Abstract
Current therapy for HIV effectively suppresses viral replication and prolongs life, but the infection persists due, at least in part, to latent infection of long-lived cells. One favored strategy towards a cure targets latent virus in resting memory CD4+ T cells by stimulating viral production. However, the existence of an additional reservoir in bone marrow hematopoietic progenitor cells has been detected in some treated HIV-infected people. This review describes approaches investigators have used to reactivate latent proviral genomes in resting CD4+ T cells and hematopoietic progenitor cells. In addition, we review approaches for clearance of these reservoirs along with other important topics related to HIV eradication.
Keywords: HIV/AIDS, HIV latency, antiviral therapy, HDAC inhibitors, resting memory CD4+ T cells, hematopoietic progenitor cells
Introduction
Over 34 million people around the world are living with HIV infection as of 2011 [1]. Without therapy, HIV infection leads to the development of AIDS and eventually death in the majority of infected people. Current therapeutic regimens effectively suppress viral replication but do not cure disease and lifelong therapy is required. Thus, treatment poses an economic burden for HIV-infected people and for health care systems. In middle and lower-income countries, over 8 million HIV-infected people received antiretroviral drugs in 2011, while another 7 million who were eligible for treatment still did not have access [1]. 2013 treatment guidelines recommended even earlier treatment, and so the number of people eligible globally jumped from 15.9 million to 28.6 million, increasing the treatment gap even further [2]. Thus, there is an urgent need for the development of a therapeutic regimen that will cure disease.
Currently, therapies prevent new infections by inhibiting viral enzymes, including reverse transcriptase, integrase, and protease or by blocking viral entry into a cell. When used in combinations for optimal treatment, referred to as combination antiretroviral therapy (cART), these highly potent drugs reduce plasma viral loads to levels below detection by sensitive clinical assays. However, more sensitive approaches still detect viral genomes in plasma samples after up to 7 years of optimal treatment in HIV-infected people [3]. Thus, despite years of viral suppression, disruption of treatment inevitably leads to a rebound in circulating virus.
The main mechanism through which HIV is believed to persist is through latent infection of long-lived cells. After viral entry, the HIV reverse transcriptase creates a DNA copy of the HIV RNA genome. The DNA provirus then integrates into the host genome within the nucleus. If the proviral genome remains latent, there is little to no transcription of viral genes due to host or viral blocks. Latent infection can be established and maintained as a result of multiple factors: host transcription factor availability, epigenetic modifications, defects in the HIV Tat protein, site and orientation of integration, and post-transcriptional regulatory mechanisms (reviewed in [4], [5], and [6]). Current cART regimens, which target entry, reverse transcription and integration, effectively prevent new viral infections, but they do not affect integrated provirus.
Resting memory CD4+ T cells are the best-studied long-lived cellular reservoir of latent HIV infection. However, recent studies implicate bone marrow hematopoietic stem and progenitors cells (HSPCs) as a potentially important latent long-lived reservoir detectable in some donors [7–9]. While other shorter-lived cell types, including monocytes/macrophages and astrocytes ([10–13], reviewed in [14,15]), have also been implicated, this review will focus primarily on cell types that are long-lived. Thus, we will compare potential therapeutic strategies for eventual clearance of latent HIV infection of memory CD4+ T cells and bone marrow HSPCs. Important questions for further investigation of HIV reservoirs and implications of the currently proposed model of therapy are also discussed.
Defining latent reservoirs
A clinically significant latent reservoir is one that has the potential to produce infectious virus that can cause rebound viremia when treatment is stopped. Thus, this reservoir should have the capacity to harbor provirus for long periods of time, given that residual virus has been detected after more than 7 years of treatment [3].
Resting CD4+ T cells
It is well established that resting memory CD4+ T cells are a stable reservoir of latent HIV infection [16,17]. One study that estimated the size of the T cell reservoir using a viral outgrowth assay found that the CD4+ T cell reservoir decays extremely slowly with a half-life of 44 months [17]. Another study examining resting memory T cells predicted no significant loss of integrated HIV DNA over time, with a predicted half-life of roughly 25 years [18].
Resting CD4+ T cells contain barriers to productive viral infection, including rigid cortical actin, which inhibits transport of the preintegration complex, expression of cellular restriction factors that inhibit reverse transcription and low transcriptional activation (reviewed in [19]). Because of these barriers to infection of resting T cells, most latent infection may occur when infected, activated T cells become quiescent. Alternatively, direct latent infection of resting T cells may be facilitated by cytokines, endothelial cells, or other environmental interactions ([20], reviewed in [21]).
The gold standard for the detection of latently infected cells utilizes an assay in which resting memory CD4+ T cells are activated and viral outgrowth is measured. However, a recent study indicates that this technique potentially underestimates latent genomes in circulating resting T cells by up to 60-fold [22]. In this study, Ho et al found a significant subset of the non-induced proviruses did not contain lethal mutations indicating that these non-induced proviruses are capable of producing new infectious virions upon reactivation. Additionally, reconstructed non-induced proviruses produced virions with similar infectivity to those reconstructed from induced proviruses. Because these proviral genomes did not appear to be activated and cleared by standard T cell activation methods, there appear to be barriers to reactivation of functional proviruses in latently infected resting T cells that are not well understood [22].
Resting memory T cells have been divided into different subtypes, including central memory (TCM), transitional memory (TTM], effector memory (TEM], and the recently-characterized stem cell memory T cells (TSCM]. TCM cells localize to lymph nodes and, upon stimulation, will become TEM cells that can move into tissues to perform inflammatory and cytotoxic functions [23]. TTM cells show an intermediate phenotype between TCM and TEM cells [24]. The contribution of each of these subtypes to the HIV-1 reservoir is variable [23–27]. A study by Chomont et al. implicated TCM and TTM cells as the major components of the CD4+ T cell reservoir [25]. TCM cells form a reservoir of reduced size that decays slowly in HIV-infected people with normal CD4+ T cell counts who started treatment early after infection. TTM cells, on the other hand, are the primary reservoir in HIV-infected people with lower CD4 counts at the time of cART initiation. Evidence was presented that these latently infected cells may be maintained over time by homeostatic proliferation due to continuous immune activation [25].
TSCM cells are the least differentiated T cell subset with the greatest capacity for self-renewal [26]. Recently, it was reported that TSCM cells are also infected by HIV [26–29]. Buzon et al. studied these long-lived cells in HIV-infected people with optimal viral suppression for a median of 7 years and found that latently infected CD4+ TSCM cells contribute a significant portion of the HIV DNA in resting memory T cells. The TSCM contribution increased over the course of therapy as more differentiated T cell subsets that initially contributed to the reservoir were lost. The authors provided a longitudinal phylogenetic analysis of plasma and resting T cell viral sequences in 3 HIV-infected people, beginning pre-therapy and continuing at multiple time points up to 13 years post-diagnosis. These data provide evidence that TSCM cells may be infected early and continue to harbor viral genomes for an extended period [27]. Thus, eradication strategies should also target TSCM cells.
Though it is widely accepted that resting CD4+ T cells are an important source of latent infection, it is not clear that this is the only reservoir contributing to HIV persistence. One study of two optimally treated HIV-infected people found that sub-genomic amplicons derived from plasma virus exactly matched the same sub-genomic amplicons derived from virus produced by reactivated resting CD4+ T cells [30]. However, other studies that have isolated residual plasma virus from optimally treated people with suppressed viral loads were not able to match viral genome sequences to any provirus found in circulating resting T cells [31–33]. The study by Brennan et al. compared provirus in resting CD4+ T cells with plasma virus, and found significant compartmentalization of sequences in circulating T cells versus the plasma in 12 out of 14 optimally treated HIV-infected people [31]. Buzon et al. reported close relationships between plasma viral sequences and provirus from T cell subsets. However they did not report any identical viral sequences that were found in both plasma and resting CD4+ T cells [27]. Thus, there may be additional cellular reservoirs besides resting CD4+ T cells that produce virus in optimally treated people.
Hematopoietic Progenitor Cells
A long-lived infected HSPC could also be an important contributor to residual HIV in treated HIV-infected people as HSPCs express HIV receptors [7,8]. HSPCs are a heterogeneous population of cells and include subsets with extensive capacity for self-renewal. Because some analyses of plasma virus found that certain identical sequences predominate in circulation over multiple time points, it was proposed that latently-infected stem cells, with the capacity for self-renewal, contributed clonal virus upon intermittent activation [3]. Indeed, a number of studies have provided evidence that HIV can infect CD34+ bone marrow progenitors [7–9,34–36]. A study of HIV-infected people in Africa revealed that HIV-1 subtype C could infect HSPCs in vitro and in vivo. Participants with HIV-infected bone marrow progenitors also had higher rates of anemia [34].
More recent studies have now shown that HIV-1 subtypes B, C, and D can all infect HSPCs in vitro [7]. Moreover, these studies demonstrate that HIV can infect multipotent progenitors that form colonies of multiple different lineages in methylcellulose assays. Notably, HIV can also infect bona fide stems cells in vitro based on engraftment and production of all major hematopoietic lineages in an irradiated immune-deficient mouse [7,8].
To study latent infection in HSPCs, Carter et al. utilized an HIV molecular clone that expresses viral proteins under the control of the viral promoter and GFP under a constitutively-active promoter [7]. Thus, it was possible to distinguish uninfected (GFP−Gag−), actively infected (GFP+Gag+) and latently infected (GFP+Gag−) cells. When latently infected HSPCs were treated with cytokines that stimulate myeloid lineage differentiation (granulocyte macrophage-colony stimulating factor [GM-CSF] and tumor necrosis factor [TNF]-α), viral gene expression was induced. These studies demonstrate that HIV can infect HSPCs and cause both active and latent infection in vitro.
In addition, HIV Gag+ CD34+ progenitors were detected in bone marrow aspirates from some HIV+ donors with high viral loads. Progenitor cells from one donor that initially lacked detectable Gag expression, expressed Gag upon culture with GM-CSF and TNF-α. Examination of HIV-infected individuals on cART with undetectable viral loads revealed no detectable Gag expression in HSPCs, but HIV genomes were amplified with quantitative PCR from 4 out of 9 donors [7]. These initial studies provided evidence supporting the conclusion that latent HIV infection occurs in bone marrow HSPCs in vivo.
