Abstract
Background
Youth with type 1 diabetes (T1D) are at risk for weight gain due to the epidemic of childhood overweight/obesity and common use of intensive insulin therapy; the latter resulted in weight gain in the Diabetes Control and Complications Trial.
Objective
To assess overweight/obesity prevalence and intensive insulin therapy use in youth with T1D over a decade and identify factors associated with weight status and glycemic control.
Methods
We obtained cross-sectional data from four unique cohorts (1999, 2002, 2006, 2009). Youth (N= 507, 49% male) were 8-16 years old with T1D duration ≥6 months, A1c 6.0-12.0% (42-108 mmol/mol), and daily insulin dose ≥0.5 u/kg.
Results
Across cohorts, age, BMI percentile, and A1c ranged from 12.0±2.2 to 12.8±2.3 years, 70±22 to 72±21, and 8.3±1.0 (67±11) to 8.5±1.1% (69±12 mmol/mol), respectively. Intensive insulin therapy use increased from 52% to 97% (p<.001) between 1999 and 2009. However, prevalence of overweight/obesity remained similar, 27% (1999), 36% (2002), 33% (2006), and 31% (2009) (p=.54), as did z-BMI. In multivariate analysis, higher A1c was related to higher insulin dose (p<.01), less frequent blood glucose monitoring (p<.001), and non-white race (p<.001); A1c was not related to z-BMI, intensive insulin therapy, or cohort. z-BMI was related to insulin dose (p<.005) but not intensive insulin therapy or cohort.
Conclusions
Despite near-universal implementation of intensive insulin therapy, overweight/obesity prevalence in youth with T1D remained stable over a decade, similar to the general pediatric population. However, A1c remained suboptimal, underscoring the need to optimize T1D treatment to reduce future complication risk.
Keywords: Overweight, Obesity, Type 1 Diabetes, Youth, Prevalence
Introduction
Intensive insulin therapy, defined as multiple daily injections or insulin pump therapy coupled with frequent blood glucose (BG) monitoring, has become the standard of care for the management of type 1 diabetes (T1D) since the Diabetes Control and Complications Trial (DCCT). The DCCT, its long-term observational follow-up, the Epidemiology of Diabetes Interventions and Complications (EDIC) Study (1;2), and other studies such as the Pittsburgh Epidemiology of Diabetes Complications (EDC) study (3), have demonstrated reduced occurrence of complications such as proliferative retinopathy, nephropathy, and cardiovascular disease in patients with T1D receiving intensive insulin therapy. Despite the beneficial outcomes in the DCCT, adults receiving intensive insulin therapy experienced a threefold higher rate of severe hypoglycemia and gained an average of 5 kg more than adults receiving conventional therapy (1;4). The availability of newer insulin analogues since 1996 has substantially reduced the occurrence of severe hypoglycemia associated with intensive insulin therapy (5-7), but the impact of intensive insulin therapy on weight gain needs additional study, especially in the pediatric population. Excessive weight gain related to intensive insulin therapy could potentially result in adverse cardiovascular outcomes, as evidenced by its association with increases in central obesity, insulin resistance, dyslipidemia, blood pressure, and atherosclerosis during follow-up of patients in the DCCT and EDIC (8;9).
Youth with T1D are at risk for untoward weight gain due to the combined influences of the current epidemic of childhood overweight/obesity and greater use of intensive insulin therapy. Encouragingly, the prevalence of overweight and obesity has leveled off in the general pediatric population in the last decade (10-12); however, such time trend data in youth with T1D are limited. Given the progressive implementation of intensive insulin therapy in pediatric T1D, we sought to evaluate a decade of temporal trends of overweight and obesity in youth with T1D at a single center, with respect to the use of intensive insulin therapy and glycemic control.
Methods
Study cohorts
We compared four unique cohorts of youth with T1D, each representing a different time point (1999, 2002, 2006, and 2009) over a period of 10 years. We systematically captured the data from the subjects enrolled in four distinct studies at a single center. The Institutional Review Board approved each of the study protocols and all parents/youth provided written informed consent/assent. Eligibility criteria included: age 8-16 years, T1D duration ≥6 months, A1c 6.0-12.0% (42-108 mmol/mol), daily insulin dose ≥0.5 u/kg, and no other major untreated chronic medical disorder, psychiatric disorder, or cognitive disability. Cross-sectional study data, including demographics, diabetes management details, height, weight, blood pressure, and A1c, were obtained from parent/youth interview and medical record review at the baseline study visit. For all study cohorts, height and weight were obtained using standardized equipment. We categorized participants into the following insulin regimens: conventional (2 injections/day), multiple daily injections (MDI, ≥3 injections/day), and pump therapy. Two participants in the 2006 cohort were using untethered pump therapy consisting of a combination of basal injection and subcutaneous insulin boluses via the pump; these participants were included in the pump group.
