Abstract
Recently, there has been a renewed interest in the development of new drugs for the treatment of leishmaniasis. This has spurred the need for pharmacodynamic markers to monitor and compare therapies specifically for visceral leishmaniasis, in which the primary recrudescence of parasites is a particularly long-term event that remains difficult to predict. We performed a systematic review of studies evaluating biomarkers in human patients with visceral, cutaneous, and post-kala-azar dermal leishmaniasis, which yielded a total of 170 studies in which 53 potential pharmacodynamic biomarkers were identified. In conclusion, the large majority of these biomarkers constituted universal indirect markers of activation and subsequent waning of cellular immunity and therefore lacked specificity. Macrophage-related markers demonstrate favorable sensitivity and times to normalcy, but more evidence is required to establish a link between these markers and clinical outcome. Most promising are the markers directly related to the parasite burden, but future effort should be focused on optimization of molecular or antigenic targets to increase the sensitivity of these markers. In general, future research should focus on the longitudinal evaluation of the pharmacodynamic biomarkers during treatment, with an emphasis on the correlation of studied biomarkers and clinical parameters.
INTRODUCTION
Significant progress has been made the past few decades in our understanding of the pathophysiology and immunological mechanisms involved in the fatal parasitic infection visceral leishmaniasis (VL) and its dermal counterpart, cutaneous leishmaniasis (CL). Despite this progress, these scientific efforts have not directly led to new and better treatment options for patients suffering from these neglected tropical diseases. Fortunately, public interest and momentum in drug discovery and development for the leishmaniases have been renewed, which is substantiated, for instance, by the Drugs for Neglected Diseases initiative (DNDi) in the last decade (1, 2). This renewed interest stipulates the need for more modalities to compare and monitor therapeutic interventions.
Classical clinical features used to evaluate individual treatment responses of patients with VL include the normalization of spleen/liver size, defervescence, and the normalization of blood cell counts (as an indicator of recovering bone marrow). Likewise, for CL, the sizes of the inner and outer borders of cutaneous lesions are used as proxy determinants of parasite biomass, although reepithelialization, crustation, and a multiplicity of skin lesions complicate interpretation. These individual clinical features are, however, rarely used in the quantitative comparison of antileishmanial therapies in the context of a clinical trial. Within such trials, the current standard confirmation of initial cure for VL is a Leishmania-negative spleen or bone marrow aspirate confirmed by microscopy, a very invasive semiquantitative technique which cannot be regularly repeated (3–7). For CL, the confirmation of initial cure is much less clear: most clinical trials have defined “cure” as the absence of all inflammatory signs (skin edema and/or hardening) and complete scarring or reepithelialization of ulcerative lesions at the 3-month follow-up (8–10).
For both VL and CL, confirmation of a final cure as a primary endpoint is even more complicated by the long time periods between initial cure and recrudescence of parasites, requiring long follow-up periods (up to 6 or 12 months) to establish final cure (11). Parasite recrudescence is a rare and slow-developing event which is difficult to predict, mainly because little is known about the causes or risk factors. To compare the efficacies of treatment regimens, sensitive and specific markers that correlate with treatment effect and can predict long-term clinical outcome, by noninvasive sampling methods, are urgently needed.
The general definition of biomarkers, a neologism for “biological markers,” was previously established by the working group on biomarkers of the U.S. National Institutes of Health (NIH) as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention” (12). The use of biomarkers as surrogate endpoints in trials for leishmaniasis may have several possible advantages. First, they can be used for additional (earlier) analyses because primary clinical endpoints are both sparse and available only after a very long period of follow-up. Second, biomarker measurements are faster and less invasive than conventional clinical evaluations. Third, the use of biomarkers may allow the design of smaller, more efficient clinical studies, thereby speeding up the regulatory evaluation and approval of drugs (13). This systematic review focuses on the identification and evaluation of biomarkers to monitor treatment response in cases of VL, CL, and post-kala-azar dermal leishmaniasis (PKDL), with a focus on the pharmacodynamic potential of these biomarkers to be used in comparative clinical trials. To our knowledge, this is the first systematic review of biomarkers in leishmaniasis.
METHODS
Literature search strategy.
Potential biomarkers for VL, CL, and PKDL were identified by a primary-literature search performed using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, querying the PubMed database, restricted to the English language, as follows: “(((Leishmaniasis[title]) or Kala-azar[title]) or PKDL[title]) and (((((((((biomarker) or biomarkers) or marker) or markers) or level) or levels) or concentration) or activity) or profile).” This electronic search was performed from November 2013 to August 2014, and the date last searched was 19 August 2014. Results were screened manually to identify relevant publications based on title and/or abstract. Publications that did not focus on the identification or evaluation of biomarkers were excluded. Selected publications were then evaluated according to the exclusion criteria as described in Table 1. If the abstract did not clearly indicate whether a study met the initial inclusion criteria, the entire publication was assessed. Secondary literature was subsequently identified using references from the identified primary literature and related publications on PubMed and by specifically querying PubMed using the term of the identified biomarker in combination with “Leishmaniasis” and/or “Kala-azar.”
TABLE 1.
Exclusion criteria
| Exclusion criteria | Rationale |
|---|---|
| Method uses ex vivo assays | Ex vivo growth of cells is not feasible in practice, and the link with clinical relevance is unclear |
| Assay is nonquantitative | Quantitation is necessary for pharmacodynamic applicability |
| Sampling methods are invasive (e.g., splenic aspiration, high blood volumes) | Not feasible/cannot be done repetitively |
| Genetic markers are associated with drug resistance | Cannot be used to monitor treatment response during treatment |
| Genetic markers are associated with susceptibility to leishmaniasis | Not in scope of this article |
| No comparison with healthy controls | No information on “healthy levels” |
| Other | Not relevant to the topic for various reasons |
Evaluation criteria.
The biological and clinical pharmacodynamic potential of biomarkers was evaluated based on five criteria: (i) time to normalcy, i.e., the time needed for the biomarker level to regress to healthy/control levels; (ii) specificity, in relation to concomitant (infectious) diseases, such as malaria and HIV; (iii) sensitivity, the marker's quantitation in (treated) patients compared to that in healthy controls and its association with treatment cure or failure; (iv) additional sensitivity, i.e., further assessment of sensitivity by more in-depth association of the marker's quantitation to standard clinical markers of disease, such as spleen and lesion size; and (v) geographical applicability. Biomarkers were given a score (−/+/++/?) for each criterion as further explained in Table 2.
TABLE 2.
Criteria to evaluate the pharmacodynamics potential of biomarkers
| Criterion | Reason for score of: |
|||
|---|---|---|---|---|
| − | + | ++ | ? | |
| Regression to normalcy | Occurs >1 mo after treatment | Occurs within 1 mo after treatment | Occurs within treatment period | Unknown |
| Specificity | The biomarker lacks specificity when coinfections are present | The biomarker is specific for leishmaniasis with at least 1 coinfection (e.g., HIV) | The biomarker is specific for leishmaniasis with >1 coinfection | The biomarker is not tested in coinfected patients |
| Sensitivity (quantitative comparison of marker levels) | Marker levels are not significantly different from those of healthy controls | Marker levels are significantly different from those of healthy controls | Marker levels are shown to reflect treatment outcome (e.g., there are significant differences between levels in refractory and recovered patients) | Marker levels are not compared to those of healthy controls |
| Additional sensitivity (correlation with clinical markers) | Marker levels show no correlation with clinical parameters | Markers show correlation with other biomarkers (e.g., IL-10 levels or comparable) | Markers show correlation with clinical parameters (e.g., spleen size) | Clinical correlation is not tested |
| Geographical applicability | There is contradicting evidence from different countries/regions | There is confirmed evidence from >1 country | There is confirmed evidence from >1 continent | Not tested in multiple countries |
LITERATURE SEARCH
The primary-literature search identified 1,875 studies for which the titles were screened and assessed for eligibility. One thousand five hundred forty-seven records were found to be nonrelevant and excluded. Thereafter, abstracts and, subsequently, the full text of the remaining studies were assessed for their eligibility; 133 articles were eventually included in this systematic review. Thirty-seven studies were additionally identified through a secondary-literature search (Fig. 1).
FIG 1.
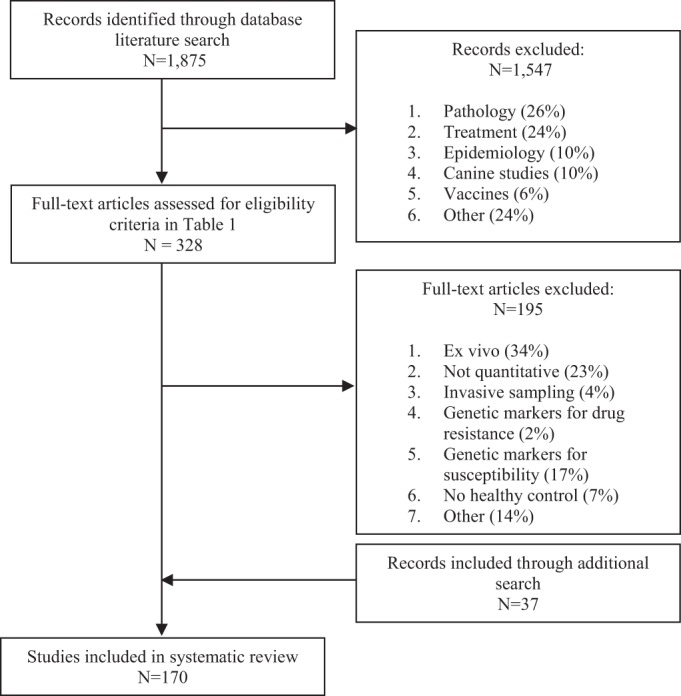
Study flow diagram.
IDENTIFIED BIOMARKERS
Fifty-three potential biomarkers were identified for VL, CL, and PKDL and are summarized in Table 3. The identified biomarkers were grouped into (a) direct markers of parasite biomass, such as parasite DNA/RNA detection and antigen-based detection, and (b) indirect markers, such as macrophage-related markers, cytokines, receptors, acute-phase proteins, and other biomarkers. Biomarkers are further discussed in this section only if they demonstrate promising potential based on the evaluation criteria (>4+). Antibodies were excluded from Table 3 because of their long elimination half-lives (see “Antibody detection” below).
TABLE 3.
