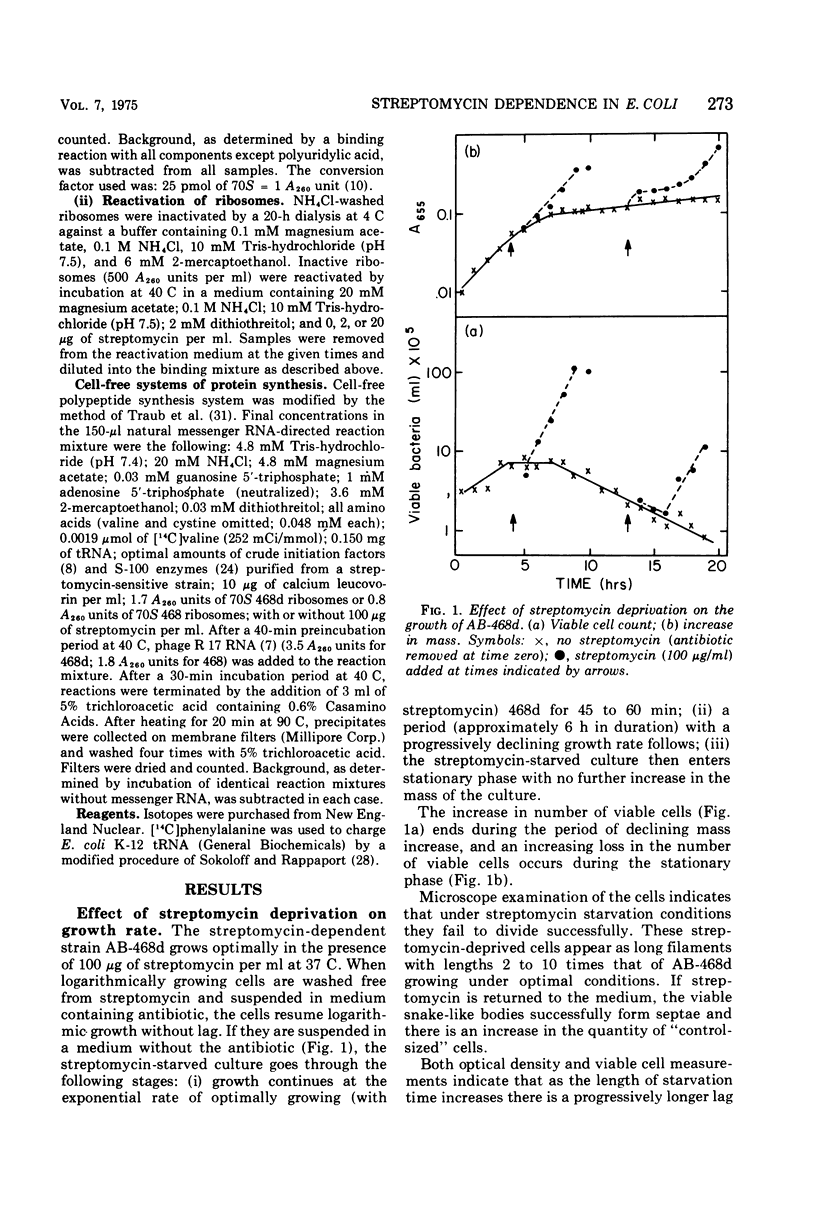
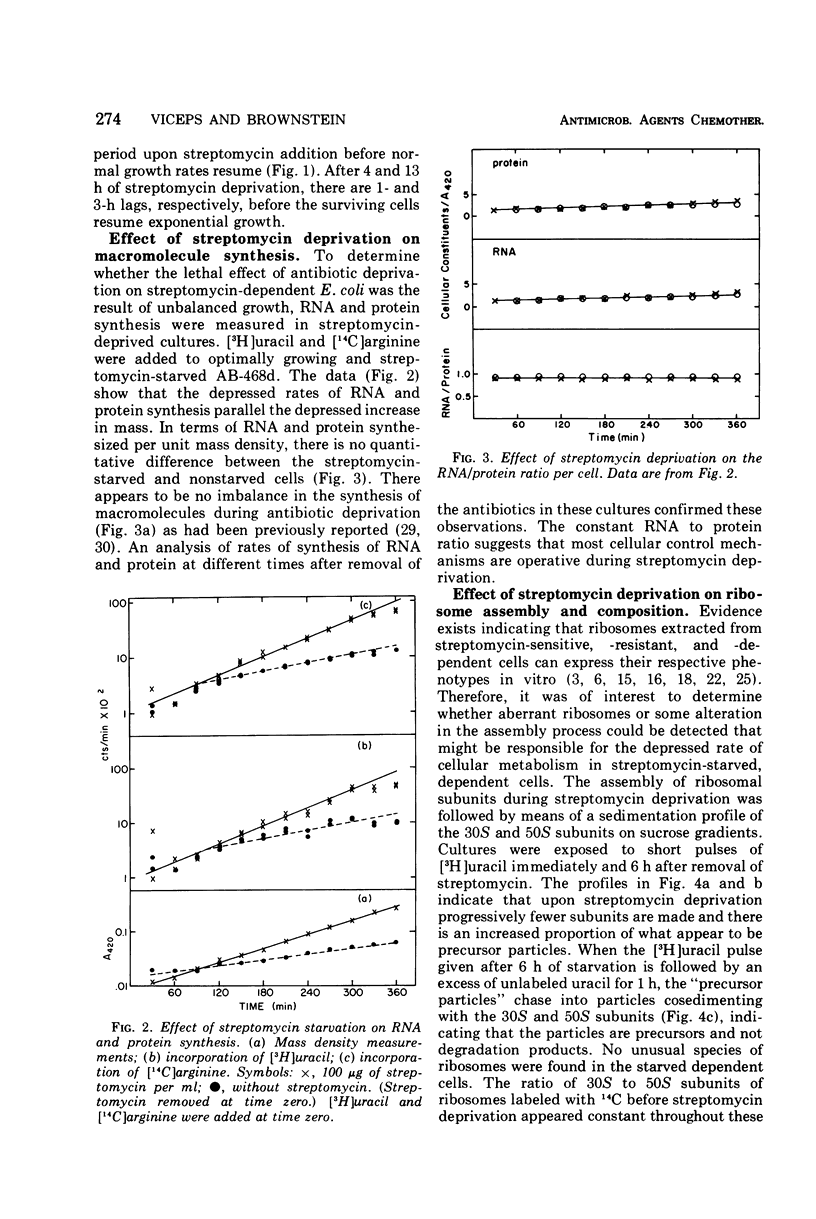
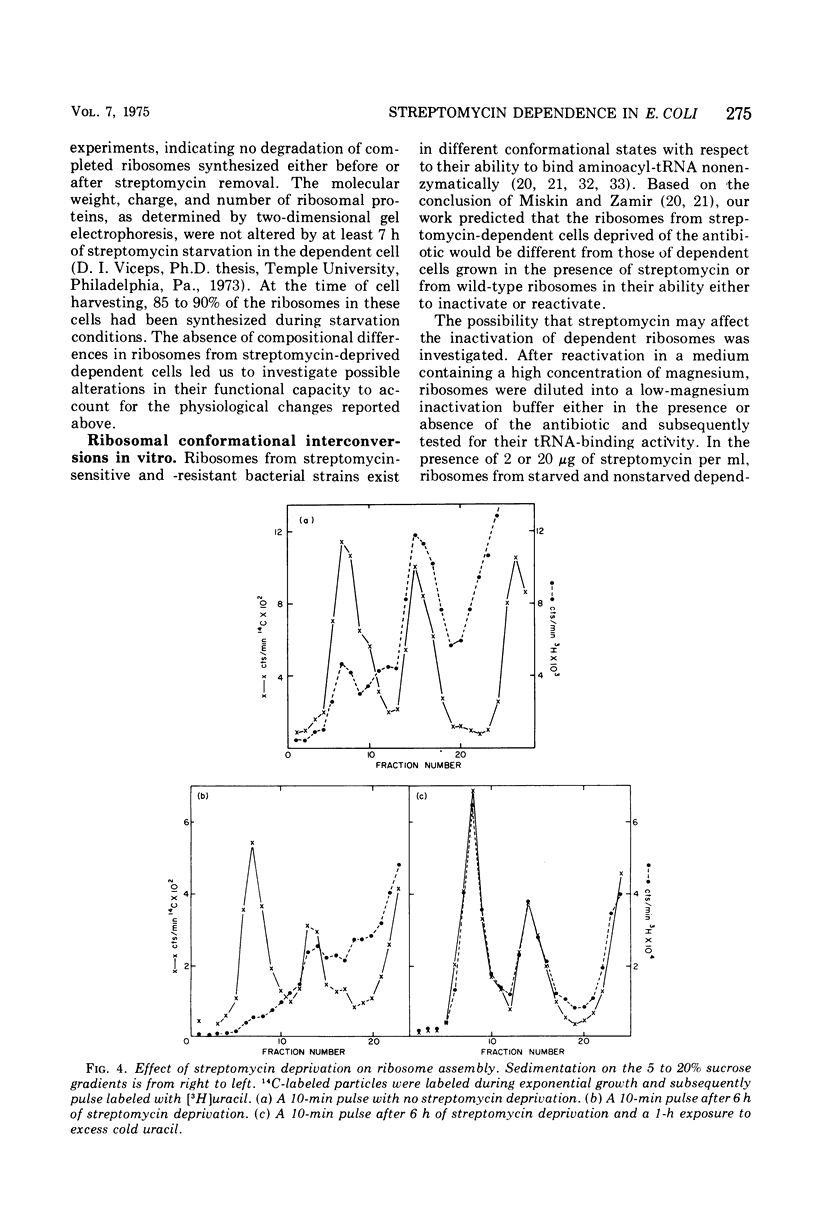
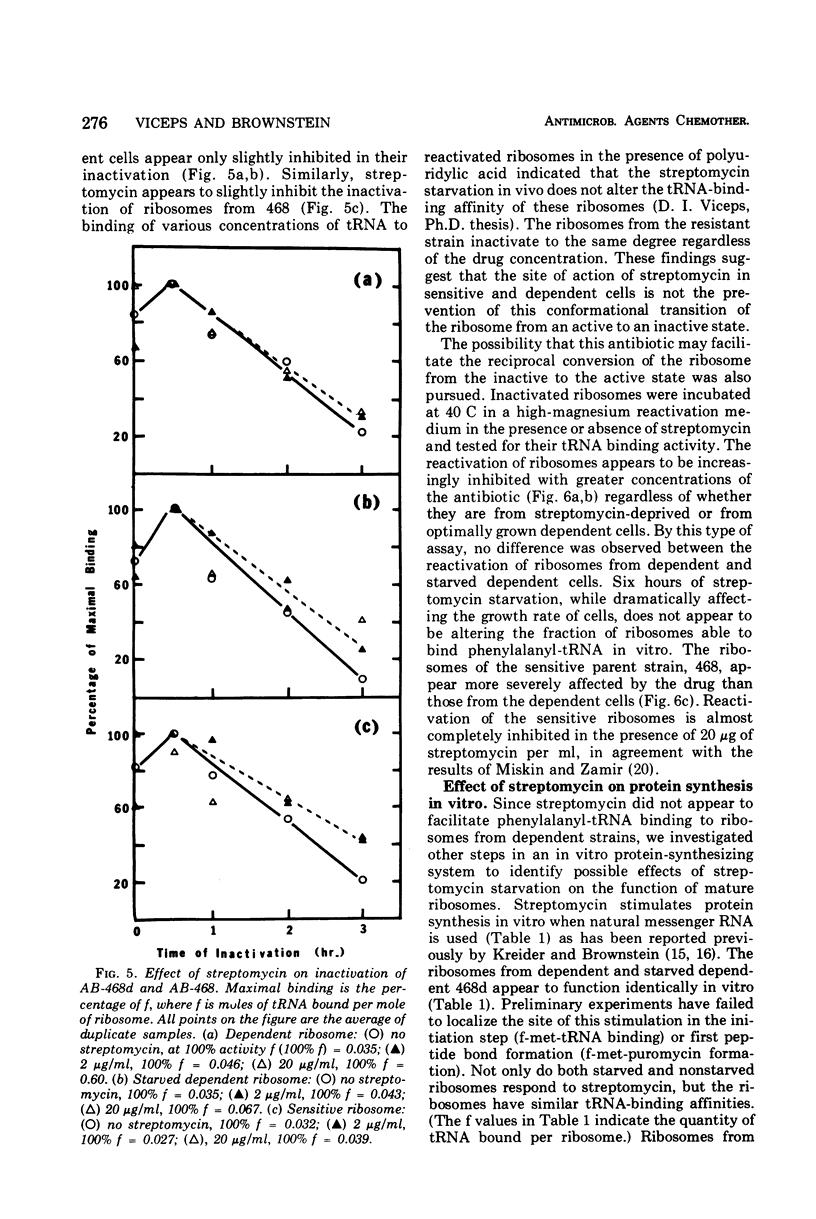
Abstract
The inhibition of cell division and the ultimate loss of viability after removal of streptomycin from growing cultures of streptomycin-dependent bacteria are not the result of “unbalanced growth” or of the breakdown of ribosomes. The streptomycin-dependent strain of Escherichia coli K-12 studied continued to synthesize ribonucleic acid (RNA) and protein during streptomycin starvation. There was no evidence of a gross imbalance in the ratio of RNA to protein synthesized or of selective degradation of either protein or RNA. Using the sedimentation of subunits in sucrose as the criterion, normal ribosomes were synthesized even after 18 h of streptomycin deprivation, although the rates of appearance of mature 30S and 50S subunits decreased with time of deprivation. Once formed, these ribosomes appeared stable, as did those synthesized before the onset of starvation. Ribosomes isolated from starved dependent cells were as “functional” as ribosomes from cells grown with streptomycin in their capacity to bind aminoacyl-transfer RNA in response to polyuridylic acid or natural messenger RNA to interconvert between active and inactive transfer RNA binding states, and to synthesize proteins in cell-free systems. The effects are consistent with an impaired rate of synthesis of ribosomal components or assembly of ribosomes resulting in a continually diminishing rate of protein synthesis. The effect on cell division may be the result of a decreased rate of protein synthesis in general and the requirement for a specific protein(s) in particular.