Abstract
During 2010, Mexico and Brazil implemented policies to enforce existing laws of restricting over-the-counter sales of antibiotics. We determined if the enforcement led to more appropriate antibiotic use by measuring changes in seasonal variation of penicillin use. We used retail quarterly sales data in defined daily doses per 1,000 inhabitant-days (DDD/TID) from IMS Health from the private sector in Mexico and Brazil from the first quarter of 2007 to the first quarter of 2013. This database contains information on volume of antibiotics sold in retail pharmacies using information from wholesalers. We used interrupted time-series models controlling for external factors with the use of antihypertensives with interaction terms to assess changes in trend, level, and variation in use between quarters for total penicillin use and by active substance. The most used penicillin was amoxicillin, followed by amoxicillin-clavulanic acid and ampicillin (minimal use in Brazil). Before the restrictions, the seasonal variation in penicillin use was 1.1 DDD/TID in Mexico and 0.8 DDD/TID in Brazil. In Mexico, we estimated a significant decrease in the seasonal variation of 0.4 DDD/TID after the restriction, mainly due to changes in seasonal variation of amoxicillin and ampicillin. In Brazil, the seasonal variation did not change significantly, overall and in the breakdown by individual active substances. For Mexico, inappropriate penicillin use may have diminished after the restrictions were enforced. For Brazil, increasing use and no change in seasonal variation suggest that further efforts are needed to reduce inappropriate penicillin use.
INTRODUCTION
The inappropriate use of antibiotics by humans is one of the main drivers of antimicrobial resistance (1, 2). Antimicrobial resistance has been increasing worldwide, but the development of new antibiotics has slowed down (3). The combination of these two factors has important public health consequences, such as long periods of treatment against resistant microbes, a switch to second-line treatments with more adverse effects, and longer duration of hospitalization, which are all associated with high costs and increased death rates (3, 4).
Antibacterial resistance is an international problem; consequently, many countries have started taking action to contain it and reduce it. In the previous 15 years, some Latin American countries, such as Chile, Colombia, Venezuela, Brazil, and Mexico, have implemented restrictions on the over-the-counter (OTC) sales of antibiotics, aiming to reduce their use and subsequently improve control of antimicrobial resistance. These OTC sales restrictions imply the requirement of a medical prescription to get antibiotics in private pharmacies and impose fines on the owners of pharmacies for noncompliance.
The impact of the earliest policies was evaluated by Wirtz et al., who showed a decrease in the consumption of antibiotics after the OTC sales restriction by approximately 1 DDD/TID (defined daily dose per 1,000 inhabitant-days) in Colombia, a decrease of 5.5 DDD/TID in Chile, and no decrease in Venezuela (5). Similarly, we evaluated the impact of these restrictions in Mexico and Brazil, where the banning of OTC sales of antibiotics was reinforced during 2010. We found a direct decrease in the level of the overall consumption of antibiotics of about 1 DDD/TID in both countries, without changes in the trends of consumption. In Mexico, penicillin use decreased by 0.86 DDD/TID and sulfonamide use decreased by 0.17 DDD/TID. In Brazil, penicillin use decreased by 0.64 DDD/TID, sulfonamide use by 0.41 DDD/TID, and macrolide use by 0.47 DDD/TID (6). In both countries, no shift toward use of other classes of antibiotics, such as quinolones, macrolides, and tetracyclines, was observed. An interesting finding in the previous evaluation was that seasonal variation appeared to change after the restrictions took place, but this was not explored in more detail. Seasonal variation in antibiotic use has been associated with short-term lowering of resistance rates in the United States (7) and Israel (8); moreover, low seasonal variation has been related to appropriate-use profiles in Europe (9, 10). Furthermore, it has been suggested that a better understanding of seasonal variation of antibiotic prescribing can be useful in the design of interventions to reduce inappropriate use of antibiotics (11).
To determine if the OTC sales restrictions led to a more appropriate use of antibiotics in Mexico and Brazil, we measured the changes in the seasonal variation in penicillin use before and after the OTC sales restrictions. We focused on the consumption of penicillins because they are the most frequently used class of antibiotics in the selected countries. Additionally, seasonal variation in their use and high rates of self-medication have been reported previously (5, 6, 12, 13).