Two other groups have searched for latent HIV genomes in CD34+ bone marrow cells from HIV+ donors on long-term cART without success. Josefsson et al. did not detect HIV amplicons in CD4− CD34+ HSPCs in a cohort of eight virally suppressed HIV-infected people: five who initiated cART during acute or early infection and three who started cART during chronic infection [37]. In this study, the authors removed CD4+ cells to deplete the sample of T lymphocytes. However, a subset of HSPCs express CD4 and CD4 is required for HIV infection of HSPCs [8]. Thus, it is likely that the negative results from this study were due to the absence of susceptible cells in the samples. The study by Durand et al. tested HSPCs from a cohort of 11 optimally treated HIV-infected people, 10 of whom were diagnosed prior to 2001 [38]. These investigators were unable to detect HIV DNA in CD34+ HSPCs by real-time PCR. Nor could they detect virus produced using a co-culture assay of HSPCs stimulated with GM-CSF and TNF-α plus activated CD4+ lymphoblasts. Based on the latter study, some investigators suggested the possibility that CD4+ T cell contamination confounded prior results [7]. However, because the Durand et al. study was not powered to detect DNA in HSPCs from donors diagnosed after 2001, an alternative explanation is that it is harder to detect HIV infection of HSPCs in people infected decades ago, before optimal therapy was available. Indeed, all donors who tested positive in the prior study were diagnosed more recently [7].
To determine whether the year of diagnosis was indeed a determinant for detection of the HSPC reservoir, McNamara et al. recruited an additional 11 virally suppressed donors who had initiated cART during chronic infection [35]. For this study, CD133+ cells, were isolated, which allowed purification of a population enriched for stem cells. HIV genomes were detected in 6 out of the 11 donors, including in two donors that had undetectable viremia for over eight years. Samples had high CD133+ HSPC purity (<1% CD3+ T cells) and for 5 out of the 6 donors positive for HIV DNA in HSPCs, the genomes detected were determined to not be due to contaminating T cells by statistical analysis in comparison to CD133-depleted bone marrow cells. Interestingly, donors with detectable HIV DNA in HSPCs received their diagnosis significantly more recently (after 2001) than the remaining donors, but had undetectable viral loads for similar periods. Further studies with larger numbers of donors are now needed to confirm that HIV-infected HSPCs are harder to detect in people who were diagnosed earlier in the pandemic before widespread use of cART. Moreover, additional studies are necessary to determine whether HSPCs harboring provirus are a clinically significant reservoir that contributes to residual plasma viremia.
Latency and Eradication
Targeting Latent Infection
As discussed above, latently infected cells do not produce viral proteins that would lead to cytopathic effects and eventual cell death. In addition, latently infected cells are not recognized and cleared by the immune system. Current anti-retroviral drugs, which target early stages of the HIV replication cycle, cannot inhibit this non-productive infection once established. Thus, to eradicate these infected cells, new latency-reversing agents (LRAs) are being developed to oppose latency and thus force the virus to reveal itself. With concurrent cART, this approach, termed ‘shock and kill,’ aims to eliminate the infected reservoir while blocking new infection events [39].
Multiple factors contribute to latent HIV infection, including host transcription factors that bind the viral promoter and epigenetic changes that affect chromatin and alter accessibility of the viral promoter to transcriptional machinery (reviewed in [4], [5], and [6]). Thus, current work has focused on strategies to counteract these factors in favor of ‘shock’ or reactivation of latent HIV. Reactivated infected cells then need to be ‘killed,’ preferably by activation of cellular death pathways or through the host immune response. Methods that have demonstrated in vitro efficacy at reactivation of latent CD4+ T cell infection have been employed in clinical trials with limited success (reviewed in [40]). Thus, more research is needed to better understand this approach. Here, we highlight a few of the major strategies for reversing HIV latency in resting CD4+ T cells, which have recently been reviewed in detail [41–44], and discuss our current understanding of the HSPC reservoir (Table 1).
Table 1.
Summary of Latency-Reversing Agents
| Category | Agent | Mechanism* | Reactivation in HPCs |
Viral outgrowth in ex vivo T cell models*** |
Other Major Latency Models Tested**** |
Clinical effect on T cell reservoir |
|---|---|---|---|---|---|---|
|
Chromatin Accessibility |
SAHA | HDAC inhibition | Yes [9] | Yes [83], No [48] | U1.ACH-2, J89[42] | No reduction of proviral DNA, increase in HIV transcription [91] |
| Romidepsin | HDAC inhibition | Not tested | No [48], Yes[45] | Primary T cell [45] | Not tested | |
| Panobinostat, Givinostat, Belinostat |
HDAC inhibition | Not tested | No [48] (Panobinostat) | U1, ACH-2, J89 (Givinostat, Belinostat) [42] |
Not tested | |
| Aza-CdR | DNA methylation inhibition |
No [9] | Not tested | J-Lat, ACH-2, U1 [42] | Not tested | |
|
Activation of Transcription Factors |
HMBA | P-TEFb activation | No [9] | Yes [83] | U1, ACH-2 [42] | Not tested |
| Prostratin, Bryostratin-1 |
PKC activation | Weak [9] (Prostratin) |
Yes [83] (Prostratin), No [83] (Bryostatin), No [48] (Bryostratin) |
J-Lat, SCID-humanized mouse, and Bcl-2 (Prostratin) [42], THP-p89 and J1.1 (Bryostratin) [42] |
Not tested | |
| Disulfiram | AKT activation | No** | No [48] | Bcl-2 [42] | No reduction of proviral DNA [65] |
|
| Cytokines | IL-7 | Cellular activation and cell cycling |
Not tested | No [83] (IL-7+IL-2), Yes [66,67] |
SCID-humanized mouse [145] | Not tested |
| TNF-alpha | NF-κB activation and histone remodeling |
Yes [7,9] | Yes [83] | ACH-2, J-Lat [57] | Not tested | |
| TLR agonists | NF-κB activation | Not tested | Not tested | Primary T cell, primary monocyte, U1 [40] |
Not tested | |
| Interferon alpha |
Unknown | Not tested | Not tested | None | Reduction in proviral DNA [81] |
See references in text.
McNamara, Ganesh, and Collins, unpublished.
AII ex vivo models test the effect of latency-reversing agents on latently infected resting CD4+ T cells isolated from optimally suppressed HIV+ donors. Study [48] added a T-lymphoblast cell line, MOLT4/CCR5, while others added allogeneic PBMCs.
Direct comparison of ex vivo versus multiple in vitro models for many of these latency-reversing agents can be found in reference [83].
Bcl-2 = model using primary CD4+ T cells transduced with Bcl-2
SCID-humanized mouse = immunodeficient mouse with human immune cells after bone marrow engraftment
Chromatin Accessibility
A major focus for reactivation studies in vitro and in vivo has been on compounds that affect the epigenetic regulation of the integrated HIV genome. Histone deacetylase complex inhibitors (HDACis), including suberoylanilide hydroxamic acid (SAHA; vorinostat), romidepsin, and panobinostat, have been at the forefront of these studies (reviewed in [43], [45]). SAHA, the best-studied HDACi, induces reactivation in both T cell lines containing integrated HIV and primary T cells [46,47]. However, a recent study using resting T cells from HIV-infected people found that SAHA primarily promotes read-through transcription from host gene promoters and only minimally activates HIV LTR-driven transcription. The result is low protein expression and little cytopathic effect [48]. Another ex vivo assay used to quantitate reactivation of latent proviruses determined that SAHA induced virion production from an average of 0.079% of the total proviruses in resting CD4+ T cells isolated from optimally treated HIV-infected people, indicating the need for stronger interventions for latency reversal [49].
Much less is known about the effect of HDACis on HIV latency in HSPCs (Table 1). In a primary cell model of HSPC latency that utilizes freshly isolated, infected and sorted cells, SAHA induced HIV gene expression, but at doses higher than 1 µM (2 to 10 µM) that are not physiologically achievable [9]. These levels of SAHA were also cytotoxic and generated less reactivation than TNF-α. Additional research is needed to determine how to enhance the efficacy and selectivity of LRAs.
DNA methylation — the de novo methylation of CpG islands in the viral genome post-integration — was thought to play an important role in the late establishment or maintenance of resting T cell latency, with many studies initially focusing on in vitro models of latency [50,51]. Studies with T cell line models of latency observed reactivation of latently infected cells with the DNA methylation inhibitor 5-aza-2’deoxycytidine (aza-CdR) and a synergistic effect of using this drug for reactivation in combination with an activator of NF-κB [50,51]. However, a recent study noted that there was little DNA methylation in latently infected resting CD4+ T cells from treated HIV-infected people with suppressed viral loads [52]. This was affirmed by a study that found only unmethylated CpG’s when assessing over half the CpG islands in HIV genomes in peripheral blood mononuclear cell (PBMC) DNA samples from a diverse cohort of HIV-infected people [53]. Aza-CdR was also tested in an HSPC model of latency. Here, Aza-CdR by itself or combined with TNF-α did not detectably reactivate transcription of latent HIV genomes [9].
Activation of Host Transcription Factors
Another potential reactivation mechanism targets transcription factors in the host cell that are important for expression of the HIV genome. Immune modulating compounds discussed below, including TNF-a and toll-like receptor (TLR) agonists, can reactivate latent HIV in some cell systems through induction of NF-κB, which binds to specific sites in the HIV promoter region of the long terminal repeat (LTR) to promote transcription [54–57]. However, in primary CD4+ T cells, TNF-α is not sufficient to reactivate viral gene expression. In addition to NF-κB, positive transcription elongation factor b (P-TEFb) is needed for HIV transcription and resting memory cells have very low levels of this factor [58]. Hexamethylbisacetamide (HMBA) activates P-TEFb in CD4+ T cells by releasing it from an inhibitory cytoplasmic complex and allowing binding at the HIV LTR [59,60]. Resting CD4+ T cells isolated from HIV-infected people on antiretroviral therapy with undetectable viremia can produce virus upon HMBA treatment [60]. In contrast, TNF-α is sufficient for induction of HIV gene expression in latently infected HSPCs cultured in vitro, which have high baseline levels of P-TEFb [9]. Correspondingly, the addition of HMBA does not reverse latency in HSPCs [9]. Prostratin also activates the NF-κB pathway through protein kinase C (PKC) activation, and has been shown to reverse HIV latency in both primary T cells and Jurkat T cell line latency models [61,62]. In latently infected HSPCs, prostratin reactivated latent HIV at high doses (1 to 5 µM), but not to the same extent as TNF-α [9].
