Abstract
Delta-aminolevulinic acid dehydratase single nucleotide polymorphism 2 (ALAD2) and peptide transporter haplotype 2*2 (hPEPT2*2) through different pathways can increase brain levels of delta-aminolevulinic acid and are associated with higher blood lead burden in young children. Past child and adult findings regarding ALAD2 and neurobehavior have been inconsistent, and the possible association of hPEPT2*2 and neurobehavior has not yet been examined. Mean blood lead level (BLL), genotype, and neurobehavioral function (fine motor dexterity, working memory, visual attention and short-term memory) were assessed in 206 males and 215 females ages 5.1 to 11.8 years. Ninety-six percent of children had BLLs < 5.0 µg/dL. After adjusting for covariates (sex, age and mother’s level of education) and sibling exclusion (N = 252), generalized linear mixed model analyses showed opposite effects for the ALAD2 and hPEPT2*2 genetic variants. Significant effects for ALAD2 were observed only as interactions with BLL and the results suggested that ALAD2 was neuroprotective. As BLL increased, ALAD2 was associated with enhanced visual attention and enhanced working memory (fewer commission errors). Independent of BLL, hPEPT2*2 predicted poorer motor dexterity and poorer working memory (more commission errors). BLL alone predicted poorer working memory from increased omission errors. The findings provided further substantiation that (independent of the genetic variants examined) lowest-level lead exposure disrupted early neurobehavioral function, and suggested that common genetic variants alter the neurotoxic potential of low-level lead. ALAD2 and hPEPT2*2 may be valuable markers of risk, and indicate novel mechanisms of lead-induced neurotoxicity. Longitudinal studies are needed to examine long-term influences of these genetic variants on neurobehavior.
Keywords: child lead exposure, neurobehavior, motor dexterity, working memory, delta-aminolevulinic acid dehydratase, proton-coupled oligopeptide transporter, hPEPT2, ALAD
1. Introduction
Lead poisoning in children has decreased dramatically over the past 40 years while low-level lead exposure continues to impact unknown numbers of children, particularly those living in lowest socio-economic conditions across the U.S. (Bernard and McGeehin, 2003). Human and primate studies have suggested that early low-level lead exposure impairs a specific cluster of neurobehavioral functions that are dependent on basal-thalamocortical-striato-pallido loop pathways linking mid-brain and cortical structures (Bolam et al., 2005; Nakano, 2000) including but not limited to fine motor dexterity (Chiodo et al., 2004; Chiodo et al., 2007; Surkan et al., 2007); visual attention (Chiodo et al., 2004; Chiodo et al., 2007; Gilbert and Rice, 1987; Min et al., 2007); and working memory and short-term memory (Chiodo et al., 2004; Lanphear et al., 2000; Min et al., 2007).
1.1 Lead-induced neurotoxicity and delta-aminolevulinic acid (δ-ALA)
Although poorly understood, there are multiple mechanisms by which lead exposure can become neurotoxic. For example, lead particles can cross the blood-brain barrier and preferentially accumulate in astroglia (Lindahl et al., 1999; Thomas et al., 1973). In astrocytes lead particles bind to and inactivate the molecular chaperone 78-kDa (glucose-regulated protein 78, GRP78) (Legare et al., 1998; Qian et al., 2000) lowering astrocytic secretion of neuroprotective IL-6, and increasing the likelihood of excitotoxic cell death (White et al., 2007). At low levels of lead exposure however, lead particle accumulation is likely to be minimal.
Neurotoxic effects can also be indirect, as in the case of increased brain δ-ALA (Kappas, 1995). In erythrocytes, lead particles are bound by δ-aminolevulinic acid dehydratase (δ-ALAD), the second enzyme in the heme biosynthesis pathway. Lead binding inactivates δ-ALAD causing a rise in levels of its substrate, δ-ALA (Klaassen, 2006). Excess brain δ-ALA disrupts the γ-aminobutyric acid (GABA)/glutamate system, in part by blocking GABA receptors and increasing the likelihood of neuroexcitotoxic events and cell death (Brennan and Cantrill, 1979; Demasi et al., 1996b; Emanuelli et al., 2003a; Villayandre et al., 2005). Sodium channel activation is altered by extra-cellular concentrations of δ-ALA as low as 0.01 pM (Lindberg et al., 1999; Wang et al., 2005) suggesting an exquisite sensitivity of neurons to very small increases of extra-cellular δ-ALA.
1.2 Two genetic variants are associated with increased blood lead burden
Variants of two genes that contribute to increased blood lead burden and with downstream indirect and direct effects on δ-ALA levels, might be expected to alter the effects of low-level lead exposure on neurobehavior. δ-ALAD (chromosome 9q34) is encoded by the variants ALAD1 and ALAD2 [ID: rs1800435] (Wetmur et al., 1991a). ALAD2 [ID: rs1800435], is estimated to occur in ten to fifteen percent of Anglo, European and Asian populations (Kelada et al., 2001; Wetmur et al., 1991a) and has a higher binding affinity for lead (Battistuzzi et al., 1981). (Homozygotes are relatively rare thus most studies have examined effects of ALAD2 in groups that combine subjects carrying one or two copies of ALAD2.) Lead-exposed adults with ALAD2 [ID: rs1800435] had higher blood lead burden (Scinicariello et al., 2007; Wetmur et al., 1991b; Zhao et al., 2007) and child studies revealed similar results. In 93 Chilean children living near a lead-contaminated site, those with ALAD2 [ID: rs1800435] had higher BLL (14.2 µg/dL vs. 9.5 µg/dL) (Pérez-Bravo et al., 2004). Similarly, in 229 children in China, mean BLL was higher in children with ALAD2 (11.7 µg/dL vs. 9.7 µg/dL) (Shen et al., 2001). Associations were also observed at lowest levels of exposure in young children (Sobin et al., 2009), and when gender effects were examined differences were found (Sobin et al., 2011b). As compared to other subgroups, mean BLL was highest among males with ALAD2 [ID: rs1800435] (3.5 µg/dL vs. 2.7 µg/dL).
Through an entirely different pathway, another genetic variant also impacts blood lead burden and brain δ-ALA. Proton-coupled oligopeptide transporter (PEPT2, aka SLC15A2, chromosome 3q21.1) protects the brain from excess peptide-bound amino acids. In kidney, PEPT2 reabsorbs di- and tri-peptides (Shen et al., 1999), and PEPT2 maintains neuropeptide homeostasis and removes potential neurotoxins at the blood-cerebrospinal fluid barrier (Ocheltree et al., 2005). Relevant to lead exposure, PEPT2 effluxes δ-ALA from cells in cerebrospinal fluid which has suggested to some that PEPT2 may act as a genetic moderator of lead-induced neurotoxicity (Hu et al., 2007).
Several single nucleotide polymorphisms in the PEPT2 gene with unknown functional impact have been described (Pinsonneault et al., 2004) however two PEPT2 haplotypes, hPEPT2*1 and hPEPT2*2, overwhelmingly predominate. The hPEPT2*2 variant has a significantly lower binding potential (Pinsonneault et al., 2004; Ramamoorthy et al., 1995). For example, hPEPT2*1 and hPEPT2*2 had significantly different Km constants (83 ± 16 and 233 ± 38 µM, respectively) with similar Vmax values for glycyl-sarcosine in hamster ovary cells (Pinsonneault et al., 2004).
Two studies thus far have examined associations between blood lead burden and hPEPT2*2 (Sobin et al., 2009; Sobin et al., 2011b). Similar to the gender effects observed for ALAD2, at lowest levels of lead exposure, males but not females homozygous for hPEPT2*2 had significantly increased BLL (4.9 µg/dL vs. 2.6 µg/dL) (Sobin et al., 2011b). (Why hPEPT2*2 may be associated with higher blood lead burden in males has not yet been determined; possible explanations are discussed in the referenced manuscript). No interaction or additive effects of these genetic variants were observed which may reflect the broadly different pathways by which these genetic variants are likely to influence blood lead burden. (The mechanisms by which hPEPT2*2 influences blood lead burden have not been identified.)
