Abstract
Decidual stromal cells (DSCs) isolated from fetal membranes of term placentas are easily expanded and are highly immunosuppressive in vitro. These cells express high levels of integrins that are of importance in homing to inflamed tissues. In this study, we investigated DSCs as a cellular therapy for chronic graft-versus-host disease (cGvHD), a severe complication after allogeneic hematopoietic stem cell transplantation. Subsequent to transplantation, three patients developed severe extensive cGvHD and were treated with DSCs (1–2.8×106 cells/kg). One-third of the DSCs administered to two patients were labeled with 111Indium, and the in vivo distribution was tracked for 48 h. The 111In-labeled DSCs were initially located in the lungs, followed by dissemination to the liver and spleen. The DSCs induced a partial response in two of the patients. Blood samples from the patients were extensively evaluated by flow cytometry, luminex, and enzyme-linked immunosorbent assay. The nonresponder had the highest proportion of T-cells with Th17 and Th2 phenotypes and the highest median plasma concentrations of IL-17 and IL-4. The same patient also had high frequencies of HLA-DR+ T-cells and regulatory T-cells. To conclude, DSCs are safe to infuse with no adverse effects. We determined how stromal cells are distributed in vivo after infusion in a cGvHD setting. The methods established for analysis of blood samples will be useful in determining the effect of DSCs in a study comprising a larger patient material. This pilot study may provide a basis for further controlled investigations with DSCs in a clinical setting.
Introduction
Chronic graft-versus-host disease (cGvHD) is a major complication after allogeneic hematopoietic stem cell transplantation (AHSCT) and is a cause of morbidity and mortality. It resembles autoimmune disorders such as sicca, keratoconjunctivitis, scleroderma, primary biliary cirrhosis, wasting, and bronchiolitis obliterans [1–3]. Severe cGvHD includes malabsorption, esophageal and vaginal stricture, and pulmonary insufficiency. First-line therapy includes steroids with or without calcineurin inhibitors, with a response in about every second patient [2]. The poor response rate is reflected by the large number of second-line therapies. This includes azathioprine, low-dose total body irradiation, thalidomide, mycophenolate mofetil, sirolimus, anti-B-cell antibodies, extracorporeal photopheresis, imatinib, and other immunosuppressive therapies [1,4,5].
Mesenchymal stromal cells (MSCs) were successfully used to reverse steroid-refractory acute GvHD in a proportion of patients [6,7]. MSCs also showed efficacy in experimental autoimmune models [8,9]. Since cGvHD resembles autoimmune disorders, MSCs were also used to treat cGvHD [7,10,11]. Using MSCs for cGvHD, responses have been seen in approximately two-thirds of patients [12].
As an alternative to bone marrow (BM)-derived MSCs, we have investigated decidual stromal cells (DSCs) [13,14]. We have successfully used DSCs for treatment of severe acute GvHD [15].
DSCs have several advantages, including a potent immunosuppressive effect in vitro [12], a great expansion potential, and they express high levels of T-cell inhibitory markers and integrins that are of importance in homing to inflamed tissues [14,15]. DSCs also differ from MSCs in several other aspects, including a reduced differentiation capacity [13], a contact-dependent suppression of allo-activated immune cells, and a constitutive production of indoleamine-2,3-dioxygenase and they do not seem to upregulate HLA-II when stimulated with IFN-γ [14]. Due to its expansion potential, DSC is the only cell type that our group produces for clinical use.
A large number of studies have been performed in order to find relevant biological markers to predict the risk of, and/or improve diagnosis of cGvHD. The two major approaches are to correlate expression of soluble factors [16–21] or the frequency different cell subsets [22–27] to the occurrence of cGvHD.
In this pilot study, we treated three steroid-refractory cGvHD patients with DSCs. Apart from clinical evaluation, we surveyed the lymphocyte subpopulations, determined the plasma concentrations of various cytokines, and studied homing using 111In-labeled DSCs.
Patients and Methods
Isolation and expansion of decidual stromal cells
This procedure was described in detail in a previous publication by Ringdén et al. [15]. Briefly, human term placentas were obtained after cesarean section from healthy mothers after obtaining their written informed consent. All donors were seronegative for syphilis, hepatitis A and B, and HIV. The fetal membranes were removed from the chorionic plate, then cut into smaller pieces, washed in phosphate-buffered saline (PBS; Thermo Fisher Scientific, Waltham, MA), and trypsinated twice (Trypsin/EDTA; Thermo Fisher Scientific). The trypsin digests and the pieces of the fetal membranes were then placed separately in several T185 flasks with Dulbecco's modified Eagle's medium (DMEM) supplemented with penicillin (100 U/mL) and streptomycin (100 μg/mL) (both from Thermo Fisher Scientific) (PEST), and 10% fetal calf serum (Thermo Fisher Scientific) (Hereon referred to as complete DMEM) and incubated in 37°C, 5% CO2. When the culture flasks were 90%–95% confluent, the cells were harvested with Trypsin/EDTA, washed in complete DMEM, re-seeded in new cultivation flasks at 2.9×103 cells/cm2, and cultured to passage 2–4. The cells were then frozen in aliquots with supplemented DMEM containing 10% dimethyl sulfoxide (DMSO; WAK-Chemie Medical GmbH, Steinbach, Germany). The DSC product was produced under good manufacturing practice conditions, including a room with reversed isolation, a sterile class-II biosafety flow-cabinet, and separate incubators for each DSC donor.
Characterization of decidual stromal cells
We have previously described how the DSCs from each donor are characterized with regard to surface marker expression, immunomodulatory properties, multipotent differentiation capabilities, karyotype at different passages, and whether the source of the cells is maternal or fetal [15]. The expansion potential of the DSCs has also been described in greater detail elsewhere [15]. To isolate and expand DSCs to passage 4 takes 5–7 weeks. The number of cells obtained from one donor is usually>109 in passage 3, even though not all cells obtained in lower passages are used for further expansion.
The surface expression of DSCs for various markers was examined by flow cytometry. The cells were positive for CD29, CD44, CD49d, CD54, CD73, CD90, CD105, CD273, CD274, and HLA-I. The DSCs were negative for other markers such as CD14, CD31, CD34, CD45, CD86, CD326, and HLA-II.
The multipotent differentiation capabilities of the DSCs were also investigated. They showed poor differentiation to osteocytes, adipocytes, and chondrocytes.
The DSCs were also able to suppress mixed lymphocyte reactions (MLRs) with every DSC donor. DSCs were added to the MLRs in a ratio of 1:10.
Karyotype analysis was performed with CTG banding, and standard cytogenetic procedures showed a normal female karyotype. The maternal origin of the DSCs was confirmed by investigating microsatellite polymorphism. Using a primer specific for the mother and the child allowed us to determine from whom the DSCs originated.