MATERIALS AND METHODS
Data source and setting.
We used retail quarterly sales data from the private sectors in Mexico and Brazil provided by IMS Health. The data were obtained by submitting a research protocol to IMS Health under their Global Health Research program explaining the objectives and methods of the present study. IMS Health constructed the database with information of surveys done regularly at various stages of the pharmaceutical chain. The results of the surveys are projected by IMS Health to approximate total volume of sales per country. More information about IMS Health methods can be found at http://www.imshealth.com/deployedfiles/ims/Global/Content/Insights/IMS Institute for Healthcare Informatics/Global Health Research Program/Data_Sources_Global_Research.pdf and http://www.pharmaceuticalpolicy.nl/Presentations/Winter Meeting 2010/Gieshoff, Andreas.pdf.
According to IMS Health reports, pharmaceutical volume coverage was 46% for Mexico and 72% for Brazil (IMS Health, Pharmaceutical Market, Mexico and Brazil 2008, unpublished report).
The data received were expressed as kilograms per active substance of antibiotics (ATC code J01) and antihypertensives (ATC codes C02 [antihypertensives], C03 [diuretics], C07 [beta blocking agents], C08 [calcium channel blockers], and C09 [agents acting on the renin-angiotensin system]) as a reference group, from the first quarter of 2007 to the first quarter of 2013.
We converted the kilograms of each antibiotic and antihypertensive sold into a daily defined dose per 1,000 inhabitant-days (DDD/TID) according to the anatomical therapeutic chemical (ATC) classification system proposed by the World Health Organization (14). We used as the denominator the entire population of each country, which was estimated based on the growth rate per year using the annual information on the population of both countries from the Pan American Health Organization records (available at http://www.paho.org/English/SHA/coredata/tabulator/newTabulator.htm).
Data analysis.
To measure the impact of the policy implementation on the use of penicillins in each country, we used an interrupted-time-series analysis (15) with robust standard errors for each of the most commonly used penicillins in both countries. The penicillins that are most commonly used were identified by calculating the percentage of use 2 years prior and 2 years after the introduction of the restrictions in both countries. In the interrupted-time-series analysis, the antihypertensive group was used as a reference to account for external changes that may affect the consumption of medicines, such as economic growth, changes in coverage of IMS health data, and modifications in the structure of health systems.
We estimated whether the difference in use between quarters (or seasons) changed after the restriction with a set of interaction terms with dummy variables with value of 1 for autumn, winter, and spring. We chose the quarter that corresponds to the summer season as a reference (dummy with value of zero) to evaluate the changes in seasonal variation (differences in the average use between autumn and winter compared to the average use during summer). The summer season takes place during the third quarter of each year in Mexico and during the first quarter of each year in Brazil.
The banning of OTC sales of antibiotics went into effect in Mexico on 25 August 2010 and in Brazil on 29 November of the same year; therefore, we marked the beginning of the regulated consumption for Mexico as the last quarter of 2010 and for Brazil as the first quarter of 2011. The data for the quarters when the restrictions started were not included in the analysis, because these periods were only partially affected by the restriction of antibiotic sales. For each model, we examined the autocorrelation of residuals and corrected it, if present, using autoregressive models. All the analyses were conducted using STATA software, version 12 (Stata Corp LP, TX).
RESULTS
General trends in the use of penicillins.
