Abstract
Background
Diffusion tensor imaging (DTI) has expanded our knowledge of corticospinal tract (CST) anatomy and development. However, previous developmental DTI studies assessed the CST as a whole, overlooking potential differences in development of its components related to control of the upper and lower extremities. The present cross-sectional study investigated age-related changes, side and gender differences in streamline volume of the leg- and hand-related segments of the CST in children.
Subjects and methods
DTI data of 31 children (1–14years; mean age: 6±4years; 17 girls) with normal conventional MRI were analyzed. Leg- and hand-related CST streamline volumes were quantified separately, using a recently validated novel tractography approach. CST streamline volumes on both sides were compared between genders and correlated with age.
Results
Higher absolute streamline volumes were found in the left leg-related CST compared to the right (p=0.001) without a gender effect (p=0.4), whereas no differences were found in the absolute hand-related CST volumes (p>0.4). CST leg-related streamline volumes, normalized to hemispheric white matter volumes, declined with age in the right hemisphere only (R=−.51; p=0.004). Absolute leg-related CST streamline volumes showed similar, but slightly weaker correlations. Hand-related absolute or normalized CST streamline volumes showed no age-related variations on either side.
Conclusion
These results suggest differential development of CST segments controlling hand vs. leg movements. Asymmetric volume changes in the lower limb motor pathway may be secondary to gradually strengthening left hemispheric dominance and is consistent with previous data suggesting that footedness is a better predictor of hemispheric lateralization than handedness.
Keywords: corticospinal tract, development, children, diffusion tensor imaging, MRI, DTI
INTRODUCTION
The corticospinal tract (CST) connects the motor cortex and the spinal cord, making voluntary motor control of the limbs possible. Our understanding of CST anatomy has been greatly expanded by the use of diffusion tensor imaging (DTI), a technique that allows the study of neural tracts via quantification of water diffusion along the axons in vivo [1–3]. Indeed, there are numerous DTI studies of adults and children examining the damage, development and reorganization of the CST as a whole, following injury. However, there are much less data exploring how this pathway changes with age in children, especially regarding specific CST segments responsible for motor control of the upper and lower limbs. The aim of the present study was to investigate the differential development of the CST segments associated with hand and leg movements in children, using a recently validated novel DTI approach that enables separation of these functionally relevant CST segments [4–8]. We hypothesized age-related differential volume changes and increasing left-right asymmetries in the hand- and/or leg-related CST segments.
SUBJECTS AND METHODS
Subjects
The study involved 31 children (age: 1–14 years, mean age: 6±4 years), including 14 boys (5±2.5 years) and 17 girls (7±4.5 years). Seventeen were typically developing right-handed healthy children (mean age 9±3 years) and 14 children had neurological condition (e.g., new onset epilepsy) without structural abnormalities on MRI (mean age 3±1 years) and without evidence of left-handedness (in some young subjects handedness could not be determined firmly). None of the children had motor weakness on neurological examination. The Human Investigations Committee (HIC) of Wayne State University granted permission for performing MRIs (without sedation) in healthy children, and parents signed an Informed Consent Form. We had also permission from the HIC to use clinically acquired MRI scans after deidentification.
MRI protocol
MR scans were performed using a 3T GE-Signa scanner (GE Healthcare, Milwaukee, WI) equipped with an 8-channel head coil and ASSET. DWI was acquired with a multi-slice single shot diffusion weighted echo-planar-imaging (EPI) sequence at repetition time (TR) = 12,500 ms, echo time (TE) = 88.7 ms, field of view (FOV) = 240 mm, 128×128 acquisition matrix (nominal resolution = 1.89 mm), contiguous 3 mm thickness in order to cover entire axial slices of the whole brain using 55 isotropic gradient directions with b= 1000s/mm2, one b=0 acquisition, and number of excitations (NEX)=1 [5]. Approximate scanning time for the acquisition was 12 minutes using double refocusing pulse sequence to reduce eddy current artifacts. Only children with neurological condition younger than age 4 years (being scanned clinically) were sedated using pentobarbital (3 mg/kg) followed by fentanyl (1 mg/kg).
