Abstract
This study further investigated the specific contributions of the medial temporal lobe structures to contextual recognition memory. Monkeys (Macaca mulatta) with either neurotoxic lesions of the hippocampus, aspiration lesions of the perirhinal cortex and parahippocampal areas TH/TF, or sham operations were tested on five conditions of a visual-paired comparison (VPC) task in which 3-dimensional objects were presented over multicolored backgrounds. In two conditions (Conditions 1 and 2: Context-changes), the sample object was presented on a new background during the retention tests, whereas in the three others (Conditions 3–5: No-context-changes) the sample object was presented over its familiar background. Novelty preference scores of control animals were weaker, but still significantly different from chance, in the Context-changes conditions than on the No-context-changes conditions. Animals in the three experimental groups showed strong preference for novelty on the No-context-change conditions, but weaker novelty preference on the Context-change conditions. Thus, animals in all three lesion types had greater difficulty recognizing an object when its background was different from that used during encoding. The data are consistent with the view that the hippocampal formation, areas TH/TF, and perirhinal cortex contribute interactively to contextual memory processes.
Keywords: Visual Paired Comparison, contextual memory, rhesus monkeys, contextual binding
1-Introduction
Context refers to the general information that is associated with a specific stimulus at the time of encoding. It includes the environment in which it occurs, the place where it is located (spatial context) and the time during which it happens (temporal context). Thus, context can be anything associated with the to-be-remembered item in an event, and as such, can vary substantially in its complexity. It can be as simple as the color of text on a word list, or as complex as the physical environment in which learning took place. Several studies in humans have demonstrated a decreased memory performance when context is changed between encoding and retrieval after changing semantic (Light and Carter-Sobel, 1970; Reder et al., 1974; Stumpfel and Kirsner, 1986; Tulving and Thompson, 1973), cue specific (Dalton, 1993; Hollingworth, 2006; Park et al., 1984, 1987; Russo et al., 1999; Smith and Vela, 1986), olfactory (Cann and Ross, 1989), auditory (Geiselman and Bjork, 1980) or environmental (Canas and Nelson, 1986; Emmerson, 1986; Smith, 1985; Smith and Vela, 1986) contexts. Further, both rodent (Dellu et al., 1997; Dix and Aggleton, 1999) and primate (Pascalis et al., 2009) studies have shown that, although animals are able to recognize objects in a changed background context, recognition memory was stronger when the familiar context was used in the retrieval phase. Thus, as in humans, recognition memory processes in animals are also modulated by memory for contextual information.
The study of the neural substrates responsible for contextual memory has received increased attention in the last decade as a result of recent theoretical considerations of the role of the medial temporal lobe (MTL) structures in memory. There is general agreement that, within the MTL, the hippocampus acts in concert with the parahippocampal and perirhinal cortex to support recognition memory. In this view, the hippocampus associates (or binds) contextual information from the parahippocampal cortex with object representations from the perirhinal cortex, and encodes and maintains relationship among stimuli (Davachi, 2006; Diana et al., 2007; Eichenbaum, 2001; Eichenbaum et al., 2007; Montaldi et al., 2010; Sutherland and Rudy, 1989). There exists growing evidence to support the role of the hippocampus and parahippocampal cortex in contextual memory, although the role of the perirhinal is still debated.
Evidence of the role of the hippocampus in contextual memory comes from both human and animal studies. Patients with amnesia resulting from either Korsakoff's syndrome or MTL damage were not able to benefit from the use of temporal (Parkin et al., 1990), semantic (Mayes et al., 1992) or visual (Chun and Phelps, 1999) contextual cues during memory tasks and showed impaired performance as compared to control subjects. This impairment is also reported in patients with more selective bilateral hippocampal lesions or with left unilateral hippocampal damage (Horner et al., 2012; Spiers et al., 2001a; 2001b). The effect of context information on memory performance is also exemplified in neuroimaging studies of normal subjects indicating hippocampal activations during either recognition of contextual information associated with objects (Burgess et al., 2001; Rugg et al., 2012) and with words (Maratos et al., 2001), or after changes in context surrounding a stimulus (Dolan and Strange, 2002). Similarly, fornix transections impaired memory performance when stimuli were either complex naturalistic scenes (Gaffan, 1993; 1994b), or objects embedded in complex scenes (Gaffan, 1994a), and hippocampal lesions impaired recognition memory performance when a change of context occurred between encoding and retrieval (Eacott and Gaffan, 2005; Kesner and Hardy, 1983; Mumby et al., 2002; Pascalis et al., 2009; Piterkin et al., 2008). Impaired memory was also found in a contextual discrimination task where the background context signaled the rewarded object (Ridley et al., 2001) and in a discrimination task for which the use of contextual background information enhanced memory performance (Dore et al., 1998). Hippocampal place fields and neuronal responses to task-relevant stimuli are also highly sensitive to changes in the context, even when the contexts are defined by abstract task demands rather than the spatial geometry of the environment, suggesting that place fields reflect a more general context processing function of the hippocampus (for review see Smith and Mizumori, 2006; Komorowski et al., 2009; Manns and Eichenbaum, 2009). Finally, molecular activation studies revealed that initial introduction of rats into a novel environment or in an environment different from that used in the exploration phase increases c-fos activation or Arc mRNA levels in the hippocampal formation (Guzowski et al., 1999; Radulovic et al., 1998; Vann et al., 2000). Thus, there exists substantial evidence for a contribution of the hippocampus in forming contextual memory representations.
Recent neuroimaging studies in humans have also implicated the parahippocampal cortex in contextual memory either during scene processing (Epstein and Kanwisher, 1998; Epstein and Higgins, 2006; Epstein and Ward, 2010; Kohler et al., 2002; Mundy et al., 2013), object identification (Bar and Aminoff, 2003), intentional retrieval of visual context information (Hayes et al., 2004) or familiarity-based recognition (Hayes et al., 2007; Martin et al., 2013). In addition, activations of the hippocampus and parahippocampal cortex have been reported in humans during binding operations between objects and context (Goh et al., 2004). More recently, Howard and colleagues (2011) provided compelling evidence that the parahippocampal cortex supports neural representations of the global context within which events occur, whereas the hippocampus plays a more specific role in the rapid creation of item-context bindings. Similarly, animal lesion studies have demonstrated that the postrhinal cortex in rodents (homologous to the parahippocampal cortex in primates; Burwell et al., 2002) is critical for learning about the within-scene position or context (Eacott and Gaffan, 2005; Norman and Eacott, 2005). Finally, molecular activation studies in rodents revealed elevated c-fos in the postrhinal cortex when the environmental context was changed between study and test (Vann et al., 2000). Thus, there is a growing support for a role of the parahippocampal cortex in contextual recognition memory.
Studies investigating the contribution of the perirhinal cortex in contextual recognition memory have given contradictory results. For example, damage to the perirhinal cortex in monkeys impaired the learning of complex scenes (Gaffan, 1994a) and object identification when the objects were embedded in complex scenes (Buckley and Gaffan, 1998). In contrast, using a spontaneous object recognition paradigm, Norman and Eacott (2005) reported that animals with perirhinal cortex were unimpaired on memory for object in context. Similarly, changing the environment between study and test in a recognition task did not lead to any change in c-fos activation in the perirhinal cortex (Vann et al., 2000). Thus, the evidence so far suggests that the hippocampus and parahippocampal cortex, but not the perirhinal cortex, may be more importantly involved in contextual memory processes. Nevertheless, one short coming of these process-specific dissociations from animal studies is that they are mostly derived from comparing findings across studies that varied widely in the specific structures damaged (some compared perirhinal/hippocampus, others compared hippocampus/parahippocampal cortex) and the types of behavioral paradigms used to assess memory (problem-solving task versus incidental memory task). Furthermore, extent of damage could also be a potential confounding factor. For example, in our earlier studies (Pascalis et al., 2009) using a visual paired-comparison task in which backgrounds onto which objects were presented changed between encoding and retrieval, animals with neonatal hippocampal lesions showed a significant decrease in novelty preference as compared to controls; however, the hippocampal lesions were performed by aspiration procedures and extended to include the parahippocampal cortex, such that the effects of lesions on memory performance could not be solely ascribed to the hippocampal damage.
Thus, the aim of the present study was to directly compare the effects of selective damage to the hippocampus, parahippocampal cortex, and perirhinal cortex in monkeys on contextual memory using an incidental recognition memory task. The visual paired-comparison (VPC) task was modified so that the backgrounds onto which objects were presented were changed between the familiarization (or encoding) phase and the retention (or retrieval) phase.
