Abstract
Idiopathic pulmonary fibrosis (IPF) is a devastating disease characterized by alveolar epithelial cell injury, accumulation of fibroblasts/myofibroblasts and deposition of extracellular matrix proteins. Levels of sphingosine-1-phosphate (S1P), a naturally occurring bioactive lipid, are elevated in bronchoalveolar fluids and lung tissues from IPF patients and animal models of pulmonary fibrosis. However, the in vivo contribution of S1P, regulated by its synthesis catalyzed by Sphingosine kinases (SphKs) 1 & 2 and catabolism by S1P phosphatases and S1P lyase (S1PL), in the pathogenesis of pulmonary fibrosis is not well defined. Microarray analysis of blood mononuclear cells from patients with IPF and SphK1-, SphK2- or S1PL-knockout mice and SphK inhibitor were used to assess the role of S1P in fibrogenesis. The expression of SphK1 negatively correlated with lung function and survival of patients with IPF. Further, the expressions of SphK1 and S1PL were increased in lung tissues from patients with IPF and bleomycin-challenged mice. Genetic knockdown of SphK1, but not SphK2, ameliorated bleomycin-induced pulmonary fibrosis in mice while deletion of S1PL (SGPL1+/−) in mice potentiated fibrosis post-bleomycin challenge. TGF-β increased the expression of SphK1 and S1PL in human lung fibroblasts and knockdown of SphK1 or treatment with SphK inhibitor attenuated S1P generation and TGF-β mediated signal transduction. Overexpression of S1PL attenuated bleomycin-induced TGF-β secretion and S1P mediated differentiation of human lung fibroblasts through regulation of autophagy. Administration of SphK1 inhibitor 8 days post-bleomycin challenge reduced bleomycin-induced mortality and pulmonary fibrosis. Our results suggest that SphK1 and S1PL play critical roles in the pathology of lung fibrosis and may be novel therapeutic targets.
Keywords: Sphingosine-1-phosphate, TGF-β, Sphingosine kinase 1, S1P lyase, Autophagy, Pulmonary fibrosis
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive interstitial lung disease of unknown etiology with an average survival of 3–5 years following diagnosis (King, 1998). The pathogenesis of IPF is not completely understood; however IPF involves alveolar epithelial cell injury, areas of type II cell hyperplasia, accumulation of fibroblasts and myofibroblasts, and the deposition of extracellular matrix (ECM) proteins such as fibronectin (FN) and collagen (Phan, 2002, Sappino et al., 1990). Fibroblast accumulation gradually leads to an excessive scarring of the lung tissue and progressive and irreversible destruction of lung architecture leading to loss of lung function, disruption of gas exchange and respiratory failure (Selman et al., 2001). Therapies for IPF are mostly ineffective and the only effective treatment available is lung transplantation. Emerging strategies to treat patients with IPF include inhibition of epithelial injury or enhancement of repair, treating with anti-cytokines, and inhibition of fibroblast proliferation or induction of fibroblast apoptosis. In light of the poor prognosis and lack of available anti-inflammatory therapies, there is a pressing need for alternative approaches including evaluation of new targets and pathways in animal models of fibrosis.
Earlier studies provide strong evidence that the bioactive lipids such as lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P) are critical for inflammation and cancer (Zhao et al., 2013, Nagahashi et al., 2014, Pyne et al., 2014). Recent studies strongly suggest that LPA and S1P also play an important role in the pathogenesis of IPF. Bronchoalveolar lavage (BAL) fluid from IPF patients showed enhanced LPA (Tager et al., 2008) and S1P (Milara et al., 2012) levels compared to control subjects. Further, LPA1/LPA2 receptor (Huang et al., 2013b, Tager et al., 2008) as well as sphingosine kinase (SphK) 1 (Huang et al., 2013a) deficient mice were protected against bleomycin-induced pulmonary fibrosis. The source of LPA and mechanism(s) of its generation in IPF are unclear. LPA could be generated from phosphatidic acid (PA) by phospholipase A1 or phospholipase A2 and/or from lysophosphatidylcholine by autotaxin (Zhao and Natarajan, 2013). Mice with mutated cytosolic phospholipase A2 exhibited protection against bleomycin-induced pulmonary fibrosis (Ghosh et al., 2004) suggesting a pivotal role for phospholipase A2 in the fibrotic process. In mammalian cells, S1P is primarily generated by phosphorylation of sphingosine catalyzed by the two isoforms of SphK, SphK1 and SphK2 (Natarajan et al., 2013), and S1P is dephosphorylated to sphingosine by S1P phosphatases 1 and 2 or non-specific lipid phosphate phosphatases. Also, S1P is irreversibly cleaved by S1P lyase (S1PL) to ethanolamine phosphate and trans-2-hexadecenal (Natarajan et al., 2013). This dysregulation of glycerophospholipid and sphingolipid metabolism leads to the development and progression of pulmonary fibrosis and other lung disorders. Thus, modulation of LPA and S1P metabolism and its G-protein coupled receptors offers a novel therapeutic approach for ameliorating human pulmonary fibrosis. In this study, the role and regulation of S1P production and its metabolism in pulmonary fibrosis is addressed.