DTI analysis of the corticospinal tract segments (hand and leg)
Whole brain ICA+BSM (independent component analysis combined with a ball-stick model) tractograpy was performed to reconstruct streamlines of white matter fibers, as described and validated recently in various pediatric patient groups using functional MRI and invasive electrical stimulation mapping symptoms [5–7]. To identify the two segments of CST streamlines associated with primary motor pathways of the hand and leg, maximum a posteriori probability classifier was applied [4], which can automatically classify individual streamlines into one of three segments, hand, leg, and face, based on their stereotactic atlases constructed from healthy children (Figure 1). We also defined “whole CST” as the sum of the fibers related to the hand and leg without the face component. For each segment, a streamline visitation map was created by using the number of streamlines passing through each voxel. Voxels having more than 5 visits were assumed to belong to each motor pathway. Streamline volume was measured by the total volume of all voxels belonging to the pathway. Finally, normalized streamline volume of the hand and leg segments as well as the whole CST (hand and leg combined, excluding facial fibers) was obtained by dividing the streamline volume to the white matter volume of its corresponding hemisphere.
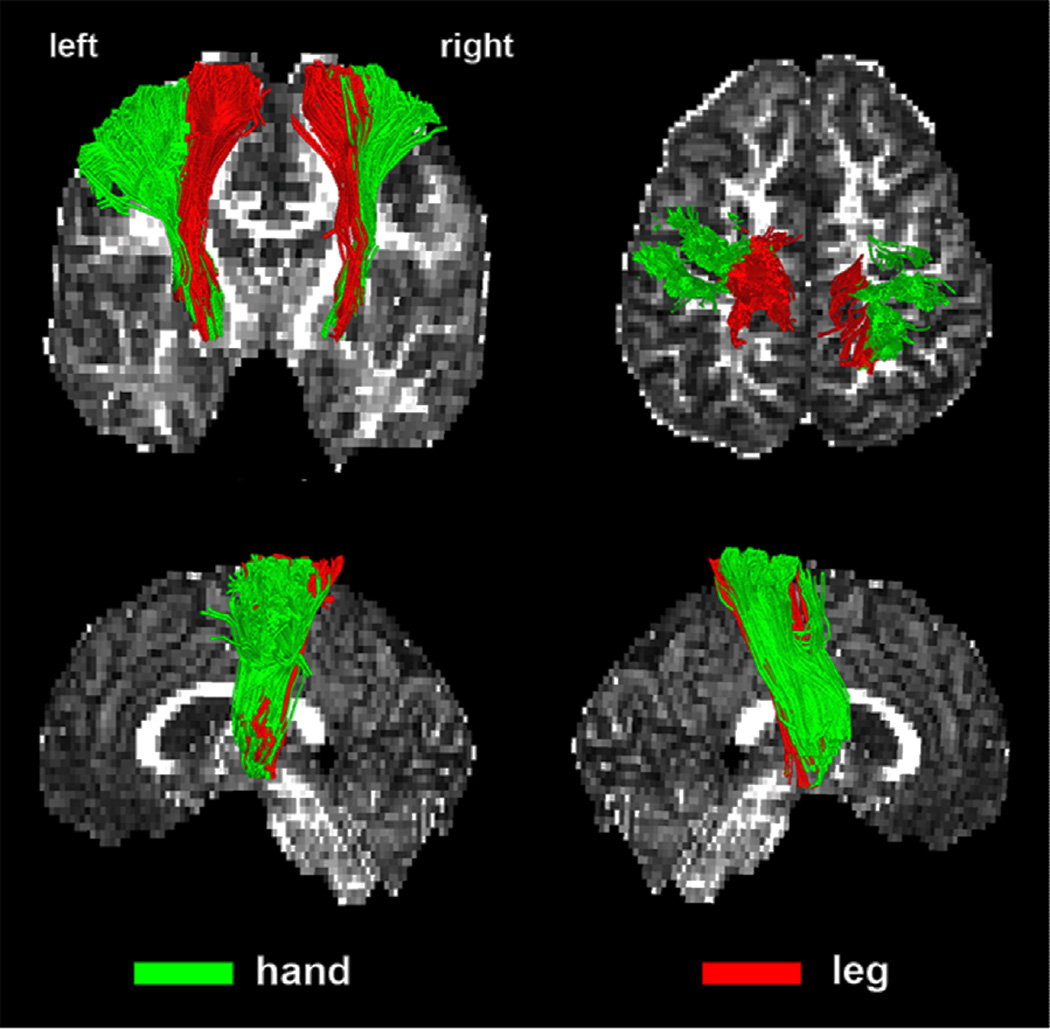
Figure 1.