Identified potential pharmacodynamic biomarkers for leishmaniasisa
| Marker category | Biomarker | Detection technique(s) | Matrix(ces) | Region(s) | Clinical presentation(s) of leishmaniasis | Biomarker evaluation score |
Reference(s) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time until normalcy | Specificity | Sensitivity (quantitative comparison) | Additional sensitivity | Geographical applicability | |||||||
| Direct markers for: | |||||||||||
| Parasite detection | Parasites in blood | (q)(RT-)PCR, NASBA(-OC), OC | Blood | India/France/Netherlands/Nepal/Italy/Sudan | VL | + | ++ | + | ? | ++ | 14–25, 27, 28 |
| Parasites in lesion biopsy specimen | qRT-PCR, QT-NASBA | Lesion biopsy specimen | Netherlands/India/Germany/Israel/Brazil | CL/PKDL | + | ++ | ++ | ? | ++ | 17, 29–37 | |
| Parasites in skin swab | q(RT-)PCR | (Extra)lesional swab | Colombia/Ecuador | CL | ? | ++ | + | ? | + | 38–40 | |
| Antigen detection | Carbohydrate antigen | Latex agglutination (KAtex), ELISA | Urine | Bangladesh/Nepal/Sudan/Brazil/Yemen/Spain/Iran | VL | + | ++ | + | ? | ++ | 26, 44–55 |
| Indirect markers for: | |||||||||||
| Macrophage-related markers | IDO | HPLC | Plasma | France | VL | – | ? | + | ? | ? | 157 |
| ADA | Colorimetric, substrate assay | Serum | India/Nepal | VL | ++ | + | + | ? | + | 59–62 | |
| Colorimetric, substrate assay | Serum | Turkey | CL | ? | ? | + | ? | ? | 63 | ||
| MIF | ELISA | Serum | Brazil | VL | ? | – | + | ? | ? | 71, 72 | |
| Neopterin | RIA | Serum | Netherlands/Kenya/Brazil | VL | + | ? | + | ? | ++ | 57, 58 | |
| C1q | Radial immunodiffusion | Serum | Kenya | VL | + | ? | + | ? | ? | 158 | |
| Cytokines | CCL3/MIP-1α | ELISA | Serum | Ethiopia | VL | ? | + | + | ? | ? | 159 |
| CCL4/MIP-1β | ELISA | Serum | Ethiopia | VL | ? | + | + | ? | ? | 159 | |
| CXCL10/IP10 | ELISA | Serum | Ethiopia | VL | – | ? | + | ? | ? | 67 | |
| CXCL9/Mig | ELISA | Serum | Ethiopia | VL | – | ? | + | ? | ? | 67 | |
| CD40L | Luminex assay | Serum | Brazil/Bangladesh | VL | + | – | + | ? | ++ | 70, 87 | |
| IL-10 | CBA/ELISA/multiplex biometric immunoassay | Serum | India/Brazil/Sicily/Ethiopia | VL/PKDL | + | – | ++ | + | ++ | 17, 64–69, 73, 74, 77, 82, 98, 160, 161 | |
| RT-PCR/qPCR/immunohistochemistry | Serum/biopsy specimen | French Guiana/Venezuela/Tunisia/Brazil | CL | ? | ? | ++ | ++ | ? | 79–82, 162–164 | ||
| IL-12 | ELISA | Serum | Iran/Brazil/Ethiopia | VL | ++ | ? | + | ? | ++ | 65, 67, 69, 73, 83, 90 | |
| RT-PCR | Biopsy specimen | Tunisia/Mexico/Brazil | CL | ? | ? | + | ++ | ++ | 76, 80, 102 | ||
| IL-15 | ELISA | Serum | Ethiopia, Sicily | VL | ? | ? | + | ? | ++ | 67, 165 | |
| IL-17A | CBA | Serum | Sudan | VL | ? | + | + | ? | ? | 100 | |
| IL-18 | ELISA | Serum/urine | Ethiopia | VL | ++ | + | + | ? | ? | 67, 103 | |
| IL-27 | ELISA | Serum | India | VL | ? | ? | + | ? | ? | 78 | |
| IL-32γ | qPCR | Biopsy specimen | Brazil | CL | ? | ? | + | ? | ? | 163 | |
| IL-33 | ELISA | Serum | France | VL | ? | ? | + | ? | ? | 166 | |
| IL-4 | ELISA | Serum | Brazil/India | VL | ? | ? | + | ? | – | 17, 66, 68, 69, 83, 84, 160, 167 | |
| RT-PCR | Biopsy specimen | India/Brazil | CL | ? | ? | + | ++ | – | 76, 79, 81, 162, 164, 168 | ||
| IL-6 | ELISA/RT-PCR/CBA | Serum | India/Brazil/Sudan/Ethiopia | VL | ++ | ? | ++ | – | ++ | 73, 75, 77, 84, 90, 101, 160 | |
| ELISA/RT-PCR/CBA | Biopsy specimen | Europe/Tunesia/Mexico | CL | ? | ? | + | ++ | ++ | 80, 92, 162, 169 | ||
| IL-8/CXCL8 | CBA | Serum | Bangladesh/Brazil | VL/CL | ? | ? | + | ? | ? | 90, 92 | |
| IFN-γ | CBA/ELISA/multiplex biometric immunoassay | Serum | India/Brazil/Sicily/Ethiopia/Sudan | VL/PKDL | + | + | ++ | ? | ++ | 65–68, 73, 74, 77, 78, 83, 97, 99, 100, 160, 161 | |
| RT-PCR | Serum/biopsy specimen | French Guiana/Mexico/Tunisia/Spain | CL | ? | ? | + | – | ? | 79, 80, 162, 164 | ||
| sFas/sFasL | ELISA | Serum | Ethiopia/Sudan/India | VL | ? | – | + | ? | ++ | 170 | |
| TGF-β | RT-PCR/immunohistology | Biopsy specimen | India/Brazil/Mexico | CL/PKDL | ? | ? | + | ? | ++ | 160, 162 | |
| TNF-α | CBA/ELISA/immunoradiometric assay kit | Serum | Brazil/India/Ethiopia/Sudan | VL/PKDL | ++ | ++ | + | ? | – | 74, 77, 83–86, 88, 90, 91, 99, 100, 160, 171 | |
| Immunoradiometric assay kit/RT-PCR/qPCR/chemiluminescence | Serum/biopsy specimen | Brazil/Mexico/Tunisia/Turkey | CL | ? | ? | + | ++ | – | 80, 92–94, 96, 160, 162, 163, 169, 172 | ||
| Cell surface molecules and circulating receptors | sHLA-G | ELISA | Serum | French | VL | ? | – | + | ? | ? | 173 |
| β2-microglobulin | ELISA | Serum | Sicily | VL | – | ? | + | ? | ? | 174 | |
| sCD14 | ELISA | Serum | Brazil | VL | ? | ? | + | ? | ? | 72 | |
| sCD26 | ELISA | Serum | Iran/India | VL/CL | ? | ? | ++ | ? | – | 175–178 | |
| sCD30 | ELISA | Serum | Iran | VL/CL | ? | ? | ++ | ? | ? | 175, 177, 178 | |
| sCD4 | ELISA | Serum | Brazil/Sicily | VL | ? | ? | + | ? | ++ | 57, 174 | |
| sCD8 | ELISA | Serum | Brazil/Sicily | VL | ? | ? | ++ | ? | ++ | 57, 174 | |
| sICAM-1 | ELISA | Serum | Brazil | VL | ? | ? | ++ | ? | ? | 57, 179 | |
| sIL-2R | ELISA | Serum | Brazil/Sicily | VL/PKDL | – | ? | ++ | + | ++ | 57, 70, 104–106 | |
| sIL-4R | ELISA | Serum | Kenya | VL | ? | + | + | ? | ? | 106 | |
| sTNFR | ELISA | Serum | Sudan/Brazil | VL | + | ? | ++ | ? | ++ | 86, 91 | |
| Acute-phase proteins | AGP | Radial immunodiffusion | Serum | Kenya | VL | ++ | ? | + | ? | ? | 107 |
| CRP | ELISA | Serum | Kenya/India/Sudan | VL | – | ? | ++ | ? | ++ | 75, 107–109 | |
| SAA | ELISA | Serum | Kenya | VL | ++ | ? | ++ | ? | ? | 107 | |
| Other proteins | Arginase | Colorimetric assay | PBMCs/lesion biopsy specimen/serum | Brazil/India/Ethiopia | VL/CL/PKDL | ++ | ? | + | – | ? | 110–113, 180 |
| Cortisol | Radioimmunoassay | Serum | Brazil/Mexico | CL | ? | ? | + | ? | – | 181, 182 | |
| CTLA-4 (CD152) | RT-PCR | Lesion biopsy specimen | India | PKDL | – | ? | + | ? | ? | 82 | |
| DHEA-S | Automated enzyme immunoassay-based techniques/radioimmunoassay | Serum | Brazil/Mexico | CL | ? | ? | + | ? | + | 181, 182 | |
| Foxp3 | RT-PCR | Lesion biopsy specimen | India | PKDL | + | ? | + | + | ? | 82, 183 | |
| MMP2 | RT-PCR | Biopsy specimen | Brazil | CL | ? | ? | ++ | ? | ? | 184 | |
| MMP9 | Luminex assay | Serum | Brazil/Bangladesh | VL | – | – | + | ? | ? | 70, 87 | |
| NOs | Griess reaction | Serum | India/Turkey/Nepal | VL | ? | ? | + | ? | – | 59, 75, 185 | |
| Griess reaction | Serum | Venezuela/Turkey | CL | ? | ? | + | ? | ++ | 89, 186–188 | ||
| Prolactin | Automated enzyme immunoassay-based techniques | Serum | Brazil | CL | ? | ? | + | ++ | ? | 181 | |
| SOD1 | ELISA | Serum | Brazil | CL | + | ? | ++ | ? | ? | 189 | |
IDO, indoleamine 2,3-dioxygenase; ADA, adenosine deaminase; MIF, migration-inhibitory factor; CCL, chemokine (C-C motif) ligand; MIP, macrophage inflammatory protein; CXCL, chemokine (C-X-C motif) ligand; Mig, monokine induced by gamma interferon; CD40L, CD40 ligand; IL, interleukin; IFN-γ, gamma interferon; sFas, soluble Fas; FasL, Fas ligand; TGF-β, transforming growth factor beta; TNF-α, tumor necrosis factor alpha; sHLA, soluble human leukocyte antigen; sICAM-1, soluble intercellular adhesion molecule-1; sTNFR, soluble tumor necrosis factor receptor; AGP, alpha-1-acid glycoprotein; CRP, C-reactive protein; SAA, serum amyloid A protein; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; DHEA-S, dehydroepiandrosterone sulfate; MMP, matrix metalloproteinases; NO, nitric oxide; SOD1, superoxide dismutase 1; RT, reverse transcription; NASBA(-OC), nucleic acid sequence-based amplification(-oligochromatography); qPCR, quantitative PCR; QT, quantitative; HPLC, high-performance liquid chromatography; RIA, radioimmunoprecipitation assay; CBA, cytometric bead array.
Direct markers. (i) Parasite detection.
Assessing the viable parasite load within a patient is probably the most direct marker of disease status for leishmaniasis, and assessing the reduction of the viable parasite biomass would allow for exact monitoring of the therapeutic response. Several target genes have been identified and used for the molecular identification and quantification of Leishmania in clinical samples, including kinetoplast DNA (kDNA, both mini- and maxicircles), small-subunit (SSU) RNA, such as 18S rRNA, and 7SL RNA. For VL patients, the measurement of the Leishmania parasite load in blood using quantitative PCR (qPCR) has been evaluated mainly for diagnosis but also as a proxy value of the overall parasite load and clinical response during and after treatment (14–26). The parasite load in blood rapidly decreases upon initiation of treatment, in parallel with clinical improvement (14–17). qPCR of blood of East African VL patients reflected differences in treatment responses to different AmBisome dosages (27); however, the sensitivity of the assay was lower than for Indian VL patients (28).
For CL, the parasite burden is localized and confined to the upper layer of the dermis, in which it is probably homogenously spread in the inflammatory zone that surrounds the necrotic ulcer (29). Confirmation of parasites by microscopy or, if available, PCR-based techniques from lesion biopsy specimens or scrapings is currently the diagnostic practice for CL (30–36). Quantitation of parasite RNA in repeated lesion biopsy specimens has been demonstrated as a technique to assess the parasite burden in CL lesions (35, 37). Treatment response was quantified in CL patients, demonstrating declines in Leishmania major parasite loads of ∼1 log/week after initiation of miltefosine treatment, which paralleled clinical improvement (29). Swabbing of lesions, which is less invasive than biopsy, was performed to determine whether parasite DNA/RNA loads were diagnostic for CL, and the sensitivity was around 90% (38–40). The pharmacodynamic use of repeated swabbing has not yet been reported. Interestingly, the presence of parasites in CL has also been shown at (unaffected) extralesional sites (38, 40), opening up other possibilities for less invasive sampling procedures. For PKDL, Leishmania DNA was also detected in lesion material before treatment; a significantly higher parasite burden was found in chronic lesions than in transient lesions, with burdens reduced to nondetectable levels posttreatment (17). The pharmacodynamic use of newer molecular tools (e.g., loop-mediated isothermal amplification [LAMP]) (41, 42) has not yet been investigated.
(ii) Antigen detection.
Disease-specific antigen detection is regularly used as a predictive biomarker, e.g., for various cancer types (43), and is potentially useful for infectious diseases as well. For leishmaniasis, however, the application of antigen tests has been limited mainly to diagnostics making use of a urine-based latex agglutination assay (KAtex), which detects a heat-stable low-molecular-weight carbohydrate antigen found in the urine of VL patients (26, 44–46). The method has been successfully evaluated and compared to other methods for diagnosis of VL patients in various geographical areas, ranging from East Africa to South Asia (3, 26, 47–55). Even though specificity was consistently high (98% to 100%) in these studies, sensitivity appeared to be very low to moderate (48% to 95.2%), with a high discrepancy between studies. Studies from India and Sudan indicated that the urine antigen detection test became negative in cured VL patients at least 30 days after the end of treatment (48, 49), indicating a possible pharmacodynamic use of this assay.
Indirect markers. (i) Macrophage-related markers.
Leishmania parasites reside and replicate inside the phagocytic cells of the reticuloendothelial system, mainly macrophages, increasing the overall macrophage biomass in the host. Since the macrophage load also decreases again with waning parasitic infection, soluble macrophage-related markers—specifically when produced by infected macrophages—are potential semidirect biomarkers. Neopterin is a heterocyclic pteridine compound which is synthesized by macrophages after gamma interferon (IFN-γ) stimulation (56). It is considered an indicator of activation of cellular immunity. Increased neopterin production is found in a broad range of diseases, e.g., in viral infections (HIV, hepatitis B and C) and infections due to intracellular bacteria (tuberculosis, malaria). Serum neopterin concentrations were elevated in VL patients compared to levels in controls and decreased to normal concentrations at the end of treatment in cured patients, whereas they were still significantly increased in refractory patients (57). Serum neopterin concentrations were not found to be elevated in CL patients (58).
Adenosine deaminase (ADA), found particularly in macrophages and lymphocytes, is a key enzyme in the breakdown of adenosine, a purine nucleoside that suppresses the inflammatory responses. For VL, serum ADA activity was increased at diagnosis and returned to almost normal concentrations at the end of therapy (day 30) in Nepalese and Indian patients (59–62). At diagnosis, activity appeared higher in VL patients than in malaria, leprosy, or tuberculosis patients (60). Also, in Turkish CL patients, adenosine deaminase was increased at the time of diagnosis (63).
(ii) Cytokines.
Recovery from VL is linked mainly to the CD4+ T-cell-mediated cellular immune response. More specifically, the Th1-mediated response is generally associated with macrophage activation, host resistance, and protection against Leishmania parasites, leading to control of disease. Conversely, the Th2-mediated response is associated with downregulation of macrophage activation and eventually progression of disease. Unfortunately, this distinction between Th1 and Th2 activation is a simplified model, and many patients demonstrate a nonspecific immune response profile.
The most studied cytokines are the proinflammatory cytokines IFN-γ and tumor necrosis factor alpha (TNF-α) and the regulatory cytokine interleukin-10 (IL-10). Plasma IL-10 was found to be increased in Indian patients with active VL and could be detected in the keratinocytes and sweat glands of patients who eventually developed PKDL (64). The increase of IL-10 concentrations in VL patients was later confirmed in a range of countries, including, among others, India, Brazil, and Ethiopia (65–77). IL-10 levels were found to drop significantly after successful treatment (66, 70, 73, 78) to near-control levels 5 to 7 days posttreatment (74). Ansari et al. found no difference in pretreatment IL-10 levels between responsive and unresponsive patients (74). For CL, IL-10 might be a possible pharmacodynamic marker indicating treatment failure, as IL-10 mRNA levels in lesion biopsy specimens were found to be positively associated with unresponsiveness to treatment (79, 80). Cured mucocutaneous leishmaniasis (MCL) patients demonstrated a higher percentage of IL-10-expressing cells pretreatment than relapsing patients (81). Interestingly, IL-10 was found to be positively correlated with the parasite load in the blood of VL patients (17, 70) and lesional tissue of PKDL patients (82).