S1P levels in IPF patients and animal models of pulmonary fibrosis
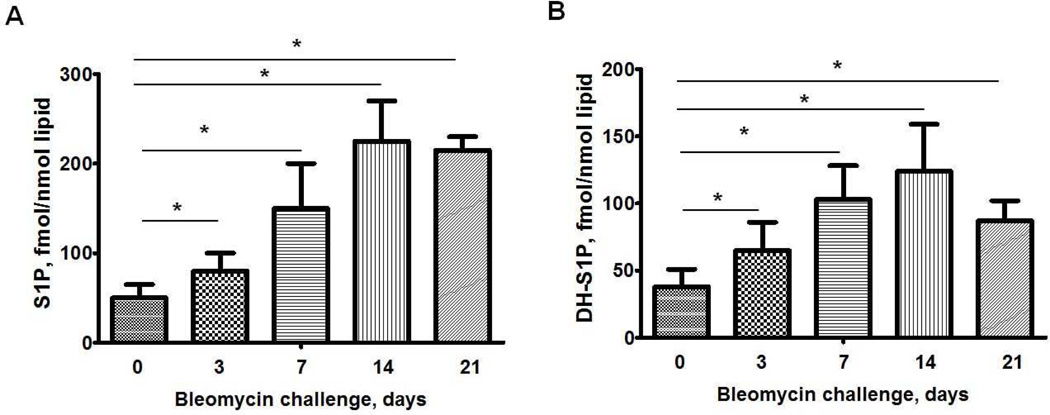
S1P is increased in serum and bronchoalveolar lavage (BAL) fluids in patients with IPF (Milara et al., 2012), which correlated with diffusion capacity of the lung for carbon monoxide, forced expiratory volume and forced vital capacity in IPF patients. Induction of inflammation and pulmonary fibrosis by bleomycin- and radiation in mice modulated sphingolipid levels in BAL fluids, lung tissues and plasma (Huang et al., 2013a). S1P and dihydro S1P levels were significantly elevated in lung tissues of mice 3, 7, and 14 days post-bleomycin challenge compared to control animals (Figure 1). Similarly, an increase in dihydro S1P and S1P levels was detected in plasma, lung tissue and BAL fluids 18 weeks after a 20 Gy thoracic irradiation of mice. Administration of a SphK inhibitor, SKI-II in mice on day 8 after bleomycin challenge decreased S1P and dihydro S1P levels in lung tissue on day 21 post bleomycin challenge, but did not affect tissue levels of sphingosine and ceramides (Huang et al., 2013a). This decrease in S1P and dihydro S1P correlated with protection against bleomycin-induced pulmonary fibrosis and mortality. Inhibition of sphingolipid de novo biosynthesis by targeting serine palmitoyltransferase with myriocin decreased levels of S1P and dihydro S1P in mouse lung and plasma and delayed the onset of radiation-induced pulmonary fibrosis (Gorshkova et al., 2012). These studies show that S1P and dihydro S1P levels are up-regulated in human IPF and animal models of pulmonary fibrosis and blocking S1P and/or dihydro S1P production or enhance their catabolism could be of potential value in therapeutic interventions of pulmonary fibrosis.
Fig. 1.
S1P and dihydro-S1P levels in lung tissues from mice post bleomycin challenge. C18-S1P (A) or C18-DHS1P (B) level in mouse lungs from C57BL/6J mice after bleomycin (2 U/kg, i.t.) treatment (0, 3, 7, 14, and 21 days). *P< 0.05, n= 4/group. S1P and dihydro-S1P levels were measured by LC/MS/MS and normalized to total lipid phosphorus in the samples.
Expression and role of SphK1 and SphK2 in the development of pulmonary fibrosis
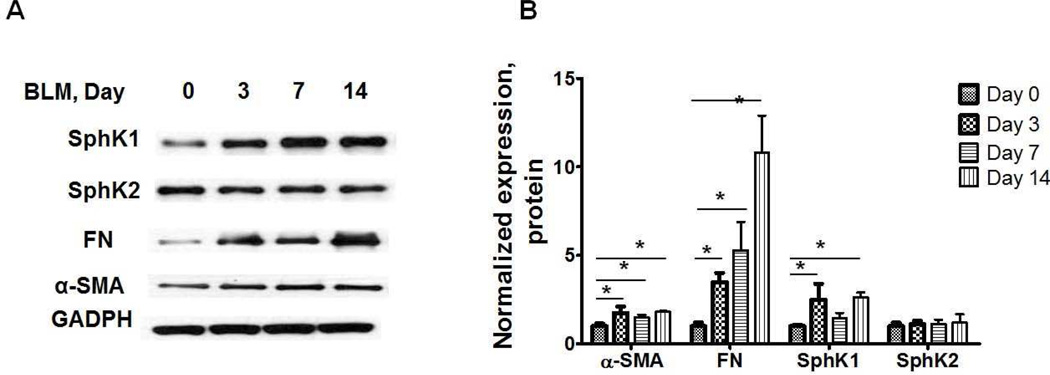
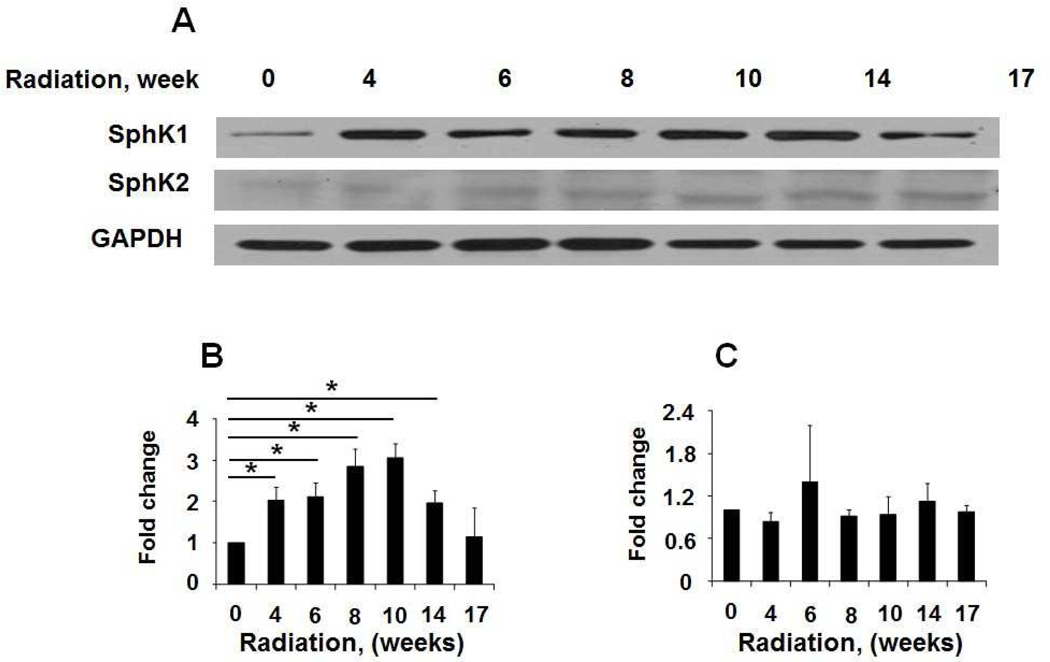
S1P accumulation in cells is a balance between its synthesis catalyzed by SphK1 and SphK2 and catabolism mediated by S1P phosphatases and S1PL. Microarray analysis of peripheral blood mononuclear cells (PBMCs) indicated increased expression of SphK1/2 in IPF patients, and the expression levels of SPHK1 and SPHK2 were negatively correlated with the lung function and survival in patients with IPF (Huang et al., 2013a). In addition to mRNA levels, protein expression of SphK1, but not SphK2, was significantly elevated in lung tissues from patients with IPF as compared to control subjects (Huang et al., 2013a), and this increased expression correlated with elevated