Disulfiram, an inhibitor of acetaldehyde dehydrogenase used for treating alcoholism, reactivates latent HIV in a primary CD4+ T cell model of HIV latency [63] but did not reactivate latent infection in the HSPC latency model (McNamara, Ganesh and Collins, unpublished studies). Disulfiram activates the protein kinase b (Akt) pathway that eventually leads to activation of NF-κB [64]. A recently published clinical trial found that 14 days of disulfiram treatment in 15 HIV-infected people on antiretroviral therapy did not decrease the size of the latent reservoir in circulating PBMCs [65]. However, the drug was well tolerated and a short-lived increase in plasma viremia immediately after receipt of disulfiram was observed. It is possible that disulfiram will demonstrate greater efficacy at higher doses or in combination with other therapies [65].
Immunomodulators
Immune-modulating molecules have been investigated as potential LRAs for latently infected cells. Interleukin-7 (IL-7) is one of the top cytokine candidates for induction of latent provirus in memory CD4+ T cells [66,67]. However, a recent study found that IL-7 might actually contribute to viral persistence in HIV-infected people. Vandergeeten et al. found that IL-7 treatment induced proliferation of cells harboring latent virus and expanded the reservoir [68]. In other studies, homeostatic proliferation of central memory T cells did not activate latent HIV [69]. Thus, cytokine induction of proliferation may increase the amount of proviral DNA within an infected individual without any clearance.
TNF-α has long been known to induce expression of HIV in T cell line models of latency [70]. In HSPC latency models, reactivation of latent infection in HSPCs was induced by TNF-α treatment via an NF-κB-dependent mechanism [9]. However, TNF promotes differentiation of progenitors towards a myeloid lineage. Thus, it is not a good candidate for general administration given its non-specific effects on HSPCs and other immune cells (reviewed in [71,72]).
TLR agonists that specifically activate innate immune pathways have also been shown to activate latent HIV in resting memory CD4+ T cells, but have not yet been tested in HSPCs ([56,73–75], reviewed in [40]). A recent double-blind randomized controlled clinical trial examined the effects of administering three doses of a pneumococcal vaccine with a TLR9 agonist in 31 HIV-infected people compared with a placebo adjuvant in 37 HIV-infected people [76]. This treatment resulted in a small, but significant, decrease in PBMC proviral load within the group treated with the TLR9 agonist compared with the mainly unchanged control group. This decline in the experimental group was accompanied by an increase in HIV-specific CD8+ T cell immunity, which points toward the potential for these agonists to both reactivate latent infection and clear the latent reservoir. HSPCs express TLRs, and signaling through these receptors occurs in mouse hematopoietic stem cells (HSC) [77,78]. In mice, activation of these receptors induces HSC proliferation, biases to myeloid differentiation, and diminishes self-renewal and engraftment capacity [72,78–80].
In a recent study, evidence was provided that pegylated (Peg) interferon-α-2a can suppress HIV replication and reduce the numbers of T lymphocytes harboring provirus in treated people [81]. In this study, the authors recruited 23 optimally treated, HIV-positive people. All subjects received Peg-interferon-α-2a therapy in addition to cART for 5 weeks; then cART, but not Peg-interferon-α-2a therapy, was interrupted for 12–24 weeks. Intriguingly, a significant decrease in the number of proviruses per CD4+ T cell was detected in 7 subjects who maintained viral suppression at week 12. Based on these results, Peg-interferon-α-2a may assist in clearance of the viral reservoir. While these data are interesting, more studies with larger numbers of participants are needed to understand the significance as it is not clear by what mechanism Peg-interferon-α-2a could clear latent reservoirs [82].
Challenges with Latency-Reversing Agents
Although many of the LRAs discussed above show potential for antagonizing HIV latency, recent studies emphasize the need for further work to understand their clinical utility; there have been variable results when the same compound is tested side-by-side in different in vitro latency models and limited success thus far as sole therapies in clinical trials. Spina et al. [83] measured the effect of a panel of LRAs on multiple widely used models of latency compared with the standard quantitative viral outgrowth assay (QVOA) that uses patient-derived latently infected resting CD4+ T cells. They found that no in vitro latency model recapitulates the ex vivo QVOA results, with many of the models seemingly biased towards reactivation by only specific classes of agents. PKC agonists generally induced latent HIV in the majority of models tested (Table 1). This paper underlines the potential difficulties of using a single in vitro model to identify the best clinical approach for ‘shocking’ latent HIV.
HDACis (SAHA, romidepsin and panobinostat) and disulfiram did not induce viral outgrowth in a newly developed ex vivo assay that may better reflect in vivo conditions because it uses cells from HIV-infected people and does not employ allogeneic T cells, which may confound results [48]. Using this assay, viral outgrowth was only observed from donor CD4+ T cells treated with T cell activating agents [48] (Table 1). T cell activation and bryostatin-1, a PKC agonist, significantly induced HIV mRNA expression whereas the HDACis and disulfiram did not.
Humanized mouse and primate models may be useful in vivo models for further trials of LRAs and other strategies for eradicating virus [84,85]. Kauffman et al. recently developed a Rhesus Macaque cART model [85]. This model recapitulates what has been observed in human studies with respect to plasma viral sequence diversity after suppression with antiretrovirals and treatment interruptions.
Based on initially promising in vitro studies, SAHA, panobinostat, disulfiram, and IL-7 have been or are currently being tested in clinical trials with no clear success as yet (reviewed in [40]). The first study using the ‘shock’ strategy examined the effect of the HDACi valproic acid plus a viral entry inhibitor over a three-month period [86]. In this study, four HIV-infected individuals on cART had declines in numbers of infected CD4+ T cells ranging from 68% to over 84%. However, subsequent trials of valproic acid failed to replicate these results [87–90]. In a separate study, SAHA treatment was found to increase HIV RNA expression in resting CD4+ T cells, but had no detectable impact on residual plasma viremia [91]. As mentioned above, a pilot study of disulfiram treatment also demonstrated no effect on the size of the circulating latent reservoir [65]. While clinical trials with single agents have not yet been successful, combinations of LRAs may prove effective in further studies [42].
The HSPC reservoir is particularly difficult to assess with respect to understanding its response to treatment as infected cells are rare and there is no clear protocol for monitoring the size of the latent reservoir in this cell population. Previous studies have utilized bone marrow aspirations, which are more invasive than peripheral blood collection [7,35,37,38]. It is also unknown how representative a single bone marrow sample is of the HIV reservoir in marrow sites throughout the body and so it may be challenging to observe the effect of reactivation strategies on this reservoir.
Clearing Infection after Reversal of Latency
Reactivating reservoirs of latent HIV is only the first step of the ‘shock and kill’ approach. Strategies to eliminate cells after reversal of latency are an equally important consideration for a cure. The two main strategies for killing a cell with reactivated infection are activation of cell-death pathways and immune-mediated clearance.
Induction of Cell Death
In response to viral infection, cell death pathways become activated to prevent further spread of an infection [92]. However, HIV encodes strategies to delay death of the cell and favor the establishment of infection [93]. Further research should consider how well LRAs of interest can induce cell death in the various cell types implicated as reservoirs for latent HIV, as this effect may be cell-type dependent. One study found that ex vivo reactivation of latent virus with a 6-day treatment of the HDACi SAHA in PBMCs from cART-treated HIV-infected people did not reduce the number of latently-infected cells by a limiting dilution viral outgrowth assay [94]. Moreover, SAHA did not promote cell death of resting CD4+ T cells in an in vitro latency model, whereas T cell activation did [94].
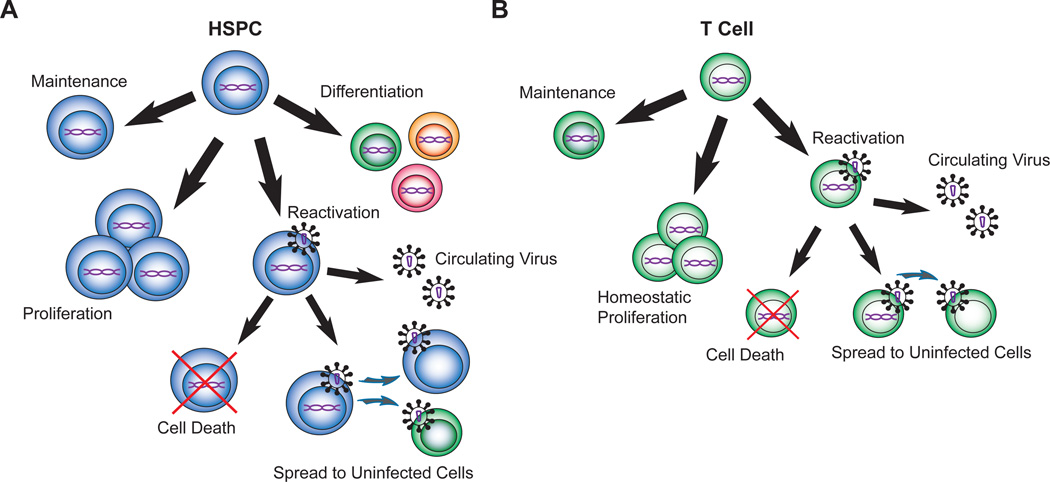
Similar to what has been observed in T lymphocytes, active infection of an HSPC induces apoptosis based on annexin V staining and loss of infected cells in culture [7]. However, it is not yet known whether reactivating latent infection in HSPCs leads to cell death in vitro or in vivo. If infected HSPCs are able to divide and differentiate without reversal of latency and activation of cell death, mature myeloid or lymphoid cells could retain latent HIV contributing to viral persistence (Figure 1) and these cell types would also need to be effectively targeted. Thus, studies are needed to better understand HIV infection of HSPCs and their progeny.
