Abstract
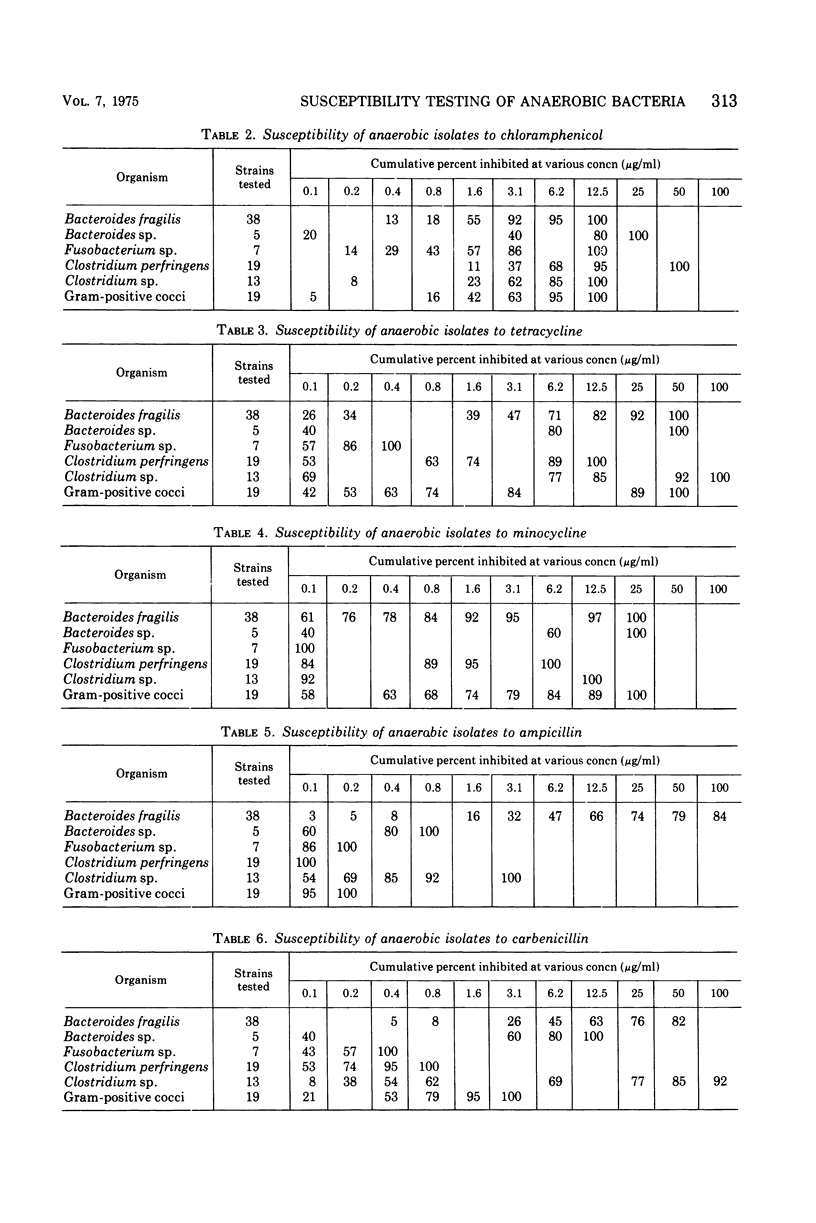
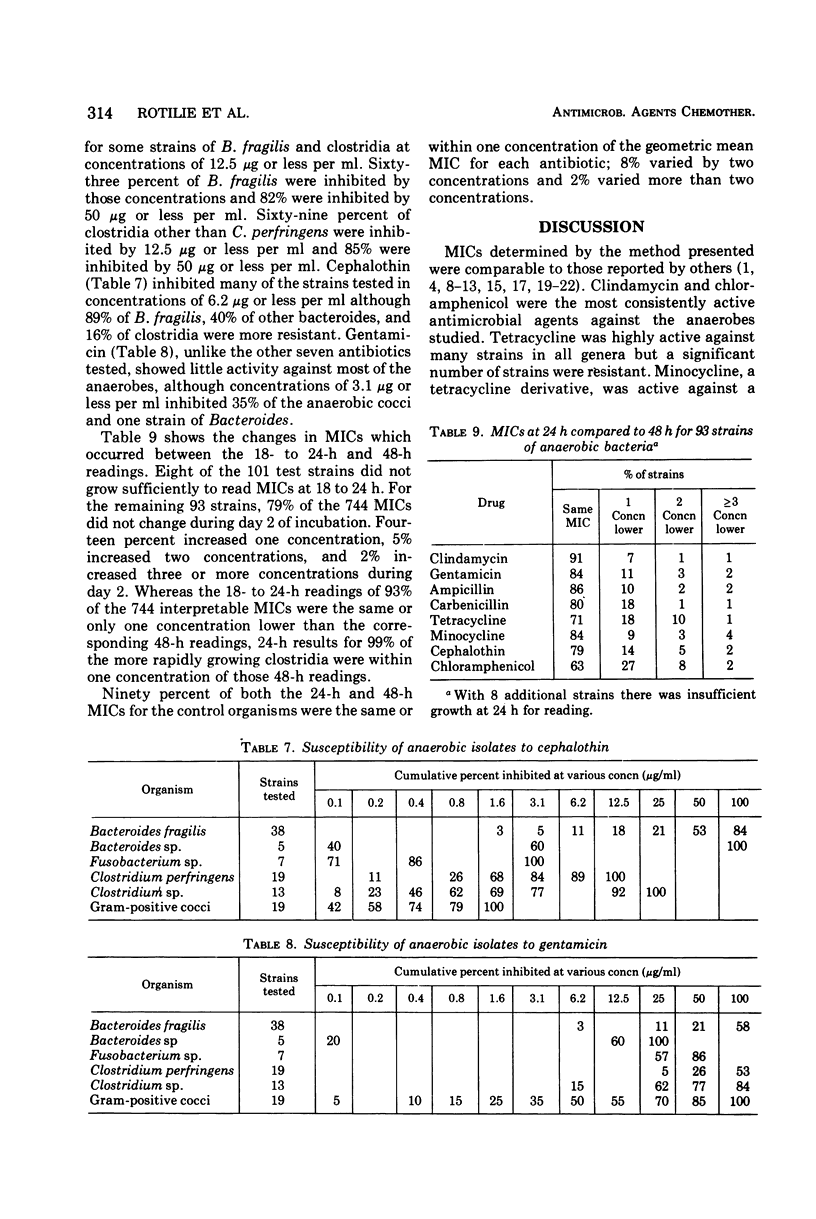
A microdilution technique using commercially available media and materials was developed and used to determine the minimal inhibitory concentrations (MICs) of clindamycin, chloramphenicol, tetracycline, minocycline, ampicillin, carbenicillin, cephalothin, and gentamicin for 101 anaerobic isolates. Representative strains of Bacteroides, Clostridium, Fusobacterium, Peptococcus, and Peptostreptococcus were tested. The use of Schaedler broth at pH 7.2, an inoculum of 105 to 107 colony-forming units per ml, and incubation at 35 C in an anaerobic glove box with an atmosphere of 80% nitrogen, 10% hydrogen, and 10% carbon dioxide resulted in good growth and easily interpretable results. After 48 h of incubation, 97% of strains tested were inhibited by 3.1 μg or less of clindamycin per ml and 98% were inhibited by 12.5 μg or less of chloramphenicol per ml. Tetracycline and minocycline inhibited 81 and 88% of strains tested in concentrations of 1.6 μg or less per ml and 1.6 μg or less per ml, respectively. Ampicillin inhibited all strains other than B. fragilis in concentrations of 3.1 μg or less per ml. Excluding certain strains of Bacteroides and Clostridium, carbenicillin in concentrations of 12.5 μg or less per ml and cephalothin in concentrations of 6.2 μg or less per ml inhibited all strains tested. Gentamicin was inactive although some strains of anaerobic cocci and Bacteroides were inhibited by 3.1 μg or less per ml. After 18 to 24 h of incubation, eight of the 101 strains had not grown sufficiently for MICs to be determined; for the 93 strains which had grown sufficiently, 93% of 744 MICs were the same or one concentration lower than the 48-h MICs.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodner S. J., Koenig M. G., Treanor L. L., Goodman J. S. Antibiotic susceptibility testing of Bacteroides. Antimicrob Agents Chemother. 1972 Aug;2(2):57–60. doi: 10.1128/aac.2.