α-smooth muscle actin (α-SMA), fibronectin (FN) and collagen type I. In murine models of bleomycin- and radiation-induced pulmonary fibrosis, the expression of SphK1, but not SphK2, was increased in lung tissues (Figures 2 & 3), and genetic knockdown of SphK1, but not SphK2, expression in mice protected against bleomycin-induced increase in lung collagen, TGF-β, fibronectin, α-SMA and lung fibrogenesis. However, depletion of SphK1 had no effect on lung inflammation on day 14 after bleomycin challenge in mice (Huang et al., 2013a). In addition to enhanced SphK1 expression, acid sphingomyelinase and acid ceramidases activities were up-regulated by bleomycin in lungs of fibrotic mice, and knockdown of acid sphingomyelinase attenuated bleomycin-induced lung inflammation and fibrosis (Dhami et al., 2010). Activation of acid sphingomyelinase and acid ceramidases could result in elevated production of ceramide and sphingosine and phosphorylation of sphingosine to S1P by SphKs (Gorshkova et al., 2012). Thus, in animal models of fibrosis, increased S1P levels in lung parenchyma are essential for fibrogenesis.
Fig. 2.
Expression of SphK1, SphK2, fibronectin and α-smooth muscle actin in lung tissues from mice challenged with bleomycin. (A) Representative Western blot, and (B) quantification of the expression of SphK1, SphK2, FN and α-SMA in mouse lung tissue after bleomycin challenge (2 U/kg, i.t.) (0, 3, 7, and 14 days). Intensity of each band was quantified and normalized to GAPDH. Data are expressed as means ± SEM. *P< 0.05, n =8/group.
Fig. 3.
Expression of SphK1 and SphK2 in lung tissues from radiation challenged mice. Mice were administered a single dose of thoracic radiation (20 Gy) and lung tissues were collected at various time points as shown. (A) Representative Western blot, and quantification of the expression of SphK1 (B) and SphK2 (C) in lung tissues from mice 0, 4, 6, 8, 10, 14, 17 week post irradiation. Intensity of each band was quantified and normalized to GAPDH. Data are expressed as means ± SEM. *P< 0.05, n =4/group.
Role of SphK1/S1P/S1P-receptor signaling axis in TGF-β mediated fibrogenesis
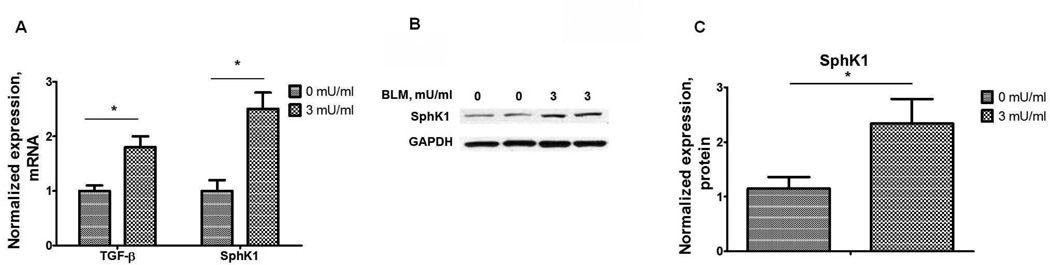
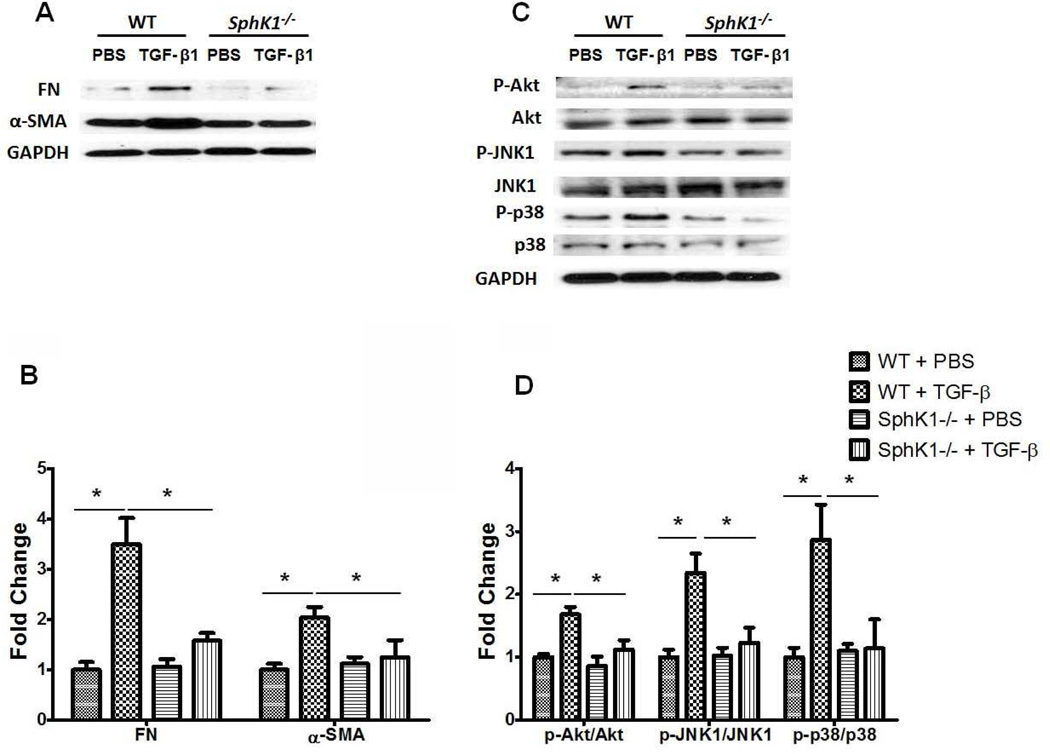
Transforming growth factor (TGF)-β is a key growth factor mediating fibroblast to myofibroblast transformation and alveolar epithelial to mesenchymal transition (EMT) both in vivo and in vitro (Willis and Borok, 2007, Wynn, 2008) and is up-regulated in IPF lungs and animal models of fibrosis (Wynn, 2011). In the murine model of fibrosis, bleomycin-induced secretion of TGF-β was suppressed by SphK1 knockdown or inhibition of activity (Huang et al., 2013a). Further, bleomycin-induced SphK1 expression in mouse lung was TGF-β dependent (Figure 4), and TGF-β regulated S1P levels in lung fibroblasts by inducing the expression of SphK1 (Kono et al., 2007). In vivo, bleomycin-induced phosphorylation of Smad2, Akt, JNK1 and p38 MAPK