Abstract
Methamphetamine (METH) is a psychomotor stimulant strongly associated with increases in sexual drive and impulsive sexual behaviors that often lead to unsafe sexual practices. In women METH users, such practices have been associated with increases in unplanned pregnancies and sexually transmitted diseases. Despite this significant heath concern, the neural mechanisms underlying this drug-sex association are not known. We previously established a rodent model of METH-facilitated female sexual behavior in which estradiol and progesterone interact with METH to increase motivational components of female behavior and neuronal activation in the posterodorsal medial amygdala (MePD) (Holder et al., 2010; Holder and Mong, 2010). The current study more directly examines the mechanisms underlying the drug-sex interaction. Here, we hypothesize that METH-induced increases in MePD dopamine signaling bridge the METH-hormone interaction. In support of this hypothesis, we found that excitotoxic lesions targeted to the MePD attenuated the METH-induced increases in proceptive behavior. Furthermore, infusion of a D1 agonist into the MePD increased proceptive behavior, while infusion of a D1 antagonist blocked the ability of METH to increase proceptive behaviors. Additionally, we found that METH-treatment increased progesterone receptor (PR)- immunoreactivity in the MePD, suggesting an interaction between dopamine and progesterone signaling. Indeed, infusions of the PR antagonist, RU486, prevented METH-induced increases in sexual behavior. Thus, taken together, the current findings suggest dopamine in the MePD modulates enhanced sexual motivation via an amplification of progesterone signaling and contributes to a better understanding of the neurobiology of drug-enhanced sexual behaviors.
Keywords: progesterone receptor, proceptive behavior, methamphetamine, medial amygdala, progesterone receptor immunoreactivity, dopamine receptor
Introduction
Methamphetamine (METH) use among women is a burgeoning health concern as its abuse is significantly associated with high-risk sexual behaviors, leading to increased rates of sexually transmitted diseases such as HIV/AIDS and unplanned pregnancies among METH-addicted women (Corsi and Booth, 2008; Mansergh et al., 2006). Self-report studies have clearly established that METH use elicits heightened sexual drives, desires and sexual activities in women (Mansergh et al., 2006; Rawson et al., 2002; Semple et al., 2004a; Semple et al., 2004b). However, self-reporting surveys do not allow direct testing of the cellular and molecular events underlying METH effects on sexual motivation; thus, the mechanisms by which METH induces female sexual motivation have remained unexplored. For this, rodent models are most appropriate as they can inform the physiological processes underlying the human experience (Blaustein, 2008; Pfaus et al., 2003).
Recently, several laboratories, including our own, have demonstrated that METH facilitates sexual motivation in female rodents (Guarraci, 2009; Winland et al., 2011). In our established model, repeated administration of METH more than doubles the frequency of proceptive (i.e. solicitation) behaviors and augments lordosis intensity. The METH-induced increase in proceptivity depends upon estradiol and progesterone (Holder et al., 2010), suggesting a convergence of ovarian steroids and METH actions.
The posterodorsal medial amygdala (MePD) is uniquely situated to act as a convergence point for METH and hormone actions as it has been implicated in the modulation of female sexual behavior (reviewed in Erskine, 1989). Our previous findings demonstrate that the combined administration of estradiol/progesterone and METH, over either treatment alone, increases neuronal activation and spinophilin protein expression (Holder et al., 2010; Holder and Mong, 2010) in the MePD. Additionally, exogenous estradiol/progesterone administration increases tyrosine hydroxylase (TH) expression in the MePD (Holder and Mong, 2010), suggesting an increase in catecholamine synthesis. METH increases the concentration of extracellular catecholamines by reversal of reuptake transporters (Fleckenstein et al., 2000; Fukui et al., 2003; Sulzer et al., 2005). Thus, increases in TH could further increase extracellular catecholamine concentrations and activation of dopamine and/or norepinephrine receptors. Activation of the dopamine D1 receptor (D1R) increases the transcriptional activation of progesterone receptors (PR) (Bai et al., 1997; Denner et al., 1990; Mani et al., 1994a; Power et al., 1991), which are required for the expression of proceptive behaviors (Beach, 1942; McEwen et al., 1987; Pfaff et al., 1994; Whalen, 1974). Additionally, activation of the noradrenergic α1 receptor (α1R) can also facilitate sexual behavior by increasing PR activation (Chu and Etgen, 1999; Chu et al., 1999; Etgen, 1990; Gonzalez-Flores et al., 2007; Gonzalez-Flores et al., 2004).
In the present study, we examined the role of the MePD in the METH-enhanced female sexual motivation. We then determined which of the candidate catecholaminergic receptors within the MePD mediated the enhanced sexual motivation. Importantly, within these analyses we established a potential mechanism through with METH and progesterone converge in the MePD to increase female sexual motivation.
Materials and Methods
Animals
Adult female Sprague-Dawley rats (275–300g) were purchased from Charles River Laboratories (Kingston, NY) and housed in the Laboratory Animal Facility of the Health Sciences Facilities at the University of Maryland, School of Medicine under a reversed 12h:12h dark: light cycle (lights off at 1000h) with food and water available ad libtum. All animals were bilaterally ovariectomized under isoflurane anesthesia and allowed a 10–14 day recovery period following surgery. All procedures were approved by the University of Maryland, Baltimore Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Hormones and Methamphetamine Treatment
All injections were administered in accordance with the treatment procedures used in our previous studies (Holder et al., 2010; Holder and Mong, 2010). Forty-eight hours before the start of the experimental assay (e.g., behavioral testing or tissue collection), the animals were administered 5μg 17-β-estradiol benzoate (EB, SC; Sigma Aldrich, St. Louis, MO) followed by 10μg EB 24h later. Four hours prior to experimental assay, the rats were injected with progesterone (P, 500μg, SC; Sigma-Aldrich). During the three days of hormonal priming rats received a daily injection of METH (5mg/kg/day, IP; Sigma-Aldrich) or saline vehicle. This dose and administration protocol of METH was previously demonstrated to facilitate female sexual motivation (Holder and Mong, 2010) and behaviors (Holder et al., 2010) without causing an increase in stereotypy or general locomotion at the time point of the experimental assay (Holder et al., 2010).
Sexual Behavior
The behavioral tests were conducted under dim red light in the dark phase of the light cycle between 1300 and 1600h, approximately 4h after the last METH administration. Experimental females were placed in a 50cm × 38cm × 25cm Plexiglas observation chamber with a sexually experienced male. Each behavioral test was recorded by a video camera and was completed when 10 mounts were received or when 15 min had elapsed. During the tests, the investigator remained at a consistent location approximately 0.5 meter away from the observation chambers during all trials. An experimenter blind to the treatment groups scored the receptive and proceptive sexual behaviors as previously described (Holder et al., 2010; Holder and Mong, 2010). Briefly, the number of proceptive behaviors (hops, darts, and ear wiggling) that occurred in 10 min was quantified. Additionally, the quantitative (lordosis quotient; LQ) and qualitative (lordosis intensity score; LS) parameters of lordosis were scored as previously described (Holder et al., 2010).
Stereotaxic Surgeries Targeting Posterodorsal Medial Amygdala
Animals were placed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA) under isofluorane anesthesia, an incision was made to expose the skull, and holes were drilled at 3.1mm posterior and ± 3.7mm lateral to Bregma on the skull surface (Guarraci et al., 2004) using a dremmel drill (Dremmel, Racine, WI). Bilateral neurotoxic lesions targeting the MePD were produced with injections of 0.4μl of ibotenic acid (10.0μg/μl in phosphate-buffered saline (PBS), Sigma) using a 5μl Hamilton syringe (700 series, Hamilton, Reno, NV) lowered 9.3mm ventral from the skull surface and delivered over a 10 min period. After each injection, the syringe was left in place for a minimum of 10 min. Sham lesions were performed using the same methods, but using vehicle injections. Following surgery, animals were allowed a 10–14 day recovery period. Animals were treated with METH (sham: n=6, lesion: n=6) or saline (sham: n=6, lesion: n=6) and EB/P as described above and tested for sexual behavior. For the cannulation experiments, chronic indwelling 25-gauge guide cannulae (Plastics One, Roanoke, VA) were bilaterally implanted into the MePD (3.1mm posterior, ± 3.7mm lateral, and 8.0mm ventral from Bregma) and affixed to the skull using dental acrylic. Dummy stylets were placed in the guide cannulae in order to keep them unobstructed.
