Abstract
PURPOSE
We undertook a study to identify distinct functional trajectories in the year before hospice, to determine how patients with these trajectories differ according to demographic characteristics and hospice diagnosis, and to evaluate the association between these trajectories and subsequent outcomes.
METHODS
From an ongoing cohort study of 754 community-living persons aged 70 years or older, we evaluated data on 213 persons who were subsequently enrolled in hospice from March 1998 to December 2011. Disability in 13 basic, instrumental, and mobility activities was assessed during monthly telephone interviews through June 2012.
RESULTS
In the year before hospice, we identified 5 clinically distinct functional trajectories, representing worsening cumulative burden of disability: late decline (10.8%), accelerated (10.8%), moderate (21.1%), progressively severe (24.9%), and persistently severe (32.4%). Participants with a cancer diagnosis (34.7%) had the most favorable functional trajectories (ie, lowest burden of disability), whereas those with neurodegenerative disease (21.1%) had the worst. Median survival in hospice was only 14 days and did not differ significantly by functional trajectory. Compared with participants in the persistently severe trajectory, those in the moderate trajectory had the highest likelihood of surviving and being independent in at least 1 activity in the month after hospice admission (adjusted odds ratio = 5.5; 95% CI, 1.9–35.9).
CONCLUSIONS
The course of disability in the year before hospice differs greatly among older persons but is particularly poor among those with neurodegenerative disease. Late admission to hospice (as shown by the short survival), coupled with high levels of severe disability before hospice, highlight potential unmet palliative care needs for many older persons at the end of life.
Keywords: longitudinal studies, hospice, palliative care, disability evaluation, aging, frail elderly, end of life care, practice-based research
INTRODUCTION
Older persons with persistent levels of severe disability have high mortality and substantial care needs.1,2 One option for addressing these needs is palliative care, a specialized medical and interdisciplinary care approach that aims to alleviate stress, pain, and other distressing symptoms among those with serious illness, independent of prognosis or treatment being pursued, with the goal of optimizing quality of life for both patients and families.3 Hospice care, a similar approach, is a second option. In the United States, hospice is largely defined by the Medicare Hospice Benefit, which restricts services to persons who have an expected survival of 6 months or less. Because hospice also limits the pursuit of curative and most life-prolonging therapies, it is often considered only for patients who have end-stage, terminal conditions, or when death is imminent.4–15 Palliative care is often confused with hospice and can be difficult to access.16 Consequently, many older persons do not receive hospice or palliative care near the end of life,17,18 which can place a high burden on caregivers19 and result in suffering.20
Although functional decline among patients in hospice has been previously evaluated,21,22 the course of disability before hospice has not been well characterized. Several prior studies23–26 have evaluated the prevalence and time course (or trajectory) of disability at the end of life, but have not focused on the functional antecedents to hospice admission. Knowledge of functional trajectories before hospice could help place this resource in the context of the broader palliative care needs of older persons.27
The objectives of the current study were to identify distinct functional trajectories in the year before hospice, to determine how older patients with these trajectories differ according to demographic characteristics and hospice admission diagnosis, and to evaluate the association between these trajectories and subsequent disability and survival outcomes. Our ultimate goal was to inform discussions about potential unmet palliative care needs at the end of life.
METHODS
Parent Population
Participants were drawn from the Precipitating Events Project, an ongoing prospective cohort study of 754 community-living persons aged 70 years and older who were initially nondisabled in 4 basic activities of daily living—bathing, dressing, walking, and transferring.28,29 The overall goal of the study is to elucidate the epidemiology of disability and recovery in older persons.30 Potential participants were identified from a large health plan in greater New Haven, Connecticut. Exclusion criteria, present in 8 individuals, included considerable cognitive impairment with no available proxy,31 plans to move out of the area, inability to speak English, or terminal illness. Only 4.6% of persons contacted declined screening, and 75.2% of those eligible agreed to participate and were enrolled from March 1998 to October 1999.
Analytic Sample
From the parent population, we identified all hospice admissions through 2011 using Medicare claims data and review of medical records.32,33 Of the 223 participants who were admitted to hospice, 10 (4.5%) had previously withdrawn from the study, leaving 213 participants for analysis.
Data Collection
Comprehensive home-based assessments were completed at baseline and 18-month intervals for 144 months (except 126 months), while telephone interviews were completed monthly through June 2012, with a completion rate of 99.1%. For participants who had considerable cognitive impairment, a proxy informant was interviewed using a rigorous protocol with demonstrated reliability and validity.34 During the comprehensive assessments, data were collected on demographic characteristics and 9 self-reported, physician-diagnosed chronic conditions. Deaths were ascertained by review of local obituaries, from an informant, or both, with a completion rate of 100%.