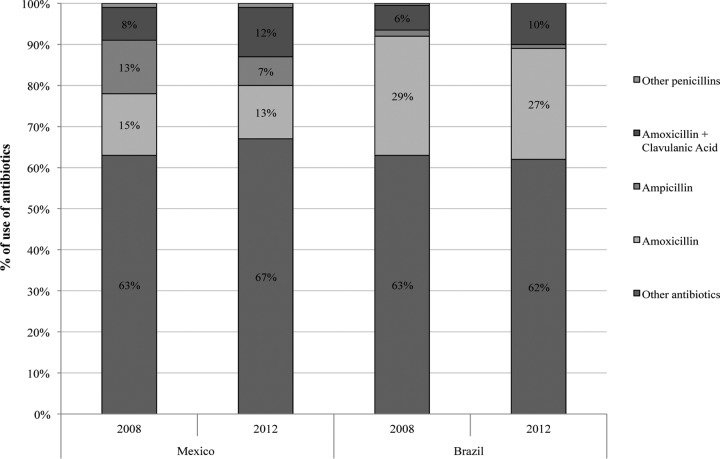
The proportion of the use of penicillins among all antibiotics was fairly similar between the two countries, being 33% to 38% of the total consumption of antibiotics (Fig. 1). Overall, amoxicillin and ampicillin were the most commonly used active substances, but the proportions of the use of these substances differed between countries. In 2008, 2 years before the implementation of the OTC restrictions, the use of amoxicillin alone represented 15% of overall antibiotic use in Mexico and 29% in Brazil; the use of amoxicillin in combination with clavulanic acid represented 8% in Mexico and 6% in Brazil. Ampicillin was frequently used in Mexico (13%), but in Brazil the use was below 2%. In 2012, 2 years after the implementation of the OTC restrictions, the proportion of the use of amoxicillin had decreased by 2%, while the use of amoxicillin with clavulanic acid had increased by 4% in both countries. The use of ampicillin had decreased by 5% in Mexico alone.
FIG 1.
Percentage of use of antibiotics by active substance 2 years before and after the OTC restrictions took effect.
A seasonal variation in the overall use of penicillins was clearly visible, with higher use during winter and autumn seasons (Fig. 2a and b). The time series analysis showed that before the OTC restriction, Mexico presented a seasonal variation (difference in the use between autumn-winter and summer) in the overall use of penicillins of 1.04 DDD/TID in winter and 1.23 DDD/TID in autumn; for Brazil, this difference was significant only in autumn, with 0.81 DDD/TID (Table 1).
FIG 2.

Overall penicillin use before and after the 2010 OTC restrictions indicating changes in level and trend of use and seasonal variation, in Mexico (a) and Brazil (b). No change in trend was observed (dotted line). Open dots indicate summer seasons, which were taken as references to calculate seasonal variation.
TABLE 1.
Difference in the use of penicillins (expressed as DDD/TID) in autumn, winter, and spring versus summer before the OTC restrictionsa
| Penicillin(s) | DDD/TID (95% CI) |
|||||
|---|---|---|---|---|---|---|
| Mexico |
Brazil |
|||||
| Winter | Spring | Autumn | Winter | Spring | Autumn | |
| All penicillins | 1.039** (0.808–1.271) | 0.060 (−0.210–0.33) | 1.226** (0.981–1.472) | 0.880 (−0.135–1.896) | 0.265 (−0.462–0.993) | 0.809* (0.017–1.601) |
| Amoxicillin | 0.472** (0.303–0.641) | 0.021 (−0.148–0.191) | 0.589** (0.456–0.721) | 0.629 (−0.106–1.363) | 0.184 (−0.344–0.713) | 0.615* (0.036–1.193) |
| Amoxicillin + clavulanic acid | 0.293** (0.200–0.386) | 0.050 (−0.029–0.129) | 0.352** (0.266–0.439) | 0.251 (−0.027–0.528) | 0.085 (−0.116–0.286) | 0.196 (−0.025–0.416) |
| Ampicillin | 0.255** (0.180–0.331) | −0.004 (−0.070–0.063) | 0.259** (0.146–0.371) | −0.001 (−0.012–0.011) | −0.003 (−0.014–0.007) | −0.003 (−0.013–0.008) |
| Other penicillins | 0.019 (−0.012–0.049) | −0.007 (−0.039–0.025) | 0.027 (−0.011–0.064) | 0.002* (0.000–0.004) | −0.001 (−0.002–0.001) | 0.001 (−0.001–0.003) |
DDD/TID, defined daily dose per 1,000 inhabitant-days; *, significant at 5%; **, significant at 1%; 95% CI, robust 95% confidence interval.
