Abstract
PURPOSE
The way in which spirometry is interpreted can lead to misdiagnosis of chronic obstructive pulmonary disease (COPD) resulting in inappropriate treatment. We compared the clinical relevance of 2 criteria for defining a low ratio of forced expiratory volume in 1 second to forced vital capacity (FEV1/FVC): the fixed ratio and the lower limit of normal.
METHODS
We analyzed data from the cross-sectional phase of the population-based Canadian Cohort of Obstructive Lung Disease (CanCOLD) study. We determined associations of the spirometric criteria for airflow limitation with patient-reported adverse outcomes, including respiratory symptoms, disability, health status, exacerbations, and cardiovascular disease. Sensitivity analyses were used to explore the impact of age and severity of airflow limitation on these associations.
RESULTS
We analyzed data from 4,882 patients aged 40 years and older. The prevalence of airflow limitation was 17% by fixed ratio and 11% by lower limit of normal. Patients classified as having airflow limitation by fixed ratio only had generally small, nonsignificant increases in the odds of adverse outcomes. Patients having airflow limitation based on both fixed ratio and lower limit of normal had larger, significant increases in odds. But strongest associations were seen for patients who had airflow limitation by both fixed ratio and lower limit of normal and also had a low FEV1, defined as one less than 80% of the predicted value.
CONCLUSIONS
Our results suggest that use of the fixed ratio alone may lead to misdiagnosis of COPD. A diagnosis established by both a low FEV1/FVC (according to fixed ratio and/or lower limit of normal) and a low FEV1 is strongly associated with clinical outcomes. Guidelines should be reconsidered to require both spirometry abnormalities so as to reduce overdiagnosis of COPD.
Keywords: clinical relevance, COPD, lung diseases, spirometry, diagnosis, fixed ratio, lower limit of normal, FEV1
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is one of the most prevalent chronic diseases and the cause of much morbidity and mortality worldwide.1 COPD has been estimated to occur in up to 25% of the population aged 40 years and older1–3; however, both overdiagnosis and underdiagnosis pose challenges in daily practice, resulting in inappropriate patient management.2–10 COPD guidelines recommend the use of a low ratio of forced expiratory volume in 1 second to forced vital capacity (FEV1/FVC) to establish the diagnosis in patients with chronic respiratory symptoms or those at risk.1,11,12
There is currently no consensus on the best criteria to be used for the spirometric confirmation of a clinical diagnosis of COPD. A lively debate revolves around 2 main measures. One measure is the FEV1/FVC fixed ratio of less than 0.7, alone or in combination with a low FEV1 set at a value less than 80% of predicted; the combination comprises Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 2 or worse disease. The other measure is the FEV1/FVC ratio below the 5th percentile, in other words, below the lower limit of normal (LLN).1,11–17 Prevalence studies have shown discordance between the fixed ratio and LLN, suggesting potential overdiagnosis by the former or underdiagnosis by the latter.3,4,8,18,19
This controversy persists because of the absence of a reference standard for the diagnosis of COPD.19 The associations of the 2 criteria with clinical outcomes have been assessed in a few studies, with unclear results on the relative superiority of 1 criterion over the other when it comes to their relationship with different clinical outcomes.20 The cross-sectional phase of the Canadian Cohort of Obstructive Lung Disease (CanCOLD) study provided us with a large population-based database to further explore the clinical relevance of these criteria by examining their associations with multiple patient-reported outcomes such as symptoms, health status, dyspnea-related disability, exacerbations, and cardiovascular comorbidity, with adjustments for age, sex, and smoking exposure.
METHODS
We analyzed data from 5,176 people from the general population aged 40 years and older to assess the clinical relevance of differing diagnostic criteria for COPD. Data were collected between August 2005 and May 2009 in a large, cross-sectional, multisite, population-based study on lung health, which constituted the first phase of the longitudinal CanCOLD study. The sampling strategy and study protocol of the baseline cross-sectional part of the study were the same as those used in the international Burden of Obstructive Lung Disease (BOLD) initiative, the full details of which have been published elsewhere.21 Briefly, random samples of noninstitutionalized adults from 9 Canadian urban sites were drawn from census data of Statistics Canada (Survey and Analysis Section), and recruitment was conducted by the NRG Research group by random telephone digit dialing to identify eligible individuals21 who were invited to attend a clinic visit to complete interviewer-administered respiratory questionnaires and to perform pre- and post-bronchodilator spirometry. The only exclusion criterion was inability or refusal to perform spirometry. The mean clinic visit participation rate was 74% (range, 63% to 87%).21 All participants gave written informed consent, and the study was approved by the respective institutional ethical review boards. CanCOLD is registered at http://www.clinicaltrials.gov (NCT00920348).