Infusion of decidual stromal cells
The DSCs were thawed and diluted in CliniMACS PBS/EDTA buffer (AMCell Miltenyi Biotec GmbH, Gladbach, Germany) supplemented with 5% human AB-plasma. The cells were washed thrice, counted, filtered through a 70-μm cell strainer (BD Biosciences, Franklin Lakes, NJ), suspended in an infusion solution containing NaCl (B. Braun Melsungen AG, Melsungen, Germany) supplemented with 10% AB-plasma, and transferred to a heparinized syringe (BD Biosciences) at a count of 2×106 cells/mL. The solution was infused intravenously for 5–10 min via a central venous line catheter. The infusion solution and the cell preparations were tested for bacterial contamination. The well-being of the patients was monitored closely for 2 h after infusion with DSCs. All three patients received DSCs from the same donor and passage (passage 3) for the first infusion. For the second treatment for two of the patients, a second donor source of DSCs was used. One patient (UPN 1265) received cells in passage 3, and one patient (UPN 1101) received cells from passage 4.
Collection of blood samples
Blood samples were collected from all three patients. For patient 1, samples were collected before, 3 days after, and 10 weeks after infusion of DSCs. Samples from patient 2 were obtained before, 1 week after, and 10 weeks after infusion. From patient 3, samples were acquired before and after 1, 2, and 4 weeks. Plasma was obtained after centrifugation, and peripheral blood mononuclear cells (PBMCs) were isolated using Lymphoprep™ (Axis-Shield, Dundee, Scotland) according to the manufacturer's instructions. The PBMCs were frozen in aliquots in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 5% AB-serum (in-house), penicillin (100 U/mL) and streptomycin (100 μg/mL), 2 mM l-glutamine (Invitrogen), and 10% DMSO until use.
Flow cytometry
Three panels were used to analyze PBMCs from the patients. The cells were stained with the following antibodies: aCD3-V450 (clone UCHT1), aCD4-Alexa Fluor 700 (RPA-T4), aCD8-APC (SK1), aCD8-Pe-Cy7 (SK1), aCD45RA-Pe-Cy7 (HI100), aCCR7-Pe-CF594 (150503), aCCR4-Pe (1G1), aCXCR3-Alexa Fluor 700 (1C6/CXCR3), 7AAD, aCD25-Pe (M-A251), aHLA-DR-V500 (G46-6) (BD Biosciences), aCD4-Chrome orange (13B8.2), aCD127-APC-Alexa Fluor 700 (R34.34) (Beckman Coulter, Pasadena, CA), aCCR6-Fitc (R6H1), aβ-7-Fitc (FIB504) (both from Biolegend, San Diego, CA), and aCD49d-APC (44H6) (Bio-Rad, Hercules, CA). Data were collected with an FACSAria (BD Biosciences) using FACSDiva software. All samples from the same patient were run simultaneously. The cytometer settings were the same for all three patients. The data were analyzed with FlowJo software (Treestar, Ashland, OR) and Graphpad Prism software (Graphpad, San Diego, CA). Fluorescence minus one controls were used for gating.
Luminex
A 27-parameter milliplex panel (Merck Millipore, Billerica, MA) was used according to the manufacturer's instructions to determine the concentrations of cytokines and chemokines in plasma samples from the three patients. Before analysis, the plasma samples were diluted 1:3.
Enzyme-linked immunosorbent assay
An enzyme-linked immunosorbent assay (ELISA; R&D systems, Minneapolis, MN) was performed on all samples to detect concentrations of B-cell activating factor (BAFF). The test was run according to the manufacturer's instructions.
111In-oxine labeling of the decidual stromal cells
For each patient, the solution containing DSCs was divided into two portions. One portion, containing one-third of the cells, was used for radiolabeling. The other portion was used for infusion of the patient without radiolabeling.
Before labeling the DSCs, 1 mL of 111In-oxinate solution (DRN 4908; Mallinckrodt, Petten, The Netherlands) was adjusted to pH 7.4. Labeling was then carried out by incubating the cells, suspended in 5 mL complete DMEM, with 111In-oxine for 15 min at room temperature. The cells were centrifuged at 200 g for 7 min, washed twice with PBS/EDTA supplemented with 5% AB-plasma, and then resuspended in infusion solution. After washing and before infusion, the radiolabeling efficiency, cell viability, and cell counts were determined. Mean labeling efficiency was 74%±11%. The activity administered was 8–15 MBq, depending on the weight of the patient.
In order to ensure long-term survival and sufficient incorporation of the isotope into the labeled DSCs, cells from one DSC donor were labeled in different media (supplemented DMEM, PBS, or NaCl), seeded in equal numbers in culture flasks, and cultured for at least 72 h. The proliferative capacity of the labeled DSCs was followed by microscopy. Unlabeled controls were incubated under the same conditions but without the 111In-oxinate solution. Initial experiments indicated that DSCs radiolabeled in supplemented DMEM were viable and could proliferate for at least 7 days in vitro.
