Abstract
Objectives
We previously demonstrated that ceftaroline enhances daptomycin against MRSA in vitro. However, prolonged combination therapy is clinically undesirable and possibly unnecessary. The purpose of this study was to determine if this combination could be de-escalated to a single agent without compromising efficacy.
Methods
We investigated the following simulated regimens against two clinical, daptomycin-non-susceptible MRSA isolates in an in vitro pharmacokinetic/pharmacodynamic hollow-fibre model over 192 h: 600 mg of ceftaroline every 12 h (fCmax 17.0 mg/L, t½ 2.66 h); 10 mg/kg/day daptomycin (fCmax 11.3 mg/L, t½ 8 h); 6 mg/kg/day daptomycin (fCmax 7.5 mg/L, t½ 8 h); ceftaroline + daptomycin; and ceftaroline + daptomycin de-escalated to ceftaroline, daptomycin or drug-free simulations.
Results
Daptomycin and ceftaroline MICs were 2 and 2 and 0.5 and 1 mg/L for strains R6063 and R5563, respectively. Ceftaroline + daptomycin (6 or 10 mg/kg/day) achieved a >5 log10 cfu/mL reduction within 96 h against both strains. Bacterial counts remained <1.5 log10 cfu/mL from 96 to 192 h regardless of de-escalation to either agent. There were no significant differences between combination or de-escalation regimens for either organism at either daptomycin dose. All combination/de-escalation to monotherapy regimens resulted in significantly improved activity compared with drug-free control, ceftaroline or daptomycin monotherapy (P < 0.01).
Conclusions
These findings confirm that ceftaroline + daptomycin is a potent combination against MRSA. The high degree of bactericidal activity observed with this combination appears sufficiently robust to allow for de-escalation to a single agent without bacterial regrowth. The equivalent activity observed with ceftaroline + daptomycin (6 and 10 mg/kg/day) suggests this combination could also be daptomycin sparing. Further research is warranted to optimize dose and de-escalation timing.
Keywords: daptomycin non-susceptibility, hVISA, VISA, refractory
Introduction
Due to increasing rates of failure for MRSA bacteraemia, the guidelines have recommended combination therapy for refractory cases.1 Combinations of β-lactams + daptomycin against Staphylococcus aureus have demonstrated a synergistic effect that appears to be the result of changes in the cell surface charge, leading to increased daptomycin binding and depolarization.2–6 Because ceftaroline demonstrates activity against MRSA, it represents a unique β-lactam option to utilize in combination with daptomycin. However, keeping patients on prolonged combination therapy could lead to adverse drug events, drug interactions and increased cost. Therefore, the objective of this study was to determine whether ceftaroline + daptomycin could be de-escalated to a single agent for the treatment of S. aureus.
Materials and methods
Bacterial strains
Two clinical MRSA bloodstream isolates (R6063 and R5563) from the Anti-Infective Research Laboratory were evaluated. Both strains are ceftaroline susceptible and daptomycin non-susceptible.
Antimicrobials
Daptomycin (Cubist Pharmaceuticals, Lexington, MA, USA) and vancomycin (Sigma Chemical Co., St Louis, MO, USA) were purchased commercially. Analytical ceftaroline powder was provided by Forest Laboratories (New York, NY, USA).
Susceptibility testing
The MICs of the study antimicrobials were determined in duplicate according to CLSI guidelines.7 Glycopeptide heteroresistance was analysed by modified population analysis as previously described.8
In vitro pharmacokinetic/pharmacodynamic model
A previously described in vitro pharmacokinetic/pharmacodynamic hollow-fibre model consisting of a two-compartment chamber (C3008; Fiber Cell Systems, Frederick, MD, USA) was utilized for all experiments.9,10 The apparatus was pre-filled with medium and antibiotics were administered as boluses over a 192 h time period with a target inoculum of ∼1 × 107 cfu/mL. The antibiotic regimens evaluated were as follows: simulations of 600 mg of ceftaroline fosamil every 12 h (fCmax 17.04 mg/L, average t½ 2.66 h, protein binding 20%), 10 mg/kg daptomycin every 24 h (fCmax 11.3 mg/L, average t½ 8 h, protein binding 92%) and 6 mg/kg daptomycin every 24 h (fCmax 7.51 mg/L, average t½ 8 h, protein binding 92%).11,12 Simulated regimens included ceftaroline + daptomycin for 8 days, ceftaroline + daptomycin for 4 days followed by daptomycin for 4 days, ceftaroline + daptomycin for 4 days followed by ceftaroline for 4 days and ceftaroline + daptomycin for 4 days followed by no antimicrobials for 4 days. Daptomycin 10 and 6 mg/kg every 24 h were evaluated. Supplemental daptomycin was added at an appropriate rate to ceftaroline combination models to compensate for the higher flow rate required to simulate ceftaroline clearance.13 Models were performed in duplicate to ensure reproducibility.