Representative examples of CST segmentation into leg- and hand-related bundles. Two CST pathways: hand (green) and leg (red) were obtained using the DWI-MAP classifier from a normal control participant (9 years old boy). Fractional anisotropy map was used as the background.
Statistics
Mixed design analyses of covariance (ANCOVA) were performed to examine the CST segmental volumes in relation to gender (inter-subject effect), left and right hemispheric location (intra-subject effect), and age included as a covariate. Separate analyses were done for the absolute and normalized leg- and hand-related CST volumes. CST segmental (hand, leg) volumes were also correlated with age using Pearson’s correlation both in the whole group and in two subgroups (healthy children and children with neurological condition). Statistical analysis was carried out using the SPSS Statistics 20.0 software (SPSS Inc, Somers, New York). P-values < .05 were considered statistically significant.
RESULTS
Side differences, gender and age effects in CST volumes
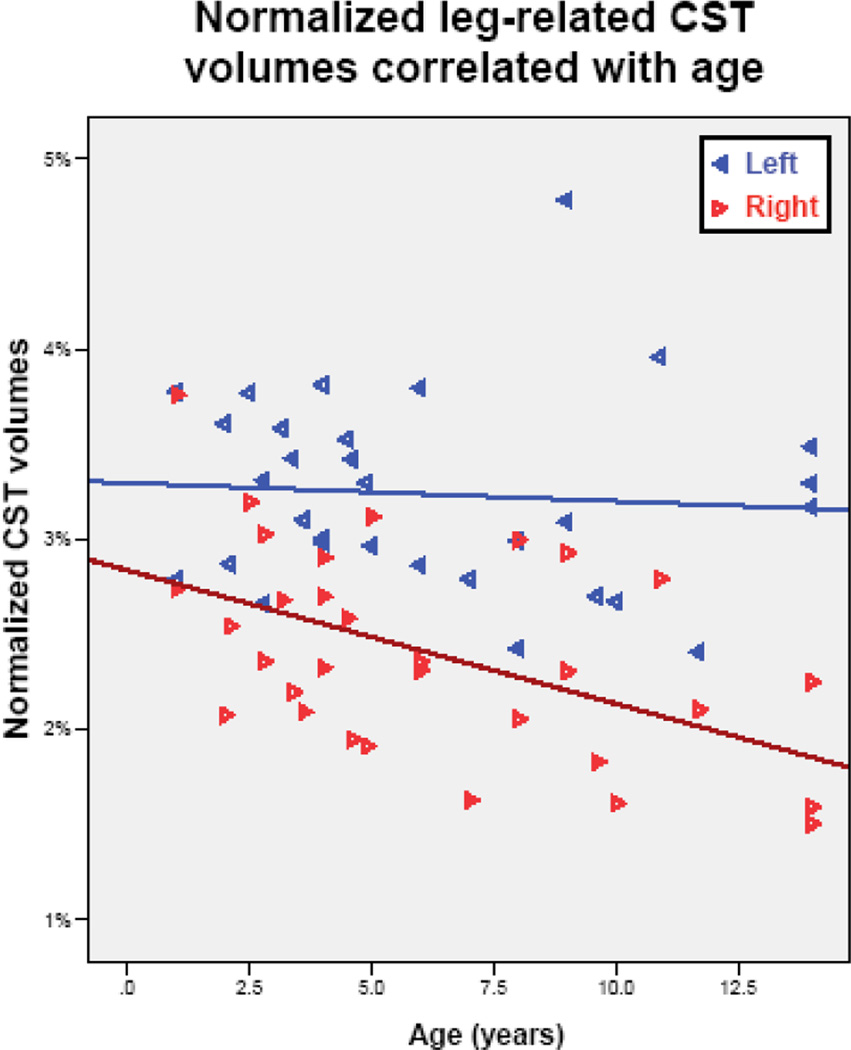
The ANCOVA revealed no gender effects related to streamline-volumes of the whole CST or its segments either before, or after normalization (p≥0.40). The absolute whole-CST streamline-volumes showed a trend towards a difference between the left and right hemispheres (F=3.03; p= 0.093) that became significant following normalization (F=4.80; p=0.037), whereas no age effect was detected in either analysis (p>0.3). The ANCOVA of the CST segments demonstrated higher leg-related CST absolute streamline volumes in the left hemisphere (F=14.8; p< 0.001) without an age effect (F=0.72; p=0.41). After normalizing the volume of this CST segment, its lateralization became even more prominent (F=21.10; p<0.001), and an age effect was revealed as well (F=4.89; p=0.035). The leg-related CST segments remained highly asymmetric in the healthy subgroup (n=17) in both the absolute (p=0.006) and normalized (p=0.005) values (Figure 2); in contrast, this asymmetry was not significant in the subgroup of children with neurological condition (p>0.1 in both absolute and normalized volumes). No side differences were found in the absolute (F= 0.02; p> 0.97) or normalized hand-related CST streamline-volumes (F<0.01; p>0.77) (Figure 2), although the absolute values of this segment showed a mild age effect (F=4.07; p=0.053) that disappeared after normalization (F= 0.63; p=0.43).