Investigation of isotope distribution
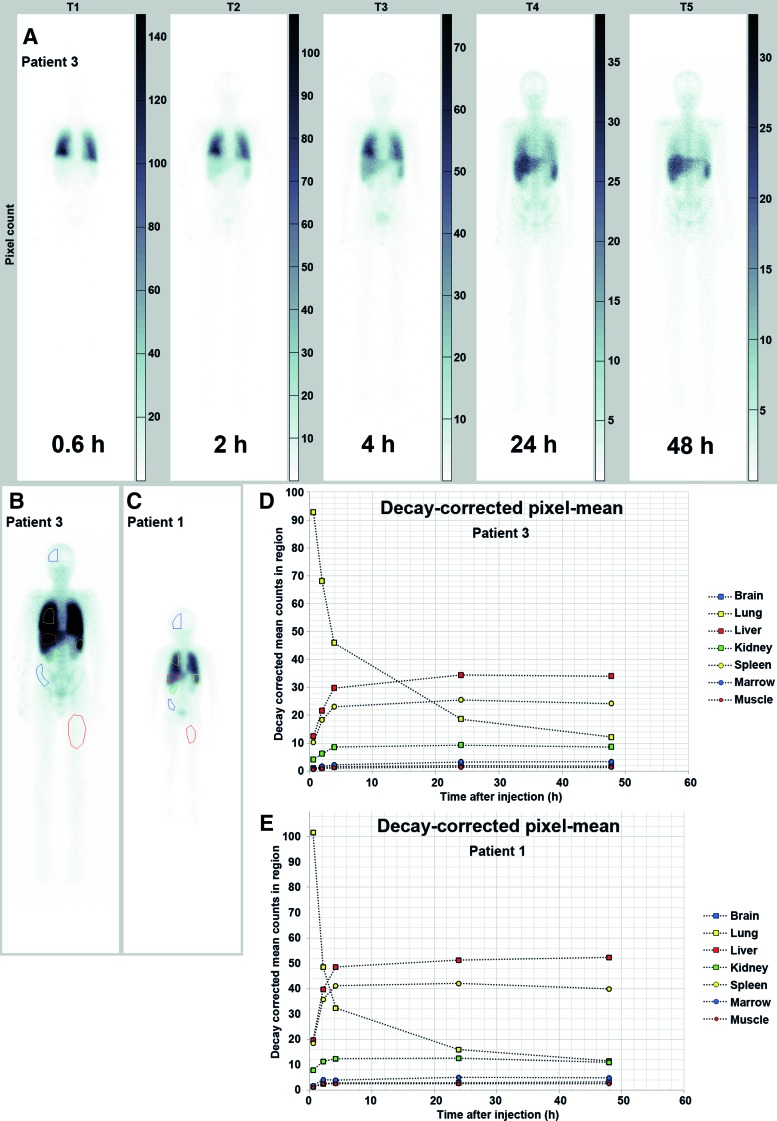
Whole-body scans were done with the patient in supine position, starting at 1, 2, 4, 24, and 48 h after an injection of 111In. The images were acquired with a dual-head gamma camera E-cam (Siemens, Erlangen, Germany). Medium-energy low-penetration collimators were used. The energy peaks were set to 172 and 242 keV with a 15% energy window centered over the photo peaks. An anterior and posterior view was acquired at a scan speed of 10 cm/min. Images were evaluated visually and quantitatively. A region of interest (ROI) was drawn over the liver, spleen, BM, lungs, brain, and kidney for each time point.
Whole-body images were acquired on a matrix of size 256×1,024 with pixels of size 2.40 mm at a speed of 1.7 mm/s. Five sets of anterior and posterior images were acquired at T1 (0.6 h), T2 (2 h), T3 (4 h), T4 (24 h), and T5 (48 h) postinjection using medium-energy collimators. An energy window over the 245.4 keV photopeak ranging from 224 to 260 keV was used.
The geometrical-mean images were calculated on a pixel-by-pixel basis for each time point as
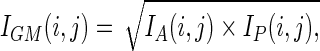 |
where IA (i, j) and IP (i, j) are the anterior and the posterior images, respectively. The IGM images for time points T2, T3, T4, and T5 were manually aligned with the IGM image for time point T1. A summed image of all time points was created for drawing ROIs. The ROIs were drawn manually over the brain, lung, liver, kidney, spleen, BM, and muscle. The mean number of counts per pixel in the regions was calculated and corrected for physical decay. The counts in each region were normalized to those at time point T1 and plotted as a function of time.
Patients
Three patients were treated for cGvHD with DSCs (Table 1) in accordance to the declaration of Helsinki. Follow up was 2 years after infusion: December 16, 2013. The study was approved by the Ethics Committee of Karolinska University Hospital Huddinge (2009/418-31/4, 2011/15/11). Patients and donors of DSCs gave written informed consent. Conditioning, immunosuppression, and supportive care has been previously described in detail [28]. Acute GvHD was graded from 0 to IV according to the grading by Glucksberg et al. in the Seattle team [29]. cGvHD was graded according to the guidelines of the National Institutes of Health (NIH) [2].
Table 1.
Characteristics of Patients Treated with DSCs for Severe Chronic GvHD
| Patient no. | UPN | Sex/age | Diagnosis | Donor | Graft source | Acute GvHD, grade | Complications | Chronic GvHD | DSCs, time after HSCT | DSC cell dose×106/kg | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A | 1011 | M/1 | Infant B-ALL | HLA-id sibling | BM | I | SOS | — | Relapse and reinduction | ||
| 1B | 1011 | M/2 | Pre-B-ALL | HLA-id sibling | BM | III | Severe | 7 years 8 months | 2.8 | Developed severe chronic GvHD involving lungs, OB, liver and GI tract, skin | |
| 7 years 10 months | 1.7 | ||||||||||
| 2 | 1265 | M/15 | AML CR2 | HLA-id sibling | PBSC | II | Severe | 3 years 9 months | 1.8 | Developed severe acute GvHD of skin, mouth, eyes, liver, malabsorption, scleroderma | |
| 3 years 11 months | 1.0 | ||||||||||
| 3 | 1475 | F/18 | CML | MUD | PBSC | II | Severe | 10 months | 1.8 | Developed moderate-to-severe chronic GvHD of skin, liver, and OB |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BM, bone marrow; CML, chronic myeloid leukemia; DSC, decidual stromal cells; GvHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplantation; MUD, matched unrelated donor; OB, obstructive bronchiolitis; PBSC, peripheral blood stem cells; SOS, sinusoidal obstructive syndrome.
Results
Patient outcomes
Patient 1 (UPN 1011)
At 1 year after the first AHSCT, the patient had recurrent leukemia and after reinduction, he underwent retransplantation (Table 1). He developed severe gastrointestinal acute GvHD grade III, which slowly responded to treatment with steroids and MSCs [7]. He developed bronchiolitis obliterans, the therapy of which included cyclosporine, corticosteroids, mycophenalate mofetil, and fluticasone for inhalation. Forced expiratory volume (FEV1) was 29%. After infusion of DSCs, he was subjectively better and he did not require oxygen when he flew back home. Before infusion, he had required oxygen sometimes at night and when flying. Prednisolone could also be tapered. Due to previous sinusoidal obstructive syndrome, he had portal hypertension and varices that needed frequent ligations. After infusion of DSCs, varices have been minimal and no ligations have been required. After the two infusions of DSCs, liver enzymes normalized (Table 2). He received In−111-labeled DSCs. At 2 years, his main problem is still bronchiolitis obliterans and he cannot climb stairs. He no longer attends school. FEV1 is 21%. Prednisolone is given at 1.4 mg/kg/day. He has been referred for lung transplantation [30].
Table 2.
Patients with Chronic GvHD in Various Organs; Scoring Before and After Treatment with DSCs and at 2-Year Follow Up Was According to NIH Guidelines
| Patient no. | UPN | Time | Skin | Mouth | Eyes | GI-tract | Liver | Lung | Joint, fascia | Global scoring |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1011 | Before | 0 | 0 | 0 | 2 | 1 | 3 | 0 | 3 |
| After | 0 | 0 | 0 | 1 | 0 | 2–3 | 0 | 3 | ||
| 2 years | 0 | 0 | 0 | 1 | 0 | 3 | 0 | 3 | ||
| 2 | 1265 | Before | 3 | 2 | 1 | 2 | 2 | 1 | 1 | 3 |
| After | 3 | 2 | 1 | 1 | 0 | 0 | 1 | 3 | ||
| 2 years | 3 | 2 | 1 | 1 | 0 | 1 | 1 | 3 | ||
| 3 | 1475 | Before | 2 | 1 | 0 | 0 | 2 | 2 | 0 | 3 |
| After | 2 | 1 | 0 | 0 | 2 | 2 | 0 | 3 | ||
| 2 years | 1 | 1 | 0 | 0 | 2 | 3 | 0 | 3 |
All patients had severe extensive chronic GvHD.
