Abstract
OBJECTIVE
Type 1 diabetes (T1D) results from the inflammatory destruction of pancreatic β-cells. In the present study, we investigated the effect of docosahexaenoic acid (DHA) supplementation on stimulated inflammatory cytokine production in white blood cells (WBC) from infants with a high genetic risk for T1D.
RESEARCH DESIGN AND METHODS
This was a multicenter, two-arm, randomized, double blind pilot trial of DHA supplementation, beginning either in the last trimester of pregnancy (41 infants) or in the first five months after birth (57 infants). Levels of DHA in infant and maternal red blood cell (RBC) membranes and in breast milk were analyzed by gas chromatography/mass spectrometry. Inflammatory cytokines were assayed from whole blood culture supernatants using the Luminex Multiplex assay after stimulation with high dose lipopolysaccharide (LPS), 1μg/mL.
RESULTS
The levels of RBC DHA were increased by 61–100% in treated compared to control infants at ages 6 to 36 months. There were no statistically significant reductions in production of the inflammatory cytokines, IL-1β, TNFα or IL-12p40 at any of the 6 time points measured. The inflammatory marker, hsCRP, was significantly lower in breast-fed DHA-treated infants compared to all formula-fed infants at age 12 months. Three infants (two received DHA) were removed from the study as a result of developing ≥ two persistently positive biochemical islet autoantibodies.
CONCLUSIONS
This pilot trial showed that supplementation of infant diets with DHA is safe and fulfilled the pre-study goal of increasing infant RBC DHA levels by at least 20%. Inflammatory cytokine production was not consistently reduced.
Keywords: Type 1 diabetes, Docosahexaenoic Acid (DHA), Cytokines, Breast Milk DHA, Red Blood Cell DHA
INTRODUCTION
Type 1 diabetes (T1D) is considered an autoimmune and inflammatory disease and is increasing at a rate of 3 to 5 % per year(1–3) although the peak age at diagnosis may be changing.(2) The majority of children who present with overt T1D before the age of 10 years seroconvert to autoantibody positivity by age 2 years.(4) Furthermore, the number of persistently positive biochemical islet autoantibodies relates to the more rapid development of T1D.(5) Recent evidence suggests increasing environmental influences and diminishing genetic roles in the etiology of the disorder.(6)
Using a case control design, cod liver oil taken either by the mother during pregnancy or by the child during the first year of life, correlated with a decreased risk of developing T1D.(7) In a subsequent study, a history of supplementation with cod liver oil during the first year of life was associated with a significantly lower risk of developing T1D before age 15 years (odds ratio [OR] of 0.7; P<0.001).(8) It was concluded that either docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), vitamin D, or all three in the cod liver oil, might have a protective effect in preventing T1D.
Food frequency questionnaires were used as part of a longitudinal, observational study called The Diabetes Autoimmunity Study in the Young (DAISY).(9) The intake of omega-3 fatty acids was significantly associated with a decreased risk of developing persistent multiple islet autoantibodies (hazard ratio [HR], 0.23; 95% CI, 0.09 – 0.58; P=0.002). In the same study, a case-cohort evaluation of the omega-3 fatty acid content of RBC membranes was also inversely associated with risk of autoantibody development (HR, 0.63; 95% CI, 0.41–0.96; P=0.03). However, further follow up did not reveal subsequent protection against development of T1D in subjects who developed autoantibodies.(10)
A recent report from the SEARCH for Diabetes in Youth Study Group evaluated 656 youth with T1D diagnosed in 2002–2005 and followed longitudinally.(11) They found that dietary intake of omega-3 fatty acids may be associated with a reduced risk of development of islet autoimmunity in children at increased genetic risk for T1D as well as with preservation of C-peptide production in youth recently diagnosed with T1D. As with most infants and children, intake of DHA and EPA were low in the DAISY and SEARCH studies. Previous studies have been epidemiologic in nature and have not included controlled randomized trials of DHA supplementation.
The purpose of the current pilot trial was to determine the effect of DHA supplementation, beginning in the last trimester of pregnancy or in the first five months after birth, on levels of RBC membrane DHA and on whole-blood stimulated inflammatory cytokine production in infants with a high genetic risk for developing T1D.
RESEARCH DESIGN AND METHODS
The research design, methods and pre-study goals of this nine-center, two-arm, randomized, double-masked controlled clinical pilot trial were described previously,(12) and a flow chart is available in Figure 1. The full protocol is available online at http://www.clinicaltrials.gov [NCT00333554]. Key aspects are summarized herein.
Figure 1.