2.0 - Method
2.1 - Subjects
Subjects were eighteen rhesus monkeys (Macaca mulatta) of both sexes. Six monkeys (all male) received selective ibotenic acid lesions of the hippocampal formation (Group H), three monkeys (1 male and 2 females) received aspiration lesions of the perirhinal cortex (Group PRh), three (3 males) received aspiration lesions of areas TH/TF of the parahippocampal gyrus (Group TH/TF) and six (all male) were sham-operated controls (Group C). Subjects weighed 5 to 12 kg and were aged 3 to 12 years at the time of testing. They were housed individually, fed ad libitum Purina Monkey Chow and water, and were maintained on a 12:12 hour light-dark cycle. Monkeys were given multi-vitamins daily and fresh fruit weekly. There were no food or water manipulations.
Monkeys in Groups PRh and TH/TF were tested on Transverse Patterning, Object VPC, Spatial VPC, DNMS and dDNMS before beginning the present experiment (Alvarado et al., 2002; Bachevalier and Nemanic, 2008; Nemanic et al., 2004). Monkeys in Groups H and C were tested on social behavior and food preference (Machado and Bachevalier, 2006, 2007a, 2007b) before beginning the present experiment.
2.2 - Neuroimaging and Surgical procedures
All procedures were approved by the Committee on Laboratory Animal Welfare of the University of Texas Health Science Center at Houston. MR imaging procedures were performed while the animals were sedated with ketamine/xylazine (7:3 mixture of Ketamine hydrochloride, 100mg/ml, and Xylazine, 20mg/ml, i.m.) and their head secured in a non-ferromagnetic stereotaxic apparatus (Crist Instruments Co., Inc., Damascus, MD). Measurements of the positions of the ear bars, mouthplate and mouthpiece on the stereotaxic apparatus were recorded to permit precise re-positioning of the animal during all subsequent surgical and neuroimaging procedures. All imaging procedures were carried out using a 1.5 T GE Signa scanner (GE Medical Systems, Milwaukee, WI) and described in details in previous publication (Nemanic et al., 2004). Briefly, a pre-surgical T1-weighted scan was acquired 1–3 weeks prior to the surgical procedures to determine coordinates of each injection site for neurotoxic hippocampal lesions (Group H) or to visualize the sulcal borders of the targeted cortical lesions for Groups PRh and TH/TF. All animals in Group H received a second scanning procedures 7–10 days after surgery, including a T1-weighed scan for structural information and a Fluid Attenuated Inversion Recovery (FLAIR) scan to visualize areas of hyperintensity caused by edema and indicative of cell death.
All surgical procedures were performed under deep anesthesia with aseptic techniques. The animal was sedated with ketamine hydrochloride (10 mg/kg, i.m.), brought to the surgical facility, intubated with an endotracheal canula and maintained on Isoflurane gas (1.0–2.0%, v/v, to effect) for the duration of the surgery. Monkeys received an intravenous fluid containing 0.45% sodium chloride to maintain hydration. Heart rate, respiration rate, blood pressure, expired CO2, and body temperature were monitored throughout the procedure and until the monkey recovered fully from anesthesia. During surgery, the animal was placed on a warm water-heating pad to prevent hypothermia. Emla cream was applied to the ear canals to reduce pain from the ear bars, and ophthalmic ointment was applied to the eyes to prevent ocular dryness. Following disinfections (Nolvasan solution) of the scalp and application of local anesthetic (Marcaine 25%, 1.5ml) along the incision line, the skin was cut from the orbit to the occiput, and connective tissue and the temporal muscles were gently retracted. Following the brain lesions, the dura openings and all tissues were closed in anatomical layers. The animal was recovered in the surgical facility until it could maintain an SPO2 of >88% for one hour and regain consciousness. The monkey was then moved to a recovery room, placed into a primate cage, and housed there for 2–3 days, after which it was returned to its home cage.
All monkeys received pre- and post-surgical treatment that began 12 hours prior to and continuing for one week after surgery. Monkeys received dexamethazone sodium phosphate (0.4 mg/kg, i.m.) and Cephazolin (Bristol-Myers Squib, 25mg/kg, i.m.) to reduce inflammation and protect against infection, respectively. For 3 days after surgery the monkeys also received an analgesic (acetaminophen 10 mg/kg, p.o.).
2.2.1 - Neurotoxic hippocampal lesions
Hippocampal lesions were produced by MRI-guided injections of ibotenic acid as previously described (Nemanic et al., 2004). A bone flap was made and small slits were cut in the dura over the location of the injection sites and injections occurred simultaneously in the two hippocampi. For the posterior two-thirds of the hippocampal formation, one injection site was selected to target the center of the body of the hippocampus and spaced 1.5-mm apart in the antero-posterior plane. For the most anterior portion of the hippocampus, where the uncus was clearly visible, two sites were selected to target the hippocampus body laterally and the uncus medially. A total of 11 sites per hippocampus were stereotaxically injected via a 10 μl Hamilton syringe, held in a Kopf electrode manipulator (David Kopf Instruments, Tujunga, CA). A total of 2.4 μl of ibotenic acid (Biosearch Technologies, Novato, Ca, 10 mg/ml in PBS, pH 7.4) was injected into each site at a rate of 0.4 μl/min. To minimize brain swelling all animals were treated with 30 ml of Mannitol intravenous (20%), delivered at a rate of 1 ml/min, before beginning the final injection.
2.2.2 - Perirhinal cortex lesions
The perirhinal cortex aspiration lesions were performed according to the procedures developed by Bachevalier and colleagues (Meunier et al., 1993). The anesthetized animal was placed in a head-holder that permitted rotation of the animal's head to 120° during surgery. After opening, the zygomatic arch was removed, followed by removal of the bone over the ventrolateral surface of the frontotemporal junction. For the rostral portion of the aspiration, the dura was cut in a crescent over the frontal and temporal lobes, and the frontal lobe was gently elevated to expose the medial temporal pole. With the aid of a surgical microscope, the pia matter on the lateral lip of the rostral portion of the rhinal sulcus was cauterized, after which the cortex within the lateral bank of the sulcus as well as a 2 mm strip of cortex lateral to the sulcus was aspirated using a small gauge sucker. This portion of the lesion was extended from the temporal pole to the floor of the temporal fossa. When the rostral portion of the removal was complete, the dura was sewn with 5.0 Vicryl suture, the animal was given 30 ml of Mannitol (20%), intravenously, at a rate of 1 ml per minute to control brain swelling. For the caudal portion of the rhinal sulcus, the head-holder was rotated until the monkey's head was tilted at an angle of 120° from the upright position, and a second incision was made in the dura over the lateral temporal lobe. The base of the temporal lobe was deflected to expose the posterior end of the first aspiration. The pia matter on the lateral lip of the rhinal sulcus was cauterized, and the lesion within the sulcus and 2-mm lateral to the sulcus was extended to the posterior tip of the sulcus. The lesion was intended to include Broadman's areas 35 and 36.
2.2.3 - Areas TH/TF lesions
Areas TH/TF lesions were performed using a supralabyrinthine approach developed by Bachevalier and colleagues (Webster et al., 1994). The head of the animal was secured in the head-holder as used for the perirhinal cortical lesions. A bone flap over the ventrolateral surface of the temporal lobe was performed, and the dura was cut in a crescent over the temporal lobes. The borders of the lesion included the lip of the medial bank of the occipitotemporal sulcus laterally, the brain stem medially, and the posterior tip of the rhinal sulcus rostrally. Caudally, the posterior middle temporal sulcus was identified, its midpoint was localized and used as the most posterior extent of the lesions. Cortical tissue between these borders was aspirated with a small gauge sucker and was intended to include both areas TH and TF (von Bonin and Bailey, 1947).
2.2.4 - Sham lesions
The anesthetized animal was placed in the non-ferromagnetic stereotaxic apparatus used for the neurotoxic hippocampal lesions. After opening, a bone flap was made as described for the neurotoxic hippocampal lesions (see above), and the bone flap re-sutured. The needles were not lowered and no drug was injected.
2.3 - Lesion Evaluation
2.3.1 - Hippocampal lesions
All lesions were evaluated using MRI techniques which have been shown to provide an accurate estimate of extent of brain damage in monkeys (Malkova et al., 2001; Nemanic et al., 2002). Post-surgical FLAIR images were used to identify regions of hyperintensity caused by cell death within the hippocampal formation as well as adjacent brain structures. Post-surgical FLAIR coronal images were matched to corresponding pre-surgical T1-weighted images and drawings of coronal sections through the intact rhesus brain. The extent of hyperintensity seen on the MR images was visually identified and plotted onto the corresponding drawings of coronal sections. Within each hemisphere, the volume of hyperintensity for each structure was evaluated using Scion Image® and expressed as a percentage of the normal volume for that region. The extent of hippocampal lesions have been described elsewhere (Nemanic et al., 2004) and is given in Table 1. The removals of the hippocampal formation were largely as intended, ranging from 66.3 – 99.1%, with mild unintended damage to the tail of the caudate and putamen in one case (H-2 and H1, respectively) and moderate damage to areas TH and TF (Mean: 22% and 24%, respectively) in all cases but one (see Table 1).
