Abstract
Prenatal maternal immune activation increases risk for schizophrenia and/or autism. Previous data suggest that maternal weight change in response to the immune activator polyinosinic-polycytidylic (Poly IC) in rats influences the severity of effect in the offspring as does the exposure period. We treated gravid Sprague-Dawley rats from E14-18 with 8 mg/kg/day Poly IC or saline. The Poly IC group was divided into those that gained the least weight or lost (Poly IC (L)) and those that gained the most (Poly IC (H)) weight. There were no effects of Poly IC on anxiety (elevated zero-maze, open-field, object burying), or Morris water maze cued learning or working memory or Cincinnati water maze egocentric learning. The Poly IC (H) group males had decreased acoustic startle whereas Poly IC (L) females had reduced startle and increased PPI. Poly IC offspring showed exaggerated hyperactivity in response to amphetamine (primarily in the Poly IC (H) groups) and attenuated hyperactivity in response to MK-801 challenge (primarily in the Poly IC (L) group). Poly IC (L) males showed reduced cued conditioned freezing; both sexes showed less time in the dark in a light-dark test, and the Poly IC groups showed impaired Morris water maze hidden platform acquisition and probe performance. The data demonstrate that offspring from the most affected dams were more affected than those from less reactive dams indicating that, degree of maternal immune activation predicts severity of effects on offspring behavior.
Keywords: Polyinosinic-polycytidylic acid, Poly IC, (+)-amphetamine-induced locomotor activity, prenatal immune activation, MK-801-induced locomotor activity, Morris water maze, rat, light-dark test, conditioned fear, prepulse inhibition of acoustic startle
Maternal infection during pregnancy is associated with increased risk of neuropsychiatric illness in their children, including autism spectrum disorder (ASD) and schizophrenia (Atladottir et al., 2010;Brown, 2006;Brown and Derkits, 2010;Brown and Susser, 2002;Ciaranello and Ciaranello, 1995;Patterson, 2002). Prenatal influenza infection specifically has been associated with increased risk for schizophrenia (Brown et al., 2004;Mednick et al., 1988) as have prenatal infections with rubella and toxoplasma (Penner and Brown, 2007). More recently a family history of psychotic behavior combined with urinary tract infection during pregnancy has been associated with increased prevalence of schizophrenia (Clarke et al., 2009).
Experimental models of prenatal influenza infection result in behavioral abnormalities in the offspring (Fatemi et al., 1999;Fatemi et al., 2002;Fatemi et al., 2005;Moreno et al., 2011;Shi et al., 2003). To determine if the transplacental effects are attributable to the virus or maternal response to the virus, experiments have used viral immune activators such as the double-stranded synthetic RNA polyinosinic-polycytidylic acid (Poly IC). Injection of Poly IC during gestation in mice induces effects on offspring brain and behavior similar to the effects seen after maternal influenza-induced infection (Bitanihirwe et al., 2010a;Bitanihirwe et al., 2010b;Cunningham et al., 2007;Ito et al., 2010;Meyer et al., 2006b;Nyffeler et al., 2006;Ozawa et al., 2006;Shi et al., 2003;Shi et al., 2009;Smith et al., 2007). In rats, Poly IC administered i.v. on embryonic day (E)14 resulted in reduced latent inhibition, exaggerated locomotor activity in response to amphetamine and MK-801, and impaired T-maze and Morris water maze (MWM) learning on reversal trials (Zuckerman et al., 2003a;Zuckerman et al., 2003b;Zuckerman and Weiner, 2003). In other studies, E14 i.p. Poly IC exposure (Bronson et al., 2011;Richtand et al., 2011) reduced locomotor activity in response to MK-801 and (+)-amphetamine challenge. In another set of experiments E14 i.v. Poly IC (Dickerson et al., 2010;Wolff et al., 2011;Wolff and Bilkey, 2008;Wolff and Bilkey, 2010) reduced prepulse inhibition (PPI) of the acoustic startle response (ASR). Similarly, E14 i.v., Poly IC decreased PPI and reduced time in open arms and open-arm entries in an elevated plus maze in rat offspring (Yee et al., 2011). E14 i.v. Poly IC increased errors on an operant set-shifting task, reduced PPI, reduced locomotor activity in response to MK-801 before puberty but not after, and reduced novel position but not novel object recognition (Howland et al., 2012; Zhang et al., 2012). However, E10–11, E15–16, and E18–19 Poly IC had no effect on adult offspring PPI in another study (Fortier et al., 2007).
Prenatal maternal immune activation as a cause of transplacental effects has been reviewed extensively (Boksa, 2010;Bronson and Richtand, 2011;Deverman and Patterson, 2009;Meyer et al., 2005;Meyer et al., 2009a;Shi et al., 2009). The weight of the evidence supports the view that maternal proinflammatory cytokine release is the source of transplacentally-induced effects on offspring brain, behavior, immune regulation, and placental function (Hsiao et al., 2012;Hsiao and Patterson, 2011;Meyer et al., 2007;Smith et al., 2007). An issue in this field is the role of litter effects. In developmental studies, litter effects, litter size, and sex affect outcome and if not controlled can result in over-estimates of significant effects (Giovanoli and Meyer, 2013;Holson and Pearce, 1992;Lazic, 2013). It is well-established that in multiparous species, over-sampling from litters and treating each offspring as if orthogonal to its littermates inflates F-ratios and overestimate effect size and significance (Holson and Pearce, 1992;Lazic and Essioux, 2013). These and other issues of study design and data analyses are discussed at length elsewhere (Meyer et al., 2005;Meyer et al., 2009a); see also(Deverman and Patterson, 2009).
Immune activation affects some animals more than others and we previously showed that maternal weight gain following Poly IC accounted for differences in behavioral outcome of the offspring if we dichotomized the data by Poly IC-treated dams that gained the least versus those that gained the most weight after treatment (Vorhees et al., 2012). In the present experiment we again stratified the Poly IC group by maternal weight exactly as we did previously. In addition, because the stage of embryonic development at which exposure occurs has been shown to affect the nature of the neurobehavioral effects in offspring after Poly IC we expanded the exposure period to later stages of embryo-fetal development. In our previous experiment we treated on E14 only (Vorhees et al., 2012). In order to determine if a longer exposure interval would have a greater or different effect, in the present experiment we treated for 5 days from E14–18. The same set of tests was used as before (Table 1). The selection of tests was based on two considerations: (1) tests predicted to be associated with a schizophrenia-related phenotype (e.g., acoustic startle with PPI, learning and memory, anxiety, latent inhibition, drug challenges using agents that affect neurotransmitters altered in schizophrenia), and (2) several reference tests included to provide basic information on the phenotype (e.g., baseline locomotor activity and marble burying) or as control procedures for other tests (e.g., straight swimming channel as necessary training before testing in the Cincinnati water maze (CWM), and Cued trials in the Morris water maze (MWM) as a procedural control ensuring that proximal cue learning is not affected).
Table 1.
Each litter was culled to 4 males (M) and 4 females (F) and randomly assigned as M/F pairs to one of 4 behavioral test sequences
| Pair | Test | Test age |
|---|---|---|
| A | Elevated zero-maze (EZM) | P65 |
| Open-field locomotor activity | P66 | |
| Marble burying | P66 | |
| Acoustic startle with prepulse inhibition (PPI) | P67 | |
| Straight channel swimming | P68 | |
| Cincinnati water maze (CWM) | P69–86 | |
| Morris water maze matching-to-sample (MWM-MTS) | P87–107 | |
| B | Conditioned fear: Tone pre-exposure (PE) | P60–62 |
| Locomotor activity with drug challenge | P64±1 | |
| C | Conditioned fear: No tone pre-exposure (NPE) | P60–62 |
| Locomotor activity with drug challenge | P64±1 | |
| D | Light/Dark test | P66±1 |
| Straight channel swimming | P68 | |
| Morris water maze (MWM)-acquisition | P70–76 | |
| MWM-reversal | P77–83 | |
| MWM-shift | P84–90 | |
| Morris water maze cued (MWM-cued) | P91–92 |
Methods
Subjects
Harlan Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) were bred in-house (females weighed 225–250 g upon arrival and were given at least 2 weeks to acclimate to the vivarium). The day a sperm plug was found was considered E0. Pregnant females were housed in polycarbonate cages (26 × 48 × 20 cm) with woodchip bedding and provided with NIH-07 diet, filtered water, and a stainless steel enclosure as partial enrichment (Vorhees et al., 2008). The day of parturition was designated postnatal day (P)0. On P1, offspring were counted, sexed, and assigned an arbitrary number. Using a random number table, 4 males and 4 females were then selected for retention. All experimental procedures were approved by the Institutional Animal Care and Use Committee and adhered to NIH and Society for Neuroscience guidelines for the humane care of animals. All efforts were taken to minimize discomfort and/or pain in accordance with National Institutes of Health guidelines for the care and use of animals in research; the vivarium is accredited by the Association for the Assessment and Accreditation of Animal Care International.
Treatment
Polyinosinic-polycytidylic acid (Poly IC) sodium (Sigma-Aldrich #P1530, St. Louis, MO) was dissolved in sterile 0.9% saline at a concentration of 8 mg/ml and administered intraperitoneally at a dose of 8 mg/kg body weight. Forty gravid dams were assigned initially to two groups: 20 dams to the Saline group and 20 dams to the Poly IC group. Dosing was from E14–18 similar to that used by (Zuckerman et al., 2003a). Zuckerman dated pregnancy as starting on E1 whereas we designate the first day as E0, hence Zuckerman’s E15 is our E14 (Vorhees et al., 2012). The Poly IC dose used is in the mid-range of those used by others in rats by the i.p. route (Fortier et al., 2004;Gilmore et al., 2005).
Experimental design
All litters had 4 males and 4 females with one male-female pair randomly assigned (using a random number table) to each of four test sequences (Table 1).
Housing and weaning
Dams were separated from their litters on P28. On P35, offspring were housed in pairs with littermates A and B of the same sex housed together and littermates C and D of the same sex housed together. Testing was conducted by personnel blind to the treatment group.
Behavioral Methods
Pair-A
Elevated zero-maze
The apparatus was elevated 72 cm above the floor and consisted of a 10-cm wide circular track 105 cm in diameter made of black Kydex divided equally in 4 quadrants, 2 enclosed by 28 cm high black side walls and 2 open with 1.3 cm high clear polyethylene curbs to prevent slipping from the edge (Braun et al., 2011). The room was lighted (11.7 lux) and rats were tested as described (Braun et al., 2011). Behavior was monitored via a video camera fed to a monitor with an experimenter who scored behavior for time-in-open, head dips, zone crossings, and stretch-attend movements from the next room.
