Abstract
Acute and/or chronic alcohol ingestion has been shown to exacerbate the morbidity and mortality rate associated with acute mechanical and/or thermal trauma. While alcohol ingestion can affect many organs and systems, clinical and preclinical studies indicate that alcohol ingestion can cause a ‘leaky gut’ syndrome which in turn contributes to infection and systemic organ dysfunction. This study investigated the acute effect of alcohol on gut barrier function. Using an in vivo isolated gut sac model of naïve male rats, each individual gut sac was injected with different concentrations (0, 5, 10, 20, and 40%, v/v) of alcohol. After different times of alcohol exposure, each isolated gut segment was harvested and intestinal permeability and mucosal surface hydrophobicity (a physiologic marker of mucus barrier function) were measured as well as luminal DNA, mucus, protein and free fatty acids. The results showed that alcohol caused dose-dependent and time-dependent increases in gut permeability and decreases in mucosal surface hydrophobicity, with significant changes to be observed 5 min after treatment with 10% alcohol. In addition, it is further found that these changes in permeability and hydrophobicity are more closely associated with increased intestinal luminal free fatty acids levels but not protein or DNA levels. These results suggest that alcohol may cause loss of gut barrier function by extracting and dissolving lipids from the mucus with a resultant decrease in mucosal surface hydrophobicity, which is a critical component of gut barrier function.
Keywords: Alcohol, Gut permeability, Mucosal hydrophobicity, Mucus, Free fatty acids
1. Introduction
Alcohol use is endemic worldwide and contributes to morbidity and mortality. According to the World Health Organization (WHO), it is estimated that about two billion people consume alcoholic beverages worldwide and that 76.3 million of these people have diagnosable alcohol-use disorders (Ghosh et al., 2012). Furthermore, in 2004, 3.8% of all global deaths and 9.0% of male deaths in the United States were attributable to alcohol (Rehm et al., 2009). Not only has alcohol use been associated with damage to multiple organs (Esper et al., 2006; Guidot and Hart, 2005; Lieber, 1995; Moss and Burnham, 2003), nearly 38% of alcohol-related deaths involve intentional and unintentional injury (Rehm et al., 2009). In fact, studies show that approximately 50% of male burn patients as well as trauma patients have positive blood alcohol concentrations at the time of admission to the hospital (Rivara et al.,1993; Scalfani et al., 2007). Because acute and/or chronic alcohol ingestion appears to exacerbate the morbidity and mortality of patients with mechanical or thermal trauma, a number of clinical and preclinical animal studies have investigated this relationship (Bird and Kovacs, 2008; Dinda et al., 1996; Kaur, 2002; Li et al., 2011). One area that has received special attention is the relationship between enteral alcohol ingestion and loss of gut barrier function, where studies have documented that an alcohol-induced increased absorption of endotoxin due to a “leaky gut” plays a critical role in alcoholic hepatitis and cirrhosis (Bjarnason et al., 1984; Keshavarzian et al., 1999; Purohit et al., 2008). Additionally, the adverse effects of an impaired gut barrier have been recognized in several non-cirrhotic patient populations including ICU patients and patients sustaining major burn or mechanical trauma (Cherpitel, 1997, 2007; Rivara et al., 1993). Because of the relationship between intestinal dysfunction and the development of the systemic sepsis (SIRS) and multiple organ dysfunction syndromes (MODS), we and others have focused investigative attention on the mechanisms leading to gut injury and gut-induced MODS (Deitch, 2001; Deitch et al., 2006; Leaphart and Tepas, 2007; Nieuwenhuijzen and Goris, 1999). Most recently, these studies have high-lighted the important role that the mucus layer plays in normal gut barrier function and how its loss can result in increased gut permeability (Qin et al., 2008, 2011; Sharpe et al., 2010). Thus, the object of this study was to begin to characterize the effects of enteral alcohol on normal small bowel function with a special focus on its physiologic effects on the mucus layer and the associated changes in intestinal permeability.
2. Materials and methods
2.1. Animals
Specific pathogen-free male Sprague-Dawley rats (Taconic Farms, Germantown, NY) weighing 300-350 g (about 10–12 weeks old) were housed under barrier-sustained conditions and kept at 25 °C with 12-h light/dark cycles. The rats had free access to water and chow (Teklad 22/5 Rodent Diet W-8640, Harlan Teklad, Madison, Wis). All rats were maintained and all experiments were conducted in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of Rutgers, etc.
2.2. Experimental procedure
To reduce the number of animals as well as the variation among animals, the small intestine after flushing was divided into several segments, with each receiving a different treatment. Below is the detailed description of the procedure.