TNF-α is a cytokine produced mainly by activated macrophages. TNF-α levels were found to be significantly increased in patients with active VL (77, 83–85); they declined during treatment (85–89) and returned to healthy-control levels at the end of treatment (84). Unresponsive patients retained elevated levels of TNF-α (85). In contrast, other studies found minimal levels of this cytokine in Indian VL patients (74, 90, 91). Moreover, TNF-α was also present in asymptomatic VL patients (83), complicating the interpretation of TNF-α in cases of VL. For CL patients, studies of TNF-α serum levels are contradictory; some studies found elevated levels of TNF-α in the plasma of CL patients that decreased posttreatment compared to healthy controls (92–95), but others could confirm this only for MCL patients (85, 96). TNF-α mRNA levels in lesion biopsy specimens were found to be positively correlated with lesion size (80).
IFN-γ is a critical soluble cytokine for innate and adaptive immunity against intracellular infections and is involved in the activation of macrophages. IFN-γ levels were found to be significantly elevated in patients with active VL, which was confirmed in a wide range of countries: India (74, 75), Bangladesh (70), Brazil (70, 72, 73, 77), Ethiopia (67, 68), Sicily (66), and Iran (65). During and after successful treatment, IFN-γ levels were found to drop significantly but remained elevated compared to levels in healthy controls (66, 70, 73, 75, 78). In contrast, Cenini et al. (84) showed that IFN-γ levels returned to healthy-control levels at the end of treatment. Moreover, IFN-γ plasma concentrations appeared to be significantly higher after the end of treatment in patients unresponsive to therapy than in responsive patients treated with sodium stibogluconate (SSG) (74, 75). Discrepant results in asymptomatic VL patients indicated that IFN-γ was elevated in 48% of asymptomatic Brazilian patients but that it was undetectable in the vast majority of asymptomatic Ethiopian patients (67, 83). Additionally, a recent study of Brazilian pediatric VL patients showed that low levels of IFN-γ were associated with signs of severity, such as jaundice or hemorrhage (97). In CL lesion biopsy specimens, no significant difference in IFN-γ levels could be found between patients with favorable and unfavorable lesion evolutions (79).
For PKDL patients, the expression of the mRNA of the three cytokines IL-10, IFN-γ, and TNF-α in lesions was found to be significantly elevated compared to that in control tissues (74, 82). After treatment, these levels were restored to near-control levels (74). Ganguly et al. found that IL-10 and IFN-γ levels were significantly higher in patients with polymorphic PKDL than in patients with macular PKDL (98).
Concerning patients with HIV-VL coinfection, only TNF-α and IFN-γ serum levels were still significantly elevated in HIV patients when they developed VL coinfection, while IL-10 levels tended to decrease (99). Also, compared to Chagas disease, dengue fever, and tuberculosis patients, leishmaniasis patients showed high levels of TNF-α (70). TNF-α and IFN-γ levels increased significantly when malaria patients developed a VL coinfection (100).
The interleukins IL-6 (74, 75, 77, 84, 101) and IL-12 (65, 67, 69, 70) (often measured as the concentration of the subunit IL-12p40) were also found to be significantly increased in the sera of VL patients. In Sudanese and Ethiopian VL patients, IL-6 returned to normal concentrations within the treatment period (84, 101) and seemed indicative of relapse events (101). However, IL-6 was not correlated with spleen/liver size (73). Also IL-12 levels were found to drop significantly within 30 days of treatment (73) and was largely absent in cured and asymptomatic cases (67, 69). In contrast, in Bangladesh and Brazil, IL-12 was shown to be elevated in asymptomatic VL cases (83, 90). Both interleukins also showed pharmacodynamic potential in CL patients. IL-12 was correlated with unfavorable lesion evolution and lesion duration (80, 102). IL-6 mRNA from biopsy specimens was correlated with lesion size, and also IL-6 serum concentrations were found to be elevated in CL patients (80, 94).
IL-18 was also increased in patients with active VL compared to levels in healthy controls (67). Interestingly, a significant decrease in urinary IL-18 levels was detected during treatment (103). Urinary detection of biomarkers would be ideal due to its noninvasive collection method.
Soluble CD40 ligand (sCD40L) (also called sCD154) was significantly decreased in VL patients at diagnosis compared to levels in controls in areas of endemicity (70, 87). During treatment, sCD40L levels increased toward healthy control levels. However, similar CD40L levels were found for Chagas disease and VL patients, which might cause specificity issues (87).
(iii) Cell surface molecules and circulating receptors.
Levels of circulating soluble cytokine receptors for IL-2 and IL-4 (sIL-2R and sIL-4R, respectively) were elevated in patients with active VL, with higher concentrations of sIL4R than in patients with other local and systemic parasitic infections (57, 104–106). Serum sIL-2R concentrations correlated with Leishmania DNA serum levels (70) and significantly decreased during treatment (57, 70) but returned to normal only after several months (105). At the start of treatment, sIL-2R levels were also significantly higher in patients developing PKDL than in patients not developing PKDL (64). Additionally, mRNA levels of the IL-2R α-chain were significantly elevated in lesions of PKDL patients before treatment and returned to control levels posttreatment (82).
Circulating sCD4 and sCD8 were increased at the start of treatment and returned to levels comparable to those in healthy controls within several months after treatment (57, 105). sCD8 was significantly decreased posttreatment in responders to therapy compared to levels in nonresponders, making it a possible suitable pharmacodynamic marker (57).
Serum levels of the soluble receptors for TNF (sTNFRs) were significantly elevated in patients with active VL compared to levels in controls in areas of endemicity and nonendemicity (91). Responding patients showed a steep decrease in sTNFR levels already at day 15 during treatment, in contrast to nonresponders (86, 91).
(iv) Acute-phase proteins.
Acute-phase proteins widely used as clinical markers of inflammation and infection, which increase during many (non)infective inflammatory diseases and malignancies, also increase during VL. C-reactive protein (CRP), serum amyloid A protein (SAA), and alpha-1-acid glycoprotein (AGP) were increased in Kenyan VL patients upon diagnosis and reached normal levels before or at the end of treatment (SAA and AGP) or at 60 days posttreatment (CRP) (107). Elevation of CRP levels was confirmed for Indian patients with active VL (75). Interestingly, pretreatment CRP levels were lower in patients responding fast to treatment than in slow-responders, with lower splenic parasite counts (107), which was confirmed in a large Indian pediatric VL cohort (108). An increased pretreatment CRP concentration in VL patients was associated with the development of PKDL (109), while CRP levels were not significantly elevated in PKDL patients. However, the specificity of acute-phase proteins in the monitoring of VL treatments is probably low, as they are increased in a myriad of other infectious and noninfectious inflammatory illnesses.
(v) Other markers.
Arginase catalyzes the metabolism of l-arginine into l-ornithine and urea. The resulting diminishing bioavailability of l-arginine is regarded as a potent mechanism of immune suppression and impairment of T-cell responses. In patients with active VL and CL, arginase activity in plasma was found to be significantly increased, and levels returned to control levels for VL patients during SSG treatment (110, 111). VL-HIV coinfection patients appeared to have increased arginase activity compared to VL patients, both in plasma and peripheral blood mononuclear cells (PBMCs) (112). In PKDL patients, arginase activity declined after miltefosine treatment but not after SSG treatment (113).
Antibody detection.
All of the current first-line diagnostic serological tests for VL are antibody detection tests (114, 115). Two serological tests are currently being used in the field: the direct agglutination test (DAT), based on numbers of killed whole L. donovani promastigotes, and the recombinant K39 (rK39)-based immunochromatographic antibody detection test. Other antigen-based assays have been developed for Leishmania antibody detection, using (recombinant) proteins rK9, rK26, rK28, Leishmania infantum cytosolic tryparedoxin peroxidase (LicTXNPx), rgp63, rLepp12, recombinant open reading frame F (rORFF), BHUP2, rKLO8, rHSP70, guanylate binding protein (GBP), galactosyl-α(1-3)galactose, 9-O-acetylated sialic acids, recombinant peroxidoxin, and amastin (116–130). Unfortunately, antibodies against Leishmania parasites exhibit a long half-life and stay detectable for several months up to several years after an infection [tested by the DAT and for galactosyl-α(1-3)galactose, LicTXNPx, rK26, rK39, and BHUP2] (49, 120, 121, 131–141), which compromises the diagnosis of a relapse case and also the pharmacodynamic application of these markers. However, it was found that for some antibodies (against the recombinant Leishmania antigens rH2A, KMP11, the “Q” protein, and 9-O-acetylated sialic acids), the levels do decrease significantly 30 to 60 days after treatment (129, 142). Furthermore, 1 week posttreatment, only ∼40 to 50% of patients gave a positive signal for rLepp12, compared to 100% for rK39 and for direct agglutination (125). Though not very sensitive (44%), Leishmania-specific immunoglobulin E (L-IgE) has been suggested to be a specific (98.3%) marker of active VL disease (L. chagasi), although it is undetectable at subclinical levels in VL patients, Chagas disease patients, and healthy controls (143–145). Moreover, increased L-IgE concentrations were demonstrated to regress to normal values during the time span of treatment (143, 145, 146). In cases of atypical cutaneous leishmaniasis, IgE levels were not significantly different from those of asymptomatic or healthy controls (147). Anam et al. (144) also hinted at a possible (diagnostic) role for L. donovani-specific IgG3 antibody isotype detection. While the IgG3 antibody level decreases significantly posttreatment (148, 149), the pharmacodynamic value of this marker is probably very low, as the time to normalcy for IgGs is longer than 3 months for both CL and VL patients (150–154).
Besides the drawback of the long half-lives of antibodies, antibody detection tests tend to be positive in a significant proportion of noninfected or otherwise asymptomatic individuals living in areas where VL is endemic (135, 155, 156). Due to these crucial limitations in the use of antibodies to monitor therapies, these markers are excluded from Table 3.
GENERAL ISSUES PERTAINING TO THE PHARMACODYNAMIC POTENTIAL OF BIOMARKERS
Our systematic literature review identified 53 biomarkers for VL, CL, and PKDL. Several general issues might limit their pharmacodynamic potential. First, the large majority of biomarkers were evaluated only for their diagnostic use. Leishmaniasis patients were generally compared to healthy controls before the start of their treatment. Only a few VL studies have focused on differentiating active, clinical disease from subclinical or asymptomatic disease, although this might potentially be an interesting approach to demonstrate the Th1/Th2 paradigm. When a biomarker was evaluated for its ability to monitor a treatment effect, it was almost always done by comparison of pre- and posttreatment concentrations, without repeated longitudinal measurements. Therefore, the pharmacodynamic potential of most biomarkers remains difficult to assess based on the available literature.
Second, most identified biomarkers for leishmaniasis are indirect markers, i.e., universal markers of activation and the subsequent waning of cellular immunity. As a result, specificity may be low compared to that for patients suffering from common concomitant infections. Interestingly, a few biomarkers (TNF-α, CCL3, and CCL4) have been shown to be specific for HIV-VL coinfection patients rather than HIV patients. Other biomarkers (ADA and sIL4R) were elevated in patients with VL, but not malaria, indicating a possible value in malaria-VL coinfection. Despite these exploratory results, the majority of markers have not been tested against the most common VL coinfections, and further research is needed to establish their specificity as biomarkers.
Third, multiple studies focused on correlating biomarker levels to clinical features of CL (e.g., lesion size), while this correlation was generally ignored for VL. In general, more emphasis should be put in future clinical trials on establishing a correlation between the studied biomarkers and clinical parameters.
Fourth, the time needed to regress to normalcy for the biomarkers (characterized by their elimination half-lives) remains a concern. For instance, almost all of the antibody-related markers have a very long elimination half-life of up to several months and stay present in the body long after the actual parasitic infection has been resolved. Their potential for pharmacodynamic monitoring of antileishmanial treatment is therefore probably negligible. Leishmania antigen detection might be more promising in that respect; however, this has been investigated mainly in the context of a diagnosis of VL, with only limited attention paid to repeated quantitative measurement during and after treatment. The less specific indirect markers, for example, AGP and TNF-α, often show preferable time-to-normalcy values.
Lastly, the practical feasibility, in terms of cost, invasiveness, and laboratory requirements, is an additional concern. The preferred sample matrix for a biomarker should be noninvasive (e.g., urine or saliva). All identified biomarkers were measured in blood or biopsy specimens, except for IL-18 and KAtex, both of which can be measured in urine. Though this review focused on biomarkers within the context of a clinical trial setting, it is important to note that equipment-free procedures not requiring a cold chain are required for the application of pharmacodynamic biomarkers in routine settings.
SELECTION OF POTENTIAL PHARMACODYNAMIC BIOMARKERS
In this section, we will highlight and critically appraise the application of a selection of potential pharmacodynamic biomarkers, with some recommendations for research priorities.
Direct biomarkers.
Recently, the quantitative application of molecular parasite detection methods as a pharmacodynamic measure was demonstrated, both in VL and CL. While this method measures the parasite directly and therefore is theoretically the most promising biomarker, there are some issues. First, the sensitivity of this marker for VL is relatively low (∼80%) and seems to vary between geographical regions (27, 28). The parasite loads appear to decrease with clinical cure but are undetectable before clinical cure can be established. The parasites reside within the spleen, bone marrow, and liver, and plasma is therefore only a proxy reservoir of the parasite. Additionally, it remains unknown what the predictive value is of blood parasite loads in relapsing patients and controls in areas of endemicity. Last, it is unsuitable for routine monitoring due to its high costs (considering the ∼€30/sample material costs and the required state-of-the-art laboratory equipment and trained technicians, this tool can be used only in a clinical trial setting).
Another direct biomarker with potential is the Leishmania carbohydrate antigen, which forms the basis for the diagnostic KAtex test. One of the biggest advantages of this biomarker is that it can be detected in urine. Its specificity is consistently high, but its sensitivity appears variable, which may make it suitable only in controlled settings. The Leishmania-specific antigen can be assessed quantitatively by enzyme-linked immunosorbent assay (ELISA), which makes it easier to adopt at primary health care facilities than the molecular detection methods.