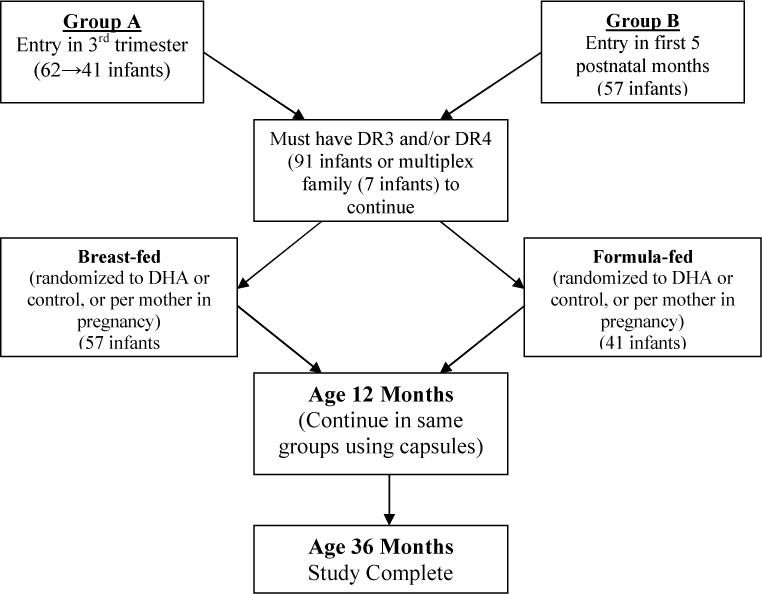
Flow Chart of Study Design.
Primary pre-study goals, in addition to safety, included showing an increase in infant RBC DHA levels of at least 20% and a reduction in the production of the inflammatory cytokine, IL-1ß, by at least 20% in DHA treated infants at age 12 months. The protocol was approved by the local institutional review boards, and all parents signed appropriate informed consent forms. All infants were required to have a first-degree relative with T1D and to have HLA DR3 and/or DR4 (91 infants) or to have multiple first-degree relatives with T1D (7 infants). It was predetermined that infants would be removed from the study if there was persistent detection of ≥ two positive islet autoantibodies.
Mothers of infants in Group A (41 infants) were enrolled in the last trimester of pregnancy (Figure 1). If the Group A neonate did not have DR3 and/or DR4 at birth (21 infants), they were discontinued from the study. Group A mothers were randomized to receive DHA (800 mg/day) or corn/soy oil (800 mg/day) in the last trimester of pregnancy and continued on this same dose after delivery if breast-feeding. Formula-fed infants received formula with 10.2 mg DHA/ounce (treatment) or 3.4 mg DHA/ounce (control).
Formula-fed infants and infants of breast-feeding mothers in Group B (57 infants) were randomized in the first five postnatal months to receive similar dosages of DHA or corn/soy oil as their counterparts in Group A. To keep infant ages consistent, Group B infants entering in the first five postnatal months had their initial follow-up blood draw at age 6 months (not 6 months after entry). Their mean age of entry was 4.0 months. If breast-feeding was discontinued in Group A or B mothers, the same treatment was continued via formula or capsules until age 12 months.
At age 12 months, all infants were treated with two 200-mg capsules per day of either DHA or corn/soy oil (per earlier randomization) until age 36 months. The DHA was in the form of DHASCO-5 oil (Martek Bioscience Corporation, Colombia, MD) and is derived from microalgae. It contains 39% of fatty acids as DHA, 15.3% as docosapentenoic acid (DPA) and 1.4% as EPA (three omega-3 fatty acids). Baseline data for the Group A and Group B infants is shown in Table 1.
Table 1.
Infant Baseline Information
| Entry A | Entry B | |
|---|---|---|
| Number of Infants | 41 | 57 |
|
| ||
| Method of Feeding** | ||
|
| ||
| Initially Breast-fed | 29 | 39 |
|
| ||
| Formula-fed | 23 | 18 |
|
| ||
| Male, % | 46 | 51 |
|
| ||
| Race/Ethnicity, % | ||
| White | 98 | 93 |
|
| ||
| Gestational Age, wk, Mean ± SD | 38 ± 1.5 | 38 ± 1.6 |
|
| ||
| Weight, kg, Mean ± SD | 3.6 ± 0.7 | 3.5 ± 0.7 |
|
| ||
| Delivery Type, % | ||
| Vaginal | 55 | 45 |
| Cesarean Section | 45 | 55 |
|
| ||
| Entry A (n = 41) | Entry B (n = 56)* | |
|
| ||
| HLA Alleles | ||
| DR3/DR4 | 4 | 10 |
| DR3/DR3 | 0 | 4 |
| DR4/DR4 | 6 | 5 |
| DR3/X | 11 | 11 |
| DR4/X | 16 | 23 |
| X/X | 4 | 3 |
Excludes infant accidentally admitted with protective gene (HLA DR0602)
Numbers do not add up to 98 as they include mothers who breast-fed at least 3 months, but then switched prior to 6 month visit to formula feeding. They were continued in the same randomized treatment group.
Infant follow-up visits for medical history and physical exam were every three months in the first year of life and every six months to age 36 months. Laboratory testing was at entry (HLA typing and islet autoantibodies) and at age six months and then every six months (islet autoantibodies, fatty acids, vitamin D, CRP, and stimulated cytokine production) up to 36 months. Fatty acids were measured by gas liquid chromatography/ mass spectrometry in maternal and infant RBC membranes and in breast milk (for mothers breast-feeding). Levels of the omega-3 fatty acids, DHA and DPA, are reported in this manuscript, whereas levels of other fatty acids will be reported in a future manuscript. Levels of stimulated IL-1ß, IL-6, IL-12p40 and TNFα cytokine production were assayed by Luminex multiplexassay from the supernatants of diluted whole blood cultured with 1 μg/mL of LPS for 16 hours. The quality control (QC) procedures included measures such as intra and inter assay variations, limits of detection and inclusion of internal run controls for low, intermediate and high levels. They also included controls for matrix and recombinant versus human peripheral blood mononucleated cell produced cytokines. Acceptable variance in cytokines was <15% CVs. The Laboratory Monitoring Committee reviewed and approved all QC reports. The hsCRP measurements were performed by Northwest Lipids Research lab (Seattle) using the Nephelometric method. Plasma vitamin D levels were determined by HPLC-tandem mass spectrometry. Three biochemical islet autoantibodies, ICA512, insulin and GAD65 were measured as previously described(13;14).