Figure 1. Potential outcomes of latent infection in a hematopoietic stem and progenitor cell versus a T cell.
A. Diagram representing conceivable fates of a hematopoietic progenitor with an integrated viral genome (purple). An infected HSPC can maintain or expand the pool of latently infected cells through remaining quiescent or proliferating without differentiation. With stimulation by cytokines or reactivation agents, the HSPC could go from a latent to an actively infected state, where cell death could be induced, virus could be produced to infect other cells, and new virions could contribute to plasma virus. An HSPC could theoretically differentiate into a mature hematopoietic cell such as a T cell and retain viral DNA. B. A latently infected T cell could persist through maintenance or homeostatic proliferation. With reversal of latency, the actively infected T cell could die, infect additional cells, and release virus into the periphery. HSPC: hematopoietic stem and progenitor cell
The importance of targeting pathways for cell death to augment clearance of HIV-infected cells or reactivated latent infection was recently covered in an excellent review [93]. The authors promote the idea of ‘prime, shock, and kill,’ in which cells would be pre-treated in a way that induced death after reactivation [93]. Two drugs already approved for clinical use, the topical antifungal ciclopirox and the iron chelator deferiprone, are promising as agents to induce cell death [95]. These drugs preferentially kill infected cells by lowering the natural threshold to apoptosis in all cells, while opposing viral proteins that prevent the induction of apoptosis in response to viral infection. Thus, the development of strategies that promote the death of cells treated with LRAs may facilitate clearance of residual HIV reservoirs.
Immune Clearance
Another strategy for clearing latent infection utilizes immune defenses to target and kill reactivated cells. According to the common definition of latency, there is little to no production of viral proteins, which makes them poor targets for cytotoxic T lymphocytes (CTLs). Anti-HIV CTLs limit replication of the virus, but these cells often show functional defects in the context of HIV infection [96]. A small group of HIV-infected people, referred to as elite controllers, have low levels of HIV replication without therapy, and these HIV-infected people have HIV-specific CTLs that can kill autologous resting CD4+ T cells that reactivate latent infection ex vivo [94]. In cART-treated HIV-infected people, latently-infected resting CD4+ T cells reactivated with SAHA ex vivo are not cleared by CTLs isolated from the same patient, unless those CTLs are pre-stimulated with HIV Gag peptides [94]. The susceptibility of infected bone marrow HSPCs to immune clearance has not yet been assessed, but is certainly an important consideration for targeting this potential reservoir.
Interestingly, in optimally suppressed HIV-infected people, there is evidence that a subset of resting CD4+ T cells, termed Gag-positive reservoir (GPR) cells, express Gag without supporting a spreading infection [97]. Though these cells are not truly latent given that transcription is occurring, Graf et at. found a correlation between the effectiveness of CTLs to clear GPR cells and lower levels of integrated HIV genomes per PBMC in vivo [98]. Thus, there is some evidence that CTLs can limit the reservoir size [98]. GPR cells are targeted more effectively by CTLs from elite controllers than non-controllers and could perhaps be killed, even without reactivating infection, by boosting the CTL function of HIV-infected people on therapy.
One problem with relying too heavily on the efficacy of CTL killing for reservoir clearance is that HIV has strategies to limit effective CTL recognition. In side-by-side assays, HIV-1-infected cells lacking Nef are more sensitive to CTL recognition and lysis than cells infected with wild type virus in vitro [99–102]. There is also evidence that the ability of Nef to promote immune evasion from CTLs by downmodulating major histocompatibility complex-class I molecules (MHC-I) is necessary for infection in vivo. Simian immunodeficiency virus (SIV) Nef alleles with difficult to revert mutations that specifically disrupt the ability of Nef to downmodulate MHC-I, rapidly evolve in vivo to acquire compensatory changes elsewhere in Nef that restore the ability of the recovered virus to downmodulate MHC-I [103]. Additionally, ex vivo analysis of Nef alleles provide evidence for the importance of this activity in HIV-1 infected people [104,105]. MHC-I downmodulation is one of the best-characterized functions of Nef and there is ample evidence that Nef enhances the ability of the virus to evade CTL clearance in vitro and in vivo.
The capacity of CTLs to recognize targets is also influenced by the availability of antigenic peptides. While there are examples in which CTLs require just a single epitope to kill a target [106], CTLs kill HIV-1 infected primary T lymphocytes more effectively when more viral antigen is available for presentation [107]. If safe and effective Nef inhibitors could be developed, combined approaches with latency antagonists and Nef inhibitors would be expected to optimize CTL recognition and clearance.
Strategies to boost immune recognition and clearance of HIV-infected cells are of interest. In addition, anti-HIV vaccinations and broadly neutralizing monoclonal antibodies as therapy to enhance the host immune response have had some promising results and these could be combined with LRAs to increase clearance of latent virus from T cell and HSPC reservoirs (reviewed in [108–110]). Recent studies using rhesus CMV-based vaccine vectors in macaque models are encouraging in this regard [111]. Another approach to immunotherapy includes stem cell-derived HIV-specific CTLs. Kitchen et al. genetically engineered and delivered a T cell receptor specific for a Gag epitope into human HSCs [112]. The transduced HSCs developed into mature T cells in human thymus implants in immunodeficient mice. These engineered CTLs could significantly suppress HIV replication in a humanized mouse HIV model, but have not yet been tested in humans.
Expert Commentary and Five-Year View
Hope for a global cure of HIV infection has been stimulated by the documented cure of an HIV-infected man following bone marrow transplantation in Berlin and the transient ‘functional cure’ of an infected baby from Mississippi [113,114]. However, there remain important questions that need to be addressed in the expedition toward a cure.
What are the important reservoirs in viral persistence?
The current focus on the well-studied reservoir of latently infected resting memory CD4+ T cells will need to be supplemented by investigations of other sources of persistent infection. Thus far, clinical trials with agents shown to reactivate infection in T cells have not been successful in eradicating the virus from HIV-infected people. This review discusses the possible contribution of bone marrow HSPCs to persistence, but additional studies may identify other reservoirs.
There is evidence that shorter-lived myeloid cells, including monocytes, macrophages, and dendritic cells are able to harbor integrated HIV and contribute to persistence (reviewed in [14,15]). Though infrequent, monocytes with integrated genomes have been recovered from HIV-infected people after many years of optimal viral suppression. Proviral genomes from these cells closely match residual plasma virus in a study of 7 HIV-infected people [115,116]. Monocyte-derived cells, including perivascular macrophages, microglial cells, and astrocytes have been implicated as reservoirs in the central nervous system ([11–13], reviewed in [15]). Because these cells are shorter-lived, their persistence may play a role in settings in which therapy is not optimal such that low level active infection can occur.
Although not discussed in depth here, current antiretroviral therapy may not completely block virus spread directly between cells and may also allow ongoing replication in anatomic sites with decreased drug penetration. Emerging evidence indicates that low level active infection can continue to occur in some people on effective antiretroviral treatment [108,117–125]. Studies in animal models have detected viral RNA in lymphoid tissue from the gastrointestinal tract, draining lymph node, spleen and in some cases, bone marrow [126]. Studies in human subjects have also revealed evidence of persistent active infection in CD14+ monocytes [124]. In addition, some intensification studies have detected unspliced HIV RNA in the ileum, suggesting ongoing productive infection in some HIV-infected people on ART [125]. Thus, infected cells in lymphoid tissue can potentially produce low levels of HIV that could re-seed the reservoir of persistent HIV. Continued virus production and infection could also lead to inflammation [117,127], which may play a role in maintaining the persistent reservoir of HIV. These additional issues may also need to be addressed for effective clearance of persistent virus.
Can a functional cure be achieved and is it enough?
In discussion of a cure, two categories have been proposed: sterilizing and functional [128]. With a sterilizing cure, there is complete eradication of all replication-competent HIV from a patient. On the other hand, with a functional cure, there is suppression of viral replication and maintenance of CD4+ T cell function without anti-retroviral therapy indefinitely. Thus far, there have been a few instances of functional cures when treatment was initiated early after initial infection. In one case, an HIV-infected woman from Mississippi who did not receive prenatal HIV treatment gave birth to a baby that immediately received cART [114]. The infant’s initial viral load decayed after treatment began, and, after treatment was stopped at 18 months of age, circulating virus remained undetectable for about 2 years without any therapy. Eventually however, the child developed detectable viremia and needed to resume treatment [129]. The extended period of virological control that occurred after therapy cessation offers hope that proviral reservoirs can be reduced with early treatment. A complementary study of infants infected perinatally found lower levels and higher decay rates of PBMC provirus in four children that began cART sooner (age 0.5–2.6 years) compared with four that began cART later (age 6–14.7 years) [130].
In adults, recent studies suggest that early treatment can lead to a higher than expected rate of post treatment controllers (PTCs). PTCs refer to treated individuals who are found to have very low levels of viral replication after interrupting therapy. A group of 14 adult PTCs were recently identified from a cohort that started treatment early during primary HIV infection, and were able to maintain viral control at least 24 months after treatment interruption [131]. These HIV-infected people generally had small HIV reservoirs in PBMCs and less infection of long-lived subsets of resting T cells. While complete eradication of HIV-infected cells would be ideal, it is practical to consider the goal of a functional cure, which could theoretically involve viral suppression without therapy after clearance of just a fraction of reservoirs. Additionally, treatments to boost immune function or prevent viral immune evasion, as with a Nef inhibitor, may be the most helpful to allow a patient’s own immune defenses to effectively control HIV replication.
What approaches besides ‘shock and kill’ should be considered?
Additional strategies towards a cure that are being considered include stem cell transplants, potentially augmented genetically to make cells resistant to infection. Gene therapy approaches that target latently infected cells are also being tested.