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G., SIX E. Inheritance of prophage P2 in bacterial crosses. Virology. 1958 Oct;6(2):357–381. doi: 10.1016/0042-6822(58)90089-8. [DOI] [PubMed] [Google Scholar]
- Bertani G. A Method for Detection of Mutations, Using Streptomycin Dependence in Escherichia Coli. Genetics. 1951 Nov;36(6):598–611. doi: 10.1093/genetics/36.6.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birge E. A., Kurland C. G. Altered ribosomal protein in streptomycin-dependent Escherichia coli. Science. 1969 Dec 5;166(3910):1282–1284. doi: 10.1126/science.166.3910.1282. [DOI] [PubMed] [Google Scholar]
- Bjare U., Gorini L. Drug dependence reversed by a ribosomal ambiguity mutation, ram, in Escherichia coli. J Mol Biol. 1971 May 14;57(3):423–435. doi: 10.1016/0022-2836(71)90101-x. [DOI] [PubMed] [Google Scholar]
- Chang F. N., Flaks J. G. Binding of dihydrostreptomycin to Escherichia coli ribosomes: characteristics and equilibrium of the reaction. Antimicrob Agents Chemother. 1972 Oct;2(4):294–307. doi: 10.1128/aac.2.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLAKS J. G., COX E. C., WITTING M. L., WHITE J. R. Polypeptide synthesis with ribosomes from streptomycin-resistant and dependent E. coli. Biochem Biophys Res Commun. 1962 May 11;7:390–393. doi: 10.1016/0006-291x(62)90321-2. [DOI] [PubMed] [Google Scholar]
- GESTELAND R. F., BOEDTKER H. SOME PHYSICAL PROPERTIES OF BACTERIOPHAGE R17 AND ITS RIBONUCLEIC ACID. J Mol Biol. 1964 Apr;8:496–507. doi: 10.1016/s0022-2836(64)80007-3. [DOI] [PubMed] [Google Scholar]
