Helfaer M.A., Smith C., and Margulies S S. (2011). Neurocritical care monitoring correlates with neuropathology in a swine model of pediatric traumatic brain injury. Neurosurgery 69, 1139–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raghupathi R. and Margulies S.S. (2002). Traumatic axonal injury after closed head injury in the neonatal pig. J. Neurotrauma 19, 843–853 [DOI] [PubMed] [Google Scholar]
- 34.Raghupathi R., Mehr M.F., Helfaer M.A., and Margulies S.S. (2004). Traumatic axonal injury is exacerbated following repetitive closed head injury in the neonatal pig. J. Neurotrauma 21., 307–316 [DOI] [PubMed] [Google Scholar]
- 35.Friess S.H., Ichord R.N., Owens K., Ralston J., Rizol R., Overall K.L., Smith C., Helfaer M.A., and Margulies S.S. (2007). Neurobehavioral functional deficits following closed head injury in the neonatal pig. Exp. Neurol. 204, 234–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friess S.H., Ichord R.N., Ralston J., Ryall K., Helfaer M.A., Smith C., and Margulies S.S. (2009). Repeated traumatic brain injury affects composite cognitive function in piglets. J. Neurotrauma 26, 1111–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou C., Eucker S.A., Durduran T., Yu G., Ralston J., Friess S.H., Ichord R.N., Margulies S.S., and Yodh A.G. (2009). Diffuse optical monitoring of hemodynamic changes in piglet brain with closed head injury. J. Biomed. Opt. 14, 034015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eucker S.A., Smith C., Ralston J., Friess S.H., and Margulies S.S. (2011). Physiological and histopathological responses following closed rotational head injury depend on direction of head motion. Exp. Neurol. 227, 79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sullivan S., Friess S.H., Ralston J., Smith C., Propert K.J., Rapp P.E., and Margulies S.S. (2013). Behavioral deficits and axonal injury persistence after rotational head injury are direction dependent. J. Neurotrauma 30, 538–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naim M.Y., Friess S., Smith C., Ralston J., Ryall K., Helfaer M.A., and Margulies S.S. (2010). Folic acid enhances early functional recovery in a piglet model of pediatric head injury. Dev. Neurosci 32, 466–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faul M, Xu L., Wald M.M., and Coronado V.G. (2010). Traumatic brain injury in the United States: Emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Available at: http://www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf Accessed: November4, 2014 [Google Scholar]
- 42.Bruins B., Kilbaugh T.J., Margulies S.S., and Friess S.H. (2013). The anesthetic effects on vasopressor modulation of cerebral blood flow in an immature swine model. Anesth. Analg. 116, 838–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rhondali O., Mahr A., Simonin-Lansiaux S., De Queiroz M., Rhzioual-Berrada K., Combet S., Cejka J.C., and Chassard D. (2013). Impact of sevoflurane anesthesia on cerebral blood flow in children younger than 2 years. Paediatr Anaesth. 23, 946–951 [DOI] [PubMed] [Google Scholar]
- 44.Li C.X., Patel S., Auerbach E.J., and Zhang X. (2013). Dose-dependent effect of isoflurane on regional cerebral blood flow in anesthetized macaque monkeys. Neurosci. Lett. 541, 58–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dagal A., and Lam A. M. (2009). Cerebral autoregulation and anesthesia. Curr. Opin. Anaesthesiol. 22, 547–552 [DOI] [PubMed] [Google Scholar]
- 46.Kilbaugh T.J., Bhandare S., Lorom D.H., Saraswati M., Robertson C.L., and Margulies S.S. (2011). Cyclosporin a preserves mitochondrial function after traumatic brain injury in the immature rat and piglet. J. Neurotrauma 28, 763–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jullienne A., and Badaut J. (2013). Molecular contributions to neurovascular unit dysfunctions after brain injuries: Lessons for target-specific drug development. Future Neurol 8, 677–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmer R.M., Ferrige A.G., and Moncada S. (1987). Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327, 524–526 [DOI] [PubMed] [Google Scholar]
- 49.Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., and Masaki T. (1988). A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332, 411–415 [DOI] [PubMed] [Google Scholar]
- 50.Armstead W.M., Riley J., and Vavilala M.S. (2013). Dopamine prevents impairment of autoregulation after traumatic brain injury in the newborn pig through inhibition of up-regulation of endothelin-1 and extracellular signal-regulated kinase mitogen-activated protein kinase. Pediatr. Crit. Care Med. 14, e103–e111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferreira L.C., Regner A., Miotto K.D., Moura S.D., Ikuta N., Vargas A.E., Chies J.A., and Simon D. (2014). Increased levels of interleukin-6, −8 and −10 are associated with fatal outcome following severe traumatic brain injury. Brain Inj. 28, 1311–1316 [DOI] [PubMed] [Google Scholar]
- 52.Gao C., Liu X., Shi H., Xu S., Ji Z., Wang C., Wu P., Liu Z., and Zhao S. (2009). Relationship between sympathetic nervous activity and inflammatory response after subarachnoid hemorrhage in a perforating canine model. Auton. Neurosci. 147, 70–74 [DOI] [PubMed] [Google Scholar]
- 53.Megyeri P., Abraham C.S., Temesvari P., Kovacs J., Vas T., and Speer C.P. (1992). Recombinant human tumor necrosis factor alpha constricts pial arterioles and increases blood-brain barrier permeability in newborn piglets. Neurosci. Lett. 148, 137–140 [DOI] [PubMed] [Google Scholar]