Stem Cell Transplants
The first person to be cured of HIV infection was treated with a bone marrow transplant for acute myeloid leukemia [113]. Often referred to as the Berlin patient, this 40-year-old man received an allogeneic bone marrow transplant (BMT) from a donor with a homozygous deletion in the CCR5 gene. Thus, the donor cells were inherently resistant to HIV infection because they lacked expression of an HIV co-receptor. At the time of the transplant, the patient stopped anti-retroviral therapy, and had no detectable viremia without antiretroviral therapy for over 5 years [113,132]. Whether the donor stem cells or the bone marrow ablation strategy, or a combination of the two, led to this cure is unknown. However, this case renewed interest in stem cell therapy as a potential cure, though with no additional successes yet. Indeed, recent studies that have examined the impact of bone marrow transplant have not replicated the conditions that led to a cure of the Berlin patient. Cillo et al. detected plasma virus and HIV DNA in 10 HIV-infected people after they had received autologous BMTs [133]. Two other HIV+ men experienced a decline of peripheral blood HIV reservoir after allogeneic transplants from wild type-CCR5+ donors [134]. After a treatment interruption, they had undetectable viral levels for a prolonged period, but eventually both experienced viral rebound [134,135].
Despite the failure of BMT as a therapy so far, an alternative approach is to transplant genetically modified hematopoietic stem cells to allow continued production of immune cells that are resistant to infection. Some studies have used genetic approaches that delete CCR5 or insert restriction factors into stem cells to prevent infection ([136], reviewed in [137]). Gene therapy has also been used to modify T cells. In a preliminary trial, re-infusion of autologous T cells that had been edited by zinc-finger nucleases to eliminate CCR5 gene expression was well tolerated in 12 HIV-infected people [138].
Gene Therapy
Additionally, gene therapy approaches have been utilized as a strategy to directly target latently infected cells. A recent study of interest utilized the clustered regularly interspaced short palindromic repeat (CRISPR)/ CRISPR-associated protein (CAS) 9 system to edit an integrated HIV genome and prevent transcription. This unique strategy aims to cure infection by permanently silencing proviral genomes [139].
Alternative Strategies
Other approaches to directly target latently infected cells include therapies specific to infected cells. For example, treatment with an HIV-targeted immunotoxin in combination with anti-retroviral therapy effectively kills cells with productive infection in a humanized mouse model [140]. Another approach utilized radiolabeled antibodies recognizing the HIV envelope protein to selectively clear HIV-infected cells in mouse models without severe toxicity [141]. If proven safe and effective, these therapies could be used to specifically target latently-infected cells, assuming a marker can be found that is uniquely expressed on cells with transcriptionally silent infection. One study found that CD2 expression is usually high on resting memory T cells harboring latent HIV [142]. However, this marker is also commonly found on uninfected cells and many infected cells were CD2− Further characterization of which subsets of cells are infected within the resting T cell and HSPC reservoirs could reveal a targetable characteristic for cell-directed therapies.
Five-Year View
Our increased understanding of HIV pathogenesis, effective anti-retroviral drugs, and viral reservoirs has transformed HIV treatment and research over the past decades. Looking forward, we can see how this knowledge may be applied to future goals and priorities of HIV clinicians, researchers, and policy-makers. Clinicians, acting on the new World Health Organization treatment guidelines proposed in 2013, will be working to begin treatment during initial stages of infection and help HIV-infected people adhere to treatment to decrease deaths and prevent new infections. A longitudinal population study in Canada and a randomized controlled trial performed in 9 countries are the most recent evidence that earlier and expanded coverage with cART can significantly decrease HIV morbidity, mortality, and transmission [143,144]. As discussed above, the case of the Mississippi baby and PTCs illustrate the possibilities that initiating treatment earlier may allow at least a portion of HIV-infected people to attain a functional cure. Thus, treatment will be expanded by the push to implement cART earlier and apply therapies targeting latent reservoirs. A major obstacle to the expansion of treatment will be the large burden on governments and health care systems to supply medications for all the HIV-infected people who should be treated, especially the majority that live in low or middle income countries. For the millions of HIV-infected people, in whom resting CD4+ T cells and other reservoirs are already established, researchers will strive to narrow the pool of plausible agents for ‘shock and kill’ or other strategies for targeting latent infection. As a deeper understanding of HIV persistence directs clinical trials, new and more economical treatment regimens could emerge. The universal goal remains to provide a cure and end the prospect of a life of illness and arduous treatment for all HIV+ people.
Key Issues.
HIV forms a latent infection that allows the virus to persist despite therapy.
The best-studied latent reservoir of HIV is in resting memory CD4+ T cells, including central memory, transitional memory, and stem central memory T cell subsets.
Bone marrow HSPCs can be latently infected and viral genomes have been detected in these cells in a subset of HIV-infected people.
HDAC and DNA methylation inhibitors have been tested to reverse latent HIV in CD4+ T cells and HSPCs, but more strategies are needed to boost the efficacy of these compounds.
Agents that result in increased availability of host factors, such as NF-κB or pTEFb, can increase transcription of the latent genome and contribute to reactivation of latent infection.
Immune-modulating compounds may antagonize latent infection, but are not ideal as treatments due to their non-specific effects on HSPCs and immune cells.
Two major strategies for clearance of reactivated latent infection are activation of cell death pathways or the patient’s own immune system.
Important questions to be considered in future studies include: Which HIV reservoirs should we target? How can we attain a functional cure? What are the alternative approaches to a cure?
Acknowledgments
Acknowledgements and Funding: Thank you to Roshan Najafi, Veronique Sebastian, Val Terry and Thomas Zaikos for careful reading of the manuscript. N.T.S was supported by the University of Michigan [Cellular and Molecular Biology Distinguished Student Fellowship], NIH Medical Scientist Training Program Grant T32-GM007863, and NIH Cellular and Molecular Biology Training Grant T32-GM007315. This work was supported by the Burroughs Wellcome Foundation and NIH RO1 AI096962.
Abbreviations
- HIV
human immunodeficiency virus
- AIDS
acquired immune deficiency syndrome
- cART
combination antiretroviral therapy
- HSPCs
hematopoietic stem and progenitor cells
- GM-CSF
granulocyte macrophage-colony stimulating factor
- TNF-alpha
tumor necrosis factor-alpha
- LRAs
latency-reversing agents
- HDACi
histone deacetylase complex inhibitor
- LTR
long terminal repeat
- IL-7
interleukin-7
- TLR
toll-like receptor
- PBMC
peripheral blood mononuclear cell
- PKC
protein kinase C
- SAHA
suberoylanilide hydroxamic acid
- MHC-I
major histocompatibility complex-class I molecules
- PTC
post treatment controller
Footnotes
Conflicts of Interest: The authors declare no potential conflict of interest.
References
Reference annotations:
* = of interest
** = of considerable interest
- 1.The Joint United Nations Programme on HIV/AIDS Global Fact Sheet. UNAIDS. 2012 [Google Scholar]
- 2.World Health Organization. Global Update on HIV Treatment 2013: Results, Impact and Opportunities. 2013 [Google Scholar]
- 3.Palmer S, Maldarelli F, Wiegand A, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci USA. 2008;105(10):3879–3884. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trono D, Van Lint C, Rouzioux C, et al. HIV persistence and the prospect of long-term drug-free remissions for HIV-infected individuals. Science. 2010;329(5988):174–180. doi: 10.1126/science.1191047. [DOI] [PubMed] [Google Scholar]
- 5.Siliciano RF, Greene WC. HIV latency. Cold Spring Harb Perspect Med. 2011;1(1):a007096. doi: 10.1101/cshperspect.a007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Der Sluis RM, Jeeninga RE, Berkhout B. Establishment and molecular mechanisms of HIV-1 latency in T cells. Curr Opin Virol. 2013;3(6):700–706. doi: 10.1016/j.coviro.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 7. Carter CC, Onafuwa-Nuga A, Mcnamara LA, et al. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat Med. 2010;16(4):446–451. doi: 10.1038/nm.2109. * First report of latent HIV infection of high purity HSPCs in vitro and in vivo providing evidence for a latent reservoir in this cell type
- 8.Carter CC, Mcnamara LA, Onafuwa-Nuga A, et al. HIV-1 utilizes the CXCR4 chemokine receptor to infect multipotent hematopoietic stem and progenitor cells. Cell Host Microbe. 2011;9(3):223–234. doi: 10.1016/j.chom.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mcnamara LA, Ganesh JA, Collins KL. Latent HIV-1 infection occurs in multiple subsets of hematopoietic progenitor cells and is reversed by NF-kappaB activation. J Virol. 2012;86(17):9337–9350. doi: 10.1128/JVI.00895-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A, Abbas W, Herbein G. HIV-1 latency in monocytes/macrophages. Viruses. 2014;6(4):1837–1860. doi: 10.3390/v6041837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narasipura SD, Kim S, Al-Harthi L. Epigenetic regulation of HIV-1 latency in astrocytes. J Virol. 2014;88(5):3031–3038. doi: 10.1128/JVI.03333-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Churchill MJ, Wesselingh SL, Cowley D, et al. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann Neurol. 2009;66(2):253–258. doi: 10.1002/ana.21697. [DOI] [PubMed] [Google Scholar]
