Abstract
Many drugs used to treat inflammatory diseases are ineffective in a substantial proportion of patients. Identifying patients that are likely to respond to specific therapies would facilitate personalized treatment strategies that could improve outcomes while reducing costs and risks of adverse events. Despite these clear benefits, there are limited examples of predictive biomarkers of drug efficacy currently implemented into clinical practice for inflammatory diseases. We review efforts to identify genetic and nongenetic biomarkers of drug response in these diseases and consider potential benefits from combining multiple sources of biological data into multifeature predictive models.
Keywords: biologics, biomarkers, immune system, inflammatory disease, personalized medicine, steroids
Background
Interindividual variation in response to medical treatments is a serious challenge for the treatment of inflammatory disorders (e.g., asthma, inflammatory bowel disease and rheumatoid arthritis). While much attention has been paid to the risk of adverse events [1-4], there is also widespread variation in drug efficacy. Many treatments that are considered effective on average do not decrease disease activity in a substantial fraction of patients. For example, glucocorticoids, thiopurines and anti-TNF monoclonal antibodies are recommended treatments for both Crohn’s disease and ulcerative colitis. All three of these types of drugs are ineffective in a large number of patients. Approximately 16–30% of patients with inflammatory bowel disease (IBD) who are treated with glucocorticoids do not experience a regression of symptoms and require alternate therapies [5,6]. Nonresponse rates as high as 55% have been seen for thiopurines in patients with Crohn’s disease [7]. Similarly, clinical trials of anti-TNF therapies showed induction of remission in <50% of patients [8]. This observation is not limited to IBD. For example, similar rates of nonresponse have been observed for glucocorticoids in asthma (~30%) [9] and anti-TNF-α antibodies in rheumatoid arthritis (30–40%) [10]. For many treatments, it is currently impossible to identify patients that are unlikely to respond. Predictive biomarkers could be used to guide treatments toward patients who are likely to have a successful response and away from those who are not.
The ideal biomarker would directly measure the factors that ultimately cause variation in response, facilitating accurate and robust prediction. Unlike therapeutic drug monitoring or pharmacodynamic biomarkers, causative factors could be assessed prior to treatment. Variation in drug response can be caused by both genetic and nongenetic factors. Genetic variants are attractive biomarkers due to standardized assays (e.g., sequencing or microarrays) and analyses (e.g., association mapping) used to identify and validate tests. Genetic biomarkers may not exist for all drug response traits or may have insufficient predictive value to be clinically useful. This is an important consideration, as nongenetic factors can make substantial contributions to interindividual variation in drug response. For example, cigarette smoking at time of treatment is strongly associated with a weaker response to treatment with anti-TNF-α antibodies in patients with rheumatoid arthritis [11-14]. While cigarette smoking can be easily recorded and incorporated into predictive models (and used as a covariate in genetic mapping experiments), other nongenetic factors that are currently unknown and difficult to identify (e.g., other environmental exposures) may also influence treatment outcome. Fortunately, many nongenetic biological measurements (e.g., gene-expression levels) can capture both genetic and nongenetic causative factors. For example, cigarette smoking is associated with changes in expression of specific genes in peripheral lymphocytes [15]. This suggests that gene-expression profiles could, presumably, identify smokers even if smoking behavior was not directly identifiable. However, nongenetic biological measurements such as gene-expression levels, circulating protein levels, and cellular assays do not always have clear connections to underlying causative factors. This makes it more difficult to avoid confounding in correlative studies between these measurements and treatment outcomes. Here we discuss the use of genetic and nongenetic biomarkers, as well as the potential benefits from leveraging the relative strengths of each to develop robust and highly accurate assays to predict drug response. We note that this review is not meant to be an exhaustive discussion of all efforts to identify predictive biomarkers for drug response in all inflammatory diseases, but rather to highlight key studies that exemplify general issues encountered by the field.
Genetic predictors: benefits & challenges
There has been considerable attention on the potential use of genetic variants as biomarkers of treatment response (i.e., pharmacogenomics). Genetic predictors have a number of benefits over other biomarkers. Germ-line genetic variation across the genome can now be assayed in a relatively cheap and comprehensive manner (e.g., using genotyping microarrays). These technologies, and corresponding analytical approaches, are agnostic to phenotype. This allows the same approach to be applied across multiple treatment outcomes. Many advantages stem from the fact that germ-line genetic variation is stable throughout a patient’s lifespan. From an implementation perspective, this allows a large number of genetic variants that influence many different treatment outcomes to be assayed in a single multiplexed test that can be performed at any time (e.g., regardless of disease status, current treatment, etc). Indeed, efforts to broadly genotype and store genetic information for future use are currently underway at multiple healthcare institutions (eg., [16,17]). This approach could be widely adopted and expanded upon in the near future; for example, preemptive whole genome sequencing upon entering a healthcare system could become routine practice. The stability of genotype over time and the random nature of chromosomal segregation in meiosis limits the potential for confounding effects in association studies allowing causal inference in well-controlled studies (reviewed in [18]). Robust statistical frameworks have been developed in the last few decades to estimate and control for relatedness and population structure in genetic association studies [19,20].