in mouse lung tissue, which was attenuated by blocking S1P generation via knockdown of SphK1 expression or inhibition of SphK1 activity using a small molecule inhibitor (Huang et al., 2013a). Also, SphK1 knockdown or inhibition suppressed the TGF-β mediated fibroblast to myofibroblast transition (Kono et al., 2007) suggesting a potential link between TGF-β expression, S1P production and its signaling through S1P receptors in pulmonary fibrosis (Figure 5). Down-regulation of S1PR2 and S1PR3, but not S1PR1, with siRNA attenuated TGF-β mediated up-regulation of fibronectin and α-SMA in human lung fibroblasts (Cencetti et al., 2010, Gellings Lowe et al., 2009; Kono et al., 2007). In contrast to human lung fibroblasts, prolonged exposure of S1PR1 agonists, FTY720 and AUY954, exacerbated bleomycin-induced vascular leak, fibrosis and mortality in mice (Shea et al., 2010, Sobel et al., 2013), suggesting that S1P/S1PR1 signaling may represent an important pathway for limiting the fibrotic response to injury. The role of S1P in fibrogenesis is not restricted to the lung. Interestingly, over-expression of SphK1 in mouse heart resulted in the development of cardiac fibrosis through reactive oxygen species mediated SphK1/S1P/S1PR3 signaling cascade (Takuwa et al., 2010). In an animal model of laser-induced retinal fibrosis, intravitreous injection of anti-S1P monoclonal antibody dramatically inhibited choroidal neovascularization (CNV) formation and sub-retinal collagen deposition compared to control group, thereby identifying S1P as a previously unrecognized mediator of angiogenesis and sub-retinal fibrosis in this model (Caballero et al., 2009). Thus, a cross-talk between TGF-β/TGF-βR and S1P/S1PR represents a molecular mechanism of dysregulated wound repair in the development and progression of pulmonary fibrosis.
Fig. 4.
Bleomycin induces TGF-β and SphK1 expression in human lung fibroblasts. (A) mRNA levels of TGF-β and SphK1 in human lung fibroblast challenged with bleomycin (0–3 mU/ml, 48 h). *P< 0.05, n =4/group. (B) Representative Western blot, and (C) quantification of the expression of SphK1 in human lung fibroblast after bleomycin challenge.
Fig. 5.
SphK1 deficiency attenuates TGF-β induced expression of fibronectin and α-smooth muscle actin and activation of Akt and MAPKs in mouse lung fibroblasts. Primary mouse lung fibroblasts were isolated from wild type (WT) and SphK1 knockout (SphK1−/−) mice (male, 8–10 weeks). Following over-night starvation, cells were treated with vehicle (PBS) or TGF-β (5ng/ml) for 48 h. (A) Representative Western blot, and (B) quantification of the expression of fibronectin (Fn) and α- smooth muscle actin (α-SMA) in mouse lung fibroblasts after PBS or TGF-β challenge (5 ng/ml, 48 h). *P< 0.05, n =4/group. (C) Representative Western blot, and (D) quantification of the activation of Akt, JNK1 and p38 MAPK in mouse lung fibroblasts after PBS or TGF-β challenge. *P< 0.05, n =4/group.