After the OTC restriction, we estimated a significant reduction in the seasonal variation in Mexico of −0.36 DDD/TID in winter and −0.47 DDD/TID in autumn (Table 2), whereas in Brazil, no significant change in the seasonal variation was observed (Table 2). In addition to this, the analyses showed a similar reduction in overall level of use, −0.94 DDD/TID in Mexico and −0.81 DDD/TID in Brazil, without a change in trend in both countries.
TABLE 2.
Changes in the difference in the use of penicillins (expressed as DDD/TID) between autumn, winter and spring versus summer after the OTC restrictions and changes in level and trend of usea
| Country and penicillin(s) | DDD/TID (95% CI) |
Trend | |||
|---|---|---|---|---|---|
| Winter | Spring | Autumn | Level | ||
| Mexico | |||||
| All penicillins | −0.359** (−0.613 to −0.105) | −0.032 (−0.309 to 0.245) | −0.469* (−0.817 to −0.121) | −0.938* (−1.825 to −0.051) | 0.027 (0.094 to 0.148) |
| Amoxicillin | −0.170 (−0.352 to 0.011) | −0.009 (−0.180 to 0.162) | −0.186* (−0.361 to −0.011) | −0.441 (−0.889 to 0.008) | 0.020 (−0.042 to 0.082) |
| Amoxicillin + clavulanic acid | 0.045 (−0.129 to 0.219) | −0.052 (−0.139 to 0.035) | −0.023 (−0.173 to 0.127) | −0.001 (−0.371 to 0.369) | 0.027 (−0.029 to 0.083) |
| Ampicillin | −0.229** (−0.364 to −0.095) | 0.021 (−0.056 to 0.098) | −0.247** (−0.398 to −0.096) | −0.474** (−0.676 to −0.272) | 0.017 (−0.010 to 0.044) |
| Other penicillins | −0.005 (−0.036 to 0.027) | 0.008 (−0.024 to 0.040) | −0.013 (−0.051 to 0.025) | −0.022 (−0.164 to 0.120) | 0.023*(0.004 to 0.043) |
| Brazil | |||||
| All penicillins | 0.077 (−1.142 to 1.297) | 0.388 (−0.532 to 1.308) | −0.000 (−1.009 to 1.008) | −0.814 (−1.670 to −0.042) | 0.023 (−0.114 to 0.160) |
| Amoxicillin | −0.012 (−0.883 to 0.858) | 0.267 (−0.407 to 0.941) | −0.088 (−0.808 to 0.631) | −0.843* (−1.474 to −0.213) | 0.032 (−0.066 to 0.130) |
| Amoxicillin + clavulanic acid | 0.085 (−0.252 to 0.421) | 0.118 (−0.117 to 0.352) | 0.086 (−0.198 to 0.370) | 0.147 (−0.519 to 0.224) | 0.034 (−0.023 to 0.091) |
| Ampicillin | 0.004 (−0.021 to 0.029) | 0.000 (−0.031 to 0.032) | 0.002 (−0.025 to 0.029) | −0.254 (−0.526 to 0.018) | 0.049* (0.011 to 0.086) |
| Other penicillins | 0.001 (−0.003 to 0.005) | 0.003 (−0.001 to 0.006) | −0.000 (−0.005 to 0.004) | −0.230 (−0.502 to 0.042) | 0.046* (0.009 to 0.084) |
DDD/TID, defined daily dose per 1,000 inhabitant-days; *, significant at 5%; **, significant at 1%; 95% CI, robust 95% confidence interval.
Seasonal variation in individual penicillin use.
Amoxicillin was the main driver of the seasonal variation in both countries. In Mexico, the seasonal variation before the OTC restriction was significant for autumn, with 0.47 DDD/TID, and for winter, with 0.59 DDD/TID. In Brazil, the seasonal variation was significant only for autumn, with a 0.62-DDD/TID difference (Table 1; also, see Fig. S1 and S2 in the supplemental material). After the OTC restriction, Mexico showed a reduction of 33% (−0.17 DDD/TID) in the seasonal variation for the autumn season only (Table 2). Brazil did not show a significant change in seasonal variation (Table 2). Mexico showed a marginally significant reduction in the level of use of 0.44 DDD/TID (P = 0.054). In contrast, Brazil had a significant reduction of 0.84 DDD/TID (P = 0.010) in the level of use of amoxicillin.