Despite these benefits, according to the Clinical Pharmacogenetics Implementation Consortium there are only currently 17 pharmacogenetic tests recommended for implementation [21]. This includes three tests with relevance for treatment of inflammatory diseases; variants in TPMT associated with azathioprine toxicity, variants in HLA-B associated with allopurinol hypersensitivity, and variants in CYP3A genes associated with tacrolimus pharmacokinetics. This relatively small number of clinically implemented tests partially reflects the general inability to identify high-confidence genetic associations that also have sufficient predictive value to be clinically useful [22]. For example, variability in response to expensive anti-TNF-α antibodies has prompted efforts to identify biomarkers that can predict response. This has included multiple genome-wide association studies (GWAS) intended to provide unbiased scans for variants associated with response. These studies have not yielded well-replicated associations. In a GWAS of clinical response in 89 rheumatoid arthritis patients, Liu et al. [23] reported 16 genome-wide significant signals (based on permutation – no association met the generally accepted, although conservative, significance threshold for GWAS of 5 × 10−8). A subsequent and larger (n = 1340, divided into three stages) GWAS in rheumatoid arthritis patients reported suggestive associations [24], but did not replicate those reported by Liu et al. [23]. An additional GWAS in 196 rheumatoid arthritis patients by Krintel et al. [25] did not replicate signals from either of these previous GWAS and did not report any novel genome-wide significant associations.
While this could be due to a number of factors, polygenic architecture is likely to play a major role. It has become clear that many biomedical traits in humans are highly polygenic, meaning that they are influenced by a large number of genetic polymorphisms with small effects [26,27]. The cumulative effects of these polymorphisms might be large enough that a multifeature model could explain a substantial proportion of the phenotypic variance [28], but confidently identifying an association at each polymorphism may be very difficult. For example, estimates of ‘chip heritability’, or the proportion of phenotypic variance explained by genetic information across all assayed polymorphisms, can be very large for phenotypes where few to no individual polymorphisms were confidently associated [28,29]. For example, a GWAS for response to albuterol in 1644 asthma patients did not identify any significant associations after correcting for multiple tests (although an interesting candidate variant was identified through follow-up analyses and experiments) [30]. However, subsequent polygenic modeling of the same dataset estimated that common polymorphisms explained 28.6% (standard error 16%; p = 0.043) of the variation in response [31].
Polygenic models, which combine information across large numbers of polymorphisms, are emerging as powerful tools to perform genetic analysis of phenotypes with polygenic architecture. This approach has not yet been widely applied to drug response traits. Despite improvements in modeling, the reliability of any genetic predictor will ultimately depend on an accurate estimate of the effect at individual polymorphisms [32], and this will likely require large sample sizes for polygenic traits. Indeed, a recent study used simulations to show that successful application of common polygenic modeling approaches would require sample sizes greater than 1000 individuals for traits with less than 50% heritability [33].
Nongenetic biomarkers of drug response
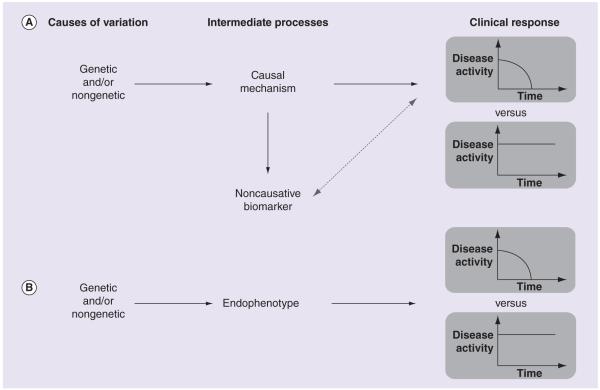
Biomarkers based on nongenetic biological measurements may have a much stronger predictive value than genetic biomarkers, as they can reflect genotype at multiple causative variants, as well as nongenetic factors that also may influence treatment outcome. Unlike genetic predictors, other biological measurements can be unstable over time depending on external factors. Even epigenetic measurements (e.g., DNA methylation and histone modification) can be influenced by environmental factors and stochastic changes throughout a patient’s lifespan (reviewed in [34]). The challenge posed by nongenetic biomarkers is that, unlike germline genetic predictors, an association with clinical outcome does not necessarily imply a causative relationship. A classical example is the relationship between serum levels of lipoproteins and cardiovascular disease (CVD) risk. As first established by the Framingham study in 1977 [35] and subsequently replicated by numerous studies (meta-analysis in [36]), high-density lipoprotein (HDL) levels are negatively correlated with risk of CVD while low-density lipoprotein (LDL) levels are positively correlated with CVD risk. These measurements are, therefore, commonly used as predictive biomarkers for CVD outcomes. Although both biomarkers show strong correlations, subsequent work has shown that LDL has a causal relationship with CVD while HDL does not. A well-powered study in 2012 found that genetic variants that effect LDL levels are associated with myocardial infarction while genetic variants that affect HDL levels are not [37]. This corroborated results from clinical trials that have shown efficacy for drugs that target LDL (notably statins, as well as newer drugs reviewed in [38]) and no demonstrable efficacy for drugs that target HDL (reviewed in [39]). Noncausative biomarkers, such as HDL, are correlated with clinical outcomes through indirect relationships (e.g., a separate biological process has independent effects on the biomarker and on the clinical outcome – Figure 1A), which may or may not be consistent across patient populations.
Figure 1. The relationship between genetic and nongenetic causes of variation, nongenetic biomarkers and clinical response to therapy.
Genetic and nongenetic factors influence treatment response (decrease in disease activity following treatment) through intermediate biological processes. (A) A nongenetic biomarker may be correlated with clinical response without being causative if a biological process exists that independently influences both the biomarker and clinical response. The dashed, two-headed arrow represents a correlation between the noncausative biomarkers and clinical response. (B) Endophenotypes are nongenetic biomarkers that directly measure biological processes that mediate the effects of causal factors.