After anesthesia with an intraperitoneal injection of sodium pentobarbital (50mg/kg) and laparotomy, the luminal content of the small intestine was flushed out through three cuts, each about three-fourths of the gut circumference and effort was made to avoid cutting visible blood vessels: first cut at about 5 cm down the ligament of treitz, the second cut about 50 cm distal from the first cut, and the third cut at about 5 cm above the end of the ileum. The gut was gently flushed with 60 ml warm saline to remove the luminal contents. Flushing was done to reduce any potential confounding effects caused by differences in the amount as well as composition (digestive proteases, bile salts, etc.) of the luminal contents between the intestinal segments to be tested and to facilitate the assay of various luminal parameters.
After gently milking out the flushing solution, the small intestine was sequentially divided into different segments with a length of up to 15 cm for each, isolated by ligation. For the time course study, each segment was injected with 20% alcohol at a volume of 0.1 ml/cm and harvested at different time points. For the dose response study, the different segments were injected with 0–40% alcohol that encompassed the alcohol content of commonly used alcoholic beverages from beer and wine to spirits like brandy, whiskey and vodka. By the end of the incubation period, the blood vessels to the segment were ligated and the segment was harvested. The luminal contents were collected and stored at −80 C. A 4 cm piece of the segment was cut off and used to measure gut mucosal hydrophobicity. Another 6 cm piece was taken to measure gut permeability. The luminal contents were subsequently assayed for DNA, protein, free fatty acids, and mucus levels.
To reduce the potential variation caused by the location of the different segments, the segments corresponding to the different treatment were rotated among the different animals as demonstrated in Table 1 for the dose-response study.
Table 1.
Treatment of the different segments from upper jejunum to middle ileum of the different animals in the dose–response study.
| Gut segments (upper jejunum to middle ileum) | |||||
|---|---|---|---|---|---|
|
| |||||
| 1 | 2 | 3 | 4 | 5 | |
| Rat #1 | 0 | 5% | 10% | 20% | 40% |
| Rat #2 | 40% | 0 | 5% | 10% | 20% |
| Rat #3 | 20% | 40% | 0 | 5% | 10% |
| Rat #4 | 10% | 20% | 40% | 0 | 5% |
| Rat #5 | 5% | 10% | 20% | 40% | 0 |
2.3. Measurement of intestinal mucosal permeability
Intestinal permeability was measured using the everted gut sac method and the fluorescent tracer, fluorescein isothiocyanate dextran (MW 4000 Da; FD4) as described in our previous study (Qin et al., 2008). Briefly, the intestinal segment was everted using a thin metal rod. One end of the segment was secured with 4–0 silk to the grooved tip of a 1-ml plastic syringe containing 0.5 ml modified Krebs–Henseleit bicarbonate buffer (KHBB, pH 7.4). A ligation was made 4 cm away from the tip and the everted gut sac was suspended in a 100-ml beaker containing 80 ml of KHBB with added FD4 (20 μg/ml). The solution in the beaker was maintained at 37 °C temperature in a water bath, and a gas mixture containing 95% O2 and 5% CO2 was bubbled continuously. A 1.0-ml sample was taken from the beaker before placing the everted gut sac to determine the initial external (mucosal surface) FD4 concentration. The everted gut sac was distended gently by injecting the 0.5 ml of KHBB and incubated for 30 min in the KHBB solution containing FD4. After that, the fluid on the serosal side was aspirated into the syringe and put into a centrifuge tube. The samples were centrifuged for 10 min at 1000 × g. Two hundred microliters of the supernatant were put into the wells of a microplate and fluorescence was measured by a PerkinElmer LS-50 fluorescence spectrophotometer (Palo Alto, CA) at an excitation wavelength of 492 (slit width, 10 nm) and an emission wavelength of 515 nm (slit width, 10 nm). Permeability was expressed as the mucosal-to-serosal clearance of FD4 calculated using the following equations:
where M is the mass (in ng) of FD4 in the gut sac at the end of the 30-min incubation period, [FD4]serosal is the FD4 concentration in the serosal fluid aspirated from the sac at the end of the 30 min incubation period, F is the flux of FD4 (in ng/min) across the mucosa, [FD4]mucosal is the FD4 concentration measured in the beaker at the beginning of the 30 min incubation period, A = ∏ LD which is the calculated area (in cm2) of the mucosal surface, and C is the clearance of FD4 (in nl min−1 cm−2) across the mucosa.
2.4. Measurement of hydrophobicity
The hydrophobicity was measured by a goniometer (Rame-Hart, Mountain Lakes, NJ), which contains an adjustable sample stage, a syringe, a light source, and a microscope linked to a computer as described in our previous study (Qin et al., 2008). In brief, the segment of the ileum was put on a piece of paper (about 5 cm long by 4 cm wide), where it was cut open, spread out and its luminal contents removed by gently rinsing the segment with saline. Then the moist supporting paper containing the intestinal segment was mounted on a slide by wrapping both edges of the paper around the slide. This maneuver ensured that the intestinal segment would remain flat during drying of the tissue. The tissue was allowed to dry until the mucosal surface showed a matted appearance. Then, the paper along with the tissue was taken off from the slide and a strip of the tissue with the underlying paper was cut and mounted onto a stand of Styrofoam with a narrow edge to ensure a good view of the droplet once it was placed on the tissue. After placing the styrofoam stand on the stage of the goniometer, 5 μl of saline was gently applied from the syringe onto the surface of the tissue and adjustments were made to create a good exposure of the droplet in the view on the computer screen. Then the contact angle of the droplet was measured and recorded by the machine. Four to five measurements were taken for each segment and their average was used in the analysis. A greater contact angle means a greater hydrophobicity.