Measurements
Spirometry was performed according to the acceptability and reproducibility criteria of the American Thoracic Society and European Respiratory Society guidelines.22 Measurements included FEV1 and FVC. Data in administered questionnaires provided information on age, sex, body mass index, educational level, and race; smoking status, including number of pack-years; and the patient report of physician-diagnosed COPD, emphysema, and chronic bronchitis.
Additional questionnaire data included patient-reported outcomes: (1) respiratory symptoms including chronic cough and chronic phlegm for most days during 3 months in the last year; chronic bronchitis (defined as chronic cough and chronic phlegm for more than 2 years); and wheeze in the last year (not only during colds); (2) disability by the Medical Research Council (MRC) dyspnea scale; (3) general health status as assessed by the Short Form 12-item health survey (SF-12); (4) self-reported exacerbations of COPD; and (5) self-reported physician-diagnosed cardiovascular disease.
We compared 2 spirometric criteria for determining airflow limitation—a postbronchodilator FEV1/FVC ratio of less than 0.70 (fixed ratio) and a postbronchodilator FEV1/FVC ratio of less than the 5th percentile (LLN)—using the reference equations from the Third National Health and Nutrition Examination Survey (NHANES III).23 We assessed diagnosis according to presence of either or both criteria.
Outcomes and Modifying Factors
We defined clinically relevant outcomes as follows: respiratory symptoms including chronic cough, chronic phlegm, chronic bronchitis, and wheeze; health status, as measured by SF-12; disability, as measured by MRC dyspnea scale; exacerbations of COPD as measured by ever having breathing problems that interfered with usual daily activities or caused one to miss work; and cardiovascular disease (heart disease and stroke).
We assessed a variety of factors for their potential modification of the relationship between the 2 diagnostic criteria and clinically relevant outcomes, including sex, age-group (younger or older than 60 years), cardiovascular disease, and smoking status. In particular, we studied the impact of the severity of airflow limitation, assessed by FEV1 as the percentage of the predicted value, on the association of fixed ratio or LLN with the outcomes.
Statistical Analysis
We analyzed the associations of the 2 main diagnostic criteria with clinically relevant outcomes. In addition, we compared 6 subgroups of individuals who satisfied various diagnostic criteria consisting of single spirometric measures or combinations of measures. Univariate analysis of variance (ANOVA) or the Kruskal-Wallis test for continuous variables, and the χ2 square test for dichotomous variables were performed to compare the subgroups on baseline characteristics. We analyzed the clinical relevance of the diagnostic criteria according to the subgroups by a multivariate regression model, controlling for confounders at baseline. Logistic models were used for binary data, and linear models were used for continuous data. In addition, we performed interaction analyses between the spirometric criteria and modifying factors to determine whether any of these factors significantly modified the association with clinical outcomes. Finally, sensitivity analyses were performed by including 2 additional subgroups with a low FEV1, defined as a value less than 80% of predicted: a subgroup meeting the fixed ratio criterion and having low FEV1 (ie, GOLD stage 2 or higher disease) and subgroup meeting the LLN criterion and having low FEV1. A P value of less than .05 was considered to be statistically significant.
RESULTS
Analyses were based on 4,882 CanCOLD study participants who had complete data on all study parameters (Table 1). Their mean age (±SD) was 57 (±11) years, with a range of 40 to 93 years; 146 (3%) were older than 80 years. Forty-three percent were male. Their mean FEV1 (±SD) was 95.0% (±17%) of the normal predicted value.
Table 1.