GI, gastrointestinal; NIH, National Institutes of Health.
Patient 2 (UPN 1265)
The patient developed extensive and severe cGvHD affecting the eyes with sicca, severe mucositis, malabsorption, extensive skin disease with lichenoid changes all over the skin, and contracture of the right axilla with 10% extension deficit. Previously failed therapy included rapamycine, extracorporal psoralene and ultraviolet light (PUVA), mycophenalate mofetil, infliximab, rituximab, imatinib, and corticosteroids. He was given two doses of DSCs 2 months apart (Table 1). Before infusion, liver enzymes were ALAT 3.65 μkat/L and ASAT 1.16 μkat/L. After DSC infusions, liver enzymes normalized. Serum albumin was 29 g/L before infusion and 32 g/L after infusion, and stabilized on a higher level. Weight was unchanged at 52 kg. After the first DSC infusion, he had transient response in skin, and erythema disappeared over the trunk, face, and legs. He had complete liver response and transient skin responses (Table 2). At 2 years, he still suffers from moderate-to-severe cGvHD of several organs, but his liver enzymes are normal.
Patient 3 (UPN 1475)
This 17-year-old girl with CML developed cGvHD of the skin with lichenoid changes and erythema all over the body, elevated liver enzymes, and bronchiolitis obliterans (FEV1 43%). She had been treated with steroids, cyclosporine and prograf, PUVA, and fluticasone inhalation with limited effect. She was, therefore, treated with DSCs, 1.8×106/kg in one infusion, with no effect. 111In-labeled DSCs were also infused. She had an upper respiratory tract infection before infusion of DSCs and till day +12. Her skin has improved with lichenoid changes affecting two-thirds of the body, but no erythema and no blisters. She feels better, but spirometry shows an FEV1 of 26%.
Clinical response to DSCs
Patients 1 and 2 had normalization of elevated liver enzymes after DSC infusion, and these remained normal at the 2-year follow up (Table 2). In patient 1, esophageal varices did not reappear and there was no need for banding. Patient 2 had transient improvement in the skin, a subjective feeling of improvement in the mouth, and improvement in serum albumin levels. Patient 3 had no improvement. Global scoring was NIH grade 3 and severe extensive GvHD in all patients and remained so at the 2-year follow up in patients 2 and 3. Patient 1 worsened and became NIH grade 3.
Radiolabeling
The IGM images for patient 3 are shown in Figure 1A. Most of the isotope could be detected in the lungs at 0.6 h. The count then rapidly decreased in the lungs and increased in the liver and spleen. The summed images with the ROIs can be seen in Figure 1B for patient 3 and in Figure 1C for patient 1. The plots of the normalized decay-corrected mean pixel counts in the regions as a function of time are given in Figure 1D for patient 3 and in Figure 1E for patient 1. In patient 1, the mean counts at 4 h were higher in the liver and spleen than in the lungs. In patient 3, this was observed at 24 h.
FIG. 1.
(A) Shows the geometric mean of images for all five time points [T1 (0.6 h), T2 (2 h), T3 (4 h), T4 (24 h), and T5 (48 h)] for patient 3. (B, C) Show the summed image of all time points for patient 3 (B) and patient 1 (C), with the regions of interest (ROIs). The image has been windowed to improve visualization of the organs. The plots in (D, E) illustrate the normalized decay-corrected mean pixel count in the ROIs for patient 3 and patient 1, respectively. Color images available online at www.liebertpub.com/scd
T-cell characterization
Except from using clinical parameters to evaluate response of the DSCs and progression of the GvHD, we have established a protocol for extensive immunosurveillence of patient peripheral blood. T-cells play a key role in the graft-versus-host reaction. We, therefore, characterized helper- and cytotoxic T-cell subsets with regard to maturation and activation.
T-cells were identified as CD3+CD4+ helper T-cells (Th-cells) and CD3+CD8+ cytotoxic T-cells (Tc-cells). The T-cells were further characterized as naïve (CD45RA+CCR7+), central memory (CD45RA−CCR7+), effector memory (CD45RA−CCR7−), and terminally differentiated (CD45RA+CCR7−) T-cells.
Furthermore, we determined the frequencies of specific central and effector memory Th subsets such as Th1 (CXCR3+CCR4−CCR6−), Th2 (CXCR3−CCR4+CCR6−), Th17 (CXCR3−CCR4+CCR6+), and Th1/Th17 (CXCR3+CCR4−CCR6+) [31], and regulatory T-cells (CD4+CD25highCD127low/−). The activation of T-cell subsets was detected by staining for HLA-DR and CD25.
No uniform effects in all three patients could be seen between the samples taken before and after infusion. No trends that differentiated the responders (patients 1 and 2) from the nonresponder (patient 3) were observed when we compared the samples taken before and after infusion with DSCs. However, when taking all time points into consideration, differences could be seen between the three patients. A complete summary of all parameters measured by flow cytometry data is presented in Table 3 (median frequency of the data from all time points for each patient).
Table 3.