1.3 Genetic variants associated with higher blood lead burden could also predict neurobehavior
Several studies have examined associations between ALAD2 [rs1800435] and neurobehavior in lead exposed children (Bellinger et al., 1994; Pawlas et al., 2012), adolescents (Krieg et al., 2009) and adults (Gao et al., 2010). Additional studies are needed however. While child studies have suggested ALAD2 is neuroprotective, studies in older adults suggest worse outcomes (Rajan et al., 2008). Moreover, no studies have examined associations between hPEPT2 genotypes and neurobehavior in low-level lead exposed children; the ALAD and hPEPT2 variants have not yet been considered in a single model; and interactions with BLL have rarely been examined. Understanding how these genetic variants are associated with neurobehavior in low-level lead exposed children could suggest novel hypotheses regarding the mechanisms by which low-level lead exposure disrupts early neurobehavior, and ultimately perhaps, provide a means for identifying subgroups of children at heightened risk for poor outcome (Levin et al., 2009).
The goal of this study was to test the possible moderating effects of ALAD and hPEPT2 genetic variants on motor dexterity, visual attention, working memory and short-term memory in young children tested for lead exposure. Significant main effects of aggregate BLL, ALAD and hPEPT2, and interaction effects for ALAD and hPEPT2 by BLL on neurobehavior were tested.
2. Materials and Methods
2.1 Participants
Permission to conduct these studies was obtained from the local school district and approved by the university Institutional Review Board. Children were tested with the full understanding and prior written consent of parents; child assent was obtained immediately prior to testing. Convenience samples were recruited from two elementary schools (sites) located in lower-income neighborhoods and included children 5.1 to 11.8 years of age. A participation invitation letter was sent to all parents from the school principal and a copy of the consent form was enclosed in the letter. Interested parents attended informational sessions during which details of the study were explained and informed consent was obtained. Participants represented 27.6 – 38.4% of enrolled students in each school. All study forms and materials were available in Spanish and English versions. Researchers on this study were fully bilingual and throughout the study interacted with parents and children in their preferred language.
2.2 Procedures
2.2.1 Genetic sample collection
Cheek cell collection, DNA extraction and single-nucleotide polymorphism detection were completed using proprietary technology developed by TrimGen Corporation (Sparks, MD). Detailed procedures were described previously (Sobin et al., 2009).
2.2.2 Blood lead analysis
Blood lead level testing was conducted at two time points, between 53 and 67 days prior to neurobehavioral testing, and at the time of neurobehavioral testing. Detailed procedures were previously described (Sobin et al., 2011a; Sobin et al., 2011b). Aggregate blood lead level was the mean of two values obtained an average of 60 days apart for each child. Four hundred and twenty-one children were tested (206 males, 215 females). Blood lead level was determined by either inductively coupled plasma mass spectrometry (331/421, 79%) (LOD, 0.022 µg/dL) or the LeadCare I (LCI) device (ESA Magellan Biosciences, Chelmsford, MA) (90/421, 21%) (LOD, 0.5 µg/dL). (Cost restricted ICP-MS analysis of all samples.) In the final sample of children included for these analyses, there were no BLL values below the LOD for either method. The two samples obtained from each child were analyzed by only one method (either ICP-MS or LCI) and only one method of detection was used at a given site. Methods for ICP-MS analyses were previously described (Sobin et al., 2011b). The LeadCare® testing device electrochemically measured the amount of lead in whole blood via a 1.4 cm × 4.2 cm colloidal gold electrode (Wang and Tian, 1992) onto which a drop of the mixed sample was applied. Methods were followed as previously described (Sobin et al., 2009).
2.2.3 Neurobehavioral assessment
The selection of neurobehavioral test domains was guided by results from previous studies of neurobehavior in low-level lead exposed children (Chiodo et al., 2004; Chiodo et al., 2007; Lanphear et al., 2000; Min et al., 2007; Surkan et al., 2007). The specific battery selected was shown in previous studies to be relatively free from association with parent demographics and family income (Fray and Robbins, 1996; Waber et al., 2007). Tests included the Grooved Pegboard (motor dexterity) (Knights and Moule, 1968) and CANTAB computer-based tests of working memory, sustained visual attention, and short-term memory (Waber et al., 2007). Stimuli and responses for all tasks were “language-free,” that is, independent of a participant’s ability to linguistically label stimuli or respond with linguistically based answers. All tasks were administered according to the standard published administration procedures by three fluent English/Spanish bi-lingual graduate-level researchers specially trained and supervised by a licensed psychologist, and with assistance from four undergraduate-level research workers in a specially designated, distraction- and noise-free testing room. Testers interacted with children and provided instructions orally in either Spanish or English, according to the child’s preference and language ability. The large majority of children spoke in both languages interchangeably and testers followed children along according to the child’s preferences. Testers were blind to child blood lead level and genetic status. All testing was completed within one 22-month period.
A brief screening task (CANTAB, Big Little Circle) was used to ensure children’s ability to follow changing instructions, and to familiarize children with use of the touch screen computer. The screening task did not replicate any of the neurobehavioral tests.
2.2.3.1 Motor dexterity
Motor dexterity was assessed with the Grooved Pegboard task. Materials for this task included a small board (h 9”× w 5”× d 1”) with ¼” long key-hole shaped slots and a well at the top of the board containing key-hole shaped pegs. Participants were instructed to pick up one peg at a time and place it in a hole, manipulating the orientation of each peg only with the fingers used to pick up the peg using only the thumb and forefinger. All children completed placement of ten pegs using dominant and non-dominant hands. Completion time and number of peg drops for each hand were recorded. (Dominant and non-dominant hand peg drops were infrequent and did not provide enough variability to be statistically analyzed.)
2.2.3.2 Working memory
The Rapid Visual Processing task (RVP) assessed working memory. In this task, random numbers appeared rapidly in a white box at the center of the computer screen. Participants were instructed to press the response key immediately after the target sequence was detected, which was the number 1, followed by the number 2, followed by the number 3. The task included a pretest acclimation phase (2 minutes) and a test phase (3 minutes). Hits, misses, false alarms and correct rejections were measured.
2.2.3.3 Visual attention
The Reaction Time test (RTI) assessed sustained visual attention and measured reaction time and movement time in one-choice and five-choice paradigms. Reaction time was the delay between the stimulus presentation and the release of the response button; movement time was the amount of time required to touch the target on the screen after key release. For the one-choice task, participants began the task by holding down the response key, released the key to touch a one-inch diameter yellow circle that appeared on the computer screen, and then returned to the key press position. For the five-choice task, the yellow spot to be touched appeared in one of five empty one-inch diameter circles arranged on the screen in a circular array, which increased the attentional challenge during timed responding. Accuracy for each response was determined according to whether the child pressed the target area within the boundary of the target circle.
2.2.3.4 Short-term memory
Short-term memory was assessed with the Spatial Span task (SSP). The task began with an arrangement of small white boxes on the computer screen. One by one, each box changed color indicating a sequence to be remembered. Immediately following the sequence presentation, participants were asked to demonstrate recall of the sequence by touching boxes in the order in which the colors had changed. The number of boxes presented increased from two to nine. Span length achieved and number of out-of sequence boxes touched (total errors) were measured.
2.3 Data analysis
SAS Version 9.3 (SAS Institute Inc., Cary, North Carolina) was used. Data were triple-checked for accuracy after entry and neurobehavioral data were examined for missing values, distribution properties and outliers. No values were out of the range of plausible responses. All analyses controlled for sex, age, mother’s level of education and site. Parent information including mother’s level of education was obtained for 336/421 (80%) of children tested, including 157 males and 179 females After sibling exclusion, the sample for analysis included 252 participants, including 121 (48%) males and 131 (52%) females (Table 1). Generalized linear mixed model analyses (GLIMM IX) with maximum likelihood estimates were used. Fixed effects included aggregate BLL (mean of blood lead levels tested approximately 60 days apart), ALAD genotype (“wild type,” without ALAD2; “heterozygous,” carrying one copy of ALAD2); hPEPT2 genotype (“wild type,” without hPEPT2*2; “heterozygous,” carrying one copy of hPEPT2*2; or “homozygous” carrying two copies of hPEPT2*2); age, sex, mother’s level of education, and the interaction effects of BLL × ALAD and BLL × hPEPT2. Site was included as a random effect. The Gaussian distribution with an identity link function was specified for continuous data; the Poisson distribution with a log link function was specified for discrete (count) data. All models were checked for convergence and the G matrix estimate.
Table 1.