Open-Field activity
Each test chamber was made of clear polyethylene ~40 × 40 cm (Accuscan Instruments, Columbus, OH) with 16 infrared LED-photodetector pairs along the X and Y-axes spaced every 2.5 cm and another 16 pairs above the floor to detect rearing. Rats were tested for 60 min and data captured in 5 min intervals for horizontal activity, distance (cm), rearing, and time spent in central and peripheral zones.
Marble burying
Testing was conducted in standard rat cages for 20 min using 18 marbles arranged symmetrically on the surface in rows. The cage was filled with bedding to a depth of 5 cm. The number of marbles at least two-thirds buried was counted at the end of the test session.
Acoustic startle response (ASR) with prepulse inhibition (PPI)
SR-LAB startle chambers were used (San Diego Instruments, San Diego, CA). Rats were separately placed in an inner cylindrical holder, that was mounted to an acrylic plate with an accelerometer force transducer affixed to the underside, inside a sound attenuated test chamber. The background noise level was 70 dB and the startle signal (SS) was a 120 dB (SPL) white noise burst lasting 20 ms. Prepulses were also white noise (20 ms) at sound levels of 73, 75, or 80 dB. Prepulses preceded the startle signal by 100 ms from prepulse (PP) onset to SS onset. A 5 min acclimation period preceded trials which were of 5 types: no signal, SS, PP-73 + SS, PP-75 + SS, or PP-80 + SS presented in a 5 × 5 Latin square repeated twice for a total of 50 trials. Responses were recorded for 100 ms after SS onset and recorded as voltage change. The dependent measure was maximum voltage change (Vmax), i.e., peak startle response amplitude and average voltage change (Vavg).
Straight channel
Testing was in a 15 × 244 × 50 cm straight swimming channel filled to a depth of 30 cm with a submerged platform at one end. Each rat received four back-to-back trials (up to 2 min/trial) to traverse from one end to the other and climb on the platform. The test teaches escape and assesses swim speed and motivation to escape.
Cincinnati Water Maze (CWM)
The CWM is a test of route-based egocentric learning that is dependent upon striatal dopamine (Braun et al., 2012). The maze, described elsewhere (Vorhees, 1987;Vorhees et al., 1991), consists of nine T-shaped cul-de-sacs that branch from a central pathway. Channels are 15 cm wide and walls 51 cm high filled with water (21 ± 1°C) to a depth of 30 cm. Testing was conducted under infrared lighting. Performance was scored by visualizing the animals using infrared LED emitters and a near infrared camera mounted above and connected to a monitor located in an adjacent room (Vorhees et al., 2011). This prevented rats from using distal cues by which to navigate. Time limit was 5 min per trial with a 5 min intertrial interval if they failed to locate the goal or immediately if they did find the goal. Animals were given 2 trials/day for 18 days. Errors and latency to escape were recorded. An error occurred if an animal entered the stem or arm of a T-shaped cul-de-sac. Occasionally an animal did not find the escape after multiple trials and stopped searching. This resulted in few errors compared with animals that actively searched. To correct this, scores on non-searching trials were given the number of errors of the worst performing animal + 1.
Morris Water Maze Matching-to-Sample (MWM-MTS)
The maze was stainless steel and 210 cm in diameter with 51 cm high sides and was filled to a depth of 30 cm with water (21 ± 1°C). The platform was 10 cm in diameter and was positioned in one of four positions halfway between the center and the wall and submerged 1.5 cm. The maze was flat black and the platform was clear acrylic and not visible at water level. Rats were tested for 21 days, 2 trials/day. Each day the start and platform were placed in new positions but remained the same for the 2 trials of the day. Performance was captured using an overhead camera connected to a computer operating ANYmaze tracking software (Stoelting Instruments, Wood Dale, IL). The data analyzed were latency, path length (cm), and cumulative distance from the platform (m).
Pair-B
Conditioned Fear/Latent Inhibition
Latent inhibition was assessed using a fear conditioning paradigm (Smith et al., 2007). Animals were placed in test chambers (Coulbourn Instruments, Allentown, PA) with a speaker and grid floor connected to a shock source, and a video camera mounted in the ceiling connected to Coulbourn Freeze Frame software. Each test chamber was positioned inside a sound-attenuating enclosure. Pair-B was the PE (pre-exposed) group. On day 1, these animals were placed in the test chambers for acclimation followed by 30 tones (82 dB, 2 kHz, 30 s duration) separated by 30 s inter-stimulus intervals which was then followed by 3 tone-footshock pairings with the shock (0.5 mA) occurring during the last 1 s of the tone interval. On Day-2, rats were returned to the test chambers to assess contextual conditioning for 6 min during which immobility intervals of >4 s were scored as freezing. On Day-3, rats were returned to the test chambers with a different floor texture and tested for 6 min; the first 3 min with no-tone and the last 3 min with tone and again scored for bouts of immobility.
Open-field with drug challenge
Rats were placed in the open-field described above for 30 min with no drug given, removed, injected subcutaneously with 1.0 mg/kg of (+)-amphetamine or 0.3 mg/kg of MK-801 (both expressed as the freebase), and returned to the apparatus for an additional 180 min.
Pair-C
Conditioned Fear/Latent Inhibition
This was as above except that Pair-C was assigned to the no-tone PE (NPE) group such that they received the same procedure except that on Day-1 they received no tone prior to the tone-shock pairings.
Open-field with drug challenge
Rats were tested the same as Pair-B above with half challenged with 1.0 mg/kg of (+)-amphetamine and half with 0.3 mg/kg of MK-801.
Pair-D
Light/Dark test
The test was conducted in the open-field apparatus described above except that a black acrylic box with lid was inserted that occupied 50% of the floor area. Rats were placed in the lighted side and data recorded for 10 min for activity and time on each side.
Straight channel
Straight channel testing on this pair of rats was the same as described above.
Morris Water Maze (MWM)
Spatial learning and memory were assessed in the MWM as described (Vorhees and Williams, 2006;Vorhees and Williams, 2014). The test consisted of 4 phases, 3 with hidden platforms and 1 with a visible platform. Phase-1 was acquisition (platform in SW quadrant), Phase-2 was reversal (platform in NE quadrant), Phase-3 was shift (platform in NW quadrant), and Phase-4 was cued with the platform randomized on each trial. Animals received 4 trials per day for 6 days in each hidden platform phase followed by a 30 s probe trial on day-7 with the platform removed. Each phase used a platform of a different size (10, 7, and 5 cm diameter, respectively). The final phase was cued using a 10 cm platform with a plastic ball mounted on top of a brass rod protruding 10 cm above the water. For cued trials, curtains were closed around the maze to minimize extramaze cues and the animals were given four trials per day for two days with the platform and start positions changed on every trial. Performance was video tracked using (ANYmaze, Stoelting Instruments, Wood Dale, IL). On platform trials, latency, cumulative distance, path length, and swim speed were analyzed. On probe trials, platform site crossovers, swim speed, and average distance from the target were analyzed. For cued, latency was analyzed.
Statistical Analysis
Data were analyzed using completely randomized block analyses of variance (ANOVA) mixed linear models (SAS V9.2, Proc Mixed, SAS Institute, Cary, NC) to control for litter effects. Treatment and sex were fixed factors within blocks. Kenward-Roger adjusted degrees of freedom were used. Prior to each ANOVA, variance-covariance matrices were tested for best fit to the data and the model of best fit applied. Where animals were tested repeatedly, repeated measure factors were included in the ANOVA model. Interactions were analyzed using slice-effect ANOVAs at each level of the repeated-measure factor. A posteriori pairwise group comparisons were analyzed using the Hochberg step-up method to control for multiple comparisons. The significance threshold was set at p ≤ 0.05 (2-tailed where no a priori prediction was made and 1-tailed where predictions were made based on our prior data (Vorhees et al., 2012)). The predicted case was that Poly IC would attenuate female weight gain during gestation relative to Saline-treated females. Data are presented as least square (LS) mean ± SEM to be consistent with the Mixed Model ANOVAs. After the significant effects of Poly IC on offspring behavior were identified by ANOVA, these data were reanalyzed by ANCOVA with maternal body weight after Poly IC treatment as the covariate. This was done to determine the extent to which maternal body weight accounted for the significant ANOVA effects.
Results
Maternal body weight
Based on the idea that changes in maternal body weight represent the degree of immune activation and the severity of the maternal response, we divided the Poly IC group into subgroups as a function of maternal weight change. Those dams that gained the most and those that gained the least (or lost) weight were divided into two equal groups (Table 2). The 20 Poly IC-treated females were divided into the 10 gaining the most and the 10 gaining the least weight. Repeated measures ANOVA showed a significant effect of treatment on maternal body weight (F(2,37.6) = 4.31, p<0.02); there was also a significant effect of day (p<0.0001), and a treatment x day interaction (F(12,179) = 4.31, p<0.0001). Slice-effect ANOVAs on each day showed significant effects on E17–20. On E17 none of the pairwise Hochberg comparison groups were significant. On E18–20, the Poly IC low gain group was significantly lighter than Saline control dams; the Poly IC high gain group did not differ from Saline.
Table 2.
Maternal body weight (g) during gestation in Saline and Poly IC treated (those that gained weight (H) and those that lost weight (L)) dams with treatment beginning on embryonic day (E)14 and ending on E18
| Embryonic day (E) | Saline | Poly IC (H) | Poly IC (L) |
|---|---|---|---|
| N | 20 | 10 | 10 |
| E14 | 308.0 ± 3.6 | 321.6 ± 5.1 | 311.4 ± 5.1 |
| E15 | 315.5 ± 3.6 | 327.7 ± 5.1 | 313.5 ± 5.1 |
| E18 | 357.9 ± 3.6 | 368.6 ± 5.1 | 345.0 ± 5.1* |
| E20 | 389.8 ± 3.6 | 399.0 ± 5.1 | 368.7 ± 5.1** |
Maternal body weight (g) during gestation (mean ± SEM)
P<0.05 vs. Saline (one-tailed)
P<0.01 vs. Saline (one-tailed)
To ensure that the weight change subdivision was reliable we determined day-by-day weight differences prior to versus after treatment (i.e., E15 minus E14, E16-14, E17-14, etc.) and summed the differences and re-divided dams into two groups and the dividing point was identical to the first approach, hence the groups were Poly IC (H) (i.e., high-gain), Poly IC (L) (i.e., low-gain), and Saline control.
Gestation Length, Litter Size, and Sex ratio
ANOVA on gestation length showed no significant treatment effects (Table 3). Litters were not disturbed on the day of birth (P0). On P1, dams were removed and offspring counted, sexed, and weighed prior to culling. There were no significant effects of treatment on litter number or the number of males or females within litters (Table 3).