Indirect biomarkers.
Of the indirect biomarkers, the most promising are the macrophage-related markers, as these are directly related to parasitic infection of macrophages. ADA activity is increased in patients with VL and CL, returns to normal during treatment, and shows promising results in patients with HIV-VL coinfection. Unfortunately, this marker has no proven geographical applicability, and there are no data on the relation between ADA activity levels and clinical outcome.
Though most cytokines demonstrated a lack of specificity, a range of them showed promising results with regard to the other evaluation criteria. IL-10 correlated with the parasite load at the time of diagnosis, decreased during treatment, and was even associated with the occurrence of PKDL. However, IL-10 was increased as well in subclinical cases, which complicates its interpretation, certainly in the context of parasite recrudescence. Although studies from different regions contradict each other on its sensitivity, TNF-α shows the highest specificity in comparison to other cytokines, indicating that it might be applicable as a biomarker in certain regions of endemicity. Levels of other indirect markers (e.g., sTNFR, IL-6) appeared predictive for clinical outcome but require further evaluation with regard to the other criteria for us to be able to draw conclusions on their potential. A practical advantage of cytokines is that their ELISA kits are relatively low in cost and may be implemented on a large scale, even though a basic laboratory is still required.
CONCLUSIONS AND FUTURE PERSPECTIVE
The biomarkers identified in this systematic review have been evaluated mainly for diagnostic purposes and do not (yet) meet the requirements for monitoring of clinical outcome as surrogate endpoints in clinical trials. Most promising for the application in pharmacodynamic evaluations are the highly specific direct biomarkers (DNA/RNA or antigenic markers), which appear to have a good correlation with clinical outcome. However, future research should specifically focus on the identification of optimal molecular and antigenic targets to increase the sensitivity of these tools. Macrophage-related markers are theoretically the most promising of the indirect markers, as they are directly linked to macrophage (and possibly parasite) load. Though neopterin and ADA have shown high sensitivity and geographical applicability as biomarkers, more evidence is needed to confirm their potential in predicting clinical outcome. Indirect markers, such as IL-10 and TNF-α, have demonstrated high sensitivity and seem to indicate clinical outcome. Nevertheless, given the lack of specificity and the complexity of the immunological response associated with VL infection, it is unlikely that a single immunological biomarker will be suitable to accurately monitor treatment response. These markers can still be of use in well-controlled trials with sufficient exclusion of concomitant diseases. However, they are not suitable for application in routine clinical care, as in that case, the biomarker should be able to discriminate clinical outcome at the level of an individual patient. Additional efforts are needed to investigate the applicability of combinations of cytokines as biomarker profiles to monitor treatment outcome at the patient level.
In general, future biomarker research should extend its focus to biomarkers' pharmacodynamic potential by correlating longitudinal quantitative assessments of the marker (i.e., the marker concentration-time profile in response to therapy) to multiple clinical parameters.
The coming of age of new treatment options for leishmaniasis was long and eagerly awaited, but now that this moment approaches, we urgently need better and more accurate tools to evaluate their potential superiority over existing regimens and rationalize their dosing schedule. Evaluation of pharmacodynamic biomarkers is therefore of crucial importance to optimize and accelerate drug development for this neglected tropical disease.
ACKNOWLEDGMENT
We have no conflicts of interest to declare.
REFERENCES
- 1.Croft SL, Olliaro P. 2011. Leishmaniasis chemotherapy—challenges and opportunities. Clin Microbiol Infect 17:1478–1483. doi: 10.1111/j.1469-0691.2011.03630.x. [DOI] [PubMed] [Google Scholar]
- 2.Chatelain E, Ioset JR. 2011. Drug discovery and development for neglected diseases: the DNDi model. Drug Des Dev Ther 5:175–181. doi: 10.2147/DDDT.S16381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boelaert M, Bhattacharya S, Chappuis F, El Safi SH, Hailu A, Mondal D, Rijal S, Sundar S, Wasunna M, Peeling RW. 2007. Evaluation of rapid diagnostic tests: visceral leishmaniasis. Nat Rev Microbiol 5:S30–S39. doi: 10.1038/nrmicro1766. [DOI] [Google Scholar]
- 4.Chulay JD, Bryceson ADM. 1983. Quantitation of amastigotes of Leishmania donovani in smears of splenic aspirates from patients with visceral leishmaniasis. Am J Trop Med Hyg 32:475–479. [DOI] [PubMed] [Google Scholar]
- 5.Kager PA, Rees PH, Manguyu FM, Bhatt KM, Bhatt SM. 1983. Splenic aspiration; experience in Kenya. Trop Geogr Med 35:125–131. [PubMed] [Google Scholar]
- 6.Thakur CP. 1997. A comparison of intercostal and abdominal routes of splenic aspiration and bone marrow aspiration in the diagnosis of visceral leishmaniasis. Trans R Soc Trop Med Hyg 91:668–670. doi: 10.1016/S0035-9203(97)90516-2. [DOI] [PubMed] [Google Scholar]
- 7.Sundar S, Rai M. 2002. Laboratory diagnosis of visceral leishmaniasis. Clin Diagn Lab Immunol 9:951–958. doi: 10.1128/CDLI.9.5.951-958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.González U, Pinart M, Rengifo-Pardo M, Macaya A, Alvar J, Tweed JA. 2009. Interventions for American cutaneous and mucocutaneous leishmaniasis. Cochrane Database Syst Rev 2:CD004834. doi: 10.1002/14651858.CD004834.pub2. [DOI] [PubMed] [Google Scholar]
- 9.González U, Pinart M, Reveiz L, Alvar J. 2008. Interventions for Old World cutaneous leishmaniasis. Cochrane Database Syst Rev 4:CD005067. doi: 10.1002/14651858.CD005067.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez U, Pinart M, Reveiz L, Rengifo-Pardo M, Tweed J, Macaya A, Alvar J. 2010. Designing and reporting clinical trials on treatments for cutaneous leishmaniasis. Clin Infect Dis 51:409–419. doi: 10.1086/655134. [DOI] [PubMed] [Google Scholar]
- 11.Rijal S, Ostyn B, Uranw S, Rai K, Bhattarai NR, Dorlo TP, Beijnen JH, Vanaerschot M, Decuypere S, Dhakal SS, Das ML, Karki P, Singh R, Boelaert M, Dujardin JC. 2013. Increasing failure of miltefosine in the treatment of kala-azar in Nepal and the potential role of parasite drug resistance, re-infection or non-compliance. Clin Infect Dis 56:1530–1538. doi: 10.1093/cid/cit102. [DOI] [PubMed] [Google Scholar]
- 12.Biomarkers Definitions Working Group. 2001. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 13.Strimbu K, Tavel JA. 2010. What are biomarkers? Curr Opin HIV AIDS 5:463–466. doi: 10.1097/COH.0b013e32833ed177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mary C, Faraut F, Drogoul MP, Xeridat B, Schleinitz N, Cuisenier B, Dumon H. 2006. Reference values for Leishmania infantum parasitemia in different clinical presentations: quantitative polymerase chain reaction for therapeutic monitoring and patient follow-up. Am J Trop Med Hyg 75:858–863. [PubMed] [Google Scholar]
- 15.Mary C, Faraut F, Lascombe L, Dumon H. 2004. Quantification of Leishmania infantum DNA by a real-time PCR assay with high sensitivity. J Clin Microbiol 42:5249–5255. doi: 10.1128/JCM.42.11.5249-5255.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Vries PJ, Van der Meide WF, Godfried MH, Schallig HD, Dinant HJ, Faber WR. 2006. Quantification of the response to miltefosine treatment for visceral leishmaniasis by QT-NASBA. Trans R Soc Trop Med Hyg 100:1183–1186. doi: 10.1016/j.trstmh.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Verma S, Kumar R, Katara GK, Singh LC, Negi S, Ramesh V, Salotra P. 2010. Quantification of parasite load in clinical samples of leishmaniasis patients: IL-10 level correlates with parasite load in visceral leishmaniasis. PLoS One 5:e10107. doi: 10.1371/journal.pone.0010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osman OF, Kager PA, Zijlstra EE, El-Hassan A, Oskam L. 1997. Use of PCR on lymph-node samples as test of cure of visceral leishmaniasis. Ann Trop Med Parasitol 91:845–850. doi: 10.1080/00034989760608. [DOI] [PubMed] [Google Scholar]
- 19.Pizzuto M, Piazza M, Senese D, Scalamogna C, Calattini S, Corsico L, Persico T, Adriani B, Magni C, Guaraldi G, Gaiera G, Ludovisi A, Gramiccia M, Galli M, Moroni M, Corbellino M, Antinori S. 2001. Role of PCR in diagnosis and prognosis of visceral leishmaniasis in patients coinfected with human immunodeficiency virus type 1. J Clin Microbiol 39:357–361. doi: 10.1128/JCM.39.1.357-361.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deborggraeve S, Boelaert M, Rijal S, De Doncker S, Dujardin JC, Herdewijn P, Büscher P. 2008. Diagnostic accuracy of a new Leishmania PCR for clinical visceral leishmaniasis in Nepal and its role in diagnosis of disease. Trop Med Int Health 13:1378–1383. doi: 10.1111/j.1365-3156.2008.02154.x. [DOI] [PubMed] [Google Scholar]
- 21.Arora SK, Gupta S, Bhardwaj S, Sachdeva N, Sharma NL. 2008. An epitope-specific PCR test for diagnosis of Leishmania donovani infections. Trans R Soc Trop Med Hyg 102:41–45. doi: 10.1016/j.trstmh.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Bossolasco S, Gaiera G, Olchini D, Gulletta M, Martello L, Bestetti A, Bossi L, Germagnoli L, Lazzarin A, Cinque P, Uberti-Foppa C. 2003. Real-time PCR assay for clinical management of human immunodeficiency virus-infected patients with visceral leishmaniasis. J Clin Microbiol 41:5080–5084. doi: 10.1128/JCM.41.11.5080-5084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salotra P, Sreenivas G, Pogue GP, Lee N, Nakhasi HL, Ramesh V, Negi NS. 2001. Development of a species-specific PCR assay for detection of Leishmania donovani in clinical samples from patients with kala-azar and post-kala-azar dermal leishmaniasis. J Clin Microbiol 39:849–854. doi: 10.1128/JCM.39.3.849-854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antinori S, Calattini S, Longhi E, Bestetti G, Piolini R, Magni C, Orlando G, Gramiccia M, Acquaviva V, Foschi A, Corvasce S, Colomba C, Titone L, Parravicini C, Cascio A, Corbellino M. 2007. Clinical use of polymerase chain reaction performed on peripheral blood and bone marrow samples for the diagnosis and monitoring of visceral leishmaniasis in HIV-infected and HIV-uninfected patients: a single-center, 8-year experience in Italy and review. Clin Infect Dis 44:1602–1610. doi: 10.1086/518167. [DOI] [PubMed] [Google Scholar]
- 25.De Doncker S, Hutse V, Rijal S, Man B, Singh Karki BM, Decuypere S, Jacquet D, Le Ray D, Boelaert M, Koirala S, Dujardin JC. 2005. A new PCR-ELISA for diagnosis of visceral leishmaniasis in blood of HIV-negative subjects. Trans R Soc Trop Med Hyg 99:25–31. doi: 10.1016/j.trstmh.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Salam MA, Khan GM, Mondal D. 2011. Urine antigen detection by latex agglutination test for diagnosis and assessment of initial cure of visceral leishmaniasis. Trans R Soc Trop Med Hyg 105:269–272. doi: 10.1016/j.trstmh.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Khalil EAG, Weldegebreal T, Younis BM, Omollo R, Musa AM, Hailu W, Abuzaid AA, Dorlo TPC, Hurissa Z, Yifru S, Haleke W, Smith PG, Ellis S, Balasegaram M, El-Hassan AM, Schoone GJ, Wasunna M, Kimutai R, Edwards T, Hailu A. 2014. Safety and efficacy of single dose versus multiple doses of AmBisome H for treatment of visceral leishmaniasis in eastern Africa: a randomised trial. PLoS Negl Trop Dis 8:e2613. doi: 10.1371/journal.pntd.0002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sudarshan M, Weirather JL, Wilson ME, Sundar S. 2011. Study of parasite kinetics with antileishmanial drugs using real-time quantitative PCR in Indian visceral leishmaniasis. J Antimicrob. Chemother 66:1751–1755. doi: 10.1093/jac/dkr185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dorlo TPC, van Thiel PP, Schoone GJ, Stienstra Y, van Vugt M, Beijnen JH, de Vries PJ. 2011. Dynamics of parasite clearance in cutaneous leishmaniasis patients treated with miltefosine. PLoS Negl Trop Dis 5:e1436. doi: 10.1371/journal.pntd.0001436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray HW, Berman JD, Davies CR, Saravia NG. 2005. Advances in leishmaniasis. Lancet 366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 31.de Oliveira CI, Báfica A, Oliveira F, Favali CBF, Correa T, Freitas LAR, Nascimento E, Costa JM, Barral A. 2003. Clinical utility of polymerase chain reaction-based detection of Leishmania in the diagnosis of American cutaneous leishmaniasis. Clin Infect Dis 37:e149–153. doi: 10.1086/379610. [DOI] [PubMed] [Google Scholar]