Table 1.
Percent intended and unintended damage in Group H.
| Intended Damage | Unintended Damage | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Subjects | Hippocampus |
TH |
TF |
|||||||||
| L% | R% | X% | W% | L% | R% | X% | W% | L% | R% | X% | W% | |
|
|
|
|
||||||||||
| H-1 | 76.5 | 97.9 | 87.2 | 74.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H-2 | 75.8 | 81.3 | 78.5 | 61.6 | 53.1 | 20.1 | 36.6 | 10.7 | 60.3 | 27.6 | 43.9 | 16.6 |
| H-3 | 67.6 | 74.1 | 70.9 | 50.1 | 26.7 | 15.3 | 21.0 | 4.1 | 30.0 | 44.0 | 37.0 | 13.2 |
| H-4 | 56.4 | 76.3 | 66.3 | 43.0 | 13.6 | 27.8 | 20.7 | 3.8 | 18.5 | 19.4 | 18.9 | 3.6 |
| H-5 | 98.8 | 99.3 | 99.1 | 98.1 | 15.2 | 15.9 | 15.6 | 2.4 | 38.8 | 8.5 | 23.7 | 3.3 |
| H-6 | 88.9 | 94.9 | 91.9 | 84.3 | 29.6 | 45.6 | 37.6 | 13.5 | 21.2 | 17.2 | 19.2 | 3.6 |
|
| ||||||||||||
| Mean | 77.4 | 87.3 | 70.6 | 58.9 | 23.0 | 20.8 | 21.9 | 5.8 | 28.1 | 19.8 | 23.8 | 6.7 |
Percent damage to the hippocampus and parahippocampal (TH/TF) areas as estimated from pre- and post-surgery coronal MR FLAIR images: L% - percent damage to the left hemisphere; R% - percent damage to the right hemisphere; X% - average damage to both hemispheres; W% - weighted average damage to both hemispheres (W% = (L% × R%)/100); Avg – average for the entire group; TH/ TF: cytoarchitectonic fields of the parahippocampal gyms as defined by von Bonin and Bailey, 1947.
2.3.2 - PRh and TH/TF lesions
Cortical lesions were evaluated via histological processing of the brain. At the end of the behavioral experiments, the animals of Groups PRh and TH/TF were given a lethal dose of sodium pentobarbital and perfused intracardially with 0.9% saline followed by 4% paraformaldehyde. The brain was removed and post-fixed in 30% sucrose-formalin, and then cut frozen at 50μm in the coronal plane. Every 10th section was mounted for staining with thionin, providing one section every 0.5mm, and used to visualize cell bodies. Every 20th section was mounted for staining with silver (Gallyas, 1979), providing one section every 1mm. The two series of sections were mounted, de-lipidated in Xylene, stained, and cover slipped. For each animal, all sections through the extent of the cortical lesions were microscopically examined. The damage seen on each section was reconstructed onto matched sections of an intact rhesus brain, and the volume of each damaged area (intended and unintended) was quantified as described previously (Nemanic et al., 2002). For each region in each section, the surface area of damage was measured and the estimated volume of damage for each region of interest was calculated by: [(Σ surface areas of damage to region in all sections * distance between sections)/ (Σ surface areas of region all sections in the intact monkey brain* distance between sections) * 100].
The extent of PRh and TH/TF lesions have been described in details elsewhere (Nemanic et al., 2004; see Figures 4–6 for PRh and Figures 7–8 for TH/TF). Table 2 lists the percent damage identified histologically for each brain region of each hemisphere of the monkeys in Group TH/TF. The TH/TF lesions were largely as intended, ranging from 67.4% – 88.9% for area TH and 65.1% – 92.1% for area TF, with minor damage to perirhinal cortex anteriorly in one case (TH/TF-1).
Table 2.
Percent intended and unintended damage in Group TH/TF.
| Intended Damage | Unintended Damage | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Subjects | TH |
TF |
PRh |
|||||||||
| L% | R% | X% | W% | L% | R% | X% | W% | L% | R% | X% | W% | |
|
|
|
|
||||||||||
| TH/TF-1 | 68.4 | 66.3 | 67.4 | 45.3 | 64.4 | 65.7 | 65.1 | 35.2 | 0.96 | 12.6 | 6.78 | 0.12 |
| TH/TF-2 | 92.8 | 85.0 | 88.9 | 78.9 | 93.0 | 89.4 | 91.2 | 83.1 | 8.1 | 8.1 | 8.1 | 0.66 |
| TH/TF-3 | 72.4 | 95.6 | 84.0 | 69.2 | 91.5 | 92.6 | 92.1 | 84.7 | 1.8 | 4.4 | 3.1 | 0.08 |
|
| ||||||||||||
| Mean | 77.9 | 82.3 | 80.1 | 64.5 | 83.0 | 82.6 | 82.8 | 67.7 | 3.62 | 8.37 | 5.99 | 0.29 |
Percent damage to the parahippocampal (TH/TF) and perirhinal (PRh) areas as estimated post-mortem histological material. Abbreviations as in Table 1 and PRh: perirhinal cortical areas 35/36.
Table 3 lists the percent damage identified histologically for each brain region of each hemisphere of monkeys in Group PRh. The perirhinal cortical removals were largely as intended, ranging from 84.0% – 91.5%, with mild to moderate damage to entorhinal cortex, and more extensive unilateral damage to TG in all cases.
Table 3.
Percent intended and unintended damage in Group PRh.
| Intended Damage | Unintended Damage | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| Subjects | PRh | ERh | TE | TG | ||||||||||||
|
|
|
|||||||||||||||
| L% | R% | X% | W% | L% | R% | X% | W% | L% | R% | X% | W% | L% | R% | X% | W% | |
|
|
|
|
||||||||||||||
| PRh-3 | 95.2 | 82.4 | 88.8 | 78.4 | 18.7 | 13.1 | 15.9 | 2.45 | 9.6 | 6.4 | 8.0 | 0.61 | 22.4 | 23.3 | 22.9 | 5.22 |
| PRh-4 | 92.8 | 90.1 | 91.5 | 83.6 | 15.6 | 15.2 | 15.4 | 2.37 | 7.8 | 6.2 | 7.0 | 0.48 | 0.0 | 0.0 | 0.0 | 0.0 |
| PRh-5 | 94.9 | 85.6 | 84.0 | 81.2 | 21.3 | 33.6 | 27.5 | 7.16 | 40.7 | 21.5 | 31.1 | 8.75 | 35.5 | 29.2 | 32.4 | 10.4 |
|
| ||||||||||||||||
| Mean | 94.3 | 86.0 | 88.1 | 81.1 | 18.4 | 20.6 | 19.6 | 3.9 | 30.9 | 11.4 | 15.4 | 3.28 | 19.3 | 17.5 | 18.4 | 5.2 |
Percent damage to the perirhinal, entorhinal, TE and TG areas as estimated post-mortem histological material. Abbreviations as in Table 1 and PRh: perirhinal cortical areas 35/36; ERh: entorhinal cortical area 28; TE and TG: cytoarchitectonic fields of the anterior temporal cortexas defined by von Bonin and Bailey, 1947.
2.4 - Visual Paired Comparison
2.4.1 - VPC Apparatus
The monkey was seated in a primate chair (Crist Instruments, Damascus, MD) with a padded headrest designed to restrict head movements. The primate chair was placed inside a testing box approximately 40 cm in front of a computer monitor connected to a computer that presented the stimuli. A video camera was mounted on a tripod behind the screen, and positioned such that the eyes of the monkey were clearly visible and their movements recorded. The output of the camera was fed into a time generator that was connected to a VCR. The output was also fed into a television so that the experimenter could monitor the monkey's eye movements during the task.
2.4.2 - Stimuli
The stimuli consisted of images displaying an object placed over a background. The objects were three-dimensional digital colored pictures of everyday objects. The backgrounds consisted of different patterned wallpapers with a resolution of 300 dpi. The stimulus pool was sufficiently large to ensure that no objects or backgrounds were repeated over the course of testing. The object-background stimulus images were created to look like 3-dimensional objects standing on a background using a customized VPC program (Kindred, Houston, TX). A mask of the object was created using the Magic Mask® plug-in of Adobe® Photoshop® 5.0, and was superimposed onto the background to create a 3-dimensional effect.
