Abstract
Background
Chronic rotator cuff tears are a common source of shoulder pain and disability, and patients with chronic cuff tears often have substantial weakness, fibrosis, inflammation and fat accumulation. Identifying therapies to prevent the development of these pathologies will likely have a positive impact on clinical outcomes. Simvastatin is a drug with demonstrated anti-inflammatory and anti-fibrotic effects in many tissues, but had not previously been studied in the context of rotator cuff tears. We hypothesized that following the induction of a massive supraspinatus tear, simvastatin would protect muscles from a loss of force production and fibrosis.
Methods
We measured changes in muscle fiber contractility, histology and biochemical markers of fibrosis and fatty infiltration in rats that received a full-thickness supraspinatus tear and were treated with either carrier alone or simvastatin.
Results
Compared to vehicle treated controls, simvastatin did not have an appreciable effect on muscle fiber size, but treatment did increase muscle fiber specific force by 20%. Simvastatin also reduced collagen accumulation by 50%, but did not effect triglyceride content of muscles. Several favorable changes in the expression of genes and other markers of inflammation, fibrosis and regeneration were also observed.
Conclusions
Simvastatin partially protected muscles from the weakness that occurs as a result of chronic rotator cuff tear. Fibrosis was also markedly reduced in simvastatin treated animals. While further studies are necessary, statin medication could potentially help to improve outcomes for patients with rotator cuff tears.
Keywords: rotator cuff, fatty degeneration, muscle atrophy, statin, myosteatosis, fibrosis, HMG-CoA reductase inhibitor
Introduction
Tears to the rotator cuff tears are among the most common and devastating upper extremity injuries, with over a quarter of a million surgical repairs performed in the US each year8. The ability to successfully repair the torn cuff and promote the return of patients to normal strength and function is often complicated by fibrosis, atrophy and fatty infiltration of the rotator cuff muscles5. These changes, termed "myosteatosis" or "fatty degeneration", increase with time and are a limiting factor for adequate repair as well as post-operative rehabilitation and recovery13; 25. The extent of fatty degeneration can be quantified with magnetic resonance imaging (MRI) or computed tomography (CT) imaging techniques, and there is a positive correlation between the amount of fatty degeneration present in a muscle and poor functional outcomes, as well as an increased risk for structural failure after repair14. Therapies that reverse or halt the progression of fatty degeneration may therefore lead to an improvement in function and greater patient satisfaction following rotator cuff tear.
Hydroxy-methyl-glutaryl (HMG) coenzyme A (CoA) reductase inhibitors, or "statins", are among the most frequently prescribed medications in the US31. These medications are most commonly used in the treatment of hypercholesterolemia, as they are very effective at lowering low-density lipoprotein cholesterol and improving clinical outcomes of patients with coronary artery disease and other cardiovascular conditions6; 31. In addition to promoting cardiovascular disease, hypercholesterolemia is associated with a greater risk for rotator cuff tendon tear and impaired tendon-bone regeneration1; 4. Aside from their efficacy in treating hypercholesterolemia, there are emerging roles for statins in the treatment of inflammatory diseases6; 31. Statins work by inhibiting the activity of the HMG-CoA reductase enzyme, which catalyzes the conversion of HMG-CoA into mevalonate, which is a precursor for cholesterol and other isoprenoids that either directly or indirectly activate pro-inflammatory signaling pathways6. Numerous studies have identified the ability of statins to prevent fibrosis and inflammation in several diseased or injured tissues, including the heart, blood vessels, lungs, kidneys, skin and articular cartilage2; 6; 23; 32. To our knowledge, the ability of statins to prevent fibrosis, atrophy, inflammation and fat accumulation in skeletal muscle tissue, and specifically the rotator cuff, has not been explored to-date.
As therapeutic interventions to prevent muscle scar tissue formation and inflammation may enhance the treatment of chronic rotator cuff disease, our objective was to evaluate the ability of a commonly used statin medication, simvastatin (Zocor), to prevent atrophy and fibrosis following rotator cuff tear. We hypothesized that, following an induction of a massive supraspinatus tear, simvastatin would enhance muscle fiber force production, and prevent fibrosis and fat accumulation. To test this hypothesis, we used a well-described rat model of full64 thickness chronic rotator cuff tear16; 26; 36, treated rats with either vehicle or simvastatin, and measured changes in muscle fiber type and contractility, and biochemical and molecular markers of fibrosis and fatty degeneration 28 days after induction of tear.
Methods
Animals and Surgical Procedures
This study used 6-month old male retired breeder Sprague-Dawley rats and was approved by the University of Michigan IACUC (protocol 10500). Animals were housed in specific pathogen free conditions and randomly assigned to either the control group (N=8 rats) or the simvastatin treatment group (N=8 rats). A bilateral full thickness supraspinatus tenectomy was performed in each rat as previously described.16; 17 Rats were anesthetized with 2% isoflurane, placed in a lateral decubitus position and the skin above the shoulder was shaved and scrubbed with chlorhexidine gluconate. A deltoid splitting transacromial approach was used to visualize the supraspinatus tendon, which was then clamped and sharply detached from its insertion on the humerus. A full-thickness incision was made just distal to the myotendinous junction, and the tendon was removed to prevent healing and scarring into the surrounding connective tissue. A splash block of 1% lidocaine was administered for analgesia, and the deltoid was closed using 4-0 chromic gut (Johnson & Johnson, New Brunswick, NJ, USA). The skin was closed using a running subcutaneous suture of 5-0 vicryl (Johnson & Johnson) that was reinforced with GLUture (Abbott Labs, Abbott Park, IL, USA). Rats also received subcutaneous buprenorphine (0.05 mg/kg) as analgesia postoperatively. After 28 days of recovery, the animals were anesthetized with sodium penobarbital (50 mg/kg) and the supraspinatus muscles on both sides were harvested and weighed. The distal ends of all muscles were mobile and showed no sign of scar or lateral adhesions of the muscle. The rats were then humanely euthanized by overdose of sodium penobarbital which was followed by creation of a bilateral pnemothorax. The left supraspinatus from each rat was used for histology and single fiber contractility, and the right supraspinatus was finely minced and used for gene expression and biochemical analysis.