Statistical Analysis
The data was examined for normality prior to testing, and log transformations were made on the values where appropriate and noted. Statistical comparisons between groups were performed using Analysis of Covariance, Mixed linear model adjusting for kit effect, t-tests and Pearson’s correlation coefficient (on the log-transformed data where needed). Adjustments for study entry point were made. Values at the visits were compared (in contrast to changes in the values). The means and standard deviations were used as summary statistics unless stated otherwise. Where log transformations were performed, the geometric means and 95% confidence intervals were presented. In certain cases the least square means are presented adjusting for kit effect from a mixed linear model. A comparison to the median value was used to evaluate the combined changes in the three inflammatory cytokines, IL-1ß, IL-12p40 and TNFα in control versus treated infants at 12 to 36 months. Data from the three infants with persistent islet autoantibodies is included in the analyses prior to reaching the predetermined end-point (following which all three rapidly became insulin-dependent).
RESULTS
Maternal RBC Membrane DHA Levels (mg/dL)
Group A control (n=16) and treatment (n=21) mothers, who began DHA or placebo in the last trimester of pregnancy, and for whom adequate blood was available for analysis (37 of 42 mothers), had similar baseline mean [95% CI] DHA levels of 45.2 [36.2, 56.3] and 47.5 [38.5, 58.6] respectively. Upon enrollment of their infants at delivery, the DHA level had declined in the control mothers to 25.3 [18.9, 34.1] and increased in the DHA-treated mothers to 56.3 [48.4, 65.4] (P<0.001 in comparison of the two groups). Baseline pretreatment values in Group B control and treatment mothers (postnatal) were similar at 22.9 [17.3, 30.3] and 23.1 [18.4, 29.4], respectively, and were similar to those of the above Group A control mothers at enrollment.
Breast Milk DHA Levels (mg/dL)
Group A mothers, who began supplements in the last trimester of pregnancy, had two- to three-fold higher levels of DHA in breast milk of the 21 treated mothers compared to the 16 control mothers starting at the baseline post-delivery sample (P=0.008; Figure 2a). In Group B the control and the treatment mothers had baseline breast milk DHA levels that were almost identical (and similar to Group A control mothers [Figure 2b]). Thereafter, values were two- to three-fold higher in breast milk from the Group A or B treated mothers compared to the control mothers.
Figure 2. Breast milk DHA levels.
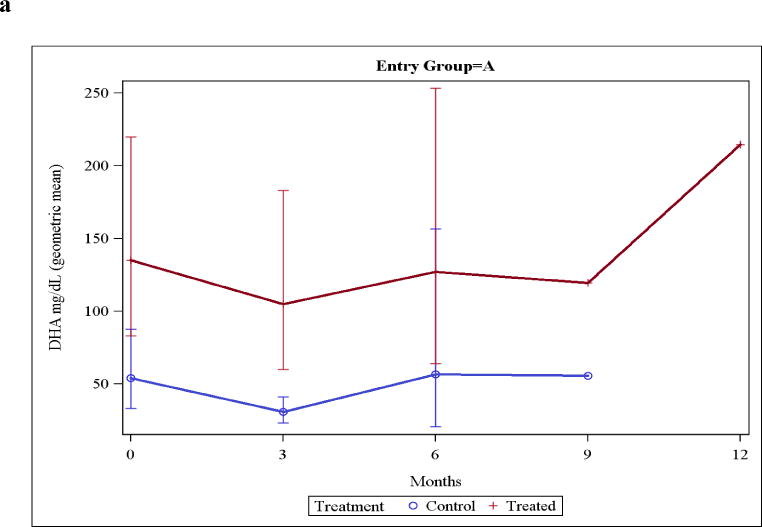
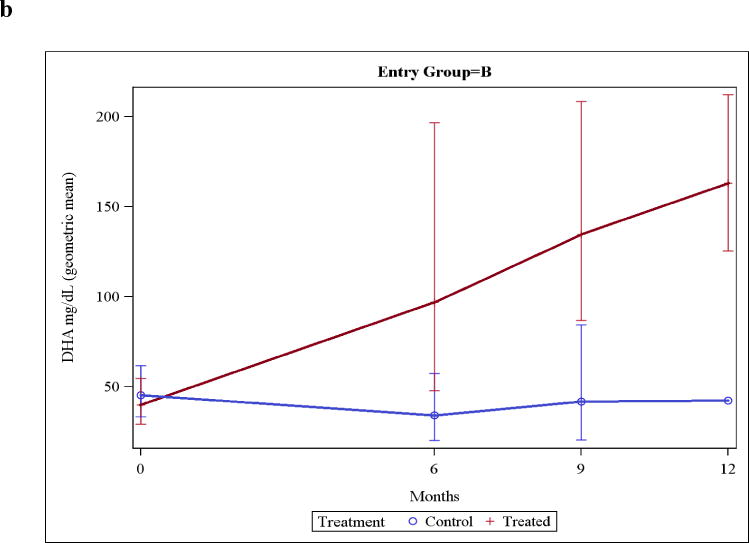
Figure 2a: Breast milk DHA levels in Group A mothers (who entered the trial in the last trimester of pregnancy). Insufficient mothers were still nursing at 12 months so a value is not provided. Values were significantly different (P<0.01) at 0 and 3 months (when adequate samples were available for analysis).