- 13.Zink MC, Brice AK, Kelly KM, et al. Simian immunodeficiency virus-infected macaques treated with highly active antiretroviral therapy have reduced central nervous system viral replication and inflammation but persistence of viral DNA. J Infect Dis. 2010;202(1):161–170. doi: 10.1086/653213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Douce V, Herbein G, Rohr O, Schwartz C. Molecular mechanisms of HIV-1 persistence in the monocyte-macrophage lineage. Retrovirology. 2010;7:32. doi: 10.1186/1742-4690-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koppensteiner H, Brack-Werner R, Schindler M. Macrophages and their relevance in Human Immunodeficiency Virus Type I infection. Retrovirology. 2012;9:82. doi: 10.1186/1742-4690-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finzi D, Blankson J, Siliciano JD, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5(5):512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 17.Siliciano JD, Kajdas J, Finzi D, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9(6):727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 18.Murray JM, Zaunders JJ, Mcbride KL, et al. HIV DNA subspecies persist in both activated and resting memory CD4+ T cells during antiretroviral therapy. J Virol. 2014;88(6):3516–3526. doi: 10.1128/JVI.03331-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan X, Baldauf HM, Keppler OT, Fackler OT. Restrictions to HIV-1 replication in resting CD4+ T lymphocytes. Cell Res. 2013;23(7):876–885. doi: 10.1038/cr.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen A, Baker JJ, Scott GL, Davis YP, Ho YY, Siliciano RF. Endothelial cell stimulation overcomes restriction and promotes productive and latent HIV-1 infection of resting CD4+ T cells. J Virol. 2013;87(17):9768–9779. doi: 10.1128/JVI.01478-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pace MJ, Agosto L, Graf EH, O’doherty U. HIV reservoirs and latency models. Virology. 2011;411(2):344–354. doi: 10.1016/j.virol.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho YC, Shan L, Hosmane NN, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155(3):540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 24.Riou C, Yassine-Diab B, Van Grevenynghe J, et al. Convergence of TCR and cytokine signaling leads to FOXO3a phosphorylation and drives the survival of CD4+ central memory T cells. J Exp Med. 2007;204(1):79–91. doi: 10.1084/jem.20061681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15(8):893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17(10):1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buzon MJ, Sun H, Li C, et al. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med. 2014;20(2):139–142. doi: 10.1038/nm.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabler CO, Lucera MB, Haqqani AA, et al. CD4+ Memory Stem Cells (TSCM) are Infected by HIV-1 in a Manner Regulated in Part by SAMHD1 Expression. J Virol. 2014 doi: 10.1128/JVI.00324-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flynn JK, Paukovics G, Cashin K, et al. Quantifying susceptibility of CD4+ stem memory T-cells to infection by laboratory adapted and clinical HIV-1 strains. Viruses. 2014;6(2):709–726. doi: 10.3390/v6020709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson JA, Archin NM, Ince W, et al. Clonal sequences recovered from plasma from patients with residual HIV-1 viremia and on intensified antiretroviral therapy are identical to replicating viral RNAs recovered from circulating resting CD4+ T cells. J Virol. 2011;85(10):5220–5223. doi: 10.1128/JVI.00284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brennan TP, Woods JO, Sedaghat AR, Siliciano JD, Siliciano RF, Wilke CO. Analysis of human immunodeficiency virus type 1 viremia and provirus in resting CD4+ T cells reveals a novel source of residual viremia in patients on antiretroviral therapy. J Virol. 2009;83(17):8470–8481. doi: 10.1128/JVI.02568-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bailey JR, Sedaghat AR, Kieffer T, et al. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J Virol. 2006;80(13):6441–6457. doi: 10.1128/JVI.00591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sahu GK, Paar D, Frost SDW, Smith MM, Weaver S, Cloyd MW. Low-Level Plasma HIVs in Patients on Prolonged Suppressive Highly Active Antiretroviral Therapy Are Produced Mostly by Cells Other Than CD4 T-cells. J Med Virol. 2009;81(1):9–15. doi: 10.1002/jmv.21366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redd AD, Avalos A, Essex M. Infection of hematopoietic progenitor cells by HIV-1 subtype C, and its association with anemia in southern Africa. Blood. 2007;110(9):3143–3149. doi: 10.1182/blood-2007-04-086314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mcnamara LA, Onafuwa-Nuga A, Sebastian NT, Riddell JT, Bixby D, Collins KL. CD133+ hematopoietic progenitor cells harbor HIV genomes in a subset of optimally treated people with long-term viral suppression. J Infect Dis. 2013;207(12):1807–1816. doi: 10.1093/infdis/jit118. ** A study of HIV-infected people with suppressed viral loads detected HIV DNA in immature HSPCs. The results demonstrated that the detected virus was not likely to come from contaminating infected T cells.
- 36.Nixon CC, Vatakis DN, Reichelderfer SN, et al. HIV-1 infection of hematopoietic progenitor cells in vivo in humanized mice. Blood. 2013;122(13):2195–2204. doi: 10.1182/blood-2013-04-496950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Josefsson L, Eriksson S, Sinclair E, et al. Hematopoietic precursor cells isolated from patients on long-term suppressive HIV therapy did not contain HIV-1 DNA . J Infect Dis. 2012;206(1):28–34. doi: 10.1093/infdis/jis301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Durand CM, Ghiaur G, Siliciano JD, et al. HIV-1 DNA is detected in bone marrow populations containing CD4+ T cells but is not found in purified CD34+ hematopoietic progenitor cells in most patients on antiretroviral therapy. J Infect Dis. 2012;205(6):1014–1018. doi: 10.1093/infdis/jir884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deeks SG, Autran B, et al. International ASSWGOHIVC. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol. 2012;12(8):607–614. doi: 10.1038/nri3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasmussen TA, Tolstrup M, Winckelmann A, Ostergaard L, Sogaard OS. Eliminating the latent HIV reservoir by reactivation strategies: advancing to clinical trials. Hum Vaccin Immunother. 2013;9(4):790–799. doi: 10.4161/hv.23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sgarbanti M, Battistini A. Therapeutics for HIV-1 reactivation from latency. Curr Opin Virol. 2013;3(4):394–401. doi: 10.1016/j.coviro.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 42.Xing S, Siliciano RF. Targeting HIV latency: pharmacologic strategies toward eradication. Drug Discov Today. 2013;18(11–12):541–551. doi: 10.1016/j.drudis.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shirakawa K, Chavez L, Hakre S, Calvanese V, Verdin E. Reactivation of latent HIV by histone deacetylase inhibitors. Trends Microbiol. 2013;21(6):277–285. doi: 10.1016/j.tim.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Remoli AL, Marsili G, Battistini A, Sgarbanti M. The development of immune-modulating compounds to disrupt HIV latency. Cytokine Growth Factor Rev. 2012;23(4–5):159–172. doi: 10.1016/j.cytogfr.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Wei DG, Chiang V, Fyne E, et al. Histone deacetylase inhibitor romidepsin induces HIV expression in CD4 T cells from patients on suppressive antiretroviral therapy at concentrations achieved by clinical dosing. PLoS Pathog. 2014;10(4):el004071. doi: 10.1371/journal.ppat.1004071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Archin NM, Espeseth A, Parker D, Cheema M, Hazuda D, Margolis DM. Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid. AIDS Res Hum Retroviruses. 2009;25(2):207–212. doi: 10.1089/aid.2008.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Contreras X, Schweneker M, Chen CS, et al. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J Biol Chem. 2009;284(11):6782–6789. doi: 10.1074/jbc.M807898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bullen CK, Laird GM, Durand CM, Siliciano JD, Siliciano RF. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med. 2014;20(4):425–429. doi: 10.1038/nm.3489. ** Provides evidence that current latency-reversing agents will not be able to reactivate latent HIV infection in HIV-infected people
- 49.Cillo AR, Sobolewski MD, Bosch RJ, et al. Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2014;111(19):7078–7083. doi: 10.1073/pnas.1402873111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kauder SE, Bosque A, Lindqvist A, Planelles V, Verdin E. Epigenetic regulation of HIV-1 latency by cytosine methylation. PLoS Pathog. 2009;5(6):el000495. doi: 10.1371/journal.ppat.1000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blazkova J, Trejbalova K, Gondois-Rey F, et al. CpG methylation controls reactivation of HIV from latency. PLoS Pathog. 2009;5(8):el000554. doi: 10.1371/journal.ppat.1000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blazkova J, Murray D, Justement JS, et al. Paucity of HIV DNA methylation in latently infected, resting CD4+ T cells from infected individuals receiving antiretroviral therapy. J Virol. 2012;86(9):5390–5392. doi: 10.1128/JVI.00040-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weber S, Weiser B, Kemal KS, et al. Epigenetic analysis of HIV-1 proviral genomes from infected individuals: predominance of unmethylated CpG’s. Virology. 2014;449:181–189. doi: 10.1016/j.virol.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duh EJ, Maury WJ, Folks TM, Fauci AS, Rabson AB. Tumor necrosis factor-alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-κB sites in the long terminal repeat. Proc Natl Acad Sci USA. 1989;86(15):5974–5974. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Osborn L, Kunkel S, Nabel GJ. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kB. Proc Natl Acad Sci USA. 1989;86(7):2336–2336. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schlaepfer E, Speck RF. TLR8 activates HIV from latently infected cells of myeloid-monocytic origin directly via the MAPK pathway and from latently infected CD4+ T cells indirectly via TNF-alpha. J Immunol. 2011;186(7):4314–4324. doi: 10.4049/jimmunol.1003174. [DOI] [PubMed] [Google Scholar]