Assessment of Disability
During the monthly interviews, participants were asked, “At the present time, do you need help from another person to (complete the task)?” for each of the 4 basic activities, 5 instrumental activities (shopping, doing housework, preparing meals, taking medications, and managing finances), and 3 mobility activities (walking one-fourth of a mile, climbing a flight of stairs, and lifting/carrying 10 pounds).29,31,35 For these 12 activities, disability was operationalized as the need for personal assistance (yes/no). Participants were also asked about a fourth mobility activity, “Have you driven a car during the past month?” Those who responded “No” were deemed to have stopped driving. To maintain consistency with the other activities, these participants were classified as being disabled in driving that month.35 The primary outcome included the number of disabilities in all 13 functional activities. The test-retest reliability of this standardized scale,32,36 which assesses the severity of disability, with 0 denoting no disability and 13 denoting complete disability, was high, with an intraclass correlation coefficient of 0.81.
Hospice Admission Diagnosis
Diagnosis codes for hospice admissions were obtained through linkage with Medicare claims data.32,33 On the basis of Centers for Medicare & Medicaid Services (CMS) International Classification of Diseases, 9th Revision (ICD-9) diagnosis codes, we classified the hospice admission diagnosis into 7 categories, described below.
Statistical Analysis
To identify distinct functional trajectories in the year before hospice, we used trajectory modeling,37–39 which is a form of latent class analysis. In contrast to traditional regression or growth-curve models, which estimate a single mean for the population, trajectory modeling simultaneously estimates each participant’s probabilities for membership in multiple trajectories, with assignment to a specific trajectory based on the highest probability of membership. We used PROC TRAJ in SAS (SAS Institute Inc), which fits a semiparametric mixture model to longitudinal data using the maximum likelihood method.37,39 We modeled the total number of disabilities, ranging from 0 to 13, for 12 months as a zero-inflated Poisson distribution. We used the Bayesian Information Criterion (BIC) to test from 2 to 6 trajectories and to determine whether each trajectory was best fit by intercept only (ie, constant) or by linear, quadratic, or cubic terms.38 For each number of trajectories, the order of the equations was varied until a best-fitting model was derived with the use of the following formula: 2(ΔBIC) > 2.39 Participants were classified according to a specific trajectory on the basis of the maximum estimated probability of assignment. We required that the average probability of group membership for each trajectory be greater than 0.9, denoting an excellent fit, and that each trajectory group include a minimum of 10% of the analytic sample.38 CIs for the observed severity of disability were calculated by bootstrapping, using sampling with replacement.40
In a series of descriptive analyses, we calculated the demographic characteristics (age, sex, race/ethnicity, educational attainment) and number of chronic conditions for each trajectory group and hospice admission diagnosis, respectively, and subsequently evaluated the distribution of the functional trajectories according to the hospice diagnosis. In bivariate analysis, we evaluated the association between the prehospice functional trajectories and overall survival after hospice admission using the Kaplan-Meier method.41 In multivariate analysis, we used Cox regression, adjusted for age and sex, to estimate the hazard ratios for survival, comparing each of the trajectory groups with the most severe disability group. Next, we used logistic regression, adjusted for age and sex, to evaluate the likelihood of being alive and not completely disabled (ie, in all 13 activities) at the first monthly interview after hospice admission. Finally, among the survivors, we completed a similar analysis with the outcome of not being completely disabled.
All analyses were performed using SAS version 9.3 (SAS Institute Inc).
RESULTS
Five distinct functional trajectories were identified in the year before hospice (Figure 1 and Supplemental Figure 1). Participants in the late decline disability group, comprising 10.8% of the analytic sample, were initially independent in most activities until about 3 months before the start of hospice when the severity of disability increased markedly. The accelerated disability group (10.8%) was also largely independent 1 year before entering hospice, but their severity of disability increased gradually before accelerating and diverging from the late decline group about 7 months before hospice. The moderate disability group (21.1%) was more disabled than the accelerated group 1 year before hospice, but had comparable levels of disability at the start of hospice. The progressively severe disability group (24.9%) had a similar trajectory as the moderate group, but had much higher levels of disability throughout the year. Participants with persistently severe disability, which was the largest group (32.4%), had very high levels of disability throughout the year before hospice.
Figure 1.
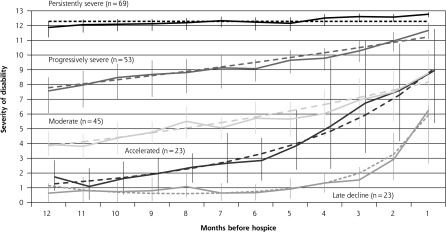
Functional trajectories in the 12 months before start of hospice (N = 213).