Clinical and demographic characteristics of male and female children
| Males 121/252 (48%) |
Females 131/252 (52%) |
|
|---|---|---|
| Time 1 mean BLL (SD) | 2.8 (1.6) | 2.5 (1.2) |
| Time 2 mean BLL (SD) | 2.5 (2.1) | 2.2 (1.2) |
| Aggregate BLL (BLL) µg/dL mean (SD) | 2.7 (1.5) | 2.4 (1.0) |
| Age mean (SD) | 7.9 (1.7) | 8.1 (1.8) |
| Hispanic | 116/121 (96%) | 127/131 (97%) |
| Housing built before 1974 | 110/121 (91%) | 122/131 (93%) |
| Household income < 20K, mean family size 4.6 | 113/121 (93%) | 121/131 (92%) |
Models were evaluated by examining fixed effect Type III F-values and significance for main effects and interactions. Main effects included BLL (aggregate of two time points), ALAD, and hPEPT2, controlling for age, sex, mother’s level of education and site; interactions included BLL × ALAD, and BLL × hPEPT2. Data coding set “wild type” as the reference group for all parameter estimate tests and parameter estimate significance values indicated difference from zero for continuous variables (i.e., aggregate BLL) or for each level of categorical variables (i.e., genotype). Significance for the Type III fixed effect tested whether the variable or interaction estimate differed significantly from zero and indicated the amount of model variance accounted for by a given (continuous or categorical) variable or interaction. When the fixed effect F-value was statistically significant, relevant post-hoc tests of least square means (for categorical effects) were evaluated; or regression coefficients for the significant continuous predictor (BLL) or interactions were determined and tested. For genotype main effects, post-hoc comparisons of least square mean differences were calculated using the Tukey-Kramer adjustment for multiple comparisons. Least square means (LSM) reflected the mean of a variable after co-varying other model factors, i.e., sex, age, mother’s level of education and site. Adjusted alpha ≤ .05, and adjusted lower and upper 95% confidence intervals were used to evaluate all post-hoc comparisons.
3. Results
Table 1 gives the demographic characteristics of the analyzed sample of 252 children, including 121 (48%) males (mean age 8.2 ± 1.9 years) and 131 (52%) females (mean age 8.0 ± 1.8 years). Ninety-seven percent of parents were of self-identified Hispanic origin. Ninety-three percent of families had an annual household income of less than twenty-thousand dollars and a mean family size of 4.6 ± 1.3.
Table 2 shows the means (SDs) of BLLs assessed at time 1, time 2 and the aggregate (average) of the two assessments by genotype. The percentages of genetic variants observed in this sample closely approximated reported frequencies from previous mixed race studies (Benkman et al., 1983; Petrucci et al., 1982; Secchi et al., 1974). Consistent with reported rates, only one child (female) was homozygous for ALAD2 and this child’s data were included with ALAD2 heterozygous females for subsequent analyses. Ninety-six percent of children (241/252) had aggregate BLLs < 5.0 µg/dL. The aggregate values for the remaining 11 children (4%) were not widely distributed (9 children had BLLs between 5 and 7 µg/dL and the remaining two had BLLs of 8.5 and 9.5 µg/dL). Log transformation did not improve the distribution and was not used.
Table 2.
Aggregate BLLs for males and female children by genotype, N = 252.
| Males | BLL Aggregate (SD) |
Females | BLL Aggregate (SD) |
|
|---|---|---|---|---|
| ALAD 1/1 (wt) | 111/121 (92%) | 2.7 ±1.5 | 116/131 (89%) | 2.4 ±1.0 |
| ALAD 1/2 (het) | 10/121 (8%) | 2.6 ±1.6 | 14/131 (11%) | 1.9 ±0.6 |
| ALAD 2/2 (hom) | 0/121 | 1/131* | 2.2 | |
| hPEPT2*1/1 (wt) | 70/121 (58%) | 2.7 ±1.3 | 61/131 (47%) | 2.2 ±0.8 |
| hPEPT2*1/2 (het) | 41/121 (34%) | 2.5 ±1.5 | 49/131 (37%) | 2.6 ±1.1 |
| hPEPT2*2/2 (hom) | 10/121 (8%) | 3.4 ±2.3 | 21/131 (16%) | 2.4 ±0.7 |
Data from one female ALAD2 homozygote was included with heterozygotes for regression analyses.
Table 3 shows the means (SDs) and range for each of the neurocognitive variables tested. Models were tested with data from 252 children. Neurobehavioral data from a total of two participants were incomplete due to fatigue. Two females were missing data from two of four CANTAB tasks. Convergence criterion was reached for all models except working memory task errors.
Table 3.
Descriptive statistics for neurocognitive performance data, N = 252 children ages 5.0 to 11.8 years of age.
| Mean ±SD | Range | ||
|---|---|---|---|
| Motor Dexterity | |||
| Grooved Pegboard | |||
| Dominant Hand Completion Time (s) | 43.9 ±17.5 | 23 – 135 | |
| Non-Dominant Hand Completion Time (s) | 50.0 ±24.3 | 24 – 223 | |
| Working Memory | |||
| Rapid Visual Processing (RVP) | |||
| Hits (number) | 18.8 ±4.4 | 0 – 24 | |
| Misses (number) | 5.2 ±4.4 | 0 – 24 | |
| False Alarms (number) | 5.1 ±7.7 | 0 – 66 | |
| Correct Rejections (number) | 258.6 ±13.9 | 200 – 276 | |
| Visual Attention | |||
| Reaction Time (RTI) | |||
| Single Choice Visual Display | |||
| Accuracy (number) | 13.9 ±1.3 | 8 – 15 | |
| Reaction Time (ms) | 449.2 ±154.2 | 244.8 – 1616.9 | |
| Movement Time (ms) | 453.3 ±150.0 | 149.6 – 1080.3 | |
| Five-Choice Visual Display | |||
| Accuracy (number) | 14.3 ±1.2 | 7 – 15 | |
| Reaction Time (ms) | 462.6 ±106.2 | 292.5 – 961.5 | |
| Movement Time (ms) | 416.5 ±92.4 | 157.9 – 722.5 | |
| Short-Term Memory | |||
| Spatial Span (SSP) | |||
| Span Length (number) | 4.5 ±1.4 | 1 – 9 | |
| Total Errors (number) | 12.2 ±5.2 | 4 – 30 | |
Table 4 below summarizes the Type III fixed effects solutions and parameter estimates for the main effects of BLL and genotype, and the interactions of BLL × genotype. Significant effects of BLL and/or genotype were found for one or more components of motor dexterity, working memory, and visual attention. These included main effects for BLL or hPEPT2, and interaction effects for BLL × ALAD. No effects were observed for short-term memory.
Table 4.
Significant Type III fixed effects and parameter estimates showing associations of ALAD and hPEPT2 genotype and/or aggregate BLL on neurobehavior controlling for sex, age, mother’s level of education and site.