2.4.3 - VPC task
Each trial began with a familiarization phase during which the monkey was required to look at the sample image for a cumulative 30 sec. After a delay of 10 sec for monkeys in Group C, H and TH/TF, the same image and a new image were displayed on the screen side-by-side for two retention trials of 5 sec each, during which the left/right position of the two objects was reversed. Monkeys in Group PRh were tested at a 1 sec delay because they had previously demonstrated attenuated preference for looking at novel objects at the 10 sec delay but showed robust novelty preference when tested with a 1-sec delay (Nemanic et al., 2004). The 5 conditions were presented in an intermixed manner within a daily session; the order was determined by a randomization protocol in the VPC computer program. The same order was run on all monkeys, and all monkeys were tested for a total of 50 trials (10 trials/condition).
In this VPC-context task, novel images used in the retention phase differed from the familiarized images by modifying either the object and/or the background (Figure 1). In two conditions (Conditions 1 and 2: Context changes), the sample object was presented on a new background during the retention tests. In Condition 1, the novel images consisted of a new object over the same novel background as the sample object. In Condition 2, the novel image consisted of a new object placed over the familiar background, such that the two images had a component of novelty (either the background or the object). Three other conditions (Conditions 3–5: No context changes) were given in which the sample object/background pair was represented unchanged during the retention test. So, in condition 3, the novel image consisted of the sample object on a new background. In condition 4, the novel image consisted of a new object presented on the familiar background. Thus, Conditions 3 and 4 served as controls to assess whether any impaired memory performance in Conditions 1 and 2 could have resulted from a difficulty encoding the background or the object separately. Finally, in Condition 5, the novel image consisted of a new object on a new background to ensure that all animals could demonstrate novelty preference when using these more complex stimuli.
Figure 1.
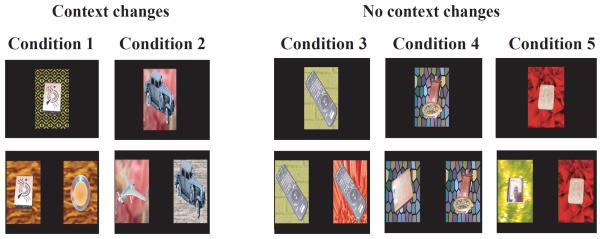
Trial Types for the Context VPC task. The familiarization phase images are composed of objects presented over backgrounds, and retention phase stimuli are five different modifications of the novelty and familiarity of the object, background or both the object and background. Conditions 1 and 2 are experimental conditions with the sample object presented over a new background in the retention tests. Conditions 3 – 5 are control conditions with sample object presented unchanged over the familiar background.
2.4.4 - Data Analyses
The time spent looking at each image during the retention tests was measured with the aid of a frame-by-frame video-recording system that allowed detailed analysis of the corneal reflection of the images, but monitoring of visual scanning patterns of the images was not possible (Pascalis and Bachevalier, 1999). Two observers analyzed samples of videotape and an inter-observer reliability was calculated (Pearson correlation, r = 0.91, p < 0.0001). During videotape analysis, observers were not informed of the group membership of the animal. Percent looking time at an image was calculated by dividing the looking time to one image by the total looking time at both images and multiplying this ratio by 100. All conditions, except Condition 3, were coded with the retention test image containing the novel object designated as the novel image. In Condition 3, there was no novel object, and this condition was coded with respect to the image containing the novel background.
To ensure that lesions did not impact overall looking patterns, the Total Retention time (i.e. the time monkeys looked at both stimuli during the two retention tests) was also calculated.
2.4.5 - Statistical Analysis
We first compared performance of control animals across the 5 conditions using a oneway ANOVA with conditions as a repeated factor, followed by post-hoc analyses using Bonferroni corrected t-tests.
The effects of lesions were then compared to controls using two-way ANOVAs (Group × Condition) with repeated measure for the last factor. When sphericity was not assumed, a Huynh-Feldt correction was used. Additionally, given the small sample size, planned comparisons (Pedhazur, 1982) were performed between the control group and the experimental groups, using a one-tailed Dunnett's test. Comparisons between each experimental group were performed using a Tukey test. These statistical analyses were carried out on the Total Retention Time and Percent Novelty scores. Finally, for the three experimental groups, percent novelty scores for each condition were correlated with the percent damage (intended or unintended) to each brain region using Pearson correlations.
2.5 - Results
2.5.1 - Performance of control animals
We first analyzed performance of control animals to assess the effects of changes in context on preference for novelty. A one-way ANOVA on percent looking at novel images across the 5 conditions indicated a significant effect of Condition [FHUYNH-FELDT (4, 20) = 5.35, p < .004]. Post-hoc analyzes revealed that novelty preference in control animals was weaker when sample objects were presented over a new background (Conditions 1 and 2) than when they were presented over the familiar background (Conditions 3–5, all ps < .05). Further novelty preference did not differ between Conditions 1 and 2 or between Conditions 3 and 5 (all ps > .05).
2.5.2 -Effects of hippocampal, perirhinal and parahippocampal lesions
2.5.2.1: Total Retention Time
We first analyzed whether the lesions affected looking patterns. The total amount of time each animal spent viewing the images in the retention tests of the VPC-Context task across all 5 conditions is listed in Table 4. There were no significant group effect or group by condition interaction [Group: F (3, 14) = 0.44, NS; Group × Condition: F (12, 56) = 1.12, NS]; but the condition effect reached significance [Condition: F (4, 56) = 3.99, p < 0.006]. For all animals, Total Retention Time was slightly greater in Condition 5 in which the sample image was re-presented unchanged and the novel image consisted of a new object on a new background [Condition 5: 7.09 ± 0.266 sec] than in the other 4 conditions in which the novel image share some features with the familiar image [Condition 1: 6.82 ± 0.256 sec; Condition 2: 6.84 ± 0.222 sec; Condition 3: 6.49 ± 0.268 sec; Condition 4: 6.68 ± 0.273 sec;], but post-hoc analyses did not reveal significant difference between groups [all ps > .05].
Table 4.
Total Retention time in the VPC-context.
| Group/Case | Total RetentionTime (sec) |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| C-1 | 5.6 | 5.0 | 4.9 | 4.9 | 5.7 |
| C-2 | 6.6 | 6.8 | 6.5 | 6.6 | 6.8 |
| C-3 | 7.6 | 7.0 | 6.7 | 8.1 | 8.3 |
| C-4 | 7.9 | 6.6 | 6.8 | 6.5 | 7.0 |
| C-5 | 7.5 | 7.3 | 7.1 | 7.2 | 7.7 |
|
| |||||
| X | 7.0 | 6.5 | 6.4 | 6.7 | 7.1 |
|
| |||||
| H-1 | 4.7 | 5.7 | 5.6 | 4.6 | 5.5 |
| H-2 | 5.2 | 6.3 | 4.4 | 4.7 | 5.9 |
| H-3 | 5.6 | 5.5 | 4.5 | 5.5 | 5.7 |
| H-4 | 7.4 | 8.6 | 7.7 | 8.4 | 7.6 |
| H-5 | 7.4 | 6.8 | 6.8 | 7.9 | 7.5 |
| H-6 | 7.1 | 8.1 | 8.1 | 7.5 | 8.2 |
|
| |||||
| X | 6.2 | 6.8 | 6.2 | 6.4 | 6.7 |
|
| |||||
| TH/TF-1 | 6.4 | 6.5 | 5.6 | 6.5 | 6.8 |
| TH/TF-2 | 6.0 | 6.2 | 6.8 | 6.7 | 7.8 |
| TH/TF-3 | 6.9 | 7.5 | 6.9 | 7.2 | 7.4 |
|
| |||||
| X | 6.4 | 6.7 | 6.4 | 6.8 | 7.3 |
|
| |||||
| PRh-1 | 7.5 | 6.9 | 7.0 | 7.0 | 7.9 |
| PRh-2 | 8.9 | 9.2 | 8.3 | 8.1 | 9.6 |
| PRh-3 | 6.4 | 5.9 | 5.9 | 6.3 | 5.5 |
|
| |||||
| X | 7.6 | 7.4 | 7.1 | 7.1 | 7.6 |
Scores are total time spent looking at the two images during the two retention tests combined.