Microinfusion Experiments
Hormonally primed animals were treated as described in Table 1 or Supplemental Table 1. Animals used in the receptor experiments received daily infusions of one of the following: the dopamine D1R/D5R agonist SKF38393 (Sigma), the D2R agonist quinpirole (Sigma), the norepinephrine α1R agonist phenylephrine (Sigma) or the dopamine D1R/D5R antagonist SCH23390 (Sigma). Animals used in the PR experiment received one infusion of RU486 (Sigma) 30 min prior to progesterone injection. During the microinfusions, the dummy stylets were removed and replaced by 33 gauge microneedles that project 1.3mm below the guide cannulae and were attached via polyethylene tubing to a 25μL Hamilton syringe (700 series, Hamilton, Reno, NV) attached to a BASi Bee pump attached to a Bee Hive controller (Bioanalytical Systems, Inc., West Lafayette, IN). Infusions (0.5μL) occurred over 5 min and the injectors remained in place for an additional 5 min to ensure diffusion away from the injector tips.
Table 1.
Experimental treatment groups for the infusion experiments. Delineation of the experimental treatment groups and the number of animals used.
| Experiment | Microinfusions | Drug Treatment | ||
|---|---|---|---|---|
| Day of Infusion | D1–D3 | |||
| D1 | D2 | D3 | ||
|
| ||||
| SKF38393 | saline | saline | saline | saline (n=6) |
| Experiment | 100ng | 100ng | 100ng | saline (n=6) |
|
| ||||
| SCH23390 | saline | saline | saline | METH (5 mg/kg; n=6); saline (n=6) |
| Experiment | 100ng | 100ng | 100ng | METH (5 mg/kg; n=6); saline (n=6) |
|
| ||||
| RU486 | oil | METH (5 mg/kg; n=7); saline (n=7) | ||
| Experiment | 200ng | METH (5 mg/kg; n=7); saline (n=7) | ||
Dose response studies were conducted in order to determine the lowest effective dose of SCH23390 and SKF38393 (Supplemental Tables 1 and 2); doses were based on those that have been previously demonstrated to attenuate or facilitate lordosis behavior when infused into the third ventricle (Mani et al., 1994a). Based on the dose response results, 100ng of both SCH23390 and SKF38393 were used in the microinfusion studies. Additionally, it should be noted that RU486 also inhibits glucocorticoid receptors at higher doses than for PR; its binding affinity is higher for PRs than for glucocorticoid receptors in the rodent brain (Etgen and Barfield, 1986; Vathy et al., 1989). Numerous studies have clearly demonstrated that the antiprogestin actions of RU486 in the ventromedial nucleus of the hypothalamus (VMN) abolish the expression of female sexual behaviors (Brown and Blaustein, 1984; Brown and Blaustein, 1986; Etgen and Barfield, 1986; Mani et al., 1996; Mani et al., 1994a; Mani et al., 1994b; Vathy et al., 1987; Vathy et al., 1989). The dose used in this study is ~100-fold less than that used to block PR action in the VMN to attenuated female sexual behavior (Mani et al., 1994a; Mani et al., 1994b) so as to avoid actions at the glucocorticoid receptors.
Placement Verification
Posterodorsal Medial Amygdala Lesions
Immediately following sexual behavior testing, animals euthanized using CO2. Brains were removed and submersion-fixed in a solution of 4% paraformaldehyde and 2.5% acrolein in potassium phosphate buffered saline (kPBS; 0.5M, pH 7.4), at 4 °C, followed by cryoprotection in 30% sucrose in kPBS. After cryoprotection, the brains were frozen on dry ice and stored at -80 °C until processed for Nissl staining. Brains were sectioned (30 μm) in the coronal plane in a cryostat and stored in a cryoprotectant solution (ethylene glycol/glucose in sodium phosphate buffer) until processed for Cresyl Violet. The sections were rinsed in kPBS, washed in 0.5% hydrogen peroxide, and washed in kPBS. They were mounted serially on 2% gelatin-coated glass slides. They were rehydrated in dH2O, stained in 0.5% Cresylecht Violet (Chroma) solution containing 1M sodium acetate, and 1M acetic acid and coverslipped.
The needle placement and the extent of the lesions were mapped using the Neurolucida software (MicroBrightField, Colchester, VT), which allows for the reconstruction of an area in three-dimensional space. Slides were anatomically matched and numerically coded so that the investigator conducting analysis was blinded to the experimental group. A contour field was used to mark the extent of the lesion, based upon the absence of neurons and presence of pyknotic cells as indicated by the Cresyl Violet staining. In the sham-lesioned animals, neurons are present at the injection site (Figure 1A), while the injection sites of lesioned animals show evidence of pyknotic cells (Figure 1A). The lesion tracings for each animal at each level of Bregma indicate that the lesions were targeted to the MePD (Figure 1B). A placement was deemed appropriate when the needle track was located within sections that corresponded to plates 56–60 in a standard brain atlas and fell dorsolateral to the optic tract. The MePD corresponding to plates 56–60 were lesioned in all animals administered ibotenic acid.
Figure 1.
Effects of lesions of the posterodorsal medial nucleus of the amygdala (MePD) on female sexual behaviors. (A) Photomicrograph of a representative excitotoxic acid lesioned MePD. ot, optic tract. Scale bar: 200 μm. Arrow points to the needle marks and the injection site. Insert: 60x magnification of the MeA lesion, showing the presence of pyknotic cells (arrowhead) and the absence of neurons. (B) Photomicrograph of a representative sham lesioned MePD. ot, optic tract. Scale bar: 200 μm. Arrow points to the needle marks, denoting the injection site. Insert: 60x magnification of the MePD, showing neurons (arrow) and some microglia (arrowhead). (C) Schematic reconstruction of the lesions for the MePD. Coronal sections through the medial amygdala (2.4 – 3.6 mm posterior to bregma). Bilateral lesions from each animal mapped using a translucent grey intensity for the entire extent of the lesion. The schematics for the lesions for each animal were then overlaid, yielding an area of dark intensity, which represents common overlap among the lesioned animals. Adapted from The Rat Brain in Stereotaxic Coordinates (5th ed.) by G. Paxinos & C. Watson, 2005, San Diego, CA: Elsevier Academic Press. Copyright 2005 by Elsevier Academic Press. (D) A two-way ANOVA revealed a significant interaction of lesion and METH treatment on the proceptive behaviors. A Bonferroni post hoc comparison revealed a significant difference between sham-lesioned animals treated with METH and with saline (*p<0.05). (E) A two-way ANOVA revealed a significant main effect of METH treatment on the lordosis intensity score. A Bonferroni post hoc comparison revealed a significant difference between sham-lesioned animals treated with METH and with saline (*p<0.05). (F) There was no effect of MePD lesions or METH treatment on the lordosis quotient. Data are represented as means ± SEM (n = 6 animals in each group).
Posterodorsal Medial Amygdala Cannulations
Cannulae placements were confirmed by infusing 1% Evan’s Blue (0.5μl) into the MePD, using the same infusion protocol as above. Immediately following the Evan’s Blue infusions, animals were asphyxiated by CO2. Brains were removed and immediately flash-frozen in dry-ice-chilled isopentane and stored at -80°C until use. Starting at approximately -2.56mm from Bregma, 30μm sections were cut on a cryostat and mounted onto 2% gelatin-coated glass slides. The slides were rehydrated in dH2O and placed in 4% formalin. Following formalin-fixation, the slides were processed for Neutral Red (5g; Sigma) staining to examine cannulae placement (Figure 2A).
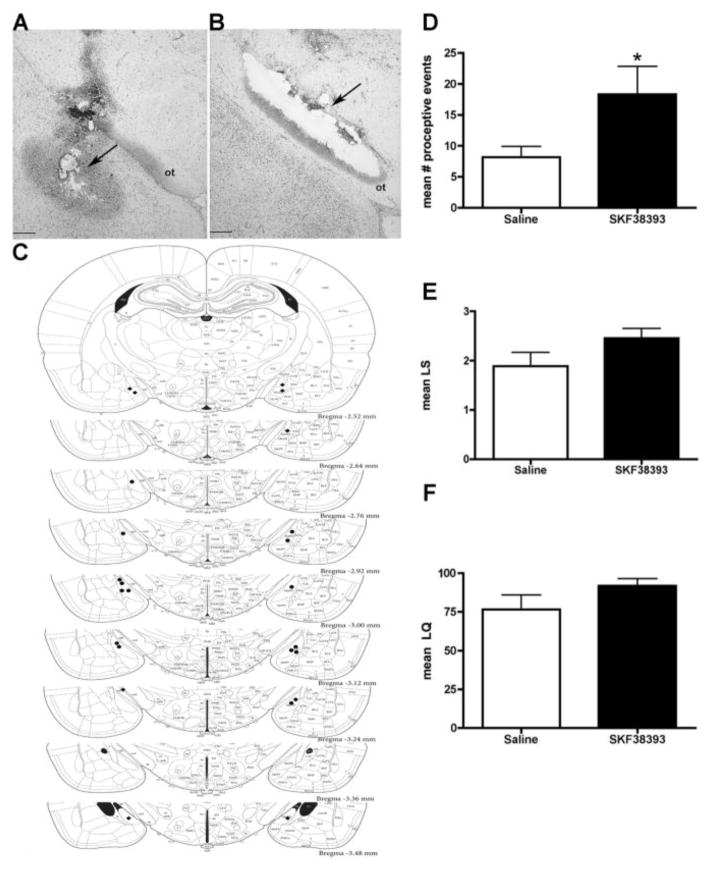
Figure 2.