Expression and role of S1PL in the development of pulmonary fibrosis
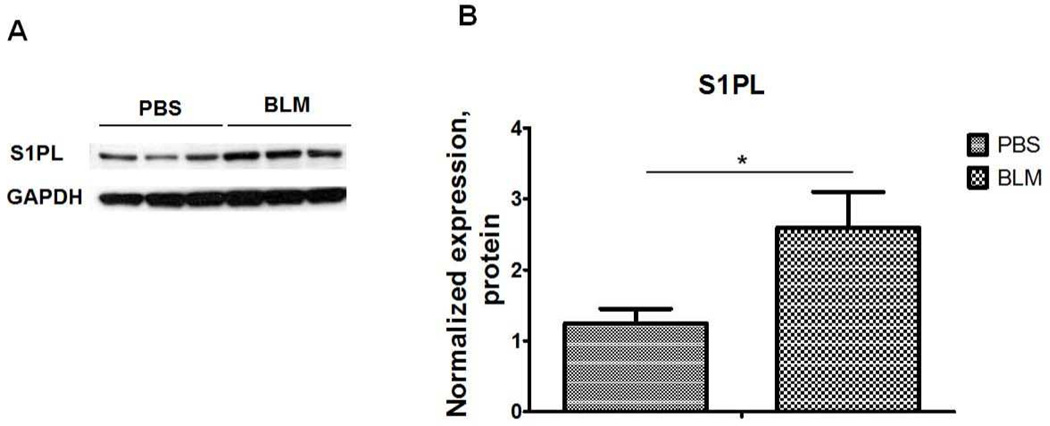
S1P can be dephosphorylated to sphingosine by S1P phosphatases, lipid phosphate phosphatases or irreversibly cleaved by S1PL to ethanolamine phosphate and hexadecenal. Micro-array analysis of human PBMCs from IPF patients revealed a direct correlation between SGPL1 expression, pulmonary functions values and overall survival in IPF patients. Lower expression of SGPL1 in patients correlated with relatively severe IPF and Kaplan-Meier survival analysis showed a marginal increase in survival of patients with higher expression levels of SGPL1 mRNA (Huang et al., 2014). Similarly, SGPL1 mRNA and protein were elevated in IPF lung homogenates and immunostaining of paraffin embedded lung tissues revealed increased S1PL expression in IPF specimens, and co-localization with α-SMA, especially in fibrotic foci, as compared to control subjects. The enhanced expression of S1PL in IPF was confirmed in murine models of bleomycin- and radiation-induced pulmonary fibrosis (Figure 6). However, there was no increase in S1PL activity in the lung tissues from radiation challenged mice (Gorshkova et al., 2012). While SphK1 expression increased during early stages of pulmonary fibrosis, changes in the S1PL expression occurred during latter stages of fibrogenesis in mice after exposure to bleomycin or radiation (Huang et al., 2014).
Fig. 6.
S1P Lyase expression in lung tissues from bleomycin challenged mice. (A) Representative Western blot, and (B) quantification of the expression of S1PL in mouse lung tissues from mice after bleomycin (2 U/kg, i.t.) challenge (14 days). Intensity of each band was quantified and normalized to GAPDH. Data are expressed as means ± SEM. *P< 0.05, n =5/group.
TGF-β mediates S1PL expression in lung fibroblast
TGF-β, a key cytokine that regulates fibroblast proliferation and differentiation, in a time-dependent manner increased the mRNA and protein expression of S1PL in human lung fibroblasts, which was abolished by pre-treatment of cells with anti-TGF-β antibody (Huang et al., 2014). In silico analysis of human SGPL1 promoter sequence indicated at least two consensus sequences for Smad2/3 binding localized in the up-stream promoter region of SGPL1 gene. ChIP assay revealed increased binding of Smad3 to the consensus sites at −75bp and −143bp after TGF-β challenge of human lung fibroblasts. Additionally, knockdown of Smad3 with siRNA attenuated the TGF-β-induced protein expression of S1PL in human lung fibroblasts, and mutation of the Smad binding site in SGPL1 promoter sequence significantly inhibited the TGF-β mediated SGPL1 luciferase reporter activity (Huang et al., 2014). These results suggest a role for TGF-β in SGPL1 expression in lung fibroblast through activation of Smad pathway.
Over-expression of S1PL attenuates TGF-β- and S1P-induced differentiation of fibroblast to myofibroblast
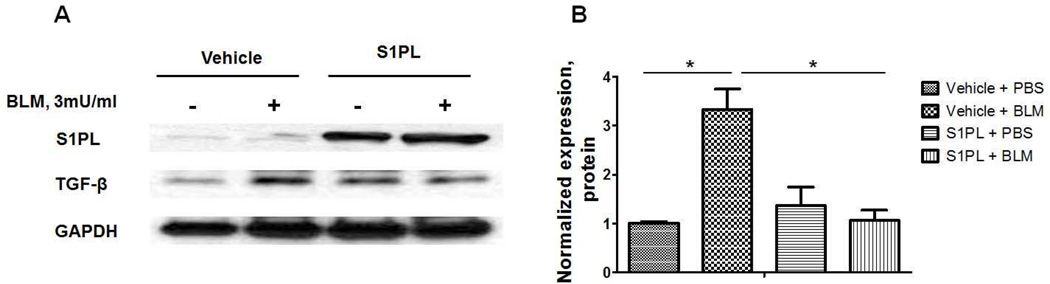
Over-expression of S1PL in human lung fibroblasts decreased intracellular S1P levels, attenuated TGF-β- or S1P- induced expression of fibronectin and α-SMA, markers of differentiation of lung fibroblasts to myofibroblasts and suppressed bleomycin induced TGF-β expression in human lung fibroblast (Figure 7). These results further provide evidence for cross-talk between TGF-β/TGF-βR and S1P/S1PR signaling in differentiation of fibroblast to myofibroblast and fibrogenesis.
