Abstract
Aim
We assessed the management and outcomes of non-ST segment elevation myocardial infarction (NSTEMI) patients randomly assigned to fractional flow reserve (FFR)-guided management or angiography-guided standard care.
Methods and results
We conducted a prospective, multicentre, parallel group, 1 : 1 randomized, controlled trial in 350 NSTEMI patients with ≥1 coronary stenosis ≥30% of the lumen diameter assessed visually (threshold for FFR measurement) (NCT01764334). Enrolment took place in six UK hospitals from October 2011 to May 2013. Fractional flow reserve was disclosed to the operator in the FFR-guided group (n = 176). Fractional flow reserve was measured but not disclosed in the angiography-guided group (n = 174). Fractional flow reserve ≤0.80 was an indication for revascularization by percutaneous coronary intervention (PCI) or coronary artery bypass surgery (CABG). The median (IQR) time from the index episode of myocardial ischaemia to angiography was 3 (2, 5) days. For the primary outcome, the proportion of patients treated initially by medical therapy was higher in the FFR-guided group than in the angiography-guided group [40 (22.7%) vs. 23 (13.2%), difference 95% (95% CI: 1.4%, 17.7%), P = 0.022]. Fractional flow reserve disclosure resulted in a change in treatment between medical therapy, PCI or CABG in 38 (21.6%) patients. At 12 months, revascularization remained lower in the FFR-guided group [79.0 vs. 86.8%, difference 7.8% (−0.2%, 15.8%), P = 0.054]. There were no statistically significant differences in health outcomes and quality of life between the groups.
Conclusion
In NSTEMI patients, angiography-guided management was associated with higher rates of coronary revascularization compared with FFR-guided management. A larger trial is necessary to assess health outcomes and cost-effectiveness.
Keywords: Acute coronary syndrome, Non-ST elevation myocardial infarction, Fractional flow reserve, Medical therapy, Coronary revascularization
See page 75 for the editorial comment on this article (doi:10.1093/eurheartj/ehu362)
Introduction
Non-ST segment elevation myocardial infarction (NSTEMI) is the commonest form of acute coronary syndrome (ACS), the most common indication for invasive coronary angiography, and a leading global cause of premature morbidity and mortality.1 Coronary angiography in ACS patients can detect obstructive coronary artery disease and identify patients who may benefit from coronary revascularization.1–3 Usual care is based on visual interpretation of coronary disease severity and management decisions include medical therapy, percutaneous coronary intervention (PCI) or coronary artery bypass surgery (CABG). Visual assessment of lesion severity with coronary angiography may be inaccurate resulting in over- or underestimation of the physiological significance of the lesion.4,5 Hence judgements made by cardiologists in every day practice are subjective, potentially leading to misdiagnosis and incorrect treatment decisions.4–6
An alternative approach involves the measurement of the myocardial fractional flow reserve (FFR) using a pressure-sensitive coronary guidewire. Fractional flow reserve assesses the physiological significance of a coronary stenosis and is expressed as the ratio of maximal blood flow in a stenotic artery to maximal flow in an unobstructed artery.7 Recent studies (DEFER,8 FAME,9 FAME-2,10 and RIPCORD11) have evaluated the value of FFR to guide treatment decisions. An FFR ≤0.80 is an evidence-based physiological threshold that correlates with the presence of inducible ischaemia on non-invasive testing.7 Fractional flow reserve values >0.80 indicate that patients can be managed safely with medical therapy without the need for coronary revascularization.
Fractional flow reserve measurements require maximal coronary hyperaemia which may be less readily achieved in patients with acute coronary disease because of coronary microvascular dysfunction.12,13 Recent clinical studies indicate that FFR in this setting may be valid14–18 but in the absence of evidence from randomized prospective trials, a routine physiological approach for the management of patients with recent MI is not recommended in guidelines.1–3 We hypothesized that management decisions in patients with NSTEMI undergoing coronary angiography guided by routine FFR measurement would be feasible and safe, and would provide additive clinical utility compared with standard care based on visual interpretation of the angiogram.
Methods
Trial design
We performed a prospective 1 : 1 randomized controlled parallel group trial in 350 NSTEMI patients enrolled from October 2011 to May 2013.19
Participants and eligibility criteria
Patients with a clinical diagnosis of recent NSTEMI1 and with at least one risk factor for coronary artery disease (e.g. diabetes mellitus) were eligible for randomization if urgent invasive management was planned within 72 h of the index episode of myocardial ischaemia or if there was a history of recurrent ischaemic symptoms within 5 days. The angiographic inclusion criteria required at least one coronary stenosis ≥30% severity with normal coronary blood flow [Thrombolysis in Myocardial Infarction (TIMI) grade III] in which FFR measurement might have a diagnostic value.
The exclusion criteria were the presence of ischaemic symptoms that were not controlled by medical therapy, haemodynamic instability, MI with persistent ST elevation, intolerance to anti-platelet drugs, ineligible for coronary revascularization, a treatment plan for non-coronary heart surgery (e.g. valve surgery), a history of prior CABG, angiographic evidence of severe (e.g. diffuse calcification) or mild (<30% severity) coronary disease, a life expectancy <1 year and an inability to give informed consent.
Setting
The study participants were enrolled in six hospitals in the UK, of which three were non-academic regional centres, and their medical and invasive management followed evidence-based guidelines, including complete revascularization during the index hospitalization, as appropriate.1–3
Informed consent
The study information sheet that had been approved by the research ethics committee was provided to each participant. Written informed consent was obtained before the diagnostic coronary angiogram and randomization.
Coronary angiogram acquisition and analyses
Coronary angiograms were acquired during usual care with standard cardiac catheter laboratory equipment. The angiograms were assessed visually by the attending clinicians who made the treatment decision for medical therapy, PCI or CABG.
Randomization, implementation, and blinding
Participants were enrolled by research staff on the ward before the angiogram was obtained. The standard care management strategy was established and recorded before FFR was measured. The treatment plan was based on all of the clinical information including the results of the angiogram and before FFR was measured. If the angiographic eligibility criteria were fulfilled, the patients were then randomized by the research nurse in the catheter laboratory to FFR-guided and angiography-guided strategies using a web-based randomization system. The randomization sequence was created using randomized permuted blocks of length 4, without stratification. The allocation sequence was on a 1 : 1 basis between the FFR-guided group and the angiography-guided group and the sequence was concealed electronically. The participants were blinded to the treatment group allocation.
Interventions
The randomized participants had FFR measured in all coronary arteries with a lesion of ≥30% diameter stenosis that were amenable to instrumentation with a pressure-sensitive coronary guidewire (St Jude Medical, Uppsala).19 Fractional flow reserve was measured during coronary hyperaemia induced by i.v. adenosine (140 µg/kg/min). The FFR intervention in this study, including the assessment of adenosine effect, the measurement of FFR, vessel selection, blinding and disclosure of the FFR results, has been previously described.19
Fractional flow reserve-guided group
Fractional flow reserve ≤0.80 was an indication for revascularization by PCI or CABG, as appropriate, and FFR >0.80 was an indication for medical therapy only. Any changes in management strategy following FFR disclosure were prospectively recorded.