Figure 2b: Breast milk DHA levels in Group B mothers, with infants entering the trial anytime between birth and the fifth post-natal month. Zero-time reflects the enrollment time, with all infants then studied at age 6 months. Values were significantly different (P<0.01) at 6, and 9 months. Inadequate control samples were available at 12 months for statistical analysis.
Infant RBC Membrane DHA Levels (mg/dL)
At baseline, the infants of mothers who had received DHA in the last trimester of pregnancy had a mean [95% CI] level of 45.5. In comparison, the combined baseline value for Group A controls and all group B infants was 21.8 (P = 0.06). Infant RBC membrane DHA levels were consistently higher in infants who received DHA compared with control infants (Figure 3 and Appendix, Table 4).
Figure 3. RBC membrane DHA levels.
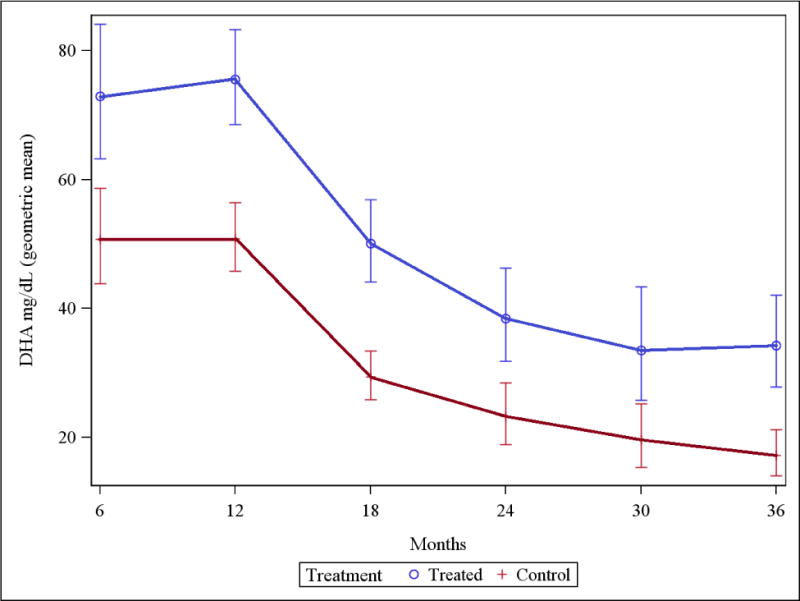
Infant RBC membrane DHA levels (mg/dL) were higher for all treatment infants compared to all control infants (P<0.001 for all comparisons except at age 30 months [P<0.01]). Data in Table 4, Appendix.
Infant RBC Membrane DPA Levels (mg/dL)
Analysis of the levels of the omega-3 fatty acid, DPA, in the infant RBC membranes showed no significant differences at ages 6 and 12 months, but were significantly lower (mean [CI] values in the treated infants at 18 months (9.9 [8.9, 11.0]) compared to the control infants (13.8 [12.4, 15.3], P<0.001). All infants had been on capsules with or without DHASCO-5 oil for the previous six months. Levels were also significantly lower in the treated infants at age 24 months (8.8 [7.3, 10.6] versus 14.1 [11.5, 17.2], P=0.001), but were not significantly lower at age 30 months (P=0.10) or 36 months(P=0.09).
Infant WBC Stimulated Inflammatory Cytokine Production
At six months, for all infants, there were no consistent effects of the increased infant RBC DHA levels on infant high-dose LPS-stimulated whole blood inflammatory cytokine production (Table 2). There were no statistical differences (P>0.05) between individual cytokine production in the control versus the treatment infants at any of the six time periods (Table 2). IL-1ß production was reduced by more than 20% (23%) at age 12 months for all DHA-treated infants only in comparison to the breast-fed control infants (the only infants who received no supplemental DHA). Their IL-1ß level at 12 months was 1,003 [278, 3621] pg/mL. At age 18 months, when all infants had received DHA or placebo from capsules for at least 6 months, the inflammatory cytokines IL-1ß, IL-12p40 and TNFα were significantly reduced as a group (P<0.05) in the DHA-treated infants. This was not true (P>0.05) for all other time periods. There was not a greater reduction at any time in inflammatory cytokine production in infants whose mothers began DHA in pregnancy (Group A) in comparison to values for the infants who began DHA in the first five months after birth (Group B) (data not shown).
Table 2. Infant Stimulated Inflammatory Cytokine Levels.