Weight categories and blood pressure
Body mass index percentiles and z-scores were calculated using age- and sex-standardized norms from the Centers for Disease Control and Prevention (CDC). Youth with T1D were categorized into three categories of weight status: normal weight (<85th percentile), overweight (≥85 to <95th percentile), and obese (≥95th percentile)(13). Only 3 participants (1 in the 2006 cohort and 2 in the 2009 cohort) were underweight according to BMI percentile (<5th percentile); we included these participants with the normal weight group. Blood pressures were measured during clinic visits in a standardized manner. Age, sex, and height standardized blood pressure percentiles were calculated according to CDC norms (14).
Statistical Analysis
Descriptive data are presented as mean ± standard deviation or percentage. Analysis of variance with Tukey adjustment provided comparisons of continuous variables across the four unique cohorts and three insulin regimens. Chi-square test/Fisher's exact test compared proportions. Bivariate and multivariate analyses were performed to determine factors associated with z-BMI and A1c. Given the collinearity between insulin regimen and study cohort, we performed multivariate analyses with two separate models; one with insulin regimen and a second model with study cohort. In the separate multivariate models, we examined the associations of z-BMI and A1c with either temporal study cohort or insulin regimen along with meaningful demographic and diabetes specific variables including age, sex, duration of T1D, total daily insulin dose (u/kg), and BG monitoring frequency. In the multivariate analysis, insulin regimen was dichotomized into intensive insulin therapy (pump + MDI) and conventional therapy. Statistical significance was defined by a p value <.05.
Results
Demographic and diabetes treatment characteristics according to temporal cohort
There were 507 youth with T1D (49% males) in the four cohorts combined. In all cohorts, participants were 8-16 years of age and had T1D for 0.5-15 years. Sex distribution, race/ethnicity distribution, and glycemic control were similar across the four cohorts (Table 1). While there were statistical differences regarding attained age among the four cohorts, from a clinical standpoint, their ages were similar (12.0±2.2 to 12.8±2.3 years). Duration of T1D was statistically and clinically dissimilar among the four cohorts, with the 1999 cohort having a substantially shorter mean duration of 2.8 years compared to approximately 6 years for the other three cohorts. These variations in demographic characteristics called for adjustments in the multivariate analyses.
Table 1. Participant characteristics according to temporal cohort.
| 1999 (n=94) |
2002 (n=144) |
2006 (n=133) |
2009 (n=136) |
|
|---|---|---|---|---|
| Age (years)a | 12.0±2.2 | 12.8±2.3 | 12.1±1.9 | 12.7±2.5 |
| Sex (% female) | 46 | 56 | 53 | 49 |
| Race/ethnicity (% non-white) | 6 | 9 | 12 | 7 |
| z-BMI (SDS) | 0.69±0.76 | 0.74±0.75 | 0.65±0.76 | 0.65±0.82 |
| BMI percentile (%) | 71±21 | 72±21 | 70±22 | 70±23 |
| Age at T1D diagnosis (years)b | 9.2±2.7 | 6.4±3.4 | 6.4±3.4 | 6.3±3.0 |
| T1D duration (years)b | 2.8±1.5 | 6.5±3.5 | 5.7±3.3 | 6.4±3.2 |
| Insulin dose (U/kg/day)c | 0.9±0.2 | 1.0±0.2 | 0.9±0.2 | 0.9±0.3 |
| BG monitoring frequency (%)d | ||||
| ≤2 times/day | 7 | 8 | 8 | 4 |
| 3 times/day | 28 | 28 | 12 | 6 |
| 4 times/day | 60 | 51 | 19 | 22 |
| ≥5 times/day | 5 | 14 | 62 | 68 |
| A1c % (mmol/mol) | 8.5±1.1 (69±12) |
8.4±1.3 (68±14) |
8.3±1.0 (67±11) |
8.3±1.1 (67±12) |
1999 vs. 2002: p=.03, 2002 vs. 2006: p=.04.
1999 vs. 2002, 1999 vs. 2006, 1999 vs. 2009: all p<.001.
1999 vs. 2002: p=.01.