Effects of a dopamine D1R agonist (SKF38393) infused into the posterodorsal medial nucleus of the amygdala (MePD) on female sexual behaviors. Cannulae implantations into the posterodorsal medial amygdala (MePD) for the dopamine agonist experiments. (A) Representative photomicrograph of a properly placed cannula, with the injection site in the MePD. ot, optic tract. Scale bar: 200 μm. Arrow points to the needle marks, denoting the injection site. (B) Representative photomicrograph of an improperly placed cannula, with the injection site outside of the MePD. ot, optic tract. Scale bar: 200 μm. Arrow points to the needle marks and the injection site. (C) Schematic reconstruction of the cannulae placements in the MePD. Coronal sections through the medial amygdala (2.6 – 3.4 mm posterior to bregma). Correctly placed bilateral cannulation sites were mapped using a black circle, while the misses were marked using a black diamond. Adapted from Paxinos & Watson (2005). SKF38393 (100 ng) infusion caused a significant increase in (D) proceptive behaviors (*p<0.05). There was no effect of SKF38393 on (E) the lordosis score or (F) the lordosis quotient. Data are represented as means ± SEM (n = 6 animals in each group).
A placement was deemed appropriate when the needle track was located within sections that corresponded to plates 56–60 in a standard brain atlas (Figure 2A) and fell dorsolateral to the optic tract (Figure 2A). Animals that did not have at least one cannula placed in the MePD were removed from the experimental group and placed within the negative control group; based on this criteria, four animals were removed in the antagonist experiments, two animals were removed in the agonist experiments, and two animals were removed from analysis in the RU486 experiments. Overall, the cannulae were placed in the MePD approximately 77% of the time. Figures 2C, 3A and 4A depict the cannulae placements in the medial amygdala for the dopamine agonists, SCH23390, and RU486 experiments, respectively.
Figure 3.
Effects of a dopamine D1R antagonist (SCH23390) infused into the posterodorsal medial nucleus of the amygdala (MePD) on female sexual behaviors. Cannulae implantations into the posterodorsal medial amygdala (MePD) for the SCH23390 experiments. (A) Schematic reconstruction of the cannulae placements in the MePD. Coronal sections through the medial amygdala (2.6 – 3.4 mm posterior to bregma). Correctly placed bilateral cannulation sites were mapped using a black circle, while the misses were marked using a black diamond. Adapted from Paxinos & Watson (2005). During the SCH23390 experiment, METH (5mg/kg/day) or saline vehicle was co-administered on each day of hormonal priming. METH treatment caused a significant increase in (D) proceptive behaviors and (E) lordosis score in animals infused with the saline vehicle (*p<0.05). Bonferroni post hoc comparisons revealed that SCH23390 prevented the METH-induced increases in both proceptive behaviors and the lordosis score (†p<0.05). (F) Animals infused with SCH23390 were highly receptive (LQ>75). Data are represented as means ± SEM (n = 6 animals in each group).
Figure 4.
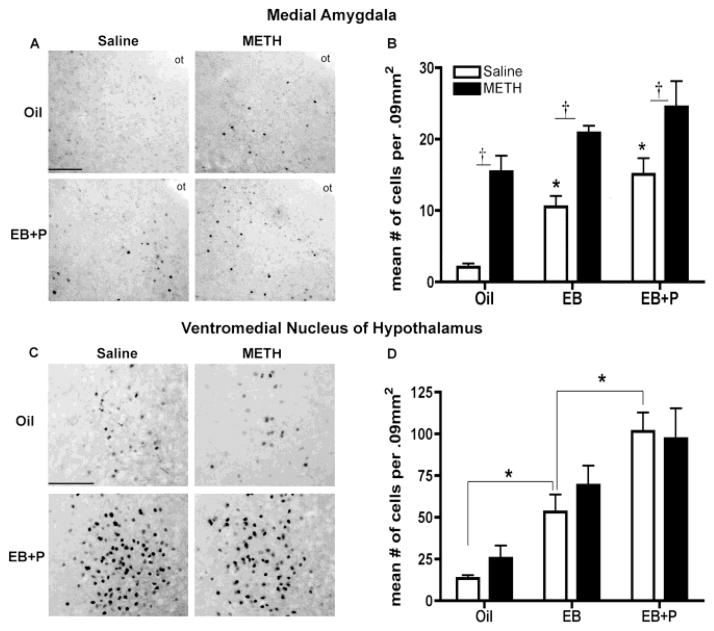
Effects of ovarian steroids and METH on progesterone receptor-immunoreactivity (PR-ir) in the (A, B) posterodorsal medial nucleus of the amygdala (MePD) and (C, D) ventromedial nucleus of the hypothalamus (VMN). (A) The photomicrographs represent the PR-ir in the MePD. ot, optic tract. Scale bar: 200 μm. (B) Quantification of PR-ir in the MePD. A two-way ANOVA revealed a significant main effect of both EB+P and METH treatment. Treatment with EB caused a significant increase PR-ir, regardless of drug treatment, compared to oil vehicle (*p<0.05). There was no significant difference between EB alone and EB+P, regardless of drug treatment. For each hormonal group, METH treatment caused a significant increase in PR-ir compared to the respective saline group (†p<0.05). (C) The photomicrographs represent the PR-ir in the VMN. Scale bar: 200 μm. (D) Quantification of PR-ir in the VMN. A two-way ANOVA revealed a significant main effect of EB+P. Treatment with EB caused a significant increase PR-ir, regardless of drug treatment, compared to oil vehicle (*p<0.05). There was also a significant difference between EB alone and EB+P. Data are represented as means ± SEM (n=6–8 per group).
Progesterone Receptor Immunoreactivity and Quantification
A separate cohort of animals treated as described in Table 2 was used to determine the effects of METH on PR expression. The animals did not undergo behavioral testing; rather, 4h after the last injection, they were transcardially perfused under ketamine anesthesia (65mg/kg, IP) with 0.9% saline containing sodium nitrate, followed by 4% paraformaldehyde and 2.5% acrolein in 0.5M kPBS. Following perfusion, the brains were extracted, post-fixed, cryoprotected, frozen and processed for immunocytochemistry as described above. Cohorts containing sections from all treatment groups were rinsed in kPBS, incubated with 0.1M Glycine, 0.5% sodium borohydride, with rinses of kPBS between incubations. Sections were then incubated in 1% Trition X-100 in kPBS and in a blocking solution containing 0.5% Trition X-100, 5% Normal Goat Serum, and 1% hydrogen peroxide in kPBS. Sections were incubated for 48 h at 4 °C with a mouse monoclonal antibody targeted to the N-terminal region of the progesterone receptor (Cat No. 18-0172 Invitrogen, Carlsbad, CA) at a dilution of 1:15000 in 0.05% Triton X-100 and 1% BSA in kPBS. After primary incubation the sections were incubated in biotinylated secondary antibody (goat anti-mouse; Vector Laboratories, Burlingame, CA), an avidin-biotin horseradish-peroxidase complex (Vectastain ABC, Elite Kit; Vector Laboratories), and visualized using nickel sulfate (25mg/ml)-enhanced 3,3′-diaminobenzidine tetrahydrochloride (0.2mg/ml; Polysciences Warrington, PA, USA) using standard techniques (Holder et al., 2010). After visualization, the sections were mounted serially on 2% gelatin-coated glass slides and coverslipped. The number of PR-positive cells was counted with the aid of the Neurolucida software (MicroBrightField, Colchester, VT) using laboratory standard methods in accordance with previously defined parameters for the MePD and VMN (Holder et al., 2010). Briefly, a standardized contour was used to demarcate the counting areas. Slides were anatomically matched and numerically coded so that the investigator conducting analysis was blinded to the experimental group. Sections containing the MeA and VMN were analyzed. Three brain sections (in series) separated by 120 μm were used. In the event that three sections from the appropriate brain region could not be obtained, the animals was excluded from that region’s analysis. The placement and size of counting contours were in accordance with previously defined parameters (Holder et al., 2010). Both sides of the bilateral nuclei were included in the analysis, resulting in six counting contours per region. From these six contours, an average PR-positive cell number per section for each region was derived.
Table 2.