Notes: The severity of disability is indicated by the mean number of disabled activities of daily living (basic, instrumental, and mobility). The solid lines indicate the observed trajectories, and the dashed lines indicate the predicted trajectories. Trajectories shown are unadjusted; only minor differences were apparent after adjustment for age and sex. The error bars indicate bootstrapped 95% CIs for the observed severity of disability. The average group membership probability for each group was greater than 0.9.
The most common hospice diagnosis was cancer, followed by neurodegenerative and cardiac disease, respectively (Table 1). Table 2 provides relevant characteristics of the analytic sample. Overall, the mean age at the start of the trajectories was 86.4 years. Participants in the progressively severe and persistently severe disability groups were considerably older than those in the other 3 groups (P <.001, t test). The proportion of women was lowest in the late decline group and highest in the persistently severe group. Participants in the late decline and accelerated groups were the most likely to be non-Hispanic white and the least likely to have low educational attainment. The mean number of chronic conditions did not differ appreciably according to functional trajectory.
Table 1.
Hospice Admission Diagnoses (N = 213)
| Diagnosis Category | Patients, No. (%) | ICD-9 Description | ICD-9 Code(s) |
|---|---|---|---|
| Cancer | 74 (34.7) | Malignant neoplasm | 140–209 |
| Uncertain or unspecified neoplasms | 235–239 | ||
| Neurodegenerative | 45 (21.1) | Mental disorders | 290–319 |
| Parkinson’s and other cerebral degenerations | 330–332 | ||
| Slow virus and prion disease of central nervous system | 046 | ||
| Cardiac | 34 (16.0) | Heart disease | 410–429 |
| Vascular | 14 (6.6) | Stroke | 430–438 |
| Aneurysm or aortic dissection | 441–442 | ||
| Respiratory | 11 (5.2) | Pulmonary diseases | 490–519 |
| Respiratory abnormality not elsewhere classified | 786.9 | ||
| Other organ failure | 15 (7.0) | Gastrointestinal and liver disease | 530–539, 555–579 |
| Renal disease | 580–589 | ||
| Frailty/debility | 20 (9.4) | Adult failure to thrive | 783.7 |
| Debility not otherwise specified | 799.3 |
ICD-9 = International Classification of Diseases, 9th Revision.
Note: As described in the Methods, the primary classification scheme was based on ICD-9 diagnosis codes. Ten decedents who did not fit this scheme were classified by review of preceding hospital discharge records: 2 cancer, 1 neurodegenerative, 3 cardiac, 2 other organ failure, and 2 frailty/debility.
Table 2.
Patient Characteristics by Functional Trajectory Before Hospice and Hospice Admission Diagnosis
| Characteristic | Patients, No. (%) | Age, Mean (SD), ya | Female Sex, No. (%) | Non-Hispanic White, No. (%) | <12 Years’ Education, No. (%) | Chronic Conditions, Mean (SD), No.b |
|---|---|---|---|---|---|---|
| Overall | 213 (100) | 86.4 (5.8) | 138 (64.8) | 192 (90.1) | 79 (37.1) | 2.5 (1.3) |
| Functional trajectoryc | ||||||
| Late decline | 23 (10.8) | 82.0 (6.2) | 12 (52.2) | 22 (95.7) | 4 (17.4) | 2.4 (1.3) |
| Accelerated | 23 (10.8) | 83.9 (4.9) | 13 (56.5) | 22 (95.7) | 6 (26.1) | 2.5 (1.2) |
| Moderate | 45 (21.1) | 83.9 (5.1) | 26 (57.8) | 38 (84.4) | 17 (37.8) | 2.6 (1.2) |
| Progressively severe | 53 (24.9) | 88.7 (5.1) | 37 (69.8) | 49 (92.5) | 25 (47.2) | 2.5 (1.2) |
| Persistently severe | 69 (32.4) | 88.6 (5.0) | 50 (72.5) | 61 (88.4) | 27 (39.1) | 2.4 (1.4) |
| Admission diagnosisd | ||||||
| Cancer | 74 (34.7) | 83.6 (5.9) | 42 (56.7) | 67 (90.5) | 24 (32.4) | 2.7 (1.3) |
| Neurodegenerative | 45 (21.1) | 87.7 (5.1) | 30 (66.7) | 38 (84.4) | 17 (37.8) | 2.0 (1.1) |
| Cardiac | 34 (16.0) | 87.9 (5.6) | 19 (55.9) | 32 (94.1) | 17 (50.0) | 2.7 (1.2) |
| Frailty/debility | 20 (9.4) | 90.9 (4.3) | 15 (75.0) | 19 (95.0) | 8 (40.0) | 2.3 (1.2) |
| Other organ failure | 15 (7.0) | 87.7 (4.7) | 12 (80.0) | 15 (100) | 5 (33.3) | 2.8 (1.5) |
| Vascular | 14 (6.6) | 85.0 (5.3) | 11 (78.6) | 11 (78.6) | 5 (35.7) | 2.0 (1.5) |
| Respiratory | 11 (5.2) | 87.1 (3.8) | 9 (81.8) | 10 (90.9) | 3 (27.3) | 3.2 (1.3) |
Age was measured at the start of the trajectories.