Following the development in the last decade of high-throughput and reliable molecular assays (e.g., microarrays and then next-generation sequencing), molecular biomarkers (especially transcript levels) can now be accurately measured in large numbers of individuals and correlated with clinical outcomes. A notable example is AlloMap, a US FDA-approved gene-expression signature for predicting rejection despite immunosuppressive therapy following heart transplantation. In 2006, Deng et al. [40] used microarrays to identify a set of 262 genes where expression differed in peripheral blood mononuclear cells (PBMCs) between patients who did and did not reject heart transplants. Validation in an additional patient population sample revealed that expression of a set of 11 of these 262 genes could predict rejection with 84% accuracy [40]. A subsequent randomized clinical trial showed that monitoring transplant recipients using this gene-expression signature was as effective as traditional monitoring approaches and much less invasive [41]. It is important to note that blood was drawn after patients had undergone transplantation. The gene-expression profile likely reflects mononuclear cells responding to physiological conditions that eventually lead to rejection, as suggested by the authors of the initial study [40]. In other words, this gene-expression profile is most strongly associated when it is measured under a specific set of conditions (e.g., post-transplant). It is also unclear if these genes directly contribute to rejection.
While not yet widely implemented into clinical practice, gene-expression profiles have shown strong correlations with other clinical outcomes in immune-related diseases. For example, several groups have explored gene-expression signatures that can predict response to costly anti-TNF-α antibodies. Arijs et al. [42] used expression microarrays to find 53 genes differentially expressed in colonic mucosal biopsies (taken at first treatment) between UC patients who did and did not respond to infliximab treatment. Collectively, the authors estimated that these genes could predict response with 95% sensitivity and 85% specificity [42]. A similar study identified a signature composed of expression of five genes in colonic mucosal biopsies that could completely distinguish infliximab responders and nonresponders in a sample of patients with Crohn’s disease [43]. Both of these studies had relatively small sample sizes (two cohorts with 21 and 24 patients for Arijs et al. [42] and one sample of 19 patients for Arijs et al. [43]), yet they were still able to identify genes with significant differences in expression pattern based on clinical outcome.
As for all biomarkers, replication of gene-expression signatures is key to moving forward with clinical implementation. This has been problematic for gene-expression signatures. Toonen et al. [44] tested whole blood gene-expression signatures identified in eight previous publications for their ability to predict infliximab or adalimumab response in 42 patients with rheumatoid arthritis and were only able to validate the signature from one study. This low validation rate could reflect a high false-positive rate in initial studies or insufficient power in the validation study. However, many of the studies used standard statistical approaches and were of similar size to the validation study. For example, Julia et al. [45] reported a signature identified in 44 patients that involved expression of eight genes. The authors used leave-one-out-cross-validation to estimate a predictive accuracy of 97% and then proceeded to validate the model in an additional sample of 14 patients with 86% predictive accuracy. This model then showed low predictive accuracy in the subsequent validation study by Toonen et al. [44]. It is possible that a lack of replication for this and other gene-expression signatures reflects the well-known sensitivity of gene-expression patterns to external perturbations (e.g., environmental exposures or medical treatments). In other words, gene-expression signatures may be inherently unstable unless measurement conditions are tightly controlled.
Measuring molecular and cellular processes following in vitro tissue culturing and treatment allows experimental conditions to be more tightly controlled as compared with measurements taken directly from biological samples without manipulation. This approach can be used to test specific biological processes relevant to treatment outcome, such as the change in expression levels upon exposure to a drug. In vitro models can be used to measure an individual’s sensitivity to a drug in a specific context, such as the ability of a drug to suppress a disease-related biological process in a particular tissue. For example, in vitro inhibition of immune processes by glucocorticoids in cultured white blood cells has been correlated with clinical response in a number of inflammatory diseases. Multiple such studies have used phytohemagglutinin or direct T-cell receptor agonists to stimulate T-cell proliferation in the presence and absence of glucocorticoids. The relative inhibition of proliferation has been shown to be correlated with clinical response in patients with severe UC [46-48], Crohn’s disease [48], rheumatoid arthritis [49], asthma [50], and organ transplant rejection [51]. This approach is not limited to glucocorticoids. Cossu et al. [52] recently found that in vitro induction of apoptosis and inhibition of IFN-γ by thiopurines (azathioprine, 6-mercaptopurine and 6-thioguanine) in CD4+ T cells activated by direct T-cell receptor binding was correlated with clinical response to thiopurine treatment in patients with Crohn’s disease.
A similar approach could be used to assay gene-expression response to in vitro treatment. This approach is promising, but has not yet been widely adopted. One exception is a study of 106 asthma patients showed that an expression signature from 15 genes in PBMCs treated in vitro with either GC or control could discriminate GC resistant patients with >80% accuracy [53]. Glucocorticoid-mediated changes in gene expression at the most strongly associated gene in this study, that is, NFKB1, has been correlated with glucocorticoid inhibition of T-cell proliferation in healthy donors [54,55]. However, validation of this association with clinical response is still needed.
Genetic mapping of endophenotypes in pharmacogenomics
Interindividual variation in drug response reflects underlying genetic and nongenetic causative factors that act through effects on biological processes at intermediate levels between those factors and clinical response to therapy. For example, a genetic variant might influence expression levels of a particular gene, which in turn might influence cellular sensitivity at the effect site, and this might ultimately determine clinical response. As discussed previously, measurements of intermediate biological processes (e.g., gene expression) can be used as biomarkers of clinical outcome (i.e., gene-expression signatures). These measurements are also phenotypes, as each intermediate process may be influenced by a combination of genetic and nongenetic factors. These molecular and cellular traits are referred to as ‘intermediate phenotypes’ or ‘endophenotypes’. The key distinction between endophenotypes and other biomarkers is that they are measurements of a process that directly mediates the effect of a causative factor on clinical response, as opposed to being only correlated with such a process (Figure 1B). This allows endophenotypes to be used to identify genetic variants that influence a corresponding clinical outcome. Molecular and cellular phenotypes are likely influenced by a smaller number of factors than clinical phenotypes and, therefore, may have simpler and more tractable genetic architecture. This is, essentially, because clinical outcome depends only partially on any given endophenotype. These, and other, issues related to genetic analysis of complex phenotypes are discussed in greater depth in Manolio et al. [56] and Gibson [57].