Experimental treatment groups from the progesterone receptor immunocytochemistry. Delineation of the experimental treatment group and the number of animals used.
| Hormone Administration (0900h) | Drug Treatment | ||
|---|---|---|---|
|
| |||
| D10 | D11 | D12 | D10–D12 |
| Oil | Oil | Oil | METH (5mg/kg; n=7) or saline (n=7) |
| EB (5μg) | EB (10μg) | Oil | METH (5 mg/kg; n=8) or saline (n=8) |
| EB (5μg) | EB (10μg) | P (500μg) | METH (5 mg/kg; n=8) or saline (n=6) |
Statistical Analyses
Results are expressed as means ± SEM. The distribution of data did not deviate significantly from normality. One-way ANOVAs followed by Newman-Keuls post hoc tests were used to analyze the dose response experiments. Two-way ANOVAs followed by Bonferroni t-test post hoc comparisons were used to analyze all data unless otherwise noted. Student’s t-tests were used to analyze the agonist experiments. All statistical tests were conducted using the Graph Pad Prism program (San Diego, CA) on a Macintosh Duo-Core computer. Effect size estimates were calculated using eta squared (η2) for ANOVAs (Levine and Hullett, 2002). Cohen’s d values were also calculated to determine effect sizes for the standardized mean differences in pair-wise comparisons (Berben et al., 2012).
Results
Female sexual behavior following MePD lesions
The aim of this first experiment was to investigate the role of the MePD in the METH-induced enhancement of sexual behavior in hormonally primed, ovariectomized female Sprague-Dawley rats. Infusions of ibotenic acid led to lesions of the MePD as indicated by the absence of neurons and the presence of pyknotic cells compared to the sterile PBS vehicle infusions (Figure 1A). The MePD was at least partially lesioned for every animal given ibotenic acid, with an area of common overlap occurring at Bregma -2.76 to -3.24mm without extending beyond the optic tract or amygdala (Figure 1B).
Motivational components of female sexual behavior are indicated by proceptive behaviors (hops, darts, and ear wiggles) that solicit the male’s attention. Quantification of proceptive events revealed a significant interaction between METH treatment and MePD lesions [F(1,20) = 10.76, p=0.0037, η2 = 0.28; Figure 1C]. As previously reported, treatment of METH increased the number of proceptive events displayed by animals receiving sham lesions by 1.9-fold (Holder et al., 2010), compared to saline treated controls (p=0.0025, d = 0.91; Figure 1C), and this increase was blocked in the lesion animals. Animals receiving MePD lesions displayed levels of proceptivity similar to the sham-saline control animals (Figure 1C). Sexual behavior in the female rats also has a reflexive or receptive component known as lordosis, a reflexive dorsoflexion of the spine. As with proceptive behavior, METH treatment resulted in higher LS in the sham-lesioned animals, compared to saline [F(1,20) = 8.99, p=0.0071, η2 = 0.27; Figure 1D], but there was no difference in LS among the MePD-lesioned animals given either METH or saline (p=0.48, d = -0.28). The MePD lesions did not decrease the receptivity of these animals as measured by the LQ [F(1,20) = 0.20, p=0.66, η2 = 0.009; Figure 1E]. Taken together, these data show that the MePD is required for the METH-induced enhancement of female sexual behavior but not the baseline receptor or proceptive behaviors.
Sexual behavior following catecholaminergic receptor agonist infusions
The medial amygdala is reported to have moderate amounts of the D1R versus undetectable amounts of the D2R subtypes (Huang et al., 1992; Mansour et al., 1990; Meador-Woodruff and Mansour, 1991; Meador-Woodruff et al., 1989; Meador-Woodruff et al., 1992; Meador-Woodruff et al., 1991; Weiner et al., 1991). Alternatively, the MePD contains a moderately high concentration of α1R (Day et al., 1997). As activation of D1R (Mani et al., 1996; Mani et al., 1994a; Meredith et al., 1997; Meredith et al., 1998) and α1R (Chu and Etgen, 1999; Chu et al., 1999; Etgen, 1990; Gonzalez-Flores et al., 2007; Gonzalez-Flores et al., 2004) are implicated in the ligand-independent phosphorylation and activation PR, we determined whether the activation of D1R, D2R, and/or α1R in the MePD was sufficient to enhance proceptive behaviors.
Animals that received the infusion of SKF38393, a D1R agonist (100ng), displayed a 2.4-fold increase in proceptive behavior, compared to the saline-treated controls. [t(10) = 2.12, p=0.03, d=0.89; Figure 2D]. This increase in proceptivity is similar to that displayed following METH treatment. Administration of SKF38393 increased the LS [t(10) = 1.67, p=0.06, d=0.96; Figure 2E] and the LQ [t(10) = 1.49, p=0.08, d=0.86; Figure 2F], but these increases did not reach statistical significance. There was no effect of the administration of quinpirole (100ng), a D2R agonist, on proceptive behaviors [t(10) = 0.27, p=0.79, d=0.21; Supplemental Figure 1A], LS [t(10) = 0.66, p=0.54, d=0.50; Supplemental Figure 1B] or the LQ [t(10) = 0.20, p=0.85, d=0.65; Supplemental Figure 1C]. The selected dose has previously been shown to facilitate solicitation behaviors when infused into the mPOA (Graham and Pfaus, 2010). Additionally there was no effect of the administration of phenylephrine (50ng), an α1R agonist, on proceptive behaviors [t(10) = 0.37, p=0.36, d=0.24; Supplemental Figure 1D], LS [t(10) = 0.72, p=0.25, d=0.16; Supplemental Figure 1E] or the LQ [t(10) = 0.39, p=0.29, d=0.39; Supplemental Figure 1F]. The selected dose has previously been shown to facilitate lordosis behaviors in infused into the VMN (Kow et al., 1992). Taken together, these data show that the activation of the D1R family of dopamine receptors in MePD is able to enhance proceptive sexual behavior.
Sexual behavior following D1R dopaminergic receptor antagonist infusions
Next, we tested the effect of the D1R antagonist SCH23390 in the presence of METH. Blocking the D1R subtype with SCH23390 (100ng) prevented the METH-induced increases in proceptive behaviors [F (1,18) = 7.93, p=0.012, η2 = 0.23]. As previously reported (Holder et al., 2010; Holder and Mong, 2010), treatment with METH caused a 1.76-fold increase in the number of proceptive events displayed by animals receiving vehicle infusions (p=0.0034, d=1.017; Figure 3B), compared to saline-treated controls. In contrast, in animals receiving infusions of SCH23390, there was no difference in the number of proceptive events displayed by those receiving METH and those receiving saline (p=0.42, d=0.29; Figure 3C). There was an interaction between METH treatment and SCH23390 administration on LS [F (1,18) = 9.17, p=0.0072, η2 = 0.24; Figure 3C]. In animals given METH, SCH23390 infusion led to a decrease in the LS compared to vehicle infusion (p=0.0062, d=1.85). There was also a significant interaction between SCH23390 and METH treatments on LQ [F (1,18) = 5.31, p=0.033, η2 = 0.19; Figure 3D], with SCH23390 infusion decreasing the LQ of animals given METH (p=0.012, d=0.40), compared to those receiving vehicle infusion. However, these animals were still considered highly receptive as the LQ was greater than 75 (86.65 ± 8.179). Taken together, these data indicate that the activation of the D1R family of dopamine receptors in MePD is necessary for the METH-induced increases in female sexual motivation.
Progesterone receptor expression in the MePD
D1R activation can also increase expression of PR (Olesen and Auger, 2008; Olesen et al., 2007; Olesen et al., 2005); therefore, we next investigated whether METH induced PR expression in the MePD in the presence or absence of estradiol/progesterone. Administration of both EB+P and METH increased the expression of PRs in the MePD. A very low level of PR-immunoreactivity was detected in the oil-saline control animals (Figure 4A, B). As expected, treatment with ovarian steroids- either EB alone or EB+P- increased the number of PR-positive cells in the MePD compared to the oil-saline controls [F(2,38) = 15.17 p<0.0001, η2 = 0.26; Figure 3A, B]. Interestingly, treatment with METH caused a significant increase in the number of PR-positive cells in the MePD of animals treated with oil vehicle, compared to the oil-saline controls [F(1,38) = 46.43, p<0.0001, η2 = 0.40; Figure 3A, B]. Animals treated with the combination of METH and either EB (p=0.027, d=1.46) or EB+P (p=0.0038, d=1.072) showed a 2- or 1.6-fold increase in PR-immunoreactivity, compared to their respective saline controls (Figure 4A, B). These data indicate that METH administration induces the expression PRs in the MePD, suggesting that METH enhances progesterone signaling to facilitate female sexual motivation and behavior.