Average based on 9 physician-diagnosed, patient-reported conditions: diabetes, hypertension, arthritis, cancer, chronic lung disease, congestive heart failure, myocardial infarction, hip fracture, and stroke.
The 95% CIs for the group membership frequencies, based on 1,000 bootstrap samples, were as follows: late decline, 5.6% to 16.0%; accelerated, 4.2% to 17.6%; moderate, 13.4% to 28.2%; progressively severe, 17.4% to 32.4%; and persistently severe, 23.9% to 42.3%.
As defined in Table 1.
The mean age was lowest among participants with cancer and highest among those with frailty/debility. Women were underrepresented in the cancer and cardiac groups, but overrepresented in the vascular, respiratory, other organ failure, and frailty/debility groups. The vascular group had the lowest proportion of participants who were non-Hispanic white. Low educational attainment was most common in the cardiac group and least common in the respiratory group. The number of chronic conditions was lowest in the neurodegenerative and vascular groups and highest in the respiratory group.
Participants with noncancer diagnoses tended to have a higher burden of disability before hospice than those with a cancer diagnosis (Table 3). Participants with cancer were unlikely to be in the persistently severe disability group, but comprised most of the late decline group (19 of 23). Among participants with neurodegenerative disease, nearly three-quarters had persistently severe disability before hospice, and only 1 participant was largely disability free 12 months before hospice. Participants admitted to hospice because of a stroke or other vascular disease were underrepresented in the late decline group, but otherwise had a relatively even distribution of functional trajectories. Participants admitted to hospice with a diagnosis of cardiac disease, respiratory disease, other organ failure, or frailty/debility generally had high levels of disability before hospice, although their distribution across the accelerated, progressively severe, and persistently severe groups differed modestly.
Table 3.
Functional Trajectory Before Hospice by Hospice Admission Diagnosis
| Functional Trajectory | Cancer, No. (%) | Neurodegenerative, No. (%) | Cardiac, No. (%) | Vascular, No. (%) | Respiratory, No. (%) | Other Organ Failure, No. (%) | Frailty/Debility, No. (%) | Total, No. (%) |
|---|---|---|---|---|---|---|---|---|
| Late decline | 9 (25.7) | 1 (2.2) | 0 (0.0) | 1 (7.14) | 2 (18.2) | 0 (0.0) | 0 (0.0) | 23 (10.8) |
| Accelerated | 2 (16.2) | 0 (0.0) | 4 (11.8) | 4 (28.6) | 0 (0.0) | 1 (6.7) | 2 (10.0) | 23 (10.8) |
| Moderate | 2 (29.7) | 5 (11.1) | 7 (20.6) | 3 (21.4) | 0 (0.0) | 5 (33.3) | 3 (15.0) | 45 (21.1) |
| Progressively severe | 5 (20.3) | 6 (13.3) | 16 (47.1) | 3 (21.4) | 6 (54.6) | 3 (20.0) | 4 (20.0) | 53 (24.9) |
| Persistently severe | 6 (8.1) | 33 (73.3) | 7 (20.6) | 3 (21.4) | 3 (27.3) | 6 (40.0) | 11 (55.0) | 69 (32.4) |
Note: Percentages may not add up to 100 because of rounding.
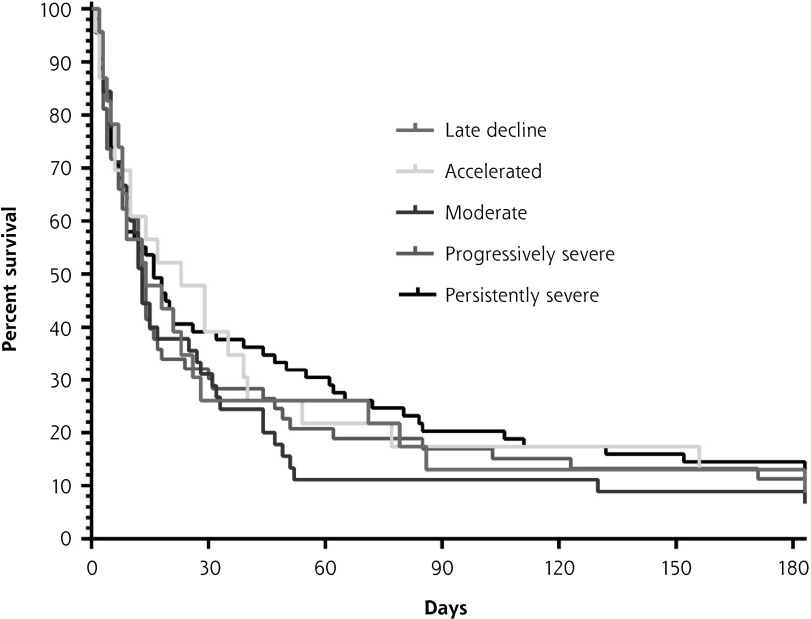
Among all participants, the median survival after hospice admission was only 14 days (interquartile range, 6–50). The survival curves did not differ significantly according to prehospice functional trajectory (Figure 2 and Supplemental Figure 2). After adjustment for age and sex, the hazard ratios, relative to the persistently severe group, for each of the other disability groups were nonsignificant and their CIs spanned 1.0.