Angiography-guided group and blinding
In patients randomized to the angiography-guided group, FFR was measured in the same way as in the FFR-guided group except that the FFR results were not disclosed. The research staff obscured the haemodynamic monitor [RadiAnalyzer Xpress (St Jude Medical, Uppsala)] from the clinicians, nurses, and patients such that it was impossible for them to observe the pressure wire information either in the catheter laboratory or afterwards. Electronic displays of distal coronary pressure on other haemodynamic monitors that may have been visible in the catheter laboratory were also disabled. Quality control checks, including assessments of equalized pressure recordings and verification of symptoms and haemodynamic changes with i.v. adenosine, were conducted in the usual way. The quality assurance procedures have been previously described.19
Outcomes
Primary outcome
The pre-specified primary outcome was the between-group difference in the proportion of patients allocated to medical management. The final treatment decision was made by the clinicians in the cardiac catheter laboratory during the index procedure or shortly afterwards if a multidisciplinary heart team review was indicated.
Secondary outcomes
The feasibility and safety of routine FFR measurement.
The relationship between FFR and coronary stenosis severity by visual assessment of the angiogram.
Major adverse cardiac events (MACE) during the follow-up over 12 months, defined as cardiac death20 or hospitalization for myocardial infarction21 or heart failure20 after randomization. Cardiovascular death, stroke, transient ischaemic attack, contrast nephropathy, and bleeding were also prospectively recorded.20 All of these events were adjudicated by a Clinical Event Committee (CEC) comprised of three cardiologists who were independent of the trial and blinded to the treatment allocations. The CEC charter and the definitions for these events are described in the Supplementary material online, Methods.19–23 Coronary revascularization, including PCI and CABG, were prospectively recorded in the clinical report form. Information on serious adverse events during the follow-up was obtained by contacting the patients by telephone and reviewing their primary and secondary care records. All complications that were potentially related to the invasive procedure were prospectively recorded.
Index hospitalization resource use including: material, procedure, hospitalization, and in-hospital event costs.
Health-related quality of life [HRQoL; EuroQol 5-Dimensions 3-Level (EQ-5D-3L)].24
Healthcare resources and costs
Costs during index hospitalization were calculated by applying resource use or events at the individual level to unit costs derived from NHS National Procurement,25 NHS Reference Costs,26 Information Services Division Scotland,27 the British National Formulary (www.bnf.org), and the Golden Jubilee National Hospital. Further details of the methods are provided in the Supplementary material online, Methods.
Trial management
The trial was conducted in line with Guidelines for Good Clinical Practice in Clinical Trials,28 and the study complies with the Declaration of Helsinki. There was a Trial Management Group for operational activity, an independent Clinical Event Committee to adjudicate on serious adverse events for safety and efficacy outcomes, an independent Data and Safety Monitoring Committee (DSMC),29 and a Trial Steering Committee to coordinate the trial and liaise with the Sponsor and Trials Unit (Supplementary material online, Methods). During the course of the trial, the DSMC met once and received two safety and progress reports from the trials unit (June 2012 and March 2013).
The Robertson Centre for Biostatistics within the Glasgow Clinical Trials Unit provided the trial-specific electronic data collection system, acted as an independent coordinating centre for randomization and data management, and conducted the statistical analyses. The trial was approved by the National Research Ethics Service (reference 11/S0703/6). The clinical trial registration numbers are NCT01764334 and ISRCTN97489534 and the trial sponsor is the National Waiting Times Centre Board, NHS Scotland. The sponsor monitored the study for safety and the study underwent an external audit commissioned by the sponsor (October 2013). All serious adverse events were prospectively reported to the Robertson Centre for Biostatistics.
Sample size
We calculated that 322 randomized subjects (161 subjects in each group) would provide 90% power at a 5% level of significance to detect a 50% relative increase in the proportion of patients assigned to medical treatment in the FFR disclosed group from 15 to 30%. This difference was based on observations made in a pilot study30 intended to inform the design of the current trial. Allowing for loss of data at the time of the procedure the number of participants in the randomized trial was increased to 350.
Statistical methods
Mean (standard deviation) or median (inter-quartile range) were used to summarize continuous data. Counts and percentages were used to summarize categorical data. All tests were two-tailed and assessed at the 5% significance level.
The primary outcome of the proportion of patients allocated to medical therapy was assessed in terms of the difference in proportions and the relative risk between groups estimated with exact 95% confidence intervals and P-values. The proportion of patients with MACEs within 12 months and other binary outcomes were analysed using the same methods, and time to events within 12 months was compared between groups using log-rank tests. Health-related quality of life was compared between groups using baseline-adjusted linear regression. Length of stay was compared between groups using a Wilcoxon-Rank Sum test. Costs were compared using bootstrapping (details in Supplementary material online, Methods). The statistical analyses were performed using R version 3.0.0 and StatXact version 5.0.3.
Results
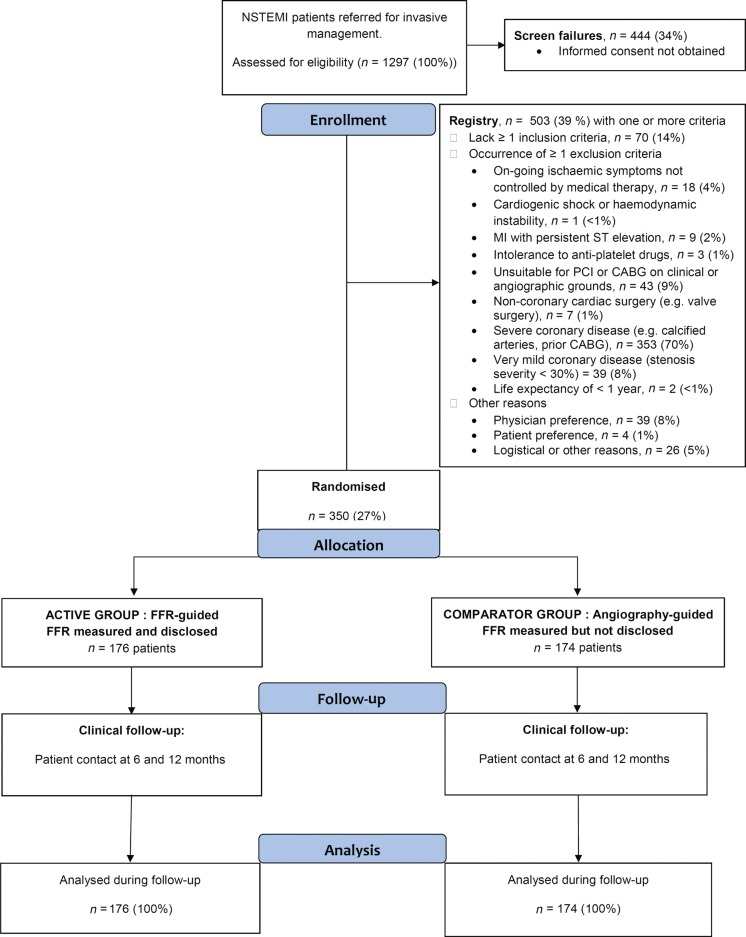
Eight hundred and fifty-three NSTEMI patients were referred for invasive management and gave informed consent from October 2011 to May 2013 (Figure 1 ; Table 1). Of these, 350 patients (mean age 62 years, 74% male) were randomized (n = 176 FFR-guided, n = 174 angiography-guided). Fractional flow reserve was measured in all (100%) participants but only disclosed in the FFR-guided group. The clinical and treatment characteristics of the patients included in the FFR-guided group and the angiography-guided group were similar (Table 1).
Figure 1.