- 32.Schönian G, Nasereddin A, Dinse N, Schweynoch C, Schallig HDFH, Presber W, Jaffe CL. 2003. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn Microbiol Infect Dis 47:349–358. doi: 10.1016/S0732-8893(03)00093-2. [DOI] [PubMed] [Google Scholar]
- 33.Mendonça MG, de Brito MEF, Rodrigues EHG, Bandeira V, Jardim ML, Abath FGC. 2004. Persistence of leishmania parasites in scars after clinical cure of American cutaneous leishmaniasis: is there a sterile cure? J Infect Dis 189:1018–1023. doi: 10.1086/382135. [DOI] [PubMed] [Google Scholar]
- 34.Schubach A, Haddad F, Oliveira-Neto MP, Degrave W, Pirmez C, Grimaldi GJ, Fernandes O. 1998. Detection of Leishmania DNA by polymerase chain reaction in scars of treated human patients. J Infect Dis 178:911–914. doi: 10.1086/515355. [DOI] [PubMed] [Google Scholar]
- 35.van der Meide WF, Schoone GJ, Faber WR, Zeegelaar JE, de Vries HJC, Ozbel Y, Lai A Fat RFM, Coelho LIARC, Kassi M, Schallig HDFH. 2005. Quantitative nucleic acid sequence-based assay as a new molecular tool for detection and quantification of Leishmania parasites in skin biopsy samples. J Clin Microbiol 43:5560–5566. doi: 10.1128/JCM.43.11.5560-5566.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar R, Bumb RA, Ansari NA, Mehta RD, Salotra P. 2007. Cutaneous leishmaniasis caused by Leishmania tropica in Bikaner, India: parasite identification and characterization using molecular and immunologic tools. Am J Trop Med Hyg 76:896–901. [PubMed] [Google Scholar]
- 37.van der Meide WF, Peekel I, Van Thiel PPAM, Schallig HDFH, de Vries HJC, Zeegelaar JE, Faber WR. 2008. Treatment assessment by monitoring parasite load in skin biopsies from patients with cutaneous leishmaniasis, using quantitative nucleic acid sequence-based amplification. Clin Exp Dermatol 33:394–399. doi: 10.1111/j.1365-2230.2007.02680.x. [DOI] [PubMed] [Google Scholar]
- 38.Figueroa RA, Lozano LE, Romero IC, Cardona MT, Prager M, Pacheco R, Diaz YR, Tellez JA, Saravia NG. 2009. Detection of Leishmania in unaffected mucosal tissues of patients with cutaneous leishmaniasis caused by Leishmania (Viannia) species. J Infect Dis 200:638–646. doi: 10.1086/600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mimori T, Matsumoto T, Calvopiña MH, Gomez EA, Saya H, Katakura K, Nonaka S, Shamsuzzaman SM, Hashiguchi Y. 2002. Usefulness of sampling with cotton swab for PCR-diagnosis of cutaneous leishmaniasis in the New World. Acta Trop 81:197–202. doi: 10.1016/S0001-706X(01)00215-7. [DOI] [PubMed] [Google Scholar]
- 40.Romero I, Téllez J, Suárez Y, Cardona M, Figueroa R, Zelazny A, Gore Saravia N. 2010. Viability and burden of Leishmania in extralesional sites during human dermal leishmaniasis. PLoS Negl Trop Dis 4:e819. doi: 10.1371/journal.pntd.0000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adams ER, Schoone GJ, Ageed AF, Safi SE, Schallig HDFH. 2010. Development of a reverse transcriptase loop-mediated isothermal amplification (LAMP) assay for the sensitive detection of Leishmania parasites in clinical samples. Am J Trop Med Hyg 82:591–596. doi: 10.4269/ajtmh.2010.09-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Itoh M, Takagi H. 2011. Mass-survey using urine and confirmation by LAMP for control of visceral leishmaniasis, p 91–98. In Jha TK, Noiri E (ed), Kala azar in South Asia: current status and challenges ahead, 1st ed. Springer, New York, NY. [Google Scholar]
- 43.Sawyers CL. 2008. The cancer biomarker problem. Nature 452:548–552. doi: 10.1038/nature06913. [DOI] [PubMed] [Google Scholar]
- 44.Sarkari B, Chance M, Hommel M. 2002. Antigenuria in visceral leishmaniasis: detection and partial characterisation of a carbohydrate antigen. Acta Trop 82:339–348. doi: 10.1016/S0001-706X(02)00043-8. [DOI] [PubMed] [Google Scholar]
- 45.Hatam GR, Ghatee MA, Hossini SM, Sarkari B. 2009. Improvement of the newly developed latex agglutination test (Katex) for diagnosis of visceral leishmaniasis. J Clin Lab Anal 205:202–205. doi: 10.1002/jcla.20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Attar ZJ, Chance ML, El-Safi S, Carney J, Azazy A, El-Hadi M, Dourado C, Hommel M. 2001. Latex agglutination test for the detection of urinary antigens in visceral leishmaniasis. Acta Trop 78:11–16. doi: 10.1016/S0001-706X(00)00155-8. [DOI] [PubMed] [Google Scholar]
- 47.Ahsan MM, Islam MN, Mollah AH, Hoque MA, Hossain MA, Begum Z, Islam MT. 2010. Evaluation of latex agglutination test (KAtex) for early diagnosis of kala-azar. Mymensingh Med J 19:335–359. [PubMed] [Google Scholar]
- 48.El-Safi SH, Abdel-Haleem A, Hammad A, El-Basha I, Omer A, Kareem HG, Boelaert M, Chance M, Hommel M. 2003. Field evaluation of latex agglutination test for detecting urinary antigens in visceral leishmaniasis in Sudan. East Mediterr Health J 9:844–855. [PubMed] [Google Scholar]
- 49.Singh DP, Goyal RK, Singh RK, Sundar S, Mohapatra TM. 2010. In search of an ideal test for diagnosis and prognosis of kala-azar. J Health Popul Nutr 28:281–285. doi: 10.3329/jhpn.v28i3.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chappuis F, Rijal S, Jha UK, Desjeux P, Karki BMS, Koirala S, Loutan L, Boelaert M. 2006. Field validity, reproducibility and feasibility of diagnostic tests for visceral leishmaniasis in rural Nepal. Trop Med Int Health 11:31–40. doi: 10.1111/j.1365-3156.2005.01533.x. [DOI] [PubMed] [Google Scholar]
- 51.Cruz I, Chicharro C, Nieto J, Bailo B, Cañavate C, Figueras MC, Alvar J. 2006. Comparison of new diagnostic tools for management of pediatric Mediterranean visceral leishmaniasis. J Clin Microbiol 44:2343–2347. doi: 10.1128/JCM.02297-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diro E, Techane Y, Tefera T, Assefa Y, Kebede T, Genetu A, Kebede Y, Tesfaye A, Ergicho B, Gebre-Yohannes A, Mengistu G, Engers H, Aseffa A, Desjeux P, Boelaert M, Hailu A. 2007. Field evaluation of FD-DAT, rK39 dipstick and KATEX (urine latex agglutination) for diagnosis of visceral leishmaniasis in northwest Ethiopia. Trans R Soc Trop Med Hyg 101:908–914. doi: 10.1016/j.trstmh.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 53.Riera C, Fisa R, Lopez P, Ribera E, Carrió J, Falcó V, Molina I, Gállego M, Portús M. 2004. Evaluation of a latex agglutination test (KAtex) for detection of Leishmania antigen in urine of patients with HIV-Leishmania coinfection: value in diagnosis and post-treatment follow-up. Eur J Clin Microbiol Infect Dis 23:899–904. doi: 10.1007/s10096-004-1249-7. [DOI] [PubMed] [Google Scholar]
- 54.Sundar S, Singh RK, Bimal SK, Gidwani K, Mishra A, Maurya R, Singh SK, Manandhar KD, Boelaert M, Rai M. 2007. Comparative evaluation of parasitology and serological tests in the diagnosis of visceral leishmaniasis in India: a phase III diagnostic accuracy study. Trop Med Int Health 12:284–289. doi: 10.1111/j.1365-3156.2006.01775.x. [DOI] [PubMed] [Google Scholar]
- 55.Sundar S, Agrawal S, Pai K, Chance M, Hommel M. 2005. Detection of leishmanial antigen in the urine of patients with visceral leishmaniasis by a latex agglutination test. Am J Trop Med Hyg 73:269–271. [PubMed] [Google Scholar]
- 56.Huber C, Batchelor JR, Fuchs D, Hausen A, Lang A, Niederwieser D, Reibnegger G, Swetly P, Troppmair J, Wachter H. 1984. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J Exp Med 160:310–316. doi: 10.1084/jem.160.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schriefer A, Barral A, Carvalho EM, Barral-Netto M. 1995. Serum soluble markers in the evaluation of treatment in human visceral leishmaniasis. Clin Exp Immunol 102:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamerlinck FF, van Gool T, Faber WR, Kager PA. 2000. Serum neopterin concentrations during treatment of leishmaniasis: useful as test of cure? FEMS Immunol Med Microbiol 27:31–34. doi: 10.1111/j.1574-695X.2000.tb01408.x. [DOI] [PubMed] [Google Scholar]
- 59.Khambu B, Mehta KD, Rijal S, Lamsal M, Majhi S, Baral N. 2007. Serum nitrite level and adenosine deaminase activity is altered in visceral leishmaniasis. Nepal Med Coll J 9:40–43. [PubMed] [Google Scholar]
- 60.Tripathi K, Kumar R, Bharti K, Kumar P, Shrivastav R, Sundar S, Pai K. 2008. Adenosine deaminase activity in sera of patients with visceral leishmaniasis in India. Clin Chim Acta 388:135–138. doi: 10.1016/j.cca.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 61.Rai AK, Thakur CP, Velpandian T, Sharma SK, Ghosh B, Mitra DK. 2011. High concentration of adenosine in human visceral leishmaniasis despite increased ADA and decreased CD73. Parasite Immunol 33:632–636. doi: 10.1111/j.1365-3024.2011.01315.x. [DOI] [PubMed] [Google Scholar]
- 62.Baral N, Mehta KD, Chandra L, Lamsal M, Rijal S, Koirala S. 2005. Adenosine deaminase activity in sera of patients with visceral leishmaniasis in Nepal. Trop Doct 35:86–88. doi: 10.1258/0049475054036887. [DOI] [PubMed] [Google Scholar]
- 63.Erel O, Kocyigit A, Gurel MS, Bulut V, Seyrek A, Ozdemir Y. 1998. Adenosine deaminase activities in sera, lymphocytes and granulocytes in patients with cutaneous leishmaniasis. Mem Inst Oswaldo Cruz 93:491–494. doi: 10.1590/S0074-02761998000400014. [DOI] [PubMed] [Google Scholar]
- 64.Gasim S, Elhassan AM, Khalil EA, Ismail A, Kadaru AM, Kharazmi A, Theander TG. 1998. High levels of plasma IL-10 and expression of IL-10 by keratinocytes during visceral leishmaniasis predict subsequent development of post-kala-azar dermal leishmaniasis. Clin Exp Immunol 111:64–69. doi: 10.1046/j.1365-2249.1998.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khoshdel A, Alborzi A, Rosouli M, Taheri E, Kiany S, Javadian MH. 2009. Increased levels of IL-10, IL-12, and IFN-γ in patients with visceral leishmaniasis. Braz J Infect Dis 13:44–46. doi: 10.1590/S1413-86702009000100010. [DOI] [PubMed] [Google Scholar]
- 66.Cillari E, Vitale G, Arcoleo F, D'Agostino P, Mocciaro C, Gambino G, Stassi G, Giordano C, Milano S. 1995. In vivo and in vitro cytokine profiles and mononuclear cell subsets in Sicilian patients with active visceral leishmaniasis. Cytokine 7:740–745. doi: 10.1006/cyto.1995.0088. [DOI] [PubMed] [Google Scholar]
- 67.Hailu A, van der Poll T, Berhe N, Kager PA. 2004. Elevated plasma levels of interferon (IFN)-γ, IFN-γ inducing cytokines and IFN-γ inducible CXC chemokines in visceral leishmaniasis. Am J Trop Med Hyg 71:561–567. [PubMed] [Google Scholar]
- 68.Hailu A, Van Baarle D, Knol GJ, Berhe N, Miedema F, Kager PA. 2005. T cell subset and cytokine profiles in human visceral leishmaniasis during active and asymptomatic or sub-clinical infection with Leishmania donovani. Clin Immunol 117:182–191. doi: 10.1016/j.clim.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 69.Costa ASA, Costa GC, De Aquino DMC, De Mendonça CRR, Barral A, Barral-Netto M, Caldas Ade J. 2012. Cytokines and visceral leishmaniasis: a comparison of plasma cytokine profiles between the clinical forms of visceral leishmaniasis. Mem Inst Oswaldo Cruz 107:735–739. doi: 10.1590/S0074-02762012000600005. [DOI] [PubMed] [Google Scholar]
- 70.Duthie MS, Guderian J, Vallur A, Bhatia A, Lima dos Santos P, Vieira de Melo E, Ribeiro de Jesus A, Todt M, Mondal D, Almeida R, Reed SG. 2014. Alteration of the serum biomarker profiles of visceral leishmaniasis during treatment. Eur J Clin Microbiol Infect Dis 33:639–649. doi: 10.1007/s10096-013-1999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santos-Oliveira JR, Regis EG, Giacoia-Gripp CBW, Valverde JG, Alexandrino-de-Oliveira P, Lindoso JÂ, Goto LH, Oliveira-Neto MP, Guerra JO, Grinsztejn B, Jerônimo SB, Morgado MG, Da-Cruz AM. 2013. Microbial translocation induces an intense proinflammatory response in patients with visceral leishmaniasis and HIV type 1 coinfection. J Infect Dis 208:57–66. doi: 10.1093/infdis/jit135. [DOI] [PubMed] [Google Scholar]