Progesterone receptor expression in the VMN
While the MePD has been implicated in the modulation of female sexual motivation and behavior, the VMN is absolutely essential for the expression of female sexual behavior. Therefore, the expression of PRs was also examined in the VMN. As expected, animals treated with saline and either EB alone or EB+P [F(2,38) = 25.53, p<0.0001, η2 = 0.56] increased the number of PR-positive cells in the VMN, compared to the oil-saline controls (Figure 4C, D). Additionally, in animals treated with saline, there is a significant increase in the expression of PR following treatment of EB and P, compared to EB alone (p=0.016, d = 0.40; Figure 4C, D). However, there was no effect of METH treatment on the PR-immunoreactivity in the VMN, irrespective of the hormone treatment. These data indicate that METH does not affect PR signaling the VMN, further suggesting that the site of hormone-drug interaction is not the VMN in the METH-induced enhancement of female sexual behavior.
Sexual behavior following progesterone receptor antagonist infusions
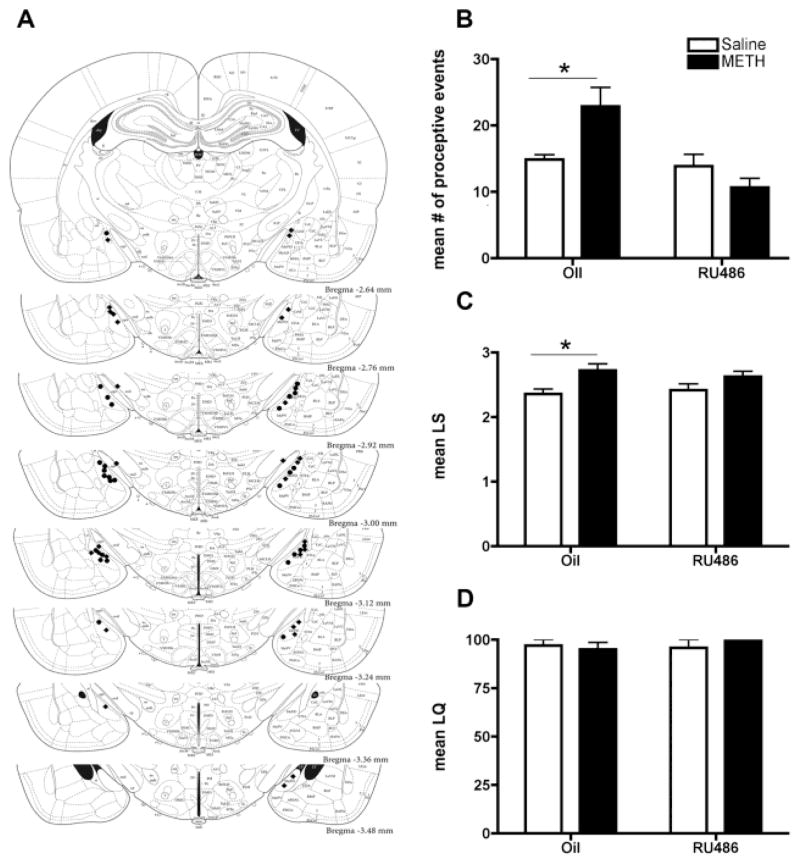
As progesterone is necessary for the METH-induced enhancement of female sexual behavior (Holder et al., 2010), we next undertook experiments to examine whether PR signaling in the MePD is necessary for METH’s effects. RU486, a PR antagonist, was infused into the MePD 30 min prior to progesterone administration on the day of behavior. Antagonism of PR prevented the METH-induced enhancements of sexual motivation as indicated by the proceptive behaviors [F(1,22) = 8.14, p=0.0074, η2 = 0.19; Figure 5B]. As previously reported, treatment with METH increased the number of proceptive events displayed by animals receiving vehicle control infusions by 1.53-fold (Holder et al., 2010), compared to saline treated controls (p=0.0028, d=1.009; Figure 5B). In contrast, in the animals receiving microinfusions of RU486, there was no difference in the number of proceptive events displayed by those receiving METH (p=0.26, d= 0.47; Figure 5B) and those receiving saline. The animals receiving RU486 displayed levels of proceptivity similar to those of the control animals (p=0.76, d=0.11; Figure 5B). As with the proceptive behavior, METH treatment resulted in higher LS in the vehicle-treated animals (Figure 5C), compared to saline [F(1,22) = 8.91, p=0.0066, η2 = 0.28], but there was no difference in LS among the RU486-infused animals given either METH or saline. The RU486 treatment did not decrease the receptivity of these animals as measured by the LQ (p=0.77, d=0.04; Figure 5D). Taken together, these data demonstrate that the activation of PRs in the MePD is necessary for the METH-induced enhancement of female sexual behavior. Additionally, the blockage of the PRs in the MePD did not abolish baseline female receptive or proceptive behaviors, indicating that while the PRs in the VMN are required for female sexual behavior, PRs in the MePD may play a modulatory role.
Figure 5.
Effects of progesterone antagonist infused into the posterodorsal medial nucleus of the amygdala (MePD) on female sexual behaviors. (A) Cannulae implantations into the posterodorsal medial amygdala (MePD) in RU486 experimental rats. Rats were ovariectomized and underwent stereotaxic surgery and cannulae were bilaterally implanted into the MePD, and allowed to recover for 7–10 days before sexual behavior testing. Schematic reconstruction of the cannulae placements in the MePD. Coronal sections through the medial amygdala (2.6 – 3.4 mm posterior to bregma). Bilateral cannulation sites for animals infused with RU486 were mapped using a black circle, while the animals receiving oil vehicle are marked using a black diamond. Adapted from Paxinos & Watson (2005). A two-way ANOVA revealed a significant interaction of RU486 and METH treatment on the (B) proceptive behaviors and (C) lordosis score. Bonferroni post hoc comparisons revealed a significant difference between oil-infused animals treated with METH and with saline on both proceptive behaviors and the lordosis score (*p<0.05). There was no effect of RU486 or METH treatment on (D) the lordosis quotient. Data are represented as means ± SEM (n = 7 animals in each group).
Discussion
The mechanism by which METH facilitates female sexual behavior has been unexplored in either humans or rodent models. The current study presents evidence to support a novel mechanism underlying METH facilitation of female sexual behavior in rats. The findings support a necessary role for the MePD. Mechanistically, within the MePD, METH exposure leads to signaling via the D1R system that is necessary and sufficient for the augmented female sexual behavior. Additionally, this augmentation also requires activation of PR in the MePD. Perhaps more intriguing is that METH alone increases PR expression in the MePD, suggesting a feed-forward mechanism that enhances PR signaling. What remains to be answered is whether D1R activation is responsible for enhanced PR signaling. Taken together, these studies demonstrate that, within the MePD, dopamine and progesterone are key mediators of the drug-sex interaction.
Previous studies in the cats, rats, and deer mice have implicated the MePD as a key site for the modulation and enhancement of female sexual motivation (Masco and Carrer, 1980; Masco and Carrer, 1984). The MePD processes and integrates sexually relevant signals such as chemosensory (Keller et al., 2009) and somatosensory signals (Erskine, 1993; Pfaus, 1993; Rowe and Erskine, 1993; Tetel et al., 1993; Veening and Coolen, 1998; Wersinger et al., 1993), suggesting that MePD may act as a decision-making node in the expression of motivated sexual behaviors. Here, we demonstrated that MePD is necessary for the METH-induced enhancement of proceptive behaviors, but not for the display of baseline sexual behavior. Coupled with our previous finding that combined administration of METH and ovarian hormones increases the number of Fos-positive cells in the MePD over either one alone in sexually naïve rats (Holder et al., 2010), these studies indicate that a greater than baseline activation of the MePD is involved in the enhancement of sexual motivation. In support, Pfaus and colleagues identified a naturally occurring variant in Long Evans rats in which females exhibit “super-solicitational” behaviors that are marked by increased proceptive events and mounting displays (Afonso et al., 2009; Afonso and Pfaus, 2006). These super-solicitational rats have a 2.5-fold increase in Fos-positive cells in the MePD compared to normal rats (Afonso et al., 2009), and MePD lesions abolish the expression of the mounting displays and proceptive behaviors (Afonso et al., 2009). It is unclear if the increased activation of the MePD in the super-solicitational rats is involved in the initiation of the motivation or if it is a response to sexual behavior. Additionally, the finding that MePD lesions do not effect baseline motivated behaviors such as paced mating (Guarraci et al., 2004) further supports a role for the MePD in mediating “enhanced” sexual behaviors.
