Abstract
Research has indicated that gonadal hormones may mediate behavioral and biological responses to cocaine. Estrogen, in particular, has been shown to increase behavioral responding to cocaine in female rats relative to male rats. The current study investigated the effect of cocaine on locomotor activity and hormonal correlates in male and female Japanese quail (Coturnix japonica). In Japanese quail, circulating hormone levels can be manipulated without surgical alterations via modifying the photoperiod. Male and female quail were housed on either an 8L:16D (light:dark) or 16L:8D (light:dark) cycle for 21 days. Blood samples were taken prior to the beginning of the experiment and assays were performed to determine levels of testosterone (T) and estradiol (E2). Quail were given injections of saline or cocaine (10 or 20 mg/kg) once a day for 10 days. Immediately after each injection, birds were placed in open field arenas and distance travelled was measured for 30 min. Results showed that male quail housed under long-light conditions exhibited cocaine-induced sensitization to 10 mg/kg cocaine which was correlated with high levels of plasma T. Female quail housed under short-light conditions demonstrated sensitization to 10 mg/kg cocaine, but this was not correlated with levels of plasma E2. The current findings suggest that cocaine-induced locomotor activity was associated with T in males but not with E2 in females.
Keywords: Cocaine, Behavioral sensitization, Estradiol, Testosterone, Sex differences, Birds, Distance travelled, Locomotor activity, Photoperiod, Japanese quail
Introduction
While the rate of cocaine abuse and dependence has remained relatively stable over the last 15 years, abuse and dependence among women has dramatically increased, such that nearly 40% of users over the age of 26 are female (Evans & Foltin, 2010; Jackson et al., 2006). Men and women are equally likely to use cocaine if given the opportunity, but women are more likely to reach dependence criteria compared to their male counterparts (Kasperski et al., 2011; Van Etten & Anthony, 1999). Women report shorter periods of abstinence (Kosten et al., 1993), enter into treatment at younger ages (Griffin et al., 1989; Mendelson et al., 1991), and once admitted for treatment, their use is more severe compared to men (Kosten et al., 1993). Additionally, drug-related cues induce higher levels of craving in cocaine-dependent women than in cocaine-dependent men (Robbins et al., 1999). Collectively, these studies suggest that women may be more sensitive to the reinforcing properties of psychostimulants and may be more vulnerable to some aspects of drug addiction than men.
Research has indicated that gonadal hormones, particularly estrogen, may be responsible for the heightened behavioral and biological responses to cocaine in females (Evans & Foltin, 2006; Hu & Becker, 2003). Women report greater subjective responses to cocaine when tested during the follicular phase compared to the luteal phase of the menstrual cycle (Collins et al., 2007; Evans et al., 2002; Sofuoglu et. al, 1999). In fact, sex differences only emerge in humans when men are compared with women in the luteal phase, as women in the follicular phase have similar responses to cocaine as men (Collins et al., 2007; See Quinones-Jenab & Jenab, 2012 for review). Rodent models have demonstrated that intact female rats acquire cocaine self-administration at faster rates and have higher breaking points than ovariectomized (OVX) female rats and male rats (Jackson et al., 2006, Lynch & Carroll, 1999). Russo et al. (2003) showed that female rats develop associations to environmental cues and to the rewarding properties of cocaine at lower doses and at faster rates than male rats. Additionally, intact and estradiol-treated OVX female rats show significantly greater locomotor activity following chronic cocaine administration compared to intact and castrated male rats and OVX female rats (Hu & Becker, 2003). It should be noted that estradiol has been shown to rapidly down regulate D2 in the striatum (Bazzett & Becker, 1994) and may, in part, account for the increased sensitivity to repeated cocaine observed in female rodents (Hu & Becker, 2003; Becker & Hu, 2008). Taken together, these studies suggest that ovarian hormones may play a role in the increased vulnerability to psychostimulants among females.
Research on the role of testosterone in cocaine-induced locomotor effects in male rodents is mixed and inconclusive. Some studies have reported no differences between castrated (CAST) and intact male rats in cocaine-induced locomotor activity (Becker et al., 2001; Forgie & Stewart, 1994; Hu & Becker, 2003; Hu et al., 2004; Robinson et al., 1981; van Haaren & Meyer, 1991). In these studies, cocaine increased locomotor activity in both CAST and intact male rats. Other studies have reported increased cocaine-induced locomotor activity and striatal dopamine in CAST male rats relative to intact rats (Camp & Robinson, 1988a, b; Hernandez et al., 1994; Purvis-Tyson et al., 2014; Robinson, 1984). In contrast, Menendez-Delmestre and Segarra (2011) observed cocaine-induced sensitization at 15 and 30 mg/kg in intact and CAST male rats with testosterone replacement but not in CAST male rats. Thus, it is difficult to decipher the role of testosterone on cocaine-induced locomotor based on the current literature.
The current study proposes to examine the effects of gonadal hormones (estradiol and testosterone) on cocaine-induced locomotor activity using a visually-oriented animal model, Japanese quail. Japanese quail have color vision and high visual acuity (Fidura & Gray, 1966) unlike rodent species. While the current study will not manipulate visual cues, it will serve to inform future studies involving the interaction between hormones, drug effects, and visual cues. There are currently no studies investigating this interaction. However, several clinical studies have shown that environmental visual cues play a role in drug addiction and relapse (e.g., Childress et al., 1988; O'Brien et al., 1992). Environmental stimuli may become associated with interoceptive drug cues and later, in the absence of the drug, trigger drug-seeking and ultimately relapse. Therefore, studying drug-hormone interactions in the context of how visual cues may induce relapse may be of importance to understanding drug addiction mechanisms.
Cocaine-induced behavioral sensitization (Akins & Geary, 2008; Geary & Akins, 2007; Levens & Akins, 2001) and cocaine reward (Akins et al., 2004; Levens & Akins, 2001) have been demonstrated in our laboratory in male Japanese quail. These studies utilized male quail that were on long-light photoperiods (functionally intact males). The current study proposes to compare cocaine effects on locomotor activity in long-light males and short-light (functionally CAST) males and then to determine whether testosterone is correlated with those effects. Similarly, no studies have examined cocaine effects on locomotor activity in female quail nor whether estradiol levels are correlated with those effects.