- 57.Bosque A, Planelles V. Studies of HIV-1 latency in an ex vivo model that uses primary central memory T cells. Methods. 2011;53(1):54–61. doi: 10.1016/j.ymeth.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tyagi M, Pearson RJ, Karn J. Establishment of HIV latency in primary CD4+ cells is due to epigenetic transcriptional silencing and P-TEFb restriction. J Virol. 2010;84(13):6425–6437. doi: 10.1128/JVI.01519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Contreras X, Barboric M, Lenasi T, Peterlin BM. HMBA releases P-TEFb from HEXIM1 and 7SK snRNA via PI3K/Akt and activates HIV transcription. PLoS Pathog. 2007;3(10):1459–1469. doi: 10.1371/journal.ppat.0030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choudhary SK, Archin NM, Margolis DM. Hexamethylbisacetamide and disruption of human immunodeficiency virus type 1 latency in CD4(+) T cells. J Infect Dis. 2008;197(8):1162–1170. doi: 10.1086/529525. [DOI] [PubMed] [Google Scholar]
- 61.Korin YD, Brooks DG, Brown S, Korotzer A, Zack JA. Effects of prostratin on T-cell activation and human immunodeficiency virus latency. J Virol. 2002;76(16):8118–8123. doi: 10.1128/JVI.76.16.8118-8123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams SA, Chen LF, Kwon H, et al. Prostratin antagonizes HIV latency by activating NF-kappaB. J Biol Chem. 2004;279(40):42008–42017. doi: 10.1074/jbc.M402124200. [DOI] [PubMed] [Google Scholar]
- 63.Xing S, Bullen CK, Shroff NS, et al. Disulfiram reactivates latent HIV-1 in a Bcl-2-transduced primary CD4+ T cell model without inducing global T cell activation. J Virol. 2011;85(12):6060–6064. doi: 10.1128/JVI.02033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Doyon G, Zerbato J, Mellors JW, Sluis-Cremer N. Disulfiram reactivates latent HIV-1 expression through depletion of the phosphatase and tensin homolog. AIDS. 2013;27(2):F7–F11. doi: 10.1097/QAD.0b013e3283570620. [DOI] [PubMed] [Google Scholar]
- 65.Spivak AM, Andrade A, Eisele E, et al. A pilot study assessing the safety and latency-reversing activity of disulfiram in HIV-1-infected adults on antiretroviral therapy. Clin Infect Dis. 2014;58(6):883–890. doi: 10.1093/cid/cit813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lehrman G, Ylisastigui L, Bosch RJ, Margolis DM. Interleukin-7 induces HIV type 1 outgrowth from peripheral resting CD4+ T cells. J Acquir Immune Defic Syndr. 2004;36(5):1103–1104. doi: 10.1097/00126334-200408150-00015. [DOI] [PubMed] [Google Scholar]
- 67.Wang FX, Xu Y, Sullivan J. IL-7 is a potent and proviral strain-specific inducer of latent HIV-1 cellular reservoirs of infected individuals on virally suppressive HAART. J Clin Invest. 2005;115(1) doi: 10.1172/JCI22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vandergeeten C, Fromentin R, Dafonseca S, et al. Interleukin-7 promotes HIV persistence during antiretroviral therapy. Blood. 2013;121(21):4321–4329. doi: 10.1182/blood-2012-11-465625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bosque A, Famiglietti M, Weyrich AS, Goulston C, Planelles V. Homeostatic proliferation fails to efficiently reactivate HIV-1 latently infected central memory CD4+ T cells. PLoS Pathog. 2011;7(10):el002288. doi: 10.1371/journal.ppat.1002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Folks TM, Clouse KA, Justement J, et al. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci USA. 1989;86(7):2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baldridge MT, King KY, Goodell MA. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol. 2011;32(2):57–65. doi: 10.1016/j.it.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boiko JR, Borghesi L. Hematopoiesis sculpted by pathogens: Toll-like receptors and inflammatory mediators directly activate stem cells. Cytokine. 2012;57(1):1–8. doi: 10.1016/j.cyto.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scheller C, Ullrich A, Mcpherson K, et al. CpG oligodeoxynucleotides activate HIV replication in latently infected human T cells. J Biol Chem. 2004;279(21):21897–21902. doi: 10.1074/jbc.M311609200. [DOI] [PubMed] [Google Scholar]
- 74.Schlaepfer E, Audige A, Joller H, Speck RF. TLR7/8 triggering exerts opposing effects in acute versus latent HIV infection. J Immunol. 2006;176(5):2888–2895. doi: 10.4049/jimmunol.176.5.2888. [DOI] [PubMed] [Google Scholar]
- 75.Thibault S, Imbeault M, Tardif MR, Tremblay MJ. TLR5 stimulation is sufficient to trigger reactivation of latent HIV-1 provirus in T lymphoid cells and activate virus gene expression in central memory CD4+ T cells. Virology. 2009;389(1–2):20–25. doi: 10.1016/j.virol.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 76.Winckelmann AA, Munk-Petersen LV, Rasmussen TA, et al. Administration of a Tolllike receptor 9 agonist decreases the proviral reservoir in virologically suppressed HIV-infected patients. PLoS One. 2013;8(4):e62074. doi: 10.1371/journal.pone.0062074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nagai Y, Garrett KP, Ohta S, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24(6):801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Megias J, Yanez A, Moriano S, O'connor JE, Gozalbo D, Gil ML. Direct Toll-like receptor-mediated stimulation of hematopoietic stem and progenitor cells occurs in vivo and promotes differentiation toward macrophages. Stem Cells. 2012;30(7):1486–1495. doi: 10.1002/stem.1110. [DOI] [PubMed] [Google Scholar]
- 79.Esplin BL, Shimazu T, Welner RS, et al. Chronic exposure to a TLR ligand injures hematopoietic stem cells. J Immunol. 2011;186(9):5367–5375. doi: 10.4049/jimmunol.1003438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yanez A, Hassanzadeh-Kiabi N, Ng MY, et al. Detection of a TLR2 agonist by hematopoietic stem and progenitor cells impacts the function of the macrophages they produce. Eur J Immunol. 2013;43(8):2114–2125. doi: 10.1002/eji.201343403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Azzoni L, Foulkes AS, Papasavvas E, et al. Pegylated Interferon alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration. J Infect Dis. 2013;207(2):213–222. doi: 10.1093/infdis/jis663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mcnamara LA, Collins KL. Interferon alfa therapy: toward an improved treatment for HIV infection. J Infect Dis. 2013;207(2):201–203. doi: 10.1093/infdis/jis667. [DOI] [PubMed] [Google Scholar]
- 83. Spina CA, Anderson J, Archin NM, et al. An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog. 2013;9(12):el003834. doi: 10.1371/journal.ppat.1003834. * Collaborative study comparing a panel of reactivation agents in multiple latency models. Results indicate different models are biased towards specific classes of agents
- 84.Choudhary SK, Archin NM, Cheema M, Dahl NP, Garcia JV, Margolis DM. Latent HIV-1 infection of resting CD4(+) T cells in the humanized Rag2(−)/(−) gammac(−)/(−) mouse. J Virol. 2012;86(1):114–120. doi: 10.1128/JVI.05590-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kauffman RC, Villalobos A, Bowen JH, Adamson L, Schinazi RF. Residual viremia in an RT-SHIV rhesus macaque HAART model marked by the presence of a predominant plasma clone and a lack of viral evolution. PLoS One. 2014;9(2):e88258. doi: 10.1371/journal.pone.0088258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lehrman G, Hogue IB, Palmer S, et al. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005;366(9485):549–555. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Siliciano JD, Lai J, Callender M, et al. Stability of the latent reservoir for HIV-1 in patients receiving valproic acid. J Infect Dis. 2007;195(6):833–836. doi: 10.1086/511823. [DOI] [PubMed] [Google Scholar]
- 88.Sagot-Lerolle N, Lamine A, Chaix ML, et al. Prolonged valproic acid treatment does not reduce the size of latent HIV reservoir. AIDS. 2008;22(10):1125–1129. doi: 10.1097/QAD.0b013e3282fd6ddc. [DOI] [PubMed] [Google Scholar]
- 89.Archin NM, Cheema M, Parker D, et al. Antiretroviral intensification and valproic acid lack sustained effect on residual HIV-1 viremia or resting CD4+ cell infection. PLoS One. 2010;5(2):e9390. doi: 10.1371/journal.pone.0009390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Routy JP, Tremblay CL, Angel JB, et al. Valproic acid in association with highly active antiretroviral therapy for reducing systemic HIV-1 reservoirs: results from a multicentre randomized clinical study. HIV Med. 2012;13(5):291–296. doi: 10.1111/j.1468-1293.2011.00975.x. [DOI] [PubMed] [Google Scholar]
- 91.Archin NM, Liberty AL, Kashuba AD, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487(7408):482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lamkanfi M, Dixit VM. Manipulation of host cell death pathways during microbial infections. Cell Host Microbe. 2010;8(1):44–54. doi: 10.1016/j.chom.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 93. Badley AD, Sainski A, Wightman F, Lewin SR. Altering cell death pathways as an approach to cure HIV infection. Cell Death Dis. 2013;4(7):e718. doi: 10.1038/cddis.2013.248. * Review on targeting cell death pathways in infected cells, especially in the context of reactivated latent infection
- 94. Shan L, Deng K, Shroff NS, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36(3):491–501. doi: 10.1016/j.immuni.2012.01.014. ** Study demonstrating that reactivation by SAHA is not sufficient for clearance by cell death or CTLs, unless CTLs are pre-stimulated with HIV peptides
- 95.Hanauske-Abel HM, Saxena D, Palumbo PE, et al. Drug-induced reactivation of apoptosis abrogates HIV-1 infection. PLoS One. 2013;8(9):e74414. doi: 10.1371/journal.pone.0074414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Migueles SA, Weeks KA, Nou E, et al. Defective human immunodeficiency virus-specific CD8+ T-cell polyfunctionality, proliferation, and cytotoxicity are not restored by antiretroviral therapy. J Virol. 2009;83(22):11876–11889. doi: 10.1128/JVI.01153-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pace MJ, Graf EH, Agosto LM, et al. Directly infected resting CD4+T cells can produce HIV Gag without spreading infection in a model of HIV latency. PLoS Pathog. 2012;8(7):el002818. doi: 10.1371/journal.ppat.1002818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Graf EH, Pace MJ, Peterson BA, et al. Gag-positive reservoir cells are susceptible to HIV-specific cytotoxic T lymphocyte mediated clearance. PLoS One. 2013;8(8):e71879. doi: 10.1371/journal.pone.0071879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391(6665):397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 100.Le Gall S, Buseyne F, Trocha A, Walker BD, Heard JM, Schwartz O. Distinct trafficking pathways mediate Nef-induced and clathrin-dependent major histocompatibility complex class I down-regulation. J Virol. 2000;74(19):9256–9266. doi: 10.1128/jvi.74.19.9256-9266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ali A, Lubong R, Ng H, Brooks DG, Zack JA, Yang OO. Impacts of epitope expression kinetics and class I downregulation on the antiviral activity of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes. J Virol. 2004;78(2):561–567. doi: 10.1128/JVI.78.2.561-567.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ali A, Ng HL, Dagarag MD, Yang OO. Evasion of cytotoxic T lymphocytes is a functional constraint maintaining HIV-1 Nef expression. Eur J Immunol. 2005;35(11):3221–3228. doi: 10.1002/eji.200535053. [DOI] [PubMed] [Google Scholar]