Frequencies of Different T-Cell Subsets
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| CD3+CD4+ lymphocytes | |||
| Naive | 24.4 (24.3–24.4) | 25.1 (11.2–26.3) | 4.8 (3.6–6.3) |
| Central memory | 4.1 (2.5–5.7) | 2.5 (1.7–3.3) | 3.9 (2.0–4.53) |
| Effector memory | 38.9 (38.6–40.1) | 37.8 (30.8–47.0) | 31.9 (23.5–38.8) |
| Terminally differentiated | 2.3 (2.1–2.6) | 7.4 (2.4–8.7) | 12.7 (10.9–23.2) |
| Th1 | 6.4 (6.6–7.5) | 11.2 (6.7–20.7) | 5.88 (5.6–7.0) |
| Th2 | 7.3 (6.5–8.6) | 12.2 (10.8–19.6) | 15.4 (11.5–17.2) |
| Th17 | 0.8 (0.5–1.7) | 4.7 (1.6–7.4) | 8.2 (4.7–11.0) |
| Th1/Th17 | 0.5 (0.5–2.9) | 1.2 (0.4–1.3) | 3.3 (1.3–2.6) |
| Treg | 6.4 (4.8–6.5) | 3.3 (2.5–4.8) | 11.5 (8.63–15.9) |
| HLA+ | 21.5 (17.6–21.9) | 36.5 (25.8–50.8), | 72.9 (72.7–73.3) |
| CD25+ | 20.6 (19.2–21.5) | 13.9 (10.6–24.1) | 21.6 (16.1–26.3) |
| CD25 intensity | 1,069 (1,006–1,231) | 1,175 (881–1,249) | 989.5 (814–1,206) |
| CD3+CD8+ lymphocytes | |||
| Naive | 33.5 (31.8–36.5) | 14.7 (8.4–15.9) | 25.9 (25.3–35.5) |
| Central memory | 4.1 (3.4–5.4) | 2.5 (1.8–3.3) | 3.9 (2.0–4.5) |
| Effector memory | 16.4 (15.7–19.1) | 19.1 (18.5–20.5) | 9.4 (7.5–10.3) |
| Terminally differentiated | 43.3 (43.0–47.3) | 63.1 (63.0–69.4) | 60.0 (51.4–65.3) |
| HLA+ | 33.6 (30.9–37.5) | 60.5 (56.7–68.1) | 80.6 (70.8–83.8) |
| CD25+ | 6.7 (5.4–10.3) | 3.2 (1.3–3.7) | 15.1 (9.2–18.6) |
| CD25 intensity | 588 (581–595) | 1,059 (996–1,445) | 254 (205–1,004) |
These are presented as the median frequency and range, with data from all time points of measurement. The specific surface marker expression of all subsets is presented in the Results section.
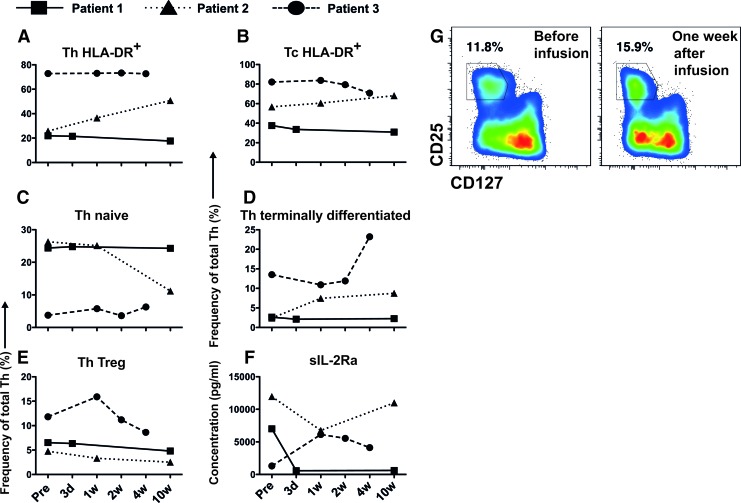
HLA-DR was used as a marker to investigate the activation of lymphocytes in peripheral blood. Patient 3 had consistently high proportions of HLA-DR+ cells in the Th- and the Tc populations. This contrasts with patients 1 and 2, both of whom had lower proportions of HLA-DR+ cells (Fig. 2A, B).
FIG. 2.
Immunocharacterization of peripheral blood from patient 1, patient 2, and patient 3. Samples were obtained before and till 10 weeks after infusion with decidual stromal cells. The figure shows the frequencies of cells that were positive for (A) HLA-DR in the CD3+CD4+ (Th) and (B) the CD3+CD8+ (Tc) lymphocyte populations. The graphs in (C, D) show the frequency of CD45RA+CCR7+ cells (naïve) and CD45RA+CCR7− cells (terminally differentiated) in the Th population, respectively. The proportion of Th-regulatory cells (Treg) is shown in (E) and the concentration in plasma of soluble interleukin-2 receptor alpha (sIL-2Ra) in (F). (G) Shows representative FACS data for all Th-cells from patient 3 at two time points. Gating shows the identification of Tregs. Color images available online at www.liebertpub.com/scd
The percentage of Th-cells with a naïve phenotype was higher in patients 1 and 2 than in patient 3 (Fig. 2C). There were no differences in the proportions of central memory Th-cells between the three patients (Table 3). However, the frequency of terminally differentiated Th-cells was higher in patient 3 than in the other two patients (Fig. 2D). In the Tc compartment, patient 2 had a lower frequency of naïve Tc-cells than patients 1 and 3, whereas patient 3 had a low proportion of effector memory cells (Table 3). Patient 1 had the lowest percentage of terminally differentiated Tc-cells (Table 3).
The frequency of Tregs was elevated in patient 3 after infusion, whereas it was lower in the other two patients (Fig. 2E). A representative FACS plot can be seen in Figure 2G.
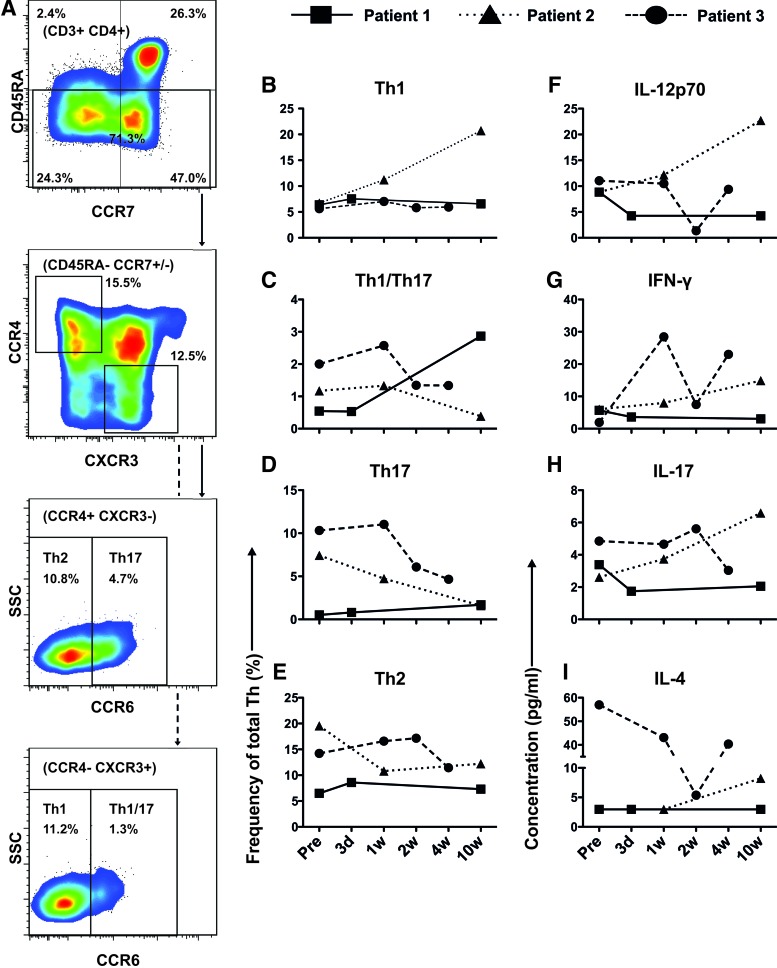
Patient 3 also had a larger proportion of Th-cells with a Th17, Th1/Th17, Th2, and Treg phenotype than the two responders (Figs. 2 and 3). Patient 3 also had the highest median plasma concentrations of cytokines that are associated with these T-cell subsets [IL-17 (Th17), IFN-γ (Th1), and IL-4 (Th2)] (Fig. 3). In contrast, patient 1 had an elevated frequency of Th1-cells and a high concentration of the Th1-inducing cytokine IL-12p70.