1999 vs. 2006, 1999 vs. 2009, 2002 vs. 2006, 2002 vs. 2009: all p<.001.
Intensive management, noted by more frequent daily BG monitoring and greater use of intensive insulin therapy, defined as 3+ injections/day or pump use, increased significantly over time by temporal cohort (Table 1 and Fig 1-a). While 48% of the 1999 cohort received conventional therapy, only 3% of the 2009 cohort received conventional therapy. BG monitoring frequency of ≥4 checks/day increased from 65% in the 1999 and 2002 cohorts to 81-90% in the 2006 and 2009 cohorts, while monitoring ≥5 times /day increased from 5-14% to 62-68% in 1999 and 2002 vs. 2006 and 2009 cohorts, respectively.
Figure 1.
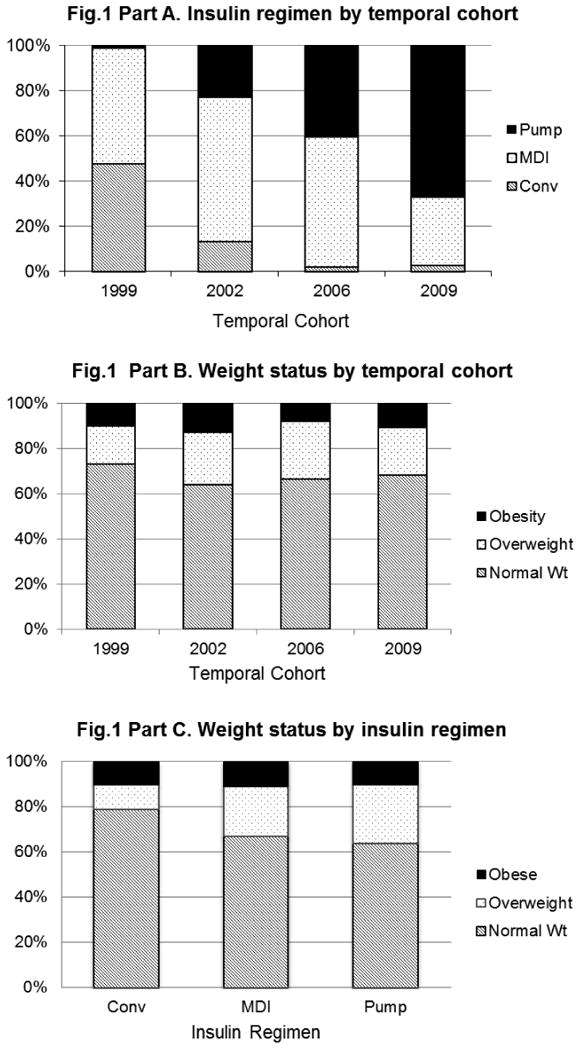
Figure 1a. Insulin regimen according to temporal cohort.
There was a significant increase in the use of intensive insulin therapy from 1999-2009.
Figure 1b. Weight status according to temporal cohort.
The prevalence of overweight/obesity in youth with type 1 diabetes remained unchanged from 1999-2009.
Figure 1c. Weight status according to insulin regimen.
There was no significant difference in weight status among youth treated with conventional therapy, multiple daily injections, or insulin pump therapy.
Conv: Conventional therapy, MDI: Multiple daily injections.
Demographic and diabetes treatment characteristics according to insulin regimen
Participant characteristics, such as age, duration of T1D, and details of diabetes management, varied according to insulin regimen (Table 2). Those using conventional insulin therapy were significantly younger (11.3±2.2 years, p<.001) and had shorter duration of diabetes (3.6±2.8 years, p<.001), reflecting the predominance of conventional insulin therapy in the 1999 cohort. As expected, diabetes management varied according to insulin regimen. Total daily insulin dose was higher in those receiving MDI (1.0±0.3 u/kg, p<.001) compared to patients using conventional treatment (0.9±0.2 u/kg) or pump therapy (0.9±0.2 u/kg). Frequency of BG monitoring also differed by insulin regimen; 70% of pump treated participants checked BG levels ≥5 times/day compared to 27% of participants receiving MDI and 7% of conventionally treated participants (p<.001). A1c differed by insulin regimen, with those receiving pump therapy having the lowest A1c of 8.0±0.9% (64±10 mmol/mol), a value that was significantly lower than the MDI group (8.6±1.1% (70±12 mmol/mol), p<.001) and non-significantly lower than the conventionally treated group (8.4±1.2% (68±13 mmol/mol), p=.07).