While genetic mapping of endophenotypes (mainly cellular sensitivity to chemotherapy or radiation) has been widely applied for response to cancer therapies [58], examples for drugs used to treat inflammatory diseases are more limited. One exception is a study that performed genetic analysis of in vitro inhibition of T-cell proliferation by glucocorticoids [55], a cellular assay that has been associated with clinical response to glucocorticoids in multiple diseases. This study revealed a genetic variant with cis-acting regulatory effects on a nearby gene (RBMS3) that was also associated in trans with changes in gene expression at other genes and with the magnitude of glucocorticoid inhibition of T-cell proliferation. While these results suggest that this genetic variant and associated gene-expression patterns may be useful biomarkers of glucocorticoid response, the relationship of these features with clinical response remains to be investigated.
Association mapping of gene-expression levels, usually called expression quantitative trait locus (or eQTL) mapping, has been successfully applied to identify loci that harbor regulatory polymorphisms in a variety of cell types and conditions (e.g., [59-65]). Based on the hypothesis that gene-expression levels (under measured conditions) influence treatment outcome, eQTLs can be used as ‘candidate’ variants to improve power in GWAS. In support of this approach, it has been shown that eQTLs are more likely than other SNPs in the genome to be associated with many complex diseases [66] and with chemotherapy-induced in vitro cytotoxicity [67]. This approach was used by Cui et al. [68] to identify genetic variants associated with etanercept response in patients with rheumatoid arthritis. Similar to other GWAS of anti-TNF-α response, this study did not identify any genome-wide significant associations (as discussed previously in the section titled ‘Genetic predictors: benefits and challenges’). When the authors integrated their GWAS results from an eQTL study in PBMCs, they found that the most strongly associated variant from the GWAS was also strongly associated with expression of CD84. In turn, CD84 expression level in PBMCs was correlated with disease activity in patients with rheumatoid arthritis and showed a trend toward association with change in disease activity following etanercept treatment.
Gene-expression levels can also be measured in the presence and absence of in vitro treatments in a population sample. This approach can be used to map genetic variants that interact with treatment to influence changes in expression (this approach is reviewed in greater depth in Maranville et al. [69] and Idaghdour et al. [70]). For example, a mapping experiment with glucocorticoid treatment in HapMap lymphoblastoid cell lines revealed 26 genes with treatment-dependent eQTLs, including one for TNIP1 that was also associated with glucocorticoid response in asthma patients [71]. A similar approach was recently used to identify a treatment (simvastatin)-specific cis-eQTL for GATM in lymphoblastoid cell lines that was also significantly associated with risk of simvastatin-induced myopathy [72]. Interestingly, GATM encodes glycine amidinotransferase, a rate-limiting enzyme in the biosynthesis of creatine, suggesting a novel mechanism for statin-related myopathy [73].
Conclusion
Genetic variants have inherent benefits over other predictive biomarkers. They can be measured well ahead of clinical need and as part of relatively inexpensive multiplexed assays. Indeed, multiple healthcare systems have already initiated programs to perform large-scale preemptive genotyping. It is likely that this approach will be more widely adopted in the coming decades. Despite benefits of this approach and widespread interindividual variation in clinical response, there are very few clinically useful pharmacogenetic biomarkers. This is especially true for drug efficacy in inflammatory diseases, where there are currently no validated genetic markers with sufficient predictive value to be used to guide treatment decisions in the clinic.
On the other hand, many studies have identified gene-expression signatures that show very strong correlations with clinical response to multiple drugs used to treat inflammatory diseases. Many of these studies report predictive values that, if accurate and robust, would enable these signatures to be clinically useful. With some notable exceptions (e.g., AlloMap), the challenge with gene-expression signatures has been a lack of replication across studies. This could reflect the ephemeral nature of gene-expression levels, where differences in patient exposures between studies strongly influence patterns of expression. This could also reflect the unclear causal relationship between expression measurements and correlated clinical outcomes. Measuring gene-expression patterns under controlled conditions in tissue samples cultured and treated in vitro may provide more robust predictors of drug response. Additional studies are warranted to explore this approach.
Leveraging the relative benefits of genetic and nongenetic measurements is a promising path forward in the effort to develop predictive biomarkers. Studies should be designed to leverage the hierarchical structure of this data, where genotype is an ultimate cause and endophenotypes (e.g., gene expression and cellular processes) mediate the effects of ultimate causes on clinical outcomes. For example, under this model genotypes are expected to show stronger associations with endophenotypes than with clinical phenotypes. This has been harnessed successfully in many studies to identify candidate genetic variants for mapping clinical outcomes by first mapping (presumably relevant) endophenotypes (e.g., [68,71,73,74]). Endophenotypes from in vitro culturing and treatment of relevant cell types may be especially informative as specific biological processes can be assayed under controlled conditions.