FIG. 3.
Immunocharacterization of peripheral blood (PB) from patients 1, 2, and 3. Samples were obtained before and several times after infusion with decidual stromal cells. (A) Shows a representative patient sample (patient 2 at 1 week after infusion) and the gating strategy for identification of the maturation of T-cells. Effector cells are then gated further for identification of Th2, Th17, Th1, and Th1/17 cell subsets. (B–E) Show the frequency of cells in the CD3+CD4+ (Th) lymphocyte population with a Th1, Th1/Th17, Th17, or Th2 phenotype, respectively, according to specific surface markers (see Results section). In (F–I), the plasma concentrations of IL-12p70, IFN-γ, IL-17, and IL-4, respectively, are presented for all three patients. Color images available online at www.liebertpub.com/scd
Parameters that showed no differences between the patients were the frequency of CD25 and the intensity of CD25 expression in both the Th and Tc compartment (Table 3). In addition, Th-cells with an effector memory phenotype and central memory cells within the Tc-compartment only showed small differences between the three patients (Table 3).
Concentrations of soluble factors
Expression of several proinflammatory cytokines and other molecules may be elevated during cGvHD. Using Luminex and ELISA, we determined the concentrations of some of these molecules in patient plasma.
Levels of the soluble alpha chain of the IL-2 receptor (sIL-2rα) increased after infusion of DSCs in patient 3 (Fig. 2F). In contrast, the concentration of sIL-2rα in patients 1 and 2 was reduced after treatment with DSCs.
Another factor implicated in cGvHD is BAFF. No difference could be seen between the responders and the nonresponder after treatment with DSCs. However, patient 3 had the highest median concentration of BAFF when we compared the data from all time points [0.8 ng/mL (range 0.6–1.3 ng/mL) as compared with 0.2 ng/mL (0.2–0.3 ng/mL) and 0.5 ng/mL (0.4–0.7 ng/mL) in patients 1 and 2, respectively]. Patient 3 also had the highest median plasma concentrations of sIL-1ra [0.3 ng/mL (0.1–1.1 ng/mL) vs. 5.7 pg/mL (5.3–8.8 pg/mL) (patient 1) and 29 pg/mL (13–60 pg/mL) (patient 2)] and IL-8 [13 pg/mL (9.2–16 pg/mL) vs. 9.7 pg/mL (6.4–11 pg/mL) (patient 1) and 10 pg/mL (7.4–21 pg/mL) (patient 2)].
Discussion
In this study, we introduced decidual stromal cells as a new cellular therapy for severe cGvHD. Two patients had a partial response and one patient did not respond. The DSCs were also traced in vivo for 48 h after infusion, using an 111In isotope. The DSCs were initially located in the lungs, followed by dissemination to the liver and spleen. Flow cytometry data also show that the nonresponder (patient 3) had high proportions of activated cells and differentiated T-cells throughout the whole time of measurement. Luminex data also indicated that patient 3 had a high concentration of several cytokines associated with cGvHD.
The major limitations of this study are the small number of patients, the short time that the isotope was tracked in vivo, and the fact that samples were obtained at different time points after infusion of DSCs. Due to the small number of patients in the study, no conclusion can be drawn regarding the immunological parameters that were measured and a potential connection to cGvHD severity or response to the treatment with DSCs. We can, however, see that the treatments do not induce acute toxicity, the isotope distribution pattern is the same in both cases, and we have established analysis methods which can be implemented in a study comprising a larger patient material.
Both patients who responded had normalization of liver enzymes, which is in accordance with the results for the first patient treated with MSCs for cGvHD [7]. Other stable improvements included healing of esophageal varices in patient 1 and elevated albumin in patient 2. Healing of tissue toxicity—such as hemorrhagic cystitis, pneumomediastinum, and perforated colon—has been previously reported for MSCs [32]. Improvements regarding oxygenation and skin features were only transient. In the context of using MSCs to treat patients with cGvHD, only 61 patients have been reported in the literature, with a complete response rate of 26% and a partial response rate of 48% [33]. The largest report—from Weng et al.—involved 19 patients; these had a similar response rate to that reported overall [10]. Since stromal cells give almost no side effects (in contrast to immunosuppressive drugs), they are an attractive treatment modality. However, after these initial pilot studies, prospective randomized studies are now required to establish this therapy. Since some patients do not respond at all, there is certainly room for improvement.
Patient 3 did not appear to be affected by the treatment. Noticeably, she differed in immunophenotypic status from the other two patients with regard to numerous parameters. These differences included enhanced expression of HLA-DR; increased frequencies of TH1/17, Th17, Th2, terminally differentiated Th-cells, and Tregs. She also had high plasma concentrations of several proinflammatory cytokines and a low frequency of naïve T-helper cells. The data suggest that patient 3 had a highly activated and/or exhausted immune system. However, it is important to note that the activated immune system in patient 3 may have other causes except different severity of the cGvHD. Patient 3 had an upper respiratory tract infection during the first 2 weeks after treatment with DSCs. This may have affected the immune parameters. Another possibility is that she received DSCs which were ineffective in her case. There is, however, no difference in the donor or passage of DSCs given to patient 3 compared with the other two patients. She also received extracorporeal PUVA treatment on the day of DSC infusion. These suggestions are speculative. Other parameters such as immune reconstitution, heterogeneity, graft composition, and other chronical inflammations may also affect the activation markers monitored in this study.
In this study, the dose ranged between 1.0 and 2.8×106 cells/kg. Determination of the doses was based on previous reports [15]. The doses do not appear to be important for response, as a similar response was seen in patients 1 and 2 after the second treatment with DSCs. The second treatments contained 1.1×106 and 0.8×106 fewer cells per kg than in the first infusion in patient 1 and patient 2, respectively. To our knowledge, there are no studies where the therapeutic window of stromal cells has been investigated. A high-dose regimen and/or repeated dosing with stromal cells might prove beneficial.