In Mexico, amoxicillin in combination with clavulanic acid presented a significant seasonal variation before the OTC restriction, with 0.35-DDD/TID-higher use in autumn and 0.29-DDD/TID-higher use in winter. In Brazil, the seasonal variation was not significant. After the OTC restrictions in both countries, this combination did not have a significant change in seasonal variation, level, or trend of use (Table 2).
The use of ampicillin was the highest in Mexico, with a level of use of about 1.2 DDD/TID; in Brazil, it was only 0.10 DDD/TID (Table 1; also, see Fig. S5 and S6 in the supplemental material). In Mexico, a seasonal variation in the use of this active substance was observed before the restriction, with a difference of almost 0.3 DDD/TID for both autumn and winter compared with use in summer. The seasonal variation completely disappeared after the restriction together with a significant change in level of 0.47 DDD/TID. In Brazil, changes in the seasonal variation and level of use were not observed, but a significant change of 0.05 DDD/TID per quarter in the trend of use was estimated.
DISCUSSION
The objective of this study was to measure the changes in the seasonal variation of penicillin use before and after the OTC restrictions in Mexico and Brazil. The seasonal variation can be seen as a proxy of appropriate use of antibiotics. We showed that after the OTC restrictions, the seasonal variation in the use of penicillins in Mexico decreased by 63%, whereas the seasonal variation in Brazil did not show significant changes. In Mexico, significant decreases in seasonal variation in the use of both amoxicillin (−34%) and ampicillin (−93%) were the main drivers of the overall seasonal reduction. In Brazil, none of the active substances had a significant change in their seasonal variation.
A low seasonal variation together with a low use has been connected to appropriate use of antibiotics and low antimicrobial resistance rates (2, 9, 10). In the present study, Mexico and Brazil showed seasonal variations in the use of penicillins with a higher use in autumn and winter than in summer. We estimated that the mean difference in use before the OTC restrictions was 1.1 DDD/TID for Mexico and 0.7 DDD/TID for Brazil, which corresponds to a 46% and 39% difference, respectively, between the mean consumption in summer and the mean consumption in winter. Previous studies have found that northern European countries, where inappropriate use of antibiotics is lower, showed a mean difference of 23% in consumption of antibiotics between winter and summer. In contrast, southern European countries, where inappropriate use of antibiotics is higher, showed a mean difference of 38% of consumption of antibiotics between seasons (2, 9). If we follow the same method for our data from Mexico and Brazil, the inappropriateness of penicillin use was similar to that in southern Europe 2 years before the OTC restrictions but decreased by 9% in Mexico and increased 1% in Brazil after the OTC restrictions.