Flow diagram of the clinical trial.
Table 1.
Baseline clinical and angiographic characteristics of all-comers
| Baseline characteristicsa | Randomly assigned groups |
|
|---|---|---|
| FFR-guided group n = 176 |
Angiography-guided group n = 174 |
|
| Clinical | ||
| Age, years | 62.3 (11.0) | 61.6 (11.1) |
| Male sex, n (%) | 133 (75.6) | 127 (73.0) |
| Heart rate, b.p.m. | 73 (15) | 74 (17) |
| ECG evidence of ischaemia at initial presentation, n (%) | 137 (77.8) | 143 (82.2) |
| Peak troponin concentration before the procedurea | ||
| >×5 upper limit of normal | 129 (73.3) | 137 (78.7) |
| >×10 upper limit of normal | 107 (60.8) | 115 (66.1) |
| GRACE score for death or myocardial infarction within 6 months of admission | 146 (131, 173) | 146 (122, 172) |
| Patients with a GRACE score for death or myocardial infarction within 6 months >140, n (%) | 102 (58.0) | 97 (55.7) |
| Diabetes mellitusb, n (%) | 26 (14.8) | 26 (14.9) |
| History of atrial fibrillation or flutter, n (%) | 12 (6.8) | 7 (4.0) |
| History of stroke or transient ischaemic attackb, n (%) | 15 (8.5) | 9 (5.2) |
| History of peripheral vascular diseaseb, n (%) | 14 (8.0) | 14 (8.0) |
| Previous myocardial infarction, n (%) | 22 (12.5) | 24 (13.8) |
| Previous percutaneous coronary intervention, n (%) | 19 (10.8) | 19 (10.9) |
| History of treated hypertensionb, n (%) | 78 (44.3) | 81 (46.6) |
| History of treated hypercholesterolaemiab, n (%) | 71 (40.3) | 56 (32.2) |
| History of smokingb, n (%) | ||
| Current | 72 (40.9) | 71 (40.8) |
| Former (stopped >3 months) | 55 (31.2) | 47 (27.0) |
| Never | 49 (27.8) | 56 (32.2) |
| Angina, Canadian Cardiovascular Society angina class at presentation, n (%) | ||
| I | 1 (0.6) | 0 (0) |
| II | 1 (0.6) | 2 (1.1) |
| III | 7 (4.0) | 15 (8.6) |
| IV | 166 (94.3) | 156 (89.7) |
| New York Heart Association functional class at presentation, n (%) | ||
| I | 154 (87.5) | 154 (88.5) |
| II | 17 (9.7) | 16 (9.2) |
| III | 2 (1.1) | 3 (1.7) |
| IV | 3 (1.7) | 1 (0.6) |
| Frailty, n (%)a | ||
| Well | 148 (87.1) | 144 (87.8) |
| Vulnerable | 20 (11.8) | 17 (10.4) |
| Frail | 2 (1.2) | 3 (1.8) |
| Health-related quality of life, EQ-5D score | 0.78 (0.29) | 0.81 (0.25) |
| Medication at procedure | ||
| Aspirin, n (%) | 175 (99.4) | 173 (99.4) |
| P2Y12 inhibitor, n (%) | 176 (100) | 173 (99.4) |
| Clopidogrel | 169 (96.0) | 168 (97.1) |
| Ticagrelor or Prasugrel | 7 (4.0) | 5 (2.9) |
| Statin, n (%) | 168 (95.5) | 167 (96.0) |
| Beta-blocker, n (%) | 161 (91.5) | 147 (84.5) |
| Calcium channel blocker, n (%) | 27 (15.4) | 25 (14.4) |
| Isosorbide mononitrate, n (%) | 18 (10.2) | 20 (11.5) |
| Nicorandil, n (%) | 18 (10.2) | 13 (7.5) |
| Intravenous nitrate, n (%) | 32 (18.2) | 21 (12.1) |
| Low molecular weight heparin, n (%) | 165 (93.8) | 168 (96.6) |
ECG, electrocardiogram.
Means ± SD or median (inter-quartile range) for normal and non-normally distributed data, respectively. Categories for peak troponin I and T concentrations were determined based on the upper limit of normal (99th centile) for each hospital.
aIn the Registry, the mean age was 63.8 (12.3) years and 328 (65.3%) were male.
bAt least one risk factor for coronary artery disease was required for eligibility. Diabetes mellitus was defined as a history of diet-controlled or treated diabetes. Frailty was assessed using the frailty index score (Supplementary material online, Methods31) and the six categories were summarized into three groups: well, vulnerable, or frail.
Data for the randomized participants were missing for the following variables: frailty index,31 n = 6 patients in the FFR-guided group and n = 10 patients in the angiography-guided group.
Three hundred and twenty-two (92%) of 350 randomized participants had a history of angina at rest (Canadian Cardiovascular Society Angina Class IV angina) and 280 (80%) patients had ECG evidence of ischaemia. The median (inter-quartile range) time from the index episode of myocardial ischaemia to the invasive angiogram was 3 (2, 5) days and 81% of the participants underwent angiography within 5 days of the index event or most recent episode of chest pain. All the patients were followed up for 12 months and all of the randomized participants were included in the analysis.
Fractional flow reserve-guided vs. Angiography-guided treatment groups
Fractional flow reserve was measured in 704 (99.7%) of 706 lesions with a stenosis severity ≥30%, and was measured in at least one artery in all (100%) patients. Of lesions with an FFR result (n = 704), 430 (61.1%) were physiologically significant (FFR ≤0.80) (Table 2 and Supplementary material online).
Table 2.
Procedure characteristics and findings
| Characteristicsa | Randomly assigned groups |
|
|---|---|---|
| FFR-guided group n = 176 |
Angiography-guided group n = 174 |
|
| Procedure | ||
| Time from index episode of myocardial ischaemia to the invasive angiogram, days | 3 (1, 4) | 4 (2, 5) |
| Procedure characteristics | ||
| Radial artery access, n (%) | 158 (89.8) | 157 (90.2) |
| Procedure time (including angiography and PCI), min | 66.5 (23.4) | 70.5 (33.5) |
| Volume of contrast used, mL | 218.7 (97.3) | 221.9 (110.4) |
| Stents | ||
| Number of stents per patient | 1.1 (1.1) | 1.4 (1.2) |
| Total stent length per patient, mm | 24.4 (24.7) | 29.4 (26.9) |
| Total number of stents | 203 | 245 |
| Angiographic findings | ||
| Total number of lesions with a stenosis ≥30% of the reference diameter of the artery | 355 | 351 |
| Total number of lesions with a stenosis ≥50% of the reference diameter of the artery (% of all lesions) | 331 (93.2) | 314 (89.5) |
| Patients with at least one lesion ≥50% severity, n (%) | 172 (97.7) | 168 (96.6) |
| Arteries with at least one angiographically significant lesion, n (%) | ||
| 0 | 4 (2.3) | 6 (3.4) |
| 1 | 62 (35.2) | 68 (39.1) |
| 2 | 72 (40.9) | 69 (39.7) |
| 3 | 33 (18.8) | 27 (15.5) |
| 4 | 5 (2.8) | 4 (2.3) |
| Patients with at least one lesion ≥50% severity in the proximal or mid left anterior descending artery, n (%) | 115 (65.3) | 110 (63.2) |
| Patients with at least one lesion ≥50% severity in the left main artery, n (%) | 2 (1.1) | 6 (3.4) |
| FFR findings | ||
| Lesions successfully measured for FFR, number/total number (%) | 355 (100) | 349 (99.4) |
| Number of physiologically significant (FFR ≤0.80) lesions (% of all lesions) | 208 (58.6) | 222 (63.6) |
| Arteries with at least one physiologically significant (FFR ≤0.80) lesion, n (%) | ||
| 0 | 34 (19.3) | 29 (16.7) |
| 1 | 91 (51.7) | 90 (51.7) |
| 2 | 39 (22.2) | 42 (24.1) |
| ≥3 | 12 (6.8) | 13 (7.5) |
| Patients with at least one physiologically significant lesion (FFR ≤0.80), n (%) | 142 (80.7) | 145 (83.3) |
| Patients with at least one physiologically significant lesion (FFR ≤0.80) in the proximal or middle left anterior descending artery, n (%) | 72 (40.9) | 86 (49.4) |
| Mean FFR in lesions with FFR ≤ 0.80 | 0.56 (0.12) | 0.58 (0.13) |
| Lesion characteristics based on visual interpretation of the angiogram | ||
| Stenosis, n (%) | ||
| 30–49% of diameter | 24 (6.8) | 37 (10.5) |
| 50–69% of diameter | 76 (21.4) | 73 (20.8) |
| 70–89% of diameter | 113 (31.8) | 88 (25.1) |
| ≥90% of diameter | 111 (31.3) | 124 (35.3) |
| Total occlusion | 31 (8.7) | 29 (8.3) |
TIMI, thrombolysis in myocardial infarction grade.