- 72.Santos-Oliveira JR, Regis ER, Leal CRB, Cunha RV, Bozza PT, Da-Cruz AD. 2011. Evidence that lipopolysaccharide may contribute to the cytokine storm and cellular activation in patients with visceral leishmaniasis. PLoS Negl Trop Dis 5:e1198. doi: 10.1371/journal.pntd.0001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Caldas A, Favali C, Aquino D, Vinhas V, Van Weyenbergh J, Brodskyn C, Costa J, Barral-Netto M, Barral A. 2005. Balance of IL-10 and interferon-γ plasma levels in human visceral leishmaniasis: implications in the pathogenesis. BMC Infect Dis 5:113. doi: 10.1186/1471-2334-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ansari NA, Saluja S, Salotra P. 2006. Elevated levels of interferon-γ, interleukin-10, and interleukin-6 during active disease in Indian kala azar. Clin Immunol 119:339–345. doi: 10.1016/j.clim.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 75.Ansari NA, Sharma P, Salotra P. 2007. Circulating nitric oxide and C-reactive protein levels in Indian kala azar patients: correlation with clinical outcome. Clin Immunol 122:343–348. doi: 10.1016/j.clim.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 76.Costa-Silva MF, Gomes LI, Martins-Filho OA, Rodrigues-Silva R, de Moura Freire J, Quaresma PF, Pascoal-Xavier MA, de Oliveira Mendes TA, Serakides R, Zauli DAG, Campi-Azevedo AC, Melo MN, Gontijo CMF, Peruhype-Magalhães V, Teixeira-Carvalho A. 2014. Gene expression profile of cytokines and chemokines in skin lesions from Brazilian Indians with localized cutaneous leishmaniasis. Mol Immunol 57:74–85. doi: 10.1016/j.molimm.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 77.Peruhype-Magalhães V, Martins-Filho OA, Prata A, De Silva AL, Rabello A, Teixeira-Carvalho A, Figueiredo RM, Guimarães-Carvalho SF, Ferrari TCA, Van Weyenbergh J, Correa-Oliveira R. 2006. Mixed inflammatory/regulatory cytokine profile marked by simultaneous raise of interferon-γ and interleukin-10 and low frequency of tumour necrosis factor-α (+) monocytes are hallmarks of active human visceral leishmaniasis due to Leishmania chagasi. Clin Exp Immunol 146:124–132. doi: 10.1111/j.1365-2249.2006.03171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ansari NA, Kumar R, Gautam S, Nylén S, Singh OP, Sundar S, Sacks D. 2011. IL-27 and IL-21 are associated with T cell IL-10 responses in human visceral leishmaniasis. J Immunol 186:3977–3985. doi: 10.4049/jimmunol.1003588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bourreau E, Prévot G, Gardon J, Pradinaud R, Launois P. 2001. High intralesional interleukin-10 messenger RNA expression in localized cutaneous leishmaniasis is associated with unresponsiveness to treatment. J Infect Dis 184:1628–1630. doi: 10.1086/324665. [DOI] [PubMed] [Google Scholar]
- 80.Louzir H, Melby PC, Ben Salah A, Marrakchi H, Aoun K, Ben Ismail R, Dellagi K. 1998. Immunologic determinants of disease evolution in localized cutaneous leishmaniasis due to Leishmania major. J Infect Dis 177:1687–1695. doi: 10.1086/515297. [DOI] [PubMed] [Google Scholar]
- 81.Tuon FF, Gomes-Silva A, Da-Cruz AM, Duarte MIS, Neto VA, Amato VS. 2008. Local immunological factors associated with recurrence of mucosal leishmaniasis. Clin Immunol 128:442–446. doi: 10.1016/j.clim.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 82.Katara GK, Ansari NA, Verma S, Ramesh V, Salotra P. 2011. Foxp3 and IL-10 expression correlates with parasite burden in lesional tissues of post kala azar dermal leishmaniasis (PKDL) patients. PLoS Negl Trop Dis 5:e1171. doi: 10.1371/journal.pntd.0001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gama MEA, Costa JML, Pereira JCR, Gomes CMC, Corbett CEP. 2004. Serum cytokine profile in the subclinical form of visceral leishmaniasis. Braz J Med Biol Res 37:129–136. doi: 10.1590/S0100-879X2004000100018. [DOI] [PubMed] [Google Scholar]
- 84.Cenini P, Berhe N, Hailu A, Mcginnes K, Frommel D. 1993. Mononuclear cell subpopulations and cytokine levels in human visceral leishmaniasis before and after chemotherapy. J Infect Dis 168:986–993. doi: 10.1093/infdis/168.4.986. [DOI] [PubMed] [Google Scholar]
- 85.Barral-Netto M, Badaró R, Barral A, Almeida RP, Santos SB, Badaró F, Pedral-Sampaio D, Carvalho EM, Falcoff E, Falcoff R. 1991. Tumor necrosis factor (cachectin) in human visceral leishmaniasis. J Infect Dis 163:853–857. doi: 10.1093/infdis/163.4.853. [DOI] [PubMed] [Google Scholar]
- 86.Medeiros IM, Reed S, Castelo A, Salomão R. 2000. Circulating levels of sTNFR and discrepancy between cytotoxicity and immunoreactivity of TNF-α in patients with visceral leishmaniasis. Clin Microbiol Infect 6:34–37. doi: 10.1046/j.1469-0691.2000.00011.x. [DOI] [PubMed] [Google Scholar]
- 87.De Oliveira FA, Vanessa Oliveira Silva C, Damascena NP, Passos RO, Duthie MS, Guderian JA, Bhatia A, De Moura TR, Reed SG, De Almeida RP, De Jesus AR. 2013. High levels of soluble CD40 ligand and matrix metalloproteinase-9 in serum are associated with favorable clinical evolution in human visceral leishmaniasis. BMC Infect Dis 13:331. doi: 10.1186/1471-2334-13-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salomão R, Casterlo Filho A, De Medeiros IM, Sicolo MA. 1996. Plasma levels of tumor necrosis factor-alpha in patients with visceral leishmaniasis (kala-azar). Association with activity of the disease and clinical remission following antimonial therapy. Rev Inst Med Trop 38:113–118. [DOI] [PubMed] [Google Scholar]
- 89.Freitas-Teixeira PM, Silveira-Lemos D, Giunchetti RC, Baratta-Masini A, Mayrink W, Peruhype-Magalhães V, Rocha RDR, Campi-Azevedo AC, Teixeira-Carvalho A, Martins-Filho O. 2012. Distinct pattern of immunophenotypic features of innate and adaptive immunity as a putative signature of clinical and laboratorial status of patients with localized cutaneous leishmaniasis. Scand J Immunol 76:421–432. doi: 10.1111/j.1365-3083.2012.02748.x. [DOI] [PubMed] [Google Scholar]
- 90.Kurkjian KM, Mahmutovic AJ, Kellar KL, Haque R, Bern C, Secor WE. 2006. Multiplex analysis of circulating cytokines in the sera of patients with different clinical forms of visceral leishmaniasis. Cytom A 358:353–358. doi: 10.1002/cyto.a.20256. [DOI] [PubMed] [Google Scholar]
- 91.Zijlstra EE, Van der Poll T, Mevissen M. 1995. Soluble receptors for tumor necrosis factor as markers of disease activity in visceral leishmaniasis. J Infect Dis 171:498–501. doi: 10.1093/infdis/171.2.498. [DOI] [PubMed] [Google Scholar]
- 92.Kocyigit A, Gur S, Gurel MS, Bulut V, Ulukanligil M. 2002. Antimonial therapy induces circulating proinflammatory cytokines in patients with cutaneous leishmaniasis. Infect Immun 70:12–15. doi: 10.1128/IAI.70.12.6589-6591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vouldoukis I, Issaly F, Fourcade C, Paul-Eugène N, Arock M, Kolb JP, Alves da Silva O, Monjour L, Poinsot H, Tselentis Y, Dugas B, Debré P, Mossalayi MD. 1994. CD23 and IgE expression during the human immune response to cutaneous leishmaniasis: possible role in monocyte activation. Res Immunol 145:17–27. doi: 10.1016/S0923-2494(94)80037-5. [DOI] [PubMed] [Google Scholar]
- 94.Kocyigit A, Gur S, Erel O, Gurel MS. 2002. Associations among plasma selenium, zinc, copper, and iron concentrations and immunoregulatory cytokine levels in patients with cutaneous leishmaniasis. Biol Trace Elem Res 90:47–55. doi: 10.1385/BTER:90:1-3:47. [DOI] [PubMed] [Google Scholar]
- 95.Castes M, Trujillo D, Rojas ME, Fernandez CT, Arava L, Cabrera M, Blackwell J, Convit J. 1993. Serum levels of tumor necrosis factor in patients with American cutaneous leishmaniasis. Biol Res 26:233–238. [PubMed] [Google Scholar]
- 96.Da-Cruz AM, De Oliveira MP, De Luca PM, Mendonça SCF, Coutinho SG. 1996. Tumor necrosis factor-α in human American tegumentary leishmaniasis. Mem Inst Oswaldo Cruz 91:225–229. doi: 10.1590/S0074-02761996000200019. [DOI] [PubMed] [Google Scholar]
- 97.Gama MEA, De Castro Gomes CM, Silveira FT, Laurenti MD, Da Graça Gonçalves G, Da Silva AR, Corbett CEP. 2013. Severe visceral leishmaniasis in children: the relationship between cytokine patterns and clinical features. Rev Soc Bras Med Trop 46:741–745. doi: 10.1590/0037-8682-0203-2013. [DOI] [PubMed] [Google Scholar]
- 98.Ganguly S, Das NK, Panja M, Pal S, Modak D, Rahaman M, Mallik S, Guha SK, Pramanik N, Goswami R, Barbhuiya JN, Saha B, Chatterjee M. 2008. Increased levels of interleukin-10 and IgG3 are hallmarks of Indian post-kala-azar dermal leishmaniasis. J Infect Dis 197:1762–1771. doi: 10.1086/588387. [DOI] [PubMed] [Google Scholar]
- 99.Medrano FJ, Rey C, Leal M, Cañavate C, Rubio A, Sánchez-Quijano A, Alvar J, Lissen E. 1998. Dynamics of serum cytokines in patients with visceral leishmaniasis and HIV-1 co-infection. Clin Exp Immunol 114:403–407. doi: 10.1046/j.1365-2249.1998.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Van den Bogaart E, Talha AB, Straetemans M, Mens PF, Adams ER, Grobusch MP, Nour BYM, Schallig HDFH. 2014. Cytokine profiles amongst Sudanese patients with visceral leishmaniasis and malaria co-infections. BMC Immunol 15:16. doi: 10.1186/1471-2172-15-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van der Poll T, Zijlstra EE, Mevissen M. 1995. Interleukin 6 during active visceral leishmaniasis and after treatment. Clin Immunol Immunopathol 77:111–114. doi: 10.1016/0090-1229(95)90144-2. [DOI] [PubMed] [Google Scholar]
- 102.Melby PC, Andrade-Narvaez F, Darnell BJ, Valencia-Pacheco G. 1996. In situ expression of interleukin-10 and interleukin-12 in active human cutaneous leishmaniasis. FEMS Immunol Med Microbiol 15:101–107. doi: 10.1111/j.1574-695X.1996.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 103.Noiri E, Hamasaki Y, Negishi K, Sugaya T, Doi K, Fujita T, Osada Y, Matsumoto Y, Jamil K. 2011. The potential of urinary tests in the management of kala-azar, p 60–90. In Jha TK, Noiri E (ed), Kala azar in South Asia: current status and challenges ahead, 1st ed. Springer, New York, NY. [Google Scholar]
- 104.Barral-Netto M, Barral A, Santos SB, Carvalho EM, Badaro R, Rocha H, Reed SG, Johnson WD Jr. 1991. Soluble IL-2 receptor as an agent of serum mediated suppression in human visceral leishmaniasis. J Immunol 147:281–284. [PubMed] [Google Scholar]
- 105.Vitale G, Reina G, Mansueto S, Gambino G, Mocciaro C, D'Agostino R, Dieli M, Cillari E. 1992. The significance of serum soluble IL-2 receptor as a marker for active visceral leishmaniasis in Sicilian patients. Clin Exp Immunol 90:219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sang DK, Ouma JH, John CC, Whalen CC, King CL, Mahmoud AAF, Heinzel FP. 1999. Increased levels of soluble interleukin-4 receptor in the sera of patients with visceral leishmaniasis. J Infect Dis 179:743–746. doi: 10.1086/314635. [DOI] [PubMed] [Google Scholar]
- 107.Wasunna KM, Raynes JG, Werel JB, Muigai R, Gachihi G, Carpenter L, McAdam KP. 1995. Acute phase protein concentrations predict parasite clearance rate during therapy for visceral leishmaniasis. Trans R Soc Trop Med Hyg 89:678–681. doi: 10.1016/0035-9203(95)90442-5. [DOI] [PubMed] [Google Scholar]
- 108.Singh UK, Patwari AK, Sinhs RK, Kumar R. 1999. Prognostic value of serum C-reactive protein in kala-azar. J Trop Pediatr 45:226–228. doi: 10.1093/tropej/45.4.226. [DOI] [PubMed] [Google Scholar]
- 109.Gasim S, Theander TG, Elhassan AM. 2000. High levels of C-reactive protein in the peripheral blood during visceral leishmaniasis predict subsequent development of post kala-azar dermal leishmaniasis. Acta Trop 75:35–38. doi: 10.1016/S0001-706X(99)00089-3. [DOI] [PubMed] [Google Scholar]