| Significant Type III Fixed Effect | Solutions for Fixed Effects | |||||||
|---|---|---|---|---|---|---|---|---|
| F | p | Est | SE | DF | t value |
p | ||
| Motor Dexterity Non-Dominant Hand Time (s) | Intercept* | 71.15 | 21.30 | 233 | 3.34 | <0.01 | ||
| BLL | 1.29 | 0.26 | BLL | 1.93 | 1.67 | 233 | 1.16 | 0.25 |
| hPEPT2 | 7.89 | < 0.01 | hPEPT2*2 Hom | 16.66 | 4.20 | 3.97 | <0.01 | |
| ALAD | 0.00 | 0.97 | hPEPT2*2 Het | 2.25 | 2.80 | 0.80 | 0.421 | |
| BLL × hPEPT2 interaction | 0.70 | 0.50 | hPEPT2*1 WT | 0 | . | . | . | |
| BLL × ALAD interaction | 0.20 | 0.65 | ALAD2 Het | −0.16 | 4.35 | −0.04 | 0.97 | |
| ALAD1 WT | 0 | . | . | . | ||||
| BLL × hPEPT2*2 Hom interaction |
−2.53 | 3.01 | −0.84 | 0.40 | ||||
| BLL × hPEPT2*2 Het interaction |
1.07 | 2.37 | 0.45 | 0.65 | ||||
| BLL × hPEPT2*1 WT interaction |
0 | . | . | . | ||||
| BLL × ALAD2 Het interaction | 1.73 | 3.85 | 0.45 | 0.65 | ||||
| BLL × ALAD1 WT interaction | 0 | . | . | . | ||||
| Working Memory Misses | Intercept | 3.14 | 0.54 | 233 | 5.87 | <0.01 | ||
| BLL | 6.16 | 0.01 | BLL | 0.11 | 0.03 | 233 | 3.39 | <0.01 |
| hPEPT2 | 0.01 | 0.99 | hPEPT2*2 Hom | 0.00 | 0.10 | −0.03 | 0.98 | |
| ALAD | 2.98 | 0.09 | hPEPT2*2 Het | 0.01 | 0.06 | 0.11 | 0.91 | |
| BLL × hPEPT2 interaction | 4.54 | 0.01 | hPEPT2*1 WT | 0 | . | . | . | |
| BLL × ALAD interaction | 0.51 | 0.48 | ALAD2 Het | −0.18 | 0.10 | −1.73 | 0.09 | |
| ALAD1 WT | 0 | . | . | . | ||||
| BLL × hPEPT2*2 Hom interaction |
0.10 | 0.05 | 1.76 | 0.08 | ||||
| BLL × hPEPT2*2 Het interaction |
−0.09 | 0.05 | −1.67 | 0.10 | ||||
| BLL × hPEPT2*1 WT interaction |
0 | . | . | . | ||||
| BLL × ALAD2 Het interaction | −0.05 | 0.07 | −0.71 | 0.48 | ||||
| BLL × ALAD1 WT interaction | 0 | . | . | . | . | |||
| Working Memory False Alarms Errors | Intercept | 4.42 | 0.38 | 233 | 11.75 | <0.01 | ||
| BLL | 1.93 | 0.17 | BLL | 0.05 | 0.03 | 233 | 1.49 | 0.14 |
| hPEPT2 | 6.32 | <0.01 | hPEPT2*2 Hom | 0.33 | 0.09 | 3.54 | <0.01 | |
| ALAD | 0.13 | 0.72 | hPEPT2*2 Het | 0.09 | 0.07 | 1.31 | 0.19 | |
| BLL × hPEPT2 interaction | 1.78 | 0.17 | hPEPT2*1 WT | 0 | . | . | . | |
| BLL × ALAD interaction | 10.95 | <0.01 | ALAD2 Het | 0.04 | 0.10 | 0.36 | 0.72 | |
| ALAD1 WT | 0 | . | . | . | ||||
| BLL × hPEPT2*2 Hom interaction |
0.00 | 0.06 | 0.04 | 0.96 | ||||
| BLL × hPEPT2*2 Het interaction |
0.09 | 0.05 | 1.78 | 0.08 | ||||
| BLL × hPEPT2*1 WT interaction |
0 | . | . | . | ||||
| BLL × ALAD2 Het interaction | −0.29 | 0.09 | −3.31 | <0.01 | ||||
| BLL × ALAD1 WT interaction | 0 | . | . | . | ||||
|
Visual Attention 5-Choice Movement Time (ms) |
Intercept | 478.34 | 96.17 | 231 | 4.97 | <0.01 | ||
| BLL | 3.03 | 0.08 | BLL | 26.07 | 7.52 | 231 | 3.46 | <0.01 |
| hPEPT2 | 0.75 | 0.47 | hPEPT2*2 Hom | −10.50 | 19.11 | −0.55 | 0.58 | |
| ALAD | 1.00 | 0.32 | hPEPT2*2 Het | −15.13 | 12.61 | −1.20 | 0.23 | |
| BLL × hPEPT2 interaction | 2.09 | 0.15 | hPEPT2*1 WT | 0 | . | . | . | |
| BLL × ALAD interaction | <0.01 | ALAD2 Het | 19.59 | 19.61 | 1.00 | 0.32 | ||
| 8.68 | ||||||||
| ALAD1 WT | 0 | . | . | . | ||||
| BLL × hPEPT2*2 Hom interaction |
−25.42 | 13.63 | −1.87 | 0.08 | ||||
| BLL × hPEPT2*2 Het interaction |
−23.71 | 10.69 | −2.22 | 0.06 | ||||
| BLL × hPEPT2*1 WT interaction |
0 | . | . | . | ||||
| BLL × ALAD2 Het interaction | −51.24 | 17.39 | −2.95 | <0.01 | ||||
| BLL × ALAD1 WT interaction | 0 | . | . | . | ||||
BLL values were centered for these analyses and the intercepts produced are directly interpretable.
3.1 Motor dexterity
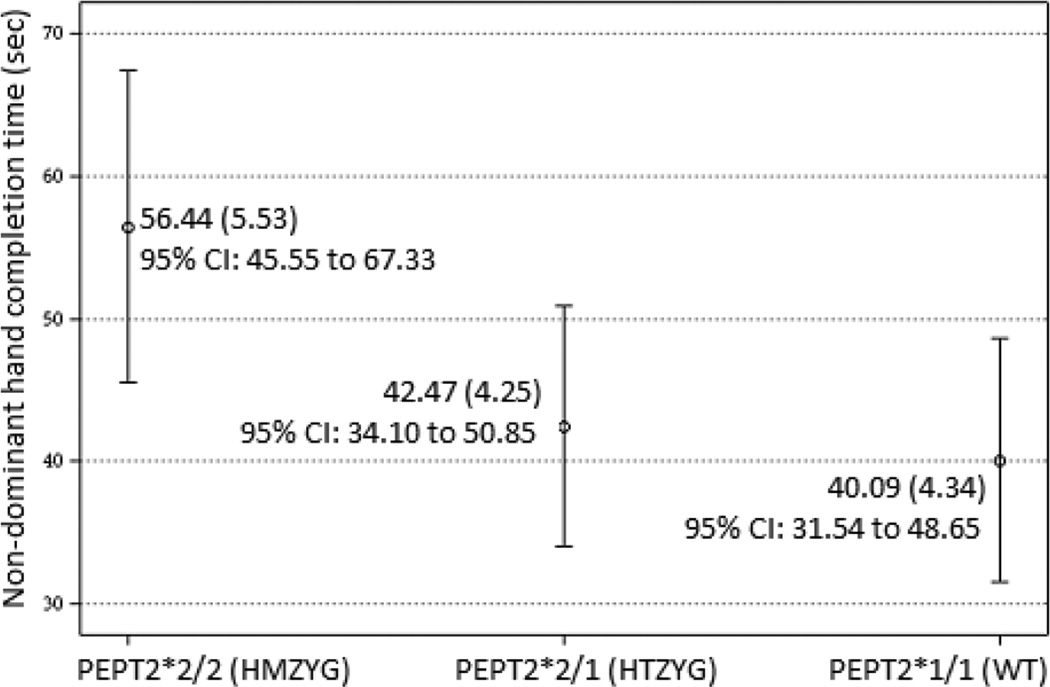
Two variables were tested including dominant and non-dominant hand completion time for the peg board task. The hPEPT2 genotype (independent of aggregate BLL) predicted non-dominant hand motor performance. Children homozygous for the hPEPT2*2 were substantially slower as compared with the other hPEPT2 genotypes (Table 4). Post-hoc comparisons of differences between hPEPT2 genotypes showed differences between homozygotes and heterozygotes (diff estimate = 13.97, SE = 4.35, t = 3.21, p < .01, 95% C.I. 5.40 to 22.55) and between homozygotes and wild types (diff estimate = 16.35, SE = 4.14, t = 3.952, p < .01, 95% C.I. 8.20 to 24.50). The LSM difference between heterozygotes and wild types was not significant. Figure 1. shows the hPEPT2 genotype LSMs with 95% confidence intervals. Children homozygous for hPEPT2*2 had substantially slower non-dominant hand completion time independent of aggregate BLL.
Fig. 1.
Least square means and 95% confidence intervals for non-dominant hand completion time by hPEPT2 genotype. Homozygotes differed significantly (p < .01) from heterozygotes and wild types; heterozygotes and wild types did not differ.
3.2 Working memory
Four variables were tested including hits, misses, correct rejections, and false alarms. As shown in Table 4, significant effects were found in the models for misses and false alarm errors.