Simvastatin Administration
Pharmaceutical grade simvastatin tablets (80 mg tablets, Cadila Pharmaceuticals, Ahmedabad, India) were finely ground with a mortar and pestle, and extensively mixed with vehicle (1% hydroxypropyl methyl cellulose, HPMC) fresh daily. Rats received once daily simvastatin at a dose of 20mg/kg or vehicle (1% HPMC) administered via oral gavage. This dosage was selected based on results from previous studies.2; 39 Treatment began two hours before the surgery to induce rotator cuff tear, and continued each day until the rats were euthanized.
Muscle Fiber Contractility
The proximal portion of the left supraspinatus muscle was used for muscle fiber contractility analysis. Tissue was prepared, and the cross-sectional area (CSA), maximum isometric force (Fo) and specific force (sFo, which is calculated by dividing Fo by CSA) was determined as described at a sarcomere length of 2.5µm16; 17; 27. Ten to twelve type II fibers were tested from each supraspinatus muscle.
Histology
Histology was performed as previously described16; 17. The distal portion of the left supraspinatus muscle was placed in Tissue-Tek (Sakura, Torrance, CA, USA) and frozen in isopentane cooled to approximately −160°C. Muscles were sectioned at a thickness of 10µm and labeled with monoclonal antibodies against myosin heavy chain (Developmental Studies Hybridoma Bank, Iowa City, IA, USA). Primary antibodies were detected with AlexaFluor conjugated secondary antibodies (Invitrogen, Grand Island, NY, USA), and the extracellular matrix (ECM) was identified with wheat germ agglutinin (WGA) lectin conjugated to AlexaFluor 488 (Invitrogen). High resolution images of slides were obtained using a Axioplan 2 (Zeiss, Jena, Germany) microscope equipped with AxioCam (Zeiss) cameras. Quantitative histomorphometry was performed using ImageJ (NIH, Bethesda, MD, USA).
Gene Expression
RNA isolation and gene expression was performed as previously described.16; 17 RNA was isolated from right supraspinatus muscles using a miRNeasy kit (Qiagen, Valencia, CA, USA) and treated with DNAse I (Qiagen) to eliminate genomic DNA. RNA was reverse transcribed using the RT2 First strand kit (Qiagen) and cDNA was amplified in a CFX96 real time thermal cycler (Bio-Rad, Hercules, CA, USA) using RT2 SYBR Green qPCR mix (Qiagen) and primers for specific mRNA species (Qiagen). Expression of mRNA transcripts (Supplementary Table 1) was normalized to the stable housekeeping gene β-actin, and the simvastatin samples were further normalized to the control samples using the 2ΔCt approach35.
Lipid Analysis
Muscle tissue was weighed, homogenized and suspended in a 0.9% NaCl solution at a concentration of 20µg/mL. Lipid was extracted according to the methods of Bligh and Dyer7 in a 2:2:1.8 chloroform:methanol:aqueous mixture. Samples were then stored in 500µL chloroform and spotted on 10 × 10cm silica HPTLC plates (EMD Millipore, San Diego, CA, USA). Plates were developed in a 60:30:5 chloroform:methanol:water solution to separate phospholipids, and then dried and further developed in a 80:20:1.5 hexane:diethyl ether:acetic acid solution to separate apolar lipids. To visualize lipid species, plates were stained rhodamine 6G (Sigma, Saint Louis, MO) and imaged in a ChemiDoc XRS system (Bio-Rad, Hercules, CA, USA). Standard of known lipid species were used as an internal control across different plates. Densitometry of triglyceride and phospholipid bands was performed using ImageJ.
MMP Activity Assay
MMP activity was measured in samples using a SensoLyte Colorimetric Assay kit (AnaSpec, Fremont, CA, USA) using techniques modified from Kumar24. Fifty milligrams of minced muscle was homogenized and sonicated in 1mL of ice cold T-PER (Pierce, Rockford, IL, USA), which was subsequently centrifuged at 12,000 × g for 10 minutes. The supernatant was collected, and the concentration of protein in samples was measured using aBCA assay (Pierce). Fifty micrograms of protein was loaded into a 96 well plate and incubated with the MMP chromogenic substrate for one hour at 37°C. The absorbance of samples was then measured at 412 nm in a SpectraMax microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Hydroxyproline Assay
Hydroxyproline is an amino acid that makes up approximately 14% of the dry mass of fibrillar collagens and is commonly used as a marker of the collagen content of tissues30. Measurements of hydroxyproline was performed as previously described28. Briefly, 25 mg portions of finely minced supraspinatus muscles were desiccated for 4 hours at 90°C, and the dry mass of samples was then recorded. Samples were then digested into free amino acids in 6.0N HCl overnight at 110°C, and neutralized in an equal volume of 6.0N NaOH. The hydroxyproline content was then determined using a colorimetric assay41 that was measured in a SpectraMax microplate reader (Molecular Devices) and normalized to the dry mass of the muscle tissue.