Table 3.
Gestation length and litter characteristics, including litter size and average number of males and females per litter
| Saline | Poly IC (H) | Poly IC (L) | |
|---|---|---|---|
| N | 20 | 10 | 10 |
| Gestation length (day) | 21.2 ± 0.2 | 22.0 ± 0.2 | 22.1 ± 0.2 |
| Litter size (P1)* | 13.7 ± 0.4 | 13.5 ± 0.6 | 12.1 ± 0.6 |
| No. males/litter | 6.8 ± 0.4 | 6.9 ± 0.6 | 5.9 ± 0.6 |
| No. females/litter | 6.9 ± 0.4 | 6.6 ± 0.6 | 6.2 ± 0.6 |
Litter size prior to culling.
Offspring Body Weight
Body weight analysis on P1 prior to culling showed no significant main effect of treatment, but there were significant effects of sex (F(1,277) = 20.31, p<0.0001) and treatment x sex (F(2,277) = 3.75, p<0.03). Slice-effect ANOVAs for treatment for each sex showed no treatment effect for males (F < 1) or females (F < 1).
After culling, offspring were weighed weekly. Analysis of preweaning body weight showed no significant main effect of treatment or treatment x sex interaction, but there was a significant treatment x week effect (F(6,839) = 11.35, p<0.0001); no other interactions were significant. Slice-effect ANOVAs on each week’s weight showed a significant treatment effect on P28 only (p<0.05). Hochberg pairwise comparisons showed that on P28, the Poly IC (H) group weighed more than the Saline or Poly IC (L) groups: (mean ± SEM): Saline = 88.7 ± 1.0 g, Poly IC (H) = 92.9 ± 1.4 g*, Poly IC (L) = 88.4 ± 1.4 g; *P<0.05).
Postweaning weekly body weights were analyzed from P35–91. Representative body weights are shown in Table 4. The treatment main effect was not significant. There were significant effects of sex (p<0.0001) and week (p<0.0001) but no significant interactions of treatment with sex, week, or sex x week.
Table 4.
Offspring body weights (g) at representative ages from weaning to the end of behavioral testing
| Age (P) | Saline | Poly IC (H) | Poly IC (L) | |||
|---|---|---|---|---|---|---|
| M | F | M | F | M | F | |
| 35 | 139.4 ± 2.4 | 118.7 ± 2.4 | 145.9 ± 3.5 | 124.2 ± 3.4 | 139.2 ± 3.5 | 118.9 ± 3.5 |
| 42 | 192.3 ± 2.4 | 153.0 ± 2.4 | 193.5 ± 3.5 | 154.1 ± 3.4 | 190.7 ± 3.4 | 151.0 ± 3.4 |
| 56 | 287.9 ± 2.4 | 199.8 ± 2.4 | 291.5 ± 3.5 | 201.3 ± 3.4 | 284.7 ± 3.5 | 196.7 ± 3.5 |
| 70 | 347.7 ± 2.4 | 231.8 ± 2.4 | 358.7 ± 3.5 | 235.3 ± 3.4 | 348.3 ± 3.4 | 232.9 ± 3.4 |
| 84 | 387.6 ± 2.4 | 253.8 ± 2.4 | 394.5 ± 3.5 | 255.8 ± 3.4 | 385.2 ± 3.5 | 255.0 ± 3.5 |
| 91 | 403.2 ± 2.4 | 262.2 ± 2.4 | 408.2 ± 3.5 | 263.3 ± 3.4 | 397.9 ± 3.5 | 262.6 ± 3.5 |
(mean ± SEM)
Non-significant outcomes
No significant treatment main effects or interactions were found on several tests; these included elevated zero-maze, open-field locomotor activity, marble burying, straight swimming channel, CWM, MWM-MTS, MWM hidden platform reversal or shift phases, or MWM cued testing. These data are summarized in Table 5.
Table 5.
Summary of behavioral tests on which no significant treatment effects of prenatal Poly IC were obtained
| Saline | Poly IC (H) | Poly IC (L) | ||||||
|---|---|---|---|---|---|---|---|---|
| Pair | Test | Variable | M | F | M | F | M | F |
| A | EZM | N | 18 | 18 | 10 | 10 | 7 | 7 |
| Open time (s) | 84.8 ± 9.5 | 88.3 ± 9.5 | 82.5 ± 12.8 | 84.4 ± 12.8 | 79.9 ± 15.3 | 88.2 ± 15.3 | ||
| Latency (s) to 1st entry |
8.3 ± 1.0 | 7.0 ± 1.6 | 6.1 ± 1.3 | 4.1 ± 1.3 | 6.3 ± 1.6 | 8.3 ± 1.6 | ||
| Open entries | 9.6 ± 1.0 | 10.4 ± 1.0 | 7.7 ± 1.3 | 11.4 ± 1.3 | 9.1 ± 1.5 | 12.0 ± 1.5 | ||
| Open-field | N | 20 | 20 | 10 | 10 | 10 | 10 | |
| Horizontal Activity |
891.3 ± 71.2 | 878.1 ± 71.2 | 1022.5 ± 101.4 | 952.3 ± 101.4 | 939.6 ± 101.4 | 967.1 ± 101.4 | ||
| Central distance (cm) |
109.2 ± 14.5 | 106.7 ± 14.5 | 128.8 ± 20.5 | 103.3 ± 20.5 | 125.6 ± 20.5 | 116.1 ± 20.5 | ||
| Peripheral distance (cm) |
316.5 ± 34.6 | 320.7 ± 34.6 | 339.3 ± 49.0 | 363.2 ± 49.0 | 353.2 ± 49.0 | 381.0 ± 49.0 | ||
| Marble burying |
No. 2/3rd buried | 5.6 ± 1.2 | 3.8 ± 1.2 | 3.8 ± 1.7 | 7.2 ± 1.7 | 3.5 ± 1.7 | 3.6 ± 1.7 | |
| Str. Chan. | N | 21 | 21 | 10 | 10 | 10 | 10 | |
| Average latency (s) |
10.5 ± 0.6 | 10.8 ± 0.6 | 11.4 ± 0.9 | 9.1 ± 0.9 | 12.1 ± 0.9 | 9.6 ± 0.9 | ||
| CWM | N | 20 | 20 | 9 | 9 | 10 | 10 | |
| Errors | 41.7 ± 3.1 | 44.4 ± 3.1 | 49.1 ± 4.6 | 38.0 ± 4.6 | 43.7 ± 4.4 | 43.8 ± 4.4 | ||
| MWM-MTS | N | 17 | 18 | 10 | 10 | 7 | 7 | |
| T1-T2 (s) | 34.2 ± 2.5 | 31.8 ± 2.5 | 30.9 ± 3.4 | 27.8 ± 3.4 | 39.9 ± 3.6 | 33.6 ± 3.6 | ||
| D | Str. Chan. | N | 19 | 19 | 9 | 10 | 10 | 10 |
| Average latency (s) |
11.7 ± 0.7 | 10.8 ± 0.7 | 11.1 ± 1.0 | 10.4 ± 1.0 | 10.1 ± 1.0 | 10.0 ± 1.0 | ||
| MWM | N | 19 | 19 | 9 | 10 | 10 | 10 | |
| Acq | Path length (m) | 10.5 ± 0.8 | 11.5 ± 0.8 | 9.4 ± 1.1 | 10.0 ± 1.1 | 11.8 ± 1.1 | 14.2 ± 1.1 | |
| Acq-Probe | Avg. dist. (m) | 0.57 ± 0.04 | 0.67 ± 0.04 | 0.62 ± 0.05 | 0.66 ± 0.05 | 0.78 ± 0.05 | 0.78 ± 0.05 | |
| Rev | Path length (m) | 9.1 ± 0.7 | 9.4 ± 0.7 | 8.2 ± 1.0 | 9.8 ± 0.9 | 9.2 ± 0.9 | 10.9 ± 0.9 | |
| Rev-Probe | Avg. dist. (m) | 0.68 ± 0.04 | 0.77 ± 0.04 | 0.65 ± 0.05 | 0.75 ± 0.05 | 0.62 ± 0.05 | 0.75 ± 0.05 | |
| Shift | Path length (m) | 6.6 ± 0.7 | 8.6 ± 0.7 | 7.0 ± 1.0 | 7.6 ± 1.0 | 7.6 ± 1.0 | 8.4 ± 1.0 | |
| Shift-probe | Avg. dist. (m) | 0.61 ± 0.04 | 0.79 ± 0.04 | 0.58 ± 0.05 | 0.76 ± 0.05 | 0.69 ± 0.05 | 0.78 ± 0.05 | |
| Cued lat (s) | 13.2 ± 1.8 | 18.2 ± 1.8 | 13.9 ± 2.6 | 18.8 ± 2.4 | 14.5 ± 2.5 | 17.9 ± 2.5 | ||
Average group performance (mean ± SEM)
P<0.05 compared with Saline within the same sex.
Significant outcomes
Significant and nearly significant effects were obtained on the following tests: ASR/PPI, Light-Dark test, conditioned fear, and open-field locomotor activity after (+)-amphetamine and MK-801 challenge.