2.5.2.2: Percent looking at novelty
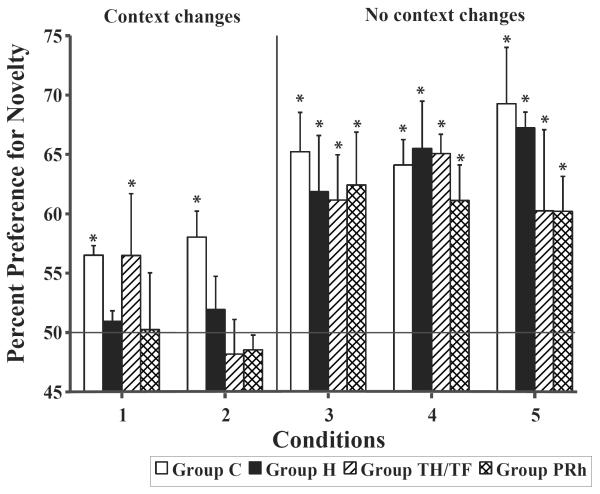
Mean percent preference for novelty obtained for all conditions for the four groups are presented in Figure 2. The two-way ANOVA indicated no significant effect of group [F(3,14) = 1.18, p > .05] and no interaction between the two factors [FHUYNH-FELDT (12, 56) = 0.84, p > .05], but the condition effect reached significance [FHUYNH-FELDT (4, 56) = 16.85, p < .001], indicating that all groups had weaker novelty scores in Conditions 1 and 2 than in Conditions 3–5. Nevertheless, only for Group C did the percent preference for novelty differ from chance on all conditions (Condition 1: t = 2.96, p < 0.004; Condition 2: t = 5.62, p < 0.001; Condition 3: t = 5.62, p < 0.0001; Condition 4: t = 5.80, p < 0.0001, Condition 5: t = 8.52, p < 0.0001), indicating novelty preference in all conditions. For Group TH/TF, percent preference for novelty differed from chance on all conditions (Condition 1: t = 2.08, p < 0.047; Condition 3: t = 3.29, p < 0.003; Condition 4: t = 5.88, p < 0.0001, Condition 5: t = 2.87, p < 0.008), except Condition 2 (Condition 2: t = 5.54, NS). Finally, for Groups H and PRH, percent novelty preference differed from chance for the three No-context-change conditions (Group H: Condition 3: t = 4.24, p < 0.0001; Condition 4: t = 6.38, p < 0.001, Condition 5: t = 8.55, p < 0.0001; Group TH/TF:; Group PRh: Condition 3: t = 3.30, p < 0.003; Condition 4: t = 2.63, p < 0.014, Condition 5: t = 2.86, p < 0.008), but not in the two conditions with context changes (H: Condition 1: t = 0.40, NS, Condition 2: t = 0.91, NS; PRh: Condition 1: t = 0.06, NS, Condition 2: t = 0.32, NS). Thus, considering the lack of novelty preference in the experimental groups in conditions in which the context changed, we separately analyzed the effects of the lesions on the two conditions (Conditions 1 and 2) with context changes and on the three conditions (Conditions 3–5) in which the context did not change.
Figure 2.
Context VPC results. Percent preference for novelty on the Context VPC task for Groups C, H, PRh and TH/TF. The solid horizontal line represents chance performance and the vertical lines indicate standard error of the mean. * indicates score differing significantly from chance (p < .05).
For conditions 1 and 2, the two-way ANOVAs revealed a significant effect of Group [Group: F (3,13) = 4.18, p < 0.026], but no effect of condition [Condition: FHUYNH-FELDT (1,13) = 0.87, NS] and no interaction between the two factors [Group × Condition: FHUYNH-FELDT (3,13) = 1.23, NS]. Planned comparisons revealed that for these two conditions Groups H, PRh and TH/TF differed from Group C [Dunnett's, p's < 0.02; p < 0.01; p < 0.08, respectively], but did not differ from each other [Tukey, p = NS]. It is important to note that the trend for a significance difference between Group TH/TF and Group C is likely due to normal novelty preference scores of one of the three animals with TH/TF lesions in Condition 1.
For Conditions 3–5, in which the familiar object/background pairing remained unchanged between the familiarization phase and the retention tests, the two way ANOVA revealed no significant effects of groups [Group: F (3,13) = 0.51, NS] or conditions [Condition: F (HUYNH-FELDT) (2, 26) = 0.28, NS], and no significant interaction between the two factors [Group × Condition: F (HUYNH-FELDT) (6, 26) = 0.74, NS]. It is interesting to note that in the control condition (Condition 5) in which there was no overlap between the two images, monkeys with perirhinal and parahippocampal lesions had weaker novelty preference than the controls and animals with hippocampal lesions. This slight reduction is most likely due the small sample size in these two groups (N = 3 in each) and to the fact that one of the three animals in the two groups performed at chance (TH/TF-3: 47.7% and PRh-3: 54.4%), whereas the other two animals showed robust novelty preference (TH/TF-1: 61.9%; TH/TF-2: 71.2%; PRh-4: 63.6%; PRh-5:62.7%) similar to that of controls. The lack of novelty preference in these two animals is not related to smaller lesion size (see Tables 2 and 3).
2.5.3 - Correlations
None of the correlations reached significance, except for a negative correlation between unintended damage to areas TH/TF and performance on the Control conditions in Group H (r = −0.92, p < 0.002), indicating that animals with hippocampal lesions that had additional damage to areas TH/TF showed slightly weaker preference for novelty in the three conditions with no context changes.
2.6 - Discussion
The main findings were that control animals showed weaker novelty preference in the two conditions with context changes than in the three conditions with no context changes, although their novelty scores remained above chance in all conditions. By contrast, lesions of the hippocampus, parahippocampal and perirhinal cortex resulted in a lack of novelty preference in conditions with context changes but not in those in which the context was not changed.
5.6.1 – Contextual memory in control animals
As expected, although control animals displayed novelty preference in all 5 conditions, recognition memory was stronger in Conditions 3–5 (No context changes) than in Conditions 1–2 (Context changes). This finding is consistent with the decrease in memory performance found in control human subjects when tested with a variety of tasks and under different types of context manipulations (Canas and Nelson, 1986; Cann and Ross, 1989; Dalton, 1993; Emmerson 1986; Geiselman and Bjork, 1980; Hollingworth, 2006; Light and Carter-Sobel, 1970; Park et al., 1984, 1987; Reder et al., 1974; Russo et al., 1999; Smith, 1985; Smith and Vela, 1986; Stumpfel and Kirsner, 1986; Tulving and Thompson, 1973;). Furthermore, the results parallel those reported in animal studies (Dellu et al., 1997; Dix and Aggleton, 1999; Mumby et al., 2002; O'Brien et al., 2006) that have used incidental recognition memory tasks similar to the VPC-context task used in the present study. The data, thus, strengthened the view that, as in humans, recognition memory processes in animals are also modulated by contextual information.
An interesting finding for control animals was their performance on Condition 2. In this specific condition, the two images contained an element of novelty, i.e. the sample object was presented on a new background on one image, and on the other a new object was presented over the familiar background. Visual attention of control animals in this type of trial was significantly biased towards the image with the new object. This attentional bias may relate to the fact that the objects in this task were presented in 3-D over the background, a procedure that may have enhanced attention towards the novel foreground object. Alternatively, this attentional bias could be explained by recent computational saliency models of attention predicting that a scene (or image) can be analyzed by two parallel pathways even in condition in which the subject have no notion of the task or context (see for review Oliva and Torralba, 2007). A global pathway represents the entire image to provide information about the expected location of a target. By contrast, a local pathway represents each elements independently to compute image saliency and perform object recognition on the basis of local appearance. Thus, in Condition 2, the image containing the expected familiar background could have attracted and, then, maintained the attention of the subjects towards the novel objects in favor of exploring the other image. Further studies, using eye-tracking technology, will be required to test this suggestion.
5.6.2 – Contextual memory after hippocampal lesions
Unlike their normal performance on the conditions with no context changes, animals with hippocampal lesions had novelty preference scores that did not differ from chance in the two conditions with context changes (Conditions 1 and 2) and that were significantly weaker than controls. In addition, their novelty preference scores differed from those of controls in Conditions 1 and 2 but not in Conditions 3–5. These findings suggest an impact of hippocampal lesions on memory for the context onto which objects are perceived.
The ability of monkeys with hippocampal lesions to display robust novelty preference in conditions in which the sample object was re-presented over the same familiar background rules out several explanations for their lack of novelty preference in the conditions in which the sample object was presented over a new background. First, the deficits in recognition cannot be attributed to differences in visual exploration of the images since animals with hippocampal lesions explored the images during the retention tests in the same amount of time than control animals. Second, the recognition impairment cannot have resulted from an inability to either discriminate the features of the objects, to recognize them, or to detect novelty. Finally, the memory deficit cannot be ascribed to an inability to encode or store representation of the backgrounds or the objects given that hippocampal-operated animals displayed robust novelty preference towards the novel background in Condition 3 and towards the novel object in Condition 4. Thus, the hippocampal lesions impacted the formation of an object/background representation or the retrieval of this representation. This contextual memory impairment after hippocampal lesions is consistent with a similar recognition memory impairment reported in rodents with damage to the hippocampus and tested in exploratory preference tasks in which context was manipulated (Ennaceur and Aggleton, 1994; Moses et al., 2002; Mumby et al., 2002; O'Brien et al., 2006; Piterkin et al., 2008; after fornix transection Eacott and Gaffan, 2005). Fornix transections in monkeys also impair performance on the DNMS task when object stimuli were embedded in complex scenes (Gaffan, 1994a) and hippocampal damage in humans impaired recognition of contextual information associated with object (Mundy et al., 2013; Spiers et al., 2001a; 2001b). Furthermore, changes in hippocampal cell firing are found when rats associate a specific object with a place or context (Komorowski et al., 2009; Manns and Eichenbaum, 2009) and accurate performance on item-context associative task are also associated with stronger coupling between theta and gamma oscillations in the CA3 field of the hippocampus (Tort et al., 2009). Additional evidence for automatic encoding of object-context associations within the hippocampus is also provided by immediate-early genes studies in rodents (Albasser et al., 2010) and neuroimaging studies in humans (Burgess et al., 2001; Dolan and Strange, 2002; Mundy et al., 2013; Rugg et al., 2012).