Future perspective
Given the tendency for small effect sizes at genetic variants and strong but unstable correlations for gene-expression signatures, it is likely that the combination of genetic and nongenetic biological information will provide the best predictive signatures of treatment outcome. This will likely include both low-level molecular features (e.g., gene-expression levels) and cellular features (e.g., in vitro measures of cellular sensitivity). The optimal method for building these models is currently unclear and will likely need to be assessed based on real data. It would likely be beneficial, however, to consider the underlying biology when constructing these models. Consider the previously mentioned study that found a regulatory variant associated with variation among healthy donors in gene-expression response at multiple genes and in glucocorticoid inhibition of T-cell proliferation [55]. Correlation analyses of gene-expression response with inhibition of T-cell proliferation revealed a set of 27 genes with significant associations. The observation of correlation does not provide evidence that these genes have a causal relationship with inhibition of T-cell proliferation. Indeed, it is impossible to exclude that these correlations reflect some unmeasured confounder that cannot be controlled for by using traditional genomic analyses (e.g., principal component analysis). Genetic analysis revealed a variant that was significantly associated with inhibition of T-cell proliferation and with gene-expression response at 14 of the 27 genes that comprised this ‘signature’, which suggests that gene-expression response at these genes mediates the effects of this variant on inhibition of T-cell proliferation. Because they are implicated in a causal pathway, one could speculate that a gene-expression signature comprised of these 14 genes versus the other 13 genes will show a more robust association with inhibition of T-cell proliferation by glucocorticoids. Carefully integrating genetic and nongenetic information along these lines may lead to multifeature models that have the predictive values of gene-expression signatures and the reliability of genetic markers. Such predictive models will also need to be evaluated in the context of other factors that may influence drug response, such as age and ancestry. Unfortunately, research in this area is often performed in narrow patient populations, with limited inclusion of non-European ancestral backgrounds and special populations (e.g., children). For example, all of the patient studies highlighted include predominately individuals of European ancestry, and only one study included patients under 18 years of age (Table 1). Broad application of predictive models would benefit greatly from future consideration of more diverse patient populations.
Table 1.
Studies of biomarkers for drug response in inflammatory diseases.
| Study | Drug class | Type of biomarker |
Sample size |
Ages | Ancestry | Disease | Ref. |
|---|---|---|---|---|---|---|---|
| Liu et al. | Anti-TNF antibodies | Genetic | 89 | >18 | European | Rheumatoid arthritis |
[23] |
| Plant et al. | Anti-TNF antibodies | Genetic | 1340 | >18 | European | Rheumatoid arthritis |
[24] |
| Krintel et al. | Anti-TNF antibodies | Genetic | 196 | European | Rheumatoid arthritis |
[25] | |
| Himes et al. | Albuterol | Genetic | 1644 | <18 (1 group), >18 (7 groups) |
European | Asthma | [30] |
| McGeachie et al. |
Albuterol | Genetic | 1644 | <18 (1 group), >18 (7 groups) |
European | Asthma | [31] |
| Deng et al. | Immunosuppressive drugs |
Gene expression |
629 | >18 (recipients: 93.6%, donors: 82.1%) |
European (recipients: 72.3%, donors: 71.4%) |
Organ transplant |
[40] |
| Arijs et al. | Anti-TNF antibodies | Gene expression |
21 | >18 | NA | Ulcerative colitis |
[42] |
| Arijs et al. | Anti-TNF antibodies | Gene expression |
19 | >18 | NA | Crohn′s disease | [43] |
| Toonen et al. | Anti-TNF antibodies | Gene expression |
42 | >18 | NA | Rheumatoid arthritis |
[44] |
| Julia et al. | Anti-TNF antibodies | Gene expression |
44 | >18 | NA | Rheumatoid arthritis |
[45] |
| Hakonarson et al. |
Glucocorticoids | Gene- expression response |
106 | >18 | European | Asthma | [53] |
| Hearing et al. | Glucocorticoids | Cellular assays | 17 | >18 | NA | Ulcerative colitis |
[46] |
| Lee et al. | Glucocorticoids | Cellular assays | 29 | >18 | European | Crohn′s disease | [47] |
| Kirkham et al. |
Glucocorticoids | Cellular assays | Rheumatoid arthritis |
[49] | |||
| Corrigan et al. |
Glucocorticoids | Cellular assays | 16 | >18 | NA | Asthma | [50] |
| Langhoff et al. |
Glucocorticoids | Cellular assays | Organ transplant |
[51] | |||
| Cossu et al. | Thiopurines | Cellular assays | 29 | >18 | NA | Crohn′s disease | [52] |
NA: Not applicable.
Executive summary.
Background
A substantial proportion of patients do not respond to treatment with many of the drugs used in inflammatory diseases.
Predictive biomarkers could guide patients to effective drugs.
Genetic predictors: benefits & challenges
-
Genetic variants have benefits over other across biomarkers:
– They can be measured well ahead of clinical need using relatively inexpensive multiplexed assays.
– Causation can be established in well-controlled studies.
Despite these benefits, there are very few examples of clinically useful pharmacogenetic biomarkers, potentially reflecting the polygenic architecture of many traits.
Nongenetic biomarkers of drug response
Nongenetic biomarkers, such as gene-expression signatures, can show very strong correlations with clinical response to drug treatment.
Efforts to develop gene-expression signatures of drug response have suffered from a lack of replication across studies.
This could reflect the ephemeral nature of gene-expression level measurements and the unclear causal relationship between biomarkers and treatment outcomes.
Genetic mapping of endophenotpyes in pharmacogenomics
Association mapping of endophenotypes can be used to identify candidate genetic variants for related clinical response traits.
Conclusion
Combining genetic and nongenetic measurements is a promising path forward in the effort to develop predictive biomarkers.
It is likely that multifeature models that include both genetic and nongenetic biological information will provide the best predictive signatures of treatment outcome.