- Ghosh H. P., Söll D., Khorana H. G. Studies on polynucleotides. LXVII. Initiation of protein synthesis in vitro as studied by using ribopolynucleotides with repeating nucleotide sequences as messengers. J Mol Biol. 1967 Apr 28;25(2):275–298. doi: 10.1016/0022-2836(67)90142-8. [DOI] [PubMed] [Google Scholar]
- Goodman R. E., Spotts C. R. Effects of streptomycin deprivation on enzyme synthesis in streptomycin-dependent Escherichia coli. J Bacteriol. 1967 Oct;94(4):1154–1161. doi: 10.1128/jb.94.4.1154-1161.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. E., Rossetti G. P., Van Holde K. E. Physical studies of ribosomes from Escherichia coli. J Mol Biol. 1969 Sep 14;44(2):263–277. doi: 10.1016/0022-2836(69)90174-0. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Isono K. Altered 30S ribosomal proteins in streptomycin resistant, dependent and independent mutants of Bacillus stearothermophilus. Mol Gen Genet. 1974;133(1):77–86. doi: 10.1007/BF00268679. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Donachie W. D. Chromosome replication, transcription and control of cell division in Escherichia coli. Nat New Biol. 1973 May 23;243(125):100–103. [PubMed] [Google Scholar]
- KJELDGAARD N. O. The kinetics of ribonucleic acid- and protein formation in Salmonella typhimurium during the transition between different states of balance growth. Biochim Biophys Acta. 1961 Apr 29;49:64–76. doi: 10.1016/0006-3002(61)90870-8. [DOI] [PubMed] [Google Scholar]
- Kreider G., Brownstein B. L. A mutation suppressing streptomycin dependence. II. An altered protein on the 30 s ribosomal subunit. J Mol Biol. 1971 Oct 14;61(1):135–142. doi: 10.1016/0022-2836(71)90211-7. [DOI] [PubMed] [Google Scholar]