Japanese quail allow for utilization of a practical laboratory technique for manipulating hormone levels. In quail, circulating hormone levels can be manipulated through alterations of photoperiod without surgical methods (Robinson & Follett, 1982). Male and female quail exposed to long photoperiods exhibit increased plasma levels of testosterone and estradiol, respectively (Adkins & Adler, 1972; Balthazart et al., 1979; Balthazart et al., 1983; Brain et al., 1988; Delville et al., 1986; Delville & Balthazart, 1987; Doi et al., 1980; Domjan, 1987; Guyomarc'h & Guyomarc'h, 1994; Mills et al., 1997; Noble, 1972). Male and female quail exposed to short photoperiods exhibit decreased plasma levels of testosterone and estradiol, respectively (Adkins & Adler, 1972; Balthazart et al., 1979; Brain et al., 1988; Delville et al., 1986; Delville & Balthazart, 1987; Doi et al., 1980; Domjan, 1987; Guyomarc'h & Guyomarc'h, 1994; Mills et al., 1997; Noble, 1972). Furthermore, exposure to short-light conditions has been shown to be comparable to surgical gonadectomy in both male and female quail (Adkins & Nock, 1976).
The present study was designed to investigate the role of gonadal sex hormones in cocaine-induced behavioral sensitization in a visual species. The overarching hypothesis was that increases in plasma sex hormones would correlate with increases in cocaine-induced locomotor activity in Japanese quail and that female quail would be more sensitive to the locomotor-activating effects of cocaine than males. Specifically, it was predicted that photostimulated (long-light) female and male quail would dose-dependently exhibit increases in locomotor activity to repeated administration of cocaine, and that photostimulated female quail would sensitize to a greater degree than male quail. Based on previous cocaine sensitization studies in male quail and the rodent literature, it was predicted that cocaine-induced locomotor effects would be correlated with testosterone in male quail and with estradiol in female quail.
Materials and Methods
Subjects
Forty-six male and 45 female Japanese quail (Coturnix japonica), approximately 5-8 months old were subjects in the experiment. Eggs were supplied by Northwest Gamebirds (Kennewick, WA) and quail chicks were hatched and then raised in mixed-sex groups until approximately 28 days of age. At 28 days of age, male quail were housed individually and female quail were group housed in wire mesh cages (GQF Manufacturing, Savannah, GA). Female quail were housed individually when selected for the experiment. Subjects were raised in a long-light (16L: 8D) cycle with food and water available ad libitum. Approximately 3 weeks (21 days) prior to the start of the experiment, twenty-two male and twenty-one female quail were transferred to a short-light (8L: 16D) cycle with lights on at 0900 (Adkins, 1973; Henare et al., 2011, Robinson & Follett, 1982). In the current experiment, the short-light cycle resulted in plasma T (0.65 ± 0.71 ng/ml) and E2 (43.72 ± 41.62 pg/ml) levels similar to previous reports (Balthazart et al., 1979, 1983; Brain et al., 1988). Thus, male and female steroid hormone levels were adequately low under short-light conditions in the current experiment.
All subjects were drug and sexually naïve prior to experimentation. All experimental procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
Plasma Testosterone and Estradiol ELISA
Blood samples were taken two days prior to the start of the experiment. Approximately 0.5 ml of blood was taken from the brachial (wing) vein of the quail and placed into heparinized tubes. To minimize corticosteroid release, the time between removal of quail from carrier box and collection did not exceed 5 min (Dallman & Bhatnager, 2001; Mizrahi et al., 2001). Blood was immediately centrifuged at 1500 RPM for 5 min and plasma was stored at −20°C until assayed.
Plasma testosterone (T) and estradiol (E2) were measured in duplicate via an enzyme-linked immunoassay kit (DRG Diagnostics; testosterone EIA-1559, estradiol EIA-2693) modified from the procedure described by Wilhelms et al. (2005). The kits were validated for use with quail plasma by testing for parallelism and recovery of added mass (standard biochemical validations). To test for parallelism, high and low T and E2 pools were pipetted at five different volumes in quadruplicate to ensure that the dose response curves were parallel to the standards under dilution and to confirm that the sample bound to the antibody had the same affinity. The test for parallelism ensures the assay maintains linearity under dilution, and recovery of exogenous T or E2 verifies accurate measurement throughout the working range of the assay. To test for recovery of added mass, three standard curve points (from the middle of the standard curve) were added to the high and low pool to ensure that the added mass could be detected, indicating that the sample was not blocking the antibodies ability to bind with the standard. The intra-assay and inter-assay coefficient of variance (CV) for the T ELISA was 8% and 10%, respectfully. The intra-assay and inter-assay CV for the E2 ELISA was 9% and 12%, respectively.
Drugs
Cocaine hydrochloride was dissolved in physiological saline (0.9%) and injected intraperitoneally (i.p.) at a volume of 1 ml/kg of body mass. Doses were 10 or 20 mg/kg and were chosen based on previous research that demonstrated cocaine-enhanced locomotor activity (e.g. Hu & Becker, 2003; Levens & Akins, 2001; Geary & Akins, 2007). Physiological saline (0.9%) was used as a vehicle and was injected i.p. at a volume of 0.1 ml.
Apparatus
Distance travelled was used as an index of locomotor activity and was measured in 8 identical open field chambers. Each chamber had white plastic walls, screen mesh ceilings, and white corrugated paper as the floor. The chambers measured approximately 45.72 cm tall and 55.88 cm in diameter. Distance travelled was collected using ANY-Maze video tracking software (San Diego Instruments, San Diego, CA).
Procedure
Male and female quail were randomly assigned to receive 20 mg/kg cocaine (n = 16 males, n = 16 females), 10 mg/kg cocaine (n = 15 males, n = 14 females), or saline (n = 15 males, n = 15 females). Subjects were assigned to the same box for each trial and were given two days of habituation for 30 min prior to the start of the experiment. Birds were weighed each day, injected i.p. with 10 or 20 mg/kg cocaine or saline and immediately placed in the open field chambers. Distance travelled was recorded in 5 min time bins for 30 min. Trials were conducted once per day for 10 days with each subject receiving the same treatment throughout the experiment.
Studies have demonstrated that male and female quail may be sensitive to auditory stimulation (Guyomarc'h & Guyomarc'h, 1984, 1989; Li & Burke, 1987; Millam et al., 1985). To ensure that vocalizations did not affect locomotor activity, male and female quail were tested separately. After all male subjects were tested, chamber walls were cleaned and papers were changed, then all female subjects were tested. On alternate days, females were tested first, followed by males to minimize any potential time-of-day confounds.