Table 2. Participant characteristics according to insulin regimen.
| CONV (n=70) |
MDI (n=258) |
PUMP (n=179) |
|
|---|---|---|---|
| Age (years)a | 11.3±2.2 | 12.7±2.2 | 12.6±2.2 |
| Sex (% female) | 59 | 48 | 54 |
| Race/ethnicity (% non-white)b | 13 | 12 | 3 |
| z-BMI (SDS) | 0.56±0.82 | 0.73±0.72 | 0.66±0.82 |
| BMI percentile (%) | 67±23 | 72±20 | 70±23 |
| Age at T1D diagnosis (years)c | 7.7±2.9 | 7.1±3.6 | 6.2±3.0 |
| T1D duration (years)d | 3.6±2.8 | 5.5±3.5 | 6.4±3.1 |
| Insulin dose (U/kg/day)e | 0.9±0.2 | 1.0±0.3 | 0.9±0.2 |
| BG monitoring frequency (%)f | |||
| ≤2 times/day | 14 | 7 | 3 |
| 3 times/day | 20 | 22 | 10 |
| 4 times/day | 59 | 43 | 17 |
| ≥5 times/day | 7 | 27 | 70 |
| A1c % (mmol/mol)g | 8.4±1.2 (68±13) | 8.6±1.1 (70±12) | 8.0±0.9 (64±10) |
CONV: Conventional therapy; MDI: Multiple daily injections
CONV vs. MDI: p<.001, CONV vs. PUMP: p<.001.
CONV vs. PUMP: p=.002, MDI vs. PUMP: p<.001.
CONV vs. PUMP: p=.003, MDI vs. PUMP: p=.01.
CONV vs. MDI: p<.001, CONV vs. PUMP: p<.001, MDI vs. PUMP: p=.02.
CONV vs. MDI: p<.001, MDI vs. PUMP: p<.001.
CONV vs. MDI: p=.002, CONV vs. PUMP: p<.001, MDI vs. PUMP: p<.001.
MDI vs. PUMP: p<.001.
z-BMI and weight status according to temporal cohort and insulin regimen
In total, one-third of participants across all four cohorts were either overweight (n=111, 22%) or obese (n=53, 10%). Neither the mean z-BMI (p=.70) nor the distribution of weight status (p=.54) (Fig 1-b) differed significantly among the four temporal cohorts. Interestingly, the distribution of weight status also did not differ by insulin regimen (Fig 1-c) although there was a modest, non-significant excess of normal weight in those on conventional therapy.
Demographic and diabetes treatment characteristics according to weight status
Diabetes treatment characteristics were similar across weight status groups (Table 3) with respect to total daily insulin dose (u/kg), BG monitoring frequency, or insulin regimen. Further, A1c did not differ by weight status, although A1c was non-significantly higher in the obese group. Not surprisingly, the mean percentiles of systolic blood pressure were higher in the overweight and obese patients compared to those with normal weight (p<.01). Overall, only 5% of the sample had blood pressures at or above the 90th percentile. Due to the small number with hypertension or prehypertension, the proportion with blood pressure ≥90th percentile across the three weight status groups was similar.
Table 3. Participant characteristics according to weight status.
| Normal Weight (n=343) |
Overweight (n=111) |
Obese (n= 53) |
|
|---|---|---|---|
| Age (years) | 12.4±2.2 | 12.7±2.2 | 12.3±2.5 |
| Sex (% female) | 52 | 50 | 53 |
| Race/ethnicity (% non-white) | 9 | 7 | 8 |
| Age at T1D diagnosis (years)a | 7.1±3.3 | 6.8±3.4 | 5.9±3.1 |
| T1D duration (years) | 5.3±3.3 | 5.9±3.4 | 6.4±3.7 |
| Systolic BP (percentile)b | 54±24 | 63±23 | 70±23 |
| Diastolic BP (percentile)c | 57±19 | 60±16 | 66±18 |
| Insulin dose (U/kg/day) | 0.9±0.2 | 1.0±0.3 | 1.0±0.3 |
| BG monitoring frequency (%) | |||
| ≤2 times/day | 7 | 7 | 6 |
| 3 times/day | 16 | 21 | 25 |
| 4 times/day | 36 | 37 | 38 |
| ≥5 times/day | 42 | 35 | 32 |
| Insulin regimen (%) | |||
| 2 injections/day | 16 | 7 | 13 |
| ≥3 injections/day | 51 | 50 | 53 |
| Pump therapy | 33 | 42 | 34 |
| A1c % ( mmol/mol) | 8.3±1.1 (67±12) | 8.4±1.1 (68±12) | 8.7±1.1 (72±12) |
Normal weight: <85th percentile, Overweight: 85th to <95th percentile, Obese: ≥95th percentile
Normal weight vs. Obese: p=.04.