ASR/PPI
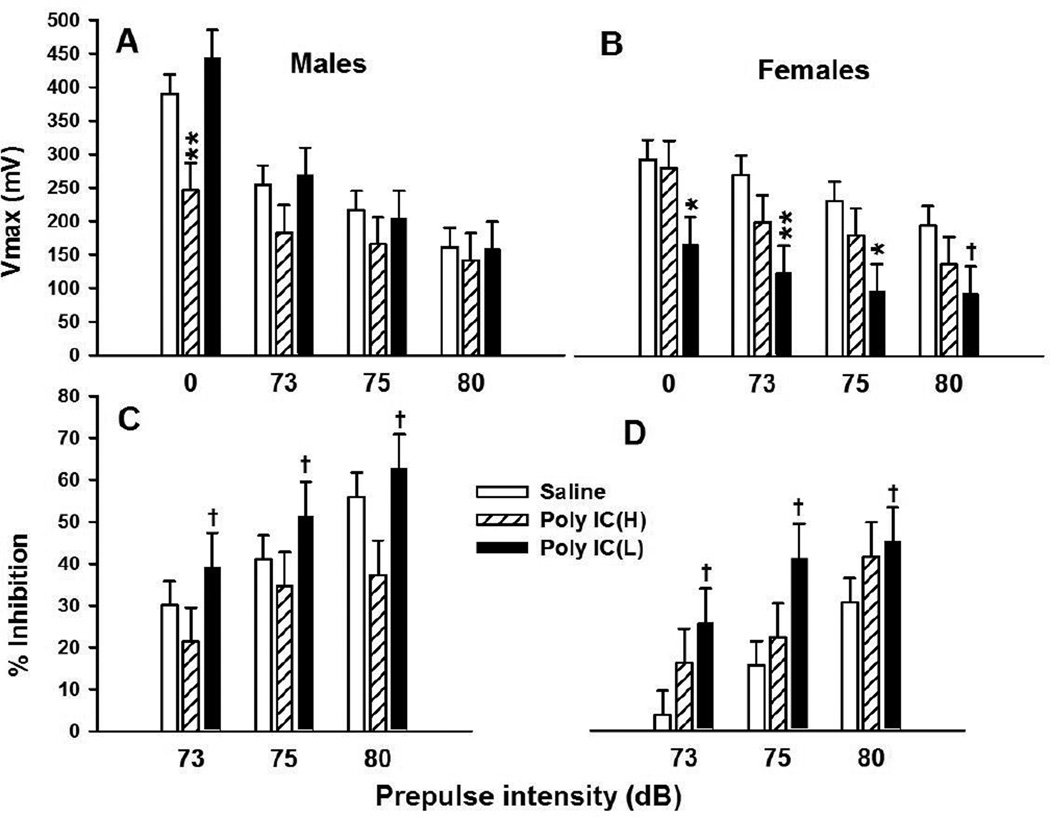
Peak startle amplitude (Vmax) data were analyzed by treatment x sex x PP ANOVA. There was no significant main effect of treatment or sex; the typical main effect of PP (p<0.0001) was obtained in which prepulses inhibited peak startle amplitude. There were no significant interactions between treatment and PP but the treatment x sex (F(2, 37) = 3.58, p<0.04) and treatment x sex x PP (F(6,222) = 3.42, p<0.01) interactions were significant. These are illustrated in Fig. 1 (A,B). Slice-effect ANOVAs showed the effects to be in the male PP-0 and female PP-0, PP-73, and PP75 conditions. Pairwise comparisons within each slice showed that for the PP-0 condition, the male Poly IC (H) group had reduced Vmax compared with Saline controls. Among females, the Poly IC (L) group showed reduced Vmax both when there was no prepulse (PP-0) and at the lower two prepulse intensities and a trend at the highest prepulse. The Vavg data were analyzed the same way with a similar outcome, i.e., the treatment x sex x PP interaction was significant (F(6,222) = 2.93, p<0.01). Slice-effect ANOVAs showed the same effect in males for Vmax, i.e., that the male Poly IC (H) group showed a significant reduction in unmodified (PP-0) ASR; however, the effect in females seen with Vmax was not significant for Vavg.
Fig. 1. Acoustic startle response/PPI.
Peak response amplitude expressed in mV of change during the 100 ms recording window averaged for all trials of the same type. Panel A, Peak startle amplitude in males; panel B, Peak startle amplitude in females; panel C, percent PPI in males; panel D, percent PPI in females. Data are mean ± SEM. Groups sizes: Total (M/F): Saline = 40 (20/20); Poly IC (H) = 20 (10/10); Poly IC (L) = 20 (10/10). †P<0.10; *P<0.05, **P<0.01 vs. Saline.
In order to determine if these effects represented a differential PPI effect, percent inhibition was calculated. ANOVA showed a treatment main effect trend (F(2,37) = 2.5, p<0.10). There were no significant interactions. Even though the percent inhibition analysis was a trend, Hochberg pairwise comparisons showed that the Poly IC (L) group had a trend toward reduced prepulse inhibition compared with Saline controls (p<0.09) whereas the Poly IC (H) group did not differ from Saline, regardless of sex (Fig. 1, C,D).
Light-Dark test
Two dependent measures were analyzed: (1) number of light-side entries and (2) time on the dark side. For number of light-side entries, the main effect of treatment showed a trend (F(2/33) = 2.71, p<0.09 (mean ± SEM: Saline = 32.8 ± 2.1; Poly IC (H) = 37.3 ± 3.0; Poly IC (L) = 27.6 ± 2.8). Neither sex nor the treatment x sex interaction was significant. For time spent on the dark side there was a significant main effect of treatment (F(2/33) = 3.41, p<0.05). Neither sex nor the treatment x sex interaction was significant. A posteriori Hochberg comparisons showed that both Poly IC groups spent less time on the dark side (both p<0.06) compared with the Saline group. Group means ± SEM (s) were: Saline = 359.4 ± 22.0; Poly IC (H) = 272.1 ± 31.1; Poly IC (L) = 287.1 ± 29.7.
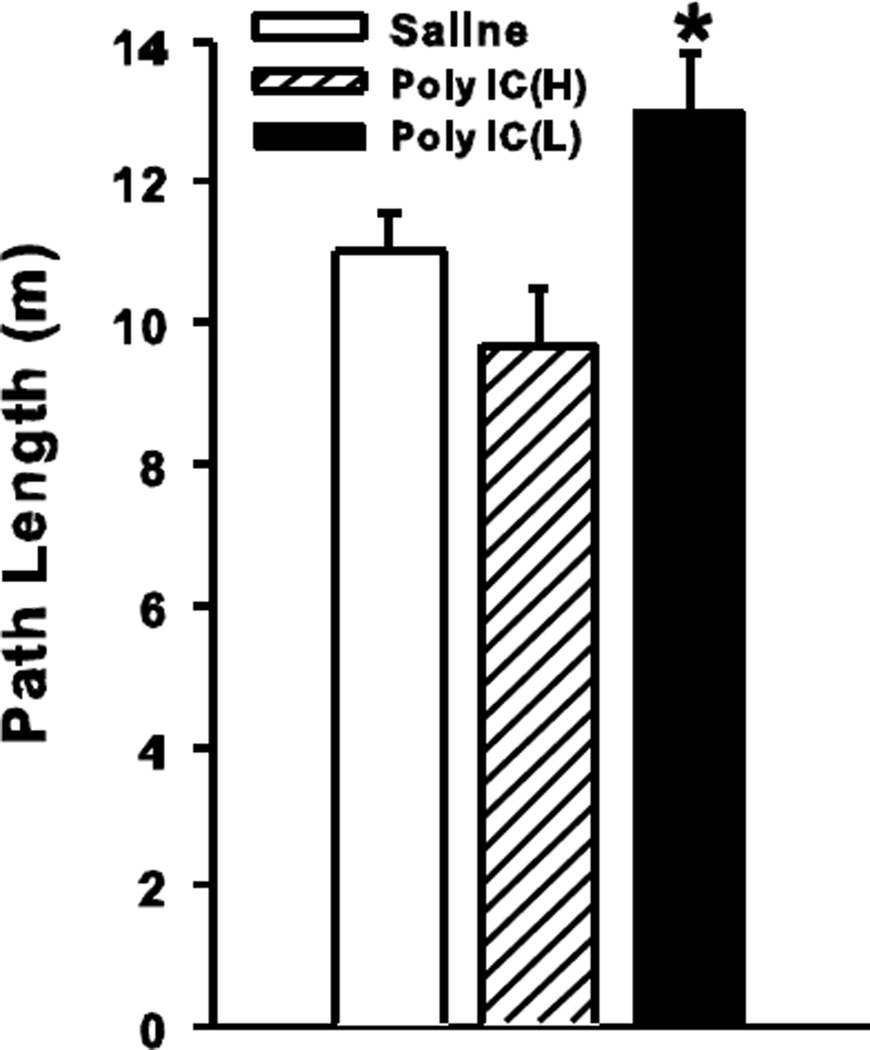
MWM
Five measures were analyzed for each phase of the test on hidden platform trials: latency, path length, cumulative distance to the target, average heading error, and swim speed. The first four reflect learning and the last performance. All learning indices showed similar patterns, therefore, path length is presented. During acquisition, there was a significant main effect of treatment (F(2/71.1) = 4.75, p<0.02). Day was also significant (p<0.0001). Sex, treatment x sex, treatment x day, and treatment x sex x day were not significant. Since there was no interaction with day, mean path length averaged across days is shown in Fig. 2. A posteriori pairwise comparisons between Saline and each Poly IC group showed longer paths to the goal in the Poly IC (L) group (p<0.05) but not in the Poly IC (H) group. Analysis of swim speed on acquisition revealed a significant main effect of treatment (F(2/71.9) = 6.53, p<0.01). Mean ± SEM swim speeds (m/s) during acquisition were: Saline = 0.29 ± 0.003; Poly IC (H) = 0.29 ± 0.005; Poly IC (L) = 0.27 ± 0.004). A posteriori comparisons between Saline and each Poly IC group showed that the Poly IC (L) group swam slightly slower than Saline controls (p<0.01), however, the difference was not sufficient to account for the path length differences in this group noted above. None of the interactions were significant. During reversal and shift phases, there were no significant effects of treatment.
Fig. 2.
Morris water maze acquisition phase: Means (± SEM) path length (m) to find the hidden platform averaged across 6 days of testing with 4 trials/day. Group sizes are as in Fig. 1. *P<0.05 vs. Saline.
A probe trial was given 24 h after the last platform trial of each phase. During a probe trial the platform was removed and the rat started from a position not used previously and allowed to search for 30 s. Indices analyzed were platform site crossovers, average distance to the platform site, percent time and percent distance in the target quadrant, average heading error, and swim speed. On the acquisition probe trial, there were no significant treatment or treatment-related interactions on crossovers or average heading error. There were treatment main effect trends on average distance to the platform site and percent time and distance in the target quadrant. For average distance, the effect approached significance (F(2,34) = 3.07, p<0.06); treatment x sex was not significant. Pairwise comparisons showed that the Poly IC (L) group had longer distances to the platform site than Saline controls (p<0.02): Mean ± SE (cm) were: Saline = 26.7 ± 0.7; Poly IC (H) = 28.6 ± 1.0; Poly IC (L) = 30.5 ± 1.0* (*P<0.05 Saline vs. Poly IC (L). The trends on percent time and percent distance in the target quadrant were similar. In addition, there was a significant main effect on swim speed (F(2,34) = 5.0, p<0.02) but there were no significant interactions. Pairwise comparisons showed that the Poly IC (L) group differed from Saline controls (p<0.01): Mean ± SE (cm/s) for the groups were: Saline = 26.7 ± 0.7; Poly IC (H) = 28.6 ± 1.0; Poly IC (L) = 30.5 ± 1.0** (**P<0.01 Saline vs. Poly IC (L). Hence, the Poly IC (L) group swam faster but was farther from the platform site than Controls indicating a dissociation between swim speed and accuracy at finding the platform. No significant treatment effects were obtained during reversal or shift probe trials.