At 18–36 months, all infants received DHA or placebo capsules. The number of infants is shown in parentheses. Results are expressed in pg/mL and represent the geometric mean and 95% confidence interval. Numbers vary due to small amounts of blood available from some infants.
| IL-1β | TNFα | IL-12p40 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Treatment | Control | Treatment | Control | Treatment | ||||
| 6 months | 907 [664, 1239] (n=36) | 751 [560, 1005] (n=41) | (P=0.38) | 624 [458, 850] (n=36) | 588 [440, 786] (n=41) | (P=0.78) | 666 [545, 815] (n=36) | 755 [623, 914] (n=40) | (P=0.37) |
| 12 months | 861 [626, 1183] (n=30) | 763 [577, 1008] (n=39) | (P=0.57) | 838 [602, 1168] (n=30) | 585 [438, 783](n=39) | (P=0.11) | 786 [657, 939] (n=29) | 641 [548, 749] (n=38) | (P=0.09) |
| 18 months | 765 [569, 1028] (n=32) | 653 [488, 873] (n=30) | (P=0.43) | 1088 [817, 1449] (n=32) | 1009 [760, 1340] (n=30) | (P=0.70) | 240 [197, 292] (n=32) | 216 [178, 262] (n=30) | (P=0.43) |
| 24 months | 634 [466, 862] (n=27) | 589 [442, 784] (n=30) | (P=0.72) | 1095[809, 1481] (n=27) | 813 [613, 1078] (n=30) | (P=0.15) | 195[159, 240] (n=27) | 171 [141 207] (n=30) | (P=0.35) |
| 30 months | 636[460, 879] (n=26) | 503[370, 684] (n=28) | (P=0.27) | 903 [617, 1321] (n=26) | 735 [542, 996] (n=28) | (P=0.32) | 160 [129, 199] (n=26) | 183 [149, 225] (n=28) | (P=0.34) |
| 36 months | 569 [390, 830] (n=18) | 514 [350, 756] (n=16) | (P=0.69) | 903 [617, 1321] (n=18) | 680 [459, 1007] (n=16) | (P=0.27) | 189 [146, 244] (n=18) | 140 [108, 183] (n=16) | (P=0.16) |
There also were no significant differences, using regression analysis, between control and treatment groups in relation to DPA levels and stimulated IL-1ß, IL-12p40 or TNFα production at the various time points, except for lower levels of IL-12p40 at 30 months (P=0.02) and of IL-1ß at 36 months (P=0.04).
IL-6 and IL-10 were also measured in all control and treated infants. There were no consistent differences between groups for either cytokine (data not shown).
hsCRP Levels (mg/dL)
The mean hsCRP level at 12 months for the breast-fed treatment infants was 0.012 [0.003, 0.048]. Breast-fed infants of all control mothers (Group A or B), who were not receiving any DHA had a mean hsCRP level at 12 months of 0.0024 [0.002, 0.315] (P>0.05 in comparison to the breast-fed treatment infants [data above]). The mean hsCRP level for all formula-fed infants (control and treatment) at 12 months was 0.086 (P=0.007 in comparison to breast-fed treatment infants [data above]). Formula-fed control infants had a mean hsCRP level of 0.130 [0.053, 0.319]. The differences in hsCRP levels between infants formerly nursed versus formula-fed were no longer significant (0.007 versus 0.033, respectively; P=0.16) at 18 months and thereafter when breast feeding had been discontinued.
Infant Vitamin D Levels (ng/mL)
Serum vitamin D levels (arithmetic mean [SD]) for all infants were 40.3 [11.9] at six months (24 samples), 37.2 [11.3] at 12 months (51 samples) and 34.4 [10.1] at 18 months (46 samples). The numbers in parenthesis reflect the number of samples available after first priority sampling for DHA and cytokine levels. At 12 months, breast-fed infants had lower vitamin D levels (31.7 [12.1]) (13 samples) than did formula-fed infants (39.5 [9.8]) (36 samples) (P=0.026). At 18 months, the DHA-treated infants mean [SD] vitamin D level (22 samples) was 37.8 [11.7] compared to a level of 31.3 [7.3] for 24 infants in the placebo group (P=0.027), although vitamin D intake was similar. After 18 months, the differences between control and treatment infants were not significant (P>0.05). Vitamin D levels for the three infants removed from the study due to persistence of two or more positive islet autoantibody values were similar to the other infants: 25.8, 49.1, and 31.5 at 12 months and 31.7, 33.4, and 32.3 at 18 months.
Infants with Two or More Persistent Islet Autoantibodies
Three infants were removed from the study, two at 12 months and one at 18 months, due to persistence of two or more positive autoantibodies. All three developed diabetes within two months. Among the three infants, infant one had high (>2SD of normal) levels of IL-12P40 and CRP, infant 2 had a high level of IL-10, and infant 3 had high levels of TNFα, IL-6 and CRP at the time of diagnosis (Table 3, Appendix). In addition, the IL-6 levels for the two infants removed at 12 months were approximately double the level of the autoantibody-negative infants (but not >2 SD of normal). Elevated cytokine levels fell following diagnosis for infants one and two who were removed at 12 months (not available for infant three – removed at 18 months). Infant 1 received formula, whereas infants 2 and 3 received formula plus supplemental DHA (Table 3, Appendix).
DISCUSSION
A pre-study goal in addition to safety, was to determine if infant RBC DHA levels could be increased by at least 20% as a result of DHA supplementation at age 12 months. An increase of 61 to 100% was found at ages six to 36 months, satisfying the first goal. A second goal was to show a decrease in production of a major inflammatory cytokine, IL-1β, by at least 20% at age 12 months as a result of DHA supplementation. When all control infants were considered (including formula-fed infants who received one-third the amount of DHA of treatment infants), there was an 11% reduction. The reduction was by 23% for all infants in the DHA treatment group compared to the breast-fed control infants (the only infants at 12 months who received no supplemental DHA).