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood L. A. Tube dilution antimicrobial susceptibility testing: efficacy of a microtechnique applicable to diagnostic laboratories. Appl Microbiol. 1969 May;17(5):707–709. doi: 10.1128/am.17.5.707-709.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold S. M., Harada N. E., Miller L. G. Antibiotic susceptibility patterns as aids in classification and characterization of gram-negative anaerobic bacilli. J Bacteriol. 1967 Nov;94(5):1443–1450. doi: 10.1128/jb.94.5.1443-1450.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavan T. L., Town M. A. A microdilution method for antibiotic susceptibility testing: an evaluation. Am J Clin Pathol. 1970 Jun;53(6):880–885. doi: 10.1093/ajcp/53.6.880. [DOI] [PubMed] [Google Scholar]
- Harwick H. J., Weiss P., Fekety F. R., Jr Application of microtitration techniques to bacteriostatic and bactericidal antibiotic susceptibility testing. J Lab Clin Med. 1968 Sep;72(3):511–516. [PubMed] [Google Scholar]
- Ingham H. R., Selkon J. B., Codd A. A., Hale J. H. A study in vitro of the sensitivity to antibiotics of Bacteroides fragilis. J Clin Pathol. 1968 Jul;21(4):432–436. doi: 10.1136/jcp.21.4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kislak J. W. The susceptibility of Bacteroides fragilis to 24 antibiotics. J Infect Dis. 1972 Mar;125(3):295–299. doi: 10.1093/infdis/125.3.295. [DOI] [PubMed] [Google Scholar]
- Martin W. J., Gardner M., Washington J. A., 2nd In vitro antimicrobial susceptibility of anaerobic bacteria isolated from clinical specimens. Antimicrob Agents Chemother. 1972 Feb;1(2):148–158. doi: 10.1128/aac.1.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastro L. J., Finegold S. M. Bactericidal activity of five antimicrobial agents against Bacteroides fragilis. J Infect Dis. 1972 Jul;126(1):104–107. doi: 10.1093/infdis/126.1.104. [DOI] [PubMed] [Google Scholar]
- Pien F. D., Thompson R. L., Martin W. J. Clinical and bacteriologic studies of anaerobic gram-positive cocci. Mayo Clin Proc. 1972 Apr;47(4):251–257. [PubMed] [Google Scholar]