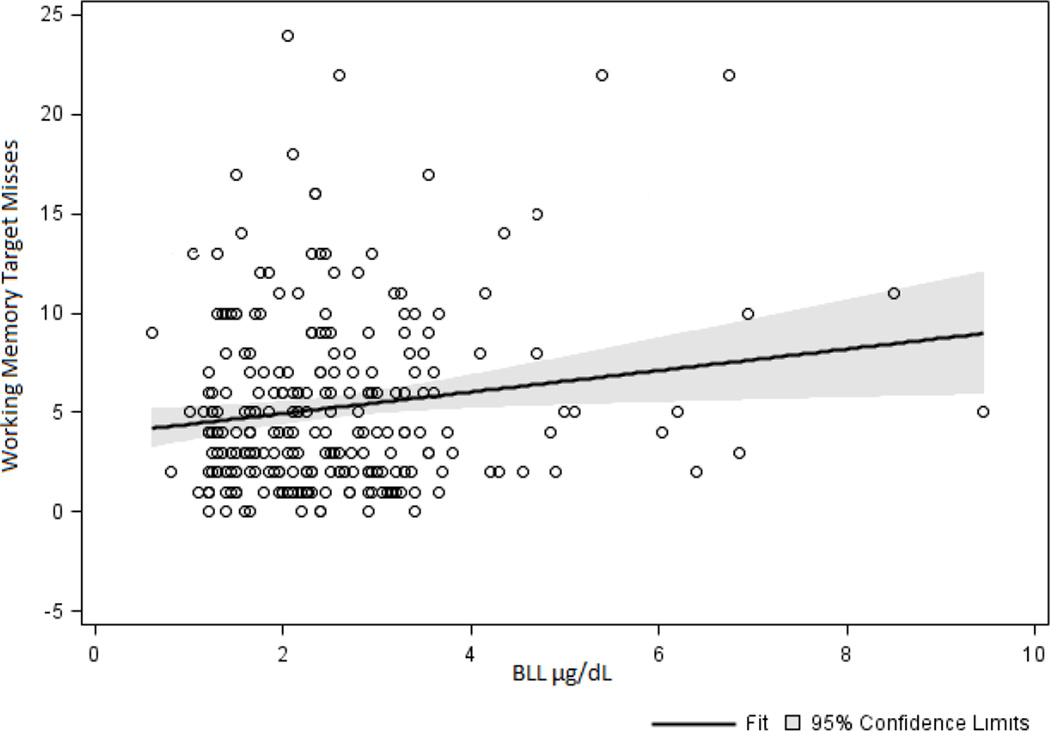
Aggregate BLL alone predicted working memory misses. As shown in Figure 2, regardless of genotype, as BLL increased number of target misses increased. The coefficient estimate for BLL was 0.09 (SE = 0.04) and differed significantly from zero (t = 2.48, p = 0.01) suggesting that as BLL increased target misses during the working memory task increased.
Fig. 2.
Independent of genotype, aggregate BLL predicted target misses on the working memory task. As BLL increased, misses during the working memory task increased.
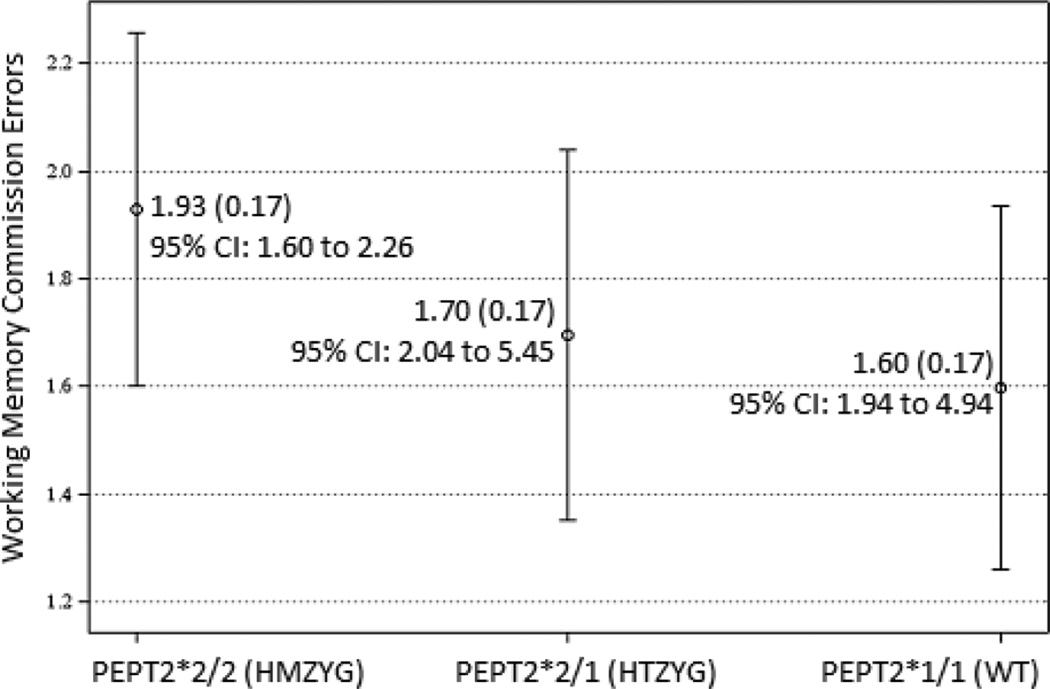
Two effects predicted false alarms errors. There was a significant main effect for hPEPT2*2. The parameter estimate indicated that children with two copies of hPEPT2*2 made significantly more commission errors than other children. Post-hoc comparisons of LSM differences between hPEPT2 genotypes showed significant differences between homozygotes and wild types (diff estimate = 0.33, SE = 0.09, t = 3.62, p < .01, 95% C.I. 0.15 to 0.51) and between homozygotes and heterozygotes (diff estimate = 0.23, SE = 0.09, t = 2.42, p < .01, 95% C.I. 0.04 to 0.42). The LSM difference between hPEPT2*2 heterozygotes and wild type was not significant. Figure 3 shows these LSM relationships.
Fig. 3.
Least square means and 95% confidence intervals for commission errors on the working memory task by hPEPT2 genotype. Homozygotes differed significantly from heterozygotes and wild types (p < .01); heterozygotes and wild types did not differ.
The interaction for BLL × ALAD was also significant. The coefficients were estimated and tested for BLL × ALAD1 wild type (0.08, SE = 0.02, t = 3.35, p < .01) and for BLL × ALAD2 heterozygotes (−0.21, SE = 0.09, t = −2.42, p = 0.02), and both differed significantly from zero. For children homozygous for ALAD1, as BLL increased false alarms increased; for children heterozygous for ALAD2, as BLL increased false alarms decreased.
3.3 Visual attention
Response reaction and movement time, and number of errors for single-item and five-choice displays were measured. Movement time towards the five-choice display was predicted by the BLL × ALAD genotype. The coefficients were estimated and tested for BLL × ALAD1 homozygotes (0.02, SE = 0.01, t = 1.57, p = 0.12) and for BLL × ALAD2 heterozygotes (−0.09, SE=0.04, t = −2.06, p = 0.04), and the effect was significant only for heterozygotes. Among children heterozygous for ALAD2, as BLL increased, movement time decreased demonstrating better visual attention in children heterozygous for ALAD2.
3.4 Short-term memory
Two variables were analyzed including span length (the longest sequence of squares correctly identified, from two to nine) and errors (total number of out-of-sequence boxes touched). No significant effects were observed.
4. Discussion
Past findings from child and adult studies of the ALAD genotype and neurobehavior in lead-exposed individuals were inconsistent, and no studies had yet examined the possible moderating effects of hPEPT2*2 on neurobehavior in young lead-exposed children. The present study examined these associations and found that in school-age children, ninety-six percent of whom had aggregate BLLs < 5 µg/dL, ALAD2 and hPEPT2*2 differently moderated neurobehavior. Consistent with the previous child literature ALAD2 appeared to be neuroprotective and, interestingly, interacted with aggregate BLL. As BLL increased, children with ALAD2 performed better on working memory and visual attention tasks. In contrast, independent of aggregate BLL, hPEPT2*2 was associated with worse performance on motor dexterity and working memory tasks.