- Kreider G., Brownstein B. L. Pleiotropic effects resulting from mutations in genes for ribosomal proteins: analysis of revertants from streptomycin dependence. J Mol Biol. 1974 Mar 25;84(1):159–171. doi: 10.1016/0022-2836(74)90219-8. [DOI] [PubMed] [Google Scholar]
- Lekover T. E., Kurland C. G. Ribosomes from a streptomycin-dependent strain of Escherichia coli. J Mol Biol. 1967 May 14;25(3):497–504. doi: 10.1016/0022-2836(67)90201-x. [DOI] [PubMed] [Google Scholar]
- Miskin R., Zamir A. Effect of streptomycin on ribosome interconversion, a possible basis for the action of the antibiotic. Nat New Biol. 1972 Jul 19;238(81):78–80. doi: 10.1038/newbio238078a0. [DOI] [PubMed] [Google Scholar]
- Miskin R., Zamir A. Inhibition of interconversion of ribosomes between forms active and inactive in 30 S subunit functions by aminoglycoside antibiotics. J Mol Biol. 1974 Jul 25;87(1):135–139. doi: 10.1016/0022-2836(74)90565-8. [DOI] [PubMed] [Google Scholar]
- Momose H., Gorini L. Genetic analysis of streptomycin dependence in Escherichia coli. Genetics. 1971 Jan;67(1):19–38. doi: 10.1093/genetics/67.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIRENBERG M. W., MATTHAEI J. H. The dependence of cell-free protein synthesis in E. coli upon naturally occurring or synthetic polyribonucleotides. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1588–1602. doi: 10.1073/pnas.47.10.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath K., Koch A. L. Protein degradation in Escherichia coli. II. Strain differences in the degradation of protein and nucleic acid resulting from starvation. J Biol Chem. 1971 Nov 25;246(22):6956–6967. [PubMed] [Google Scholar]