Compared with BM-MSCs, DSCs have a higher expression of integrins that are important for homing to damaged tissue [14]. Still, the DSCs do not appear to have increased homing to other affected organs such as the intestine, esophagus, or skin within the first 48 h after treatment with DSCs. It has been shown in several studies that BM-MSCs are capable of homing to damaged tissue in animal models [34,35]. However, in vivo tracking of BM-MSCs in a human GvHD setting is limited and there is little proof of homing to inflamed tissues [7,36]. The viability of the cells cannot be determined after infusion. Studies by both Li and Lin and Moll et al. have shown that BM-MSCs are vulnerable to the complement system [37,38]. This may also have complications for the survival of DSCs when infused to patients.
Cultured BM-MSCs have been tracked in patients with liver cirrhosis, and the distribution pattern resembles our findings [39]. A disadvantage of 111In-oxine labeling is the short half life of the isotope, which limits the time for monitoring. Long-term engraftment of DSCs should, therefore, be monitored by different methods. One study using autopsy materials showed little proof of homing to inflamed tissue and limited long-term engraftment [36].
In this study, we have established a protocol for examining how cytokines and different types of T-cells are affected by treatment with DSCs in a cGVHD setting. This analysis includes many of the factors shown to be of importance in cGvHD [16–27]. An extensive evaluation protocol as the one presented will be useful in prospective, more comprehensive studies to investigate how DSCs might modulate GvHD progression. The reliability of the methods is convincing; luminex and flow cytometry data seem to correlate in all three patients regarding plasma concentrations of cytokines and the presence of specific T-cell subsets associated with those cytokines. For instance, Patient 2 has a high frequency of cells with a Th1 phenotype and a high concentration of the Th1-inducing cytokine IL-12p70 (Fig. 3). In addition, Patient 3 has a high frequency of T-cells with a Th1/Th17 and Th17 phenotype, as well as the highest median concentration of IL-17 (Fig. 3).
To summarize, this initial pilot study using DSCs as treatment for cGvHD shows that the treatment is safe, and a partial response could be seen in two out of three patients. There was no difference in distribution of labeled DSCs between the two patients examined. The results also indicate that there was a distinct disparity in cytokine concentrations and T-cell populations between the nonresponder and the two partial responders.
Acknowledgments
The authors thank the midwives at the Department of Obstetrics and Gynecology, Karolinska University Hospital Huddinge, for recruiting parents and collecting placentas. Special thanks are due to Berit Jansson for Indium labeling the DSCs. The authors also thank the nurses at the Center for Allogeneic Stem Cell Transplantation and the Department of Pediatrics for collecting samples. O.R. was supported by grants from the Swedish Cancer Society, the Children's Cancer Foundation, the Swedish Research Council, the Cancer Society in Stockholm, and Karolinska Institutet. H.K. was supported by grants from the Swedish Society of Medicine, the Swedish Research Council, the Children's Cancer Foundation, the Cancer Society in Stockholm, Clas Groschinskys foundation, and Karolinska Institutet.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ringden O. and Deeg HJ. (1996). Clinical spectrum of graft-versus-host disease. In: Graft vs Host Disease. Ferrara JLM, Deeg HJ, Burakoff SJ, eds. Marcel Dekker, Inc., New York, pp. 525–559 [Google Scholar]
- 2.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D, et al. (2005). National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 11:945–956 [DOI] [PubMed] [Google Scholar]
- 3.Lee SJ, Klein JP, Barrett AJ, Ringden O, Antin JH, Cahn JY, Carabasi MH, Gale RP, Giralt S, et al. (2002). Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood 100:406–414 [DOI] [PubMed] [Google Scholar]
- 4.Flowers ME, Apperley JF, van Besien K, Elmaagacli A, Grigg A, Reddy V, Bacigalupo A, Kolb HJ, Bouzas L, et al. (2008). A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood 112:2667–2674 [DOI] [PubMed] [Google Scholar]
- 5.Socie G, Devergie A, Cosset JM, Pierga JY, Esperou H, Girinski T. and Gluckman E. (1990). Low-dose (one gray) total-lymphoid irradiation for extensive, drug-resistant chronic graft-versus-host disease. Transplantation 49:657–658 [DOI] [PubMed] [Google Scholar]
- 6.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, et al. (2008). Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371:1579–1586 [DOI] [PubMed] [Google Scholar]
- 7.Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, Marschall HU, Dlugosz A, Szakos A, et al. (2006). Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation 81:1390–1397 [DOI] [PubMed] [Google Scholar]
- 8.Parekkadan B, Tilles AW. and Yarmush ML. (2008). Bone marrow-derived mesenchymal stem cells ameliorate autoimmune enteropathy independently of regulatory T cells. Stem Cells 26:1913–1919 [DOI] [PubMed] [Google Scholar]
- 9.Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, Xu T, Le A. and Shi S. (2009). Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells 27:1421–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weng JY, Du X, Geng SX, Peng YW, Wang Z, Lu ZS, Wu SJ, Luo CW, Guo R, et al. (2010). Mesenchymal stem cell as salvage treatment for refractory chronic GVHD. Bone Marrow Transplant 45:1732–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou H, Guo M, Bian C, Sun Z, Yang Z, Zeng Y, Ai H. and Zhao RC. (2010). Efficacy of bone marrow-derived mesenchymal stem cells in the treatment of sclerodermatous chronic graft-versus-host disease: clinical report. Biol Blood Marrow Transplant 16:403–412 [DOI] [PubMed] [Google Scholar]
- 12.Ringden O. (2013). Mesenchymal stem cells for treatment and prevention of graft-versus-host disease and graft failure after hematopoietic stem cell transplantation and future challenges. In: Mesenchymal Stem Cell Therapy. Chase IG, Vemuri MC, eds. Springer Verlag, Humana Press, New York, pp. 173–206 [Google Scholar]
- 13.Erkers T, Nava S, Yosef J, Ringden O. and Kaipe H. (2013). Decidual stromal cells promote regulatory T cells and suppress alloreactivity in a cell contact-dependent manner. Stem Cells Dev 22:2596–2605 [DOI] [PubMed] [Google Scholar]