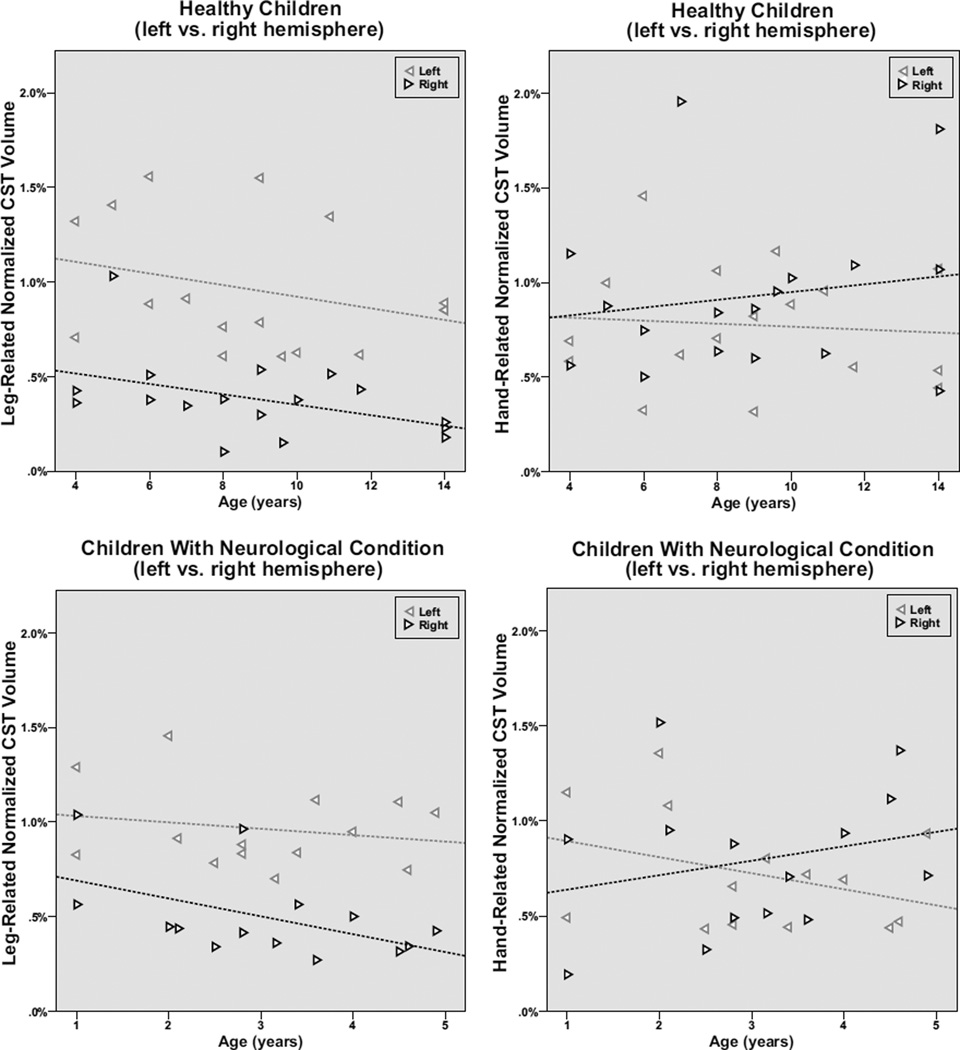
Figure 2.