Normal weight vs. Overweight: p=.003, Normal weight vs. Obese: p<.001.
Normal weight vs. Obese: p=.003.
Factors associated with z-BMI and glycemic control in multivariate models
We examined the associations of z-BMI (models 1a and 1b) and A1c (models 2a and 2b) in separate multivariate models with either temporal cohort (model a) or insulin regimen (model b), as insulin regimen was so tightly linked with temporal cohort. Covariates included in the models predicting z-BMI were age, sex, race/ethnicity, duration of diabetes, total daily insulin dose (u/kg), A1c, and cohort (model 1a) or insulin regimen (model 1b). Only 4% of the variance in z-BMI (model p value ≤.01) was explained in each of the two models. In each model, the only significant factor associated with z-BMI was total daily insulin dose (p<.005). Higher total daily insulin dose was associated with a higher z-BMI; for each 0.1 u/kg increase in total daily insulin dose, there was a 0.04 increase in z-BMI. Neither cohort (model 1a) nor insulin regimen (model 1b) were significant predictors of z-BMI.
Covariates included in the models predicting A1c were age, sex, race/ethnicity, duration of diabetes, z-BMI, total daily insulin dose (u/kg), BG monitoring frequency, and cohort (model 2a) or insulin regimen (model 2b). In the multivariate model including temporal cohort (model 2a), the independent variables accounted for 12% of the variance in A1c (model p value <.001). In the multivariate model including insulin regimen (model 2b), the independent variables accounted for 11% of the variance in A1c (model p value <.001). In both models, more frequent BG monitoring was associated with lower A1c (p<.001) after adjustment for covariates (Figure 2). Additionally, in both models, total daily insulin dose and race/ethnicity were independently associated with A1c. For each 0.1 u/kg increase in total daily insulin dose, there was a significant increase in A1c of 0.05%(0.5 mmol/mol) (p<.01) in both the insulin regimen and temporal cohort models. Nonwhite race was associated with 0.7% (7.7 mmol/mol) higher A1c (p<.001) in both models. Neither cohort (model 2a) nor insulin regimen (model 2b) were significant predictors of A1c.
Figure 2.
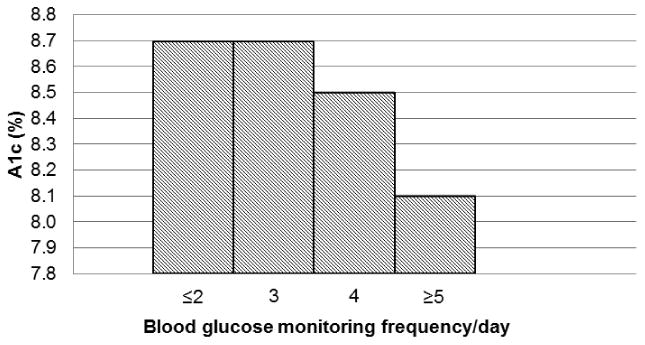
Multivariate analysis depicting A1c% according to BG monitoring frequency/day in the models with either temporal cohort or insulin regimen.
More frequent BG monitoring was significantly associated with lower A1c (p<.001).
Discussion
The prevalence of overweight and obesity in the pediatric population has increased dramatically over the past half century and, currently, 15% and 17% of U.S. youth ages 2-19 years old are overweight and obese, respectively (10). Encouragingly, recent studies indicate that the prevalence of overweight and obesity has plateaued in the last decade in multiple countries including the U.S. (10-12). Typically, youth with T1D exhibit significant weight loss at the time of diagnosis. Yet there has been a continuous rise in z-BMI at onset of T1D in children (15); the prevalence of overweight and obesity at T1D onset has increased three-fold across two decades (16). In the Pediatric Diabetes Consortium (PDC), 21% of youth with T1D were overweight or obese at diagnosis, although the mean z-BMI (-0.13) of youth with T1D was less than that of the 2000 CDC population (p=.04) (17). On the other hand, prevalence of overweight and obesity in youth with established T1D is 35%, comparable to that of the general U.S. pediatric population (18). A study of adults with established T1D showed an approximate 50% increase in overweight prevalence and seven-fold increase in obesity prevalence over 18 years (19). With a progressive increase in intensive insulin therapy use since the DCCT, particularly in the pediatric population, and a need to understand temporal trends of overweight and obesity in youth with established T1D, we investigated trends in overweight and obesity in youth with T1D over a 10-year period.