2.5. Measurement of DNA in the lumen
The amount of DNA in the lumen was measured by diphenyl-amine (DPA) method (Natarajan et al., 1994). In brief, 25 μl of the homogenized flushing solutions were added to the wells of a microplate, followed by the addition of 25 μl of mixture of 40% perchloric acid and 0.32% acetaldehyde at a ratio of 5:1, and 100 μl of 4% diphenylamine (in glacial acetic acid). After mixing and sealing the cover with parafilm, the microplate was incubated at room temperature overnight. The next day, the microplate was read with a microplate reader at 595 and 750 nm, with the reading at 750 nm as the background. Harp sperm DNA was used as the standard.
2.6. Measurement of mucus in the lumen
Mucus in the lumen was measured by alcian blue. In brief, 5 μl luminal solution was added with 295 μl saline and 100 μl 0.1% alcian blue in 0.1 mM acetic acetate buffer (pH 5.8) with 20 mM MgCl2. After mix, the samples were put at room temperature overnight. After centrifugation at 15000 × g for 5 min, 100 μl of the supernatant was transferred to 96 microplate and the absorbance was measured at 620nm.
2.7. Measurement of protein in the lumen
The amount of protein in the lumen was measured using the protein assay reagent from Bio-Rad according to the manufacturer's protocol. In brief, after thawing the flushing solution was homogenized and an aliquot of the solution was diluted 10 times with distilled water. Then 5 μl of these dilutions and protein standard (bovine serum albumin) were added to the wells of a microplate, followed by the addition of 195 μl of 1X reagent. 5 min later, the microplate was read with a microplate reader at 595 nm.
2.8. Measurement of free fatty acids in the lumen
Free fatty acids in the luminal collection were measured using kits from Wako Pure Chemical Industries and Randox Laboratories.
2.9. Statistics
Data were analyzed using SAS software (SAS Institute, Carey, NC). Differences between alcohol-treated versus non-treated groups were tested by student's t test. A difference of p < 0.05 was considered statistically significant. Data were expressed as means ± SEM.
3. Results
3.1. Effect of acute alcohol exposure on segmental gut permeability
We first investigate the relative resistance of different parts of the small intestine to alcohol by treating the upper, middle and lower small intestine with 40% alcohol for 5 min, then measuring gut permeability. As shown in Fig. 1A, the permeability was highest in the ileum, while there was no significant difference between the proximal and middle small intestine. Therefore, in the following experiments, the terminal portion of the small intestine was avoided to keep the homogeneity. The randomization by rotating the different alcohol treatment among the different intestinal segments in different rats, as shown in Table 1, further avoided the potential confounding effects of any intrinsic differences among the different locations of the small intestine. Next, we performed a time course study of the effects of alcohol on gut permeability and found that the increase in gut permeability after a 5 min incubation with 20% alcohol persisted for 30 min but had returned to normal by 3h (Fig. 1B). Then, we performed a dose–response study and found that an incubation period of 5 min with an alcohol concentration of 10% or greater was sufficient to increases gut permeability (Fig. 1C). Furthermore, the increase in permeability increased as the alcohol concentration increased (see the insert in Fig. 1C).
Fig. 1.