4.1 Low-level lead exposure was associated with poorer working memory
In this sample of children with very low blood lead, regardless of genotype, as aggregate BLL increased, working memory performance decreased as evidenced by increased numbers of target misses. This finding of poorer working memory adds to the current child clinical literature showing the ill effects of low-level lead exposure on IQ, academic achievement and neurobehavior in the domains of visual attention, problem-solving and motor dexterity. Of the four performance components of working memory assessed in this task (hits, misses, false alarms and correct rejections), misses provided the most direct evidence of poor working memory and suggested that small increases in blood lead reduced a child’s ability to consistently retain and use sequenced information to identify and complete a correct response. Working memory is often referred to as a “meta-cognitive” function because of its central importance for success in a broad array of core academic domains including, from reading comprehension to abstract problem-solving in math and the sciences. Working memory is dependent on dentate gyrus (Aimone et al., 2011; Friedman and Goldman-Rakic, 1988; Xavier et al., 1999). Thus, this finding is consistent with the animal literature showing vulnerability of dentate gyrus to higher (Gilbert et al., 1996; Gilbert and Mack, 1998; Gilbert et al., 2005; Gilbert and Lasley, 2007) and lowest levels (Sobin et al., 2013) of lead exposure. Dentate gyrus is one of only three brain regions that supports neurogenesis throughout the lifespan and studies have also shown how higher levels of lead exposure alter neurogenesis (Gilbert et al., 2005). Studies are needed to examine whether early exposure to low-level lead has long-term effects on neurogenesis in adult and aging brains.
4.2 The moderating influences of ALAD2 on low-level lead exposure may be neuroprotective
The influences of ALAD2 were observed only as interactions with aggregate BLL. As aggregate BLL increased, the performance of heterozygotes also increased in two specific ways. With regard to working memory function, false alarm errors (responding to a non-target) were fewer suggesting a higher level inhibitory control during a working memory challenge. During the visual attention task, response time to the five-choice stimuli for ALAD2 heterozygotes was faster suggesting better visual attention to a complex display.
Interpretations of the ALAD interactions however must be informed by the cross-sectional nature of these data. The data represent differences across children at different levels of lead exposure. Longitudinal studies are needed to examine the influence of ALAD2 on working memory and visual attention in individual children with fluctuating levels of lead exposure, to clarify how the effects of ALAD2 may or may not follow blood lead burden.
ALAD encodes the δ-ALAD protein which actuates the second step of heme biosynthesis. ALAD gene variants 1 and 2 produce proteins with lower (ALAD1) and higher (ALAD2) lead binding properties. Higher blood lead burden has been observed among children (and adults) with ALAD2 because the protein produced binds lead more tightly. Current knowledge regarding the characteristics of these binding mechanisms however is limited. For example, it is not yet known whether ALAD2 binds a greater number of lead particles in erythrocytes, and/or sequesters lead for longer periods of time, and the effects of either or both of these processes on δ-ALA levels in erythrocytes or brain also are not yet known.
4.3 The influences of hPEPT2*2 appear to be neurotoxic
Motor dexterity and response inhibition (during a working memory task) are critically dependent on inhibitory control mechanisms. Regardless of aggregate BLL, children homozygous for the hPEPT2*2 haplotype had poorer motor dexterity, and poorer working memory as evidenced by more false alarm errors. Motor dexterity is integral for early learning, for the development of eye-hand coordination, and for the acquisition of daily living skills which in turn contribute to a child’s growing sense of competence, confidence and emerging independence. The combination of poor motor dexterity and poor response inhibition during a working memory task, suggested that in children with hPEPT2*2, inhibitory control was diminished across task domains.
For the benefit of ongoing and future studies, it may be useful to consider the possible pathways and mechanisms that could underlie the observed effects. Poor inhibitory control suggests disrupted and perhaps inconsistent regulation of the prefrontal, cerebrocerebellar and basal ganglia pathways known to support inhibitory control (Rosenbaum, 1980). Glutamate and GABA predominate in the basal ganglia loops that overlap these pathways (Alexander et al., 1986). PEPT2 (aka SLC15A2) is a protective endogenous transporter that removes excess peptide-bound amino acids, including δ-ALA, at the blood-cerebrospinal fluid barrier (Ocheltree et al., 2005). The PEPT2 protein produced by hPEPT2*2 as compared with hPEPT2*1 has substantially lower binding potential (Pinsonneault et al., 2004; Ramamoorthy et al., 1995). In children with the hPEPT2*2 variant, greater amounts of δ-ALA, and perhaps other peptide-bound amino acids with harmful effects, might be expected to enter the brain at choroid plexus. Increased brain δ-ALA has several ill effects. δ-ALA is an ω-amino acid with a five-carbon chain (Brennan and Cantrill, 1979) and resembles the structure of GABA. δ-ALA stimulates glutamate release (Brennan and Cantrill, 1979) and irreversibly alters glutamate transporter GLT-1 thereby inhibiting glutamate uptake by astrocytes (Emanuelli et al., 2003b). As discussed in section 1.1, miniscule increases in extra-cellular δ-ALA concentrations (e.g., 0.01 pM) alter sodium channel activation in neurons (Wang et al., 2005). δ-ALA activates GABAA auto-receptors and damages GABAA receptor sites (Demasi et al., 1996a); chronic excess of δ-ALA decreases NMDA receptor density (Villayandre et al., 2005). Human studies of the effects on neurobehavior of the hPEPT2*2 genotype are just beginning.
4.4 Limitations
This study examined influences on neurobehavior of the ALAD and hPEPT2 genetic variants. The choice of these genetic variants was guided by the shared effects of these genotypes on δ-ALA, and was not intended to imply that these are the only genetic variants that could modify neurobehavior in low-level lead exposed children. Future studies are needed to examine additional genetic variants that could impact vulnerability to low-level lead exposure during development.
The numbers of children with each genotype detected in our studies closely approximated previous reports but these rates yielded unbalanced numbers of children for each genotype. This limited the analyses in three ways. First, while unbalanced subgroup sizes and fewer numbers in some subgroups would be expected to diminish the likelihood of significant effects, fewer numbers might also produce unstable results. The findings require replication. Second, sex effects were not statistically detectable however informal examination of the data suggested that neurobehavior in low-level lead exposed males and females may differ in the genotypes examined. Greater numbers of male and female children carrying the ALAD2 and hPEPT2*2 genotypes are needed before these possible differences can be tested statistically. Third, the numbers of children with each genotype allowed tests of only individual genotype effects and their possible interactions with aggregate BLL and not genotype interactions. While previous studies did not show interactions or additive influences of ALAD and hPEPT2 genotypes on blood lead level, for studies of the possible impact of these genotypes on neurobehavior, genotype interactions need to be tested.
This was a convenience sample and included children from a broad range of ages (5.1–11.8 years). The assessed neurobehaviors develop rapidly during these years. Age was co-varied in all models and the amount of age-related variability observed may have limited the detection of differences conferred by these genotypes at different ages. Additional studies of children in one year cohorts each including a balanced number of children for each genotype, are needed. Approximately 20% of parents chose not to provide detailed demographic information which reduced the numbers of children that could be included for analysis.
δ-ALA was not measured in this study because urine sample collection in a public school setting was likely to reduce compliance. Since the association between urine and brain δ-ALA is not known, particularly in cases of lowest-level lead exposure and with regard to hPEPT2*2, the value of collecting these samples was questionable, given the expected impact on compliance. When it is feasible to do so, future studies would benefit from the measurement of urinary δ-ALA.
4.5 Conclusions
ALAD2 and hPEPT2*2 gene variants influence neurobehavioral outcome in children with blood lead levels below 5 µg/dL. Consistent with previous child studies, ALAD2 appeared to be neuroprotective, and interacted with BLL such that, as BLL (across children) increased, working memory and visual attention were enhanced. In contrast, independent of BLL, hPEPT2*2 was associated with markedly poorer motor dexterity and poorer working memory. Children carrying the ALAD1 and hPEPT2*2 variants may be at higher risk of low-level lead induced neurotoxicity. Studies are needed to determine how the Pb- and δ-ALA binding mechanisms altered by these genetic variants influence immediate- and longer-term effects of low-level lead exposure on neurobehavior.
Supplementary Material
Highlights.