Figure 2.
Association between functional trajectory before hospice and overall survival after hospice admission (N = 213).
Notes: For each prehospice trajectory group, the product-limit survival function estimate is plotted by the Kaplan-Meier method. The median overall survival was 14 days; no significant difference in survival was observed across the trajectory groups (P = .79, log-rank test). The 26 (12.2%) participants who were still alive were censored at 180 days.
Of the 210 participants who continued in the study, only 28 (13.3%) were alive and without complete disability in the month after starting hospice. These 28 participants represented 29.5% of those who survived to the first monthly interview after hospice. In an analysis adjusted for age and sex, participants in the moderate disability group before starting hospice had the highest likelihood of being alive without complete disability the month after hospice, relative to those in the persistently severe disability group (Table 4). Comparable results were observed among the 95 survivors at 1 month.
Table 4.
Multivariate Associations of Functional Trajectories Before Hospice With Outcomes in the Month After Hospice Admission
| All Participants (N = 210): Alive and Not Completely Disabled | Surviving Participants (n = 95): Not Completely Disabled | |||
|---|---|---|---|---|
|
|
||||
| Functional Trajectory | With Outcome/At Risk, Nos. | Adjusted OR (95% CI) | With Outcome/At Risk, Nos. | Adjusted OR (95% CI) |
| Late decline | 4/23 | 3.5 (0.8–23.3) | 4/10 | 3.9 (0.8–32.2) |
| Accelerated | 5/23 | 4.4 (0.7–27.1) | 5/13 | 4.4 (0.6–52.8) |
| Moderate | 11/44 | 5.5 (1.9–35.9) | 11/18 | 10.5 (3.3–134.2) |
| Progressively severe | 4/53 | 1.3 (0.3–9.9) | 4/21 | 1.7 (0.4–13.6) |
| Persistently severe | 4/67 | Ref | 4/33 | Ref |
OR = odds ratio; Ref = reference group.
Note: Three participants (1.4%) who had withdrawn from the study before hospice admission were omitted from these analyses. Results from the logistic regression models are adjusted for age and sex.
DISCUSSION
In this study, we found that the course of disability in the year before hospice differed greatly among older persons, but was particularly poor among those with neurodegenerative disease. Moreover, older persons with a noncancer diagnosis tended to have a higher burden of disability in the year before hospice than those with a cancer diagnosis. Median survival after hospice admission was only 2 weeks and did not differ by functional trajectory before hospice. Late admission to hospice (as shown by the short survival), coupled with the high burden of disability before hospice, highlights potential unmet palliative care needs for many older persons at the end of life.
The availability of prospective longitudinal data on functional status at monthly intervals allowed us to identify 5 distinct trajectories of disability before the start of hospice, ranging from late decline to persistently severe. To our knowledge, no other study has provided comparable data. In prior work, trajectories of dying have been simulated through the use of data from Medicare claims42 and annual surveys.25 In 2 other studies, the assessment of disability at the end of life was based on retrospective proxy reports completed several weeks to months after death43 and an average of 4.2 years after death.44 One community-based study of decedents included prospective assessments of functional status at intervals of less than a year, but was restricted to frail older persons who met eligibility criteria for long-term care/nursing home placement.24 None of these earlier studies evaluated disability in the setting of a hospice admission.
We found that the distribution of the functional trajectories differed considerably according to the admission diagnosis for hospice. It is possible that these differences were due, at least in part, to differences in demographic and clinical characteristics. The largest differences were observed for age, which was about 6 years younger, on average, among participants enrolled in hospice for cancer than among those enrolled in hospice for frailty/debility.
For each of the functional trajectories, outcomes after admission to hospice were poor, with no differences observed in survival. The absence of any differences across the functional trajectories could be attributable to the short median survival. Alternatively, physicians may not weigh functional trajectories heavily in their decision making about hospice referral, despite evidence that functional status is one of the strongest predictors of mortality among older persons.45–47 Efforts to educate physicians, patients, and families about the poor prognosis conferred by progressively and persistently severe disability could facilitate earlier admission to hospice when appropriate. Relative to those in the persistently severe disability group before the start of hospice, participants in the moderate disability group had the best functional outcomes, although power was low to detect statistically significant differences for the other functional trajectories.