Conditioned Fear/Latent Inhibition
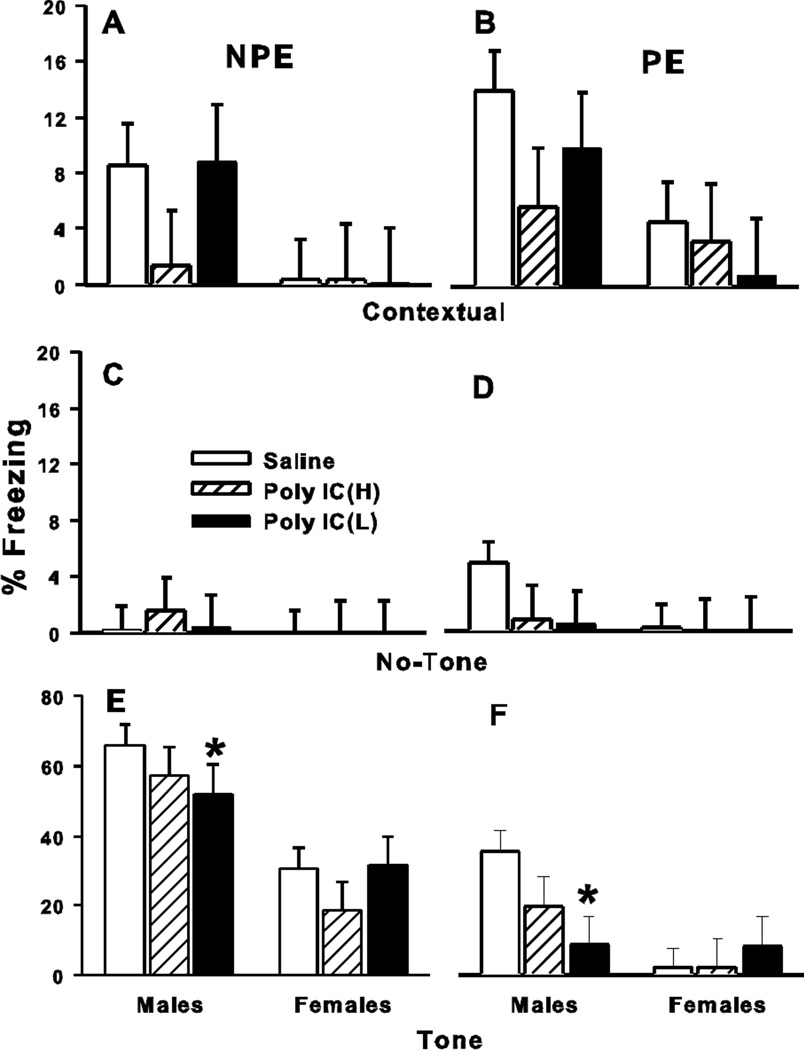
Groups were subdivided into those with tone pre-exposure prior to tone-shock pairing (PE) and those not pre-exposed (NPE) to the tone prior to tone-shock pairing (Day-1). On Day-2, contextual fear was assessed and on Day-3 cued fear. The difference in freezing time between Day-2 and Day-3 between the PE and NPE subgroups was used as an index of latent inhibition. The ANOVA included factors of treatment, condition (PE vs. NPE), and sex. On Day-2, contextual freezing (Fig. 3, panel A & B), there were no significant effects of treatment or condition, but there was an effect of sex (p<0.01); males showed more freezing than females. There were no significant interactions. For cued freezing on Day-3, there was no significant main effect of treatment; however, condition (p<0.0001) and sex (p<0.0001) were significant; rats in the NPE condition showed more freezing than those in the PE condition and males showed more freezing than females. Fig. 3C and D show that there were no significant group differences prior to tone onset on Day 3. There was a significant treatment x sex interaction (F(2,109) = 3.13, p<0.05). Slice effects for treatment on each sex showed a significant effect in males (p<0.05) but not females. Pairwise comparisons, showed that the male Poly IC (L) group differed from male Saline controls (Fig. 3, panel E & F); this effect occurred regardless of condition (p<0.02). There were no latent inhibition effects of treatment given that there was no interaction between treatment and condition (NPE vs. PE).
Fig. 3. Conditioned Fear/Latent Inhibition.
One male/female pair per litter received fear conditioning with no exposure to the tone prior to tone-shock pairing (NPE) and a second male/female pair per litter received fear conditioning with tone exposure prior to tone-shock pairing (PE). Day-1: training; day-2: test of contextual freezing; day-3 test of cued freezing. Panels: A, day-2, contextual freezing in NPE subgroup; B, day-2 contextual freezing in PE subgroup; C, day-3 freezing prior to tone onset in NPE subgroup; D, day-3 freezing prior to tone onset in PE subgroup; E, day-3 cued freezing after tone onset in NPE subgroup; F, day-3 cued freezing after tone onset in PE subgroup. Data are mean ± SEM. Groups sizes: Total (M/F): Saline = 79 (39/40); Poly IC (H) = 39 (19/20); Poly IC (L) = 40 (20/20) where half of each group of each sex were in the NPE subgroup and half in the PE subgroup. *P<0.05 vs. Saline for the combined NPE-PE Poly IC (L) group males vs. the combined NPE-PE Saline group males.
Open-field with drug challenge
Offspring were evenly divided among tests and then further divided for drug challenge. The second order subdivision, however, did not result in balanced subgroups since the assignment to subgroups was done randomly. Therefore, we analyzed the data for the drug challenges two ways: With and without the subdivision by maternal weight gain.
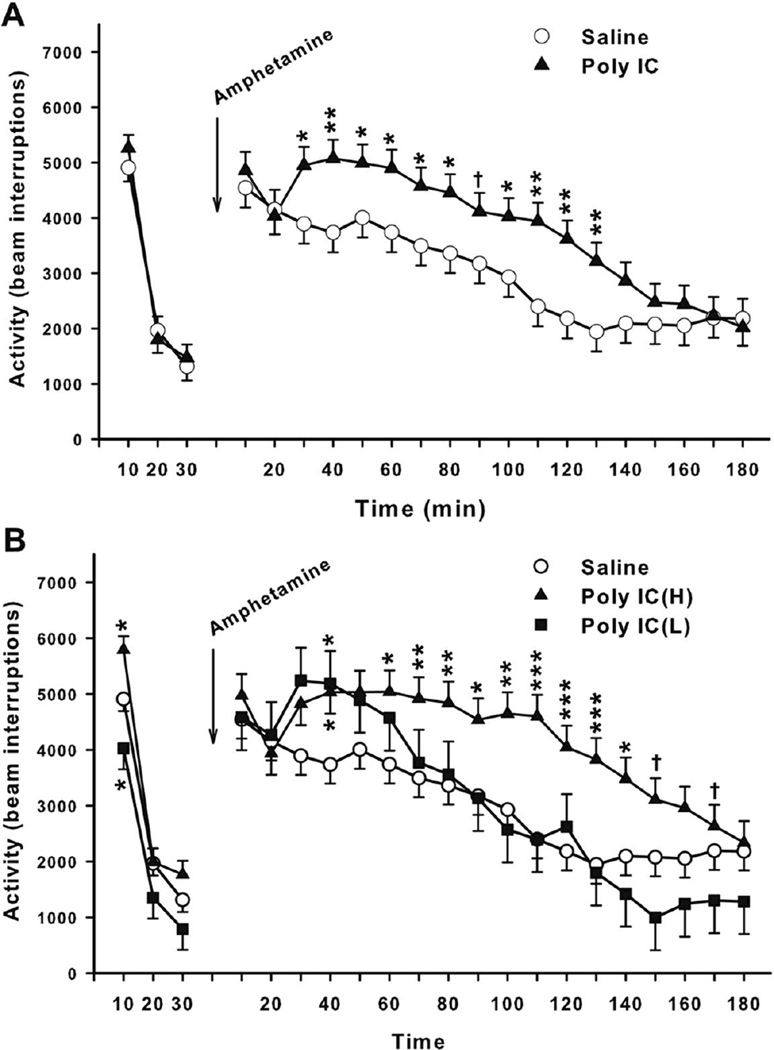
The results after amphetamine challenge in the Poly IC groups combined are shown in Fig. 4. Prior to drug administration, there was no significant main effect of treatment or treatment-related interaction.
Fig. 4. Open-field after amphetamine.
A, Overall locomotor activity before and after (+)-amphetamine: Total number of photobeam interruptions (mean ± SEM) per 10 min interval for 30 min prior to drug treatment and 180 min post-treatment with 1.0 mg/kg (+)-amphetamine given s.c. Group sizes: Total (M/F): Saline = 35 (18/17); Poly IC = 40 (20/20). B, Locomotor activity after amphetamine by subgroup. Groups sizes: Total (M/F): Saline = 35 (18/17); Poly IC (H) = 28 (14/14); Poly IC (L) = 12 (6/6). †P<0.10; *P<0.05; **P<0.01; ***P<0.001 vs. Saline.
Post-drug, there were treatment (F(2,18.6) = 4.75, p<0.05) and treatment x interval effects (F(17,1172) = 2.58, p<0.001). Slice effect ANOVAs on each interval showed significant effects on slices from 30–130 min (Fig. 4A). At these intervals the merged Poly IC group showed exaggerated hyperactivity in response to amphetamine compared with that shown by Saline controls. When these data were analyzed by the maternal weight gain subgroups, the results are as shown in Fig. 4B. In this 3-group analysis there was a significant main effect of treatment (F(2,17.5) = 5.00, p<0.02) and a significant treatment x interval interaction (F(34,1156) = 2.02, p<0.001). As can be seen Poly IC-treated offspring over-responded to amphetamine and most of the over-response was driven by the Poly IC (H) group while the Poly IC (L) group significantly over-responded only at the beginning of the test. Later, the Poly IC (L) group returned to baseline faster than Saline controls but this difference was not significant.