The role of dopamine in female sexual behavior has been primarily investigated in the VMN, a critical output nuclei for female sexual behavior (Daniels et al., 1999; Pfaff and Sakuma, 1979; Polston et al., 2001). In the VMN, D1R activation facilitates sexual receptivity (Mani et al., 1994a), presumably by increasing the phosphorylation and transcriptional activation of PRs (Ahlenius, 1993; Apostolakis et al., 1996; Auger et al., 1997; Bai et al., 1997; Denner et al., 1990; Mani et al., 1996; Mani et al., 1994a; Meredith et al., 1997; Power et al., 1991). METH increases the extracellular concentration of catecholamines, particularly dopamine, by a reversal of the reuptake transporter that ultimately spills the presynaptic dopamine contents into the synapse (Fleckenstein et al., 2000; Fukui et al., 2003). In the MePD, but not the VMN, treatment with EB+P increases protein levels of tyrosine hydroxylase, the rate-limiting enzyme in dopamine production (Holder et al., 2010), suggesting that in the presence of METH there is increased dopamine available to enhance signaling in the MePD.
Unlike the VMN, the role of dopamine signaling in the MePD on female sex behavior has not been well elucidated. The current findings indicate that dopamine acting via D1R is both sufficient to increase in proceptive behavior and necessary for the METH-induced increase in sexual behavior. To our knowledge, this is the first demonstration that D1R activation in the MePD enhances proceptive behaviors. Moreover, the ability of the D1R antagonist, SCH23390, to block increased sexual behavior by METH, but not baseline sex behaviors when infused into the MePD supports the possibility that enhanced dopamine signaling underlies METH facilitation of female sexual behavior. Interestingly, the administration of SCH23390 decreased the measures of lordosis, both the LS and the LQ, but the administration of the D1R agonist, SKF38393, did not significantly increase these measures in manner similar to METH. In this study, METH was administered via intraperitoneal injection whereas SKF38393 was infused into the MePD; thus, the possibility exists that METH was more efficacious than the D1R agonist in increasing the LS and LQ because of potential actions throughout the brain and via different neurotransmitter systems. In fact, while this study presents data supporting the hypothesis that dopamine mediates the enhancements of female sexual behaviors by METH, these findings do not exclude the possibility that other noradrenergic receptors play a role in the METH-enhancement of sexual behaviors.
It has long been known that progesterone acting via its cognate receptor is required to induce solicitation and proceptive behaviors in the female rat (Blaustein, 2008; Erskine, 1989; Whalen, 1974). Moreover, progesterone is necessary for METH facilitation of female sexual behavior, and the combination of progesterone and METH has a synergistic effect on proceptive behaviors (Holder et al., 2010). Our current findings provide evidence that PRs in the MePD are involved in the METH-induced augmentation of proceptive behaviors. First, the combination of METH and EB+P increased the number of PR immunoreactive cells to levels greater than either one alone, suggesting that enhanced PR signaling in the MePD may underlie the facilitation of proceptive behaviors. Indeed, a positive correlation has been reported between PR expression in the VMN and degree proceptive behaviors; female rats that display more proceptive behaviors have a higher induction of PR in the VMN following estradiol-treatment (Sakhai et al., 2011). Second, administration of RU486 into the MePD prevented the enhancement of female sexual behavior by METH without affecting basal proceptive or receptive behaviors, indicating that METH interacts with ovarian steroids at the level of the PR. Finally, the finding that METH increases PR protein in the MePD but not the VMN suggests a specific site of action. However, the direct investigation of the VMN’s role in METH facilitated female sexual behavior is severely limited by the critical role of the VMN in the expression of baseline sexual behavior.
While induction of PR expression primarily depends upon estradiol binding to its cognate receptors (Delville and Blaustein, 1991; Parsons et al., 1982; Romano et al., 1989), METH in the absence of estradiol increased PR-ir in the MePD, suggesting that METH is acting independently from estradiol when both are administered together. Dopamine has been reported to increase PR protein expression in the hypothalamus and amygdala in the absence of estradiol (Olesen et al., 2007; Olesen et al., 2005). In the amygdala of juvenile rats, changes in PR expression are a consequence of ligand-independent activation of the estrogen receptor by dopamine (Olesen et al., 2007; Olesen et al., 2005). Thus, a high likelihood exists that the METH-induced increases in PR are the result of ligand-independent activation of the estrogen receptor.
In contrast to the generally accepted actions of progesterone administration resulting in a down-regulation of its receptor (Auger et al., 2000; Blaustein and Turcotte, 1990; Parsons et al., 1981), we found that in the VMN, but not in the MePD, progesterone administration increased PR over that of estradiol treatment. One possible explanation for this finding may be a function of the dose and time course of progesterone administration used. In the VMN of female rats and guinea pigs, down-regulation of PRs by exogenous progesterone was observed 12 h after administration but not earlier (Auger et al., 2000; Blaustein and Turcotte, 1990). The current study quantified PR-ir 4 h after progesterone administration. Additionally, increased antigenicity of the PR based on the change in location or stabilization of PR following binding of progesterone (Isola, 1987; Perrot-Applanat et al., 1986; West et al., 1987) may have contributed to our observed increase in PR-ir following progesterone. Indeed, in the VMN of guinea pigs, increases in PR-ir following progesterone administrations have been attributed to changes in the antigenicity of PR (DonCarlos et al., 1989).
Overall, these data suggest that METH may modulate female sexual behaviors via a feed-forward mechanism in the MePD whereby ovarian steroids increase dopamine levels via increases in TH expression. Following release by METH, the increased dopamine contributes to increased PR expression that ultimately leads to enhanced PR signaling underlying the facilitation of female sexual behaviors and, especially, proceptive behaviors.
While the current study focuses on female sexual behaviors, recent studies in male rats show that repeated METH treatment leads to compulsive sexual behavior (Frohmader et al., 2010) and an increase in sexual reward, as measured by conditioned placed preference (Frohmader et al., 2011). Taken together with the current set of studies, our data demonstrate that the rodent model can be used to further examine the molecular and neurobiological mechanisms of METH’s effects on sexual behaviors. Furthermore, a better understanding of the neurobiology that links sexual motivation and METH use may elucidate a novel foundation for the biological basis of drug use that is sex-specific and lead to the development of more effective therapeutic strategies to reduce high-risk sexual behaviors.
Supplementary Material
Highlights.
A mechanism for enhanced proceptivity by methamphetamine is proposed
The role of the posterodorsal medial amygdala is examined
Dopamine receptors are necessary and sufficient for the enhanced proceptivity
Methamphetamine increases progestin receptors in medial amygdala
Progestin receptors are necessary for the enhanced proceptivity
Acknowledgments
This research was supported by NIH grant DA024943 awarded to Mary K. Holder and by NIH grant R01DA030517 awarded to Jessica A. Mong. NIDA played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The authors thank Margaret M. McCarthy for her comments on a previous version of the manuscript. The data presented herein were previously published in abstract form.