Baseline RBC DHA levels in the infants of the mothers receiving DHA during pregnancy were significantly higher than for control neonates. Maternal fish oil supplementation during pregnancy has previously been shown to promote higher concentrations of DHA in the blood of newborn infants.(15;16) Similarly, in another randomized clinical trial, supplementation with DHA (200 mg/day of the same DHASCO™ oil used in the current study) for two weeks during the 4th to 6th post partum weeks resulted in almost two-fold higher DHA levels in the 5 supplemented versus the 5 control mother’s breast milk.(17) Breast milk DHA levels in the current study were consistently higher in milk from the treatment mothers compared to the control mothers. The higher DHA levels in the present study were important both in showing compliance and in evaluating a possible effect on inflammatory cytokine production.
A previous study by Endres, et. al.(18) demonstrated a significant reduction of IL-1ß, a major inflammatory cytokine, in normal adults as a result of 18 grams/day of fish oil supplementation. They noted a five-fold increase in monocellular membrane phospholipid fatty acid, in contrast to the 50% increase in RBC membrane DHA levels found in the current study. Although these levels are not directly comparable, it is possible that even higher dosages of DHA than used in the present study are needed to further reduce IL-1β production. The Endres study was a stimulus for the current study. In addition, animal studies have shown increased levels of omega-3 fatty acids to confer strong resistance to cytokine-induced pancreatic β-cell destruction.(19)
Inflammatory cytokine production from high-dose LPS-stimulated whole blood cultures from infants supplemented with DHA over an extended period of time (three years) has not been previously reported. It is important to point out that the in vitro assay used to stimulate inflammation, 1ug/ml of LPS, is a high dose and greater than used in most studies of this type. High dose LPS was chosen for this study as reports suggest infants are poor responders to this Toll-like receptor 4 ligand. However, the subjects in this study showed a high-level response, much greater than infants with no family history of autoimmunity (data not shown; manuscript in preparation). In addition, we used a supplementation scheme that delivered a dose equivalent to two grams of DHA per day in an adult. It may be that higher doses of DHA are required to attain higher levels of cytokine suppression in T1D at-risk subjects. Future studies will need to address both assay format and DHA dosing. Alternatively, if DHA supplementation has an effect on reducing the likelihood of autoantibodies or of T1D, as suggested in epidemiologic studies,(7–11) it may be related to a mechanism other than reduction of inflammatory cytokine production. It is important that there were no recognized adverse events as a result of the study (one infant reportedly had a “body odor”).
The finding of significantly reduced DPA, also an omega 3 fatty acid, in the treated infants at 18, 24 and 36 months is unexplained. The lipid supplement had 15.3% DPA, which may not get taken up into the RBC membrane. Alternatively, DHA could reduce DPA due to its inhibitory effects on the elongase enzyme necessary to convert EPA to DPA (20:5 n-3 to 22:5 n-3). This inhibition has been described in mice fed high DHA(20) and in a patient with adrenomyeloneuropathy (AMN) treated with DHA.(21)
A major limitation of this study was the inclusion of small amounts of DHA in the control formula. This was included to make entry into the system more acceptable. This may have related to not finding differences in cytokine production at six months between control and treatment infants. However, the production from control breast-fed infants (true controls) also showed no differences at six months compared to DHA-treated infants. Between ages 12 and 36 months, all infants received only control or treatment capsules, and differences in cytokine production were still not consistently observed. A second limitation relates to no measures of immune cell function or types, which might help to support (or not) the findings on inflammatory cytokine production. Another limitation of this study was the relatively small number (98) of infants. The study was designed as a pilot trial and a much larger study would be required to evaluate protection from development of T1D.
Inflammation, as measured by elevated high-sensitivity C – reactive protein (hsCRP) levels, was evaluated as a secondary inflammatory mediator in this study (changes in cytokine production being primary). It is unknown at this time if changes in either of these inflammatory mediators is more relevant. Elevated hsCRP levels were previously suggested to be a factor when infants with a high genetic risk for T1D were followed to the development of diabetes.(22) The level of hsCRP was significantly lower (P=0.007) at age 12 months in the present study in breast-fed infants whose mothers received DHA compared to all formula-fed infants (with or without DHA). (Breast-feeding was discontinued in all infants after age 12 months.) It is possible that the nutritional matrix of breast milk, particularly with increased DHA levels, has an anti-inflammatory effect. Alternatively, formulas may enhance inflammatory responses and cows’ milk protein and/or bovine insulin have been suggested as possible environmental inflammatory agents in the etiology of T1D.(23) Although the differences may pertain only to HLA DR3 and/or DR4 infants, the protection against inflammation as a result of breast feeding and DHA deserves further investigation. Additional studies of the role of nutrition in the etiology of islet autoimmunity and of T1D will continue to be important.
Acknowledgments
This study was conducted by the Type 1 Diabetes TrialNet Study Group, a clinical trials network funded by NIH through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Center for Research Resources with support of the Juvenile Diabetes Research Foundation International, the American Diabetes Association and the Children With Diabetes Foundation.