- Sapico F. L., Kwok Y. Y., Sutter V. L., Finegold S. M. Standardized antimicrobial disc susceptibility testing of anaerobic bacteria: in vitro susceptibility of Clostridium perfringens to nine antibiotics. Antimicrob Agents Chemother. 1972 Oct;2(4):320–325. doi: 10.1128/aac.2.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalons D. R., Thornsberry C., Dowell V. R., Jr Effect of culture medium and carbon dioxide concentration on growth of anaerobic bacteria commonly encountered in clinical specimens. Appl Microbiol. 1974 Jun;27(6):1098–1104. doi: 10.1128/am.27.6.1098-1104.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staneck J. L., Washington J. A., 2nd Antimicrobial susceptibilities of anaerobic bacteria: recent clinical isolates. Antimicrob Agents Chemother. 1974 Sep;6(3):311–315. doi: 10.1128/aac.6.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter V. L., Finegold S. M. Antibiotic disc susceptibility tests for rapid presumptive identification of Gram-negative anaerobic bacilli. Appl Microbiol. 1971 Jan;21(1):13–20. doi: 10.1128/am.21.1.13-20.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter V. L., Kwok Y., Finegold S. M. Susceptibility of Bacteroides fragilis to six antibiotics determined by standardized antimicrobial disc susceptibility testing. Antimicrob Agents Chemother. 1973 Feb;3(2):188–193. doi: 10.1128/aac.3.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikins T. D., Holdeman L. V., Abramson I. J., Moore W. E. Standardized single-disc method for antibiotic susceptibility testing of anaerobic bacteria. Antimicrob Agents Chemother. 1972 Jun;1(6):451–459. doi: 10.1128/aac.1.6.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins T. D., Thiel T. Modified broth-disk method for testing the antibiotic susceptibility of anaerobic bacteria. Antimicrob Agents Chemother. 1973 Mar;3(3):350–356. doi: 10.1128/aac.3.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabransky R. J., Johnston J. A., Hauser K. J. Bacteriostatic and bactericidal activities of various antibiotics against Bacteroides fragilis. Antimicrob Agents Chemother. 1973 Feb;3(2):152–156. doi: 10.1128/aac.3.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]