Statistical Analysis
Data is presented as mean±SD. Differences between vehicle-treated control samples and simvastatin-treated samples were tested using unpaired t-tests (α=0.05) in Prism 6.0 (GraphPad, La Jolla, CA, USA).
Results
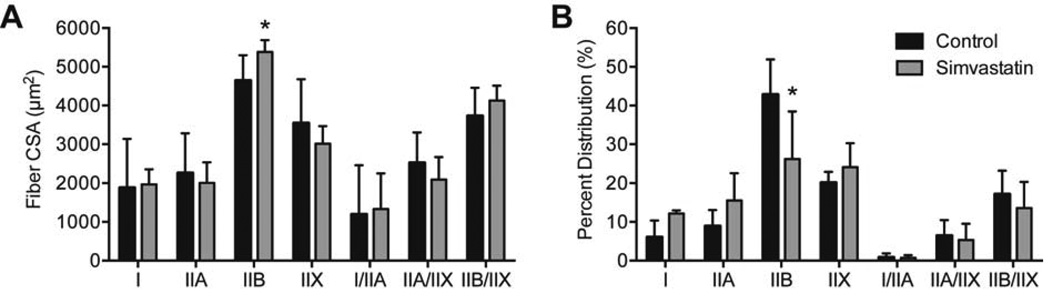
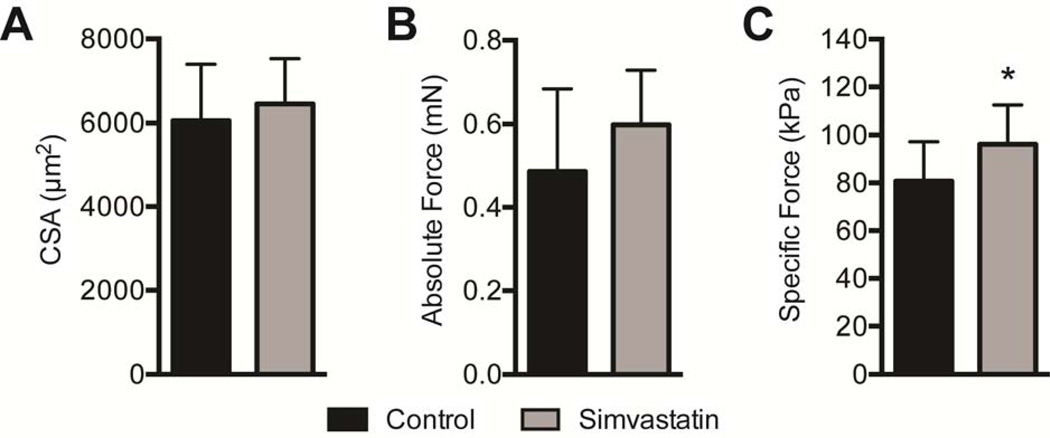
Twenty eight days after inducing a tear, no differences in body mass (643±84.8g for control rats and 673±69.2g for simvastatin rats, P=0.22) nor wet mass of supraspinatus muscles (402±41.4mg for control rats and 444±120mg for simvastatin rats, P=0.19) were observed. The cross-sectional area (CSA) of muscle fibers was generally similar, with a slight increase in the size of pathological type IIB muscle fibers in simvastatin treated rats (P=0.04, Figure 1A), although the percentage of type IIB fibers decreased by 38% (P=0.02, Figure 1B). For muscle fiber contractility, no differences in fiber CSA (P=0.27, Figure 2A) or Fo were observed (P=0.10, Figure 2B), but simvastatin treatment resulted in an approximately 20% increase in sFo compared to controls (P=0.04, Figure 2C).
Figure 1.
Muscle fiber size and percent myosin heavy chain isoform composition. (A) Cross sectional area (CSA) and (B) percent distribution of myosin heavy chain isoform of muscle fibers from control and simvastatin treated rotator cuff muscles. Values shown are mean±SD. N=8 muscles from each group. *, significantly different from control group (P<0.05).
Figure 2.
Permeabilized muscle fiber contractile measurements. (A) Permeabilized muscle fiber cross sectional area (CSA), (B) absolute maximum isometric force (Fo) and (C) specific force sFo) of control and simvastatin treated rotator cuff muscles. Values shown are mean±SD. N=8 muscles from each group. *, significantly different from control group (P<0.05).