The limitation of using this method to assess seasonality is the impossibility of determining whether the changes (if any) in the seasonal variation were significant after the OTC restrictions. Therefore, in the present work, we measured changes in the seasonal variation by adding interactions with dummy variables to an interrupted-time-series model. Following this method, we found that for Mexico the significant change in seasonal variation of overall use of penicillin from 1.1 DDD/TID to 0.7 DDD/TID was mainly due to the reduction in the seasonal variation of two active substances; amoxicillin, with a decrease of 34%, and ampicillin, with a decrease of 93%, indicating a more appropriate use of these medicines after the OTC restriction. A key question is what might explain these findings. A possible explanation is that self-medication with antibiotics is expected to take place to solve upper respiratory infections (URI). The use of amoxicillin in combination with clavulanic acid is recommended in international and national guidelines to treat URI caused by bacteria, in both children and adults (16, 17), because this combination increases the coverage to include both ampicillin-resistant Haemophilus influenzae and Moraxella catarrhalis (16). Therefore, the use of this combination would increase to some extent during autumn-winter seasons, when a high incidence of this type of infection occurs. Our analyses did not show changes in the level of use and seasonal variation in both countries, suggesting that this combination was not frequently sold OTC before the restriction of sales. Moreover, amoxicillin alone is also included in the clinical guidelines to treat URI (16), though it seemed that this active substance was consumed inappropriately before the OTC restriction in Mexico, since this country exhibited a decrease in the seasonal variation (just for autumn) and a marginal reduction in the level of use. Contrarily, ampicillin—not widely recommended for URI, because several bacteria such as H. influenzae, are already resistant to it (16)—showed large seasonal variation before the restriction of antibiotic sales was enforced. The seasonal variation of this active substance vanished after the OTC restriction in Mexico, suggesting that this drop in use was mainly due to the reduction of self-medication and inappropriate use. This result is in line with previous reports of a high rate (78%) of self-medication in Mexico (http://opinionpublicauvm.mx/automedicacion/infografia) and with the finding that ampicillin was one of the most consumed medicines in 2010 (http://www.eluniversal.com.mx/notas/668756.html) (18), as a consequence of which a high rate of resistance to this active substance has been observed (19).
Contrary to our expectations, we did not find a significant difference in the overall use of penicillins between seasons in Brazil or by active substance. This unexpected result, together with the increasing trend in the use of penicillins in Brazil over time, may be due to the inclusion of the Farmacia Popular program, which has the objective of facilitating access to medicines for the entire population (20). The effect of this program was partially controlled for by taking into account the trend of the use of antihypertensives. However, given that the program had differential subsidies for medicines for chronic diseases, this could cause a differential effect in the use between therapeutic groups. Therefore, more research is needed to evaluate the effect of the Farmacia Popular program in Brazil in the use of different therapeutic groups. Furthermore, in 2013, Brazil tightened its regulations of antibiotic sales by registering the sales of antibiotics on the controlled-medicines electronic system, indicating the difficulties of enforcing the OTC restrictions between 2010 and 2013 (21). Contrary to what occurred in Brazil, there was not further enforcement such as this in Mexico.
In preliminary stages of this research, we also explored seasonal variation in other frequently used therapeutic groups in both countries. We did not find seasonal variation in the use of tetracyclines and sulfonamides in Mexico and tetracyclines, quinolones, and sulfonamides in Brazil. The seasonal variation in quinolones in Mexico and macrolides in Brazil did not show significant changes after the restrictions took effect. In Mexico, macrolides had a decrease in the seasonal variation of 0.052 DDD/TID in autumn compared to summer. We expected these results, because these classes of antibiotics are less frequently used for self-medication (12, 13).
A possible limitation of the data is the underestimation of antibiotic use in the whole country, since we focused just on private-sector consumption. Nevertheless, the use of these data from the private sector allowed us to assess the changes in use of antibiotics in the sector where self-medication is relevant and where the policy was implemented; self-medication is less likely to happen in the public sector, where pharmacies dispense only with demonstration of a prescription. Since we looked at relative changes over time and because we corrected for changes in the coverage by adjusting for antihypertensive use focusing only on the private sector, this limitation does not affect the overall results. The use of IMS Health data allowed us to make a comparison of the same type of sales restrictions between countries.
To the best of our knowledge, no previous work has estimated the impact of OTC restrictions on seasonal variation in the use of penicillins. This measure could be helpful to determine the impact of policy changes on the rational use of antibiotics, which could be reinforced using information campaigns to guide patients to seek treatment and avoid self-medication. Information campaigns have been a key factor in the success of the results of a similar policy in Chile (22, 23), but there was no implementation of such campaigns in Mexico and Brazil. Our results have important policy implications, because the evaluation of these policies can help decision makers to take corrective actions if needed as well as monitoring the progress of this policy change. The main objective of OTC restrictions implemented in Mexico and Brazil was to reduce the use of antibiotics in the general population. The requirement of a medical prescription to get antibiotics in private pharmacies was aimed at preventing self-medication with this therapeutic group and consequently controlling antibacterial resistance. We suggest that, in addition to the evaluation of changes in level and trend of use, it is important to examine changes in seasonal variation, because this adds information on inappropriateness of use, such as self-medication behavior after a restriction of OTC sales of antibiotics goes into effect.