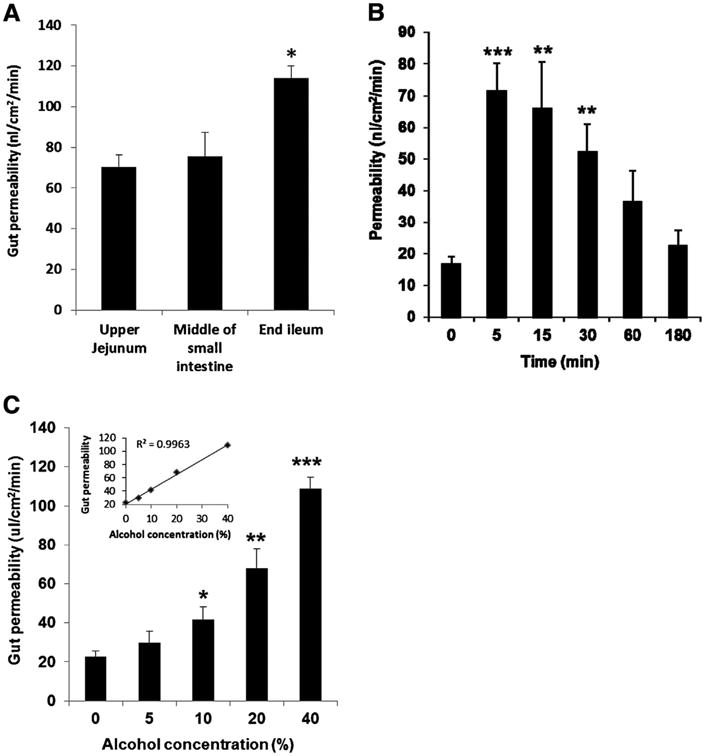
(A) Permeability of different parts of the small intestine treated with 40% alcohol for 5 min. *P < 0.05 versus jejunum and middle of small intestine; (B) time course of the changes in gut permeability after treatment with 20% alcohol. **P < 0.01, ***P < 0.001 versus time 0 (without treatment). (C) Permeability of gut segments treated with different concentrations of alcohol for 5 min. *P < 0.05, **P < 0.01, ***P < 0.001 versus without (0%) alcohol. The insert shows the correlation between gut permeability and alcohol concentration. Data were expressed as mean ± SEM (n = 5 or 6).
3.2. Effect of acute alcohol exposure on intestinal mucosal hydrophobicity
Similar to the gut permeability results, a time course study on mucosal hydrophobicity indicated that alcohol-induced a decrease in hydrophobicity which persisted for up to 15 min after alcohol exposure and than returned towards normal (Fig. 2A). In the dose–response study, it was observed that incubation with alcohol concentrations of 10% or higher decreased mucosal hydrophobicity (Fig. 2B). To investigate if a relationship existed between increased gut permeability and decreased hydrophobicity, a linear correlation analysis was performed between these two variables (Fig. 2C). This analysis showed that these two variables were correlated with an r of −0.537 (p < 0.01).
Fig. 2.
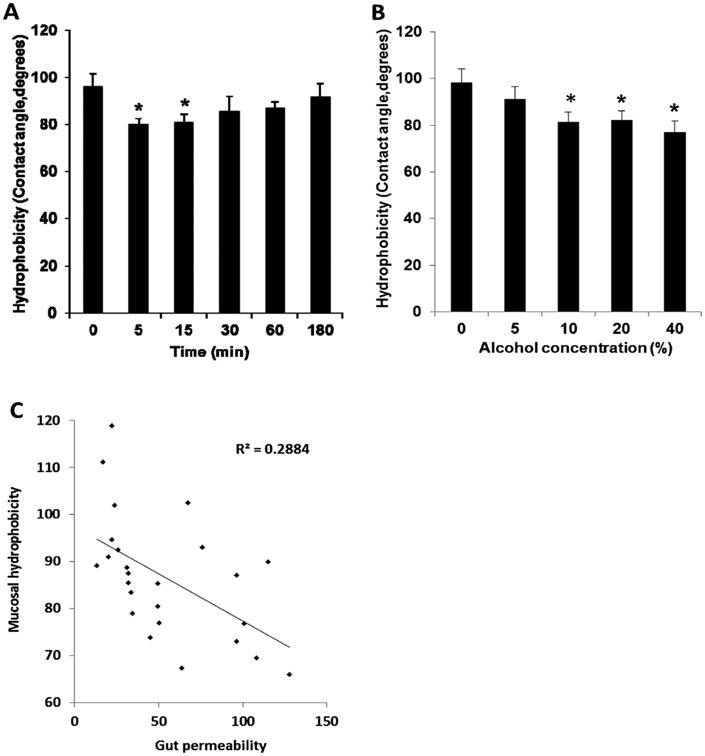
(A) Time course of the changes in mucosal surface hydrophobicity after treatment with 20% alcohol. *P < 0.05 versus time 0 (without treatment). Data were expressed as mean ± SEM (n = 6). (B) Hydrophobicity of the mucosal surface of gut segments treated with different concentrations of alcohol for 5 min. *P < 0.05 versus without (0%) alcohol. (C) Correlation between the changes in gut permeability and mucosal surface hydrophobicity after treatment with different concentrations of alcohol for 5 min. Data were expressed as mean ± SEM (n = 5 or 6).
3.3. Effect of alcohol on luminal DNA, protein, mucus and free fatty acid levels in the lumen of gut segments
Using DNA and protein as markers of gut injury, we found that only at an alcohol concentration of 40% was luminal DNA increased (Fig. 3A) while luminal protein levels were increased at alcohol concentrations of 20 and 40% (Fig. 3B). Likewise, only at an alcohol concentration of 40% was luminal mucus levels increased (Fig. 3C), while free fatty acid levels were increased at alcohol concentrations of 10% or greater (Fig. 3D). Since mucus and free fatty acids are present in the intestinal mucus layer, we tested whether either of these values correlated with intestinal hydrophobicity. A significant inverse correlation was found between hydrophobicity and luminal free fatty acid levels (r = −0.479; 95% confident interval [CI], −0.735 to −0.104, p < 0.05), but not between hydrophobicity and luminal mucus levels (r = −0.256; 95% CI, −0.591 to 0.154, p > 0.05). There was no statistical significant correlation between hydrophobicity and luminal markers of gut injury such as DNA (r = −0.341; 95% CI, −0.648 to 0.062, p > 0.05) or protein (r = 0.377; 95% CI, −0.672 to 0.021, p > 0.05).