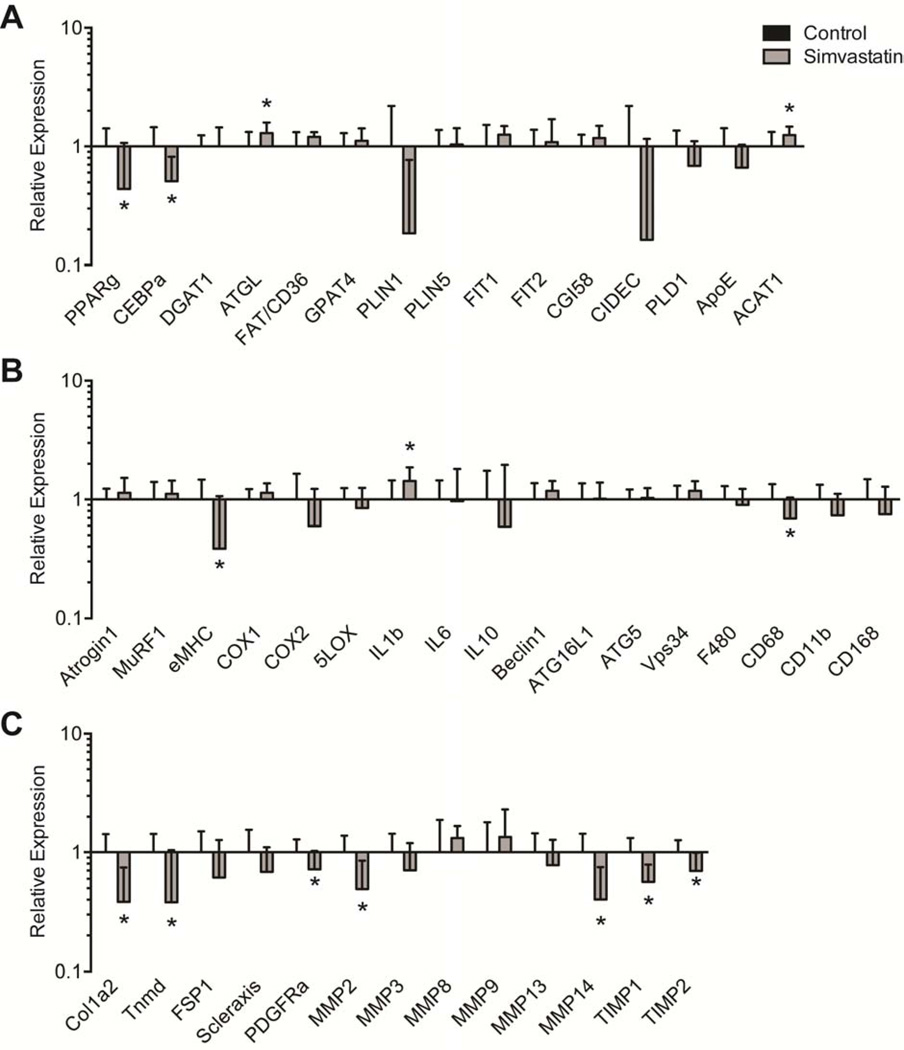
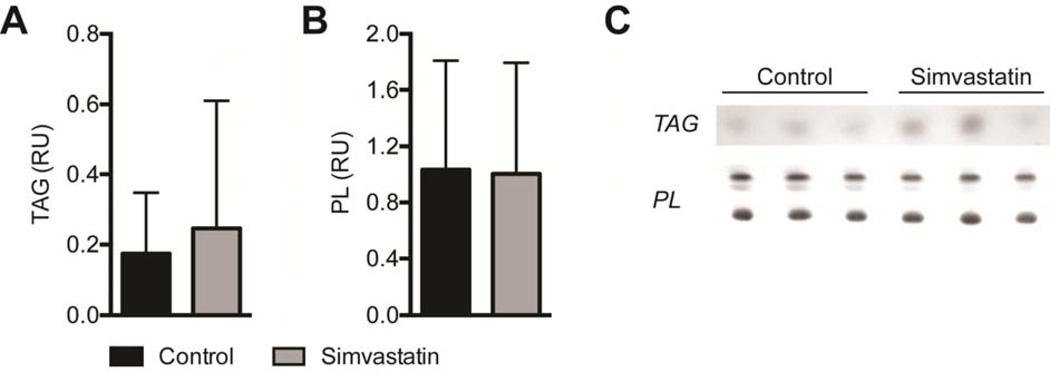
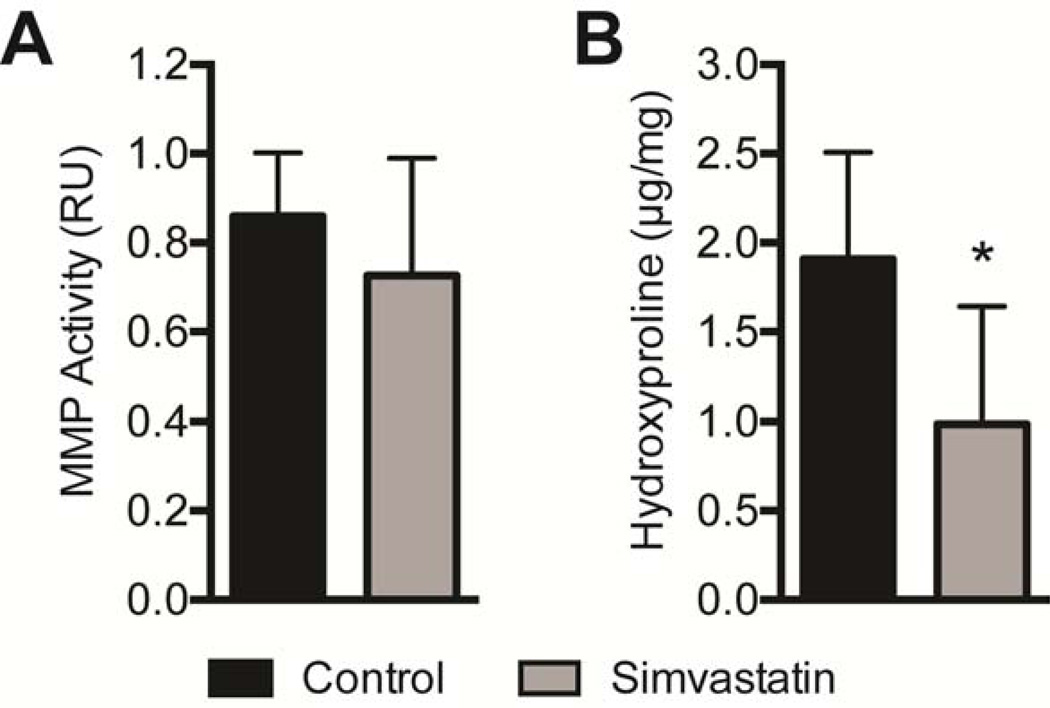
Differences in molecular and biochemical markers of fatty degeneration and fibrosis were then measured. For genes related to adipogenesis and lipid accumulation, simvastatin treatment reduced the expression of PPAR-γ (PPARg, P=0.03) and c/EBP-α (CEBPa, P=0.01), and also increased the expression of ACAT1 (P=0.04, Figure 3A). Although there was a downregulation in PPAR-γ and c/EBP-α, no differences in total triglyceride content were observed (P=0.32, Figure 4A). Phospholipids, which are lipid species that are mainly found in the plasma membranes and were therefore not anticipated to change based on the drug treatment, were present in similar levels between control and treatment groups (P=0.47, Figure 4B). Simvastatin also substantially reduced the expression of the early muscle regeneration marker embryonic myosin heavy chain (eMHC, P=0.03) and decreased the expression of the M1 macrophage marker CD68 (P=0.04), with a modest increase in the expression of the pro-inflammatory cytokine IL-1β (IL1B, P=0.03, Figure 3B). Simvastatin treatment reduced the expression of most ECM synthesis, fibrosis and fibroblast proliferation genes measured (Figure 3C), including type Icollagen (Col1a2, P=0.01), tenomodulin (Tnmd, P=0.02), PDGFR-α (PDGFRa, P=0.04), MMP-2 (P=0.01), MMP-14 (P=0.01), TIMP-1 (P=0.01) and TIMP-2 (P=0.02). While MMP and TIMP expression levels changed, on the whole no change in overall MMP activity was detected using a broad-spectrum MMP assay (P=0.11, Figure 5A). However, consistent with the decrease in type I collagen gene expression, there was a nearly 50% decrease in hydroxyproline content of simvastatin treated muscles (P=0.01, Figure 5B).
Figure 3.
Gene expression. Expression of genes associated with (A) adipogenesis and lipid storage, (B) atrophy, inflammation and autophagy, and (C) extracellular matrix synthesis and fibrosis. The expression of each gene was normalized to β-actin, and then further normalized to the control group. Values are mean±SD. N=8 supraspinatus muscles from each group. *, significantly different from control group (P<0.05).
Figure 4.
Lipid content. The content of (A) triglycerides (TAG) and (B) phospholipids (PL) in control and simvastatin treated muscles. Values are expressed as relative units (RU) of pixel density. (C) Representative rhodamine 6G stained TLC plates. Values for A and B are mean±SE. N=7 muscles for controls and N=8 muscles for simvastatin. No significant differences were found from control group (P<0.05).
Figure 5.