A diseased artery was defined as an epicardial artery with one or more lesions ≥30% of the reference vessel diameter and amenable to PCI or CABG. An angiographically significant artery was defined as an artery with one or more lesions ≥50% of the reference vessel diameter.
aMean ± SD or median (inter-quartile range) for normal and non-normally distributed data, respectively.
Ten participants (2.9%) had no lesions (stenosis severity <50%) when assessed by angiography and 63 (18.0%) patients had no lesions when subsequently assessed by FFR (>0.80). The number of patients with 0, 1, 2, or ≥3 vessel coronary disease is shown in Supplementary material online, Figure S1.
Primary outcome
The proportion of patients treated by medical therapy was higher in the FFR-guided group than in the angiography-guided group [40 (22.7%) vs. 23 (13.2%), difference 9.5% (95% CI: 1.4%, 17.7%), P = 0.022; relative risk 1.72 (1.08, 2.82)] (Table 3).
Table 3.
Primary and secondary outcomes and procedure characteristics
| Outcomea | Randomly assigned groups |
Risk difference (95% CI) | P-valueb | |
|---|---|---|---|---|
| FFR-disclosure group n = 176 |
Angiography group n = 174 |
|||
| Primary outcomec | ||||
| Medical management, n (%) | 40 (22.7) | 23 (13.2) | 9.5% (1.4, 17.7%) | 0.022 |
| Coronary revascularization during the index admission | 136 (77.3) | 151 (86.8) | ||
| Percutaneous coronary intervention, n (%) | 125 (71.0) | 139 (79.9) | −8.9% (−18.1, 0.2%) | 0.057 |
| Coronary artery bypass graft, n (%) | 11 (6.2) | 12 (6.9) | −0.7% (−6.2, 4.8%) | 0.87 |
| In-hospital adverse events | ||||
| Contrast nephropathy | 2 (1.1) | 1 (0.6) | 0.6% (−2.2, 3.5%) | 0.69 |
| Major bleeding | 2 (1.1) | 1 (0.6) | 0.6% (−2.2, 3.5%) | 0.69 |
| Health outcomes at 12 months, n (%) | ||||
| Cardiovascular death, non-fatal myocardial infarction, unplanned hospitalization for stroke or transient ischaemic attack (MACCE) | 13 (7.4) | 16 (9.2) | −1.8% (−7.9, 4.2%) | 0.56 |
| Cardiac death, non-fatal myocardial infarction or unplanned hospitalization for heart failure (MACE) | 14 (8.0) | 15 (8.6) | −0.7% (−6.7, 5.3%) | 0.89 |
| MACE, excluding procedure-related myocardial infarctiond | 10 (5.7) | 5 (2.9) | 2.8% (−1.6, 7.6%) | 0.25 |
| All-cause death | 5 (2.8) | 3 (1.7) | 1.1% (−2.4, 5.0%) | 0.54 |
| Fatal or non-fatal myocardial infarctiond | 11 (6.2) | 15 (8.6) | −2.4% (−8.2, 3.3%) | 0.49 |
| Myocardial infarction related to coronary revascularization (Type 4a, Type 4b and Type 5 myocardial infarction) | 5 (2.8) | 11 (6.3) | −3.5% (−8.5, 1.1%) | 0.12 |
| Spontaneous myocardial infarction | 7 (4.0) | 5 (2.9) | 1.1% (−3.1, 5.5%) | 0.69 |
| Heart failure | 1 (0.6) | 0 (0.0) | 0.6% (−1.6, 3.2%) | 0.51 |
| Stroke or TIA | 0 (0.0) | 1 (0.6) | −0.6% (−3.2, 1.5%) | 0.52 |
| Other secondary outcomes | Mean difference (95% CI) | P-value | ||
| Health-related quality of life: EQ-5D health status at 12 months | 0.844 (0.236) | 0.804 (0.284) | ||
| Change from baseline health status at 12 months | 0.066 (0.357) | −0.010 (0.276) | 0.055 (−0.010, 0.120) | 0.095 |
| Cost through index hospitalization, mean (SE) £ | 7289 (608) | 7484 (632) | −194 (−961 to 575) | 0·61 |
| Material cost, mean (SE) £e | 1095 (39) | 822 (46) | 274 (157 to 389) | <0.01 |
| Procedure cost, mean (SE) £e | 467 (111) | 502 (118) | −35 (−307 to 227) | 0.78 |
| Hospitalization cost, mean (SE) £e | 5701 (585) | 6117 (611) | −415 (−1069 to 239) | 0.21 |
| In-hospital event cost, mean (SE) £e | 25 (19) | 43 (19) | −18 (−69 to 37) | 0.46 |
| Duration of hospital stay at baseline admission, days | 6.1 (3.3) | 6.5 (3.1) | −0.44 (−9.41 to 8.51) | 0.09 |
FFR, fractional flow reserve.
aMeans ± SD and median (interquartile range) are used for normal and non-normally distributed data. Cost data are reported as mean ± SE.
bFFR was measured in all participants and disclosed in the FFR-guided group but not disclosed in the angiography-guided group. The P-value is the comparison between the FFR-guided group and angiography-guided group.
cThe index treatment decision as per randomized strategy occurred in 171 (97.2%) of the participants in the FFR-guided group and 173 (99.4%) of the participants in the angiography-guided group.
dOf the 10 patients with spontaneous MACE in the FFR group, FFR disclosure changed the initial treatment plan from PCI to medical therapy for the culprit artery in four patients. These events happened from 3 to 11 months after randomization. The excess of five patients with a spontaneous MACE in the FFR group is due to two deaths, two patients with spontaneous MI and one patient with a heart failure hospitalization.Myocardial infarction: 28 non-fatal MI events and 2 fatal MI events occurred within 12 months of randomization in 26 patients, including 17 procedure-related MIs in 16 patients [one standard care patient had two procedure-related MIs (index procedure and a subsequent procedure during the follow-up)] and 11 spontaneous MIs in 10 patients (one FFR patient had two of these events)]. Twenty-six patients had at least one MI event. Four patients had two MI events (n = 2 FFR-guided group, n = 2 angiography-guided group). In summary, one patient had two procedure-related MIs, one patient had two spontaneous MIs and two patients had both types of MI.
eMaterial costs includes: guide catheters, ordinary guidewires, pressure wires, adenosine, balloon catheters, drug-eluting stents, bare metal stents, GP inhibitors, and bivalirudin; Procedure costs includes: CABG, intravascular ultrasound, optical coherence tomography, echocardiogram and chest X-ray; hospitalization costs includes catheterization laboratory time, CCU days, ITU days and general ward days; in-hospital events included MI and stroke.