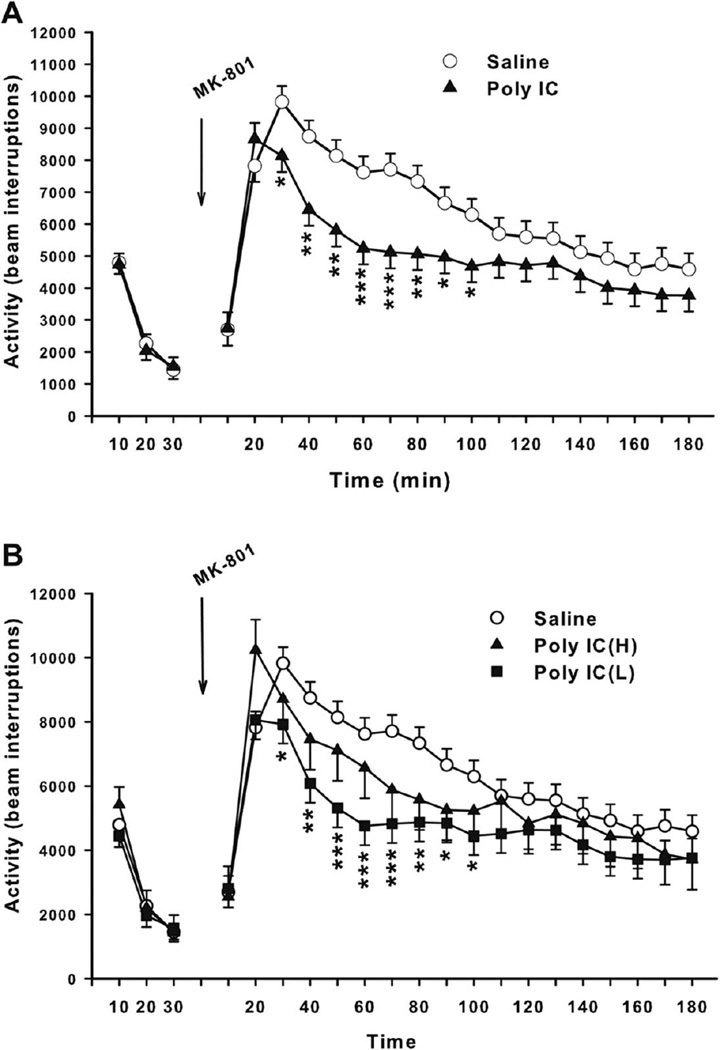
There was no significant treatment main effect or treatment x interval or sex interactions during pre-drug test period among animals in the subgroups used for MK-801 challenge. Post MK-801, there was a significant treatment main effect (F(1,19) = 5.41, p<0.05) and a significant treatment x interval effect (F(17,1240) = 3.54, p<0.0001) when the Poly IC subgroups were merged. Slice effect ANOVAs for interval showed significant effects from 30–100 min. As can be seen (Fig. 5A), the Poly IC group showed significantly attenuated hyperactivity compared with Saline controls in response to the activating effect of MK-801. When these data were analyzed by the maternal weight gain subgroups, the basic pattern remained in that Poly IC offspring under-responded to MK-801, but the difference is that in this case the Poly IC (L) group drive most of the difference even though both Poly IC groups changed in the same direction, i.e., under-responded to MK-801 (Fig. 5B). Specifically, in the 3-group analysis the main effect of treatment was only a trend (F(2,18) = 3.07, p<0.07) whereas the treatment x interval interaction was significant (F(34,1138) = 2.53, p<0.0001). Slice-effect ANOVAs on each interval showed treatment effects on intervals 30–80 min and nearly significant effect on intervals 20, 90, and 100 min. A posteriori pairwise comparisons among groups at these intervals showed all to be attributable to the significantly lower response to MK-801 of the Poly IC (L) group.
Fig. 5. Open-field after MK-801.
A, Overall locomotor activity before and after MK-801: Total number of photobeam interruptions (mean ± SEM) per 10 min interval for 30 min prior to drug treatment and 180 min post-treatment with 0.2 mg/kg s.c. MK-801. Groups sizes: Total (M/F): Saline = 40 (20/20); Poly IC (H) = 39 (20/19). B, Locomotor activity before and after MK-801 by subgroup. Groups sizes: Total (M/F): Saline = 40 (20/20); Poly IC (H) = 11 (6/5); Poly IC (L) = 28 (14/14). *P<0.05; **P<0.01;***P<0.001 vs. Saline.
Follow-up analyses
In order to evaluate the extent to which maternal body weight is correlated with Poly IC effects found on acoustic startle and locomotor activity with drug challenge, these data were reanalyzed by analysis of covariance (ANCOVA). If a covariate changes what an ANOVA shows, it indicates that the covariate is related to the outcome of interest. For acoustic startle, the ANOVA showed treatment x sex (p<0.04) and treatment x sex x prepulse (p<0.003) effects. The ANCOVA F-ratios for these same factors were not significant, i.e., treatment x sex (P>0.10) and treatment x sex x prepulse (p>0.6). In the case of locomotor activity with amphetamine challenge, the ANOVA showed significant effects of treatment (p<0.02) and treatment x interval (p<0.001) and the ANCOVA showed that both factors remained significant but with a slightly lower p-value for treatment (p<0.04) and similar p-value for treatment x interval (p<0.001). In the case of locomotor activity with MK-801 challenge, the ANOVA showed a significant treatment x interval interaction (p<0.0001) and the ANCOVA was similar (p<0.0001). Hence, maternal body weight did account for much of the Poly IC-associated variance on acoustic startle but very little variance for locomotor activity with amphetamine or MK-801 drug challenge.
Discussion
There is growing evidence that maternal immune activation during gestation with the viral mimic Poly IC or the bacterial mimic lipopolysaccharide (LPS) induces transplacental adverse effects on offspring brain development and behavior. The effects are only partially consistent with those associated with schizophrenia A number of studies have examined the effects of prenatal Poly IC in rats on offspring behavior (Dickerson et al., 2010;Fortier et al., 2007;Gilmore et al., 2005; Howland et al., 2012; Ito et al., 2010;Klein et al., 2013;Mattei et al., 2014;Oh-Nishi et al., 2010;Sangha et al., 2014;Wolff and Bilkey, 2008;Wolff and Bilkey, 2010;Zhang et al., 2012;Zuckerman et al., 2003a;Zuckerman and Weiner, 2003;Zuckerman and Weiner, 2005). We previously evaluated E14 i.p. treatment with Poly IC (8 mg/kg) in rats and showed changes in locomotor behavior in response to drug challenges (Bronson et al., 2011;Richtand et al., 2011;Vorhees et al., 2012). One of the characteristics seen in these experiments was that the response of offspring to dopaminergic and glutamatergic drug challenges was influenced by the maternal response to Poly IC reflected by maternal body weight changes following treatment. Offspring from dams that showed weight loss or small increases after treatment showed different responses compared with offspring from dams that gained weight similarly to controls. In the first of these experiments (Bronson et al., 2011), maternal starting weight predicted weight change in response to Poly IC which in turn predicted responses in the progeny to drug challenge. However, another group found that maternal weight change after prenatal Poly IC was not associated with differences in acoustic startle amplitude or prepulse inhibition (Wolff and Bilkey, 2010) . We tested the relationship to a one day (E14) Poly IC treatment and found no effects on baseline ASR or PPI but found altered activity in the open-field to drug challenge (Vorhees et al., 2012). In the present experiment with longer Poly IC exposure, spanning more of in utero brain development, we found baseline amplitude and PPI effects and replicated open-field responses to pharmacological challenge. In addition, in the previous study the Poly IC (H) group showed increased errors in the CWM and reduced ASR in females whereas in the present study, the Poly IC (H) group offspring showed no changes in CWM together with reduced unmodified ASR in males (and increased PPI), the latter also occurring in females. There was also a trend toward reduced time in the dark in the light-dark test (both sexes). Further analyses of the ASR data by ANCOVA using maternal body weight of dams following Poly IC treatment as the covariate, showed that the significant effects of Poly IC on startle were no longer significant by ANCOVA, indicating that acoustic startle changes in the offspring associated with Poly IC treatment were related to the degree of maternal immune activation caused by Poly IC.
In our previous study, the Poly IC (L) group offspring showed greater locomotor hyperactivity (without stereotypy) in response to (+)-amphetamine and in the present experiment this was again found in the merged Poly IC group but when sorted the effect was predominantly in the Poly IC (H) group. Also consistent was that the Poly IC (L) group previously and both the merged and Poly IC (L) group here showed attenuated hyperactivity in response to the NMDA receptor antagonist MK-801. Effects in the present study that differed from the previous one were impaired MWM acquisition and probe trial performance here but not previously, reduced cued fear conditioning here in males but not previously observed, and a trend toward reduced time in the dark in the light-dark test here but not previously. Overall, however, the similarities across our two experiments were more striking than the differences, especially for the drug challenge findings; however, one factor that should be kept in mind is that the drug challenges were done after the animals had gone through conditioned fear with foot shock and this may have affected how the animals responded to the drugs. When the drug challenge data were further analyzed by ANCOVA, the changes in locomotor patterns attributable to Poly IC remained, indicating that despite slightly different patterns in the two Poly IC groups, the outcomes were strongly associated with Poly IC and not to the maternal weight gain changes induced by Poly IC. Hence, Poly IC has a large effect on the CNS response to dopaminergic agonistic and NMDA receptor antagonistic effects that are independent of the acute effects of maternal immune response to Poly IC as reflected by changes in maternal body weight during pregnancy.
Possible reasons for the differences between studies include (1) more treatment days that increased the exposure period to maternal immune activation (Ashdown et al., 2006;Boksa, 2010;Deverman and Patterson, 2009;Meyer et al., 2005;Meyer et al., 2009b;Patterson, 2002;Patterson, 2009;Smith et al., 2007); (2) maternal immune response on gestational days where no exposure occurred in the previous study (Bitanihirwe et al., 2010a;Meyer et al., 2006b;Meyer et al., 2007); or (3) the effect of repeated injections to the dams here compared with only one injection previously. Because we held all other factors as constant as possible, the most likely cause of the differences was the exposure period. Remarkably, however, both studies showed the same attenuated locomotor response to MK-801 and the same exaggerated response to amphetamine. Since MK-801 is an NMDA-R antagonist, this implies that this receptor-mediated pathway had reduced function after prenatal Poly IC exposure, a finding consistent with the hypothesis concerning the pathophysiology of schizophrenia being, in part, caused by glutamatergic hypofunction (Moghaddam and Javitt, 2012). Other prenatal immune activation models have also found changes in glutamatergic-mediated behavioral responses or changes to glutamatergic markers in brain (see (Boksa, 2010)). Similarly, the exaggerated locomotor response to amphetamine in the Poly IC offspring is consistent with the related hypothesis of schizophrenia pathophysiology that it is partly attributable to dopaminergic hyperfunction.