MMP Activity and Hydroxyproline Content. (A) MMP proteolytic activity expressed as relative units of absorbance (RU), and (B) hydroxyproline content (µg of hydroxyproline per mg dry muscle mass) of control and simvastatin treated rotator cuff muscles. Values aremean±SD. N=8 supraspinatus muscles from each group. *, significantly different from control group (P<0.05).
Discussion
Statin medications are commonly used for the treatment of hypercholesterolemia, and emerging studies have suggested that statins can be efficacious in the treatment of chronic inflammatory conditions and in the acceleration of wound healing2; 6; 23; 32. The current study is the first to-date to evaluate the use of statin medication in the prevention of myosteatosis following rotator cuff tear. We hypothesized that simvastatin would enhance muscle fiber force production, and prevent fibrosis and fat accumulation after rotator cuff tear. The combined results from this study partially support our hypothesis, in that simvastatin protected against a loss in muscle fiber sFo production and markedly reduced the accumulation of ECM after chronic rotator cuff tear; however, no impact of simvastatin on total triglyceride levels was observed.
Muscle weakness is a common complaint for patients with chronic rotator cuff tears5. In rats, there is a reduction in both Fo and sFo of infraspinatus muscle fibers one month after rotator cuff tear16; 17. In the current study, both groups had sFo values lower than the values for healthy, adult rats which is around 130 kPa16. However, simvastatin treatment increased sFo by approximately 20% over controls. The accretion of type IIB muscle fibers is a common indicator of chronic muscle injuries or diseases34, and type IIB muscle fibers accumulate after chronic rotator cuff tear16. While simvastatin treatment slightly increased the size of type IIB fibers, it also dramatically decreased the percentage of type IIB fibers after chronic tear. No other changes in fiber CSA or fiber type distribution were noted. Consistent with these findings, no differences in the muscle-specific E3 ubiquitin ligases atrogin-1 and MuRF-1, which are the major rate limiting steps in skeletal muscle protein degradation18; 33, and no changes in the expression of autophagy related genes beclin 1, ATG16L1, ATG5 or Vps34, were observed. While simvastatin treatment resulted in a slight increase in IL-1β expression, no differences in the expression of other pro-inflammatory genes such as COX-1, COX-2, 5-LOX or IL-6 were noted. Simvastatin, however, did decreased the expression of eMHC, suggesting an acceleration of regeneration after tear. These results suggest that simvastatin is able to enhance sFo production after chronic rotator cuff tear, likely through an acceleration of regeneration as opposed to an inhibition of inflammation.