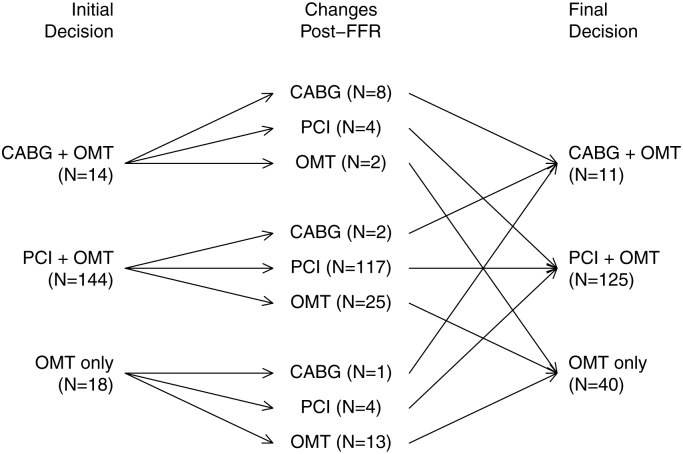
The initial treatment decisions before randomization and after FFR disclosure in the FFR-guided group are shown in Figure 2. Fractional flow reserve-disclosure resulted in a change in treatment plan in 38 (21.6%) of 176 patients. The relationship between FFR and stenosis severity is shown in Figure 3.
Figure 2.
Fractional flow reserve-guided group: treatment decisions initially based on angiography alone and then finally after fractional flow reserve disclosure.
Figure 3.
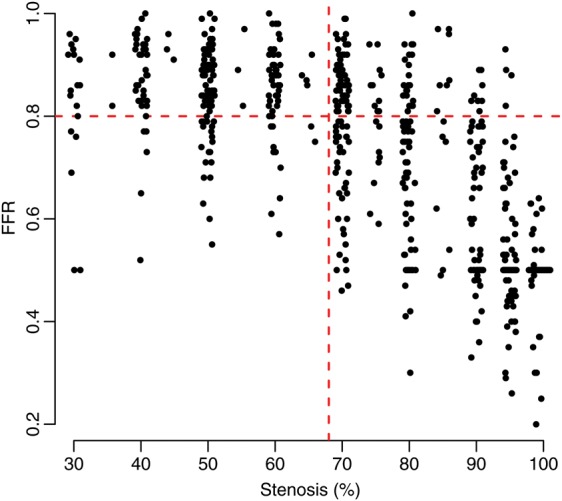
Relationship between angiographic stenosis severity assessed visually before randomization and fractional flow reserve.
Resource use and material costs during the index hospitalization
The duration of the index invasive procedure, the volume of radiographic contrast medium, and the number, type (drug-eluting stent vs. bare metal stent) and length of stents were similar in the FFR-guided group and angiography-guided groups (Table 3). Mean material costs were higher in the FFR-guided group (£1095, 95% confidence interval £1021 to £1171) compared with the angiography-guided group (£822, 95% confidence interval £737 to £914). Mean in-hospital healthcare costs were similar in the FFR-guided group (£7289, 95% confidence interval £6173 to £8549) and the angiography-guided group (£7484, 95% confidence interval £6325 to £8777) (Table 3).
Clinical events and safety
In-hospital adverse events relating to procedure safety are described in Table 3. According to independent adjudication based on review of the coronary angiograms, 8 coronary artery dissections occurred in 7 (2.0%) of 350 patients during the index procedure. Six dissections were attributed to coronary instrumentation during PCI and 2 were attributed to the pressure wire.
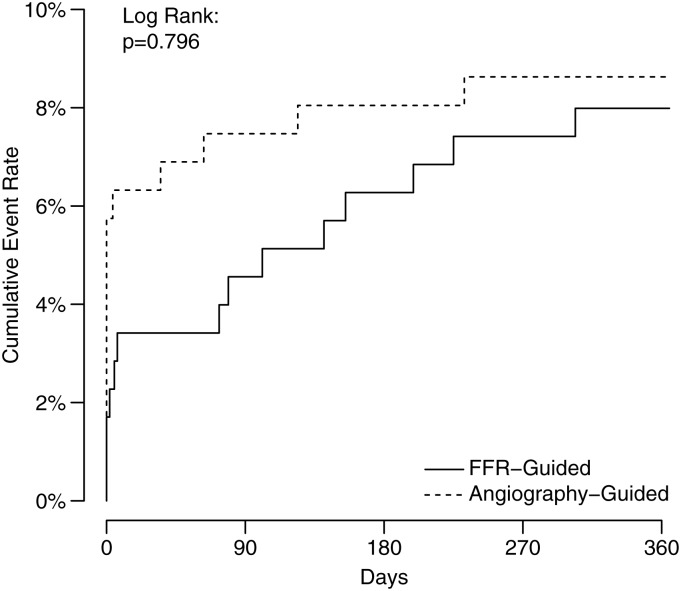
The follow-up assessments were completed in June 2014. Vital status at 12 months was obtained for all (100%) participants (Table 3). Fourteen (8.0%) of 176 patients in the FFR-guided group and 15 (8.6%) of 174 in the angiography-guided group experienced cardiac death, non-fatal myocardial infarction or heart failure hospitalization (P = 0.89) (Table 3; Figure 4). Myocardial infarction relating to PCI (Type 4a or Type 4b) or CABG (Type 5) occurred in 5 (2.8%) patients in the FFR-guided group and 11 (6.3%) patients in the angiography-guided group (P = 0.12) (Table 3). Major adverse cardiac events excluding MI related to revascularization occurred in 10 (5.7%) patients in the FFR-guided group and 5 (2.9%) patients in the angiography-guided group (P = 0.25) (Table 3; Supplementary material online, Figure S3).
Figure 4.
Kaplan–Meier plots for major adverse cardiac events during 12-month follow-up in the FFR-guided group and angiography-guided group.
Health outcomes in patients treated initially with medical therapy alone
Sixty three (18.0%) of 350 randomized participants were initially managed medically without revascularization. Of these, 3/40 (7.5%) in the FFR-guided group had a MACE event during 12 months follow-up vs. 0/23 (0%) in the angiography-guided group (P = 0.22; Supplementary material online, Table S2).
Revascularization within 12 months
Compared with the angiography-guided group, the percentage of patients who were free from coronary revascularization remained higher in the FFR disclosure group at 12 months [37 (21.0%) vs. 23 (13.2%), difference 7.8% (−0.2%, 15.8%), P = 0.054; relative risk 1.59 (0.99, 2.62)] (Supplementary material online, Table S3).
Health-related quality of life
Health-related quality of life scores were similar in each group at 12 months (Table 2).