-
-
The influence of ALAD and hPEPT2 genetic variants on motor function, memory and attention was examined
-
-
Ninety-six percent of children tested had blood lead levels below 5 µg/dL
-
-
ALAD2 was associated with improved performance in low-level lead exposed children
-
-
hPEPT2*2 was associated with poorer performance regardless of lead exposure
-
-
ALAD and hPEPT2 variants may be useful markers of neurobehavioral risk in lead exposed children
Acknowledgements
This research was made possible by grants from the National Institute of Child Health and Human Development (NICHD), National Institutes of Health, (R21HD060120, CS, PI); the National Center for Research Resources, a component of the National Institutes of Health (5G12MD007592); the Center for Clinical and Translational Science, The Rockefeller University, New York, New York; the Paso del Norte Health Foundation, El Paso, Texas; the University Research Institute, University of Texas, El Paso; and from the J. Edward and Helen M. C. Stern Professorship in Neuroscience, University of Texas, El Paso (CS). The funders had no role in the design, implementation, data analysis, or manuscript preparation for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Battistuzzi G, et al. delta-Aminolevulinate dehydrase: a new genetic polymorphism in man. Annals Of Human Genetics. 1981;45:223–229. doi: 10.1111/j.1469-1809.1981.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Bellinger D, et al. Attentional correlates of dentin and bone lead levels in adolescents. Arch Environ Health. 1994;49:98–105. doi: 10.1080/00039896.1994.9937461. [DOI] [PubMed] [Google Scholar]
- Benkman HG, Gogdaanski P, Goedde HW. Polymorphism of delta-aminolevulinic acid dehydratase in various populations. Human Heredity. 1983;33:62–64. doi: 10.1159/000153351. [DOI] [PubMed] [Google Scholar]
- Bernard SM, McGeehin MA. Prevalence of blood lead levels >or= 5 micro g/dL among US children 1 to 5 years of age and socioeconomic and demographic factors associated with blood of lead levels 5 to 10 micro g/dL, Third National Health and Nutrition Examination Survey, 1988–1994. Pediatrics. 2003;112:1308–1313. doi: 10.1542/peds.112.6.1308. [DOI] [PubMed] [Google Scholar]
- Bolam JP, et al. The Basal Ganglia VIII. Advances in Behavioral Biology. Vol. 56. Springer US: 2005. The Basal Ganglia-Thalamocortical Circuit Originating in the Ventral Premotor area (PMv) of the Macaque Monkey; pp. 585–592. ed.^eds. [Google Scholar]
- Brennan MJW, Cantrill RC. [delta]-Aminolaevulinic acid is a potent agonist for GABA autoreceptors. 1979;280:514–515. doi: 10.1038/280514a0. [DOI] [PubMed] [Google Scholar]
- Chiodo LM, Jacobson SW, Jacobson JL. Neurodevelopmental effects of postnatal lead exposure at very low levels. Neurotoxicology and Teratology. 2004;26:359–371. doi: 10.1016/j.ntt.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Chiodo LM, et al. Blood lead levels and specific attention effects in young children. Neurotoxicology and Teratology. 2007;29:538–546. doi: 10.1016/j.ntt.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Demasi M, et al. The prooxidant effect of 5-aminolevulinic acid in the brain tissue of rats: implications in neuropsychiatric manifestations in prophyries. Fre Radical Biological Medicine. 1996a;20:291–299. doi: 10.1016/0891-5849(95)02035-7. [DOI] [PubMed] [Google Scholar]
- Demasi M, et al. The prooxidant effect of 5-aminolevulinic acid in the brain tissue of rats: Implications in neuropsychiatric manifestations in porphyrias. Free Radical Biology and Medicine. 1996b;20:291–299. doi: 10.1016/0891-5849(95)02035-7. [DOI] [PubMed] [Google Scholar]
- Emanuelli T, et al. Effects of 5-aminolevulinic acid on the glutamatergic neurotransmission. Neurochemistry International. 2003a;42:115–121. doi: 10.1016/s0197-0186(02)00074-8. [DOI] [PubMed] [Google Scholar]
- Emanuelli T, et al. Effects of 5-aminolevulinic acid on the glutamatergic neurotransmission. Neurochemistry International. 2003b;42:115–121. doi: 10.1016/s0197-0186(02)00074-8. [DOI] [PubMed] [Google Scholar]
- Fray PJ, Robbins TW. CANTAB battery: Proposed utility in neurotoxicology. Neurotoxicology and Teratology. 1996;18:499–504. doi: 10.1016/0892-0362(96)00027-x. [DOI] [PubMed] [Google Scholar]
- Friedman HR, Goldman-Rakic PS. Activation of the hippocampus and dentate gyrus by working-memory: a 2-deoxyglucose study of behaving rhesus monkeys. J Neurosci. 1988;8:4693–4706. doi: 10.1523/JNEUROSCI.08-12-04693.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao A, et al. Effect of the delta-aminolevulinic acid dehydratase gene polymorphism on renal and neurobehavioral function in workers exposed to lead in China. Sci Total Environ. 2010;408:4052–4055. doi: 10.1016/j.scitotenv.2010.04.024. Epub 2010 May 26. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Mack CM, Lasley SM. Chronic developmental lead exposure increases the threshold for long-term potentiation in rat dentate gyrus in vivo. Brain Res. 1996;736:118–124. doi: 10.1016/0006-8993(96)00665-8. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Mack CM. Chronic lead exposure accelerates decay of long-term potentiation in rat dentate gyrus in vivo. Brain Res. 1998;789:139–149. doi: 10.1016/s0006-8993(97)01517-5. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, et al. Chronic developmental lead exposure reduces neurogenesis in adult rat hippocampus but does not impair spatial learning. Toxicol Sci. 2005;86:365–374. doi: 10.1093/toxsci/kfi156. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Lasley SM. Developmental lead (Pb) exposure reduces the ability of the NMDA antagonist MK-801 to suppress long-term potentiation (LTP) in the rat dentate gyrus, in vivo. Neurotoxicology and Teratology. 2007;29:385–393. doi: 10.1016/j.ntt.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Gilbert SG, Rice DC. Low-level lifetime lead exposure produces behavioral toxicity (Spatial discrimination reversal) in adult monkeys. Toxicology and Applied Pharmacology. 1987;91:484–490. doi: 10.1016/0041-008x(87)90070-6. [DOI] [PubMed] [Google Scholar]
- Hu Y, et al. Peptide transporter 2 (PEPT2) expression in brain protects against 5-aminolevulinic acid neurotoxicity. Journal of Neurochemistry. 2007;103:2058–2065. doi: 10.1111/j.1471-4159.2007.04905.x. [DOI] [PubMed] [Google Scholar]
- Kappas A, Sassa S, Galbraith RA, Nordmann Y. The porphyrias. In: Scriver CR, editor. The Metabolic Basis of Inherited Disease. Vol. 56. New York: McGraw Hill; 1995. pp. 2103–2160. ed.^eds. [Google Scholar]
- Kelada SN, et al. δ-Aminolevulinic Acid Dehydratase Genotype and Lead Toxicity: A HuGE Review. American Journal of Epidemiology. 2001;154:1–13. doi: 10.1093/aje/154.1.1. [DOI] [PubMed] [Google Scholar]
- Klaassen CD. Heavy metals and heavy-metal antagonists. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman's The Phamacological Basis of Therapeutics. New York: McGraw Hill; 2006. pp. 1753–1775. Vol., ed.^eds. [Google Scholar]
- Knights RM, Moule AD. Normative data on the motor steadiness battery for children. Perception and Motor Skills. 1968;26:643–650. doi: 10.2466/pms.1968.26.2.643. [DOI] [PubMed] [Google Scholar]
- Krieg EF, Jr., et al. Lead and cognitive function in ALAD genotypes in the third National Health and Nutrition Examination Survey. Neurotoxicol Teratol. 2009;31:364–371. doi: 10.1016/j.ntt.2009.08.003. Epub 2009 Aug 15. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, et al. Cognitive deficits associated with blood lead concentrations <10 microg/dL in US children and adolescents. Public Health Rep. 2000;115:521–529. doi: 10.1093/phr/115.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legare ME, et al. Analysis of Pb2± entry into cultured astroglia. Toxicol Sci. 1998;46:90–100. doi: 10.1006/toxs.1998.2492. [DOI] [PubMed] [Google Scholar]