Fig. 7.
Over-expression of S1P Lyase attenuates bleomycin-induced expression of TGF-β in human lung fibroblasts. Representative western blot (A) and quantification of TGF-β (B) in human lung fibroblasts after bleomycin challenge (3 mU/ml, 48 h). *P<0.05, n =4/group.
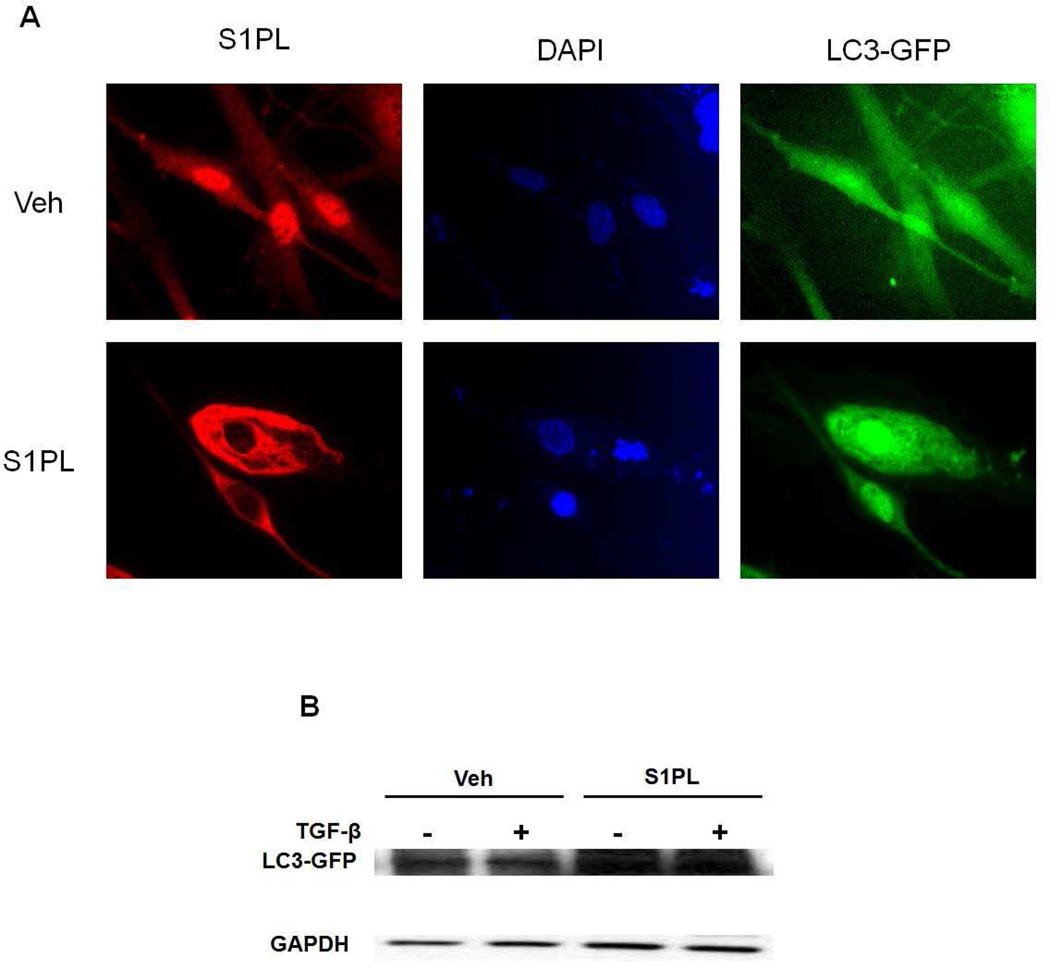
Over-expression of S1PL augments autophagy in human lung fibroblasts
Recent studies suggest a potential link between TGF-β induced activation and differentiation of lung fibroblasts and autophagy (Araya et al., 2013). Down-regulation of LC3 or Beclin1 expression, markers of autophagy, augmented TGF-β induced expression of fibronectin and α-SMA in human lung fibroblast (Patel et al., 2012). In human lung fibroblast, over-expression of S1PL enhanced expression of LC3 (Figure 8). These results show that over-expression of S1PL attenuated TGF-β-induced intracellular S1P level, and expression of fibronectin and α-SMA in lung fibroblasts by up-regulation of autophagy in human lung fibroblasts. In the murine model of pulmonary fibrosis, knockdown of S1PL exacerbated bleomycin-induced pulmonary fibrosis (Huang et al., 2014). This finding supports the notion that S1P signaling via its G-protein coupled receptors plays an important role in regulating autophagy and the development of pulmonary fibrosis mediated by TGF-β. Increased expression of S1PL may be a pathophysiological response to injury and repair during fibrogenesis and may serve as an endogenous suppressor of pulmonary fibrosis.
Fig. 8.
S1P Lyase over-expression enhances LC3 expression in human lung fibroblasts. Human lung fibroblasts were transfected with LC3-GFP plasmid (3 µg/ml/35-mm dish) and S1P lyase (S1PL) adenovirus (20 MOI/35-mm dish) for 48 h. (A), is a representative immunofluorescence micrograph and (B) Western blot of human lung fibroblasts transfected with LC3-GFP and S1PL adenovirus for 48 h followed by TGF-β challenge (5 ng/ml) for 48 h. *P< 0.05, n =4/group.