Pharmacodynamic analysis
Samples from each model were collected at 0, 4, 8, 24, 48, 72, 96, 100, 104, 120, 144, 168 and 192 h in duplicate and diluted in cold 0.9% saline. Colony counts were determined using an automatic spiral plater (WASP; DW Scientific, West Yorkshire, UK) or by vacuum filtration, if the anticipated dilution was near the MIC, to enumerate cfu/mL. For both methods, plates were incubated at 35°C for 24 h before colonies were counted and the lower limit of detection was 1 log10 cfu/mL.
Pharmacokinetic analysis
Pharmacokinetic samples were obtained at 0.5, 4, 8, 24, 32, 48, 72, 96, 100, 104, 120, 144, 168 and 192 h to verify antimicrobial concentrations. Ceftaroline concentrations were determined by bioassay as previously described.2,9,10 Blank 0.25 inch discs were placed on pre-swabbed (Bacillus subtilis ATCC 6633) agar plates and spotted with 10 μL of the standards (1.88, 3.75, 7.5, 15 and 30 mg/L) or experimental samples. Daptomycin concentrations were determined using a validated HPLC assay that conforms to the guidelines set forth by the College of American Pathologists.14,15 Both assays demonstrated an interday coefficient of variation <5% for high, medium and low standards. The t½, fCmax, fCmin and AUC were calculated using PK Analyst (version 1.10; MicroMath Scientific Software, Salt Lake City, UT, USA). The AUC was determined by the trapezoidal method.
Resistance
Development of or increased resistance was evaluated at 192 h by plating samples on agar containing 3× MIC of ceftaroline or daptomycin. If resistance was detected, earlier time points were screened to determine the time of resistance emergence. Plates were examined for growth after 48 h of incubation at 35°C. The MICs for colonies capable of growing on resistance plates were determined by broth microdilution.
Statistical analysis
Changes in cfu/mL at 96 and 192 h were compared by one-way analysis of variance with Tukey's post hoc test. All statistical analyses were performed using SPSS statistical software (release 21.0; SPSS, Chicago, IL, USA).
Results
Susceptibility testing
The MIC results for the isolates tested are summarized in Table 1. One isolate (R6063) was confirmed to be heterogeneous vancomycin-intermediate S. aureus (hVISA) by population analysis with a ratio to Mu3 of 0.99.
Table 1.
MICs for test isolates and post-drug-exposure resistant mutants
| Isolate | MIC (mg/L) |
||
|---|---|---|---|
| ceftaroline | daptomycin | vancomycin | |
| R6063 | 1 | 2 | 2 (hVISA) |
| T24 mutanta | NC | 16 | NC |
| R5563 | 0.5 | 2 | 2 (VSSA) |
| T24 mutanta | NC | 8 | NC |
VSSA, vancomycin-susceptible S. aureus; NC, no change.
aMutant derived after 24 h of exposure to daptomycin monotherapy.
In vitro pharmacokinetic/pharmacodynamic model
The observed fCmax and t½ values for ceftaroline were 16.77 ± 0.41 mg/L (targeted 17.04 mg/L) and 2.63 ± 0.07 h (targeted 2.66 h). The fT>MIC was maintained for 84.9% of the 12 h dosing interval for R6063 and 100% for R5563. The observed fCmax value for daptomycin was 11.02 ± 0.21 mg/L (targeted 11.29 mg/L) for the 10 mg/kg regimens and 7.14 ± 0.09 mg/L (targeted 7.51 mg/L) for the 6 mg/kg regimens. The observed t½ values for 10 and 6 mg/kg/day daptomycin runs were 6.7 ± 0.7 and 7.1 ± 0.1 h (targeted 8 h), respectively.