Fig. 3.
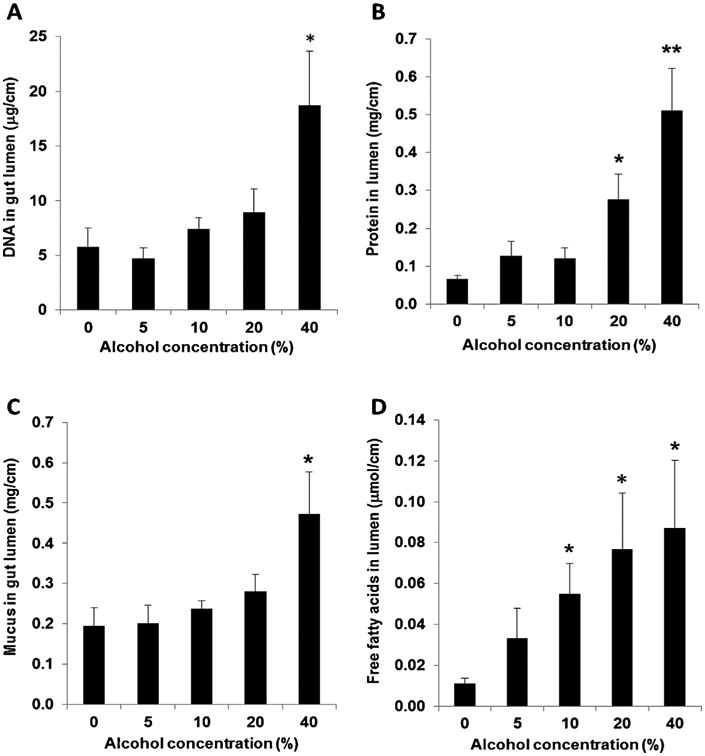
(A) DNA; (B) protein; (C) mucus; (D) free fatty acids in the luminal collection of gut segments treated with different concentrations of alcohol for 5 min. *P < 0.05, **P <0.01 versus without (0%) alcohol. Data were expressed as mean ± SEM (n = 5).
4. Discussion
It is well established that acute or chronic alcohol ingestion can result in structural and functional injuries to the gastrointestinal tract in both patients and animals (Bode and Bode, 1997, 2003; Keshavarzian et al., 1994; Persson, 1991). These alcohol-induced intestinal injuries have been characterized morphologically and include a range of findings from gastric mucosal erosions to injury of the small intestinal villous tips (Bode and Bode, 1997, 2003; Persson, 1991). Although studies indicate that the acute gut-injurious effects of isolated alcohol ingestion are relatively transient and resolve within 4–24 h (Persson, 1991), there is abundant clinical information indicating that alcohol ingestion can potentiate complications, including infection, sepsis and prolonged hospital stays in trauma patients (Greiffenstein and Molina, 2008). Furthermore, the intestinal injurious effect of alcohol potentiates the gut injurious effects of other intestinal stressors, such as the intestinal ischemia-reperfusion injury observed after major thermal or mechanical trauma (Rendon et al., 2013).
While these studies have documented that alcohol ingestion can result in mucosal damage and an increase in gut permeability, they have largely focused on the enterocyte barrier while neglecting the mucus layer that covers the enterocytes. Yet we and others have documented that the intestinal mucus layer is a key barrier that protects the underlying enterocytes from injury (Fishman et al., 2013; Sharpe et al., 2008, 2010). In fact, it is becoming increasingly recognized that the viscoelastic properties of mucus secreted from gastric and intestinal goblet cells forms a gel layer covering the mucosal surface of the gastrointestinal tract where it acts as a semi-permeable barrier between the lumen and epithelium. The stability of the mucus layer has been shown to be essential for the preservation of the integrity of the intestinal epithelium and any breakdown of this protective mucus barrier may lead to mucosal injury (Sharpe et al., 2010). Thus, a key goal of the current work was to investigate the effect of acute alcohol ingestion on the mucus layer and correlate these findings with other markers of gut injury and barrier dysfunction. Consequently, one key observation of this study was that alcohol increases gut permeability in a dose-dependent fashion and that this alcohol-induced increase in gut permeability was associated with decreased mucus hydrophobicity and increased luminal levels of FFA, protein and DNA. A second major observation was that a 10% concentration of alcohol was sufficient to increase gut permeability, reduce mucus hydrophobicity and increase luminal FFA but not luminal protein or DNA levels. In contrast, it required alcohol concentrations of 40% to cause significant enterocyte injury as reflected by increased luminal DNA and protein levels. This suggests that lower concentrations of alcohol are able to affect the mucus layer and increase gut permeability without causing enterocyte injury as reflected in increased luminal protein and DNA levels. This observation that a 10% concentration of alcohol can lead to intestinal barrier dysfunction is clinically relevant, since studies have shown that 80–90% of orally ingested alcohol can reach the small intestine (Zernig and Battista, 2000) with alcohol levels of 10% being detected in the proximal small bowel of volunteers ingesting ethanol (Millan et al., 1980).