Nearly 60% of our sample, comprising participants in the progressively and persistently severe groups, had high levels of disability during the year before hospice. Together, the progressively and persistently severe disability groups mapped most tightly to the noncancer admission diagnoses, especially those representing organ failure or frailty/debility. Prior reports have raised concerns that the Medicare Hospice Benefit may not adequately address the palliative care needs of persons whose illnesses result in a prolonged period of severe disability.3,15,48 Hospice may be delayed for these persons because of a desire to continue curative or disease-modifying therapies or because of an uncertain prognosis. Our results indicate that the Medicare Hospice Benefit may be best equipped to meet the palliative care needs of older persons in the late decline group, who predominantly had cancer as the hospice admission diagnosis. These persons, in contrast to those in the progressively and persistently severe groups, had comparatively low levels and short duration of disability before hospice.
In the setting of progressive and persistent levels of severe disability, the care needs of older persons are substantial and mortality is high.1,2 Greater access to palliative care, independent of prognosis and treatment decisions, could address these needs, while also offering symptom management, family support, and advance care planning. Although not yet widely available,49 palliative care programs can reduce costs, while providing services that are consistent with the wishes of older persons and their families.50 The recently announced Medicare Care Choices Model (http://innovation.cms.gov/initiatives/Medicare-Care-Choices/) represents one attempt to enhance the availability of palliative care services among Medicare beneficiaries who wish to continue receiving curative services.
In addition to the monthly assessments of functional status, strengths of our study include the high participation rate, completeness of data collection, low attrition for reasons other than death, and use of Medicare data to ascertain hospice use and admission diagnoses. In contrast to an earlier study that also evaluated functional trajectories at the end of life,26 the current study focused specifically on older persons admitted to hospice and included a comprehensive array of functional activities.
Despite these strengths, our results should be interpreted in the context of several potential limitations. First, our study sample was relatively small, leading to low statistical power for some comparisons. Prospective longitudinal assessment of functional status at monthly intervals would be difficult to replicate in larger populations over an extended period of time. Second, information on receipt of palliative care before the start of hospice was not available in the current study. Although it is possible that late admission to hospice was due to prior receipt of palliative care, the high burden of restricting symptoms during the last year of life among decedents in the same cohort supports the possibility of unmet palliative care needs.51 Third, because they were not included in the current study, we cannot comment on the burden of disability and potential unmet needs at the end of life among decedents who had not been admitted to hospice. Additional research is needed to evaluate the relationship between the presence and burden of disability at the end of life and the receipt of palliative care, hospice care, or both. Fourth, because the parent study excluded 8 persons who had a terminal illness, the number of hospice cases in the current study may have been slightly diminished. Fifth, most participants died within a month of starting hospice, limiting the inferences that could be drawn about subsequent functional outcomes. Sixth, although participants were assigned a single admission diagnosis for hospice, it is possible that some may have met more than 1 admission criterion, for example, having both dementia and frailty/debility. Finally, because our study participants were members of a single health plan in a small urban area, our results may not be generalizable to older persons in other settings. The demographic characteristics of our cohort reflect those of older persons in New Haven County, Connecticut, which are similar to the characteristics of the US population as a whole, with the exception of race/ethnicity.52
In summary, the care needs of many older persons, especially those with noncancer diagnoses, are substantial in the year before hospice. New models of palliative care, informed by functional trajectories before hospice, are needed to complement the Medicare Hospice Benefit.
Acknowledgments
We thank Terrence E. Murphy, PhD, and Ling Han, MD, PhD, for assistance with the trajectory analysis; Denise Shepard, BSN, MBA, Andrea Benjamin, BSN, Barbara Foster, and Amy Shelton, MPH, for assistance with data collection; Wanda Carr and Geraldine Hawthorne, BS, for assistance with data entry and management; Peter Charpentier, MPH, for design and development of the study database and participant tracking system; and Joanne McGloin, MDiv, MBA, for leadership and advice as the Project Director.
Footnotes
Conflicts of interest: authors report none.
Funding support: The work for this report was funded by a grant from the National Institute on Aging (R37AG17560). The study was conducted at the Yale Claude D. Pepper Older Americans Independence Center (P30AG21342). Dr Stabenau was supported by the Medical Student Training in Aging Research (MSTAR) Program, sponsored by the American Federation for Aging Research. Dr Gill is the recipient of an Academic Leadership Award (K07AG043587) from the National Institute on Aging.
Supplementary materials: Available at http://www.AnnFamMed.org/content/13/1/33/suppl/DC1/.