Discussion
In this trial, we assessed a routine physiological approach combined with coronary angiography to diagnose and treat coronary artery disease in patients with recent NSTEMI undergoing invasive management.
Compared with an anatomical approach based on visual interpretation of the coronary angiogram (standard care), FFR-guided management was feasible and safe in the catheter laboratory. The FFR-guided approach resulted in changes in stenosis classification and patient management in one-fifth of the patients. The rate of coronary revascularization was reduced at the index procedure and most of this difference was maintained at 12 months. Material costs during the index procedure increased because of the cost of the pressure wire but overall healthcare costs during the index hospitalization were similar. Myocardial infarction related to revascularization tended to be more frequent in the standard care group, whereas MACE events unrelated to revascularization tended to be more common in the FFR group. There was no evidence for differences in the other health outcomes or in health-related quality of life between the randomized groups.
The results of this trial have several implications. Firstly, routine FFR measurement in appropriately selected NSTEMI patients was feasible in all of the participants and relatively safe. Radial artery access was the norm and bleeding complications were rare. Secondly, on an individual patient basis, FFR disclosure commonly changed patient management (Figure 2), and overall, revascularization was reduced. Thirdly, compared with the angiography-guided group, the increased adoption of medical therapy at the expense of revascularization in the FFR disclosure group was associated with similar overall health outcomes and quality of life at 1 year. Representing a balance of competing risks, the reduction in procedure-related MI events in the FFR group should be considered against the increase in spontaneous cardiac events during the follow-up. Finally, based on the combination of coronary angiography and the use of FFR, the diagnostic work-up of patients admitted with NSTEMI could be simplified ruling out the need for deferred management and non-invasive stress testing in NSTEMI patients with a broad range of stenosis severities (≥30%).
In invasively managed NSTEMI patients, the standard care approach involves visual interpretation of the anatomical severity of disease disclosed by the coronary angiogram. Adoption of a physiological approach to inform treatment decisions in invasively managed NSTEMI patients is not the standard of care mainly because of a lack of evidence. The specific uncertainties for FFR adoption relate to a lack of evidence for FFR measurement in culprit arteries.1–3 When coronary resistance is reduced by vasodilator drugs, such as adenosine, the curvilinear relationship between coronary pressure and flow becomes approximately linear in the physiological range of blood pressure.32,33 Following STEMI, vascular injury may limit microvascular vasodilatation12 and this may limit the validity of FFR which is by definition a hyperaemic index. In NSTEMI, the pathophysiology of the culprit artery is typically non-occlusive thrombotic plaque rupture and subendocardial infarction.34 Since FFR was measured in coronary arteries with normal blood flow, microvascular dysfunction may have been limited, transient or absent in the participants in this trial.18 The post hoc analysis of medically stabilized ACS patients in the FAME trial also supports the validity of FFR.17
A further area of uncertainty that was addressed in this trial relates to the management of NSTEMI patients with non-obstructive culprit lesions and potentially rupture-prone non-culprit lesions. Stenting to seal a non-flow limiting ruptured coronary plaque might reduce the risk of recurrent MI. Alternatively, optimal medical therapy might suffice and unnecessary stenting can be harmful (e.g. stent thrombosis, restenosis). The likelihood of MI increases with coronary stenosis severity and revascularization guided by FFR reduces this risk in stable patients.7–10 Whether FFR-guided management has prognostic benefits in ACS patients is uncertain and controversial. On the one hand, a reduction in revascularization may reduce procedure-related MI. On the other hand, the risk of spontaneous MI might increase in the longer-term in non-revascularized patients since plaque with rupture-prone biology may be non-flow limiting (FFR >0.80). In our trial, 4 of the 10 patients with spontaneous MACE in the FFR group had an initial treatment plan for PCI in a culprit artery changed to medical therapy based on an FFR >0.80. The spontaneous MACE events in these patients occurred later during the follow-up (3–11 months) in keeping with remodelling in the culprit artery and late spontaneous MI rather than a false-negative FFR result. The FFR results in the other patients with spontaneous MACE in the FFR group did not influence the initial management of these patients based on angiography alone implying the FFR strategy was not associated with the MACE events. In the FAME trial, nearly one-third of participants in the FAME trial had a history of recent MI.8
The potential for FFR disclosure to impact on physicians' treatment decisions in patients with recent unstable coronary disease is also uncertain.19,30 We found that FFR disclosure changed the treatment plan in over one in five patients with a reduction in revascularization on a patient basis. However, late spontaneous MACE tended to be more common in the FFR-group, calling into question the longer-term safety of an FFR-guided change from PCI to medical therapy in culprit arteries. These observations place emphasis on the need for a larger trial with a design that is informed by these results and powered to definitively assess health outcomes and cost-effectiveness.
The FAMOUS-NSTEMI trial differed from recent trials of FFR-guided management (DEFER,7 FAME,8 FAME-2,9 and RIPCORD10) in a number of important ways. Firstly, the primary diagnosis of the patients differed between the trials. DEFER, FAME, and FAME-2 trials enrolled patients with stable coronary artery disease. In FAME,8 NSTEMI patients were included within 5 days of the index event provided the peak creatine kinase was <1000 U per L. In FAME-2,9 patients with Canadian Cardiovascular Society angina class IV or an NSTEMI were only included if the symptoms had been controlled for >7 days. Secondly, the treatment strategies in these trials were not the same. In DEFER,7 FAME,8 and FAME-2,9 the patients were selected for PCI, whereas FAMOUS patients were randomized upstream at an earlier stage in the treatment pathway when all treatment options were possible, including medical therapy, PCI and CABG. Thirdly, the angiographic criteria for FFR measurement differed between the trials. In the FAME trials, FFR was measured in stenoses assessed visually to be at least intermediate (≥50% reference diameter) in severity, whereas in FAMOUS even very mild narrowings (≥30% reference diameter) were included. The characteristics of the participants in the FAMOUS trial were similar to those of other ACS trials, such as TIMACS35 (e.g. 80% of participants in both trials had an ischaemic ECG). Finally, compared with standard care, health outcomes were improved by FFR-guided management in the FAME trials, whereas in FAMOUS, MI events were different and overall MACE were similar at 12 months.
Balancing against the potential benefits, use of a diagnostic coronary guidewire may come at the expense of cost and potential harm, including procedure-related coronary dissections. In this study, two coronary artery dissections were due to pressure wire instrumentation, as attributed by an independent clinical event committee which reviewed the angiograms. In the RIPCORD study,10 in which all coronary arteries were instrumented, three clinically important complications attributable to the pressure wire occurred in 200 patients.
In the angiography-guided group, the proportion of patients revascularized at baseline (86.8%, Table 3) were lower than the proportion of patients with an angiographically significant stenosis (96.6%, Table 2). In contrast, in the FFR-guided group, the proportion of patients with at least one physiologically significant lesion (FFR ≤0.80; 80.7%, Table 2) and the rate of revascularization at baseline (81.7%, Table 3) were similar. In the sample size calculation (Supplementary material online, Methods), we had anticipated a 15% difference in medical management between the randomized groups. The smaller actual difference (10.1%, Table 3) could be in part be explained by the lower than expected rate of revascularization in the angiographic control group.