Statistical Analysis
For locomotor activity, the total amount of distance-travelled (in meters) during the 30 min test period for each trial was recorded. A repeated measures analysis of variance (ANOVA) was performed with trials as a repeated measure and sex, treatment, and photoperiod as between-subjects variables. To further probe interactions, independent ANOVAs served as post-hoc analyses where appropriate.
Quail were considered to be sensitized to cocaine if their distance travelled was significantly greater on trial 10 compared to trial 1 (e.g. Hu & Becker, 2003). A repeated- measures ANOVA with trials as a repeated measure and sex, treatment, and photoperiod as between-subjects variables was conducted. For significant interactions, independent ANOVAs were conducted where appropriate.
To analyze whether activity was correlated with hormone levels, a univariate analysis of covariance (ANCOVA) was conducted with total distance-travelled activity on trial 10 and hormone plasma levels. Trial 10 was selected as the dependent measure because sensitization effects were most evident on that trial. Treatment (cocaine or saline) served as a fixed factor independent variable and hormone level as a covariate. Following a significant ANCOVA, linear regression analyses were performed for each treatment group.
All data were analyzed using SPSS software version 20 (International Business Machines Corp. (IBM), Armonk, NY). Effect sizes were estimated by calculating eta squared (η2) using the formulas, η2 = SSbetween/SStotal for between-subjects effects and η2 = SSinteraction/SStotal for within-subjects effects. For all analyses, the statistical significance level was set at p < 0.05.
Results
Locomotor Activity
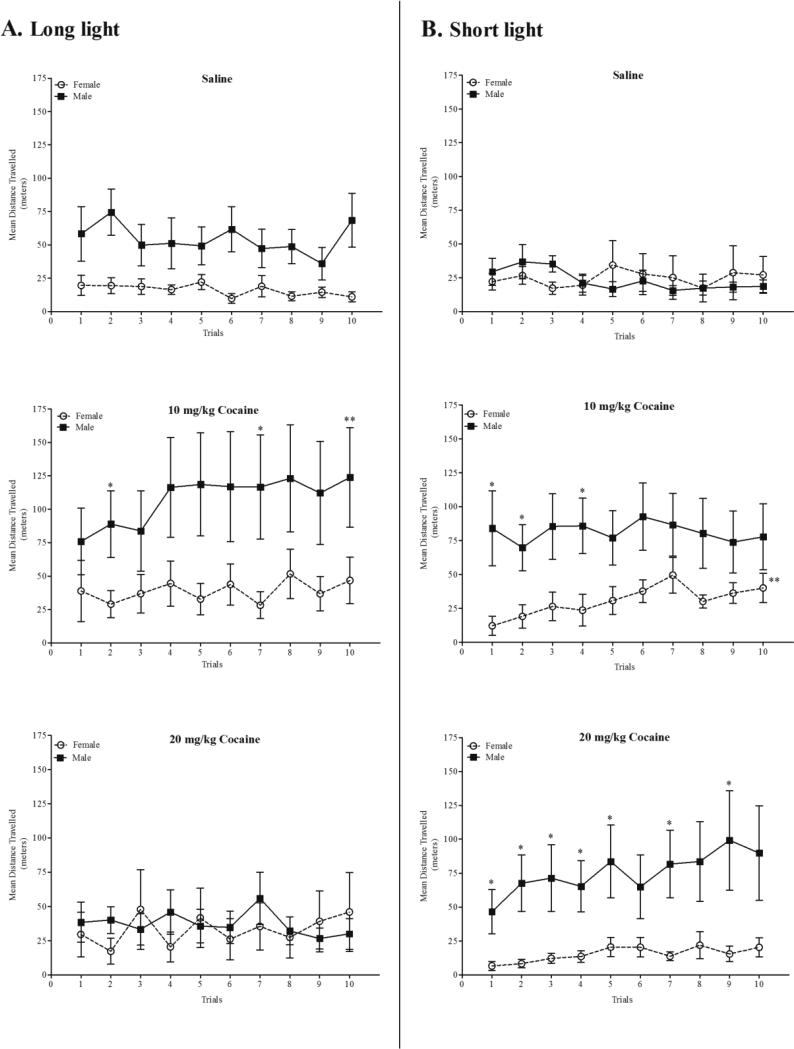
Figure 1 shows the mean distance travelled for male and female quail housed in long-light (panel A) or short-light (panel B) conditions across all 10 trials for saline, 10 and 20 mg/kg cocaine. Overall, male quail had significantly higher activity levels (M = 63.21, S.E.M. = 6.95) than female quail (M = 26.41, S.E.M. = 7.05) as revealed by a main effect of sex, F(1, 79) = 13.81, p = 0.0004, η2 = 0.13. Quail treated with 10 mg/kg cocaine (M = 64.0, S.E.M. = 8.80) had significantly greater locomotor activity relative to saline-treated quail (M = 29.59, S.E.M. = 8.61) and quail treated with 20 mg/kg cocaine (M = 40.23, S.E.M. = 8.32) as indicated by a main effect of treatment, F(2, 79) = 4.24, p = 0.018, η2 = 0.09. No other main effects were evident. A repeated measures ANOVA revealed a significant Sex × Treatment × Photoperiod × Trials interaction, F (18, 711) = 2.18, p = 0.003, η2 = 0.05. Further analyses were then conducted for each treatment.
Figure 1.
Mean (± S.E.M.) distance travelled across trials for males and females that received saline, 10, or 20 mg//kg cocaine housed in long-light (panel A) and short-light (panel B) conditions. * indicates significance from female quail (p < 0.05). ** indicates significance from trial 1 (p < 0.05).
For saline-treated quail, a significant Sex × Trials interaction indicated that males had significantly higher activity levels than females across trials, F(9, 234) = 2.25, p = 0.02, η2 = 0.07 (see Figure 1). There was not a significant Sex × Photoperiod × Trials interaction, F(9, 234) = 1.42, p = 0.179, η2 = 0.05. Therefore, no further analyses were conducted for saline-treated quail.