Acknowledgments
JC Maranville was supported by a Clinical Therapeutics training grant to the University of Chicago (T32GM007019). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Wei CY, Lee MT, Chen YT. Pharmacogenomics of adverse drug reactions: implementing personalized medicine. Hum. Mol. Genet. 2012;21(R1):R58–R65. doi: 10.1093/hmg/dds341. [DOI] [PubMed] [Google Scholar]
- 2.Karlin E, Phillips E. Genotyping for severe drug hypersensitivity. Curr. Allergy Asthma Rep. 2014;14(3):418. doi: 10.1007/s11882-013-0418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaniwa N, Saito Y. Pharmacogenomics of severe cutaneous adverse reactions and drug-induced liver injury. J. Hum. Genet. 2013;58(6):317–326. doi: 10.1038/jhg.2013.37. [DOI] [PubMed] [Google Scholar]
- 4.Hosford DA, Lai EH, Riley JH, Xu CF, Danoff TM, Roses AD. Pharmacogenetics to predict drug-related adverse events. Toxicol. Pathol. 2004;32(Suppl. 1):9–12. doi: 10.1080/01926230490424743. [DOI] [PubMed] [Google Scholar]
- 5.Creed TJ, Probert CS. Review article: steroid resistance in inflammatory bowel disease – mechanisms and therapeutic strategies. Aliment Pharmacol. Ther. 2007;25(2):111–122. doi: 10.1111/j.1365-2036.2006.03156.x. [DOI] [PubMed] [Google Scholar]
- 6.Sidoroff M, Kolho KL. Glucocorticoid sensitivity in inflammatory bowel disease. Ann. Med. 2012;44(6):578–587. doi: 10.3109/07853890.2011.590521. [DOI] [PubMed] [Google Scholar]
- 7.Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut. 2002;50(4):485–489. doi: 10.1136/gut.50.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stidham RW, Lee TC, Higgins PD, et al. Systematic review with network meta-analysis: the efficacy of anti-TNF agents for the treatment of Crohn’s disease. Aliment Pharmacol. Ther. 2014;39(12):1349–1362. doi: 10.1111/apt.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan MT, Leung DY, Szefler SJ, Spahn JD. Difficult-to-control asthma: clinical characteristics of steroid-insensitive asthma. J. Allergy Clin. Immunol. 1998;101(5):594–601. doi: 10.1016/S0091-6749(98)70165-4. [DOI] [PubMed] [Google Scholar]
- 10.Zink A, Strangfeld A, Schneider M, et al. Effectiveness of tumor necrosis factor inhibitors in rheumatoid arthritis in an observational cohort study: comparison of patients according to their eligibility for major randomized clinical trials. Arthritis Rheum. 2006;54(11):3399–3407. doi: 10.1002/art.22193. [DOI] [PubMed] [Google Scholar]
- 11.Saevarsdottir S, Wedren S, Seddighzadeh M, et al. Patients with early rheumatoid arthritis who smoke are less likely to respond to treatment with methotrexate and tumor necrosis factor inhibitors: observations from the Epidemiological Investigation of Rheumatoid Arthritis and the Swedish Rheumatology Register cohorts. Arthritis Rheum. 2011;63(1):26–36. doi: 10.1002/art.27758. [DOI] [PubMed] [Google Scholar]
- 12.Abhishek A, Butt S, Gadsby K, Zhang W, Deighton CM. Anti-TNF-alpha agents are less effective for the treatment of rheumatoid arthritis in current smokers. J. Clin. Rhematol. 2010;16(1):15–18. doi: 10.1097/RHU.0b013e3181ca4a2a. [DOI] [PubMed] [Google Scholar]
- 13.Canhao H, Rodrigues AM, Mourao AF, et al. Comparative effectiveness and predictors of response to tumour necrosis factor inhibitor therapies in rheumatoid arthritis. Rheumatology (Oxford) 2012;51(11):2020–2026. doi: 10.1093/rheumatology/kes184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyrich KL, Watson KD, Silman AJ, Symmons DP. Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford) 2006;45(12):1558–1565. doi: 10.1093/rheumatology/kel149. [DOI] [PubMed] [Google Scholar]
- 15.Charlesworth JC, Curran JE, Johnson MP, et al. Transcriptomic epidemiology of smoking: the effect of smoking on gene expression in lymphocytes. BMC Med. Genomics. 2010;3:29. doi: 10.1186/1755-8794-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman JM, Haidar CE, Wilkinson MR, et al. PG4KDS: a model for the clinical implementation of pre-emptive pharmacogenetics. Am. J. Med. Genet. C Semin. Med. Genet. 2014;166C(1):45–55. doi: 10.1002/ajmg.c.31391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’donnell PH, Danahey K, Jacobs M, et al. Adoption of a clinical pharmacogenomics implementation program during outpatient care--initial results of the University of Chicago “1,200 Patients Project”. Am. J. Med. Genet. C Semin. Med. Genet. 2014;166C(1):68–75. doi: 10.1002/ajmg.c.31385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vansteelandt S, Lange C. Causation and causal inference for genetic effects. Hum. Genet. 2012;131(10):1665–1676. doi: 10.1007/s00439-012-1208-9. [DOI] [PubMed] [Google Scholar]
- 19.Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2(12):e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou X, Stephens M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 2012;44(7):821–824. doi: 10.1038/ng.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caudle KE, Klein TE, Hoffman JM, et al. Incorporation of pharmacogenomics into routine clinical practice: the clinical pharmacogenetics implementation consortium (CPIC) guideline development process. Curr. Drug Metab. 2014;15(2):209–217. doi: 10.2174/1389200215666140130124910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George AL., Jr Appraising the value of genomic association studies. J. Am. Soc. Nephrol. 2008;19(10):1840–1842. doi: 10.1681/ASN.2008050524. [DOI] [PubMed] [Google Scholar]