One mechanism by which alcohol decreased mucus hydrophobicity and increased intestinal permeability might have involved alcohol's direct effect on the mucus layer. This possibility is supported by the observation that a 10% concentration of alcohol, which was the lowest concentration of alcohol tested that was able to increase gut permeability, was also the lowest alcohol concentration sufficient to impair mucus hydrophobicity and increase luminal FFA levels. This notion is supported by studies showing that the hydrophobicity of mucus is largely determined by its lipid content (Hills et al., 1983; Lichtenberger, 1995). This barrier promoting effect of lipids contained in the mucus gel is largely related to the fact that lipids are hydrophobic and not water soluble. Consequently, they limit the absorption of aqueous materials. Furthermore, studies have found that lipids bound within the mucus gel shielded the mucin from oxygen-radical injury as well as contributed to the barrier function of the mucus (Gong et al., 1990). Likewise, basic work has shown that free fatty acids are readily dissolved in alcohol (Lewkowitsch, 1909) and the loss of the FFA in turn reduces the hydrophobicity and barrier properties of the remaining mucus gel. In light of these studies, the current work suggests that a relatively under-appreciated potential mechanism by which alcohol contributes to gut injury is through its ability to extract FFA and lipid from the mucus layer thereby reducing intestinal hydrophobicity and increasing gut permeability. This is just an acute study with alcohol solution injected into the emptied gut sac. Further studies would be worthwhile to see how the dietary components such as the amount and type of fat in the food may affect the interactions among the alcohol, lipids, hydrophobicity of the mucosal surface and gut permeability. Nevertheless, this new finding proposed a new mechanism of action by alcohol on gut barrier function.
In summary, it is well recognized that alcohol ingestion may adversely affect many other systems in addition to the intestine, including the hemodynamic, neuroendocrine, metabolic, and counter-regulatory responses as well as the immuno-inflammatory systems (Goral et al., 2008; Greiffenstein and Molina, 2008). Because of the clinically deleterious effects of alcohol ingestion superimposed on trauma (Gentilello et al., 1993; Jurkovich et al., 1993), characterizing and understanding the host's response to alcohol has been an area of intense study. We believe that studying the effect of alcohol on the intestinal mucus layer is of potential clinical relevance based on our recent studies (Fishman et al., 2013; Qin et al., 2008, 2011; Sharpe et al., 2008, 2010) and the work from Schmid-Schonbein's laboratory (Chang et al., 2012) showing that trauma and shock-induced gut injury and gut-induced SIRS and MODS involves breakdown of the mucus barrier and subsequent auto-digestion of the gut wall by luminal pancreatic proteases. That is, these studies suggest that an early initial step in the pathogenesis of gut-induced sepsis after trauma involves dysfunction of the mucus layer lining the intestine. This study proposed a new mechanism as how ingestion of alcohol may exert an prompt detrimental impact on gut barrier functions and subsequently adverse effect on multiple organs of the body (Chart 1).
Chart 1.

A possible mechanism for the adverse effect of alcohol on gut barrier and multiple organs.
Highlights.
Alcohol causes prompt increase in gut permeability.
Alcohol causes prompt decrease in mucosal surface hydrophobicity.
Changes above are related to dissolution of lipids from mucus by alcohol.
Acknowledgments
This work was supported by NIH grants GM 59841.
Footnotes
Conflict of interest: The authors declare that there are no conflicts of interest.
Transparency document: The Transparency document associated with this article can be found in the online version.