Normalized leg- and hand-related CST volumes correlated with age in the healthy children vs. children with neurological disease. The left CST volumes are represented by the leftward pointing grey triangles, whereas right CST volumes are designated by the rightward pointing black triangles. The leg-related CST showed a consistent left>right asymmetry throughout the age range in the healthy children (p=0.005); the side difference was present but not significant in children with neurological condition. Both the combined group and the two subgroups also showed an age-related decline in the right leg-related CST normalized volumes; this was significant in the combined group (r= −0.46; p=0.01) and showed a strong trend in the smaller subgroups (healthy controls: r= −0.44; p=0.08, children with neurological condition: r=−0.51; p=0.06). In contrast, the hand-related CST segments showed no consistent asymmetry or age-related changes in either subgroup.
Correlations with age
Whole-CST streamline-volumes did not show any significant change with age before or after normalization (p≥ 0.40). The leg-related CST streamline-volumes showed a right hemispheric decline with age (r= −0.39; p= 0.033) that became more prominent after normalization (r= −0.46; p=0.01). In contrast, left hemispheric absolute leg-related streamline-volumes remained constant with age (r= 0.01; p=0.95), with only a mild (non-significant) decrease after normalization (r= −0.19; p=0.31). The hand-related CST streamline-volumes of the left hemisphere showed no correlation with age (p>0.40), while the right hemisphere presented a trend for an age-related increase (r=−0.33; p=0.067) that disappeared following normalization (r=0.24; p=0.20).
When the two groups of subjects were analyzed separately, the decline in right-sided normalized leg-related CST streamline-volumes was noted in both the healthy controls (r= −0.44; p=0.08) and the children with neurological conditions (r=−0.51; p= 0.06) (Figure 2), although neither reached significance probably due to the low sample sizes in these subgroups. None of the hand-related CST streamline-volumes segments showed any trends with age in these subgroups.
DISCUSSION
Diffusion imaging of the corticospinal tract
Several previous studies have investigated diffusion metric changes within the CST in children and adolescents, reporting an association between brain maturation and increasing fractional anisotropy (FA) as well as decreasing mean diffusivity (MD) [9–12]. This association has been corroborated by functional evidence connecting increasing FA within the CST with increasing consistency in reaction time in children compared to adolescents [13]. A few studies also showed hemispheric FA asymmetries with higher values within the left CST, with or without the presence of an effect of gender or handedness [12, 14, 15]. In contrast, the existence of volumetric asymmetries of the whole CST in children and young adults is much less evident. Consistent with our present findings, DTI studies of infants to early adults revealed no [9, 16] or only very subtle hemispheric differences of whole CST volumes [12, 17]. Our dataset demonstrates clear differences in asymmetries of leg (asymmetries present) vs. hand (no or minimal asymmetries) in CST segments, thus suggesting that the whole CST volume may mask the increasingly prominent volumetric differences of the left and right leg-related CST segments.
Asymmetries in the cortical motor control
Both fMRI and transcranial magnetic stimulation (TMS) data from adults suggest hemispheric asymmetries of brain function related to the execution of movement in the upper limbs. fMRI studies revealed bilateral signal changes including ipsilateral decrease of signal during movement of one hand. The decrease was more pronounced when the non-dominant hand was used [18, 19]. Furthermore, TMS experiments revealed lower thresholds for evoked potentials in the motor cortex controlling the dominant hand, and motor performance of the ipsilateral hand improved following unilateral inhibition of the motor cortex induced by TMS [20, 21]. The above-mentioned fMRI and TMS findings emphasize the role of interhemispheric inhibition in the control of unilateral movements of the upper limb; this could contribute to the development of hemispheric dominance and the leg-related CST asymmetries discovered in our present study.
A recent DTI study investigated CST segments associated with finger and toe movement in 11 healthy adults using a DTI approach where target regions (for CST fiber tracking) were selected based on fMRI motor activations [22]. They found no hemispheric asymmetry of either diffusion metrics or volume related to these segments. However, they did reveal greater than 7-fold higher transcallosal connectivity in the toe vs. the finger-controlling CST segments. Although these findings do not completely match our results, they do imply stronger interhemispheric interaction related to the control of the lower limbs, which may explain the gradual decrease of leg-related CST volume we found in the non-dominant hemisphere throughout the developmental ages when the process of hemispheric lateralization is ongoing.