Patient Characteristics at Baseline, According to Criteria Used to Define Airflow Limitation
| Characteristic | Total (N = 4,882) | No Airflow Limitationa (n = 4,038) | Airflow Limitation
|
||||
|---|---|---|---|---|---|---|---|
| FR+/LLN− (n = 297) | FR−/LLN+ (n = 15) | FR+/LLN+ (n = 532) | FR+ and Low FEV1b,c (n = 363) | LLN+ and Low FEV1b (n = 304) | |||
| Age, mean (SD), y | 57 (11) | 56 (11) | 68 (10) | 45 (3) | 62 (12) | 64 (11) | 63 (11) |
| Sex (male), No. (%) | 2,093 (43) | 1,668 (41) | 186 (63) | 1 (7) | 238 (45) | 177 (49) | 138 (45) |
| Postbronchodilator FEV1, mean (SD), % predicted | 95 (17) | 98 (15) | 92 (16) | 77 (18) | 77 (18) | 65 (12) | 64 (12) |
| Postbronchodilator FVC, mean (SD), % predicted | 97 (15) | 96 (15) | 101 (17) | 99 (12) | 98 (19) | 85 (14) | 86 (14) |
| Body mass index, kg/m2 | 28 (6) | 28 (6) | 27 (5) | 28 (5) | 28 (5) | 28 (6) | 28 (6) |
| Tobacco smoking status, No. (%)d | |||||||
| Never smoker | 2,091 (43) | 1,852 (46) | 95 (32) | 8 (53) | 136 (26) | 73 (20) | 100 (33) |
| Former smoker | 2,058 (42) | 1,657 (41) | 158 (53) | 1 (7) | 242 (46) | 178 (49) | 142 (47) |
| Current smoker | 729 (15) | 527 (13) | 43 (15) | 6 (40) | 153 (29) | 111 (31) | 61 (20) |
| Education ≤12 years, No. (%) | 493 (10) | 366 (9) | 43 (15) | 0 (0) | 83 (16) | 65 (18) | 55 (18) |
| Race (white), No. (%) | 4,464 (91) | 3,663 (91) | 277 (93) | 14 (93) | 510 (96) | 343 (94) | 290 (95) |
| Conditions, No. (%) | |||||||
| Chronic cough | 619 (13) | 432 (11) | 45 (15) | 6 (40) | 136 (26) | 113 (31) | 102 (34) |
| Chronic phlegm | 467 (10) | 312 (8) | 35 (12) | 1 (7) | 119 (22) | 96 (26) | 85 (28) |
| Chronic bronchitise | 213 (4) | 136 (3) | 17 (6) | 1 (7) | 59 (11) | 51 (14) | 65 (21) |
| Wheeze | 1,273 (29) | 939 (25) | 78 (31) | 8 (53) | 248 (52) | 192 (60) | 166 (61) |
| MRC dyspnea scalef | 1.4 (0.8) | 1.3 (0.7) | 1.4 (0.8) | 1.7 (1.2) | 1.8 (1.1) | 2 (1) | 2.03 (1.2) |
| SF-12 scores, mean (SD)g | |||||||
| Physical scale | 50 (9) | 51 (9) | 50 (9) | 46 (14) | 47 (11) | 45 (11) | 44 (12) |
| Mental scale | 52 (9) | 52 (9) | 54 (8) | 49 (11) | 52 (9) | 53 (9) | 52 (10) |
| Exacerbation, No. (%) | 996 (20) | 779 (19) | 58 (20) | 4 (27) | 155 (29) | 119 (33) | 100 (33) |
| Cardiovascular disease, No. (%) | 605 (12) | 436 (11) | 76 (26) | 1 (7) | 92 (17) | 81 (22) | 66 (22) |
FEV1 = forced expiratory volume in 1 second; FR = fixed ratio; FVC = forced vital capacity; LLN = lower limit of normal; MRC = Medical Research Council; SF-12 = Short Form 12-item health survey.
Notes: Continuous data are presented as mean (SD), dichotomous values as number (%). P value: univariate analysis of variance (ANOVA) or Kruskal-Wallis test for continuous variables and χ2 test for dichotomous variables.
FR−/LLN−.
FEV1 <80% of predicted.
Collectively, these 2 criteria constitute Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 2 or higher disease.
Tobacco smoking status includes both cigarette and pipe smoking.
Chronic cough and chronic phlegm for more than 2 years.
MRC dyspnea scale scores range from 0 to 5; higher scores indicate worse health/dyspnea.
SF-12 Physical and Mental scale scores range from 0 to 100; higher scores indicate better health.
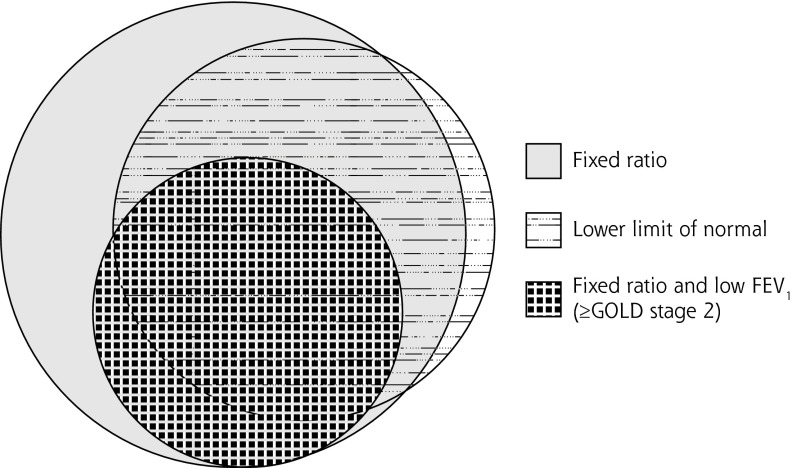
The prevalence of spirometric airflow limitation was 17% by fixed ratio and 11% by lower limit of normal (Table 1). The majority of patients with airflow limitation satisfied both spirometric criteria as there was considerable overlap (concordance) between individuals meeting the fixed ratio and LLN cutoffs for diagnosis as shown in the Venn diagram depicting the proportions satisfying various criteria (Figure 1). The number of patients with airflow limitation by LLN but not by fixed ratio (discordance) was small, at just 15 (0.3%); these patients were on average younger and almost always female (Table 1). The prevalence of airflow limitation by fixed ratio was greater than that by LLN, except among young women; the greatest differences were seen among older adults (Figures 2 and 3).