- 23.Liu C, Batliwalla F, Li W, et al. Genome-wide association scan identifies candidate polymorphisms associated with differential response to anti-TNF treatment in rheumatoid arthritis. Mol. Med. 2008;14(9–10):575–581. doi: 10.2119/2008-00056.Liu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plant D, Bowes J, Potter C, et al. Genome-wide association study of genetic predictors of anti-tumor necrosis factor treatment efficacy in rheumatoid arthritis identifies associations with polymorphisms at seven loci. Arthritis Rheum. 2011;63(3):645–653. doi: 10.1002/art.30130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krintel SB, Palermo G, Johansen JS, et al. Investigation of single nucleotide polymorphisms and biological pathways associated with response to TNFalpha inhibitors in patients with rheumatoid arthritis. Pharmacogenet. Genomics. 2012;22(8):577–589. doi: 10.1097/FPC.0b013e3283544043. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Benyamin B, McEvoy BP, et al. Common SNPs explain a large proportion of the heritability for human height. Nat. Genet. 2010;42(7):565–569. doi: 10.1038/ng.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SH, Wray NR, Goddard ME, Visscher PM. Estimating missing heritability for disease from genome-wide association studies. Am. J. Hum. Genet. 2011;88(3):294–305. doi: 10.1016/j.ajhg.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jostins L, Barrett JC. Genetic risk prediction in complex disease. Hum. Mol. Genet. 2011;20(R2):R182–R188. doi: 10.1093/hmg/ddr378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang J, Cho J, Zhao H. Practical issues in building risk-predicting models for complex diseases. J. Biopharm. Stat. 2010;20(2):415–440. doi: 10.1080/10543400903572829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Himes BE, Jiang X, Hu R, et al. Genome-wide association analysis in asthma subjects identifies SPATS2L as a novel bronchodilator response gene. PLoS Genet. 2012;8(7):e1002824. doi: 10.1371/journal.pgen.1002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mcgeachie MJ, Stahl EA, Himes BE, et al. Polygenic heritability estimates in pharmacogenetics: focus on asthma and related phenotypes. Pharmacogenet. Genomics. 2013;23(6):324–328. doi: 10.1097/FPC.0b013e3283607acf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans DM, Visscher PM, Wray NR. Harnessing the information contained within genome-wide association studies to improve individual prediction of complex disease risk. Hum. Mol. Genet. 2009;18(18):3525–3531. doi: 10.1093/hmg/ddp295. [DOI] [PubMed] [Google Scholar]
- 33.Marshall SL, Guennel T, Kohler J, Man M, Fossceco S. Estimating heritability in pharmacogenetic studies. Pharmacogenomics. 2013;14(4):369–377. doi: 10.2217/pgs.13.20. [DOI] [PubMed] [Google Scholar]
- 34.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 2003;33(Suppl.):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 35.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am. J. Med. 1977;62(5):707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 36.Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380(9841):572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sahebkar A, Watts GF. New LDL-cholesterol lowering therapies: pharmacology, clinical trials, and relevance to acute coronary syndromes. Clin. Ther. 2013;35(8):1082–1098. doi: 10.1016/j.clinthera.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 39.Wright RS. Recent clinical trials evaluating benefit of drug therapy for modification of HDL cholesterol. Curr. Opin. Cardiol. 2013;28(4):389–398. doi: 10.1097/HCO.0b013e328362059d. [DOI] [PubMed] [Google Scholar]
- 40.Deng MC, Eisen HJ, Mehra MR, et al. Noninvasive discrimination of rejection in cardiac allograft recipients using gene expression profiling. Am. J. Transplant. 2006;6(1):150–160. doi: 10.1111/j.1600-6143.2005.01175.x. [DOI] [PubMed] [Google Scholar]
- 41.Pham MX, Teuteberg JJ, Kfoury AG, et al. Gene-expression profiling for rejection surveillance after cardiac transplantation. N. Engl. J. Med. 2010;362(20):1890–1900. doi: 10.1056/NEJMoa0912965. [DOI] [PubMed] [Google Scholar]
- 42.Arijs I, Li K, Toedter G, et al. Mucosal gene signatures to predict response to infliximab in patients with ulcerative colitis. Gut. 2009;58(12):1612–1619. doi: 10.1136/gut.2009.178665. [DOI] [PubMed] [Google Scholar]
- 43.Arijs I, Quintens R, Van Lommel L, et al. Predictive value of epithelial gene expression profiles for response to infliximab in Crohn’s disease. Inflamm. Bowel Dis. 2010;16(12):2090–2098. doi: 10.1002/ibd.21301. [DOI] [PubMed] [Google Scholar]
- 44.Toonen EJ, Gilissen C, Franke B, et al. Validation study of existing gene expression signatures for anti-TNF treatment in patients with rheumatoid arthritis. PLoS ONE. 2012;7(3):e33199. doi: 10.1371/journal.pone.0033199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Julia A, Erra A, Palacio C, et al. An eight-gene blood expression profile predicts the response to infliximab in rheumatoid arthritis. PLoS ONE. 2009;4(10):e7556. doi: 10.1371/journal.pone.0007556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hearing SD, Norman M, Smyth C, Foy C, Dayan CM. Wide variation in lymphocyte steroid sensitivity among healthy human volunteers. J. Clin. Endocrinol. Metab. 1999;84(11):4149–4154. doi: 10.1210/jcem.84.11.6156. [DOI] [PubMed] [Google Scholar]
- 47.Lee RW, Creed TJ, Schewitz LP, et al. CD4+CD25(int) T cells in inflammatory diseases refractory to treatment with glucocorticoids. J. Immunol. 2007;179(11):7941–7948. doi: 10.4049/jimmunol.179.11.7941. [DOI] [PubMed] [Google Scholar]
- 48.Maranville JC, Micic D, Hanauer SB, Di Rienzo A, Kupfer SS. In vitro sensitivity assays and clinical response to glucocorticoids in patients with inflammatory bowel disease. J. Crohns Colitis. 2014 doi: 10.1016/j.crohns.2014.06.013. doi:10.1016/j.crohns.2014.06.013. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirkham BW, Corkill MM, Davison SC, Panayi GS. Response to glucocorticoid treatment in rheumatoid arthritis: in vitro cell mediated immune assay predicts in vivo responses. J. Rhematol. 1991;18(6):821–825. [PubMed] [Google Scholar]