Patients with chronic rotator cuff tears often have substantial fibrosis37, and this accumulation of fibrotic connective tissue is believed to decrease the elasticity and reparability of chronically torn rotator cuff muscles12. Fibroblasts are thought to be the predominant cell type in muscle that secretes type I collagen9, and we therefore evaluated changes in markers of fibroblasts and their precursors. While no differences in the expression of the fibroblast markers FSP-1 or scleraxis were observed, we did note a robust decrease in the fibroblast proliferation marker10 tenomodulin, as well as a slight decrease in the expression of the fibroblast precursor marker21 PDGFR-α. To quantitatively measure ECM abundance, we used hydroxyproline as a marker for fibrillar collagen content, and observed a nearly two-fold reduction in hydroxyproline with simvastatin treatment. Consistent with this finding, there was a decrease in type I collagen expression of similar magnitude. In addition to directly downregulating type I collagen expression29, simvastatin can also indirectly regulate the ECM content of tissues by modulating MMP expression and activity levels. Aktas and colleagues2 reported that simvastatin reduced the levels of MMP-3 in articular chondrocytes after induction of an ACL tear, and Yao42 reported simvastatin decreased MMP-9 expression in the vasculature of rats with experimentally induced pulmonary hypertension. While we anticipated a similar finding, no differences in MMP-3 or MMP-9 expression were observed in the current study. As we did observe changes in MMP-2 and MMP-14 expression, along with changes in the expression of TIMP-1 and TIMP-2 which regulate the activity of MMP enzymes9, we performed a MMP assay to evaluate functional changes in MMP activity. No differences were observed in functional MMP activity between control and simvastatin treated animals. Together, these results suggest that simvastatin is able to reduce fibrosis after rotator cuff tear primarily through the downregulation of type I collagen production.
An accumulation of ectopic lipid is also commonly observed after rotator cuff tear15. PPAR-γ and c/EBP-α are two transcription factors with well established roles in promoting adipogenesis40, and our group and others have observed increases in PPAR-γ and c/EBP-α expression after rotator cuff tear11; 16; 17; 20; 22. In the current study, a downregulation in PPAR-γ and c/EBP-α expression was observed in the simvastatin treated group. While we did observe slight increases in other markers of lipid accumulation such as ATGL and ACAT1, other markers of lipid synthesis and storage such as DGAT1, CD36, GPAT4, perilipin-1, perilipin-5, FIT-1, FIT-2, CGI58, CIDEC, PLD1 and ApoE were not different between the two groups. Despite the changes in the expression of genes related to adipogenesis and lipid storage, surprisingly no differences in total triglyceride levels were observed. These results indicate that, although some molecular markers of fatty infiltration were different, simvastatin treatment did not have an effect on the fat content of torn rotator cuff muscles.
While the current study identified a positive role for simvastatin in the prevention of muscle weakness and fibrosis, statin medication can also have a detrimental effect on skeletal muscle function. Between 5% and 10% of patients who take statins develop a myopathy, and a small number of these patients will go on to develop frank rhabdomyolysis38. These conditions are thought to be brought on by a decrease in cholesterol levels, as a marked reduction in cholesterol can destabilize muscle fiber plasma membranes38. Another mechanism of statin induced myopathy involves a dramatic upregulation in atrogin-1 expression, which then triggers widespread proteolysis in the muscle19. Based on the results of Hanai,19 we anticipated a potential upregulation in atrogin-1 in simvastatin treated muscles in the current study, but found no difference. The discrepancies between our two studies may be the choice of statin medication, as Hanai used pravastatin (Pravachol) in their work.19 Indeed, while statin medications are often grouped together, they do have somewhat different mechanisms of action and phamacokinetics, and appear to have different safety profiles38. There also appears to be genetic variation in the way in which different statins are metabolized by different patients, and different medical co morbidities also appear to influence statin sensitivity, which may further explain the non-uniform response of patients to statin therapy.
There are several limitations to this study. Although the rat is widely utilized as an animal model for the study of cuff tears, rats do not develop the severity of fatty degeneration that is observed in humans. We did not measure the contractility of type I muscle fibers due to their relatively low abundance. We only utilized a single time point to evaluate early chronic changes in torn cuff muscles, and did not evaluate acute or long-term changes in muscle function and morphology. The effect of simvastatin on pre-existing chronic rotator cuff tears was not studied, and the ability of simvastatin to reverse fatty degeneration that was already present was not determined. While we measured the expression of numerous mRNA molecules, we did not directly measure protein levels, and changes in gene expression may not reflect changes in protein abundance. Based on previous studies in rodents, we chose a single dose of drug and did not determine whether there were dose-dependent effects of simvastatin treatment. Despite these limitations, however, this work provided new insight into the pathophysiology of rotator cuff tears and identified simvastatin as a potential therapy to limit the development of weakness and fibrosis after rotator cuff tear.