Figure 1.
Venn diagram showing groups meeting various criteria for airflow limitation: the fixed ratio, the lower limit of normal, and the fixed ratio plus a low FEV1.
FEV1 = forced expiratory volume in 1 second; GOLD = Global Initiative for Chronic Obstructive Lung Disease.
Note: Low FEV1 is a value less than 80% of the predicted value.
Figure 2.
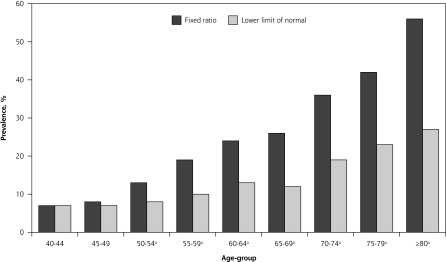
Prevalence of airflow limitation among men as determined by fixed ratio and by lower limit of normal.
aPrevalence differs significantly by fixed ratio vs lower limit of normal (P <.05).
Figure 3.
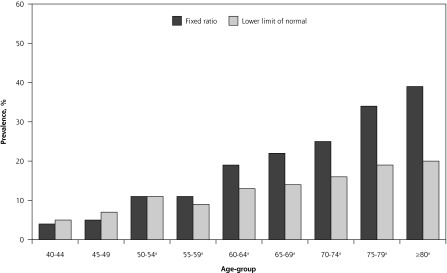
Prevalence of airflow limitation among women determined by fixed ratio and by lower limit of normal.
aPrevalence differs significantly by fixed ratio vs lower limit of normal (P <.05).
Associations With Outcomes
Compared with peers who met neither criterion for airflow limitation, patients having airflow limitation based on fixed ratio only were significantly more likely to have physician-diagnosed wheeze (odds ratio [OR] = 1.54) and cardiovascular disease (OR = 1.52); associations with other outcomes showed only nonsignificant trends (Table 2). In contrast, patients who met both the fixed ratio and LLN criteria for airflow limitation were at significantly increased risk for 7 of the outcomes studied, with particularly strong associations seen for wheeze (OR = 3.25), chronic bronchitis (OR = 3.14), and chronic phlegm (OR = 3.04). Additionally, these patients meeting both criteria also had elevated risk when compared with peers meeting the fixed ratio criterion alone (Table 3).
Table 2.
Associations Between Airflow Limitation According to Various Criteria and Outcomes
| Outcome | No Airflow Limitationa (n = 4,038) | Airflow Limitation
|
|
|---|---|---|---|
| FR+/LLN− (n = 297) | FR+/LLN+ (n = 532) | ||
| Chronic cough | 1.00 | 1.40 (0.99–1.98) | 2.54 (2.03–3.18) |
| Chronic phlegm | 1.00 | 1.43 (0.97–2.10) | 3.04 (2.39–3.87) |
| Chronic bronchitis | 1.00 | 1.59 (0.93–2.72) | 3.14 (2.25–4.37) |
| Wheeze | 1.00 | 1.54 (1.15–2.06) | 3.25 (2.65–3.97) |
| MRC dyspnea scaleb | 1.00 | 1.14 (0.86–1.52) | 2.33 (1.92–2.82) |
| SF-12 Physical component scalec | 1.00 | −0.78 (.17) | −3.31 (<.01) |
| SF-12 Mental component scalec | 1.00 | 0.47 (.40) | −0.30 (.49) |
| Exacerbation | 1.00 | 1.33 (0.98–1.81) | 1.90 (1.54–2.34) |
| Cardiovascular disease | 1.00 | 1.52 (1.14–2.04) | 1.21 (0.93–1.56) |
FR = fixed ratio; LLN = lower limit of normal; MRC = Medical Research Council; SF-12 = Short Form 12-item health survey.