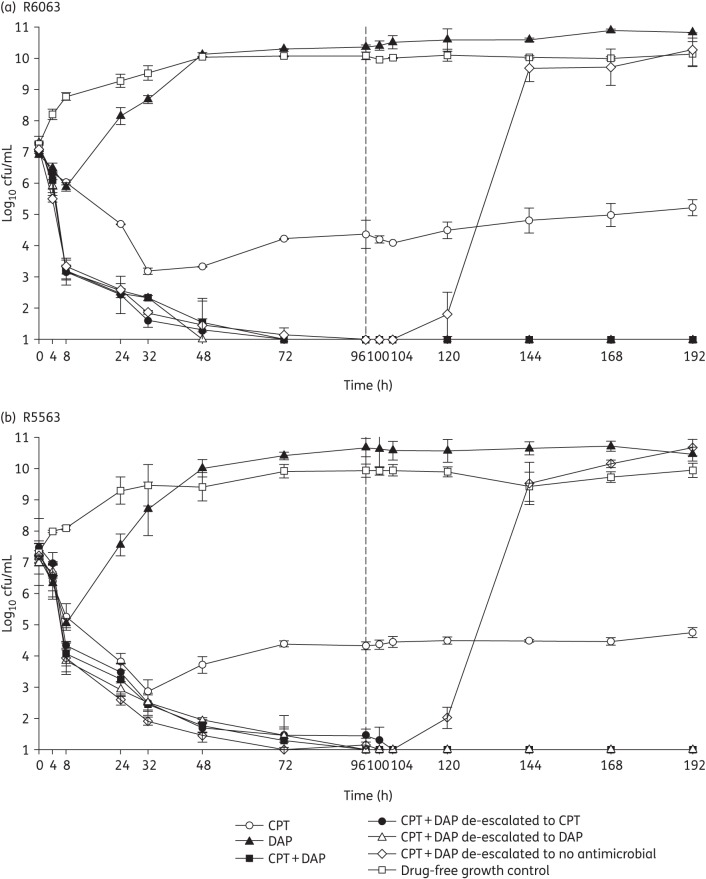
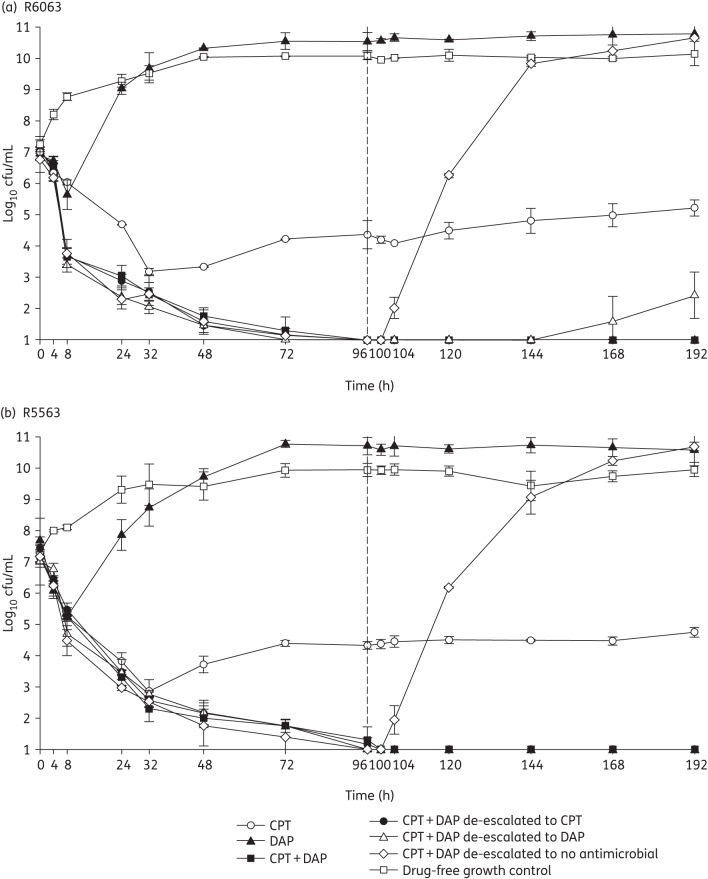
The changes in log10 cfu/mL for the tested regimens against the two strains are displayed in Figures 1 and 2. Against both strains, ceftaroline in combination with either 6 or 10 mg/kg/day daptomycin produced >5 log10 cfu/mL reductions by 96 h (4 days). This bactericidal activity was maintained through 192 h (8 days) for the combination regimen and with de-escalation to ceftaroline or daptomycin at 10 or 6 mg/kg/day. Upon de-escalation to no antimicrobial exposure, regrowth was noted within 8 h for both strains, regardless of the initial daptomycin dose. Ceftaroline alone was bactericidal with a 4.26 ± 0.5 and 3.67 ± 0.04 log10 cfu/mL reduction within 32 h against R5563 and R6063. Daptomycin monotherapy was not bactericidal against either strain at either dose tested. All combination and de-escalation to monotherapy regimens were significantly more active than monotherapy regimens or growth control at 96 and 192 h (P < 0.001). No significant differences between the combination or de-escalation simulations, regardless of daptomycin dose, were observed. No organisms with increased ceftaroline MIC values were recovered for ceftaroline or combination regimens. However, elevated daptomycin MICs were observed as early as 24 h for both strains treated with daptomycin monotherapy at either dose, with MICs as high as 16 and 8 mg/L for R6063 and R5563, respectively.
Figure 1.
In vitro hollow-fibre model results utilizing daptomycin dosages of 10 mg/kg. CPT, ceftaroline; DAP, daptomycin.
Figure 2.
In vitro hollow-fibre model results utilizing daptomycin dosages of 6 mg/kg. CPT, ceftaroline; DAP, daptomycin.
Discussion
Resistance to glycopeptides and lipopeptides has emerged in MRSA leading to increasing rates of clinical failures. With few new antimicrobial agents in the pipeline, it is necessary to explore potential combinations of currently available antimicrobials as an alternative approach to fighting resistance. The MRSA guidelines recommend that patients with refractory bacteraemia or those who have failed vancomycin could receive a β-lactam in combination with high-dose daptomycin; however, duration of combination therapy or potential for de-escalation are not clearly addressed.1 Currently, there are limited data to suggest that a specific β-lactam in combination with daptomycin is superior to