For quail treated with 10 mg/kg cocaine, a main effect of sex indicated that male quail (M = 94.48, S.E.M. = 16.23) had significantly greater cocaine-induced activity than female quail (M = 34.72, S.E.M. = 16.94), F(1, 25) = 6.49, p = 0.017, η2 = 0.20 (see Figure 1). Additionally for quail treated with 10 mg/kg, there was a significant Sex × Photoperiod × Trials interaction, F(9, 225) = 2.41, p = 0.013, η2 = 0.08. Further analyses within each sex revealed a significant Photoperiod × Trials interaction for female quail, F(9, 108) = 2.39, p = 0.016, η2 = 0.17, but not for male quail, F(9, 117) = 1.71, p = 0.095, η2 = 0.12. Analyses within each photoperiod were not significant.
For quail treated with 20 mg/kg cocaine, a significant Sex × Photoperiod × Trials interaction was revealed, F(9, 252) = 2.00, p = 0.04, η2 = 0.07 (see Figure 1). Further analyses within each sex were not significant. Analyses within each photoperiod indicated that a Sex × Trials interaction was significant for long-light quail, F(9, 126) = 1.99, p = 0.045, η2 = 0.13, but not for short-light quail, F(9, 126) = 0.93, p = 0.502, η2 = 0.06. However, analysis with short-light quail revealed a main effect of sex which indicated that male quail had significantly greater activity (M = 75.38, S.E.M. = 16.99) than female quail (M = 15.25, S.E.M. = 16.99), F(1, 14) = 6.26, p = 0.025, η2 = 0.31.
To test for cocaine-induced sensitization, trial 10 was compared to trial 1 (refer to Figure 1). A repeated measures ANOVA revealed a significant Sex × Treatment × Photoperiod × Trials interaction, F(2, 79) = 7.06, p = 0.002, η2 = 0.15. Further analyses were then conducted for each treatment. An analysis within the 10 mg/kg cocaine group showed a Sex × Photoperiod × Trials interaction, F(1, 25) = 7.86, p = 0.01, η2 =0.24. Further analyses within each sex revealed a significant Photoperiod × Trials interaction for male quail, F(1, 13) = 7.42, p = 0.017, η2 = 0.36, indicating that male quail housed in long-light conditions exhibited significantly greater activity for Trial 10 (M = 123.82, S.E.M. = 37.12) than for Trial 1 (M = 75.94, S.E.M. = 24.78), F(1, 7) = 8.23, p = 0.024, η2 = 0.54. Analyses within each photoperiod showed a significant Sex × Trials interaction for short-light quail treated with 10 mg/kg cocaine, F(1, 11) = 7.05, p = 0.02, η2 =0.39, revealing that female quail showed significantly greater activity on Trial 10 (M = 40.04, S.E.M. = 10.88) than for Trial 1 (M = 12.12, S.E.M. = 7.11), F(1, 5) = 10.12, p = 0.025, η2 =0.67. No other interactions were found when comparing trial 10 to trial 1.
Hormone Correlational Analyses
Testosterone
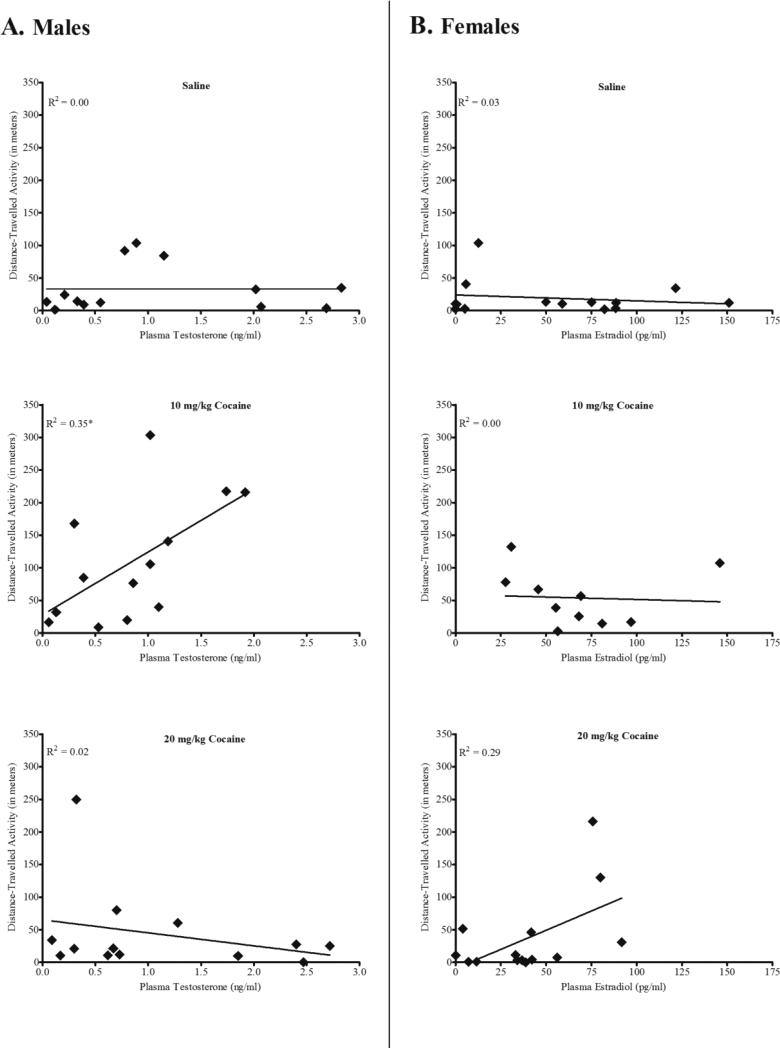
A univariate analysis of covariance (ANCOVA) revealed a significant Treatment × Hormone interaction for male quail on Trial 10, F(3, 39) = 7.09, p = 0.001, η2 = 0.37. Linear regression analyses were then conducted to determine whether testosterone levels were correlated with males’ activity for saline, 10 mg/kg cocaine, and 20 mg/kg cocaine groups (see Figure 2, Panel A). Results indicated that levels of testosterone significantly predicted activity levels for male quail treated with 10 mg/kg cocaine, R2 = 0.35, F(1, 12) = 5.96, p = 0.033. Activity levels did not correlate with testosterone levels for saline-treated males, R2 = 0.00, F (1, 12) = 0.00, p = 0.99, nor for males treated with 20 mg/kg cocaine, R2 = 0.02, F(1, 13) = 0.24, p = 0.63.
Figure 2.