- Levin ED, et al. Genetic aspects of behavioral neurotoxicology. Neurotoxicology. 2009;30:741–753. doi: 10.1016/j.neuro.2009.07.014. Epub 2009 Jul 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl L, et al. Differential ability of astroglia and neuronal cells to accumulate lead: dependence on cell type and on degree of differentiation. Toxicol. Sci. 1999;50:236–243. doi: 10.1093/toxsci/50.2.236. [DOI] [PubMed] [Google Scholar]
- Lindberg RLP, et al. Motor neuropathy in porphobilinogen deaminase-deficient mice imitates the peripheral neuropathy of human acute porphyria. J. Clin. Invest. 1999;103:1127–1134. doi: 10.1172/JCI5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J-Y, et al. Neurobehavioral function in children with low blood lead concentrations. Neurotoxicology. 2007;28:421–425. doi: 10.1016/j.neuro.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Nakano K. Neural circuits and topographic organization of the basal ganglia and related regions. Brain and Development. 2000;22:5–16. doi: 10.1016/s0387-7604(00)00139-x. [DOI] [PubMed] [Google Scholar]
- Ocheltree SM, et al. Role and Relevance of Peptide Transporter 2 (PEPT2) in the Kidney and Choroid Plexus: In Vivo Studies with Glycylsarcosine in Wild-Type and PEPT2 Knockout Mice. J Pharmacol Exp Ther. 2005;315:240–247. doi: 10.1124/jpet.105.089359. [DOI] [PubMed] [Google Scholar]
- Pawlas N, et al. Modification by the genes ALAD and VDR of lead-induced cognitive effects in children. Neurotoxicology. 2012;33:37–43. doi: 10.1016/j.neuro.2011.10.012. Epub 2011 Nov 9. [DOI] [PubMed] [Google Scholar]
- Pérez-Bravo F, et al. Association between aminolevulinate dehydrase genotypes and blood lead levels in children from a lead-contaminated area in Antofagasta, Chile. Archives Of Environmental Contamination And Toxicology. 2004;47:276–280. doi: 10.1007/s00244-004-2215-1. [DOI] [PubMed] [Google Scholar]
- Petrucci R, Leonardi A, Battistuzzi G. The genetic polymorphism of delta-aminolevulinic acid dehydratase in Italy. Human Genetics. 1982;60:289–290. doi: 10.1007/BF00303023. [DOI] [PubMed] [Google Scholar]
- Pinsonneault J, Nielsen CU, Sadee W. Genetic variants of the human H±/dipeptide transporter PEPT2: analysis of haplotype functions. J Pharmacol Exp Ther. 2004;311:1088–1096. doi: 10.1124/jpet.104.073098. Epub 2004 Jul 28. [DOI] [PubMed] [Google Scholar]
- Qian Y, et al. Lead Targets GRP78, a Molecular Chaperone, in C6 Rat Glioma Cells. Toxicology and Applied Pharmacology. 2000;163:260–266. doi: 10.1006/taap.1999.8878. [DOI] [PubMed] [Google Scholar]
- Rajan P, et al. Interaction of the delta-aminolevulinic acid dehydratase polymorphism and lead burden on cognitive function: the VA normative aging study. J Occup Environ Med. 2008;50:1053–1061. doi: 10.1097/JOM.0b013e3181792463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S, et al. Proton/peptide cotransporter (PEPT 2) from human kidney: Functional characterization and chromosomal localization. Biochimica et Biophysica Acta (BBA) -Biomembranes. 1995;1240:1–4. doi: 10.1016/0005-2736(95)00178-7. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DA. Human movement initiation: Specification of arm, direction, and extent. Journal of Experimental Psychology: General. 1980;109:444–474. doi: 10.1037//0096-3445.109.4.444. [DOI] [PubMed] [Google Scholar]
- Scinicariello F, et al. Lead and delta-aminolevulinic acid dehydratase polymorphism: where does it lead? A meta-analysis. Environmental Health Perspectives. 2007;115:35–41. doi: 10.1289/ehp.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secchi GC, Erba L, Cambiaghi G. delta-Aminolevulinic acid dehydratase activity of erythrocytes and liver tissue in man. Archives of Environmental Health. 1974;28:130–132. doi: 10.1080/00039896.1974.10666453. [DOI] [PubMed] [Google Scholar]
- Shen H, et al. Localization of PEPT1 and PEPT2 proton-coupled oligopeptide transporter mRNA and protein in rat kidney. Am J Physiol Renal Physiol. 1999;276:F658–F665. doi: 10.1152/ajprenal.1999.276.5.F658. [DOI] [PubMed] [Google Scholar]
- Shen XM, et al. Delta-aminolevulinate dehydratase polymorphism and blood lead levels in Chinese children. Environ Res. 2001;85:185–190. doi: 10.1006/enrs.2000.4230. [DOI] [PubMed] [Google Scholar]
- Sobin C, Gutierrez M, Alterio H. Polymorphisms of delta-aminolevulinic acid dehydratase (ALAD) and peptide transporter 2 (PEPT2) genes in children with low-level lead exposure. Neurotoxicology. 2009;30:881–887. doi: 10.1016/j.neuro.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin C, et al. A Bland-Altman comparison of the Lead Care(R) System and inductively coupled plasma mass spectrometry for detecting low-level lead in child whole blood samples. J Med Toxicol. 2011a;7:24–32. doi: 10.1007/s13181-010-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin C, et al. delta-Aminolevulinic acid dehydratase single nucleotide polymorphism 2 and peptide transporter 2*2 haplotype may differentially mediate lead exposure in male children. Arch Environ Contam Toxicol. 2011b;61:521–529. doi: 10.1007/s00244-011-9645-3. Epub 2011 Feb 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin C, et al. Microglial disruption in young mice with early chronic lead exposure. Toxicol Lett. 2013;15:00151–00153. doi: 10.1016/j.toxlet.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surkan PJ, et al. Neuropsychological function in children with blood lead levels <10 [mu]g/dL. Neurotoxicology. 2007;28:1170–1177. doi: 10.1016/j.neuro.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JA, Dallenbach FD, Thomas M. The distribution of radioactive lead in the cerebellum of developing rats. The Journal of Pathology. 1973;109:45–50. doi: 10.1002/path.1711090106. [DOI] [PubMed] [Google Scholar]
- Villayandre BM, et al. Effect of delta-aminolevulinic acid treatment on N-methyl-D-aspartate receptor at different ages in the rat brain. Brain Res. 2005;1061:80–87. doi: 10.1016/j.brainres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Waber DP, et al. The NIH MRI study of normal brain development: Performance of a population based sample of healthy children aged 6 to 18 years on a neuropsychological battery. Journal of the International Neuropsychological Society. 2007;13:729–746. doi: 10.1017/S1355617707070841. [DOI] [PubMed] [Google Scholar]
- Wang J, Tian B. Screen-printed stripping voltammetric/potentiometric electrodes for decentralized testing of trace lead. Anal Chem. 1992;64:1706–1709. [Google Scholar]
- Wang L, et al. Effects of extracellular -aminolaevulinic acid on sodium currents in acutely isolated rat hippocampal CA1 neurons. European Journal of Neuroscience. 2005;22:3122–3128. doi: 10.1111/j.1460-9568.2005.04471.x. [DOI] [PubMed] [Google Scholar]
- Wetmur JG, et al. Molecular characterization of the human delta-aminolevulinate dehydratase 2 (ALAD2) allele: implications for molecular screening of individuals for genetic susceptibility to lead poisoning. Am J Hum Genet. 1991a;49:757–763. [PMC free article] [PubMed] [Google Scholar]
- Wetmur JG, Lehnert G, Desnick RJ. The delta-aminolevulinate dehydratase polymorphism: higher blood lead levels in lead workers and environmentally exposed children with the 1–2 and 2–2 isozymes. Environmental Research. 1991b;56:109–119. doi: 10.1016/s0013-9351(05)80001-5. [DOI] [PubMed] [Google Scholar]
- White LD, et al. New and evolving concepts in the neurotoxicology of lead. Toxicol Appl Pharmacol. 2007;225:1–27. doi: 10.1016/j.taap.2007.08.001. Epub 2007 Aug 16. [DOI] [PubMed] [Google Scholar]
- Xavier GF, Oliveira-Filho FJ, Santos AM. Dentate gyrus-selective colchicine lesion and disruption of performance in spatial tasks: difficulties in "place strategy" because of a lack of flexibility in the use of environmental cues? Hippocampus. 1999;9:668–681. doi: 10.1002/(SICI)1098-1063(1999)9:6<668::AID-HIPO8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Zhao Y, et al. Association Between δ-Aminolevulinic Acid Dehydratase (ALAD) Polymorphism and Blood Lead Levels: A Meta-regression Analysis. Journal of Toxicology and Environmental Health, Part A. 2007;70:1986–1994. doi: 10.1080/15287390701550946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.