Notes: Multiple logistic regression analysis, except as otherwise noted. All models adjusted for age-group (<60 years), sex, and ever smoking. Data are presented as odds ratios (95% CIs) or parameter estimates (P values) from regression analysis. Number of FR−/LLN+ patients was too small for inclusion.
Reference group (FR−/LLN−).
Ordinal logistic regression analysis.
Multiple linear regression analysis.
Table 3.
Associations Between Airflow Limitation According to Various Definitions and Outcomes
| Outcome | FR+/LLN−a (n = 297) | FR+/LLN+ (n = 532) |
|---|---|---|
| Chronic cough | 1.00 | 1.81 (1.24–2.65) |
| Chronic phlegm | 1.00 | 2.13 (1.41–3.21) |
| Chronic bronchitis | 1.00 | 1.98 (1.12–3.48) |
| Wheeze | 1.00 | 2.11 (1.52–2.93) |
| MRC dyspnea scaleb | 1.00 | 2.04 (1.48–2.81) |
| SF-12 Physical scalec | 1.00 | −2.39 (<.01) |
| SF-12 Mental scalec | 1.00 | −1.10 (.11) |
| Exacerbation | 1.00 | 1.43 (1.01–2.02) |
| Cardiovascular disease | 1.00 | 0.79 (0.55–1.13) |
FR = fixed ratio; LLN = lower limit of normal; MRC = Medical Research Council; SF-12 = Short Form 12-item health survey.
Notes: Multiple logistic regression, except as otherwise noted. All models adjusted for age-group (<60 years), sex, and ever smoking. Data are presented as odds ratios (95% CI) or parameter estimate (P value) from regression analysis.
Reference group.
Ordinal logistic analysis.
Multiple linear regression analysis.
Modifying Factors and Sensitivity Analysis
Analyses did not reveal any significant interaction between the spirometric criteria for airflow limitation and potential modifying factors such as sex, age, and smoking status. Furthermore, excluding patients with cardiovascular disease did not affect any of the observed associations.
Table 4 shows the impact of the severity of airflow on the associations with patient-reported outcomes in 6 groups of patients stratified by the FEV1/FVC criteria—fixed ratio or LLN—and further by FEV1 status (normal vs low). The results indicated that patients meeting either FEV1/FVC criterion had higher risks of some clinical outcomes, but the addition of a low FEV1 to either or both criteria greatly strengthened the associations; patients having such airflow limitation had 2 to 5 times higher odds of most adverse outcomes relative to counterparts having no airflow limitation. Notably, only individuals satisfying either the fixed ratio or LLN criterion (or both) who also had a low FEV1, indicating moderate to severe airflow limitation, had significantly elevated odds of cardiovascular disease (ORs = 1.51–1.56).
Table 4.
Associations Between Airflow Limitation Criteria Alone and Further Refined by FEV1 and Outcomes
| Outcome | No Airflow Limitationa (n = 4,038) | Airflow Limitation
|
||||
|---|---|---|---|---|---|---|
| FR+ and Normal FEV1 (n = 466) | LLN+ and Normal FEV1 (n = 248) | FR+ and Low FEV1b,c (n = 363) | LLN+ and Low FEV1b (n = 304) | FR+ and LLN+ and Low FEV1b (n = 299) | ||
| Chronic cough | 1.00 | 1.32 (1.0–1.7) | 1.59 (1.12–2.25) | 3.32 (2.6–4.3) | 3.65 (2.8–4.8) | 3.64 (2.78–4.77) |
| Chronic phlegm | 1.00 | 1.55 (1.1–2.1) | 1.83 (1.25–2.67) | 3.65 (2.8–4.8) | 3.91 (2.9–5.2) | 4.10 (3.08–5.46) |
| Chronic bronchitis | 1.00 | 1.51 (1.0–2.4) | 1.60 (0.90–2.82) | 3.97 (2.8–5.7) | 4.31 (3.0–6.3) | 4.48 (3.09–6.51) |
| Wheeze | 1.00 | 1.59 (1.3–2.0) | 2.15 (1.63–2.86) | 4.50 (3.5–5.7) | 4.60 (3.5–6.0) | 4.58 (3.51–5.96) |
| MRC dyspnea scaled | 1.00 | 1.06 (0.8–1.3) | 1.27 (0.95–1.69) | 3.65 (2.9–4.6) | 3.92 (3.1–5.0) | 3.86 (3.03–4.92) |
| SF-12 Physical scalee | 1.00 | −0.083 (.85) | –0.85 (.15) | −5.45 (<.01) | −5.56 (<.01) | −5.52 (<.01) |
| SF-12 Mental scalee | 1.00 | −0.030 (.95) | −0.22 (.71) | −0.041 (.93) | −0.42 (.44) | −0.42 (.45) |
| Exacerbation | 1.00 | 1.25 (1.0–1.6) | 1.37 (1.01–1.86) | 2.39 (1.9–3.0) | 2.32 (1.8–3.0) | 2.39 (1.84–3.11) |
| Cardiovascular disease | 1.00 | 1.20 (0.9–1.6) | 0.79 (0.52–1.21) | 1.51 (1.1–2.0) | 1.53 (1.1–2.1) | 1.56 (1.15–2.12) |
FEV1 = forced expiratory volume in 1 second; FR = fixed ratio; LLN = lower limit of normal; MRC = Medical Research Council; SF-12 = Short Form 12-item health survey.