Scatterplots correlating the mean distance travelled on trial 10 for males and females that received saline, 10, or 20 mg/kg cocaine with plasma testosterone in male quail (panel A) or plasma estradiol in female quail (panel B). * indicates a significant correlation between activity and hormone level (p < 0.05).
Estradiol
There was not a significant Treatment × Hormone interaction for female quail as indicated by a univariate ANCOVA, F (3, 37) = 2.53, p = 0.074, η2 = 0.18 (see Figure 2, Panel B). Therefore, no further analyses were conducted. In addition, the effect size was not large enough to warrant further consideration.
Discussion
Contradictory to our original hypothesis, long-light cycle females did not show greater cocaine sensitization than long-light cycle males. Following chronic exposure to 10 mg/kg cocaine, long-light cycle male quail demonstrated cocaine-induced sensitization. Conversely, short-light cycle female quail demonstrated behavioral sensitization to chronic administration of 10 mg/kg cocaine. Testosterone levels were positively correlated with cocaine-induced sensitization in male quail at the 10 mg/kg dose but estradiol levels were not correlated with cocaine-induced sensitization in female quail.
In the present study, the findings with male quail are in agreement with some rodent studies that demonstrated cocaine-induced sensitization and involvement of testosterone. Martinez-Sanchis et al. (2002) found that exogenous testosterone significantly enhanced activity at 4 mg/kg cocaine and 10 mg/kg cocaine in intact male mice. Similar to the current experiment, Martinez-Sanchis et al. (2002) did not find an effect of testosterone at doses greater than 10 mg/kg cocaine. Furthermore, Menendez-Delmestre and Segarra (2011) found that 15 and 30 mg/kg of cocaine progressively increased locomotor activity in intact and CAST male rats with testosterone replacement but not in CAST male rats. In contrast, several studies examining the role of testosterone in the behavioral effects of cocaine have failed to find differences between castrated (CAST) and intact male rodents (Becker et al., 2001; Forgie & Stewart, 1994; Hu & Becker, 2003; Hu et al., 2004; Robinson et al., 1981; van Haaren & Meyer, 1991). Therefore, while the current findings implicate the role of testosterone in cocaine sensitization in male quail, other findings are inconsistent with this.
In the current study, sensitization to 10 mg/kg cocaine in female Japanese quail did not appear to correlate with circulating levels of estradiol. The majority of studies in rodents have indicated that estradiol contributes to a greater locomotor response to cocaine following chronic or acute administration in intact or estradiol-treated OVX females compared to OVX females (Camp & Robinson, 1988a, b; Hu & Becker, 2003; Peris et al., 1991; Robinson et al., 1982; Robinson, 1984; Sircar & Kim, 1999; van Haaren & Meyer, 1991). One possible explanation for the lack of correlation between estradiol and cocaine-induced activity in the present study may be ethological in that circulating estradiol may have a suppressant effect on activity in female Japanese quail. In nature, Japanese quail breed during the summer months and this is mimicked in the laboratory by long-light conditions (see Mills et al., 1997 for review). Quail maintained on long-light photoperiods have increased plasma sex steroids (Delville et al., 1986). Female quail with increased levels of plasma estradiol display low activity levels enabling them to nest, lay eggs, and to be sexually receptive to a male conspecific (see Mills et al., 1997 for review). While this explanation is speculative, it is possible that the locomotor-suppressant effects of estradiol were sufficiently strong to override any cocaine-locomotor enhancing effects.
An equally surprising finding in the current report was that short-light cycle female quail showed cocaine sensitization to chronic cocaine exposure (10 mg/kg). Plasma estradiol was substantially lower in short-light females compared to long-light females, therefore it was not expected that females housed on a short-light cycle would have shown sensitization. However, since estradiol may suppress locomotor activity in female Japanese quail, as discussed above, it may be that the reduction of estradiol in the short-light females was sufficient to allow cocaine (10 mg/kg) to increase locomotor activity. Another possibility is that corticosterone (CORT) may have played a role. CORT release is seasonally modulated in birds with maximal levels occurring during short-light periods (Romero et al., 1998). Additionally, female Japanese quail have greater plasma concentrations of CORT compared to male Japanese quail when housed in short-light conditions (Peczely and Pethes, 1981). Therefore, even with low levels of estradiol, CORT levels may have been sufficiently high in short-light females to elicit a greater response to cocaine compared to long-light females. This is speculative, however, but might suggest that CORT levels need to be measured in future studies that utilize female birds on short-light cycles.
In general, the differences between the current findings and those of rodents may be due to inherent species differences in the dopaminergic system. It has been shown that the general organization of the dopamine (DA) system is conserved between birds and mammals (Smeets & Reiner, 1994). However, the distribution and density of D1 and D2 receptors have been found to be different (Kleitz et al., 2009; Richfield et al., 1987). In an autoradiography labeling study, Kleitz and et al. (2009) found a species difference in the D2:D1 ratio in target sites for DA between Japanese quail and rats. Specifically, quail had a higher D2:D1 receptor ratio in the striatal regions of the brain compared to rats (Kleitz et al., 2009). While speculative, the current findings may suggest that increased stimulation of D2 receptors in female quail resulted in an opposite effect on behavior compared to female rodents. D2 receptor activation has been shown to decrease cocaine-induced locomotor activity in rats (Schindler & Carmona, 2002), and decrease cocaine-seeking behaviors in non-human primates (Czoty et al., 2004). Thus, an increase in D2 receptors in female quail may, in part, explain the lack of cocaine-induced locomotor activity in long-light females observed in the present study.
Estrogens have been shown to influence dopaminergic activity in the mesolimbic and nigrostriatal systems (Cyr et al., 2000, 2002; Di Paolo, 1994). In female rats, estradiol has been shown to rapidly downregulate D2 in the striatum, shifting the D2:D1 ratio towards D1 (Bazzett & Becker, 1994). Thus, a decrease in D2 receptors may, in part, explain the increased sensitivity to repeated cocaine observed in female rodents (Hu & Becker, 2003; Becker & Hu, 2008). Studies with Japanese quail have suggested that a sex difference in the availability of D1 or D2 receptors may not exist (Ball et al., 1995). Therefore, it is possible that in female quail, estradiol may not interact with dopamine receptors in the same way as rodents. However, this is speculative. Further neurobiological studies are needed in Japanese quail but the current study adds to our knowledge of cocaine-hormone interactions in behavioral sensitization in a visually-oriented species.