Recent work has eloquently explored the epidemiological relationships between statin medication and the development of a rotator cuff tear, as well as the mechanistic impact of hypercholesterolemia on tendon material properties and susceptibility to tendon tears. Patients with rotator cuff tears had an increase in circulating triglycerides, LDL and total cholesterol, and no difference in HDL levels1. In mice, chronic hypercholesterolemia decreased the elastic modulus of patellar tendons, which can increase the susceptibility of tendons to rupture3. For rats that received a rotator cuff tear and repair, a high cholesterol diet was associated with decreased healing stiffness one month after repair4. While Abboud and colleagues did not directly explore statins in their studies, they postulated that these medications may have a positive impact on tendon-bone healing. Combined with the results from the current study, it is possible that statin medication would improve muscle regeneration after repairing a chronically torn rotator cuff to its original anatomical footprint. Despite these optimistic results, further studies that evaluate statin medications in the context of acute and chronic rotator cuff injury and repair models that assess the muscle, tendon and enthesis, and that provide further information on the molecular mechanisms of action of these medications will help to inform potential clinical studies that may be conducted down the road.
Conclusions
Identifying new therapies to prevent muscle weakness and fibrosis formation are likely to improve the treatment of patients with chronic rotator cuff tears. The results of the current study demonstrated that simvastatin partially protected muscles against the loss in active force production that occurs after rotator cuff tear, and dramatically reduced fibrosis as well. While these findings are encouraging, future studies that explore statin medication in the context of acute and chronic rotator cuff repair should be conducted to determine whether a clinical trial to evaluate statin medication in patients with rotator cuff tears is warranted.
Supplementary Material
Acknowledgement
The authors would like to thank Mr. Stuart M. Roche and Mr. Michael D. Flood for technical assistance.
This work was supported by a research fellowship from the Alpha Omega Alpha Honor Medical Society, a research fellowship from Blue Cross Blue Shield of Michigan, and grants R01-AR063649 and T32-GM008322 from the National Institutes of Health. The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
This study was approved by the University of Michigan IACUC (protocol 10500).
References
- 1.Abboud JA, Kim JS. The effect of hypercholesterolemia on rotator cuff disease. Clin Orthop Relat Res. 2010;468:1493–1497. doi: 10.1007/s11999-009-1151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aktas E, Sener E, Gocun PU. Mechanically induced experimental knee osteoarthritis benefits from anti-inflammatory and immunomodulatory properties of simvastatin via inhibition of matrix metalloproteinase-3. J Orthop Traumatol. 2011;12:145–151. doi: 10.1007/s10195-011-0154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beason DP, Abboud JA, Kuntz AF, Bassora R, Soslowsky LJ. Cumulative effects of hypercholesterolemia on tendon biomechanics in a mouse model. J Orthop Res. 2011;29:380–383. doi: 10.1002/jor.21255. [DOI] [PubMed] [Google Scholar]
- 4.Beason DP, Tucker JJ, Lee CS, Edelstein L, Abboud JA, Soslowsky LJ. Rat rotator cuff tendon-to-bone healing properties are adversely affected by hypercholesterolemia. J Shoulder Elbow Surg. 2013 doi: 10.1016/j.jse.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedi A, Dines J, Warren RF, Dines DM. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92:1894–1908. doi: 10.2106/JBJS.I.01531. [DOI] [PubMed] [Google Scholar]
- 6.Biasucci LM, Biasillo G, Stefanelli A. Inflammatory markers, cholesterol and statins: pathophysiological role and clinical importance. Clin. Chem. Lab. Med. 2010;48:1685–1691. doi: 10.1515/CCLM.2010.277. [DOI] [PubMed] [Google Scholar]
- 7.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian journal of biochemistry and physiology. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 8.Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94:227–233. doi: 10.2106/JBJS.J.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis ME, Gumucio JP, Sugg KB, Bedi A, Mendias CL. MMP inhibition as a potential method to augment the healing of skeletal muscle and tendon extracellular matrix. J Appl Physiol. 2013;115:884–891. doi: 10.1152/japplphysiol.00137.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Docheva D, Hunziker EB, Fässler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. 2005;25:699–705. doi: 10.1128/MCB.25.2.699-705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frey E, Regenfelder F, Sussmann P, Zumstein M, Gerber C, Born W, et al. Adipogenic and myogenic gene expression in rotator cuff muscle of the sheep after tendon tear. J Orthop Res. 2009;27:504–509. doi: 10.1002/jor.20695. [DOI] [PubMed] [Google Scholar]
- 12.Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, Von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;86-A:1973–1982. doi: 10.2106/00004623-200409000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Gerber C, Schneeberger AG, Hoppeler H, Meyer DC. Correlation of atrophy and fatty infiltration on strength and integrity of rotator cuff repairs: a study in thirteen patients. J Shoulder Elbow Surg. 2007;16:691–696. doi: 10.1016/j.jse.2007.02.122. [DOI] [PubMed] [Google Scholar]
- 14.Goutallier D, Postel J-M, Gleyze P, Leguilloux P, Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg. 2003;12:550–554. doi: 10.1016/s1058-2746(03)00211-8. [DOI] [PubMed] [Google Scholar]
- 15.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;304:78–83. [PubMed] [Google Scholar]