Appreciation is expressed to Martek Biosciences Corporation (Columbia, MD for the DHA (DHASCO-S) and to Mead Johnson Nutrition (Evansville, IN) for the two infant formulas. Study Group Members are listed elsewhere(12).
D.D. and H.R. cared for the research subjects, at their institutions and reviewed/edited the manuscript. D.B. and J.K. did all of the statistical analyses and reviewed/edited the manuscript. L.R.M, M.C.S. and S.C. helped in the development of the initial protocol, study conduct oversight, researched data, and reviewed/edited the manuscript. H.P.C. helped develop the initial protocol, researched data, study conduct oversight, and wrote the original manuscript.
Appendix
Table 3.
Inflammatory Cytokine and CRP Levels in Infants Removed from the Study**
| All islet autoantibody-negative infants* |
Infant 1 (Formula) |
Infant 2 (Formula + DHA) |
Infant 3 (Formula + DHA) |
|||||
|---|---|---|---|---|---|---|---|---|
| Cytokine | 12 mo | 18 mo | 12 mo | 18 mo | 12 mo | 18 mo | 12 mo | 18 mo |
| IL-1β | 1,065 (744) | 636 (507) | 1,295 | 353 | 1,155 | 281 | 797 | 1,640 |
| IL-6 | 28,765 (24,820) | 14,618 (10,540) | 66,160 | 25,680 | 63,280 | 22,560 | 25440 | 94,400 |
| IL-12p40 | 786 (425) | 790 (994) | 1,885 | 613 | 436 | 432 | 672 | 872.5 |
| IL-10 | 521 (35) | 381 (193) | 505 | 216 | 760 | 141 | 894 | 542.5 |
| TNFα | 920 (590) | 636 (450) | 1,575 | 335 | 464 | 517 | 788 | 2,050 |
| CRP | 0.03 (0.71) | 0.08 (0.13) | 2.01 | 0.24 | 0.02 | 0.02 | 0.09 | 4.10 |
Results are expressed as the mean (SD). Cytokine levels are in pg/mL and CRP levels in mg/dL. Infants are included irrespective of treatment groups.
Infants removed due to presence of two or more positive biochemical islet autoantibodies on two occasions.
Table 4.
Infant RBC Membrane DHA Levels
| Age | Treatment Group† | Control Group† |
|---|---|---|
| 6 months | 72.9 (n=42)‡** [63.2, 84.1] |
50.7 (n=40) [43.8, 58.7] |
| 12 months | 75.5 (n=45)** [68.5, 83.3] |
50.8 (n=40) [45.8, 56.4] |
| 18 months | 50.1 (n=35)** [44.1, 56.9] |
29.4 (n=35) [25.9, 33.4] |
| 24 months | 38.4 (n=31)** [31.8, 46.3] |
23.2 (n=26) [18.9, 28.5] |
| 30 months | 33.5 (n=28)* [25.8, 43.4] |
19.6 (n=30) [15.3, 25.2] |
| 36 months | 34.2 (n=22)** [27.8, 42.1] |
17.2 (n=22) [14.0, 21.2] |
Values represent the mean [95% CI]
Numbers in parentheses represent the number of samples available for analysis
p<0.01
p<0.001 in relation to control infants
Footnotes
No conflicts of interest were reported by any co-investigators.
Reference List
- 1.Green A, Patterson CC. Trends in the incidence of childhood-onset diabetes in Europe 1989–1998. Diabetologia. 2001 Oct;44(Suppl 3):B3–B8. doi: 10.1007/pl00002950. [DOI] [PubMed] [Google Scholar]
- 2.Hummel K, McFann KK, Realsen J, Messer LH, Klingensmith GJ, Chase HP. The increasing onset of type 1 diabetes in children. J Pediatr. 2012 Oct;161(4):652–7. doi: 10.1016/j.jpeds.2012.03.061. [DOI] [PubMed] [Google Scholar]
- 3.Onkamo P, Vaananen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of Type I diabetes–the analysis of the data on published incidence trends. Diabetologia. 1999 Dec;42(12):1395–403. doi: 10.1007/s001250051309. [DOI] [PubMed] [Google Scholar]
- 4.Knip M, Virtanen SM, Akerblom HK. Infant feeding and the risk of type 1 diabetes. Am J Clin Nutr. 2010 May;91(5):1506S–13S. doi: 10.3945/ajcn.2010.28701C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, Chase HP, Eisenbarth GS. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes. 1996 Jul;45(7):926–33. doi: 10.2337/diab.45.7.926. [DOI] [PubMed] [Google Scholar]
- 6.Vehik K, Hamman RF, Lezotte D, Norris JM, Klingensmith GJ, Rewers M, Dabelea D. Trends in high-risk HLA susceptibility genes among Colorado youth with type 1 diabetes. Diab care. 2008 Jul;31(7):1392–6. doi: 10.2337/dc07-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stene LC, Ulriksen J, Magnus P, Joner G. Use of cod liver oil during pregnancy associated with lower risk of Type I diabetes in the offspring. Diabetologia. 2000 Sep;43(9):1093–8. doi: 10.1007/s001250051499. [DOI] [PubMed] [Google Scholar]
- 8.Stene LC, Joner G. Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: a large, population-based, case-control study. Am J Clin Nutr. 2003 Dec;78(6):1128–34. doi: 10.1093/ajcn/78.6.1128. [DOI] [PubMed] [Google Scholar]