Remarkably, contextual memory impairment in monkeys with hippocampal lesions is associated with deficits observed in the same animals in an object-place association VPC task (Bachevalier and Nemanic, 2008), indicating that selective damage to the hippocampal complex impaired covert recognition memory for both object/context and object/spatial associations.
5.6.3 – Contextual memory after perirhinal lesions
Monkeys with perirhinal lesions showed a pattern of deficits similar to that described above after hippocampal lesions. Thus, although the perirhinal lesions spared novelty preference in the three conditions with no context changes, they severely impacted novelty preference in the two conditions with context changes. As above, the normal performance of animals with perirhinal lesions on the no-context-changes conditions indicates that their impairment in Conditions 1 and 2 cannot be explained by an inability to explore the images during the retention tests, to discriminate the features of the objects and recognize them, to detect novelty or to encode or store representations of either the backgrounds or the objects. These findings are consistent with the impairment in contextual memory following perirhinal lesions in rodents tested in contextual memory tasks. Bucci and colleagues (2000, 2002) reported that perirhinal lesions impaired contextual conditioning. Furthermore, using a spontaneous recognition task, Norman and Eacott, (2005) demonstrated that perirhinal lesions impaired memory of a familiar object when mounted over a familiar, but incongruent, background. The role of the perirhinal cortex in contextual memory has also been demonstrated in neuroimaging studies in humans reporting increase perirhinal activation during perceptual learning of scenes (Mundy et al., 2013).
One explanation for the impaired contextual memory after perirhinal lesions may relate to the ambiguity between elements in the two images. A series of recent studies have led to the proposal that the contribution of the perirhinal cortex to visual discrimination becomes critical when tasks require representation of items that share many similar features (Bussey and Saksida, 2002, 2005). However, this account is unlikely given that in Condition 1 the two images have as many overlapping features as the images in Conditions 3 and 4 for which animals with perirhinal lesions performed normally. Another possibility is that the perirhinal cortex is required to configure elements within objects to form meaningful entity (Bussey et al., 2002; Norman and Eacott, 2004), thus it is possible that after perirhinal lesions the object and its background may have been processed as a gestalt (or whole object) during the familiarization phase and could be recognized as familiar only in conditions in which the same object/background pairing reappeared in the retention test. This is specifically the pattern of results observed in the VPC-context task; i.e. impairment in the Context-Changes Conditions 1 and 2 but not in in the No-Context-Changes Conditions 3–5. Given that the same animals were also impaired in a VPC task measuring object-in-place association, the data suggest that the perirhinal cortex provides or maintains the representations of objects that are required by other neural structures, such as the hippocampal formation and/or areas TH/TF, to generate object-context or object-spatial associations, but does not form these associations (Cowell et al., 2010; Gaffan, 1994a; Gaffan and Parker, 1996; Norman and Eacott, 2005; Murray et al., 2007).
5.6.3 – Contextual memory after parahippocampal lesions
For animals with parahippocampal lesions, the dissociation of performance between the context-change conditions (1 and 2) and no context-change conditions (3–5) was not as clear cut as that described above for animals with hippocampal or perirhinal lesions. This was due to the fact that performance of animals with parahippocampal lesions showed a more robust deficit in contextual memory in Condition 2 than in Condition 1. This pattern of results arises because two of the three animals displayed novelty preference scores at chance in both conditions (Condition 1: 54.4% and 48.7%; Condition 2: 51.7% and 50.5%), whereas the third one showed significantly stronger novelty preference in Condition 1 (66.4%) but not in Condition 2 (42. 4%). It is also interesting to note that, although performance of Group TH/TF in Conditions 1 and 2 did not correlate with extent of TH/TF lesions, unintended damage to areas TH/TF in animals of Group H correlated with novelty scores, such that greater unintended TH/TF damage resulted in poorer novelty scores in Conditions 1 and 2 (see results section above). Thus, taken together, the data suggest that areas TH/TF appear to be critical for memory for object-context associations as well.
The contextual memory deficit after TH/TF lesions was also associated with impairment in object-in-place memory found earlier in the same animals (Bachevalier and Nemanic, 2008). This effect of TH/TF damage on contextual memory is consistent with deficits in object-context associations following postrhinal (homologous to parahippopcampal cortex of primate, Burwell et al., 2002) lesions in rodents (see for review Eacott and Gaffan, 2005). Similarly, recent neuroimaging studies in humans have reported that the parahippocampal cortex is engaged in scene novelty (Howard et al., 2011; Kohler et al., 2002), scene layout (Epstein and Higgins, 2006; Epstein and Ward, 2010), and object-background binding (Goh et al., 2004). Finally, Hayes and colleagues (2007) demonstrated greater activity in the parahippocampal cortex during successful memory of objects previously presented in a scene even when the subjects were not required to intentionally retrieve contextual information. The results thus suggest that the parahippocampal cortex may reinstate visual context to mediate successful recognition memory. Thus, in the absence of a functional parahippocampal cortex, only conditions of the VPC-context task in which the familiar image (object/background pairings) are re-presented unchanged during the retention tests will trigger faster recognition of the familiar image and shift the animal attention towards the novel image.
6.0 – Conclusions
The present results suggest that all three medial temporal lobe structures are critical for memory for object-context associations; though these data will need to be replicated with a larger sample size. In addition, the use of an eye-tracker to follow visual scanning patterns of the animals will likely provide additional information on the specific perceptual features onto which the operated animals direct their attention in each condition.
In addition, although clear-cut double dissociations between task conditions and lesions were not found in the present study, the pattern of results indicate that, after damage to any one of the medial temporal lobe structures, the others cannot compensate for the lost function. Hence, all three structures are critical for incidental contextual memory, yet their specific role may differ. Thus, according to recent accounts of the contribution of the medial temporal lobe structures in recognition memory (see above), the results suggest that the loss of incidental contextual memory after perirhinal cortex may be due to its critical participation in ambiguous object representations, that after parahippocampal lesions may be due to its participation in scene or spatial representation, and that after the hippocampus may be due to its critical participation in object-context representation. Together with our previous data on the same animals, the different outcomes of the three lesion types on memory for object-context (this study), spatial recognition memory (Bachevalier and Nemanic, 2008) and item-specific memory (Nemanic et al., 2004) suggest that the medial temporal structures have qualitatively different, but integrative, role in recognition memory. These findings support models of MTL functions that emphasize the role of the MTL structures in either spatial (O'Keefe and Nadel, 1978) or relational (Cohen and Eichenbaum, 1993; Davachi, 2006) memory, as well as those highlighting a hierarchical organization of representational complexity (Cowell et al., 2010).
Highlights.