another. Our data support the findings from previous studies suggesting that the combination of daptomycin + ceftaroline is highly bactericidal even against strains with reduced susceptibility to daptomycin.2 In addition, this combination appears to be beneficial in decreasing the emergence or selection of resistant subpopulations.6 Therefore, this combination may be of particular value in the setting of difficult-to-treat infections where isolates with reduced susceptibility to glycopeptides or lipopeptides have emerged on therapy.
Our results suggest that the synergistic combination of ceftaroline + daptomycin can be de-escalated to a single agent with no loss of efficacy. This potential ability to de-escalate while maintaining activity may provide clinicians with the option to discontinue one agent due to adverse events, antimicrobial stewardship considerations, cost limitations or challenging dosing strategies. Furthermore, the finding that 6 mg/kg/day daptomycin + ceftaroline was approximately equal to 10 mg/kg/day daptomycin, in terms of bactericidal activity, suggests a daptomycin-sparing effect. Nevertheless, precaution is warranted since 6 mg/kg/day daptomycin + ceftaroline de-escalated to daptomycin alone allowed some minor regrowth. This finding may suggest that ceftaroline is the more appropriate agent to de-escalate to in the setting of daptomycin non-susceptibility. Potential limitations of this study include the utilization of only two strains. Additionally, the de-escalation at 4 days was somewhat arbitrary and the decision to de-escalate in the clinical setting should be made cautiously and based on clinical response.
In conclusion, we observed that the combination of ceftaroline and daptomycin was rapidly bactericidal and prevented further resistance to daptomycin. In addition, the ability to de-escalate from this combination to monotherapy may facilitate the streamlining of patients' antimicrobial therapy to more narrow-spectrum regimens, potentially decreasing drug and hospital costs and unnecessary antimicrobial exposure. Our data also indicate that combination therapy may not be necessary for the entire course of treatment. De-escalation therapy may be considered a reasonable alternative to long-term combination therapy in patients with early clinical response. However, additional investigation is warranted to determine the optimal timing of de-escalation.