- 9.Norris JM, Yin X, Lamb MM, Barriga K, Seifert J, Hoffman M, Orton HD, Baron AE, Clare-Salzler M, Chase HP, Szabo NJ, Erlich H, Eisenbarth GS, Rewers M. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA. 2007 Sep 26;298(12):1420–8. doi: 10.1001/jama.298.12.1420. [DOI] [PubMed] [Google Scholar]
- 10.Miller MR, Yin X, Seifert J, Clare-Salzler M, Eisenbarth GS, Rewers M, Norris JM. Erythrocyte membrane omega-3 fatty acid levels and omega-3 fatty acid intake are not associated with conversion to type 1 diabetes in children with islet autoimmunity: the Diabetes Autoimmunity Study in the Young (DAISY) Pediatr Diabetes. 2011 Dec;12(8):669–75. doi: 10.1111/j.1399-5448.2011.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer-Davis EJ, Dabelea D, Crandell JL, Crume T, D’Agostino RB, Jr, Dolan L, King IB, Lawrence JM, Norris JM, Pihoker C. The N. Nutritional Factors and Preservation of C-Peptide in Youth With Recently Diagnosed Type 1 Diabetes: SEARCH Nutrition Ancillary Study. Diab care. 2013 Jul;36(7):1842–50. doi: 10.2337/dc12-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chase HP, Lescheck E, Rafkin-Mervis L, Krause-Steinrauf H, Chritton S, Smith A, Adams SSJ, Clare-Salzler M. Nutritional Intervention to Prevent (NIP) Type 1 Diabetes a Pilot Trial. ICAN: Infant, Child & Adolescent Nutrition. 2013:98–107. [Google Scholar]
- 13.Bonifacio E, Yu L, Williams AK, Eisenbarth GS, Bingley PJ, Marcovina SM, Adler K, Ziegler AG, Mueller PW, Schatz DA, Krischer JP, Steffes MW, Akolkar B. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab. 2010 Jul;95(7):3360–7. doi: 10.1210/jc.2010-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skyler JS, Greenbaum CJ, Lachin JM, Leschek E, Rafkin-Mervis L, Savage P, Spain L. Type 1 Diabetes TrialNet–an international collaborative clinical trials network. Ann N Y Acad Sci. 2008 Dec;1150:14–24. doi: 10.1196/annals.1447.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connor WE, Lowensohn R, Hatcher L. Increased docosahexaenoic acid levels in human newborn infants by administration of sardines and fish oil during pregnancy. Lipids. 1996 Mar;31(Suppl):S183–S187. doi: 10.1007/BF02637073. [DOI] [PubMed] [Google Scholar]
- 16.van Houwelingen AC, Sorensen JD, Hornstra G, Simonis MM, Boris J, Olsen SF, Secher NJ. Essential fatty acid status in neonates after fish-oil supplementation during late pregnancy. Br J Nutr. 1995 Nov;74(5):723–31. doi: 10.1079/bjn19950175. [DOI] [PubMed] [Google Scholar]
- 17.Fidler N, Sauerwald T, Pohl A, Demmelmair H, Koletzko B. Docosahexaenoic acid transfer into human milk after dietary supplementation: a randomized clinical trial. J Lipid Res. 2000 Sep;41(9):1376–83. [PubMed] [Google Scholar]
- 18.Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JW, Cannon JG, Rogers TS, Klempner MS, Weber PC. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989 Feb 2;320(5):265–71. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 19.Wei D, Li J, Shen M, Jia W, Chen N, Chen T, Su D, Tian H, Zheng S, Dai Y, Zhao A. Cellular production of n-3 PUFAs and reduction of n-6-to-n-3 ratios in the pancreatic beta-cells and islets enhance insulin secretion and confer protection against cytokine-induced cell death. Diabetes. 2010 Feb;59(2):471–8. doi: 10.2337/db09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berger A, Mutch DM, German JB, Roberts MA. Dietary effects of arachidonate-rich fungal oil and fish oil on murine hepatic and hippocampal gene expression. Lipids Health Dis. 2002 Oct 21;1:2. doi: 10.1186/1476-511X-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terre’Blanche G, van der Walt MM, Bergh JJ, Mienie LJ. Treatment of an adrenomyeloneuropathy patient with Lorenzo’s oil and supplementation with docosahexaenoic acid–a case report. Lipids Health Dis. 2011;10:152. doi: 10.1186/1476-511X-10-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chase HP, Cooper S, Osberg I, Stene LC, Barriga K, Norris J, Eisenbarth GS, Rewers M. Elevated C-reactive protein levels in the development of type 1 diabetes. Diabetes. 2004 Oct;53(10):2569–73. doi: 10.2337/diabetes.53.10.2569. [DOI] [PubMed] [Google Scholar]
- 23.Akerblom HK, Virtanen SM, Ilonen J, Savilahti E, Vaarala O, Reunanen A, Teramo K, Hamalainen AM, Paronen J, Riikjarv MA, Ormisson A, Ludvigsson J, Dosch HM, Hakulinen T, Knip M. Dietary manipulation of beta cell autoimmunity in infants at increased risk of type 1 diabetes: a pilot study. Diabetologia. 2005 May;48(5):829–37. doi: 10.1007/s00125-005-1733-3. [DOI] [PubMed] [Google Scholar]