- 103.Swigut T, Alexander L, Morgan J, et al. Impact of Nef-mediated downregulation of major histocompatibility complex class I on immune response to simian immunodeficiency virus. J Virol. 2004;78(23):13335–13344. doi: 10.1128/JVI.78.23.13335-13344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lewis MJ, Balamurugan A, Ohno A, Kilpatrick S, Ng HL, Yang OO. Functional adaptation of Nef to the immune milieu of HIV-1 infection in vivo. J Immunol. 2008;180(6):4075–4081. doi: 10.4049/jimmunol.180.6.4075. [DOI] [PubMed] [Google Scholar]
- 105.Lewis MJ, Lee P, Ng HL, Yang OO. Immune selection in vitro reveals human immunodeficiency virus type 1 Nef sequence motifs important for its immune evasion function in vivo. J Virol. 2012;86(13):7126–7135. doi: 10.1128/JVI.00878-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4(6):565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 107.Bobbitt KR, Addo MM, Altfeld M, et al. Rev activity determines sensitivity of HIV-1-infected primary T cells to CTL killing. Immunity. 2003;18(2):289–299. doi: 10.1016/s1074-7613(03)00031-1. [DOI] [PubMed] [Google Scholar]
- 108.Massanella M, Martinez-Picado J, Blanco J. Attacking the HIV reservoir from the immune and viral perspective. Curr HIV/AIDS Rep. 2013;10(1):33–41. doi: 10.1007/s11904-012-0150-8. [DOI] [PubMed] [Google Scholar]
- 109.Carcelain G, Autran B. Immune interventions in HIV infection. Immunol Rev. 2013;254(1):355–371. doi: 10.1111/imr.12083. [DOI] [PubMed] [Google Scholar]
- 110.Barouch DH, Deeks SG. Immunologic strategies for HIV-1 remission and eradication. Science. 2014;345(6193):169–174. doi: 10.1126/science.1255512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hansen SG, Piatak M, Jr, Ventura AB, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502(7469):100–104. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kitchen SG, Levin BR, Bristol G, et al. In vivo suppression of HIV by antigen specific T cells derived from engineered hematopoietic stem cells. PLoS Pathog. 2012;8(4):el002649. doi: 10.1371/journal.ppat.1002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hutter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360(7):692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 114.Persaud D, Gay H, Ziemniak C, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369(19):1828–1835. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lambotte O, Taoufik Y, De Goër MG, Wallon C, Goujard C, Delfraissy JF. Detection of infectious HIV in circulating monocytes from patients on prolonged highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;23:114–119. doi: 10.1097/00126334-200002010-00002. [DOI] [PubMed] [Google Scholar]
- 116.Zhu T, Muthui D, Holte S, et al. Evidence for Human Immunodeficiency Virus Type 1 Replication In Vivo in CD14+ Monocytes and Its Potential Role as a Source of Virus in Patients on Highly Active Antiretroviral Therapy. J Virol. 2002;76(2):707–716. doi: 10.1128/JVI.76.2.707-716.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Buzon MJ, Massanella M, Llibre JM, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16(4):460–465. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 118.Buzon MJ, Codoner FM, Frost SD, et al. Deep molecular characterization of HIV-1 dynamics under suppressive HAART. PLoS Pathog. 2011;7(10):el002314. doi: 10.1371/journal.ppat.1002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sharkey M, Babic DZ, Greenough T, Gulick R, Kuritzkes DR, Stevenson M. Episomal viral cDNAs identify a reservoir that fuels viral rebound after treatment interruption and that contributes to treatment failure. PLoS pathogens. 2011;7(2):el001303. doi: 10.1371/journal.ppat.1001303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vallejo A, Gutierrez C, Hernandez-Novoa B, et al. The effect of intensification with raltegravir on the HIV-1 reservoir of latently infected memory CD4 T cells in suppressed patients. AIDS. 2012;26(15):1885–1894. doi: 10.1097/QAD.0b013e3283584521. [DOI] [PubMed] [Google Scholar]
- 121.Lewin SR, Vesanen M, Kostrikis L, et al. Use of real-time PCR and molecular beacons to detect virus replication in human immunodeficiency virus type 1-infected individuals on prolonged effective antiretroviral therapy. J Virol. 1999;73(7):6099–6103. doi: 10.1128/jvi.73.7.6099-6103.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tobin NH, Learn GH, Holte SE, et al. Evidence that low-level viremias during effective highly active antiretroviral therapy result from two processes: expression of archival virus and replication of virus. J Virol. 2005;79(15):9625–9634. doi: 10.1128/JVI.79.15.9625-9634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang L, Chung C, Hu BS, et al. Genetic characterization of rebounding HIV-1 after cessation of highly active antiretroviral therapy. J Clin Invest. 2000;106(7):839–845. doi: 10.1172/JCI10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhu T, Muthui D, Holte S, et al. Evidence for human immunodeficiency virus type 1 replication in vivo in CD14(+) monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J Virol. 2002;76(2):707–716. doi: 10.1128/JVI.76.2.707-716.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yukl SA, Shergill AK, Mcquaid K, et al. Effect of raltegravir-containing intensification on HIV burden and T-cell activation in multiple gut sites of HIV-positive adults on suppressive antiretroviral therapy. AIDS. 2010;24(16):2451–2460. doi: 10.1097/QAD.0b013e32833ef7bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.North TW, Higgins J, Deere JD, et al. Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. Journal of virology. 2010;84(6):2913–2922. doi: 10.1128/JVI.02356-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Massanella M, Esteve A, Buzon MJ, et al. Dynamics of CD8 T-cell activation after discontinuation of HIV treatment intensification. J Acquir Immune Defic Syndr. 2013;63(2):152–160. doi: 10.1097/QAI.0b013e318289439a. [DOI] [PubMed] [Google Scholar]
- 128.Dieffenbach CW, Fauci AS. Thirty years of HIV and AIDS: future challenges and opportunities. Ann Intern Med. 2011;154(11):766–771. doi: 10.7326/0003-4819-154-11-201106070-00345. [DOI] [PubMed] [Google Scholar]
- 129.“Mississippi Baby” Now Has Detectable HIV, Researchers Find. [Accessed August 9, 2014];NIH News. 2014 http://www.niaid.nih.gov/news/newsreleases/2014/pages/mississippibabyhiv.aspx.
- 130.Luzuriaga K, Tabak B, Garber M, et al. HIV-1 Proviral Reservoirs Decay Continously Under Sustained Virologic Control in Early-Treated HIV-1- Infected Children. J Infect Dis. 2014 doi: 10.1093/infdis/jiu297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Saez-Cirion A, Bacchus C, Hocqueloux L, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9(3):el003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Allers K, Hutter G, Hofmann J, et al. Evidence for the cure of HIV infection by CCR5Delta32/Delta32 stem cell transplantation. Blood. 2011;117(10):2791–2799. doi: 10.1182/blood-2010-09-309591. * First report of a cure of HIV infection in a patient who underwent a bone marrow transplant from a donor homozygous for a deletion in the HIV co-receptor CCR5
- 133.Cillo AR, Krishnan A, Mitsuyasu RT, et al. Plasma viremia and cellular HIV-1 DNA persist despite autologous hematopoietic stem cell transplantation for HIV-related lymphoma. J Acquir Immune Deflic Syndr. 2013;63(4):438–441. doi: 10.1097/QAI.0b013e31828e6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Henrich TJ, Hu Z, Li JZ, et al. Long-term reduction in peripheral blood HIV type 1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation. J Infect Dis. 2013;207(11):1694–1702. doi: 10.1093/infdis/jit086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hayden EC. Hopes of HIV cure in ‘Boston patients’ dashed. [last accessed 4 Jan 2014];Nature News and Comment. 2013 Dec 6; Available from: http://www.nature.com.proxy.lib.umich.edu/news/hopes-of-hiv-cure-in-boston-patients-dashed-1.14324.
- 136.Walker JE, Chen RX, Mcgee J, et al. Generation of an HIV-1-resistant immune system with CD34(+) hematopoietic stem cells transduced with a triple-combination anti-HIV lentiviral vector. J Virol. 2012;86(10):5719–5729. doi: 10.1128/JVI.06300-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhen A, Kitchen S. Stem-cell-based gene therapy for HIV infection. Viruses. 2014;6(1):1–12. doi: 10.3390/v6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tebas P, Stein D, Tang WW, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014;370(10):901–910. doi: 10.1056/NEJMoa1300662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ebina H, Misawa N, Kanemura Y, Koyanagi Y. Harnessing the CRISPR/Cas9 system to disrupt latent HIV-1 provirus. Sci Rep. 2013;3:2510. doi: 10.1038/srep02510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Denton PW, Long JM, Wietgrefe SW, et al. Targeted cytotoxic therapy kills persisting HIV infected cells during ART. PLoS Pathog. 2014;10(1) doi: 10.1371/journal.ppat.1003872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dadachova E, Kitchen SG, Bristol G, et al. Pre-clinical evaluation of a 213Bi-labeled 2556 antibody to HIV-1 gp41 glycoprotein in HIV-1 mouse models as a reagent for HIV eradication. PLoS One. 2012;7(3):e31866. doi: 10.1371/journal.pone.0031866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Iglesias-Ussel M, Vandergeeten C, Marchionni L, Chomont N, Romerio F. High levels of CD2 expression identify HIV-1 latently infected resting memory CD4+ T cells in virally suppressed subjects. J Virol. 2013;87(16):9148–9158. doi: 10.1128/JVI.01297-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Montaner JS, Lima VD, Harrigan PR, et al. Expansion of HAART coverage is associated with sustained decreases in HIV/AIDS morbidity, mortality and HIV transmission: the “HIV Treatment as Prevention” experience in a Canadian setting. PLoS One. 2014;9(2):e87872. doi: 10.1371/journal.pone.0087872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14(4):281–290. doi: 10.1016/S1473-3099(13)70692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Scripture-Adams DD, Brooks DG, Korin YD, Zack JA. Interleukin-7 induces expression of latent human immunodeficiency virus type 1 with minimal effects on T-cell phenotype. J Virol. 2002;76(24):13077–13082. doi: 10.1128/JVI.76.24.13077-13082.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]