Summary/Conclusions
In sum, male quail housed under long-light conditions demonstrated cocaine-induced sensitization to 10 mg/kg cocaine. This was correlated with increased levels of plasma testosterone. Female quail housed under short-light conditions demonstrated sensitization to 10 mg/kg cocaine, but this did not appear to be correlated with levels of plasma estradiol. Our results suggest that male sex hormones might be contributing to the greater magnitude of cocaine sensitization in Japanese quail. Further investigation of the quail dopamine system may lead to a better understanding of the underlying neurobiological mechanisms mediating this sex difference.
Highlights.
Male quail housed under long-light conditions demonstrated sensitization to 10 mg/kg cocaine which was correlated with testosterone.
Female quail housed under short-light conditions demonstrated sensitization to 10 mg/kg cocaine, but this was not correlated with levels of plasma estradiol.
The current findings suggest that cocaine-induced locomotor activity was associated with testosterone in males but not with estradiol in females.
Acknowledgements
Financial support for this research was provided by NIDA grant #DA022451 awarded to CKA. The authors would like to thank Luke Cornett, Joshua Shouse, Beth Ann Rice, B. Levi Bolin, Charlie Deese, and Aaron Barr for their help with data collection, animal care, and technical support. The authors would also like to thank Dr. Gregory F. Ball and his lab associates for their collaboration and guidance on this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins EK. Functional castration of the female Japanese quail. Physiol Behav. 1973;10(3):619–621. doi: 10.1016/0031-9384(73)90232-1. [DOI] [PubMed] [Google Scholar]
- Adkins EK, Adler NT. Hormonal control of behavior in the Japanese quail. J Comp Physiol Psychol. 1972;81(1):27–36. doi: 10.1037/h0033315. [DOI] [PubMed] [Google Scholar]
- Adkins EK, Nock B. Behavioural responses to sex steroids of gonadectomized and sexually regressed quail. J Endocrinol. 1976;68(1):49–55. doi: 10.1677/joe.0.0680049. [DOI] [PubMed] [Google Scholar]
- Akins CK, Geary EH. Cocaine-induced behavioral sensitization and conditioning in male Japanese quail. Pharmacol Biochem Behav. 2008;88(4):432–437. doi: 10.1016/j.pbb.2007.09.020. doi: 0.1016/j.pbb.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akins CK, Levens N, Prather R, Cooper B, Fritz T. Dose-dependent cocaine place conditioning and D1 dopamine antagonist effects in male Japanese quail. Physiol Behav. 2004;82(2-3):309–315. doi: 10.1016/j.physbeh.2004.03.035. doi: 10.1016/j.physbeh.2004.03.035. [DOI] [PubMed] [Google Scholar]
- Ball GF, Casto JM, Balthazart J. Autoradiographic localization of D1-like dopamine receptors in the forebrain of male and female Japanese quail and their relationship with immunoreactive tyrosine hydroxylase. J Chem Neuroanat. 1995;9(2):121–133. doi: 10.1016/0891-0618(95)00075-i. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Massa R, Negri-Cesi P. Photoperiodic control of testosterone metabolism, plasma gonadotrophins, cloacal gland growth, and reproductive behavior in the Japanese quail. Gen Comp Endocrinol. 1979;39(2):222–235. doi: 10.1016/0016-6480(79)90227-2. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Schumacher M, Ottinger MA. Sexual differences in the Japanese quail: behavior, morphology, and intracellular metabolism of testosterone. Gen Comp Endocrinol. 1983;51(2):191–207. doi: 10.1016/0016-6480(83)90072-2. [DOI] [PubMed] [Google Scholar]
- Bazzett TJ, Becker JB. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res. 1994;637(1-2):163–172. doi: 10.1016/0006-8993(94)91229-7. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29(1):36–47. doi: 10.1016/j.yfrne.2007.07.003. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann N Y Acad Sci. 2001;937:172–187. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- Brain PC, Onagbesan OM, Peddie MJ, Taylor TG. Changes in plasma concentrations of reproductive steroids in female Japanese quail (Coturnix coturnix japonica) raised on long or short photoperiods. Gen Comp Endocrinol. 1988;69(2):174–180. doi: 10.1016/0016-6480(88)90003-2. [DOI] [PubMed] [Google Scholar]
- Camp DM, Robinson TE. Susceptibility to sensitization. I. Sex differences in the enduring effects of chronic D-amphetamine treatment on locomotion, stereotyped behavior and brain monoamines. Behav Brain Res. 1988a;30(1):55–68. doi: 10.1016/0166-4328(88)90008-3. [DOI] [PubMed] [Google Scholar]
- Camp DM, Robinson TE. Susceptibility to sensitization. II. The influence of gonadal hormones on enduring changes in brain monoamines and behavior produced by the repeated administration of D-amphetamine or restraint stress. Behav Brain Res. 1988b;30(1):69–88. doi: 10.1016/0166-4328(88)90009-5. [DOI] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, Ehrman R, O'Brien CP. Classically conditioned responses in opioid and cocaine dependence: a role in relapse? NIDA Res Monogr. 1988;84:25–43. [PubMed] [Google Scholar]
- Collins SL, Evans SM, Foltin RW, Haney M. Intranasal cocaine in humans: effects of sex and menstrual cycle. Pharmacol Biochem Behav. 2007;86(1):117–124. doi: 10.1016/j.pbb.2006.12.015. doi: 10.1016/j.pbb.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr M, Calon F, Morissette M, Di Paolo T. Estrogenic modulation of brain activity: implications for schizophrenia and Parkinson's disease. J Psychiatry Neurosci. 2002;27(1):12–27. [PMC free article] [PubMed] [Google Scholar]
- Cyr M, Calon F, Morissette M, Grandbois M, Di Paolo T, Callier S. Drugs with estrogen-like potency and brain activity: potential therapeutic application for the CNS. Curr Pharm Des. 2000;6(12):1287–1312. doi: 10.2174/1381612003399725. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Morgan D, Shannon EE, Gage HD, Nader MA. Characterization of dopamine D1 and D2 receptor function in socially housed cynomolgus monkeys self-administering cocaine. Psychopharmacology (Berl) 2004;174(3):381–388. doi: 10.1007/s00213-003-1752-z. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Bhatnagar S. Chronic stress and energy balance: role of the hypothalamo–pituitary–adrenal axis. In: McEwen BS, Goodman HM, editors. Handbook of Physiology; Section 7: The Endocrine System; Volume IV: Coping with the Environment: Neural and Endocrine Mechanisms. New York. Oxford University Press; 2001. pp. 179–210. [Google Scholar]