- 16.Gumucio JP, Davis ME, Bradley JR, Stafford PL, Schiffman CJ, Lynch EB, et al. Rotator cuff tear reduces muscle fiber specific force production and induces macrophage accumulation and autophagy. J Orthop Res. 2012;30:1963–1970. doi: 10.1002/jor.22168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gumucio JP, Korn MA, Saripalli AL, Flood MD, Phan AC, Roche SM, et al. Aging-associated exacerbation in fatty degeneration and infiltration after rotator cuff tear. J Shoulder Elbow Surg. 2014;23:99–108. doi: 10.1016/j.jse.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gumucio JP, Mendias CL. Atrogin-1, MuRF-1, and sarcopenia. Endocrine. 2013;43:12–21. doi: 10.1007/s12020-012-9751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanai J-I, Cao P, Tanksale P, Imamura S, Koshimizu E, Zhao J, et al. The muscle-specific ubiquitin ligase atrogin-1/MAFbx mediates statin-induced muscle toxicity. J Clin Invest. 2007;117:3940–3951. doi: 10.1172/JCI32741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itoigawa Y, Kishimoto KN, Sano H, Kaneko K, Itoi E. Molecular mechanism of fatty degeneration in rotator cuff muscle with tendon rupture. J Orthop Res. 2011;29:861–866. doi: 10.1002/jor.21317. [DOI] [PubMed] [Google Scholar]
- 21.Joe AWB, Yi L, Natarajan A, Le Grand F, So L, Wang J, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HM, Galatz LM, Lim C, Havlioglu N, Thomopoulos S. The effect of tear size and nerve injury on rotator cuff muscle fatty degeneration in a rodent animal model. J Shoulder Elbow Surg. 2012;21:847–858. doi: 10.1016/j.jse.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko JH, Kim PS, Zhao Y, Hong SJ, Mustoe TA. HMG-CoA reductase inhibitors (statins) reduce hypertrophic scar formation in a rabbit ear wounding model. Plast Reconstr Surg. 2012;129:252e–261e. doi: 10.1097/PRS.0b013e31823aea10. [DOI] [PubMed] [Google Scholar]
- 24.Kumar A, Bhatnagar S, Kumar A. Matrix metalloproteinase inhibitor batimastat alleviates pathology and improves skeletal muscle function in dystrophin-deficient mdx mice. Am J Pathol. 2010;177:248–260. doi: 10.2353/ajpath.2010.091176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laron D, Samagh SP, Liu X, Kim HT, Feeley BT. Muscle degeneration in rotator cuff tears. J Shoulder Elbow Surg. 2012;21:164–174. doi: 10.1016/j.jse.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Manzano G, Kim HT, Feeley BT. A rat model of massive rotator cuff tears. J Orthop Res. 2011;29:588–595. doi: 10.1002/jor.21266. [DOI] [PubMed] [Google Scholar]
- 27.Mendias CL, Kayupov E, Bradley JR, Brooks SV, Claflin DR. Decreased specific force and power production of muscle fibers from myostatin-deficient mice are associated with a suppression of protein degradation. J Appl Physiol. 2011;111:185–191. doi: 10.1152/japplphysiol.00126.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendias CL, Marcin JE, Calerdon DR, Faulkner JA. Contractile properties of EDL and soleus muscles of myostatin-deficient mice. J Appl Physiol. 2006;101:898–905. doi: 10.1152/japplphysiol.00126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mun J-H, Kim Y-M, Kim B-S, Kim J-H, Kim M-B, Ko H-C. Simvastatin inhibits transforming growth factor-β1-induced expression of type I collagen, CTGF, and α-SMA in keloid fibroblasts. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2014;22:125–133. doi: 10.1111/wrr.12136. [DOI] [PubMed] [Google Scholar]
- 30.Neuman RE, Logan MA. The determination of hydroxyproline. J Biol Chem. 1950;184:299–306. [PubMed] [Google Scholar]
- 31.Parker BA, Thompson PD. Effect of statins on skeletal muscle: exercise, myopathy, and muscle outcomes. Exerc Sport Sci Rev. 2012;40:188–194. doi: 10.1097/JES.0b013e31826c169e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rupérez M, Rodrigues-Díez R, Blanco-Colio LM, Sánchez-López E, Rodríguez-Vita J, Esteban V, et al. HMG-CoA reductase inhibitors decrease angiotensin II-induced vascular fibrosis: role of RhoA/ROCK and MAPK pathways. Hypertension. 2007;50:377–383. doi: 10.1161/HYPERTENSIONAHA.107.091264. [DOI] [PubMed] [Google Scholar]
- 33.Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda, Md) 2008;23:160–170. doi: 10.1152/physiol.00041.2007. [DOI] [PubMed] [Google Scholar]
- 34.Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- 35.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 36.Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5:383–392. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 37.Steinbacher P, Tauber M, Kogler S, Stoiber W, Resch H, Sänger AM. Effects of rotator cuff ruptures on the cellular and intracellular composition of the human supraspinatus muscle. Tissue & cell. 2010;42:37–41. doi: 10.1016/j.tice.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Taha DA, De Moor CH, Barrett DA, Gershkovich P. Translational insight into statin-induced muscle toxicity: from cell culture to clinical studies. Transl Res. 2014 doi: 10.1016/j.trsl.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Tsuchiya A, Nagotani S, Hayashi T, Deguchi K, Sehara Y, Yamashita T, et al. Macrophage infiltration, lectin-like oxidized-LDL receptor-1, and monocyte chemoattractant protein-1 are reduced by chronic HMG-CoA reductase inhibition. Current neurovascular research. 2007;4:268–273. doi: 10.2174/156720207782446333. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y-X. PPARs: diverse regulators in energy metabolism and metabolic diseases. Cell Res. 2010;20:124–137. doi: 10.1038/cr.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 42.Yao J, Xiong M, Tang B, Chen G, Liang M, Ma X, et al. Simvastatin attenuates pulmonary vascular remodelling by down-regulating matrix metalloproteinase-1 and −9 expression in a carotid artery-jugular vein shunt pulmonary hypertension model in rats. Eur J Cardiothorac Surg. 2012;42:e121–e127. doi: 10.1093/ejcts/ezs445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.