Funding
This work was funded by an investigator-initiated grant from Forest Laboratories (TEF-IT-09). M. J. R. is supported in part by grant R21AI092055 from the NIAID.
Transparency declarations
M. J. R. has received grant support from, consulted for or provided lectures for Cepheid, Cerexa, Cubist, Durata, Forest, Novartis, Theravance and Trius. K. E. B. and B. J. W.: none to declare.
Acknowledgements
This study was presented in part at the Fifty-third Interscience Conference on Antimicrobial Agents and Chemotherapy, Denver, CO, USA, 2013 (Abstract A-467).
References
- 1.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285–92. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 2.Werth BJ, Sakoulas G, Rose WE, et al. Ceftaroline increases membrane binding and enhances the activity of daptomycin against daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus in a pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother. 2013;57:66–73. doi: 10.1128/AAC.01586-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang SJ, Xiong YQ, Boyle-Varva S, et al. Daptomycin-oxacillin combinations in treatment of experimental endocarditis caused by daptomycin-nonsusceptible strains of methicillin-resistant Staphylococcus aureus with evolving oxacillin susceptibility (the ‘seesaw effect’) Antimicrob Agents Chemother. 2010;54:3161–9. doi: 10.1128/AAC.00487-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leonard SN, Rolek KM. Evaluation of the combination of daptomycin and nafcillin against vancomycin-intermediate Staphylococcus aureus. J Antimicrob Chemother. 2013;68:644–7. doi: 10.1093/jac/dks453. [DOI] [PubMed] [Google Scholar]
- 5.Berti AD, Sakoulas G, Nizet V, et al. β-Lactam antibiotics targeting PBP1 selectively enhance daptomycin activity against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2013;57:5005–12. doi: 10.1128/AAC.00594-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berti AD, Wergin JE, Girdaukas GG, et al. Altering the proclivity towards daptomycin resistance in methicillin-resistant Staphylococcus aureus using combinations with other antibiotics. Antimicrob Agents Chemother. 2012;56:5046–53. doi: 10.1128/AAC.00502-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Fifteenth Informational Supplement M100-S15. Wayne, PA, USA: CLSI; 2005. [Google Scholar]
- 8.Wootton M, Howe RA, Hillman R, et al. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J Antimicrob Chemother. 2001;47:399–403. doi: 10.1093/jac/47.4.399. [DOI] [PubMed] [Google Scholar]
- 9.Vidaillac C, Leonard SN, Rybak MJ. In vitro activity of ceftaroline against methicillin-resistant Staphylococcus aureus and heterogeneous vancomycin-intermediate S. aureus in a hollow fiber model. Antimicrob Agents Chemother. 2009;53:4712–7. doi: 10.1128/AAC.00636-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Werth BJ, Steed ME, Kaatz GW, et al. Evaluation of ceftaroline activity against heteroresistant vancomycin-intermediate Staphylococcus aureus and vancomycin-intermediate methicillin-resistant S. aureus strains in an in vitro pharmacokinetic/pharmacodynamic model: exploring the ‘seesaw effect’. Antimicrob Agents Chemother. 2013;57:2664–8. doi: 10.1128/AAC.02308-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benvenuto M, Benziger DP, Yankelev S, et al. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob Agents Chemother. 2006;50:3245–9. doi: 10.1128/AAC.00247-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saravolatz LD, Stein GE, Johnson LB. Ceftaroline: a novel cephalosporin with activity against methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2011;52:1156–63. doi: 10.1093/cid/cir147. [DOI] [PubMed] [Google Scholar]
- 13.Blaser J. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J Antimicrob Chemother. 1985;15(Suppl A):125–30. doi: 10.1093/jac/15.suppl_a.125. [DOI] [PubMed] [Google Scholar]
- 14.Martens-Lobenhoffer J, Kielstein JT, Oye C, et al. Validated high performance liquid chromatography-UV detection method for the determination of daptomycin in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;875:546–50. doi: 10.1016/j.jchromb.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Dvorchik BH, Brazier D, DeBruin MF, et al. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob Agents Chemother. 2003;47:1318–23. doi: 10.1128/AAC.47.4.1318-1323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]