The policies to restrict OTC sales of antibiotics led to a decrease in seasonal variation in Mexico but not in Brazil, which may indicate that inappropriate use of penicillins diminished after the restrictions were enforced in Mexico. For Brazil, the increasing use of penicillins together with no change in seasonal variation suggests that further efforts are needed to reduce their inappropriate use.
Supplementary Material
ACKNOWLEDGMENTS
We are very grateful to IMS Health for data provision as well to ANVISA staff for data finding clarification.
WHO Collaborating Centre for Pharmaceutical Policy & Regulation receives no direct funding or donations from private parties, including the pharmaceutical industry. Research funding from public-private partnerships, e.g., IMI, TI Pharma (www.tipharma.nl) is accepted under the condition that no company-specific product or company-related study is conducted. The Centre has received unrestricted research funding from public sources, e.g., The Netherlands Organisation for Health Research and Development (ZonMW), the Dutch Health Care Insurance Board (CVZ), EU 7th Framework Program (FP7), the Dutch Medicines Evaluation Board (MEB), and the Dutch Ministry of Health.
This work was supported by CONACYT (National Council of Science and Technology Mexico) (YS scholarship 200794).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.03629-14.
REFERENCES
- 1.Austin DJ, Kristinsson KG, Anderson RM. 1999. The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proc Natl Acad Sci U S A 96:1152–1156. doi: 10.1073/pnas.96.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goossens H, Ferech M, Vander Stichele R, Elseviers M. 2005. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 365:579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 3.Laxminarayan R, Duse A, Wattal C, Zaidi AKM, Wertheim HFL, Sumpradit N, Vlieghe E, Hara GL, Gould IM, Goossens H, Greko C, So AD, Bigdeli M, Tomson G, Woodhouse W, Ombaka E, Peralta AQ, Qamar FN, Mir F, Kariuki S, Bhutta ZA, Coates A, Bergstrom R, Wright GD, Brown ED, Cars O. 2013. Antibiotic resistance-the need for global solutions. Lancet Infect Dis 13:1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 4.Wise R, Hart T, Cars O, Streulens M, Helmuth R, Huovinen P, Sprenger M. 1998. Antimicrobial resistance. BMJ 317:609–610. doi: 10.1136/bmj.317.7159.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wirtz VJ, Herrera-Patino JJ, Santa-Ana-Tellez Y, Dreser A, Elseviers M, Vander Stichele RH. 2013. Analysing policy interventions to prohibit over-the-counter antibiotic sales in four Latin American countries. Trop Med Int Health 18:665–673. doi: 10.1111/tmi.12096. [DOI] [PubMed] [Google Scholar]
- 6.Santa-Ana-Tellez Y, Mantel-Teeuwisse AK, Dreser A, Leufkens HGM, Wirtz VJ. 2013. Impact of over-the-counter restrictions on antibiotic consumption in Brazil and Mexico. PLoS One 8:e75550. doi: 10.1371/journal.pone.0075550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun L, Klein EY, Laxminarayan R. 2012. Seasonality and temporal correlation between community antibiotic use and resistance in the United States. Clin Infect Dis 55:687–694. doi: 10.1093/cid/cis509. [DOI] [PubMed] [Google Scholar]
- 8.Dagan R, Barkai G, Givon-Lavi N, Sharf AZ, Vardy D, Cohen T, Lipsitch M, Greenberg D. 2008. Seasonality of antibiotic-resistant streptococcus pneumoniae that causes acute otitis media: a clue for an antibiotic-restriction policy? J Infect Dis 197:1094–1102. doi: 10.1086/528995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elseviers MM, Ferech M, Vander Stichele RH, Goossens H. 2007. Antibiotic use in ambulatory care in Europe (ESAC data 1997-2002): trends, regional differences and seasonal fluctuations. Pharmacoepidemiol Drug Saf 16:115–123. doi: 10.1002/pds.1244. [DOI] [PubMed] [Google Scholar]