- Delville Y, Balthazart J. Hormonal control of female sexual behavior in the Japanese quail. Horm Behav. 1987;21(3):288–309. doi: 10.1016/0018-506x(87)90016-x. [DOI] [PubMed] [Google Scholar]
- Delville Y, Sulon J, Balthazart J. Diurnal variations of sexual receptivity in the female Japanese quail (Coturnix coturnix japonica). Horm Behav. 1986;20(1):13–33. doi: 10.1016/0018-506x(86)90026-7. [DOI] [PubMed] [Google Scholar]
- Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev Neurosci. 1994;5(1):27–41. doi: 10.1515/revneuro.1994.5.1.27. [DOI] [PubMed] [Google Scholar]
- Doi O, Takai T, Nakamura T, Tanabe Y. Changes in the pituitary and plasma LH, plasma and follicular progesterone and estradiol, and plasma testosterone and estrone concentrations during the ovulatory cycle of the quail (Coturnix coturnix japonica). Gen Comp Endocrinol. 1980;41(2):156–163. doi: 10.1016/0016-6480(80)90139-2. [DOI] [PubMed] [Google Scholar]
- Domjan M. Photoperiodic and endocrine control of social proximity behavior in male Japanese quail (Coturnix coturnix japonica). Behav Neurosci. 1987;101(3):385–392. doi: 10.1037//0735-7044.101.3.385. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31(3):659–674. doi: 10.1038/sj.npp.1300887. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002;159(4):397–406. doi: 10.1007/s00213-001-0944-7. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Fidura FG, Gray JA. Visual discrimination of color, pattern, and form in the Japanese quail. Psychon. Sci. 1966;5:437–438. [Google Scholar]
- Forgie ML, Stewart J. Sex differences in the locomotor-activating effects of amphetamine: role of circulating testosterone in adulthood. Physiol Behav. 1994;55(4):639–644. doi: 10.1016/0031-9384(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Geary EH, Akins CK. Cocaine sensitization in male quail: temporal, conditioning, and dose-dependent characteristics. Physiol Behav. 2007;90(5):818–824. doi: 10.1016/j.physbeh.2007.01.010. doi: 10.1016/j.physbeh.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Mirin SM, Lange U. A comparison of male and female cocaine abusers. Arch Gen Psychiatry. 1989;46(2):122–126. doi: 10.1001/archpsyc.1989.01810020024005. [DOI] [PubMed] [Google Scholar]
- Guyomarc'h C, Guyomarc'h JC. The influence of social factors on the onset of egg production in Japanese quail (Coturnix japonica). Biol. Behav. 1984;9:333–342. [Google Scholar]
- Guyomarc'h C, Guyomarc'h JC. Stimulation of sexual development in female Japanese quail by male song: influence of ecoethological variables. Biol. Behav. 1989;14:52–65. [Google Scholar]
- Guyomarc'h C, Guyomarc'h JC. Testosterone levels and the free running rhythm of feeding activity in Japanese quail in darkness. Gen Comp Endocrinol. 1994;96(2):165–171. doi: 10.1006/gcen.1994.1170. doi: 10.1006/gcen.1994.1170. [DOI] [PubMed] [Google Scholar]
- Henare SJ, Kikuchi M, Talbot RT, Cockrem JF. Changes in plasma gonadotrophins, testosterone, prolactin, thyroxine and triiodothyronine concentrations in male Japanese quail (Coturnix coturnix japonica) of a heavy body weight line during photo-induced testicular growth and regression. Br Poult Sci. 2011;52(6):782–791. doi: 10.1080/00071668.2011.639341. doi: 10.1080/00071668.2011.639341. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Gonzalez L, Murzi E, Paez X, Gottberg E, Baptista T. Testosterone modulates mesolimbic dopaminergic activity in male rats. Neurosci Lett. 1994;171(1-2):172–174. doi: 10.1016/0304-3940(94)90632-7. [DOI] [PubMed] [Google Scholar]
- Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23(2):693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29(1):81–85. doi: 10.1038/sj.npp.1300301. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31(1):129–138. doi: 10.1038/sj.npp.1300778. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Kasperski SJ, Vincent KB, Caldeira KM, Garnier-Dykstra LM, O'Grady KE, Arria AM. College students' use of cocaine: results from a longitudinal study. Addict Behav. 2011;36(4):408–411. doi: 10.1016/j.addbeh.2010.12.002. doi: 10.1016/j.addbeh.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleitz HK, Cornil CA, Balthazart J, Ball GF. Species differences in the relative densities of D1- and D2-like dopamine receptor subtypes in the Japanese quail and rats: an in vitro quantitative receptor autoradiography study. Brain Behav Evol. 2009;73(2):81–90. doi: 10.1159/000209864. doi: 10.1159/000209864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. J Subst Abuse Treat. 1993;10(1):63–66. doi: 10.1016/0740-5472(93)90100-g. [DOI] [PubMed] [Google Scholar]
- Levens N, Akins CK. Cocaine induces conditioned place preference and increases locomotor activity in male Japanese quail. Pharmacol Biochem Behav. 2001;68(1):71–80. doi: 10.1016/s0091-3057(00)00439-1. [DOI] [PubMed] [Google Scholar]
- Li ZZ, Burke WH. Influence of 12 hours of sound stimuli on gonad development and plasma luteinizing hormone in Japanese quail (Coturnix coturnix japonica) exposed to 6 hours of daily light. Poult Sci. 1987;66(6):1045–1052. doi: 10.3382/ps.0661045. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 1999;144(1):77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Martinez-Sanchis S, Aragon CM, Salvador A. Cocaine-induced locomotor activity is enhanced by exogenous testosterone. Physiol Behav. 2002;76(4-5):605–609. doi: 10.1016/s0031-9384(02)00764-3. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Teoh SK, Mello NK, Ellingboe J, Rhoades E. Acute effects of cocaine on plasma adrenocorticotropic hormone, luteinizing hormone and prolactin levels in cocaine-dependent men. J Pharmacol Exp Ther. 1992;263(2):505–509. [PubMed] [Google Scholar]