Summary
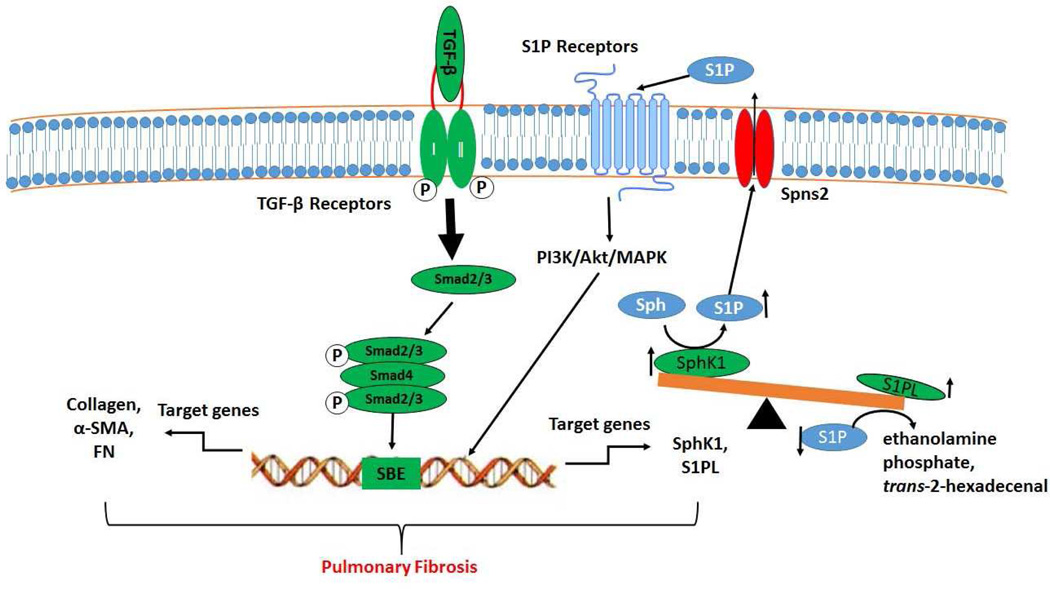
In summary, the balance between SphK1 and S1PL expression regulating S1P signaling axis is a novel pathway that promotes proliferation and differentiation of lung fibroblasts and development of fibrogenesis. While SphK1 deficiency protected mice against bleomycin-induced pulmonary fibrosis, S1PL deficiency exacerbated the fibrogenesis. Most importantly, cross-talk between TGF-β/TGF-receptor and S1P/S1P receptor signaling suggest that SphK1, S1P and S1PL are novel potential therapeutic targets for pulmonary fibrosis that should be explored in future pre-clinical and clinical studies (Figure 9).
Fig. 9.
Schematic diagram illustrating the effects of TGF-β and S1P and transforming on fibrotic gene expression, differentiation, and activation in human lung fibroblasts. TGF-β binding to TGF-β receptor 1 and 2 induces activation, translocation and binding of Smad2/3 to Smad binding elements (SBE) in the promoter of target genes, and subsequent induction of the expression of fibrotic genes (collagen, fibronectin (FN) and α-smooth muscle actin (α-SMA) and S1P metabolizing enzymes (SphK1 and S1PL). In fibroblasts, SphK1 stimulates S1P generation through phosphorylation of sphingosine (Sph), and S1PL degrades S1P to ethanolamine phosphate and trans-2-hexadecenal. S1P generated intracellularly is transported outside the cell through the S1P transporter, Spns2. The intracellularly transported S1P binds to G-protein coupled S1P receptors (S1P1-5) and stimulates the activation of PI3K/Akt/MAPK to regulate the development of pulmonary fibrosis.
Acknowledgments
This work was supported by research grants from National Institutes of Health NIH/HLBI P01 Hl98050 to V.N. The authors acknowledge collaboration with Drs. Joe G.N. Garcia and Biji Mathew on studies related to radiation-induced pulmonary fibrosis and Dr. E. Berdyshev for measuring sphingoid bases by mass spectrometry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
Reference
- Araya J, Kojima J, Takasaka N, Ito S, Fujii S, Hara H, et al. Insufficient autophagy in idiopathic pulmonary fibrosis. American journal of physiology Lung cellular and molecular physiology. 2013;304:L56–L69. doi: 10.1152/ajplung.00213.2012. [DOI] [PubMed] [Google Scholar]
- Caballero S, Swaney J, Moreno K, Afzal A, Kielczewski J, Stoller G, et al. Anti-sphingosine-1-phosphate monoclonal antibodies inhibit angiogenesis and sub-retinal fibrosis in a murine model of laser-induced choroidal neovascularization. Experimental eye research. 2009;88:367–377. doi: 10.1016/j.exer.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cencetti F, Bernacchioni C, Nincheri P, Donati C, Bruni P. Transforming growth factor-beta1 induces transdifferentiation of myoblasts into myofibroblasts via up-regulation of sphingosine kinase-1/S1P3 axis. Molecular biology of the cell. 2010;21:1111–1124. doi: 10.1091/mbc.E09-09-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhami R, He X, Schuchman EH. Acid sphingomyelinase deficiency attenuates bleomycin-induced lung inflammation and fibrosis in mice. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2010;26:749–760. doi: 10.1159/000322342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellings Lowe N, Swaney JS, Moreno KM, Sabbadini RA. Sphingosine-1-phosphate and sphingosine kinase are critical for transforming growth factor-beta-stimulated collagen production by cardiac fibroblasts. Cardiovascular research. 2009;82:303–312. doi: 10.1093/cvr/cvp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Stewart A, Tucker DE, Bonventre JV, Murphy RC, Leslie CC. Role of cytosolic phospholipase A(2) in prostaglandin E(2) production by lung fibroblasts. American journal of respiratory cell and molecular biology. 2004;30:91–100. doi: 10.1165/rcmb.2003-0005OC. [DOI] [PubMed] [Google Scholar]
- Gorshkova I, Zhou T, Mathew B, Jacobson JR, Takekoshi D, Bhattacharya P, et al. Inhibition of serine palmitoyltransferase delays the onset of radiation-induced pulmonary fibrosis through the negative regulation of sphingosine kinase-1 expression. Journal of lipid research. 2012;53:1553–1568. doi: 10.1194/jlr.M026039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LS, Berdyshev E, Mathew B, Fu P, Gorshkova IA, He D, et al. Targeting sphingosine kinase 1 attenuates bleomycin-induced pulmonary fibrosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013a;27:1749–1760. doi: 10.1096/fj.12-219634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LS, Fu P, Patel P, Harijith A, Sun T, Zhao Y, et al. Lysophosphatidic acid receptor-2 deficiency confers protection against bleomycin-induced lung injury and fibrosis in mice. American journal of respiratory cell and molecular biology. 2013b;49:912–922. doi: 10.1165/rcmb.2013-0070OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Ma W, Zhou T, He D, Feghali-Bostwick C, Raddy S, Garcia J, et al. Sphingosine 1 Phosphate Lyase Functions As An Endogenous Suppressor Of Pulmonary Fibrosis In Humans And In A Mouse Model. Am J Respir Crit Care Med. 2014;189:A1251. [Google Scholar]