In our study, the prevalence of overweight and obesity in four unique cohorts remained stable over the past decade with 22% of the subjects overweight and 10% obese. In total, one third of the youth were either overweight or obese, again mirroring the prevalence of overweight and obesity in the general pediatric population (10). Our results are consistent with the SEARCH study (18) (22.1% overweight and 12.6% obesity), indicating a higher prevalence of overweight youth compared to obese youth with T1D, while the general pediatric population yields a greater proportion of obese youth compared to overweight youth (17% vs. 15%) (10).
In our analyses, the only factor that differed significantly by weight status was blood pressure, which was higher in obese youth, suggesting an adverse weight-related cardiovascular risk profile. Diabetes management was similar according to weight status groups and z-BMI was also similar across insulin regimen groups. Thus, the prevalence of overweight and obesity in youth with T1D today likely reflects the background prevalence of overweight and obesity in the general pediatric population and no adverse impact of intensive insulin therapy on weight as was seen in the DCCT.
From 1999 to 2009, we confirmed a dramatic increase in use of intensive insulin therapy in pediatric patients with T1D. Despite the near doubling and ubiquitous use of intensive insulin therapy in our recent cohort compared with the earlier cohorts, z-BMI and prevalence of overweight and obesity remained stable during the 10-year interval. Our findings suggest that intensive insulin therapy in the post-DCCT era was not associated with untoward weight gain. On the other hand, the Hvidore study group demonstrated higher z-BMI with increased penetration of intensive therapy from 1995-1998 (20), which was also associated with higher total daily insulin dose, although glycemic control, similarly, did not improve during that time interval. These investigators reported greatest weight gain in individuals switching from twice daily injections regimens to MDI regimens. While it is encouraging that weight status has remained stable over time in our cohorts, it is discouraging that glycemic control did not improve significantly with greater use of intensive insulin therapy, a finding similar to that of the Hvidore study group. However, we observed that those using insulin pump therapy had the lowest A1c, as reported in other observational studies (21;22). Several studies have demonstrated the important role of more frequent BG monitoring in achieving better glycemic control (23-27). In our study, BG monitoring frequency was the major predictor of A1c after adjusting for either temporal cohort or insulin regimen in separate multivariate models. This suggests that the lower A1c observed in those using insulin pump therapy may be the result of greater overall adherence and specifically to more frequent BG monitoring in this group.
Another factor associated with glycemic control was total daily insulin dose (u/kg); higher u/kg was associated with a higher A1c. One can interpret this observation a number of ways. Uncontrolled diabetes, identified by higher A1c levels, might lead providers to increase the insulin dose. The resulting higher insulin dose may reflect either underlying insulin resistance with a true need for more insulin or non-adherence with missed insulin doses leading to reflexive increases in the insulin dose. Again, in the multivariate analyses predicting z-BMI, daily insulin dose was the only significant factor associated with z-BMI; a higher insulin dose was associated with a higher z-BMI. However, in these cross-sectional analyses, we cannot determine whether higher z-BMI leads to a higher daily insulin dose requirement or if higher insulin dose promotes weight gain. While insulin dose was directly associated with A1c and z-BMI, there was no significant association between z-BMI and A1c. Lastly, non-white patients had a higher A1c in our cohorts compared with white patients, a finding reported in previous studies (23;28).
There are many limitations to our findings. The main limitation is the cross-sectional nature of the study; the associations we demonstrate cannot be considered causal. Another potential limitation to our study is the dissimilarity in patient characteristics related to duration of T1D in our 1999 study cohort, which was significantly shorter compared to the other cohorts. To account for this variation, we controlled for diabetes duration in the multivariate analyses. In addition, we do not have information regarding whether patients were taking medications that are known to impact weight.
Our study highlights several important findings. First we confirmed that the use of intensive insulin therapy has nearly doubled in youth with T1D over a decade. Next our findings revealed a stable prevalence of overweight and obesity in youth with T1D despite the increased use of intensive insulin therapy. We also demonstrated no significant association between weight status and glycemic control. Finally, glycemic control remained relatively stable across the temporal cohorts, despite the increased penetration of intensive insulin therapy.