-
-
Control animals showed weaker contextual memory after a context change
-
-
Hippocampal lesions alter contextual memory
-
-
Perirhinal cortex lesions alter contextual memory
-
-
Parahippocampal lesions alter contextual memory
Acknowledgments
National Institute of mental Health MH-58846 to JB, and National Research Service Award F31 MH-12106 to SN and F32 MH-10929 to M.C.A. The authors wish to express their appreciation to the Veterinary Staff for their expertise and valuable help in the pre- and post-surgical care of the monkeys, Roger E. Price and Belinda Rivera for the care and handling of the animals during the MR imaging procedures, and Edward F. Jackson for his expert assistance in neuroimaging scanning techniques. Thanks are also due to Mortimer Mishkin for providing some of the monkeys participating in the experiments, Anupa Prabhudesai and Andy Kazama for scoring behavioral data as second observers, and Fernando Latunio for histological preparation of the brains.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albasser MM, Chapman RJ, Amin E, Iordanova MD, Vann SD, Aggleton JP. New behavioral protocols to extend our knowledge of rodent recognition memory. Learn Mem. 2010;17:407–419. doi: 10.1101/lm.1879610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado MC, Wright AA, Bachevalier J. Object and spatial relational memory in adult rhesus monkeys is impaired by neonatal lesions of the hippocampal formation but not the amygdaloid complex. Hippocampus. 2002;12:421–433. doi: 10.1002/hipo.1115. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Nemanic S. Spatial memory in monkeys as measured with the visual paired-comparison task: effects of selective hippocampal, perirhinal and areas TH/TF lesions. Hippocampus. 2008;18:64–80. doi: 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–358. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Phillips RG, Burwell RD. Contributions of postrhinal and perirhinal cortex to contextual information processing. Behav Neurosci. 2000;114:882–894. doi: 10.1037//0735-7044.114.5.882. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Saddoris MP, Burwell RD. Contextual fear discrimination Is impaired by damage to the postrhinal or perirhinal cortex. Behav Neurosci. 2002;116:479–488. [PubMed] [Google Scholar]
- Buckley MJ, Gaffan D. Perirhinal cortex ablation impairs visual object identification. J Neurosci. 1998;18:2268–2275. doi: 10.1523/JNEUROSCI.18-06-02268.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, Spiers HJ, O'Keefe J. A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage. 2001;14:439–453. doi: 10.1006/nimg.2001.0806. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Witter MP, Amaral DG. Perirhinal and postrhinal cortices of the rat: a review of the neuroanatomical literature and comparison with findings from the monkey brain. Hippocampus. 2002;5:390–408. doi: 10.1002/hipo.450050503. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida L. The organization of visual object representations: A connectionist model of the effects of lesions in perirhinal cortex. Eur J Neurosci. 2002;15:355–364. doi: 10.1046/j.0953-816x.2001.01850.x. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida L. Object memory and perception in the medial temporal lobe: An altenative approach. Curr Opin Neurobiol. 2005;15:730–737. doi: 10.1016/j.conb.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Canas J, Nelson D. Recognition and environmental context: the effect of testing by phone. Bull Psychonomic Soc. 1986;24:407–409. [Google Scholar]
- Cann A, Ross D. Olfactory stimuli as context cues in human memory. Am J Psychol. 1989;102:91–102. [PubMed] [Google Scholar]
- Chun MM, Phelps EA. Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nat Neurosci. 1999;2:844–847. doi: 10.1038/12222. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia and the hippocampal system. MIT; Cambridge, MA: 1993. [Google Scholar]
- Cowell RA, Bussey TJ, Saksida L. Components of recognition memory: Dissociable cognitive processes or just differences in representational complexity? Hippocampus. 2010;20:1245–1262. doi: 10.1002/hipo.20865. [DOI] [PubMed] [Google Scholar]
- Dalton P. The role of stimulus familiarity in context-dependent recognition. Mem Cognit. 1993;21:223–234. doi: 10.3758/bf03202735. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context, and relational episodic encoding in humans. Curr Op Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Dellu F, Fauchey V, Le Moal M, Simon H. Extension of a new two-trial memory task in the rat: influence of environmental context on recognition processes. Neurobiol Learn Mem. 1997;67:112–120. doi: 10.1006/nlme.1997.3746. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: A three-component model. Trends Cogn Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Dix SL, Aggleton JP. Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behav Brain Res. 1999;99:191–200. doi: 10.1016/s0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Strange BA. Hippocampal novelty responses studied with functional imaging. In: Squire LR, Schacter DL, editors. Neuropsychology of memory. Guilford; New York: 2002. pp. 204–214. [Google Scholar]
- Dore FY, Thornton JA, White NM, Murray EA. Selective hippocampal lesions yield nonspatial memory impairments in rhesus monkeys. Hippocampus. 1998;8:323–329. doi: 10.1002/(SICI)1098-1063(1998)8:4<323::AID-HIPO2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Gaffan EA. The roles of perirhinal cortex, postrhinal cortex, and the fornix in memory for objects, contexts, and events in the rat. Quart J Exp Psychol. 2005;58B:202–217. doi: 10.1080/02724990444000203. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behav Brain Res. 2001;127:199–207. doi: 10.1016/s0166-4328(01)00365-5. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson P. Effects on environmental context on recognition memory in an unusual environment. Percept Mot Skills. 1986;63:1047–1050. [Google Scholar]
- Ennaceur A, Aggleton JP. Spontaneous recognition of object configurations in rats: effects of fornix lesions. Exp Brain Res. 1994;100:85–92. doi: 10.1007/BF00227281. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Higgins JS. Differential parahippocampal and retrosplenial involvement in three types of visual scene recognition. Cer Cor. 2006;17:1680–1693. doi: 10.1093/cercor/bhl079. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Ward EJ. How reliable are visual context effects in the parahippocampal place area? Cer Cor. 2010;20:294–303. doi: 10.1093/cercor/bhp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffan D. Normal forgetting, impaired acquisition in memory for complex naturalistic scenes by fornix-transected monkeys. Neuropsychologia. 1993;31:403–406. doi: 10.1016/0028-3932(93)90163-t. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Dissociated effects of perirhinal cortex ablation, fornix transection and amygdalectomy: evidence for multiple memory systems in the primate temporal lobe. Exp Brain Res. 1994a;99:411–422. doi: 10.1007/BF00228977. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Scene-specific memory for objects: a model of episodic memory impairment in monkeys with fornix transections. J Cogn Neurosci. 1994b;6:605–620. doi: 10.1162/jocn.1994.6.4.305. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Parker A. Interaction of perirhinal cortex with fornix-fimbria: memory for objects and “object-in-place” memory. J Neurosci. 1996;16:5864–5869. doi: 10.1523/JNEUROSCI.16-18-05864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallyas F. Silver staining of myelin by means of physical development. Neurol Res. 1979;1:203–209. doi: 10.1080/01616412.1979.11739553. [DOI] [PubMed] [Google Scholar]
- Geiselman E, Bjork R. Primary versus secondary rehearsal in imagined voices: differential effects on recognition. Cog Psych. 1980;12:188–205. doi: 10.1016/0010-0285(80)90008-0. [DOI] [PubMed] [Google Scholar]
- Goh JOS, Siong SC, Park D, Gutchess A, Hebrank A, Chee MWL. Cortical areas involved in object, background, and object-background processing revealed with functional magnetic resonance adaptation. J Neurosci. 2004;24:10223–10228. doi: 10.1523/JNEUROSCI.3373-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Hayes SM, Ryan L, Schnyer DM, Nadel L. An fMRI study of episodic memory: Retrieval of object, spatial, and temporal information. Behav Neurosci. 118:885–896. doi: 10.1037/0735-7044.118.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Nadel L, Ryan L. The effect of scene context on episodic object recognition: parahippocampal cortex mediates memory encoding and retrieval success. Hippocampus. 2007;17:873–889. doi: 10.1002/hipo.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth A. Scene and position specificity in visual memory of objects. J Exp Psychol Learn Mem Cogn. 2006;32:58–69. doi: 10.1037/0278-7393.32.1.58. [DOI] [PubMed] [Google Scholar]
- Horner AJ, Gadian DG, Fuentemilla L, Jentschke S, Vargha-Khadem F, Duzel E. A rapid, hippocampus-dependent, item-memory signal that initiates context memory in humans. Curr Biol. 2012;22:2369–2374. doi: 10.1016/j.cub.2012.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard LR, Kumaran D, Ólafsdóttir HF, Spiers HJ. Double dissociation between hippocampal and parahippocampal responses to object-background context and scene novelty. J Neurosci. 2011;31:5253–5261. doi: 10.1523/JNEUROSCI.6055-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Hardy JD. Long-term memory for contextual attributes: dissociation of amygdala and hippocampus. Behav Brain Res. 1983;8:139–149. doi: 10.1016/0166-4328(83)90050-5. [DOI] [PubMed] [Google Scholar]
- Köhler S, Crane J, Milner B. Differential contributions of the parahippocampal place area and the anterior hippocampus in human memory for scenes. Hippocampus. 2002;12:718–723. doi: 10.1002/hipo.10077. [DOI] [PubMed] [Google Scholar]