The selective CST volume lateralization in the lower limb segment is an unexpected finding in our study. We speculate that this may be due to the different control and utilization of the upper and lower limbs. Whereas the upper limbs are able to move and function quite independently from each other, and their mirror movements are attainable, the activity of the lower limbs is strongly coupled and most commonly involves reciprocal movements with emphasis on proximal muscles with more pronounced unilateral innervation. Functionally, most right-handed individuals (left hemispheric dominant) are able to perform tasks using the left hand far better than when using the left leg. In addition, footedness was proposed to be a better predictor of hemispheric lateralization than handedness [23, 24]. Although the extent of lateralization in fMRI studies was higher in the hands, there was higher left hemispheric activation in the left compared to the right primary motor cortex and basal ganglia related to the movement of the lower limbs [25, 26]. The strong link between hemispheric lateralization and footedness could explain our findings.
Despite our findings on selective asymmetry and age-related variations in leg-related CST segments, the study had several limitations. First, the number of subjects was limited, and the age range of the healthy controls and children neurological condition was different. Second, the study was retrospective, as the data have all been collected by the time this new CST analysis method became available; therefore, we were not able to collect formal handedness/footedness measures for all subjects. Still, we made sure that we included only patients who had no evidence for left-handedness (or footedness) based on their medical charts (for the subgroup with neurological conditions) and/or reported right-hand preference before the MRI (healthy control group). Based on the presented results, it would be interesting to design a prospective study including formal evaluation of both hand and foot preference, to address the effect of handedness/footedness on CST segmental development.
Conclusion
We found age-associated differences between leg- and hand-related segments of the CST in children. The volume of the right CST showed an age-related decline in both healthy children and children with neurological condition (without motor impairment), possibly secondary to brain maturation with development of hemispheric dominance lateralizing toward the left hemisphere. This DTI methodology can be used in future studies to explore functional correlates of differential development of CST segments in healthy subjects and disease conditions.
ACKNOWLEDGEMENTS
We thank Cathie Germain, MA, for assisting patient recruitment and scheduling, Majid Janabi MD, Jane Cornett RN and Anne Deboard RN for performing sedation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, et al. Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci U S A. 1999;96:10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazar M. Mapping brain anatomical connectivity using white matter tractography. NMR Biomed. 2010;23:821–835. doi: 10.1002/nbm.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mori S, Kaufmann WE, Pearlson GD, Crain BJ, Stieltjes B, Solaiyappan M, et al. In vivo visualization of human neural pathways by magnetic resonance imaging. Ann Neurol. 2000;47:412–414. [PubMed] [Google Scholar]
- 4.Jeong JW, Asano E, Brown EC, Tiwari VN, Chugani DC, Chugani HT. Automatic detection of primary motor areas using diffusion MRI tractography: comparison with functional MRI and electrical stimulation mapping. Epilepsia. 2013;54:1381–1390. doi: 10.1111/epi.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeong JW, Asano E, Juhasz C, Chugani HT. Quantification of primary motor pathways using diffusion MRI tractography and its application to predict postoperative motor deficits in children with focal epilepsy. Hum Brain Mapp. 2014;35:3216–3226. doi: 10.1002/hbm.22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeong JW, Asano E, Yeh FC, Chugani DC, Chugani HT. Independent component analysis tractography combined with a ball-stick model to isolate intravoxel crossing fibers of the corticospinal tracts in clinical diffusion MRI. Magn Reson Med. 2013;70:441–453. doi: 10.1002/mrm.24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong JW, Chugani HT, Juhasz C. Localization of function-specific segments of the primary motor pathway in children with Sturge-Weber syndrome: a multimodal imaging analysis. J Magn Reson Imaging. 2013;38:1152–1161. doi: 10.1002/jmri.24076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamson DO, Juhász C, Shin J, Behen ME, Guy WC, Chugani HT, et al. Patterns of structural reorganization of the corticospinal tract in children with Sturge-Weber syndrome. Pediatr Neurol. 