- 110.Abebe T, Takele Y, Weldegebreal T, Cloke T, Closs E, Corset C, Hailu A, Hailu W, Sisay Y, Corware K, Corset M, Modolell M, Munder M, Tacchini-Cottier F, Müller I, Kropf P. 2013. Arginase activity—a marker of disease status in patients with visceral leishmaniasis in Ethiopia. PLoS Negl Trop Dis 7:e2134. doi: 10.1371/journal.pntd.0002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Franca-Costa J, Van Weyenbergh J, Boaventura V, Luz NF, Malta-Santos H, Oliveira MC, Santos de Campos DC, Saldanha AC, Dos-Santos WL, Bozza PT, Barral-Netto M, Barral A, Costa JM, Borges VM. 14 August 2014. Arginase I, polyamine and prostaglandin E2 pathways suppress the inflammatory response and contribute to diffuse cutaneous leishmaniasis. J Infect Dis doi: 10.1093/infdis/jiu455. [DOI] [PubMed] [Google Scholar]
- 112.Takele Y, Abebe T, Weldegebreal T, Hailu A, Hailu W, Hurissa Z, Ali J, Diro E, Sisay Y, Modolell M, Munder M, Tacchini-Cottier F, Müller I, Kropf P. 2013. Arginase activity in the blood of patients with visceral leishmaniasis and HIV infection. PLoS Negl Trop Dis 7:e1977. doi: 10.1371/journal.pntd.0001977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mukhopadhyay D, Das NK, Roy S, Kundu S, Barbhuiya JN, Chatterjee M. 2011. Miltefosine effectively modulates the cytokine milieu in Indian post kala-azar dermal leishmaniasis. J Infect Dis 204:1427–1436. doi: 10.1093/infdis/jir551. [DOI] [PubMed] [Google Scholar]
- 114.Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, Alvar J, Boelaert M. 2007. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol 5:873–882. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- 115.Srividya G, Kulshrestha A, Singh R, Salotra P. 2012. Diagnosis of visceral leishmaniasis: developments over the last decade. Parasitol Res 110:1065–1078. doi: 10.1007/s00436-011-2680-1. [DOI] [PubMed] [Google Scholar]
- 116.Abass E, Bollig N, Reinhard K, Camara B, Mansour D, Visekruna A, Lohoff M, Steinhoff U. 2013. rKLO8, a novel Leishmania donovani-derived recombinant immunodominant protein for sensitive detection of visceral leishmaniasis in Sudan. PLoS Negl Trop Dis 7:e2322. doi: 10.1371/journal.pntd.0002322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Souza AP, Soto M, Costa JML, Boaventura VS, de Oliveira CI, Cristal JR, Barral-Netto M, Barral A. 2013. Towards a more precise serological diagnosis of human tegumentary leishmaniasis using leishmania recombinant proteins. PLoS One 8:e66110. doi: 10.1371/journal.pone.0066110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Raj VS, Ghosh A, Dole VS, Madhubala R, Myler PJ, Stuart KD. 1999. Serodiagnosis of leishmaniasis with recombinant ORFF antigen. Am J Trop Med Hyg 61:482–487. [DOI] [PubMed] [Google Scholar]
- 119.Pattabhi S, Whittle J, Mohamath R, El-Safi S, Moulton GG, Guderian JA, Colombara D, Abdoon AO, Mukhtar MM, Mondal D, Esfandiari J, Kumar S, Chun P, Reed SG, Bhatia A. 2010. Design, development and evaluation of rK28-based point-of-care tests for improving rapid diagnosis of visceral leishmaniasis. PLoS Negl Trop Dis 4:e822. doi: 10.1371/journal.pntd.0000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Santarém N, Tomás A, Ouaissi A, Tavares J, Ferreira N, Manso A, Campino L, Correia JM, Cordeiro-da-Silva A. 2005. Antibodies against a Leishmania infantum peroxiredoxin as a possible marker for diagnosis of visceral leishmaniasis and for monitoring the efficacy of treatment. Immunol Lett 101:18–23. doi: 10.1016/j.imlet.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 121.Avila JL, Rojas M, Garcia L. 1988. Persistence of elevated levels of galactosyl-alpha (1-3)galactose antibodies in sera from patients cured of visceral leishmaniasis. J Clin Microbiol 26:1842–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jensen ATR, Gaafar A, Ismail A, Christensen CBV, Kemp M, El Hassan AM, Kharazmi A, Theander. 1996. Serodiagnosis of cutaneous leishmaniasis: assessment of an enzyme-linked immunosorbent assay using a peptide sequence from gene B protein. Am J Trop Med Hyg 55:490–495. [DOI] [PubMed] [Google Scholar]
- 123.Bhatia A, Daifalla NS, Jen S, Badaro R, Reed SG, Skeiky YA. 1999. Cloning, characterization and serological evaluation of K9 and K26: two related hydrophilic antigens of Leishmania chagasii. Mol Biochem Parasitol 102:249–261. doi: 10.1016/S0166-6851(99)00098-5. [DOI] [PubMed] [Google Scholar]
- 124.Mohapatra TM, Singh DP, Sen MR, Bharti K, Sundar S. 2010. Comparative evaluation of rK9, rK26 and rK39 antigens in the serodiagnosis of Indian visceral leishmaniasis. J Infect Dev Ctries 4:114–117. doi: 10.3855/jidc.544. [DOI] [PubMed] [Google Scholar]
- 125.Kumar D, Srividya G, Verma S, Singh R, Negi NS, Fragaki K, Kubar J, Salotra P. 2008. Presence of anti-Lepp12 antibody: a marker for diagnostic and prognostic evaluation of visceral leishmaniasis. Trans R Soc Trop Med Hyg 102:167–171. doi: 10.1016/j.trstmh.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 126.Fragaki K, Ferrua B, Mograbi B, Waldispühl J, Kubar J. 2003. A novel Leishmania infantum nuclear phosphoprotein Lepp12 which stimulates IL1-beta synthesis in THP-1 transfectants. BMC Microbiol 3:7. doi: 10.1186/1471-2180-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rafati S, Hassani N, Taslimi Y, Movassagh H, Rochette A, Papadopoulou B. 2006. Amastin peptide-binding antibodies as biomarkers of active human visceral leishmaniasis. Clin Vaccine Immunol 13:1104–1110. doi: 10.1128/CVI.00188-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sharma V, Chatterjee M, Mandal C, Sen S, Basu D. 1998. Rapid diagnosis of Indian visceral leishmaniasis using AchatininH, a 9-O-acetylated sialic acid binding lectin. Am J Trop Med Hyg 58:551–554. [DOI] [PubMed] [Google Scholar]
- 129.Bandyopadhyay S, Chatterjee M, Pal S, Waller RF, Sundar S, McConville MJ, Mandal C. 2004. Purification, characterization of O-acetylated sialoglycoconjugates-specific IgM, and development of an enzyme-linked immunosorbent assay for diagnosis and follow-up of Indian visceral leishmaniasis patients. Diagn Microbiol Infect Dis 50:15–24. doi: 10.1016/j.diagmicrobio.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 130.Menezes-Souza D, de Oliveira Mendes TA, Nagem RAP, de Oliveira Santos TT, Silva ALT, Santoro MM, de Carvalho SFG, Coelho EAF, Bartholomeu DC, Fujiwara RT. 2014. Mapping B-cell epitopes for the peroxidoxin of Leishmania (Viannia) braziliensis and its potential for the clinical diagnosis of tegumentary and visceral leishmaniasis. PLoS One 9:e99216. doi: 10.1371/journal.pone.0099216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hailu A. 1990. Pre- and post-treatment antibody levels in visceral leishmaniasis. Trans R Soc Trop Med Hyg 84:673–675. doi: 10.1016/0035-9203(90)90141-Z. [DOI] [PubMed] [Google Scholar]
- 132.Houghton RL, Petrescu M, Benson DR, Skeiky YA, Scalone A, Badaró R, Reed SG, Gradoni L. 1998. A cloned antigen (recombinant K39) of Leishmania chagasii diagnostic for visceral leishmaniasis in human immunodeficiency virus type 1 patients and a prognostic indicator for monitoring patients undergoing drug therapy. J Infect Dis 177:1339–1344. doi: 10.1086/515289. [DOI] [PubMed] [Google Scholar]
- 133.De Almeida Silva L, Romero HD, Prata A, Costa RT, Nascimento E, Carvalho SFG, Rodrigues V. 2006. Immunologic tests in patients after clinical cure of visceral leishmaniasis. Am J Trop Med Hyg 75:739–743. [PubMed] [Google Scholar]
- 134.Pereira de Oliveira A, Brelaz de Castro MCA, Ferreira de Almeida A, de Assis Souza M, Coutinho de Oliveira B, Reis LC, Goto H, Felinto de Brito ME, Celeste BJ, Martins-Filho OA, Pereira VRA. 2013. Comparison of flow cytometry and indirect immuno fluorescence assay in the diagnosis and cure criterion after therapy of American tegumentary leishmaniasis by anti-live Leishmania (Viannia) braziliensis immunoglobulin G. J Immunol Methods 387:245–253. doi: 10.1016/j.jim.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 135.Vallur AC, Duthie MS, Reinhart C, Tutterrow Y, Hamano S, Bhaskar KRH, Coler RN, Mondal D, Reed SG. 2013. Biomarkers for intracellular pathogens: establishing tools as vaccine and therapeutic endpoints for visceral leishmaniasis. Clin Microbiol Infect 20:374–383. doi: 10.1111/1469-0691.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Singh DP, Sundar S, Mohapatra TM. 2009. The rK39 strip test is non-predictor of clinical status for kala-azar. BMC Res Notes 2:187. doi: 10.1186/1756-0500-2-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Goswami RP, Das S, Ray Y, Rahman M. 2012. Testing urine samples with rK39 strip as the simplest non-invasive field diagnosis for visceral leishmaniasis: an early report from eastern India. J Postgrad Med 58:180–184. doi: 10.4103/0022-3859.101378. [DOI] [PubMed] [Google Scholar]
- 138.Kumar S, Kumar D, Chakravarty J, Sundar S. 2011. Identification and characterization of a novel, 37-kilodalton Leishmania donovani antigen for diagnosis of Indian visceral leishmaniasis. Clin Vaccine Immunol 18:772–775. doi: 10.1128/CVI.00559-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zijlstra EE, Nur Y, Desjeux P, Khalil EAG, El-Hassan AM, Groen J. 2001. Diagnosing visceral leishmaniasis with the recombinant K39 strip test: experience from the Sudan. Trop Med Int Health 6:108–113. doi: 10.1046/j.1365-3156.2001.00680.x. [DOI] [PubMed] [Google Scholar]
- 140.Kumar R, Pai K, Pathak K, Sundar S. 2001. Enzyme-linked immunosorbent assay for recombinant K39 antigen in diagnosis and prognosis of Indian visceral leishmaniasis. Clin Diagn Lab Immunol 8:1220–1224. doi: 10.1128/CDLI.8.6.1220-1224.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bhattarai NR, Van der Auwera G, Khanal B, De Doncker S, Rijal S, Das ML, Uranw S, Ostyn B, Praet N, Speybroeck N, Picado A, Davies C, Boelaert M, Dujardin J. 2009. PCR and direct agglutination as Leishmania infection markers among healthy Nepalese subjects living in areas endemic for Kala-azar. Trop Med Int Health 14:404–411. doi: 10.1111/j.1365-3156.2009.02242.x. [DOI] [PubMed] [Google Scholar]
- 142.Passos S, Carvalho LP, Orge G, Jerônimo SM, Bezerra G, Soto M, Alonso C, Carvalho EM. 2005. Recombinant Leishmania antigens for serodiagnosis of visceral leishmaniasis. Clin Diagn Lab Immunol 12:1164–1167. doi: 10.1128/CDLI.12.10.1164-1167.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Atta AM, D'Oliveira AJ, Correa J, Atta MLB, Almeida RP, Carvalho EM. 1998. Anti-leishmanial IgE antibodies: a marker of active disease in visceral leishmaniasis. Am J Trop Med Hyg 59:426–430. [DOI] [PubMed] [Google Scholar]
- 144.Anam K, Afrin F, Banerjee D, Pramanik N, Guha SK, Goswami RP, Gupta PN, Saha SK, Ali N. 1999. Immunoglobulin subclass distribution and diagnostic value of Leishmania donovani antigen-specific immunoglobulin G3 in Indian kala-azar patients. Clin Diagn Lab Immunol 6:231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Afchain D, Desjeux P, La Fuente C, Le Ray D, Cesbron JY, Neyrinck JL, Capron A. 1983. Specific IgE antibodies to leishmania braziliensis in patients with mucocutaneous leishmaniasis. Ann Immunol 134:311–319. [DOI] [PubMed] [Google Scholar]
- 146.Saha S, Mazumdar T, Anam K, Ravindran R, Bairagi B, Saha B, Goswami R, Pramanik N, Guha SK, Kar S, Banerjee D, Ali N. 2005. Leishmania promastigote membrane antigen-based enzyme-linked immunosorbent assay and immunoblotting for differential diagnosis of Indian post-kala-azar dermal leishmaniasis. J Clin Microbiol 43:1269–1277. doi: 10.1128/JCM.43.3.1269-1277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rodriguez B, Beatty R, Belli A, Barreto A, Palacios X, Marin F, Harris E. 2007. Atypical cutaneous leishmaniasis cases display elevated. Parasite Immunol 29:277–282. doi: 10.1111/j.1365-3024.2007.00944.x. [DOI] [PubMed] [Google Scholar]