- Ozaki M., Mizushima S., Nomura M. Identification and functional characterization of the protein controlled by the streptomycin-resistant locus in E. coli. Nature. 1969 Apr 26;222(5191):333–339. doi: 10.1038/222333a0. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Steady-state measurement of the turnover of amino acid in the cellular proteins of growing Escherichia coli: existence of two kinetically distinct reactions. J Bacteriol. 1970 Jul;103(1):207–215. doi: 10.1128/jb.103.1.207-215.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine M. J. Turnover of intracellular proteins. Annu Rev Microbiol. 1972;26:103–126. doi: 10.1146/annurev.mi.26.100172.000535. [DOI] [PubMed] [Google Scholar]
- SPOTTS C. R. Physiological and biochemical studies on streptomycin dependence in Escherichia coli. J Gen Microbiol. 1962 Jun;28:347–365. doi: 10.1099/00221287-28-2-347. [DOI] [PubMed] [Google Scholar]
- SPOTTS C. R., STANIER R. Y. Mechanism of streptomycin action on bacteria: a unitary hypothesis. Nature. 1961 Nov 18;192:633–637. doi: 10.1038/192633a0. [DOI] [PubMed] [Google Scholar]
- Sokolff L., Rappaport H. P. Isolation of isoaccepting tRNAs. Arch Biochem Biophys. 1972 Dec;153(2):788–796. doi: 10.1016/0003-9861(72)90400-6. [DOI] [PubMed] [Google Scholar]
- Zamir A., Miskin R., Elson D. Inactivation and reactivation of ribosomal subunits: amino acyl-transfer RNA binding activity of the 30 s subunit of Escherichia coli. J Mol Biol. 1971 Sep 14;60(2):347–364. doi: 10.1016/0022-2836(71)90299-3. [DOI] [PubMed] [Google Scholar]
- Zamir A., Miskin R., Elson D. Interconversions between inactive and active forms of ribosomal subunits. FEBS Lett. 1969 Apr;3(1):85–88. doi: 10.1016/0014-5793(69)80103-1. [DOI] [PubMed] [Google Scholar]