The changes in PPI differed between experiments, perhaps because of differences in exposure period but we note that in both studies the startle changes were in the same direction, i.e., reductions in startle amplitude. Changes in PPI have been reported after prenatal Poly IC exposure (see (Boksa, 2010) (Klein et al., 2013;Mattei et al., 2014). PPI has been used as a marker of CNS dysfunction associated with psychotic disorders (Geyer et al., 1990;Geyer et al., 1993;Geyer et al., 2001;Geyer, 2006;Swerdlow et al., 2006;Swerdlow and Geyer, 1993), which lends credence to the connection between pregnancy infection and transplacental injury in the progeny in association with neuropsychiatric outcome. We note, however, that in the present study we found increased percent PPI in the Poly IC (L) group versus the more often reported reduced PPI (Boksa, 2010;Klein et al., 2013;Mattei et al., 2014). It is noteworthy that percent PPI is a ratio and is therefore sensitive to changes to the denominator, i.e., the amplitude of the unmodified startle response. Since baseline startle was significantly reduced in the Poly IC (L) group and the prepulses produced less change than they did in controls the net effect was increased percent PPI which makes the effect appear different than reported by others. This is a limitation of using percent inhibition whenever a treatment affects the baseline against which inhibition is calculated. The more important point is that Poly IC significantly reduced the basic startle reflex. Curiously, most reports of Poly IC-induced reduced PPI do not mention whether baseline startle was or was not affected making the inhibition effect difficult to interpret.
We tested learning and memory since cognitive deficits are part of the schizophrenia endophenotype. We used the MWM to test two types of memory. In the MWM-MTS procedure, we tested trial-dependent learning using new platform and start positions every day and found no differences in working memory. We tested reference memory in the fixed platform MWM procedure and found an effect on acquisition in the Poly IC (L) group but no differences on reversal or shift phases. However, the acquisition effect on path length, cumulative distance, and latency was accompanied by slower swim speeds, making it unclear whether the Poly IC effect was influenced by reduced swim speed; this seems unlikely, however, since path length is largely unaffected by speed, unlike latency. We tested reference memory on probe trials and found a trend on the acquisition probe trial in which the Poly IC group performed below Saline controls; this effect was not accompanied by slower, but rather by faster swim speed, making it difficult to see how swim speed could account for the learning and memory differences given that the swim speed differences were in the opposite direction in the Poly IC group during acquisition vs. probe. No differences were found on reversal or shift learning or memory trials. Two other prenatal immune activation studies in rats also used the MWM (Samuelsson et al., 2006;Zuckerman and Weiner, 2005). In these experiments, no effects of Poly IC were found on acquisition or probe. However, during reversal trials, Poly IC offspring were reported to have shorter latencies to find the platform on the first block of reversal trials (2 trials/block) but not during the second block of reversal trials (Zuckerman and Weiner, 2005). Zuckerman and Weiner (2005) gave Poly IC intravenously and this may be an important difference. Another study administered 9 µg/kg IL-6 rather than Poly IC on E7, 9, and 11, or E15, 17, and 19 to rats and found increased MWM latencies to find the goal (Samuelsson et al., 2006), consistent with the present data.
We tested egocentric learning in the CWM; a small effect was found in our previous experiment but not here. Again, this could be because of the difference in exposure length but this requires further study. We also tested conditioned fear and found effects with the E14–18 exposure but not with the E14 exposure. The E14–18 effects were on cued, not contextual, conditioning, suggesting amygdala-related, rather than hippocampally-related effects. Changes in conditioned fear using latent inhibition paradigms have been found (Meyer et al., 2006a;Meyer et al., 2006c;Piontkewitz et al., 2011;Smith et al., 2007;Zuckerman et al., 2003a;Zuckerman and Weiner, 2003;Zuckerman and Weiner, 2005). In the present experiment, we found no interaction between treatment and NPE vs. PE conditions as a reflection of latent inhibition. The data from other studies using mice and rats were after different ages of exposure to Poly IC. Of those studies finding latent inhibition effects, some them to be sex- and/or age-dependent. One study found deficits in males only at certain ages (P70 and P90), and in females at P90 and no effects in males or females at P35, P45, or P56 (Piontkewitz et al., 2011). In addition, these experiments did not use fear conditioning but other procedures for inducing latent inhibition. One used a drinking suppression method after tone-shock pairing (Piontkewitz et al., 2011;Zuckerman et al., 2003a;Zuckerman and Weiner, 2003;Zuckerman and Weiner, 2005) versus those in which the CR was freezing after tone-shock pairing (Meyer et al., 2006a;Meyer et al., 2006c;Smith et al., 2007).
Limitations of the present experiment include: (1) No fostering-crossfostering to determine if the Poly IC effects were the result of changes to maternal behavior; (2) no assessment of cytokine release; (3) no assessments of brain neurochemical markers; (4) Poly IC was administered intraperitoneally whereas a number of recent studies give it intravenously, a route that may be more efficacious; (5) limited statistical power: we assigned 20 litters to each group, but because we subdivided the Poly IC group it may have under-powered the study; (6) we tested only a moderate dose of Poly IC (8 mg/kg), whereas others have given up to 20 mg/kg (Smith et al., 2007). (7) The direction of the changes we observed were, in some cases, not in the same as those reported in patients with schizophrenia. Despite such limitations, it is apparent that Poly IC-induced maternal immune activation has significant adverse effects on offspring behavior. Hence, the data suggest that maternal immune activation may be a “first hit” to fetal brain development that sets the stage for later effects when triggered by other adverse events such as stress, as recently shown to be one such enhancing factor (Giovanoli et al., 2013).
Supplementary Material
Highlights.
Maternal immune activation (MIA) is associated with psychiatric disorders in children
We gave Poly IC to pregnant rats to induce MIA on embryonic days 14–18
Dams were divided by maternal weight gain in response to Poly IC
Offspring from Poly IC lower weight gain dams were the most affected
MIA reduced learning, PPI, and MK-801 activity but increased activity to amphetamine
Acknowledgment
The authors thank Mary Moran for data analyses. This research was supported by NIH research grants DA021394 (CVV), ES015689 (MTW), MH083192 (NMR), and T32 ES007051 predoctoral (AAB) and postdoctoral (DLG) NRSA support, and by the Department of Veterans Affairs Medical Research Service.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions Statement
C.V., M.W., and N.R. designed the study; C.V. and M.W. supervised its conduct, determined what data analyses were to be performed, interpreted the findings, wrote, and edited the manuscript with further input by N.R. A.B., T.S., D.G., and M.S. along with technicians bred and treated the rats and did the behavioral testing. All authors reviewed the manuscript.
Competing Financial Interest Statement
There is a potential Competing Interest for one author as follows: Neil M. Richtand discloses the following: Consultant to Bristol-Meyers Squibb, Gerson Lehrman Group, Sunovion Pharmaceuticals Inc./Sepracor. Speaker’s Bureau: Bristol-Meyers Squibb, Otsuka America Pharmaceutical, Schering-Plough Corporation/Merck, Novartis Pharmaceuticals, Sunovion Pharmaceuticals Inc./Sepracor, and Grant/Research Support: Ortho-McNeil Janssen Scientific Affairs, LLC; AstraZeneca Pharmaceuticals.
All other authors report: None (i.e., no competing financial interest).
References
- Ashdown H, Dumont Y, Ng M, Poole S, Boksa P, Luheshi GN. The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol Psychiatry. 2006;11:47–55. doi: 10.1038/sj.mp.4001748. [DOI] [PubMed] [Google Scholar]
- Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40:1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- Bitanihirwe BK, Peleg-Raibstein D, Mouttet F, Feldon J, Meyer U. Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology. 2010a;35:2462–2478. doi: 10.1038/npp.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BK, Weber L, Feldon J, Meyer U. Cognitive impairment following prenatal immune challenge in mice correlates with prefrontal cortical AKT1 deficiency. Int J Neuropsychopharmacol. 2010b;13:981–996. doi: 10.1017/S1461145710000192. [DOI] [PubMed] [Google Scholar]
- Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun. 2010;24:881–897. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Braun AA, Graham DL, Schaefer TL, Vorhees CV, Williams MT. Dorsal striatal dopamine depletion impairs both allocentric and egocentric navigation in rats. Neurobiol Learn Mem. 2012 doi: 10.1016/j.nlm.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun AA, Skelton MR, Vorhees CV, Williams MT. Comparison of the elevated plus and elevated zero mazes in treated and untreated male Sprague-Dawley rats: effects of anxiolytic and anxiogenic agents. Pharmacol Biochem Behav. 2011;97:406–415. doi: 10.1016/j.pbb.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson SL, Ahlbrand R, Horn PS, Kern JR, Richtand NM. Individual differences in maternal response to immune challenge predict offspring behavior: contribution of environmental factors. Behav Brain Res. 2011;220:55–64. doi: 10.1016/j.bbr.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson SL, Richtand NM. In: Handbook of Schizophrenia Spectrum Disorders. Vol. 1. New York: Springer Science-Business Media B.V; 2011. Developmental consequences of prenatal maternal immune activation; pp. 1–25. [Google Scholar]
- Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32:200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, Susser ES. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Susser ES. In utero infection and adult schizophrenia. Ment Retard Dev Disabil Res Rev. 2002;8:51–57. doi: 10.1002/mrdd.10004. [DOI] [PubMed] [Google Scholar]
- Ciaranello AL, Ciaranello RD. The neurobiology of infantile autism. Annu Rev Neurosci. 1995;18:101–128. doi: 10.1146/annurev.ne.18.030195.000533. [DOI] [PubMed] [Google Scholar]
- Clarke MC, Tanskanen A, Huttunen M, Whittaker JC, Cannon M. Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am J Psychiatry. 2009;166:1025–1030. doi: 10.1176/appi.ajp.2009.08010031. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Campion S, Teeling J, Felton L, Perry VH. The sickness behaviour and CNS inflammatory mediator profile induced by systemic challenge of mice with synthetic double-stranded RNA (poly I:C) Brain Behav Immun. 2007;21:490–502. doi: 10.1016/j.bbi.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Dickerson DD, Wolff AR, Bilkey DK. Abnormal long-range neural synchrony in a maternal immune activation animal model of schizophrenia. J Neurosci. 2010;30:12424–12431. doi: 10.1523/JNEUROSCI.3046-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Earle J, Kanodia R, Kist D, Emamian ES, Patterson PH, Shi L, Sidwell R. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol Neurobiol. 2002;22:25–33. doi: 10.1023/A:1015337611258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Emamian ES, Kist D, Sidwell RW, Nakajima K, Akhter P, Shier A, Sheikh S, Bailey K. Defective corticogenesis and reduction in Reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Mol Psychiatry. 1999;4:145–154. doi: 10.1038/sj.mp.4000520. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Pearce DA, Brooks AI, Sidwell RW. Prenatal viral infection in mouse causes differential expression of genes in brains of mouse progeny: a potential animal model for schizophrenia and autism. Synapse. 2005;57:91–99. doi: 10.1002/syn.20162. [DOI] [PubMed] [Google Scholar]