Conclusion
The prevalence of overweight/obesity in youth with T1D has remained stable over a decade despite the increasing penetration of intensive insulin therapy. While it is encouraging that the rates of overweight and obesity have been stable, more than a third of youth with T1D continue to be either overweight or obese. Further, despite nearly universal use of intensive insulin therapy, glycemic control in youth with T1D remains suboptimal. These findings underscore the need for innovative clinical interventions to improve glycemic control and normalize weight status in pediatric patients with T1D in order to reduce the risk for future micro- and macro-vascular complications.
Acknowledgments
Charu Baskaran and Lori Laffel had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis
This study was supported by funding from: NIH grants T32DK007260, R01DK046887, P30DK036836; the Charles H. Hood Foundation; the Eleanor Chesterman Beatson Fund; the Katherine Adler Astrove Youth Education Fund; and the Maria Griffin Drury Pediatric Fund.
Contributor Information
Charumathi Baskaran, Genetics and Epidemiology Section, Joslin Diabetes Center, One Joslin Place, Boston, MA 02215.
Lisa K. Volkening, Genetics and Epidemiology Section, Joslin Diabetes Center, One Joslin Place, Boston, MA 02215.
Monica Diaz, SUNY Downstate College of Medicine, 450 Clarkson Ave, Brooklyn, NY 11203.
Lori Laffel, Genetics and Epidemiology Section, Joslin Diabetes Center, One Joslin Place, Boston, MA 02215.
Reference List
- 1.The DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Control and Complications Trial Epidemiology of Diabetes Interventions and Complications (DCCT EDIC) Research Group. Nathan DM, Zinman B, Cleary PA, Backlund JY, Genuth S, et al. Modern-day clinical course of type 1 diabetes mellitus after 30 years' duration: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications and Pittsburgh Epidemiology of Diabetes Complications Experience (1983-2005) Arch Intern Med. 2009;169(14):1307–1316. doi: 10.1001/archinternmed.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes. 2006;55(5):1463–1469. doi: 10.2337/db05-1423. [DOI] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial Research Group. Influence of intensive diabetes treatment on body weight and composition of adults with type 1 diabetes in the Diabetes Control and Complications Trial. Diabetes Care. 2001;24(10):1711–1721. doi: 10.2337/diacare.24.10.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katz ML, Volkening LK, Anderson BJ, Laffel LM. Contemporary rates of severe hypoglycaemia in youth with type 1 diabetes: variability by insulin regimen. Diabet Med. 2012;29(7):926–932. doi: 10.1111/j.1464-5491.2012.03646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunelle BL, Llewelyn J, Anderson JH, Jr, Gale EA, Koivisto VA. Meta-analysis of the effect of insulin lispro on severe hypoglycemia in patients with type 1 diabetes. Diabetes Care. 1998;21(10):1726–1731. doi: 10.2337/diacare.21.10.1726. [DOI] [PubMed] [Google Scholar]
- 7.Murphy NP, Keane SM, Ong KK, Ford-Adams M, Edge JA, Acerini CL, et al. Randomized cross-over trial of insulin glargine plus lispro or NPH insulin plus regular human insulin in adolescents with type 1 diabetes on intensive insulin regimens. Diabetes Care. 2003;26(3):799–804. doi: 10.2337/diacare.26.3.799. [DOI] [PubMed] [Google Scholar]
- 8.Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. Diabetes Control and Complications Trial. JAMA. 1998;280(2):140–146. doi: 10.1001/jama.280.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purnell JQ, Zinman B, Brunzell JD. The effect of excess weight gain with intensive diabetes mellitus treatment on cardiovascular disease risk factors and atherosclerosis in type 1 diabetes mellitus: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) study. Circulation. 2013;127(2):180–187. doi: 10.1161/CIRCULATIONAHA.111.077487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olds TS, Tomkinson GR, Ferrar KE, Maher CA. Trends in the prevalence of childhood overweight and obesity in Australia between 1985 and 2008. Int J Obes (Lond) 2010;34(1):57–66. doi: 10.1038/ijo.2009.211. [DOI] [PubMed] [Google Scholar]