Notes: Multiple logistic regression analysis, except as otherwise noted. All models adjusted for age-group (<60 years), sex, and ever smoking. Data are presented as adjusted odds ratios (95% CI) or parameter estimate (P value) from regression analysis.
Reference group (FR−/LLN−).
FEV1 <80% of predicted.
Collectively, these 2 criteria constitute Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 2 or higher disease.
Ordinal logistic regression analysis.
Multiple linear regression analysis.
DISCUSSION
Key Findings
In this study, we found that airflow limitation defined solely by either of the basic criteria for FEV1/FVC, namely, the fixed ratio or the LLN, was weakly and variably associated with patient-reported poor outcomes such as symptoms, disability, impaired health status, and exacerbations, with stronger associations seen for those meeting the latter criterion. Patients meeting both of these criteria had more consistent and greater increases in risk. But patients meeting either criterion and in addition having a low FEV1 were the most likely to experience poor outcomes. Cardiovascular disease risk was most elevated for patients who had moderate airflow limitation as determined by either FEV1/FVC cutoff criterion plus a low FEV1.
Interpretation
Overall, our results concur with findings from previous studies on patient-reported outcomes. These studies showed that patients with airflow limitation determined by an unqualified fixed ratio (meaning irrespective of FEV1 level) had higher risks of several patient-reported outcomes relative to unaffected peers. When compared with patients having limited airflow defined similarly using unqualified LLN, these associations were somewhat weaker.20
Our results differ from those of previous studies in several aspects. First, the results indicated that overdiagnosis could be a problem when using a single criterion, either fixed ratio or LLN, as patients with overdiagnosis, having a normal FEV1, (one of at least 80% of predicted), appear to experience few patient-reported outcomes; that is, they do not appear to have clinically relevant disease. This lack of clinical relevance may be related to the fact that COPD found in the population may be milder than that reported in patient cohorts. The diagnosis of COPD in patients with mild disease has been debated before.14,24,25 Second, we found that the associations were modified by the level of lung function. Excluding patients with mild airflow limitation as evidenced by a normal FEV1, the use of either fixed ratio or LLN showed clear and firm associations with symptoms and exacerbations and worsening health status and disability. Third, the significant association between cardiovascular disease and airflow limitation determined by an unqualified fixed ratio criterion may suggest a misdiagnosis of COPD in these patients. Heart failure could potentially mimic airflow obstruction in such patients and raises the question as to whether clinicians should suspect undiagnosed cardiovascular disease in those patients with no or mild airflow limitation (FEV1 ≥80%), especially in the elderly, as determined by fixed ratio only.
Additionally, falsely applying the COPD label to patients with dyspnea who do not have undisputed evidence of airflow obstruction can lead to unnecessary and inappropriate treatments. It is conceivable that a high mortality for Group B in the new GOLD classification reflected the circumstance wherein patients had heart disease but were receiving care only for COPD.26 Finally, our results from this study support the use of either fixed ratio or LLN, further qualified by a low FEV1 in the diagnostic confirmation of COPD.
Strengths and Limitations
Although the relationship between diagnostic criteria for COPD and patient-reported outcomes has been previously reported,20 we believe that this is the first study that also includes the evaluation of health status as measured by the SF-12 and examines potential factors that could modify the associations with outcomes. In particular, we systematically assessed the effect of airflow limitation. Although GOLD stage 2 or higher classification has been previously compared with fixed ratio and LLN criteria, our study is the only one to use postbronchodilator spirometry data.27,28 Another strength lies in the derivation of data from the general population and not from a convenience sample of patients as in other published studies. The data come from a large multicenter study that used strict protocols and central data management to ensure that similar measurements across study centers.