- Komorowski RW, Manns JR, Eichenbaum H. Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens where. J Neurosci. 2009;29:9918–9929. doi: 10.1523/JNEUROSCI.1378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light L, Carter-Sobel L. Effects of changed semantic context on recognition memory. J verbal Learn Verbal Beh. 1970;9:1–11. [Google Scholar]
- Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex or hippocampal formation lesions on established social relationships in monkeys. Behav Neurosci. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The effects of selective amygdala, orbital frontal cortex or hippocampal formation lesions on reward assessment in nonhuman primates. Eur J Neurosci. 2007a;25:2885–2904. doi: 10.1111/j.1460-9568.2007.05525.x. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. Measuring reward assessment in a semi-naturalistic context: The effects of selective amygdala, orbital frontal or hippocampal lesions. Neuroscience. 2007b;148:599–611. doi: 10.1016/j.neuroscience.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova L, Lex CK, Mishkin M, Saunders RC. MRI-Based evaluation of locus and extent of neurotoxic lesions in monkeys. Hippocampus. 2001;11:361–370. doi: 10.1002/hipo.1050. [DOI] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. A cognitive map for object memory in the hippocampus. Learn Mem. 2009;16:616–624. doi: 10.1101/lm.1484509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maratos EJ, Dolan RJ, Morris JS, Henson RN, Rugg MD. Neural activity associated with episodic memory for emotional context. Neuropsychologia. 2001;39:910–920. doi: 10.1016/s0028-3932(01)00025-2. [DOI] [PubMed] [Google Scholar]
- Martin CB, McLean DA, O'Neil EB, Köhler S. Distinct familiarity-based response patterns for faces and buildings in perirhinal and parahippocampal cortex. J Neurosci. 2013;33:10915–10923. doi: 10.1523/JNEUROSCI.0126-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes AR, MacDonald C, Donlan L, Pears J, Meudell PR. Amnesics have a disproportionately severe memory deficit for interactive context. Quart J Exp Psychol. 1992;45A:265–297. doi: 10.1080/14640749208401327. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaldi D, Mayes AR. The role of recollection and familiarity in the functional differentiation of the medial temporal lobes. Hippocampus. 2010;20:1291–1314. doi: 10.1002/hipo.20853. [DOI] [PubMed] [Google Scholar]
- Moses SN, Sutherland RJ, McDonald RJ. Differential involvement of amygdala and hippocampus in responding to novel objects and contexts. Brain Res Bull. 2002;58:517–527. doi: 10.1016/s0361-9230(02)00820-1. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: Memory of objects, places and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy ME, Downing PE, Dwyer DM, Honey RC, Graham KS. A critical role for the hippocampus and perirhinal cortex in perceptual learning of scenes and faces: Complementary findings from amnesia and fMRI. J Neurosci. 2013;33:10490–10502. doi: 10.1523/JNEUROSCI.2958-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Saksida L. Visual perception and memory: A new view of medial temporal lobe function in primates and rodents. Ann Rev Neurosci. 2007;30:99–122. doi: 10.1146/annurev.neuro.29.051605.113046. [DOI] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Price RE, Jackson E, Bachevalier J. Assessment of locus and extent of neurotoxic lesions in monkeys using neuroimaging techniques: a replication. J Neurosci Meth. 2002;121:1–11. doi: 10.1016/s0165-0270(02)00264-9. [DOI] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Bachevalier J. The hippocampal/parahippocampal regions and recognition memory: insights from visual paired-comparison versus object delayed nonmatching in monkeys. J Neurosci. 2004;24:2013–2026. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman G, Eacott MJ. Dissociable effects of lesions to the perirhinal cortex and the postrhinal cortex on memory for context and objects in rats. Behav Neurosci. 2005;119:557–566. doi: 10.1037/0735-7044.119.2.557. [DOI] [PubMed] [Google Scholar]
- O'Brien N, Lehmann H, Lecluse V, Mumby DG. Enhanced context-dependency of object recognition in rats with hippocampal lesions. Behav Brain Res. 2006;170:156–162. doi: 10.1016/j.bbr.2006.02.008. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The hippocampus as a cognitive map. Clarendon Press; Oxford: 1978. [Google Scholar]
- Oliva A, Torralda A. The role of context in object recognition. Trends Cogn Sci. 2007;11:520–527. doi: 10.1016/j.tics.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Park DC, Puglisi JT, Sovacool M. Picture memory in older adults: effects of contextual detail at encoding and retrieval. J Gerontol. 1984;39:213–215. doi: 10.1093/geronj/39.2.213. [DOI] [PubMed] [Google Scholar]
- Park DC, Puglisi JT, Smith AD, Dudley WN. Cue utilization and encoding specificity in picture recognition in older adults. J Gerontol. 1987;42:423–425. doi: 10.1093/geronj/42.4.423. [DOI] [PubMed] [Google Scholar]
- Parkin AJ, Leng NR, Hunkin NM. Differential sensitivity to context in diencephalic and temporal lobe amnesia. Cortex. 1990;26:373–380. doi: 10.1016/s0010-9452(13)80087-1. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Bachevalier J. Neonatal aspiration lesions of the hippocampal formation impair visual recognition memory when assessed by paired-comparison task but not by delayed nonmatching-to-sample task. Hippocampus. 1999;9:609–616. doi: 10.1002/(SICI)1098-1063(1999)9:6<609::AID-HIPO1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Hunkin NM, Bachevalier J, Mayes AR. Change in background context disrupts performance on visual paired comparison following hippocampal damage. Neuropsychologia. 2009;47:2107–2113. doi: 10.1016/j.neuropsychologia.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Pedhazur EJ. Multiple regression in behavioral research: Explanation and prediction. 2nd ed. Holt, Rinehart and Winston; New York: 1992. [Google Scholar]
- Piterkin P, Cole E, Cossette M-P, Gaskin S, Mumbu DG. A limited role for the hippocampus in the modulation of novel-object preference by contextual cues. Learn Mem. 2008;15:785–791. doi: 10.1101/lm.1035508. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Kammermeier J, Spiess J. Relationship between fos production and classical fear conditioning: effects of novelty, latent inhibition, and unconditioned stimulus preexposure. J Neurosci. 1998;18:7452–7461. doi: 10.1523/JNEUROSCI.18-18-07452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reder L, Anderson J, Bjork R. A semantic interpretation of encoding specificity. J Exp Psych. 1974;14:632–649. [Google Scholar]
- Ridley RM, Hardy A, Maclean CJ, Baker HF. Non-spatial acquisition and retention deficits following small excitotoxic lesions within the hippocampus in monkeys. Neuroscience. 2001;107:239–248. doi: 10.1016/s0306-4522(01)00358-x. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Vilberg KL, Mattson JT, Yu SS, Johnson JD, Suzuki M. Item memory, context memory and the hippocampus: fMRI evidence. Neuropsychologia. 2012;50:3070–3079. doi: 10.1016/j.neuropsychologia.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R, Ward G, Geurts H, Scheres A. When unfamiliarity matters: changing environmental context between study and test affects recognition memory for unfamiliar stimuli. Learn Mem Cog. 1999;25:488–499. [Google Scholar]
- Smith DM, Mizumori SJY. Hippocampal cells, context, and episodic memory. Hippocampus. 2006;16:716–729. doi: 10.1002/hipo.20208. [DOI] [PubMed] [Google Scholar]
- Smith S. Environmental context and recognition memory reconsidered. Bull Psychonomic Soc. 1985;23:173–176. [Google Scholar]
- Smith S. Environmental context dependent recognition memory using short term memory for task input. Mem Cognit. 1986;14:347–354. doi: 10.3758/bf03202513. [DOI] [PubMed] [Google Scholar]
- Smith SM, Vela E. Outshining-The relative effectiveness of cues. Bull Psychon Soc. 1986;24:30. [Google Scholar]
- Spiers HJ, Burgess N, Hartley T, Vargha-Khadem F, O'Keefe J. Bilateral hippocampal pathology impairs topographical and episodic memory but not visual pattern matching. Hippocampus. 2001a;11:715–725. doi: 10.1002/hipo.1087. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Burgess N, Maguire EA, Baxendale SA, Hartley T, Thompson PJ, O'Keefe J. Unilateral temporal lobectomy patients show lateralized topographical and episodic memory deficits in a virtual town. Brain. 2001b;124:2476–2489. doi: 10.1093/brain/124.12.2476. [DOI] [PubMed] [Google Scholar]
- Stumpfel V, Kirsner K. Context effects in word identification and episodic recognition: a single dissociation. Bull Psychonomic Soc. 1986;24:175–178. [Google Scholar]
- Sutherland RJ, Rudy JW. Configural association theory: The role of the hippocampal formation in learning, memory, and amnesia. Psychobiology. 1989;17:129–144. [Google Scholar]
- Tort AB, Komorwoski RW, Manns JR, Kopell NJ, Eichenbaum H. Theta-gamma coupling increases during the learning of item-context associations. Proc Nat Acad Sci USA. 2009;106:20942–20947. doi: 10.1073/pnas.0911331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Thompson D. Encoding specificity and retrieval processes in episodic memory. Psych Review. 1973;80:353–370. [Google Scholar]
- Vann SD, Brown MW, Erichsen JT, Aggleton JP. Fos imaging reveals differential patterns of hippocampal and parahippocampal subfield activation in rats in response to different spatial memory tests. J Neurosci. 2000;20:2711–2718. doi: 10.1523/JNEUROSCI.20-07-02711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bonin G, Bailey P. The neocortex of Macaca mulatta. University of Illinois; Urbana, IL: 1947. [Google Scholar]
- Webster MJ, Ungerleider LG, Bachevalier J. Connections of inferior temporal areas TE and TEO with medial temporal- lobe structures in infant and adult monkeys. J Neurosci. 1994;11:1095–1116. doi: 10.1523/JNEUROSCI.11-04-01095.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]