References
- Bird MD, Kovacs EJ. Organ-specific inflammation following acute ethanol and burn injury. J Leukocyte Biol. 2008;84:607–613. doi: 10.1189/jlb.1107766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet. 1984;1:179–182. doi: 10.1016/s0140-6736(84)92109-3. [DOI] [PubMed] [Google Scholar]
- Bode C, Bode JC. Alcohol's role in gastrointestinal tract disorders. Alcohol Health Res World. 1997;21:76–83. [PMC free article] [PubMed] [Google Scholar]
- Bode C, Bode JC. Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol. 2003;17:575–592. doi: 10.1016/s1521-6918(03)00034-9. [DOI] [PubMed] [Google Scholar]
- Chang M, Alsaigh T, Kistler EB, Schmid-Schonbein GW. Breakdown of mucin as barrier to digestive enzymes in the ischemic rat small intestine. PloS One. 2012;7:e40087. doi: 10.1371/journal.pone.0040087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherpitel CJ. Alcohol and violence-related injuries in the emergency room. Recent Dev Alcohol. 1997;13:105–118. doi: 10.1007/0-306-47141-8_6. [DOI] [PubMed] [Google Scholar]
- Cherpitel CJ. Alcohol and injuries: a review of international emergency room studies since 1995. Drug Alcohol Rev. 2007;26:201–214. doi: 10.1080/09595230601146686. [DOI] [PubMed] [Google Scholar]
- Deitch EA. Role of the gut lymphatic system in multiple organ failure. Curr Opin Crit Care. 2001;7:92–98. doi: 10.1097/00075198-200104000-00007. [DOI] [PubMed] [Google Scholar]
- Deitch EA, Xu D, Kaise VL. Role of the gut in the development of injury-and shock induced SIRS and MODS: the gut-lymph hypothesis, a review. Front Biosci: J Virtual Lib. 2006;11:520–528. doi: 10.2741/1816. [DOI] [PubMed] [Google Scholar]
- Dinda PK, Wasan S, Beck IT, Kossev P. Adaptive cytoprotection against ethanol-induced small intestinal mucosal injury. Can J Physiol Pharmacol. 1996;74:598–602. [PubMed] [Google Scholar]
- Esper A, Burnham EL, Moss M. The effect of alcohol abuse on ARDS and multiple organ dysfunction. Minerva Anestesiol. 2006;72:375–381. [PubMed] [Google Scholar]
- Fishman JE, Levy G, Alli V, Sheth S, Lu Q, Deitch EA. Oxidative modification of the intestinal mucus layer is a critical but unrecognized component of trauma hemorrhagic shock-induced gut barrier failure. Am J Physiol Gastrointest Liver Physiol. 2013;304:G57–63. doi: 10.1152/ajpgi.00170.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentilello LM, Cobean RA, Walker AP, Moore EE, Wertz MJ, Dellinger EP. Acute ethanol intoxication increases the risk of infection following penetrating abdominal trauma. J Trauma. 1993;34:669–674. doi: 10.1097/00005373-199305000-00009. discussion 674-665. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Samanta A, Mukherjee S. Patterns of alcohol consumption among male adults at a slum in Kolkata India. J Health Popul Nutr. 2012;30:73–81. doi: 10.3329/jhpn.v30i1.11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong DH, Turner B, Bhaskar KR, Lamont JT. Lipid binding to gastric mucin: protective effect against oxygen radicals. Am J Physiol. 1990;259:G681–686. doi: 10.1152/ajpgi.1990.259.4.G681. [DOI] [PubMed] [Google Scholar]
- Goral J, Karavitis J, Kovacs EJ. Exposure-dependent effects of ethanol on the innate immune system. Alcohol. 2008;42:237–247. doi: 10.1016/j.alcohol.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiffenstein P, Molina PE. Alcohol-induced alterations on host defense after traumatic injury. J Trauma. 2008;64:230–240. doi: 10.1097/TA.0b013e318158a4ad. [DOI] [PubMed] [Google Scholar]
- Guidot DM, Hart CM. Alcohol abuse and acute lung injury: epidemiology and pathophysiology of a recently recognized association. J Invest Med. 2005;53:235–245. doi: 10.2310/6650.2005.53506. [DOI] [PubMed] [Google Scholar]
- Hills BA, Butler BD, Lichtenberger LM. Gastric mucosal barrier: hydrophobic lining to the lumen of the stomach. Am J Physiol. 1983;244:G561–568. doi: 10.1152/ajpgi.1983.244.5.G561. [DOI] [PubMed] [Google Scholar]
- Jurkovich GJ, Rivara FP, Gurney JG, Fligner C, Ries R, Mueller BA, Copass M. The effect of acute alcohol intoxication and chronic alcohol abuse on outcome from trauma. JAMA. 1993;270:51–56. [PubMed] [Google Scholar]
- Kaur J. Chronic ethanol feeding affects intestinal mucus lipid composition and glycosylation in rats. Ann Nutr Metab. 2002;46:38–44. doi: 10.1159/000046751. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Fields JZ, Vaeth J, Holmes EW. The differing effects of acute and chronic alcohol on gastric and intestinal permeability. Am J Gastroenterol. 1994;89:2205–2211. [PubMed] [Google Scholar]
- Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94:200–207. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- Leaphart CL, Tepas JJ., 3rd The gut is a motor of organ system dysfunction. Surgery. 2007;141:563–569. doi: 10.1016/j.surg.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Lewkowitsch J. Physical methods of examining oils, fats, and waxes: 10. Solubility. In: Lewkowitsch J, editor. Chemical Technology and Analysis of Oils, Fats and Waxes. Macmillan and Co., Ltd.; 1909. pp. 370–380. [Google Scholar]
- Li X, Akhtar S, Kovacs EJ, Gamelli RL, Choudhry MA. Inflammatory response in multiple organs in a mouse model of acute alcohol intoxication and burn injury. J Burn Care Res. 2011;32:489–497. doi: 10.1097/BCR.0b013e3182223c9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberger LM. The hydrophobic barrier properties of gastrointestinal mucus. Annu Rev Physiol. 1995;57:565–583. doi: 10.1146/annurev.ph.57.030195.003025. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Medical disorders of alcoholism. N Engl J Med. 1995;333:1058–1065. doi: 10.1056/NEJM199510193331607. [DOI] [PubMed] [Google Scholar]
- Millan MS, Morris GP, Beck IT, Henson JT. Villous damage induced by suction biopsy and by acute ethanol intake in normal human small intestine. Dig Dis Sci. 1980;25:513–525. doi: 10.1007/BF01315213. [DOI] [PubMed] [Google Scholar]
- Moss M, Burnham EL. Chronic alcohol abuse, acute respiratory distress syndrome, and multiple organ dysfunction. Crit Care Med. 2003;31:S207–212. doi: 10.1097/01.CCM.0000057845.77458.25. [DOI] [PubMed] [Google Scholar]
- Natarajan N, Shambaugh GE, 3rd, Elseth KM, Haines GK, Radosevich JA. Adaptation of the diphenylamine (DPA) assay to a 96-well plate tissue culture format and comparison with the MTT assay. BioTechniques. 1994;17:166–171. [PubMed] [Google Scholar]
- Nieuwenhuijzen GA, Goris RJ. The gut: the ‘motor’ of multiple organ dysfunction syndrome? Curr Opin Clin Nutr Metab Care. 1999;2:399–404. doi: 10.1097/00075197-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Persson J. Alcohol and the small intestine. Scand J Gastroenterol. 1991;26:3–15. doi: 10.3109/00365529108996478. [DOI] [PubMed] [Google Scholar]
- Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, Kang YJ, Keshavarzian A, Rao R, Sartor RB, Swanson C, Turner JR. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol. 2008;42:349–361. doi: 10.1016/j.alcohol.2008.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Caputo FJ, Xu DZ, Deitch EA. Hydrophobicity of mucosal surface and its relationship to gut barrier function. Shock. 2008;29:372–376. doi: 10.1097/shk.0b013e3181453f4e. [DOI] [PubMed] [Google Scholar]
- Qin X, Sheth SU, Sharpe SM, Dong W, Lu Q, Xu D, Deitch EA. The mucus layer is critical in protecting against ischemia-reperfusion-mediated gut injury and in the restitution of gut barrier function. Shock. 2011;35:275–281. doi: 10.1097/SHK.0b013e3181f6aaf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Rendon JL, Li X, Akhtar S, Choudhry MA. Interleukin-22 modulates gut epithelial and immune barrier functions following acute alcohol exposure and burn injury. Shock. 2013;39:11–18. doi: 10.1097/SHK.0b013e3182749f96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivara FP, Jurkovich GJ, Gurney JG, Seguin D, Fligner CL, Ries R, Raisys VA, Copass M. The magnitude of acute and chronic alcohol abuse in trauma patients. Arch Surg. 1993;128:907–912. doi: 10.1001/archsurg.1993.01420200081015. discussion 912-903. [DOI] [PubMed] [Google Scholar]
- Scalfani MT, Chan DM, Murdoch EL, Kovacs EJ, White FA. Acute ethanol exposure combined with burn injury enhances IL-6 levels in the murine ileum. Alcohol Clin Exp Res. 2007;31:1731–1737. doi: 10.1111/j.1530-0277.2007.00468.x. [DOI] [PubMed] [Google Scholar]
- Sharpe S, Doucet D, Qin X, Deitch E. Role of intestinal mucus and pancreatic proteases in the pathogenesis of trauma-hemorrhage shock-induced gut barrier failure and multiple organ dysfunction syndrome. J Org Dys. 2008;4:168–176. [Google Scholar]
- Sharpe SM, Qin X, Lu Q, Feketeova E, Palange DC, Dong W, Sheth SU, Lee MA, Reino D, Xu DZ, Deitch EA. Loss of the intestinal mucus layer in the normal rat causes gut injury but not toxic mesenteric lymph nor lung injury. Shock. 2010;34:475–481. doi: 10.1097/SHK.0b013e3181dc3ff5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernig G, Battista H. Basic pharmacokinetics of alcohol. In: Zernig G, Saria A, Kurz M, O'Malley S, editors. Handbook of Alcoholism. CRC Press LLC; Boca Raton, Florida: 2000. pp. 421–423. [Google Scholar]