- 10.Achermann R, Suter K, Kronenberg A, Gyger P, Mühlemann K, Zimmerli W, Bucher HC. 2011. Antibiotic use in adult outpatients in Switzerland in relation to regions, seasonality and point of care tests. Clin Microbiol Infect 17:855–861. doi: 10.1111/j.1469-0691.2010.03348.x. [DOI] [PubMed] [Google Scholar]
- 11.Suda KJ, Hicks LA, Roberts RM, Hunkler RJ, Taylor TH. 2014. Trends and seasonal variation in outpatient antibiotic prescription rates in the United States, 2006 to 2010. Antimicrob Agents Chemother 58:2763–2766. doi: 10.1128/AAC.02239-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grigoryan L, Monnet DL, Haaijer-Ruskamp FM, Bonten MJM, Lundborg S, Verheij TJM. 2010. Self-medication with antibiotics in Europe: a case for action. Curr Drug Saf 5:329–332. doi: 10.2174/157488610792246046. [DOI] [PubMed] [Google Scholar]
- 13.Al-Azzam S, Al-Husein B, Alzoubi F, Masadeh M, Al-Horani Mohammad Ali. 2007. Self-medication with antibiotics in Jordanian population. Int J Occup Med Environ Health 20:373–380. doi: 10.2478/v10001-007-0038-9. [DOI] [PubMed] [Google Scholar]
- 14.WHO International Working Group for Drug Statistics Methodology WCC for DSM, WHO Collaborating Centre for Drug Utilization Research and Clinical Pharmacological Services. 2003. Introduction to drug utilization research. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 15.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. 2002. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 16.Chow AW, Benninger MS, Brook I, Brozek JL, Goldstein EJC, Hicks LA, Pankey GA, Seleznick M, Volturo G, Wald ER, File TM, Infectious Diseases Society of America . 2012. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis 54:e72–e112. doi: 10.1093/cid/cis370. [DOI] [PubMed] [Google Scholar]
- 17.CENETEC. 2009. Guía de Práctica Clinica—Infección Aguda de Vías Aéreas Superiores. CENETEC, Mexico City, Mexico. [Google Scholar]
- 18.Wirtz VJ, Dreser A, Silva J, Echaniz G, Velazquez ME, Garza U, Llerenas A, Ruelas G, Leyva-Flores R. 2007. Patrones de prescripción médica y de auto prescripción de antibióticos en Morelos 2006. Comisión Coordinadora de Institutos Nacionales de Salud y Hospitales de Alta Especialidad, Mexico City, Mexico. [Google Scholar]
- 19.Arredondo-García JL, Amábile-Cuevas CF. 2008. High resistance prevalence towards ampicillin, co-trimoxazole and ciprofloxacin, among uropathogenic Escherichia coli isolates in Mexico City. J Infect Dev Ctries 2:350–353. doi: 10.3855/jidc.195. [DOI] [PubMed] [Google Scholar]
- 20.Santos-Pinto CDB, do Rosário-Costa N, García Serpa Osorio-de-Castro C. 2011. The “Farmácia Popular do Brasil” Program and aspects of public provision of medicines in Brazil. Cien Saude Colet 16:2963–2973. (In Portuguese.) doi: 10.1590/S1413-81232011000600034. [DOI] [PubMed] [Google Scholar]
- 21.ANVISA. 17 January 2013, posting date Definida nova data para controle eletrônico de antibióticos. ANVISA, Brasilia, Brazil. [Google Scholar]
- 22.Bavestrello L, Cabello A, Casanova D. 2002. Impact of regulatory measures in the trends of community consumption of antibiotics in Chile. Rev Med Chil 130:1265–1272. doi: 10.4067/S0034-98872002001100009. [DOI] [PubMed] [Google Scholar]
- 23.Bavestrello FL, Cabello MÁ. 2011. Consumo comunitario de antimicrobianos en Chile, 2000-2008. Rev Chil Infectol 28:107–112. doi: 10.4067/S0716-10182011000200001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.