- King TE., Jr Update in pulmonary medicine. Annals of internal medicine. 1998;129:806–812. doi: 10.7326/0003-4819-129-10-199811150-00012. [DOI] [PubMed] [Google Scholar]
- Kono Y, Nishiuma T, Nishimura Y, Kotani Y, Okada T, Nakamura S, et al. Sphingosine kinase 1 regulates differentiation of human and mouse lung fibroblasts mediated by TGF-beta1. American journal of respiratory cell and molecular biology. 2007;37:395–404. doi: 10.1165/rcmb.2007-0065OC. [DOI] [PubMed] [Google Scholar]
- Milara J, Navarro R, Juan G, Peiro T, Serrano A, Ramon M, et al. Sphingosine-1-phosphate is increased in patients with idiopathic pulmonary fibrosis and mediates epithelial to mesenchymal transition. Thorax. 2012;67:147–156. doi: 10.1136/thoraxjnl-2011-200026. [DOI] [PubMed] [Google Scholar]
- Nagahashi M, Hait NC, Maceyka M, Avni D, Takabe K, Milstien S, Spiegel S. Sphingosine-1-phosphate in chronic intestinal inflammation and cancer. Adv Biol Regul. 2014;54:112–120. doi: 10.1016/j.jbior.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan V, Dudek SM, Jacobson JR, Moreno-Vinasco L, Huang LS, Abassi T, et al. Sphingosine-1-phosphate, FTY720, and sphingosine-1-phosphate receptors in the pathobiology of acute lung injury. American journal of respiratory cell and molecular biology. 2013;49:6–17. doi: 10.1165/rcmb.2012-0411TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AS, Lin L, Geyer A, Haspel JA, An CH, Cao J, et al. Autophagy in idiopathic pulmonary fibrosis. PloS one. 2012;7:e41394. doi: 10.1371/journal.pone.0041394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan SH. The myofibroblast in pulmonary fibrosis. Chest. 2002;122:286S–289S. doi: 10.1378/chest.122.6_suppl.286s. [DOI] [PubMed] [Google Scholar]
- Pyne NJ, Ohotski J, Bittman R, Pyne S. The role of sphingosine 1-phosphate in inflammation and cancer. Adv Biol Regul. 2014;54:121–129. doi: 10.1016/j.jbior.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Sappino AP, Schurch W, Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Laboratory investigation; a journal of technical methods and pathology. 1990;63:144–161. [PubMed] [Google Scholar]
- Selman M, King TE, Pardo A American Thoracic S, European Respiratory S, American College of Chest P. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Annals of internal medicine. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- Shea BS, Brooks SF, Fontaine BA, Chun J, Luster AD, Tager AM. Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury. American journal of respiratory cell and molecular biology. 2010;43:662–673. doi: 10.1165/rcmb.2009-0345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel K, Menyhart K, Killer N, Renault B, Bauer Y, Studer R, et al. Sphingosine 1-phosphate (S1P) receptor agonists mediate pro-fibrotic responses in normal human lung fibroblasts via S1P2 and S1P3 receptors and Smad-independent signaling. The Journal of biological chemistry. 2013;288:14839–14851. doi: 10.1074/jbc.M112.426726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nature medicine. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- Takuwa N, Ohkura S, Takashima S, Ohtani K, Okamoto Y, Tanaka T, et al. S1P3-mediated cardiac fibrosis in sphingosine kinase 1 transgenic mice involves reactive oxygen species. Cardiovascular research. 2010;85:484–493. doi: 10.1093/cvr/cvp312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. American journal of physiology Lung cellular and molecular physiology. 2007;293:L525–L534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. The Journal of pathology. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Integrating mechanisms of pulmonary fibrosis. The Journal of experimental medicine. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Natarajan V. Lysophosphatidic acid (LPA) and its receptors: role in airway inflammation and remodeling. Biochimica et biophysica acta. 2013;1831:86–92. doi: 10.1016/j.bbalip.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]