- 50.Corrigan CJ, Brown PH, Barnes NC, et al. Glucocorticoid resistance in chronic asthma. Glucocorticoid pharmacokinetics, glucocorticoid receptor characteristics, and inhibition of peripheral blood T cell proliferation by glucocorticoids in vitro. Am. Rev. Respir. Dis. 1991;144(5):1016–1025. doi: 10.1164/ajrccm/144.5.1016. [DOI] [PubMed] [Google Scholar]
- 51.Langhoff E, Ladefoged J, Jakobsen BK, et al. Recipient lymphocyte sensitivity to methylprednisolone affects cadaver kidney graft survival. Lancet. 1986;1(8493):1296–1297. doi: 10.1016/s0140-6736(86)91220-1. [DOI] [PubMed] [Google Scholar]
- 52.Cossu A, Biancone L, Ascolani M, Pallone F, Boirivant M. “In vitro” azathioprine-induced changes in peripheral T cell apoptosis and IFN-gamma production associate with drug response in patients with Crohn’s Disease. J. Crohns Colitis. 2013;7(6):441–450. doi: 10.1016/j.crohns.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 53.Hakonarson H, Bjornsdottir US, Halapi E, et al. Profiling of genes expressed in peripheral blood mononuclear cells predicts glucocorticoid sensitivity in asthma patients. Proc. Natl Acad. Sci. USA. 2005;102(41):14789–14794. doi: 10.1073/pnas.0409904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maranville JC, Baxter SS, Torres JM, Di Rienzo A. Inter-ethnic differences in lymphocyte sensitivity to glucocorticoids reflect variation in transcriptional response. Pharmacogenomics J. 2011 doi: 10.1038/tpj.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maranville JC, Baxter SS, Witonsky DB, Chase MA, Di Rienzo A. Genetic mapping with multiple levels of phenotypic information reveals determinants of lymphocyte glucocorticoid sensitivity. Am. J. Hum. Genet. 2013;93(4):735–743. doi: 10.1016/j.ajhg.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manolio TA, Collins FS, Cox NJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461(7265):747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gibson G. Rare and common variants: twenty arguments. Nat. Rev. Genet. 2011;13(2):135–145. doi: 10.1038/nrg3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wheeler HE, Dolan ME. Lymphoblastoid cell lines in pharmacogenomic discovery and clinical translation. Pharmacogenomics. 2012;13(1):55–70. doi: 10.2217/pgs.11.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pickrell JK, Marioni JC, Pai AA, et al. Understanding mechanisms underlying human gene expression variation with RNA sequencing. Nature. 464(7289):768–772. doi: 10.1038/nature08872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stranger BE, Nica AC, Forrest MS, et al. Population genomics of human gene expression. Nat. Genet. 2007;39(10):1217–1224. doi: 10.1038/ng2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Montgomery SB, Sammeth M, Gutierrez-Arcelus M, et al. Transcriptome genetics using second generation sequencing in a Caucasian population. Nature. 2010;464(7289):773–777. doi: 10.1038/nature08903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nica AC, Parts L, Glass D, et al. The architecture of gene regulatory variation across multiple human tissues: the MuTHER. study. PLoS Genet. 2011;7(2):e1002003. doi: 10.1371/journal.pgen.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grundberg E, Adoue V, Kwan T, et al. Global analysis of the impact of environmental perturbation on cis-regulation of gene expression. PLoS Genet. 2011;7(1):e1001279. doi: 10.1371/journal.pgen.1001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morley M, Molony CM, Weber TM, et al. Genetic analysis of genome-wide variation in human gene expression. Nature. 2004;430(7001):743–747. doi: 10.1038/nature02797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013;45(6):580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nicolae DL, Gamazon E, Zhang W, Duan S, Dolan ME, Cox NJ. Trait-associated SNPs are more likely to be eQTLs: annotation to enhance discovery from GWAS. PLoS Genet. 6(4):e1000888. doi: 10.1371/journal.pgen.1000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gamazon ER, Huang RS, Cox NJ, Dolan ME. Chemotherapeutic drug susceptibility associated SNPs are enriched in expression quantitative trait loci. Proc. Natl Acad. Sci. USA. 2010;107(20):9287–9292. doi: 10.1073/pnas.1001827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cui J, Stahl EA, Saevarsdottir S, et al. Genome-wide association study and gene expression analysis identifies cd84 as a predictor of response to etanercept therapy in rheumatoid arthritis. PLoS Genet. 2013;9(3):e1003394. doi: 10.1371/journal.pgen.1003394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maranville JC, Luca F, Stephens M, Di Rienzo A. Mapping gene-environment interactions at regulatory polymorphisms: insights into mechanisms of phenotypic variation. Transcription. 2012;3(2):56–62. doi: 10.4161/trns.19497. [DOI] [PubMed] [Google Scholar]
- 70.Idaghdour Y, Awadalla P. Exploiting gene expression variation to capture gene-environment interactions for disease. Front. Genet. 2012;3:228. doi: 10.3389/fgene.2012.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maranville JC, Luca F, Richards AL, et al. Interactions between glucocorticoid treatment and cis-regulatory polymorphisms contribute to cellular response phenotypes. PLoS Genet. 2011;7(7):e1002162. doi: 10.1371/journal.pgen.1002162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mangravite LM, Engelhardt BE, Medina MW, et al. A statin-dependent QTL for GATM expression is associated with statin-induced myopathy. Nature. 2013;502(7471):377–380. doi: 10.1038/nature12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ballard KD, Thompson PD. Does reduced creatine synthesis protect against statin myopathy? Cell Metab. 2013;18(6):773–774. doi: 10.1016/j.cmet.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cox NJ, Gamazon ER, Wheeler HE, Dolan ME. Clinical translation of cell-based pharmacogenomic discovery. Clin. Pharmacol. Ther. 2012;92(4):425–427. doi: 10.1038/clpt.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]