2014;50:337–342. doi: 10.1016/j.pediatrneurol.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giorgio A, Watkins KE, Chadwick M, James S, Winmill L, Douaud G, et al. Longitudinal changes in grey and white matter during adolescence. Neuroimage. 2010;49:94–103. doi: 10.1016/j.neuroimage.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Kumar A, Juhasz C, Asano E, Sundaram SK, Makki MI, Chugani DC, et al. Diffusion tensor imaging study of the cortical origin and course of the corticospinal tract in healthy children. AJNR Am J Neuroradiol. 2009;30:1963–1970. doi: 10.3174/ajnr.A1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222:212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westerhausen R, Huster RJ, Kreuder F, Wittling W, Schweiger E. Corticospinal tract asymmetries at the level of the internal capsule: is there an association with handedness? Neuroimage. 2007;37:379–386. doi: 10.1016/j.neuroimage.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 13.Tamnes CK, Fjell AM, Westlye LT, Ostby Y, Walhovd KB. Becoming consistent: developmental reductions in intraindividual variability in reaction time are related to white matter integrity. J Neurosci. 2012;32:972–982. doi: 10.1523/JNEUROSCI.4779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing-Cobbs L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex. 2007;17:2760–2768. doi: 10.1093/cercor/bhm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takao H, Abe O, Yamasue H, Aoki S, Sasaki H, Kasai K, et al. Gray and white matter asymmetries in healthy individuals aged 21–29 years: a voxel-based morphometry and diffusion tensor imaging study. Hum Brain Mapp. 2011;32:1762–1773. doi: 10.1002/hbm.21145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roze E, Harris PA, Ball G, Elorza LZ, Braga RM, Allsop JM, et al. Tractography of the corticospinal tracts in infants with focal perinatal injury: comparison with normal controls and to motor development. Neuroradiology. 2012;54:507–516. doi: 10.1007/s00234-011-0969-5. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Baleriaux D, Kavec M, Metens T, Absil J, Denolin V, et al. Structural asymmetries in motor and language networks in a population of healthy preterm neonates at term equivalent age: a diffusion tensor imaging and probabilistic tractography study. Neuroimage. 2010;51:783–788. doi: 10.1016/j.neuroimage.2010.02.066. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi MJ, Saito DN, Aramaki Y, Asai T, Fujibayashi Y, Sadato N. Hemispheric asymmetry of frequency-dependent suppression in the ipsilateral primary motor cortex during finger movement: a functional magnetic resonance imaging study. Cereb Cortex. 2008;18:2932–2940. doi: 10.1093/cercor/bhn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newton JM, Sunderland A, Gowland PA. fMRI signal decreases in ipsilateral primary motor cortex during unilateral hand movements are related to duration and side of movement. Neuroimage. 2005;24:1080–1087. doi: 10.1016/j.neuroimage.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi M, Hutchinson S, Theoret H, Schlaug G, Pascual-Leone A. Repetitive TMS of the motor cortex improves ipsilateral sequential simple finger movements. Neurology. 2004;62:91–98. doi: 10.1212/wnl.62.1.91. [DOI] [PubMed] [Google Scholar]
- 21.Triggs WJ, Calvanio R, Levine M. Transcranial magnetic stimulation reveals a hemispheric asymmetry correlate of intermanual differences in motor performance. Neuropsychologia. 1997;35:1355–1363. doi: 10.1016/s0028-3932(97)00077-8. [DOI] [PubMed] [Google Scholar]
- 22.Yeo SS, Jang SH. Differences between the somatotopic corticospinal tract for the fingers and toes in the human brain. NeuroRehabilitation. 2012;31:395–399. doi: 10.3233/NRE-2012-00809. [DOI] [PubMed] [Google Scholar]
- 23.Elias LJ, Bryden MP. Footedness is a better predictor of language lateralisation than handedness. Laterality. 1998;3:41–51. doi: 10.1080/713754287. [DOI] [PubMed] [Google Scholar]
- 24.Elias LJ, Bryden MP, Bulman-Fleming MB. Footedness is a better predictor than is handedness of emotional lateralization. Neuropsychologia. 1998;36:37–43. doi: 10.1016/s0028-3932(97)00107-3. [DOI] [PubMed] [Google Scholar]
- 25.Kapreli E, Athanasopoulos S, Papathanasiou M, Van Hecke P, Strimpakos N, Gouliamos A, et al. Lateralization of brain activity during lower limb joints movement. An fMRI study. Neuroimage. 2006;32:1709–1721. doi: 10.1016/j.neuroimage.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 26.Luft AR, Smith GV, Forrester L, Whitall J, Macko RF, Hauser TK, et al. Comparing brain activation associated with isolated upper and lower limb movement across corresponding joints. Hum Brain Mapp. 2002;17:131–140. doi: 10.1002/hbm.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]