- 14.Karlsson H, Erkers T, Nava S, Ruhm S, Westgren M. and Ringden O. (2012). Stromal cells from term fetal membrane are highly suppressive in allogeneic settings in vitro. Clin Exp Immunol 167:543–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ringden O, Erkers T, Nava S, Uzunel M, Iwarsson E, Conrad R, Westgren M, Mattsson J. and Kaipe H. (2013). Fetal membrane cells for treatment of steroid-refractory acute graft-versus-host disease. Stem Cells 31:592–601 [DOI] [PubMed] [Google Scholar]
- 16.Sarantopoulos S, Stevenson KE, Kim HT, Cutler CS, Bhuiya NS, Schowalter M, Ho VT, Alyea EP, Koreth J, et al. (2009). Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood 113:3865–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitko CL, Levine JE, Storer BE, Chai X, Fox DA, Braun TM, Couriel DR, Martin PJ, Flowers ME, et al. (2013). Plasma CXCL9 elevations correlate with chronic GVHD diagnosis. Blood 123:786–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi S, Imamura M, Hashino S, Tanaka J. and Asaka M. (1997). Clinical relevance of serum soluble interleukin-2 receptor levels in acute and chronic graft-versus-host disease. Leuk Lymphoma 28:159–169 [DOI] [PubMed] [Google Scholar]
- 19.Imamura M, Hashino S, Kobayashi H, Kubayashi S, Hirano S, Minagawa T, Tanaka J, Fujii Y, Kobayashi M, et al. (1994). Serum cytokine levels in bone marrow transplantation: synergistic interaction of interleukin-6, interferon-gamma, and tumor necrosis factor-alpha in graft-versus-host disease. Bone Marrow Transplant 13:745–751 [PubMed] [Google Scholar]
- 20.Fujii H, Cuvelier G, She K, Aslanian S, Shimizu H, Kariminia A, Krailo M, Chen Z, McMaster R, et al. (2008). Biomarkers in newly diagnosed pediatric-extensive chronic graft-versus-host disease: a report from the Children's Oncology Group. Blood 111:3276–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pidala J, Sarwal M, Roedder S. and Lee SJ. (2014). Biologic markers of chronic GVHD. Bone Marrow Transplant 49:324–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dander E, Balduzzi A, Zappa G, Lucchini G, Perseghin P, Andre V, Todisco E, Rahal D, Migliavacca M, et al. (2009). Interleukin-17-producing T-helper cells as new potential player mediating graft-versus-host disease in patients undergoing allogeneic stem-cell transplantation. Transplantation 88:1261–1272 [DOI] [PubMed] [Google Scholar]
- 23.Yamashita K, Choi U, Woltz PC, Foster SF, Sneller MC, Hakim FT, Fowler DH, Bishop MR, Pavletic SZ, et al. (2004). Severe chronic graft-versus-host disease is characterized by a preponderance of CD4(+) effector memory cells relative to central memory cells. Blood 103:3986–3988 [DOI] [PubMed] [Google Scholar]
- 24.D'Asaro M, Dieli F, Caccamo N, Musso M, Porretto F. and Salerno A. (2006). Increase of CCR7- CD45RA+CD8 T cells (T(EMRA)) in chronic graft-versus-host disease. Leukemia 20:545–547 [DOI] [PubMed] [Google Scholar]
- 25.Clark FJ, Gregg R, Piper K, Dunnion D, Freeman L, Griffiths M, Begum G, Mahendra P, Craddock C, Moss P. and Chakraverty R. (2004). Chronic graft-versus-host disease is associated with increased numbers of peripheral blood CD4+CD25high regulatory T cells. Blood 103:2410–2416 [DOI] [PubMed] [Google Scholar]
- 26.Arpinati M, Chirumbolo G, Marzocchi G, Baccarani M. and Rondelli D. (2008). Increased donor CD86+CD14+ cells in the bone marrow and peripheral blood of patients with chronic graft-versus-host disease. Transplantation 85:1826–1832 [DOI] [PubMed] [Google Scholar]
- 27.Miura Y, Thoburn CJ, Bright EC, Phelps ML, Shin T, Matsui EC, Matsui WH, Arai S, Fuchs EJ, et al. (2004). Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood 104:2187–2193 [DOI] [PubMed] [Google Scholar]
- 28.Ringden O, Remberger M, Persson U, Ljungman P, Aldener A, Andstrom E, Aschan J, Bolme P, Dahllof G, Dalianis T, et al. (1995). Similar incidence of graft-versus-host disease using HLA-A, -B and -DR identical unrelated bone marrow donors as with HLA-identical siblings. Bone Marrow Transplant 15:619–625 [PubMed] [Google Scholar]
- 29.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG. and Thomas ED. (1974). Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 18:295–304 [DOI] [PubMed] [Google Scholar]
- 30.Holm AM, Riise GC, Hansson L, Brinch L, Bjortuft O, Iversen M, Simonsen S. and Floisand Y. (2013). Lung transplantation for bronchiolitis obliterans syndrome after allo-SCT. Bone Marrow Transplant 48:703–707 [DOI] [PubMed] [Google Scholar]
- 31.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F. and Napolitani G. (2007). Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 8:639–646 [DOI] [PubMed] [Google Scholar]
- 32.Ringden O, Uzunel M, Sundberg B, Lonnies L, Nava S, Gustafsson J, Henningsohn L. and Le Blanc K. (2007). Tissue repair using allogeneic mesenchymal stem cells for hemorrhagic cystitis, pneumomediastinum and perforated colon. Leukemia 21:2271–2276 [DOI] [PubMed] [Google Scholar]
- 33.Ringdén O. (2013). Mesenchymal stem cells for clinical/therapeutic interventions of graft-vesrus-host disease. In: Stem Cell-Dependent Therapies: Mesenchymal Stem Cells in Chronic Inflammatory Disorders. Gross G. and Häupl T, eds. De Gruyter, Berlin, pp. 101–124 [Google Scholar]
- 34.Karp JM. and Leng Teo GS. (2009). Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 4:206–216 [DOI] [PubMed] [Google Scholar]
- 35.Chapel A, Bertho JM, Bensidhoum M, Fouillard L, Young RG, Frick J, Demarquay C, Cuvelier F, Mathieu E, et al. (2003). Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med 5:1028–1038 [DOI] [PubMed] [Google Scholar]
- 36.von Bahr L, Batsis I, Moll G, Hagg M, Szakos A, Sundberg B, Uzunel M, Ringden O. and Le Blanc K. (2012). Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells 30:1575–1578 [DOI] [PubMed] [Google Scholar]
- 37.Li Y. and Lin F. (2012). Mesenchymal stem cells are injured by complement after their contact with serum. Blood 120:3436–3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moll G, Rasmusson-Duprez I, von Bahr L, Connolly-Andersen AM, Elgue G, Funke L, Hamad OA, Lonnies H, Magnusson PU, et al. (2012). Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem cells 30:1565–1574 [DOI] [PubMed] [Google Scholar]
- 39.Gholamrezanezhad A, Mirpour S, Bagheri M, Mohamadnejad M, Alimoghaddam K, Abdolahzadeh L, Saghari M. and Malekzadeh R. (2011). In vivo tracking of 111In-oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nucl Med Biol 38:961–967 [DOI] [PubMed] [Google Scholar]