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afonso VM, et al. Estrogen and the neural mediation of female-male mounting in the rat. Behav Neurosci. 2009;123:369–81. doi: 10.1037/a0014121. [DOI] [PubMed] [Google Scholar]
- Afonso VM, Pfaus JG. Hormonal and experiential control of female-male mounting in the female rat. Horm Behav. 2006;49:30–37. doi: 10.1016/j.yhbeh.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Ahlenius S. Brain monoaminergic neurotransmission in the mediation of lordosis behavior in the female rat. Neurosci Biobehav Rev. 1993;17:43–9. doi: 10.1016/s0149-7634(05)80229-5. [DOI] [PubMed] [Google Scholar]
- Apostolakis EM, et al. Dopaminergic regulation of progesterone receptors: Brain D5 dopamine receptors mediate induction of lordosis by D1-like agonists in rats. J Neurosci. 1996;16:4823–4834. doi: 10.1523/JNEUROSCI.16-16-04823.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger AP, et al. Progesterone, but not progesterone-independent activation of progestin receptors by a mating stimulus, rapidly decreases progestin receptor immunoreactivity in female rat brain. Horm Behav. 2000;37:135–144. doi: 10.1006/hbeh.1999.1565. [DOI] [PubMed] [Google Scholar]
- Auger AP, et al. Progesterone-independent activation of rat brain progestin receptors by reproductive stimuli. Endocrinology. 1997;138:511–514. doi: 10.1210/endo.138.1.4986. [DOI] [PubMed] [Google Scholar]
- Bai W, et al. Differential phosphorylation of chicken progesterone receptor in hormone-dependent and ligand-independent activation. J Biol Chem. 1997;272:10457–10463. doi: 10.1074/jbc.272.16.10457. [DOI] [PubMed] [Google Scholar]
- Beach FA. Importance of progesterone to induction of sexual receptivity in spayed female rats. Proc Soc Exp Biol Med. 1942;51:369–371. [Google Scholar]
- Berben L, et al. Effect size estimation: methods and examples. Int J Nurs Stud. 2012;49:1039–47. doi: 10.1016/j.ijnurstu.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. Neuroendocrine regulation of feminine sexual behavior: lessons from rodent models and thoughts about humans. Ann Rev Psych. 2008;59:93–118. doi: 10.1146/annurev.psych.59.103006.093556. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Turcotte JC. Down-regulation of progestin receptors in guinea pig brain: new findings using an immunocytochemical technique. J Neurobiol. 1990;21:675–85. doi: 10.1002/neu.480210502. [DOI] [PubMed] [Google Scholar]
- Brown TJ, Blaustein JD. Inhibition of sexual behavior in female guinea pigs by a progestin receptor antagonist. Brain Res. 1984;301:343–9. doi: 10.1016/0006-8993(84)91103-x. [DOI] [PubMed] [Google Scholar]
- Brown TJ, Blaustein JD. Abbreviation of the period of sexual behavior in female guinea pigs by the progesterone antagonist RU 486. Brain Res. 1986;373:103–13. doi: 10.1016/0006-8993(86)90320-3. [DOI] [PubMed] [Google Scholar]
- Chu H-P, Etgen AM. Ovarian hormone dependence of α1-adrenoceptor activation of the nitric oxide-cGMP pathway: Revelance for hormonal facilitation of lordosis behavior. J Neurosci. 1999;19:7191–7197. doi: 10.1523/JNEUROSCI.19-16-07191.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H-P, et al. Cyclic GMP may potentiate lordosis behaviour by progesterone receptor activation. J Neuroendocrinol. 1999;11:107–113. doi: 10.1046/j.1365-2826.1999.00298.x. [DOI] [PubMed] [Google Scholar]
- Corsi KF, Booth RE. HIV sex risk behaviors among heterosexual methamphetamine users: literature review from 2000 to present. Curr Drug Abuse Rev. 2008;1:292–6. doi: 10.2174/1874473710801030292. [DOI] [PubMed] [Google Scholar]
- Daniels D, et al. Central neuronal circuit innervating the lordosis-producing muscles defined by transneuronal transport of pseudorabies virus. J Neurosci. 1999;19:2823–33. doi: 10.1523/JNEUROSCI.19-07-02823.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, et al. Distribution of alpha 1a-, alpha 1b- and alpha 1d-adrenergic receptor mRNA in the rat brain and spinal cord. J Chem Neuroanat. 1997;13:115–39. doi: 10.1016/s0891-0618(97)00042-2. [DOI] [PubMed] [Google Scholar]
- Delville Y, Blaustein JD. A site for estradiol priming of progesterone-facilitated sexual receptivity in the ventrolateral hypothalamus of female guinea pigs. Brain Res. 1991;559:191–9. doi: 10.1016/0006-8993(91)90002-d. [DOI] [PubMed] [Google Scholar]
- Denner LA, et al. Regulation of progesterone receptor-mediated transcription by phosphorylation. Science. 1990;250:1740–1743. doi: 10.1126/science.2176746. [DOI] [PubMed] [Google Scholar]
- DonCarlos LL, et al. Estrogen plus progesterone increases progestin receptor immunoreactivity in the brain of ovariectomized guinea pigs. Neuroendocrinology. 1989;50:613–23. doi: 10.1159/000125290. [DOI] [PubMed] [Google Scholar]
- Erskine MS. Solicitation behavior in the estrous female rat: A review. Horm Behav. 1989;23:473–502. doi: 10.1016/0018-506x(89)90037-8. [DOI] [PubMed] [Google Scholar]
- Erskine MS. Mating-induced increases in FOS protein in preoptic area and medial amygdala of cycling female rats. Brain Res Bull. 1993;32:447–451. doi: 10.1016/0361-9230(93)90289-n. [DOI] [PubMed] [Google Scholar]
- Etgen AM. Intrahypothalamic implants of noradrenergic antagonists disrupt lordosis behavior in female rats. Physiol Behav. 1990;48:31–6. doi: 10.1016/0031-9384(90)90256-4. [DOI] [PubMed] [Google Scholar]
- Etgen AM, Barfield RJ. Antagonism of female sexual behavior with intracerebral implants of antiprogestin RU 38486: correlation with binding to neural progestin receptors. Endocrinology. 1986;119:1610–7. doi: 10.1210/endo-119-4-1610. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, et al. Differential effects of stimulants on monoaminergic transporters: Pharmacological consequences and implication for neurotoxicy. Eur J Pharmacol. 2000;406:1–13. doi: 10.1016/s0014-2999(00)00639-7. [DOI] [PubMed] [Google Scholar]
- Frohmader KS, et al. Effects of methamphetamine on sexual performance and compulsive sex behavior in male rats. Psychopharmacology (Berl) 2010;212:93–104. doi: 10.1007/s00213-010-1930-8. [DOI] [PubMed] [Google Scholar]
- Frohmader KS, et al. Concurrent exposure to methamphetamine and sexual behavior enhances subsequent drug reward and causes compulsive sexual behavior in male rats. J Neurosci. 2011;31:16473–82. doi: 10.1523/JNEUROSCI.4013-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui R, et al. Effect of methylphenidate on dopamine/DARPP signalling in adult, but not young, mice. J Neurochem. 2003;87:1391–401. doi: 10.1046/j.1471-4159.2003.02101.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Flores O, et al. Facilitation of estrous behavior by vaginal cervical stimulation in female rats involves alpha1-adrenergic receptor activation of the nitric oxide pathway. Behav Brain Res. 2007;176:237–43. doi: 10.1016/j.bbr.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Flores O, et al. Regulation of lordosis by cyclic 3′,5′-guanosine monophosphate, progesterone, and its 5alpha-reduced metabolites involves mitogen-activated protein kinase. Endocrinology. 2004;145:5560–7. doi: 10.1210/en.2004-0823. [DOI] [PubMed] [Google Scholar]
- Graham MD, Pfaus JG. Differential regulation of female sexual behaviour by dopamine agonists in the medial preoptic area. Pharmacol Biochem Behav. 2010;97:284–92. doi: 10.1016/j.pbb.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Guarraci FA. “Sex, drugs and the brain”: The interaction between drugs of abuse and sexual behavior in the female rat. Horm Behav. 2010;58:138–48. doi: 10.1016/j.yhbeh.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, et al. Paced mating behavior in the female rat following lesions of three regions responsive to vaginocervical stimulation. Brain Res. 2004;999:40–52. doi: 10.1016/j.brainres.2003.10.056. [DOI] [PubMed] [Google Scholar]
- Holder MK, et al. Methamphetamine facilitates female sexual behavior and enhances neuronal activation in the medial amygdala and ventromedial nucleus of the hypothalamus. Psychoneuroendocrinology. 2010;35:197–208. doi: 10.1016/j.psyneuen.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder MK, Mong JA. Methamphetamine enhances paced mating behaviors and neuroplasticity in the medial amygdala of female rats. Horm Behav. 2010;58:519–525. doi: 10.1016/j.yhbeh.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, et al. Immunohistochemical localization of the D1 dopamine receptor in rat brain reveals its axonal transport, pre- and postsynaptic localization, and prevalence in the basal ganglia, limbic system, and thalamic reticular nucleus. Proc Natl Acad Sci U S A. 1992;89:11988–11992. doi: 10.1073/pnas.89.24.11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isola JJ. The effect of progesterone on the localization of progesterone receptors in the nuclei of chick oviduct cells. Cell Tissue Res. 1987;249:317–23. doi: 10.1007/BF00215514. [DOI] [PubMed] [Google Scholar]
- Keller M, et al. The main and the accessory olfactory systems interact in the control of mate recognition and sexual behavior. Behav Brain Res. 2009;200:268–76. doi: 10.1016/j.bbr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Kow LM, et al. Alpha 1-adrenergic agonists act on the ventromedial hypothalamus to cause neuronal excitation and lordosis facilitation: electrophysiological and behavioral evidence. Brain Res. 1992;588:237–45. doi: 10.1016/0006-8993(92)91581-x. [DOI] [PubMed] [Google Scholar]