References
- 1.Ferrucci L, Guralnik JM, Simonsick E, Salive ME, Corti C, Langlois J. Progressive versus catastrophic disability: a longitudinal view of the disablement process. J Gerontol A Biol Sci Med Sci. 1996;51(3):M123–M130. [DOI] [PubMed] [Google Scholar]
- 2.Alpert JS, Powers PJ. Who will care for the frail elderly? Am J Med. 2007;120(6):469–471. [DOI] [PubMed] [Google Scholar]
- 3.Meier DE. When pain and suffering do not require a prognosis: working toward meaningful hospital-hospice partnership. J Palliat Med. 2003;6(1):109–115. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Haley WE, Robinson BE, Schonwetter RS. Decisions for hospice care in patients with advanced cancer. J Am Geriatr Soc. 2003;51(6):789–797. [DOI] [PubMed] [Google Scholar]
- 5.Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;291(1):88–93. [DOI] [PubMed] [Google Scholar]
- 6.Kapo J, Harrold J, Carroll JT, Rickerson E, Casarett D. Are we referring patients to hospice too late? Patients’ and families’ opinions. J Palliat Med. 2005;8(3):521–527. [DOI] [PubMed] [Google Scholar]
- 7.Casarett D, Van Ness PH, O’Leary JR, Fried TR. Are patient preferences for life-sustaining treatment really a barrier to hospice enrollment for older adults with serious illness? J Am Geriatr Soc. 2006;54(3):472–478. [DOI] [PubMed] [Google Scholar]
- 8.Fried TR, O’Leary J, Van Ness P, Fraenkel L. Inconsistency over time in the preferences of older persons with advanced illness for life-sustaining treatment. J Am Geriatr Soc. 2007;55(7):1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried TR, Van Ness PH, Byers AL, Towle VR, O’Leary JR, Dubin JA. Changes in preferences for life-sustaining treatment among older persons with advanced illness. J Gen Intern Med. 2007;22(4):495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casarett D, Fishman J, O’Dwyer PJ, Barg FK, Naylor M, Asch DA. How should we design supportive cancer care? The patient’s perspective. J Clin Oncol. 2008;26(8):1296–1301. [DOI] [PubMed] [Google Scholar]
- 11.Fishman J, O’Dwyer P, Lu HL, Henderson HR, Asch DA, Casarett DJ. Race, treatment preferences, and hospice enrollment: eligibility criteria may exclude patients with the greatest needs for care. Cancer. 2009;115(3):689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salz T, Brewer NT. Offering chemotherapy and hospice jointly: one solution to hospice underuse. Med Decis Making. 2009;29(4):521–531. [DOI] [PubMed] [Google Scholar]
- 13.Conner NE. Predictive factors of hospice use among blacks: applying Andersen’s Behavioral Model. Am J Hosp Palliat Care. 2012;29(5):368–374. [DOI] [PubMed] [Google Scholar]
- 14.Fauci J, Schneider K, Walters C, et al. The utilization of palliative care in gynecologic oncology patients near the end of life. Gynecol Oncol. 2012;127(1):175–179. [DOI] [PubMed] [Google Scholar]
- 15.Casarett DJ. Rethinking hospice eligibility criteria. JAMA. 2011;305(10):1031–1032. [DOI] [PubMed] [Google Scholar]
- 16.Morrison RS, Augustin R, Souvanna P, Meier DE. America’s care of serious illness: a state-by-state report card on access to palliative care in our nation’s hospitals. J Palliat Med. 2011;14(10):1094–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenq G, Tinetti ME. Changes in end-of-life care over the past decade: more not better. JAMA. 2013;309(5):489–490. [DOI] [PubMed] [Google Scholar]
- 18.Teno JM, Gozalo PL, Bynum JP, et al. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Covinsky KE, Goldman L, Cook EF, et al. ; SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. The impact of serious illness on patients’ families. JAMA. 1994;272(23):1839–1844. [DOI] [PubMed] [Google Scholar]
- 20.Desbiens NA, Wu AW. Pain and suffering in seriously ill hospitalized patients. J Am Geriatr Soc. 2000;48(5 Suppl):S183–S186. [DOI] [PubMed] [Google Scholar]
- 21.Downing GM, Lesperance M, Lau F, Yang J. Survival implications of sudden functional decline as a sentinel event using the palliative performance scale. J Palliat Med. 2010;13(5):549–557. [DOI] [PubMed] [Google Scholar]
- 22.Harris P, Wong E, Farrington S, et al. Patterns of functional decline in hospice: what can individuals and their families expect? J Am Geriatr Soc. 2013;61(3):413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith AK, Earle CC, McCarthy EP. Racial and ethnic differences in end-of-life care in fee-for-service Medicare beneficiaries with advanced cancer. J Am Geriatr Soc. 2009;57(1):153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Covinsky KE, Eng C, Lui LY, Sands LP, Yaffe K. The last 2 years of life: functional trajectories of frail older people. J Am Geriatr Soc. 2003;51(4):492–498. [DOI] [PubMed] [Google Scholar]