- Mendelson JH, Weiss R, Griffin M, Mirin SM, Teoh SK, Mello NK, Lex BW. Some special considerations for treatment of drug abuse and dependence in women. NIDA Res Monogr. 1991;106:313–327. [PubMed] [Google Scholar]
- Menendez-Delmestre R, Segarra AC. Testosterone is essential for cocaine sensitization in male rats. Physiol Behav. 2011;102(1):96–104. doi: 10.1016/j.physbeh.2010.09.025. doi:10.1016/j.physbeh.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millam JR, El Halawani ME, Burke WH. Effect of cyclic sound cues on sexual development in nonphotostimulated Japanese quail. Poult Sci. 1985;64(1):169–175. doi: 10.3382/ps.0640169. [DOI] [PubMed] [Google Scholar]
- Mills AD, Crawford LL, Domjan M, Faure JM. The behavior of the Japanese or domestic quail Coturnix japonica. Neurosci Biobehav Rev. 1997;21(3):261–281. doi: 10.1016/s0149-7634(96)00028-0. [DOI] [PubMed] [Google Scholar]
- Mizrahi DS, Holberton RL, Gauthreaux SA., Jr. Patterns of corticosterone secretion in migrating semipalmated sandpipers at a major spring stopover site. Auk. 2001;118:79–91. [Google Scholar]
- Noble R. The effects of estrogen and progesterone on copulation in female quail (Coturnix coturnix japonica) housed in continuous dark. Horm Behav. 1972;3(3):199–204. doi: 10.1016/0018-506x(72)90032-3. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, McLellan AT, Alterman A, Childress AR. Psychotherapy for cocaine dependence. Ciba Found Symp. 1992;166:207–216. doi: 10.1002/9780470514245.ch12. discussion 216-223. [DOI] [PubMed] [Google Scholar]
- Peczely P, Pethes G. Effect of ovariectomy, thyroidectomy and of thyroxine treatment on the plasma level of corticosterone of the female Japanese quail. Acta Biol Acad Sci Hung. 1981;32(1):1–6. [PubMed] [Google Scholar]
- Peris J, Decambre N, Coleman-Hardee ML, Simpkins JW. Estradiol enhances behavioral sensitization to cocaine and amphetamine-stimulated striatal [3H]dopamine release. Brain Res. 1991;566(1-2):255–264. doi: 10.1016/0006-8993(91)91706-7. [DOI] [PubMed] [Google Scholar]
- Purvis-Tyson TD, Owens SJ, Double KL, Desai R, Handelsman DJ, Weickert CS. Testosterone induces molecular changes in dopamine signaling pathway molecules in the adolescent male rat nigrostriatal pathway. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0091151. doi:10.1371/journal.pone.0091151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones-Jenab V, Jenab S. Influence of sex differences and gonadal hormones on cocaine addiction. ILAR J. 2012;53(1):14–22. doi: 10.1093/ilar.53.1.14. [DOI] [PubMed] [Google Scholar]
- Richfield EK, Young AB, Penney JB. Comparative distribution of dopamine D-1 and D-2 receptors in the basal ganglia of turtles, pigeons, rats, cats, and monkeys. J Comp Neurol. 1987;262:446–463. doi: 10.1002/cne.902620308. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O'Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999;53(3):223–230. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- Robinson JE, Follett BK. Photoperiodism in Japanese quail: the termination of seasonal breeding by photorefractoriness. Proc R Soc Lond B Biol Sci. 1982;215(1198):95–116. doi: 10.1098/rspb.1982.0030. [DOI] [PubMed] [Google Scholar]
- Robinson TE. Behavioral sensitization: characterization of enduring changes in rotational behavior produced by intermittent injections of amphetamine in male and female rats. Psychopharmacology (Berl) 1984;84(4):466–475. doi: 10.1007/BF00431451. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB, Presty SK. Long-term facilitation of amphetamine-induced rotational behavior and striatal dopamine release produced by a single exposure to amphetamine: sex differences. Brain Res. 1982;253(1-2):231–241. doi: 10.1016/0006-8993(82)90690-4. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Camp DM, Becker JB. Gonadectomy attenuates turning behavior produced by electrical stimulation of the nigrostriatal dopamine system in female but not male rats. Neurosci Lett. 1981;23(2):203–208. doi: 10.1016/0304-3940(81)90041-0. [DOI] [PubMed] [Google Scholar]
- Romero LM, Soma KK, Wingfield JC. Hypothalamic–pituitary–adrenal axis changes allow seasonal modulation of corticosterone in a bird. Am. J. Physiol. 1998;274:1338–1344. doi: 10.1152/ajpregu.1998.274.5.R1338. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 2003;970(1-2):214–220. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Carmona GN. Effects of dopamine agonists and antagonists on locomotor activity in male and female rats. Pharmacol Biochem Behav. 2002;72(4):857–863. doi: 10.1016/s0091-3057(02)00770-0. doi: S0091305702007700 [pii] [DOI] [PubMed] [Google Scholar]
- Sircar R, Kim D. Female gonadal hormones differentially modulate cocaine-induced behavioral sensitization in Fischer, Lewis and Sprague-Dawley rats. J Pharmacol exp Ther. 1999;289:54–65. [PubMed] [Google Scholar]
- Smeets WJAJ, Reiner A. Phylogeny and Development of Catecholamine Systems in the CNS of Vertebrates. Cambridge University Press; Cambridge: 1994. [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7(3):274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Van Etten ML, Anthony JC. Comparative epidemiology of initial drug opportunities and transitions to first use: marijuana, cocaine, hallucinogens and heroin. Drug Alcohol Depend. 1999;54(2):117–125. doi: 10.1016/s0376-8716(98)00151-3. [DOI] [PubMed] [Google Scholar]
- van Haaren F, Meyer ME. Sex differences in locomotor activity after acute and chronic cocaine administration. Pharmacol Biochem Behav. 1991;39(4):923–927. doi: 10.1016/0091-3057(91)90054-6. [DOI] [PubMed] [Google Scholar]
- Wilhelms KW, Cutler SA, Proudman JA, Anderson LL, Scanes CG. Atrazine and the hypothalamo-pituitary-gonadal axis in sexually maturing precocial birds: studies in male Japanese quail. Toxicol Sci. 2005;86(1):152–160. doi: 10.1093/toxsci/kfi170. [DOI] [PubMed] [Google Scholar]