- Levine TR, Hullett CR. Eta squared, partial eta squared, and misreporting of effect size in communication research. Hum Commun Res. 2002;28:612–625. [Google Scholar]
- Mani SK, et al. Dopamine requires the unoccupied progesterone receptor to induce sexual behavior in mice. Mol Endocrinol. 1996;10:1728–1737. doi: 10.1210/mend.10.12.8961281. [DOI] [PubMed] [Google Scholar]
- Mani SK, et al. Convergent pathways for steroid hormone-and neurotransmitter-induced rat sexual behavior. Science. 1994a;265:1246–1249. doi: 10.1126/science.7915049. [DOI] [PubMed] [Google Scholar]
- Mani SK, et al. Inhibition of rat sexual behavior by antisense oligonucleotides to the progesterone receptor. Endocrinology. 1994b;135:1409–1414. doi: 10.1210/endo.135.4.7925102. [DOI] [PubMed] [Google Scholar]
- Mansergh G, et al. CDC consultation on methamphetamine use and sexual risk behavior for HIV/STD infection: Summary and suggestions. Public Health Rep. 2006;121:127–132. doi: 10.1177/003335490612100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, et al. Localization of dopamine D2 receptor mRNA and D1 and D2 receptor binding in the rat brain and pituitary: an in situ hybridization-receptor autoradiographic analysis. J Neurosci. 1990;10:2587–600. doi: 10.1523/JNEUROSCI.10-08-02587.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masco DH, Carrer HF. Sexual receptivity in female rats after lesion or stimulation in different amygdaloid nuclei. Physiol Behav. 1980;24:1073–80. doi: 10.1016/0031-9384(80)90050-5. [DOI] [PubMed] [Google Scholar]
- Masco DH, Carrer HF. Pathways conducting amygdaloid influence on feminine sexual behavior in the rat. Behav Brain Res. 1984;11:205–212. doi: 10.1016/0166-4328(84)90212-2. [DOI] [PubMed] [Google Scholar]
- McEwen BS, et al. Hormonal control of sexual behavior in the female rat: Molecular, cellular and neurochemical studies. Biol Reprod. 1987;36:37–45. doi: 10.1095/biolreprod36.1.37. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Mansour A. A. E. Bennett Award paper. Expression of the dopamine D2 receptor gene in brain. Biol Psychiatry. 1991;30:985–1007. doi: 10.1016/0006-3223(91)90120-b. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, et al. Distribution of D2 dopamine receptor mRNA in rat brain. Proc Natl Acad Sci U S A. 1989;86:7625–8. doi: 10.1073/pnas.86.19.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador-Woodruff JH, et al. Distribution of D5 dopamine receptor mRNA in rat brain. Neurosci Lett. 1992;145:209–12. doi: 10.1016/0304-3940(92)90024-2. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, et al. Comparison of the distributions of D1 and D2 dopamine receptor mRNAs in rat brain. Neuropsychopharmacology. 1991;5:231–42. [PubMed] [Google Scholar]
- Meredith JM, et al. D1 dopamine receptor agonist (SKF-38393) induction of Fos immunoreactivity in progestin receptor-containing areas of female rat brain. J Neuroendocrinol. 1997;9:385–394. doi: 10.1046/j.1365-2826.1997.00594.x. [DOI] [PubMed] [Google Scholar]
- Meredith JM, et al. Mating-related stimulation induces phosphorylation of dopamine-and cyclic AMP-regulated phosphoprotein-32 in progestin receptor-containing areas in the female brain. J Neurosci. 1998;18:10189–10195. doi: 10.1523/JNEUROSCI.18-23-10189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen KM, Auger AP. Dopaminergic activation of estrogen receptors induces fos expression within restricted regions of the neonatal female rat brain. PLoS One. 2008;3:e2177. doi: 10.1371/journal.pone.0002177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen KM, et al. Regulation of progestin receptor expression in the developing rat brain by a dopamine d1 receptor antagonist. J Neuroendocrinol. 2007;19:481–8. doi: 10.1111/j.1365-2826.2007.01554.x. [DOI] [PubMed] [Google Scholar]
- Olesen KM, et al. Dopaminergic activation of estrogen receptors in neonatal brain alters progestin receptor expression and juvenile social play behavior. Endocrinology. 2005;146:3705–12. doi: 10.1210/en.2005-0498. [DOI] [PubMed] [Google Scholar]
- Parsons B, et al. Sequential inhibition of progesterone: effects on sexual receptivity and associated changes in brain cytosol progestin binding in the female rat. Brain Res. 1981;221:149–60. doi: 10.1016/0006-8993(81)91069-6. [DOI] [PubMed] [Google Scholar]
- Parsons B, et al. Progestin receptor levels in rat hypothalamic and limbic nuclei. J Neurosci. 1982;2:1446–52. doi: 10.1523/JNEUROSCI.02-10-01446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot-Applanat M, et al. Ultrastructural localization of the progesterone receptor by an immunogold method: effect of hormone administration. J Cell Biol. 1986;102:1191–9. doi: 10.1083/jcb.102.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y. Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:189–202. [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, et al. Cellular and molecular mechanisms of female reproductive behaviors. In: Knobil E, et al., editors. The Physiology of Reproduction. Raven; New York: 1994. pp. 107–197. [Google Scholar]
- Pfaus JG. Sexual stimulation activates c-fos within estrogen-concentrating regions of the female rat forebrain. Brain Res. 1993;624:253–267. doi: 10.1016/0006-8993(93)90085-2. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, et al. What Can Animal Models Tell Us About Human Sexual Response? Annu Rev Sex Res. 2003;14:1–63. [PubMed] [Google Scholar]
- Polston EK, et al. NMDA-mediated activation of the medial amygdala initiates a downstream neuroendocrine memory responsible for pseudopregnancy in the female rat. J Neurosci. 2001;21:4104–4110. doi: 10.1523/JNEUROSCI.21-11-04104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power RF, et al. Dopaminergic and ligand-independent activation of steroid hormone receptors. Science. 1991;254:1636–1639. doi: 10.1126/science.1749936. [DOI] [PubMed] [Google Scholar]
- Rawson RA, et al. Drugs and sexual effects: Role of drug type and gender. J Subst Abuse Treat. 2002;22:103–108. doi: 10.1016/s0740-5472(01)00215-x. [DOI] [PubMed] [Google Scholar]
- Romano GJ, et al. Expression and estrogen regulation of progesterone receptor mRNA in neurons of the mediobasal hypothalamus: an in situ hybridization study. Mol Endocrinol. 1989;3:1295–300. doi: 10.1210/mend-3-8-1295. [DOI] [PubMed] [Google Scholar]
- Rowe DW, Erskine MS. c-Fos proto-oncogene activity induced by mating in the preoptic area, hypothalamus and amygdala in the female rat: role of afferent input via the pelvic nerve. Brain Res. 1993;621:25–34. doi: 10.1016/0006-8993(93)90294-w. [DOI] [PubMed] [Google Scholar]
- Sakhai SA, et al. Maternal programming of sexual attractivity in female Long Evans rats. Psychoneuroendocrinology. 2011;36:1217–25. doi: 10.1016/j.psyneuen.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple SJ, et al. Female methamphetamine users: Social characteristics and sexual risk behavior. Women Health. 2004a;40:35–50. doi: 10.1300/j013v40n03_03. [DOI] [PubMed] [Google Scholar]
- Semple SJ, et al. The context of sexual risk behavior among heterosexual methamphetamine users. Addict Behav. 2004b;29:807–10. doi: 10.1016/j.addbeh.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Sulzer D, et al. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–33. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Tetel MJ, et al. Fos expression in the rat brain following vaginal-cervical stimulation by mating and manual probing. J Neuroendocrinol. 1993;5:397–404. doi: 10.1111/j.1365-2826.1993.tb00500.x. [DOI] [PubMed] [Google Scholar]
- Vathy IU, et al. Actions of progestins on estrous behaviour in female rats. Physiol Behav. 1987;40:591–5. doi: 10.1016/0031-9384(87)90102-8. [DOI] [PubMed] [Google Scholar]
- Vathy IU, et al. Actions of RU 38486 on progesterone facilitation and sequential inhibition of rat estrous behavior: correlation with neural progestin receptor levels. Horm Behav. 1989;23:43–56. doi: 10.1016/0018-506x(89)90073-1. [DOI] [PubMed] [Google Scholar]
- Veening JG, Coolen LM. Neural activation following sexual behavior in the male and female rat brain. Behav Brain Res. 1998;92:181–93. doi: 10.1016/s0166-4328(97)00190-3. [DOI] [PubMed] [Google Scholar]
- Weiner DM, et al. D1 and D2 dopamine receptor mRNA in rat brain. Proc Natl Acad Sci U S A. 1991;88:1859–63. doi: 10.1073/pnas.88.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger SR, et al. Mating-induced FOS-like immunoreactivity in the rat forebrain: a sex comparison and a dimorphic effect of pelvic nerve transection. J Neuroendocrinol. 1993;5:557–68. doi: 10.1111/j.1365-2826.1993.tb00522.x. [DOI] [PubMed] [Google Scholar]
- West NB, et al. Immunocytochemistry versus binding assays of the estrogen receptor in the reproductive tract of spayed and hormone-treated macaques. Endocrinology. 1987;121:1789–800. doi: 10.1210/endo-121-5-1789. [DOI] [PubMed] [Google Scholar]
- Whalen RE. Estrogen-progesterone induction of mating behavior in female rats. Horm Behav. 1974;5:157–162. doi: 10.1016/0018-506x(74)90040-3. [DOI] [PubMed] [Google Scholar]
- Winland C, et al. Methamphetamine enhances sexual behavior in female rats. Pharmacol Biochem Behav. 2011;98:575–582. doi: 10.1016/j.pbb.2011.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.