- 25.Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. JAMA. 2003;289(18):2387–2392. [DOI] [PubMed] [Google Scholar]
- 26.Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med. 2010;362(13):1173–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapo J, Morrison LJ, Liao S. Palliative care for the older adult. J Palliat Med. 2007;10(1):185–209. [DOI] [PubMed] [Google Scholar]
- 28.Gill TM, Desai MM, Gahbauer EA, Holford TR, Williams CS. Restricted activity among community-living older persons: incidence, precipitants, and health care utilization. Ann Intern Med. 2001;135(5):313–321. [DOI] [PubMed] [Google Scholar]
- 29.Hardy SE, Gill TM. Recovery from disability among community-dwelling older persons. JAMA. 2004;291(13):1596–1602. [DOI] [PubMed] [Google Scholar]
- 30.Gill TM. Disentangling the disabling process: insights from the precipitating events project. Gerontologist. 2014;54(4):533–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gill TM, Hardy SE, Williams CS. Underestimation of disability in community-living older persons. J Am Geriatr Soc. 2002;50(9):1492–1497. [DOI] [PubMed] [Google Scholar]
- 32.Gill TM, Murphy TE, Gahbauer EA, Allore HG. The course of disability before and after a serious fall injury. JAMA Intern Med. 2013;173(19):1780–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolinsky FD, Miller TR, An H, et al. Hospital episodes and physician visits: the concordance between self-reports and Medicare claims. Med Care. 2007;45(4):300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 35.Gill TM, Gahbauer EA, Murphy TE, Han L, Allore HG. Risk factors and precipitants of long-term disability in community mobility: a cohort study of older persons. Ann Intern Med. 2012;156(2):131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gill TM, Gahbauer EA, Lin H, Han L, Allore HG. Comparisons between older men and women in the trajectory and burden of disability over the course of nearly 14 years. J Am Med Dir Assoc. 2013;14(4):280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixed models for estimating developmental trajectories. Sociol Methods Res. 2001;29(3):374–393. [Google Scholar]
- 38.Nagin DS. Group-Based Modeling of Development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 39.Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Methods Res. 2007;35(4):542–571. [Google Scholar]
- 40.Efron B, Tibshirani R. An Introduction to the Bootstrap. New York, NY: Chapman & Hall; 1993. [Google Scholar]
- 41.Kaplan EL, Meier PM. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 42.Lunney JR, Lynn J, Hogan C. Profiles of older Medicare decedents. J Am Geriatr Soc. 2002;50(6):1108–1112. [DOI] [PubMed] [Google Scholar]
- 43.Liao Y, McGee DL, Cao G, Cooper RS. Quality of the last year of life of older adults: 1986 vs 1993. JAMA. 2000;283(4):512–518. [DOI] [PubMed] [Google Scholar]
- 44.Romoren TI, Blekeseaune M. Trajectories of disability among the oldest old. J Aging Health. 2003;15(3):548–566. [DOI] [PubMed] [Google Scholar]
- 45.Harrold J, Rickerson E, Carroll JT, et al. Is the palliative performance scale a useful predictor of mortality in a heterogeneous hospice population? J Palliat Med. 2005;8(3):503–509. [DOI] [PubMed] [Google Scholar]
- 46.Inouye SK, Peduzzi PN, Robison JT, Hughes JS, Horwitz RI, Concato J. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279(15):1187–1193. [DOI] [PubMed] [Google Scholar]
- 47.Carey EC, Walter LC, Lindquist K, Covinsky KE. Development and validation of a functional morbidity index to predict mortality in community-dwelling elders. J Gen Intern Med. 2004;19(10):1027–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Unroe KT, Meier DE. Quality of hospice care for individuals with dementia. J Am Geriatr Soc. 2013;61(7):1212–1214. [DOI] [PubMed] [Google Scholar]
- 49.Unroe KT, Meier DE. Research priorities in geriatric palliative care: policy initiatives. J Palliat Med. 2013;16(12):1503–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manfredi PL, Morrison RS, Morris J, Goldhirsch SL, Carter JM, Meier DE. Palliative care consultations: how do they impact the care of hospitalized patients? J Pain Symptom Manage. 2000;20(3):166–173. [DOI] [PubMed] [Google Scholar]
- 51.Chaudhry SI, Murphy TE, Gahbauer E, Sussman LS, Allore HG, Gill TM. Restricting symptoms in the last year of life: a prospective cohort study. JAMA Intern Med. 2013;173(16):1534–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.US Census Bureau. American FactFinder. http://factfinder2.census.gov/faces/nav/jsf/pages/index.xhtml Accessed Jul 9, 2013.