There are also several limitations to this study. First, the questionnaire responses in this large epidemiologic study were not clinically confirmed. Second, as the analyzed data were cross-sectional, the diagnostic criteria could not predict outcomes. Last, the subgroup of patients with airflow limitation determined by LLN only was too small for analysis.
Future Directions
Underdiagnosis and overdiagnosis remain challenges to the adequate management of COPD and to the optimal use of health care resources. Given recommendations that diagnosis of COPD be based on the triple criteria of presence of risk factors, symptoms, and spirometric evidence of airflow limitation (a reduced FEV1/FVC) and that the main rationale of pharmacologic treatment of COPD is the reducing symptoms, it could be argued that overdiagnosis rather than underdiagnosis is a more pressing issue in clinical practice. The results of this large cross-sectional study have suggested that a clinically relevant diagnosis of COPD is best based on the use of either cutoff criterion for a low FEV1/FVC coupled with a low FEV1 as percentage of predicted. This impression awaits definitive clarification from longitudinal studies such as the follow-up phase of the ongoing CanCOLD study, in which clinical outcomes in patients having COPD diagnosed using different criteria can be further evaluated.
Conclusions
There is persistent controversy regarding the most appropriate cutoff values for FEV1/FVC in the diagnosis of chronic airflow limitation in COPD. The results from this population-based Canadian study on clinically relevant patient-reported outcomes showed that the use of a single criterion alone—FEV1/FVC either less than 0.7 or less than the LLN—was inadequate and may misdiagnose patients with COPD (in particular those with cardiovascular symptoms), putting them at risk for inappropriate or unnecessary treatments. Our results indicated that a low FEV1/FVC ratio by either the fixed ratio or LLN criterion coupled with a low FEV1 (<80% of predicted) is the most clinically relevant diagnostic criterion for COPD. Future guidelines may reconsider amending the current diagnostic spirometric cutoff to include this additional specification.
Footnotes
Conflicts of interest: See funding support. The authors report no other conflicts of interest.
CanCOLD Study Group: Shawn Aaron, Kenneth Chapman, Mark J. FitzGerald, Roger Goldstein, Paul Hernandez, François Maltais, Darcy Marciniuk, Dennis O’Donnell, Donald Sin, Robert Cowie, Harvey Coxson, Jonathon Leipsic, Cameron Hague, Jeremy Road, Juli Atherton, Carolyn Baglole, Styliani Daskalopoulou, J. Pierre Després, Andrea Gershon, Kim Lavoie, David Miedinger, Remi Rabasa, Machelle Wilchesky, Ross Andersen, MarieEve Doucet, Jessica Evans, Carlo Marra, John Kimoff, Peter Pare, James C. Hogg, Hélène Perrault, Bryna Shatenstein, Bill Shell, Tanja Taivassalo, Teresa To, Carole Jabet, and Maria Sedeno.
Funding support: The Canadian Cohort Obstructive Lung Disease (CanCOLD) study is funded by the Canadian Institute of Heath Research (CIHR/Rx&D Collaborative Research Program Operating Grants- 93326); industry partners AstraZeneca Canada Ltd, Boehringer-Ingelheim Canada Ltd, GlaxoSmithKline Canada Ltd, Merck, Novartis Pharma Canada Inc, Nycomed Canada Inc, Pfizer Canada Ltd; the Respiratory Health Network of the FRQS; and the Research Institute of the McGill University Health Centre.
Disclaimer: The funding sponsors had no role in the design of the study; the collection, analysis, or interpretation of the data; or the writing of the article.
References
- 1.Global Initiative for Chronic Obstructive Pulmonary Disease. Global strategy for diagnosis, management, and prevention of COPD. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2014_Jun11.pdf Updated January 2014. Accessed July 1, 2014.