- Fortier ME, Kent S, Ashdown H, Poole S, Boksa P, Luheshi GN. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2004;287:R759–R766. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- Fortier ME, Luheshi GN, Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav Brain Res. 2007;181:270–277. doi: 10.1016/j.bbr.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Geyer MA. The family of sensorimotor gating disorders: comorbidities or diagnostic overlaps? Neurotox Res. 2006;10:211–220. doi: 10.1007/BF03033358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Swerdlow NR, Mansbach RS, Braff DL. Startle response models of sensorimotor gating and habituation deficits in schizophrenia. Brain Res Bull. 1990;25:485–498. doi: 10.1016/0361-9230(90)90241-q. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Wilkinson LS, Humby T, Robbins TW. Isolation rearing of rats produces a deficit in prepulse inhibition of acoustic startle similar to that in schizophrenia. Biol Psychiat. 1993;34:361–372. doi: 10.1016/0006-3223(93)90180-l. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Jarskog LF, Vadlamudi S. Maternal poly I:C exposure during pregnancy regulates TNF alpha, BDNF, and NGF expression in neonatal brain and the maternal-fetal unit of the rat. J Neuroimmunol. 2005;159:106–112. doi: 10.1016/j.jneuroim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, Winter C, Riva MA, Mortensen PB, Feldon J, Schedlowski M, Meyer U. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013;339:1095–1099. doi: 10.1126/science.1228261. [DOI] [PubMed] [Google Scholar]
- Giovanoli S, Meyer U. Response to comment on "Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice". Science. 2013;340:811. doi: 10.1126/science.1238060. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Howland JG, Cazakoff BN, Zhang Alterned object-in-place recognition memory, prepulse inhibition, and locomotor activity in the offspring of rats exposed to a viral mimetic during pregnancy. Neuroscience. 2012;201:184–198. doi: 10.1016/j.neuroscience.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc Natl Acad Sci U S A. 2012;109:12776–12781. doi: 10.1073/pnas.1202556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun. 2011;25:604–615. doi: 10.1016/j.bbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito HT, Smith SE, Hsiao E, Patterson PH. Maternal immune activation alters nonspatial information processing in the hippocampus of the adult offspring. Brain Behav Immun. 2010;24:930–941. doi: 10.1016/j.bbi.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J, Hadar R, Gotz T, Manner A, Eberhardt C, Baldassarri J, Schmidt TT, Kupsch A, Heinz A, Morgenstern R, Schneider M, Weiner I, Winter C. Mapping brain regions in which deep brain stimulation affects schizophrenia-like behavior in two rat models of schizophrenia. Brain Stimul. 2013;6:490–499. doi: 10.1016/j.brs.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Lazic SE. Comment on "Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice". Science. 2013;340:811. doi: 10.1126/science.1237793. [DOI] [PubMed] [Google Scholar]
- Lazic SE, Essioux L. Improving basic and translational science by accounting for litter-to-litter variation in animal models. BMC Neurosci. 2013;14:37. doi: 10.1186/1471-2202-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei D, Djodari-Irani A, Hadar R, Pelz A, de Cossio LF, Goetz T, Matyash M, Kettenmann H, Winter C, Wolf SA. Minocycline rescues decrease in neurogenesis, increase in microglia cytokines and deficits in sensorimotor gating in an animal model of schizophrenia. Brain Behav Immun. 2014;38:175–184. doi: 10.1016/j.bbi.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988;45:189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Fatemi SH. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev. 2009a;33:1061–1079. doi: 10.1016/j.neubiorev.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Immunological stress at the maternal-foetal interface: a link between neurodevelopment and adult psychopathology. Brain Behav Immun. 2006a;20:378–388. doi: 10.1016/j.bbi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull. 2009b;35:959–972. doi: 10.1093/schbul/sbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006b;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Schwendener S, Feldon J, Yee BK. Prenatal and postnatal maternal contributions in the infection model of schizophrenia. Exp Brain Res. 2006c;173:243–257. doi: 10.1007/s00221-006-0419-5. [DOI] [PubMed] [Google Scholar]
- Meyer U, Yee BK, Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse? Neuroscientist. 2007;13:241–256. doi: 10.1177/1073858406296401. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Kurita M, Holloway T, Lopez J, Cadagan R, Martinez-Sobrido L, Garcia-Sastre A, Gonzalez-Maeso J. Maternal influenza viral infection causes schizophrenia-like alterations of 5-HTA and mGlu receptors in the adult offspring. J Neurosci. 2011;31:1863–1872. doi: 10.1523/JNEUROSCI.4230-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyffeler M, Meyer U, Yee BK, Feldon J, Knuesel I. Maternal immune activation during pregnancy increases limbic GABAA receptor immunoreactivity in the adult offspring: implications for schizophrenia. Neuroscience. 2006;143:51–62. doi: 10.1016/j.neuroscience.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Oh-Nishi A, Obayashi S, Sugihara I, Minamimoto T, Suhara T. Maternal immune activation by polyriboinosinic-polyribocytidilic acid injection produces synaptic dysfunction but not neuronal loss in the hippocampus of juvenile rat offspring. Brain Res. 2010;1363:170–179. doi: 10.1016/j.brainres.2010.09.054. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Maternal infection: window on neuroimmune interactions in fetal brain development and mental illness. Curr Opin Neurobiol. 2002;12:115–118. doi: 10.1016/s0959-4388(02)00299-4. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Penner JD, Brown AS. Prenatal infectious and nutritional factors and risk of adult schizophrenia. Expert Rev Neurother. 2007;7:797–805. doi: 10.1586/14737175.7.7.797. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I. Abnormal trajectories of neurodevelopment and behavior following in utero insult in the rat. Biol Psychiatry. 2011;70:842–851. doi: 10.1016/j.biopsych.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Richtand NM, Ahlbrand R, Horn P, Stanford K, Bronson SL, McNamara RK. Effects of risperidone and paliperidone pre-treatment on locomotor response following prenatal immune activation. J Psychiatr Res. 2011;220:55–64. doi: 10.1016/j.jpsychires.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson AM, Jennische E, Hansson HA, Holmang A. Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABA(A) dysregulation and impaired spatial learning. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1345–R1356. doi: 10.1152/ajpregu.00268.2005. [DOI] [PubMed] [Google Scholar]
- Sangha S, Greba Q, Robinson PD, Ballendine SA, Howland JG. Heightened fear in response to a safety cue and extinguished fear cue in a rat model of maternal immune activation. Front Behav Neurosci. 2014;8:168. doi: 10.3389/fnbeh.2014.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Smith SE, Malkova N, Tse D, Su Y, Patterson PH. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav Immun. 2009;23:116–123. doi: 10.1016/j.bbi.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA. Cozapine and haloperidol in an animal model of sensorimotor gating deficits in schizophrenia. Pharmacol Biochem Behav. 1993;44:741–744. doi: 10.1016/0091-3057(93)90193-w. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch Gen Psychiatry. 2006;63:1325–1335. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. Maze learning in rats: A comparison of performance in two water mazes in progeny prenatally exposed to different doses of phenytoin. Neurotoxicol Teratol. 1987;9:235–241. doi: 10.1016/0892-0362(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Graham DL, Braun AA, Schaefer TL, Skelton MR, Richtand NM, Williams MT. Prenatal immune challenge in rats: altered responses to dopaminergic and glutamatergic agents, prepulse inhibition of acoustic startle, and reduced route-based learning as a function of maternal body weight gain after prenatal exposure to poly IC. Synapse. 2012;66:725–737. doi: 10.1002/syn.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, He E, Skelton MR, Graham DL, Schaefer TL, Grace CE, Braun AA, Amos-Kroohs R, Williams MT. Comparison of (+)-methamphetamine, +/−- methylenedioxymethamphetamine, (+)-amphetamine and +/−-fenfluramine in rats on egocentric learning in the Cincinnati water maze. Synapse. 2011;65:368–378. doi: 10.1002/syn.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Herring NR, Schaefer TL, Grace CE, Skelton MR, Johnson HL, Williams MT. Effects of neonatal (+)-methamphetamine on path integration and spatial learning in rats: effects of dose and rearing conditions. Int J Dev Neurosci. 2008;26:599–610. doi: 10.1016/j.ijdevneu.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Weisenburger WP, Acuff-Smith KD, Minck DR. An analysis of factors influencing complex water maze learning in rats: Effects of task complexity, path order and escape assistance on performance following prenatal exposure to phenytoin. Neurotoxicol Teratol. 1991;13:213–222. doi: 10.1016/0892-0362(91)90013-m. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protocols. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Assessing spatial learning and memory in rodents. ILAR J. 2014;55:310–332. doi: 10.1093/ilar/ilu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff AR, Bilkey DK. Immune activation during mid-gestation disrupts sensorimotor gating in rat offspring. Behav Brain Res. 2008;190:156–159. doi: 10.1016/j.bbr.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Wolff AR, Bilkey DK. The maternal immune activation (MIA) model of schizophrenia produces pre-pulse inhibition (PPI) deficits in both juvenile and adult rats but these effects are not associated with maternal weight loss. Behav Brain Res. 2010;213:323–327. doi: 10.1016/j.bbr.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Wolff AR, Cheyne KR, Bilkey DK. Behavioural deficits associated with maternal immune activation in the rat model of schizophrenia. Behav Brain Res. 2011;225:382–387. doi: 10.1016/j.bbr.2011.07.033. [DOI] [PubMed] [Google Scholar]
- Yee N, Ribic A, de Roo CC, Fuchs E. Differential effects of maternal immune activation and juvenile stress on anxiety-like behaviour and physiology in adult rats: no evidence for the "double-hit hypothesis". Behav Brain Res. 2011;224:180–188. doi: 10.1016/j.bbr.2011.05.040. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cazakoff BN, Thai CA, Howland JG. Prenatal exposure to a viral mimetic alters behavioural flexibility in male, but not female, rats. Neuropharmacology. 2012;62:1299–1307. doi: 10.1016/j.neuropharm.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003a;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Rimmerman N, Weiner I. Latent inhibition in 35-day-old rats is not an “adult” latent inhibition: implications for neurodevelopmental models of schizophrenia. Psychopharmacology (Berl) 2003b;169:298–307. doi: 10.1007/s00213-003-1460-8. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Weiner I. Post-pubertal emergence of disrupted latent inhibition following prenatal immune activation. Psychopharmacology (Berl) 2003;169:308–313. doi: 10.1007/s00213-003-1461-7. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res. 2005;39:311–323. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.