The rate of change of the initial treatment plan in our trial was lower than in other studies.7,8,10,30 This discrepancy is explained by the lower rate of lesion re-classification by FFR disclosure in patients with very mild (<50%) or very severe (>90%) lesions (Figure 3). Lesions at the extremes of coronary stenosis severity were included by design in order to assess the diagnostic impact of FFR across the full range of stenosis severities. The relationship between lesion severity and the health economic value of the FFR-guided strategy should inform whether this strategy has more economic value within an intermediate range of coronary stenosis severities (e.g. 50–90%). The health economic implications of this trial will be assessed in a future planned analysis.19
Limitations
The randomized participants in this trial were included because the cardiologist believed coronary instrumentation with the pressure wire was feasible, but this decision is subjective and some patients may not have been included due to operator preference. Even though some features of severe coronary disease were exclusion criteria (e.g. a severely calcified coronary artery), some patients with severe coronary disease were still included supporting generalizability of the trial findings. For example, 6.6% of the participants were referred for CABG; 19% of the participants underwent angiography five or more days from the index episode of myocardial ischaemia. This time interval is explained by clinical service pressures that delayed access to the catheter laboratory in some of the hospitals in this trial.
Most of the participants in our trial received clopidogrel whereas ticagrelor, which improves cardiovascular outcomes in ACS patients compared with clopidogrel,36 is now recommended in NSTEMI patients.1 We have reported the cardiologists’ visual interpretation of the angiogram as actually performed in the study participants. A quantitative coronary analysis by blinded observers is currently on-going.
Our study was designed (but not powered) to assess between-group differences in health outcomes. There are too few cardiac events to draw firm conclusions and the prognostic significance of FFR-guided management in patients with optimal dual anti-platelet therapy should be further assessed in a larger trial with longer-term follow-up.
Conclusions
The FAMOUS-NSTEMI trial provides information on the feasibility, safety, and clinical utility of a routine physiological approach to guide the management of NSTEMI patients. We have shown that compared with angiography-guided standard care, routine FFR measurement is feasible and safe, and FFR disclosure resulted in a change in treatment plan in more than one-fifth of patients and revascularization was reduced overall. There were no differences in health outcomes and quality of life between the randomized groups. In the FFR group, procedure-related MI tended to be reduced but spontaneous MACE during the follow-up tended to be more common during 12-month follow-up. A large randomized trial is needed to definitively assess the cost-effectiveness of an FFR-guided management strategy in invasively managed NSTEMI patients.
Supplementary material
Supplementary material is available at European Heart Journal online.
Authors’ contributions
J.L. coordinated the study, collected data, interpreted the results and contributed to the manuscript. K.G.O., N.C., A.S., K.B., R.D., S.J., and A.O. obtained consent and randomized patients, collected data, interpreted the results, and contributed to the manuscript. A.M. contributed to study design, analysed, and interpreted the data and contributed to the manuscript. R.H.: chaired the Trial Steering Committee and contributed to the interpretation of the data and the manuscript. N.A. and A.S. collected and interpreted the data and contributed to the manuscript. M.L. contributed to recruitment, data collection including follow-up assessments, analysis of the electrocardiograms, data interpretation, and contributed to the manuscript. C.B. conceived the study, obtained the funding, recruited and randomized patients, collected data, interpreted the results and wrote the manuscript. J.N. and A.B. analysed resource utilization and related costs during the index hospitalization and contributed to the manuscript. Prof. Berry is chief investigator and Sood and Prof. Briggs, Curzen, and Oldroyd are co-applicants on the British Heart Foundation Project Grant (PG/11/55/28999) which supported this project.
Funding
This work was supported by a Project Grant from the British Heart Foundation Grant (PG/11/55/28999) and St Jude Medical provided the pressure wires. Professor Berry was supported by a Senior Fellowship from the Scottish Funding Council. Funding to pay the Open Access publication charges for this article was provided by the University of Glasgow.
Conflict of interest: Prof. Berry has acted as a consultant for St Jude Medical based on a contract with the University of Glasgow. Prof. Oldroyd has received consultant and speaker fees from St Jude Medical and Volcano Corporation which manufacture pressure wires. Prof. Curzen has received an unrestricted research grant and fees for lectures and consultancy from St Jude Medical and an educational grant with Volcano Corporation. None of the other authors have any potential conflict of interest to declare. These organizations had no other involvement in the study or in any aspect of this manuscript. The results were not disclosed to these organizations before the manuscript submission. The Chief Investigator had full access to all of the study data and had final responsibility for the decision to submit for publication.
Acknowledgements
The Clinical event committee: Andrew Hannah (Chair), Andrew Stewart, Malcolm Metcalfe (all Department of Cardiology, Aberdeen Royal Infirmary, UK). The Data and Safety Monitoring Committee: John Norrie, University of Aberdeen, UK (Chair); Saqib Chowdhary, University Hospital of South Manchester, UK; Andrew Clark, University of Hull, UK. The Trial Steering Committee members: Robert Henderson (Chair), Kanarath Balachandran, Colin Berry (Principal Investigator), Gordon Baird (Patient Advocate), Anna O'Donnell, Arvind Sood, Nick Curzen, Raj Das, Ian Ford, Jamie Layland, Shahid Junejo, and Keith Oldroyd. We thank Richard Papworth PhD for data management and quality assurance. We gratefully acknowledge the patients who participated in this study and the staff in our hospitals who supported it. We thank Ahmed Mahrous who helped with data collection.