- 12.Peneau S, Salanave B, Maillard-Teyssier L, Rolland-Cachera MF, Vergnaud AC, Mejean C, et al. Prevalence of overweight in 6- to 15-year-old children in central/western France from 1996 to 2006: trends toward stabilization. Int J Obes (Lond) 2009;33(4):401–407. doi: 10.1038/ijo.2009.31. [DOI] [PubMed] [Google Scholar]
- 13.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11(246):1–190. [PubMed] [Google Scholar]
- 14.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2):555–576. [PubMed] [Google Scholar]
- 15.Knerr I, Wolf J, Reinehr T, Stachow R, Grabert M, Schober E, et al. The ‘accelerator hypothesis’: relationship between weight, height, body mass index and age at diagnosis in a large cohort of 9,248 German and Austrian children with type 1 diabetes mellitus. Diabetologia. 2005;48(12):2501–2504. doi: 10.1007/s00125-005-0033-2. [DOI] [PubMed] [Google Scholar]
- 16.Libman IM, Pietropaolo M, Arslanian SA, LaPorte RE, Becker DJ. Changing prevalence of overweight children and adolescents at onset of insulin-treated diabetes. Diabetes Care. 2003;26(10):2871–2875. doi: 10.2337/diacare.26.10.2871. [DOI] [PubMed] [Google Scholar]
- 17.Kaminski BM, Klingensmith GJ, Beck RW, Tamborlane WV, Lee J, Hassan K, et al. Body mass index at the time of diagnosis of autoimmune type 1 diabetes in children. J Pediatr. 2013;162(4):736–740. doi: 10.1016/j.jpeds.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Liu LL, Lawrence JM, Davis C, Liese AD, Pettitt DJ, Pihoker C, et al. Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth study. Pediatr Diabetes. 2010;11(1):4–11. doi: 10.1111/j.1399-5448.2009.00519.x. [DOI] [PubMed] [Google Scholar]
- 19.Conway B, Miller RG, Costacou T, Fried L, Kelsey S, Evans RW, et al. Temporal patterns in overweight and obesity in Type 1 diabetes. Diabet Med. 2010;27(4):398–404. doi: 10.1111/j.1464-5491.2010.02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holl RW, Swift PG, Mortensen HB, Lynggaard H, Hougaard P, Aanstoot HJ, et al. Insulin injection regimens and metabolic control in an international survey of adolescents with type 1 diabetes over 3 years: results from the Hvidore study group. Eur J Pediatr. 2003;162(1):22–29. doi: 10.1007/s00431-002-1037-2. [DOI] [PubMed] [Google Scholar]
- 21.Retnakaran R, Hochman J, DeVries JH, Hanaire-Broutin H, Heine RJ, Melki V, et al. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1c. Diabetes Care. 2004;27(11):2590–2596. doi: 10.2337/diacare.27.11.2590. [DOI] [PubMed] [Google Scholar]
- 22.Paris CA, Imperatore G, Klingensmith G, Petitti D, Rodriguez B, Anderson AM, et al. Predictors of insulin regimens and impact on outcomes in youth with type 1 diabetes: the SEARCH for Diabetes in Youth study. J Pediatr. 2009;155(2):183–189. doi: 10.1016/j.jpeds.2009.01.063. [DOI] [PubMed] [Google Scholar]
- 23.Redondo MJ, Connor CG, Ruedy KJ, Beck RW, Kollman C, Wood JR, et al. Pediatric Diabetes Consortium Type 1 Diabetes New Onset (NeOn) Study: factors associated with HbA1c levels one year after diagnosis. Pediatr Diabetes. 2013 doi: 10.1111/pedi.12061. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller KM, Beck RW, Bergenstal RM, Goland RS, Haller MJ, McGill JB, et al. Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care. 2013;36(7):2009–2014. doi: 10.2337/dc12-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine BS, Anderson BJ, Butler DA, Brackett J, Laffel L. Predictors of glycemic control and short-term adverse outcomes in youth with type 1 diabetes. J Pediatr. 2001;139(2):197–203. doi: 10.1067/mpd.2001.116283. [DOI] [PubMed] [Google Scholar]
- 26.Driscoll KA, Johnson SB, Tang Y, Yang F, Deeb LC, Silverstein JH. Does blood glucose monitoring increase prior to clinic visits in children with type 1 diabetes? Diabetes Care. 2011;34(10):2170–2173. doi: 10.2337/dc11-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pihoker C, Badaru A, Anderson A, Morgan T, Dolan L, Dabelea D, et al. Insulin regimens and clinical outcomes in a type 1 diabetes cohort: the SEARCH for Diabetes in Youth study. Diabetes Care. 2013;36(1):27–33. doi: 10.2337/dc12-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klingensmith GJ, Miller KM, Beck RW, Cruz E, Laffel LM, Lipman TH, et al. Racial disparities in insulin pump therapy and hemoglobin A1c (A1c) among T1D Exchange participants. Diabetes. 2012;61(Suppl 1):A358. [Google Scholar]