- 2.Schirnhofer L, Lamprecht B, Vollmer WM, et al. COPD prevalence in Salzburg, Austria: results from the Burden of Obstructive Lung Disease (BOLD) Study. Chest. 2007;131(1):29–36. [DOI] [PubMed] [Google Scholar]
- 3.Soriano JB, Miravitlles M, Borderías L, et al. Geographical variations in the prevalence of COPD in Spain: relationship to smoking, death rates and other determining factors [in Spanish]. Arch Bronconeumol. 2010;46(10):522–530. [DOI] [PubMed] [Google Scholar]
- 4.Waked M, Khayat G, Salameh P. Chronic obstructive pulmonary disease prevalence in Lebanon: a cross-sectional descriptive study. Clin Epidemiol. 2011;3:315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill K, Goldstein RS, Guyatt GH, et al. Prevalence and underdiagnosis of chronic obstructive pulmonary disease among patients at risk in primary care. CMAJ. 2010;182(7):673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han MK, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132(2):403–409. [DOI] [PubMed] [Google Scholar]
- 7.Vanfleteren LE, Franssen FM, Wesseling G, Wouters EF. The prevalence of chronic obstructive pulmonary disease in Maastricht, the Netherlands. Respir Med. 2012;106(6):871–874. [DOI] [PubMed] [Google Scholar]
- 8.Joo MJ, Au DH, Fitzgibbon ML, McKell J, Lee TA. Determinants of spirometry use and accuracy of COPD diagnosis in primary care. J Gen Intern Med. 2011;26(11):1272–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Güder G, Brenner S, Angermann CE, et al. “GOLD or lower limit of normal definition? A comparison with expert-based diagnosis of chronic obstructive pulmonary disease in a prospective cohort-study.” Respir Res. 2012;13(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fromer L. Diagnosing and treating COPD: understanding the challenges and finding solutions. Int J Gen Med. 2011;4:729–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. [DOI] [PubMed] [Google Scholar]
- 12.National Clinical Guideline Centre. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care partial update). http://www.nice.org.uk/guidance/CG101 Published June 2010. Accessed July 1, 2014.
- 13.Swanney MP, Ruppel G, Enright PL, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008;63(12):1046–1051. [DOI] [PubMed] [Google Scholar]
- 14.Quanjer PH, Furberg CD. GOLD COPD stage I is not associated with increased risk of death. Respir Med. 2012;106(1):153. [DOI] [PubMed] [Google Scholar]
- 15.Quanjer PH, Enright PL, Miller MR, et al. The need to change the method for defining mild airway obstruction. Eur Respir J. 2011;37(3):720–722. [DOI] [PubMed] [Google Scholar]
- 16.Enright PL, Kaminsky DA. Strategies for screening for chronic obstructive pulmonary disease. Respir Care. 2003;48(12):1194–1201, discussion 1201–1203. [PubMed] [Google Scholar]
- 17.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. [DOI] [PubMed] [Google Scholar]
- 18.Vanfleteren LE, Franssen FM, Wesseling G, Wouters EF. The prevalence of chronic obstructive pulmonary disease in Maastricht, the Netherlands. Respir Med. 2012;106(6):871–874. [DOI] [PubMed] [Google Scholar]
- 19.Mohamed Hoesein FA, Zanen P, Lammers JW. Lower limit of normal or FEV1/FVC < 0.70 in diagnosing COPD: an evidence-based review. Respir Med. 2011;105(6):907–915. [DOI] [PubMed] [Google Scholar]
- 20.van Dijk WD, Gupta N, Tan WC, Bourbeau J. Clinical relevance of diagnosing COPD by fixed ratio or lower limit of normal: a systematic review. COPD. 2014:11(1):113–120. [DOI] [PubMed] [Google Scholar]
- 21.Tan WC, Bourbeau J, FitzGerald JM, et al. Can age and sex explain the variation in COPD rates across large urban cities? A population study in Canada. Int J Tuberc Lung Dis. 2011;15(12):1691–1698. [DOI] [PubMed] [Google Scholar]
- 22.Miller MR, Hankinson J, Brusasco V, et al. ; ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. [DOI] [PubMed] [Google Scholar]
- 23.Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky KH, Barr RG. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the multi-ethnic study of atherosclerosis (MESA) lung study. Chest. 2010;137(1):138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bridevaux PO, Gerbase MW, Probst-Hensch NM, Schindler C, Gaspoz JM, Rochat T. Long-term decline in lung function, utilisation of care and quality of life in modified GOLD stage 1 COPD. Thorax. 2008;63(9):768–774. [DOI] [PubMed] [Google Scholar]
- 25.Enright PL. GOLD stage I is not a COPD risk factor. Thorax. 2007;62(12):1107, author reply 1108–1109. [PMC free article] [PubMed] [Google Scholar]
- 26.Lange P, Marott JL, Vestbo J, et al. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med. 2012;186(10):975–981. [DOI] [PubMed] [Google Scholar]
- 27.Mannino DM, Diaz-Guzman E. Interpreting lung function data using 80% predicted and fixed thresholds identifies patients at increased risk of mortality. Chest. 2012;141(1):73–80. [DOI] [PubMed] [Google Scholar]
- 28.Mannino DM, Sonia Buist A, Vollmer WM. Chronic obstructive pulmonary disease in the older adult: what defines abnormal lung function? Thorax. 2007;62(3):237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]