- 148.Elassad AMS, Younisi SA, Siddig M, Grayson J, Petersen E, Ghalib HW. 1994. The significance of blood levels of IgM, IgA, IgG and IgG subclasses in Sudanese visceral leishmaniasis patients. Clin Exp Immunol 95:294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Anam K, Afrin F, Banerjee D, Guha SK, Goswami RP, Saha SK, Ali N. 1999. Differential decline in Leishmania membrane antigen-specific immunoglobulin G (IgG), IgM, IgE, and IgG subclass antibodies in Indian kala-azar patients after chemotherapy. Infect Immun 67:6663–6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gomes IT, Carvalho SFG, Rocha RDR, Peruhype-Magalhães V, Dietze R, Martins-Filho ODA, Lemos EM. 2010. Anti-Leishmania chagasi immunoglobulin G3 detected by flow cytometry for early cure assessment in American visceral leishmaniasis. J Immunol Methods 360:76–83. doi: 10.1016/j.jim.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 151.Fagundes-Silva GA, Vieira-Goncalves R, Nepomuceno MP, de Souza MA, Favoreto S Jr, Oliveira-Neto MP, Da-Cruz AM, Gomes-Silva A. 2012. Decrease in anti-Leishmania IgG3 and IgG1 after cutaneous leishmaniasis lesion healing is correlated with the time of clinical cure. Parasite Immunol 34:486–491. doi: 10.1111/j.1365-3024.2012.01379.x. [DOI] [PubMed] [Google Scholar]
- 152.Ghosh AK, Dasgupta S, Ghose AC. 1995. Immunoglobulin G subclass-specific antileishmanial antibody responses in Indian kala-azar and post-kala-azar dermal leishmaniasis. Clin Diagn Lab Immunol 2:291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Junqueira Pedras M, Orsini M, Castro M, Passos VMA, Rabello A. 2003. Antibody subclass profile against Leishmania braziliensis and Leishmania amazonensis in the diagnosis and follow-up of mucosal leishmaniasis. Diagn Microbiol Infect Dis 47:477–485. doi: 10.1016/S0732-8893(03)00141-X. [DOI] [PubMed] [Google Scholar]
- 154.Mansueto P, Pepe I, Seidita A, Scozzari F, Vitale G, Arcoleo F, Elvira I, Cillari E, Rini GB, Napoli N, Di Rosa S, Mansueto S, Di Fede G. 2012. Significance of persistence of antibodies against Leishmania infantum in Sicilian patients affected by acute visceral leishmaniasis. Clin Exp Immunol 12:127–132. doi: 10.1007/s10238-011-0150-9. [DOI] [PubMed] [Google Scholar]
- 155.Hasker E, Malaviya P, Gidwani K, Picado A, Ostyn B, Kansal S, Singh RP, Singh OP, Chourasia A, Kumar Singh A, Shankar R, Wilson ME, Khanal B, Rijal S, Boelaert M, Sundar S. 2014. Strong association between serological status and probability of progression to clinical visceral leishmaniasis in prospective cohort studies in India and Nepal. PLoS Negl Trop Dis 8:e2657. doi: 10.1371/journal.pntd.0002657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Romero HD, Silva LDA, Silva-Vergara ML, Rodrigues V, Costa RT, Guimarães SF, Alecrim W, Moraes-Souza H, Prata A. 2009. Comparative study of serologic tests for the diagnosis of asymptomatic visceral leishmaniasis in an endemic area. Am J Trop Med Hyg 81:27–33. [PubMed] [Google Scholar]
- 157.Gangneux JP, Poinsignon Y, Donaghy L, Amiot L, Tarte K, Mary C, Robert-Gangneux F. 2013. Indoleamine 2,3-dioxygenase activity as a potential biomarker of immune suppression during visceral leishmaniasis. Innate Immun 19:564–568. doi: 10.1177/1753425912473170. [DOI] [PubMed] [Google Scholar]
- 158.Kager PA, Hack CE, Hannema AJ, Rees PH, Von dem Borne AEGKR. 1982. High C1q levels, low C1s/C1q ratios, and high levels of circulating immune complexes in kala azar. Clin Immun Immunopathol 23:86–93. doi: 10.1016/0090-1229(82)90073-3. [DOI] [PubMed] [Google Scholar]
- 159.Sisay Z, Berhe N, Petros B, Tegbaru B, Messele T, Hailu A, Wolday D. 2011. Serum chemokine profiles in visceral leishmaniasis, HIV and HIV/ visceral leishmaniasis co-infected Ethiopian patients. Ethiop Med J 49:179–186. [PubMed] [Google Scholar]
- 160.Ansari NA, Ramesh V, Salotra P. 2006. Interferon (IFN)-γ, tumor necrosis factor-α, interleukin-6, and IFN-γ receptor 1 are the major immunological determinants associated with post-kala azar dermal leishmaniasis. J Infect Dis 194:958–965. doi: 10.1086/506624. [DOI] [PubMed] [Google Scholar]
- 161.Costa DL, Rocha RL, Carvalho RMA, Lima-Neto AS, Harhay MO, Costa CHN, Barral-Neto M, Barral AP. 2013. Serum cytokines associated with severity and complications of kala-azar. Pathog Glob Health 107:78–87. doi: 10.1179/2047773213Y.0000000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Melby PC, Andrade-Narvaez FJ, Darnell BJ, Valencia-Pacheco G, Tryon VV, Palomo-Cetina A. 1994. Increased expression of proinflammatory cytokines in chronic lesions of human cutaneous leishmaniasis. Infect Immun 62:837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Galdino H Jr, Mandaner AE, Pessoni LL, Soriani FM, Pereira LI, Pinto SA, Duarte FB, Gomes CM, Fleuri AK, Dorta ML, de Oliveira MA, Taxeira MM, Batista AC, Joosten LA, Vieira LQ, Ribeiro-Dias F. 2014. Interleukin 32γ (IL-32γ) is highly expressed in cutaneous and mucosal lesions of American tegumentary Leishmaniasis patients: association with tumor necrosis factor (TNF) and IL-10. BMC Infect Dis 14:249. doi: 10.1186/1471-2334-14-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hoseini SG, Javanmard SH, Hejazi SH, Rafiei L, Zarkesh SH, Karbalaii K, Khamesipour A. 2014. Comparison of immune regulatory factors in acute and chronic lesions of cutaneous leishmaniasis due to Leishmania major. J Res Med Sci 19:S36–S40. [PMC free article] [PubMed] [Google Scholar]
- 165.Milano S, Di Bella G, D'Agostino P, Barbera C, Caruso R, La Rosa M, Ferlazzo V, Vitale G, La Russa C, Gambino G, Chifari N, Mansueto S, Cillari E. 2002. IL-15 in human visceral leishmaniasis caused by Leishmania infantum. Clin Exp Immunol 127:360–365. doi: 10.1046/j.1365-2249.2002.01749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rostan O, Gangneux JP, Piquet-Pellorce C, Manuel C, McKenzie AN, Guiguen C, Samson M, Robert-Gangneux F. 2013. The IL-33/ST2 axis is associated with human visceral leishmaniasis and suppresses Th1 responses in the livers of BALB/c mice infected with Leishmania donovani. mBio 4(5):e00383-13. doi: 10.1128/mBio.00383-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sundar S, Reed SG, Sharma S, Mehrotra A, Murray AW. 1997. Circulating T helper 1 (TH1) cell- and TH2 cell-associated cytokines in Indian patients with visceral leishmaniasis. Am J Trop Med Hyg 56:522–525. [DOI] [PubMed] [Google Scholar]
- 168.Kumar R, Bumb RA, Salotra P. 2009. Correlation of parasitic load with interleukin-4 response in patients with cutaneous leishmaniasis due to Leishmania tropica. FEMS Immunol Med Microbiol 57:239–246. doi: 10.1111/j.1574-695X.2009.00607.x. [DOI] [PubMed] [Google Scholar]
- 169.Latifynia A, Khamesipour A, Bokaie S, Khansari N. 2012. Antioxidants and proinflammatory cytokines in the sera of patients with cutaneous leishmaniasis. Iran J Immunol 9:208–214. [PubMed] [Google Scholar]
- 170.Eidsmo L, Wolday D, Berhe N, Sabri F, Satti I, El Hassan AM, Sundar S, Chiodi F, Akuffo H. 2002. Alteration of Fas and Fas ligand expression during human visceral leishmaniasis. Clin Exp Immunol 130:307–313. doi: 10.1046/j.1365-2249.2002.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zwingenberger K, Harms G, Pedrossa C, Pessoa MC, Scheibenbogen C, Andreesen R. 1991. Generation of cytokines in human visceral leishmaniasis: dissociation of endogenous TNF-alpha and IL-1 beta production. Immunobiology 183:125–132. doi: 10.1016/S0171-2985(11)80192-0. [DOI] [PubMed] [Google Scholar]
- 172.Amato VS, Andrade HFJ, Amato Neto V, Duarte MIS. 2003. Persistence of tumor necrosis factor-alpha in situ after lesion healing in mucosal leishmaniasis. Am J Trop Med Hyg 68:527–528. [DOI] [PubMed] [Google Scholar]
- 173.Donaghy L, Gros F, Amiot L, Mary C, Maillard A, Guiquen C, Gangneux JP. 2007. Elevated levels of soluble non-classical major histocompatibility class I molecule human leucocyte antigen (HLA)-G in the blood of HIV-infected patients with or without visceral leishmaniasis. Clin Exp Immunol 147:236–240. doi: 10.1111/j.1365-2249.2006.03268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vitale G, Mocciaro C, Gambino G, Spinelli A, Giordano C, Stassi G, Arcoleo F, Milano S, Cillari E. 1994. Evaluation of serum levels of soluble CD4, CD8 and beta 2-microglobulin in visceral human leishmaniasis. Clin Exp Immunol 97:280–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ajdary S, Riazi-Rad F, Jafari-Shakib R, Mohebbali RM. 2006. Soluble CD 26/CD 30 levels in visceral leishmaniasis: markers of disease activity. Clin Exp Immunol 145:44–47. doi: 10.1111/j.1365-2249.2006.03105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rai AK, Thakur CP, Kumar P, Mitra DK. 2012. Impaired expression of CD26 compromises T-cell recruitment in human visceral leishmaniasis. Eur J Immunol 42:2782–2791. doi: 10.1002/eji.201141912. [DOI] [PubMed] [Google Scholar]
- 177.Ajdary S, Jafari-Shakib R, Riazi-Rad F, Khamesipour A. 2007. Soluble CD26 and CD30 levels in patients with anthroponotic cutaneous leishmaniasis. J Infect 55:75–78. doi: 10.1016/j.jinf.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 178.Jafari-Shakib R, Shokrgozar MA, Nassiri-Kashani M, Malakafzali B, Nikbin B, Khamesipour A. 2009. Plasma sCD26 and sCD30 levels in cutaneous leishmaniasis. Acta Trop 109:61–63. doi: 10.1016/j.actatropica.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 179.Sipsas N, Sfikakist PP, Sfikakist P, Choremi H, Kordossis T. 1994. Serum concentrations of soluble intercellular adhesion molecule-1 and progress towards disease in patients infected with HIV. J Infect 29:271–282. doi: 10.1016/S0163-4453(94)91151-7. [DOI] [PubMed] [Google Scholar]
- 180.Abebe T, Hailu A, Woldeyes M, Mekonen W, Bilcha K, Cloke T, Fry L, Seich Al Basatena N-K, Corware K, Modolell M, Munder M, Tacchini-Cottier F, Müller I, Kropf P. 2012. Local increase of arginase activity in lesions of patients with cutaneous leishmaniasis in Ethiopia. PLoS Negl Trop Dis 6:e1684. doi: 10.1371/journal.pntd.0001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Baccan GC, Oliveira F, Sousa AD, Cerqueira NA, Costa JML, Barral-Netto M, Barral A. 2011. Hormone levels are associated with clinical markers and cytokine levels in human localized cutaneous leishmaniasis. Brain Behav Immun 25:548–554. doi: 10.1016/j.bbi.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 182.Galindo-Sevilla N, Soto N, Mancilla J, Cerbulo A, Zambrano E, Chavira R, Huerto J. 2007. Low serum levels of dehydroepiandrosterone and cortisol in human diffuse cutaneous leishmaniasis by Leishmania mexicana. Am J Trop Med Hyg 76:566–572. [PubMed] [Google Scholar]
- 183.Bourreau E, Ronet C, Darsissac E, Lise M-C, Sainte Marie D, Clity E, Tacchini-Cottier F, Couppie P, Launois P. 2009. In leishmaniasis due to Leishmania guyanensis infection, distinct intralesional interleukin-10 and Foxp3 mRNA expression are associated with unresponsiveness to treatment. J Infect Dis 199:576–579. doi: 10.1086/596508. [DOI] [PubMed] [Google Scholar]
- 184.Maretti-Mira AC, de Oliveira-Neto MP, Da-Cruz AM, de Oliveira MP, Craft N, Pirmez C. 2011. Therapeutic failure in American cutaneous leishmaniasis is associated with gelatinase activity and cytokine expression. Clin Exp Immunol 163:207–214. doi: 10.1111/j.1365-2249.2010.04285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mukhopadhyay D, Das NK, De Sarkar S, Manna A, Ganguly DN, Barbhuiya JN, Maitra AK, Hazra A, Chatterjee M. 2012. Evaluation of serological markers to monitor the disease status of Indian post kala-azar dermal leishmaniasis. Trans R Soc Trop Med Hyg 106:668–676. doi: 10.1016/j.trstmh.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 186.Serarslan G, Yilmaz HR, Söğüt S. 2005. Serum antioxidant activities, malondialdehyde and nitric oxide levels in human cutaneous leishmaniasis. Clin Exp Dermatol 30:267–271. doi: 10.1111/j.1365-2230.2005.01758.x. [DOI] [PubMed] [Google Scholar]
- 187.Erel O, Kocyigit A, Bulut V, Gurel MS. 1999. Reactive nitrogen and oxygen intermediates in patients with cutaneous leishmaniasis. Mem Inst Oswaldo Cruz 94:179–183. [DOI] [PubMed] [Google Scholar]
- 188.Cabrera M, Rodriguez O, Monsalve I, Tovar R, Hagel I. 2003. Variations in the serum levels of soluble CD23, nitric oxide and IgE across the spectrum of American cutaneous leishmaniasis. Acta Trop 88:145–151. doi: 10.1016/S0001-706X(03)00198-0. [DOI] [PubMed] [Google Scholar]
- 189.Khouri R, Santos GS, Soares G, Costa JM, Barral A, Barral-Netto M, Van Weyenbergh J. 2014. SOD1 plasma level as a biomarker for therapeutic failure in cutaneous leishmaniasis. J Infect Dis 210:306–310. doi: 10.1093/infdis/jiu087. [DOI] [PMC free article] [PubMed] [Google Scholar]