References
- 1.Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D ESC Committee for Practice Guidelines. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 2.Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE, Jr, Ettinger SM, Fesmire FM, Ganiats TG, Jneid H, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP, Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, II, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS. 2011 ACCF/AHA focused update of the Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction (updating the 2007 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American College of Emergency Physicians, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;57:e215–e367. doi: 10.1016/j.jacc.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa Uva M, Taggart D Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS); European Association for Percutaneous Cardiovascular Interventions (EAPCI) Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501–2555. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 4.White CW, Wright CB, Doty DB, Hiratza LF, Eastham CL, Harrison DG, Marcus ML. Does visual interpretation of the coronary arteriogram predicts the physiologic importance of a coronary stenosis? N Engl J Med. 1984;310:819–824. doi: 10.1056/NEJM198403293101304. [DOI] [PubMed] [Google Scholar]
- 5.Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, Maccarthy PA, Van't Veer M, Pijls NH. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55:2816–2821. doi: 10.1016/j.jacc.2009.11.096. [DOI] [PubMed] [Google Scholar]
- 6.Selby JV, Fireman BH, Lundstrom RJ, Swain BE, Truman AF, Wong CC, Froelicher ES, Barron HV, Hlatky MA. Variation among hospitals in coronary-angiography practices and outcomes after myocardial infarction in a large health maintenance organization. N Engl J Med. 1996;335:1888–1896. doi: 10.1056/NEJM199612193352506. [DOI] [PubMed] [Google Scholar]
- 7.Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek J, Koolen JJ, Koolen JJ. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334:1703–1708. doi: 10.1056/NEJM199606273342604. [DOI] [PubMed] [Google Scholar]
- 8.Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van't Veer M, Bär F, Hoorntje J, Koolen J, Wijns W, de Bruyne B. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105–2111. doi: 10.1016/j.jacc.2007.01.087. [DOI] [PubMed] [Google Scholar]
- 9.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 10.De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Möbius-Winkler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engström T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Jüni P, Fearon WF FAME 2 Trial Investigators. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991–1001. doi: 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 11.Curzen N, Rana O, Nicholas Z, Golledge P, Zaman A, Oldroyd K, Hanratty C, Banning A, Wheatcroft S, Hobson A, Chitkara K, Hildick-Smith D, McKenzie D, Calver A, Dimitrov BD, Corbett S. Does routine pressure wire assessment influence management strategy at coronary angiography for diagnosis of chest pain?: the RIPCORD study. Circ Cardiovasc Interv. 2014 doi: 10.1161/CIRCINTERVENTIONS.113.000978. Epub ahead of print) PubMed PMID: 24642999. [DOI] [PubMed] [Google Scholar]
- 12.Uren NG, Crake T, Lefroy DC, de Silva R, Davies GJ, Maseri A. Reduced coronary vasodilator function in infarcted and normal myocardium after myocardial infarction. N Engl J Med. 1994;331:222–227. doi: 10.1056/NEJM199407283310402. [DOI] [PubMed] [Google Scholar]
- 13.Ragosta M, Powers ER, Samady H, Gimple LW, Sarembock IJ, Beller GA. Relationship between extent of residual myocardial viability and coronary flow reserve in patients with recent myocardial infarction. Am Heart J. 2001;141:456–462. doi: 10.1067/mhj.2001.113074. [DOI] [PubMed] [Google Scholar]
- 14.Potvin JM, Rodés-Cabau J, Bertrand OF, Gleeton O, Nguyen CN, Barbeau G, Proulx G, De Larochellière R, Déry JP, Batalla N, Dana A, Facta A, Roy L. Usefulness of fractional flow reserve measurements to defer revascularization in patients with stable or unstable angina pectoris, non-ST-elevation and ST-elevation acute myocardial infarction, or atypical chest pain. Am J Cardiol. 2006;98:289–297. doi: 10.1016/j.amjcard.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 15.Leesar MA, Abdul-Baki T, Akkus NI, Sharma A, Kannan T, Bolli R. Use of fractional flow reserve versus stress perfusion scintigraphy after unstable angina. Effect on duration of hospitalization, cost, procedural characteristics, and clinical outcome. J Am Coll Cardiol. 2003;41:1115–1121. doi: 10.1016/s0735-1097(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 16.Ntalianis A, Sels JW, Davidavicius G, Tanaka N, Muller O, Trana C, Barbato E, Hamilos M, Mangiacapra F, Heyndrickx GR, Wijns W, Pijls NH, De Bruyne B. Fractional flow reserve for the assessment of nonculprit coronary artery stenoses in patients with acute myocardial infarction. JACC Cardiovasc Interv. 2010;3:1274–1281. doi: 10.1016/j.jcin.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Sels JW, Tonino PA, Siebert U, Fearon WF, Van't Veer M, De Bruyne B, Pijls NH. Fractional flow reserve in unstable angina and non-ST-segment elevation myocardial infarction experience from the FAME (Fractional flow reserve versus Angiography for Multivessel Evaluation) study. JACC Cardiovasc Interv. 2011;4:1183–1189. doi: 10.1016/j.jcin.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Layland J, Carrick D, McEntegart M, Ahmed N, Payne A, McClure J, Sood A, McGeoch R, MacIsaac A, Whitbourn R, Wilson A, Oldroyd K, Berry C. The vasodilatory capacity of the coronary microcirculation is preserved in selected patients with NSTEMI. Circ Cardiovasc Intervention. 2013;6:231–236. doi: 10.1161/CIRCINTERVENTIONS.112.000180. [DOI] [PubMed] [Google Scholar]
- 19.Berry C, Layland J, Sood A, Curzen NP, Balachandran KP, Das R, Junejo S, Henderson RA, Briggs AH, Ford I, Oldroyd KG. Fractional flow reserve versus angiography in guiding management to optimize outcomes in non-ST-elevation myocardial infarction (FAMOUS-NSTEMI): rationale and design of a randomized controlled clinical trial. Am Heart J. 2013;166:662–668. doi: 10.1016/j.ahj.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hicks KA, Hung HMJ, Mahaffrey KW, et al. Standardized definitions for end point events in cardiovascular trials. http://www.cdisc.org/stuff/contentmgr/files/0/2356ae38ac190ab8ca4ae0b222392b37/misc/cdisc_november_16__2010.pdf .
- 21.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Thygesen K, Alpert JS, White HD, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Chaitman BA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasché P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S, editors. Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction, ESC Committee for Practice Guidelines (CPG) Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–2567. [Google Scholar]
- 22.Barrett BJ, Parfrey PS. Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med. 2006;354:379–386. doi: 10.1056/NEJMcp050801. [DOI] [PubMed] [Google Scholar]
- 23.Stone GW, Bertrand M, Colombo A, Dangas G, Farkouh ME, Feit F, Lansky AJ, Lincoff AM, Mehran R, Moses JW, Ohman M, White HD. Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial: study design and rationale. Am Heart J. 2004;148:764–775. doi: 10.1016/j.ahj.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 24.The EQ-5D-3L health questionnaire. http://www.euroqol.org/eq-5d-products/eq-5d-3l.html .
- 25.NHS Scotland; National Procurement, Procurement, Commissioning and Facilities. http://www.nhsscotlandprocurement.scot.nhs.uk/ [Google Scholar]
- 26.Leeds: Department of Health; 2012. NHS Reference Costs 2011–2012. https://www.gov.uk/government/publications/nhs-reference-costs-financial-year-2011-to-2012 . [Google Scholar]
- 27.Information Services Division Scotland. ISD Scotland; 2012. Theatre - direct cost per hour, by specialty [R142X] [Online] http://www.isdscotland.org/Health-Topics/Finance/Costs/Detailed-Tables/Theatres.asp . [Google Scholar]
- 28.Guidelines for Good Clinical Practice in Clinical Trials. http://www.mrc.ac.uk/Utilities/Documentrecord/index.htm?d=MRC002416 .
- 29.DAMOCLES Study Group, NHS Health Technology Assessment Programme. A proposed charter for clinical trial data monitoring committees: helping them to do their job well. Lancet. 2005;365:711–722. doi: 10.1016/S0140-6736(05)17965-3. [DOI] [PubMed] [Google Scholar]
- 30.Carrick D, Behan M, Foo F, Christie J, Hillis WS, Norrie J, Oldroyd KG, Berry C. Influence of fractional flow reserve measurement on treatment-decisions in patients with non-ST elevation myocardial infarction. Am J Cardiol. 2013;111:45–50. doi: 10.1016/j.amjcard.2012.08.046. [DOI] [PubMed] [Google Scholar]
- 31.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pijls NH, van Son JA, Kirkeeide RL, De Bruyne B, Gould KL. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation. 1993;87:1354–1367. doi: 10.1161/01.cir.87.4.1354. [DOI] [PubMed] [Google Scholar]
- 33.Spaan JA, Piek JJ, Hoffman JI, Siebes M. Physiological basis of clinically used coronary hemodynamic indices. Circulation. 2006;113:446–455. doi: 10.1161/CIRCULATIONAHA.105.587196. [DOI] [PubMed] [Google Scholar]
- 34.Uren NG, Crake T, Lefroy DC, de Silva R, Davies GJ, Maseri A. Reduced coronary vasodilator function in infarcted and normal myocardium after myocardial infarction. N Engl J Med. 1994;331:222–227. doi: 10.1056/NEJM199407283310402. [DOI] [PubMed] [Google Scholar]
- 35.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 36.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA. Freij A, Thorsén M, editors. PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]